Metabolic Profiling of Fermented Products of the Ethanolic Extract of Acanthopanax sessiliflorus Fruit and Evaluation of Its Immune Enhancement Effect in RAW 264.7 Macrophages and BV2 Microglia
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of ASE and Its Fermented Products and Evaluation of Probiotic Growth Using Fermentation with ASE
2.3. Cell Culture
2.4. MTT Assay for Cell Viability
2.5. Determination of Nitrite (NO Production)
2.6. Assays for IL-6 and TNF-α
2.7. Western Blot Analysis
2.8. Preparation of Cytosolic and Nuclear Extract
2.9. Statistical Analysis
2.10. UPLC-QTOF/MS-Based Secondary Metabolite Profiling of ASE and Its Fermented Products
3. Results
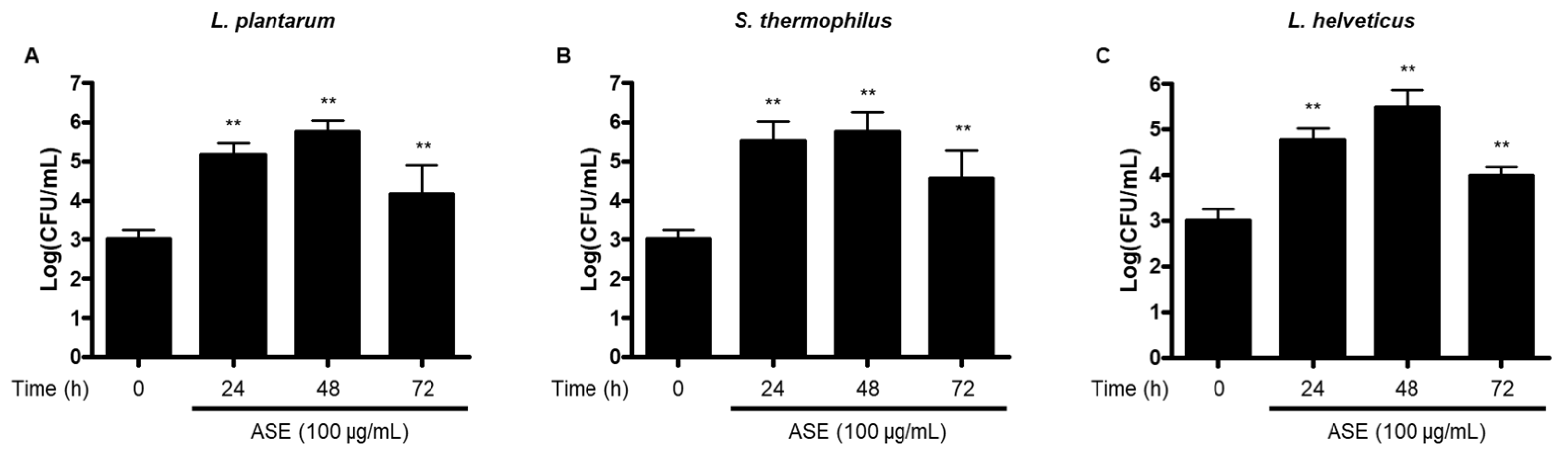
3.1. Growth of Probiotic Strains Using Fermentation with ASE
3.2. Effect of ASE and Its Fermented Products on Viability of RAW264.7 Macrophages and BV2 Microglial Cells
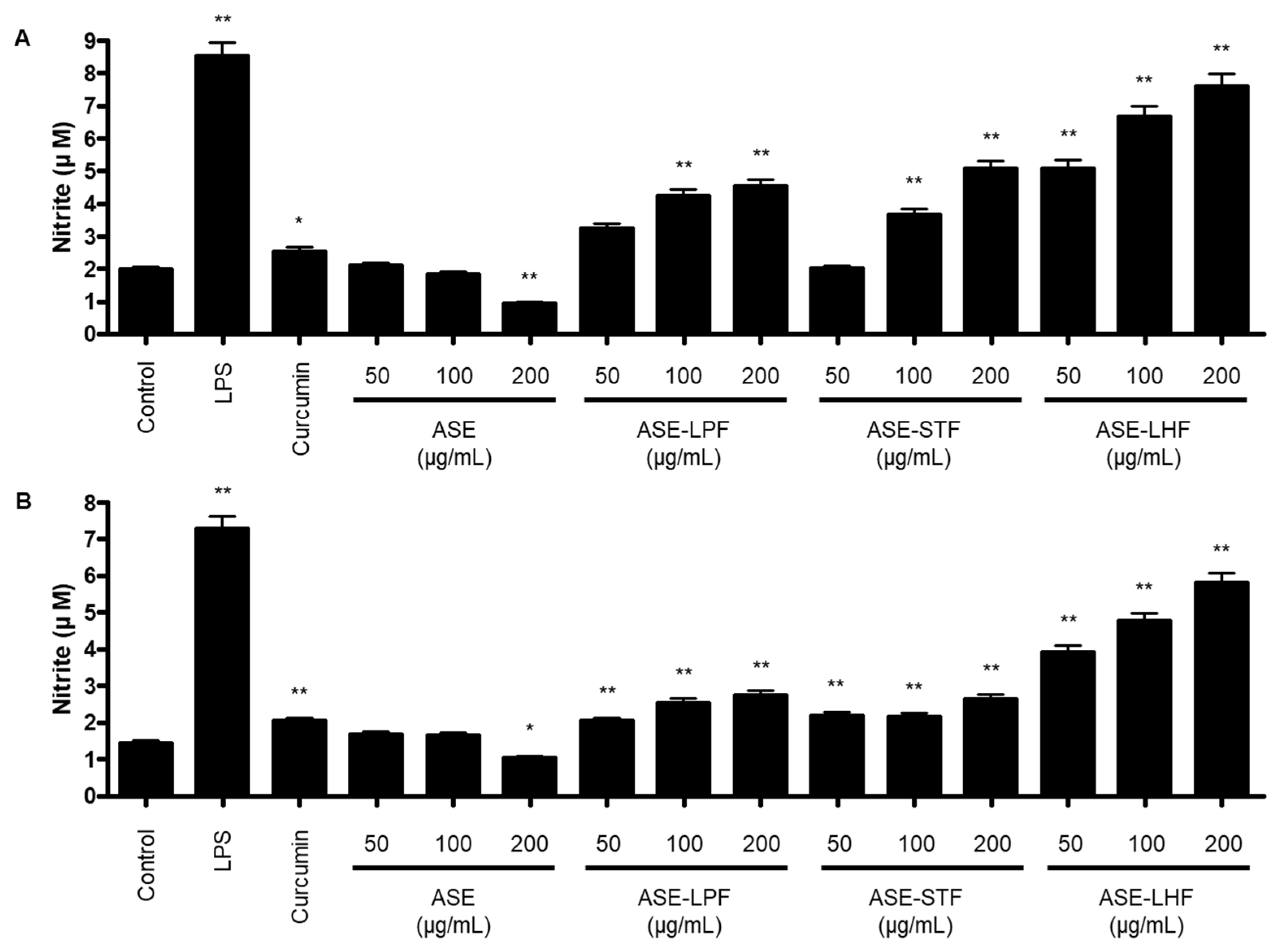
3.3. Effect of ASE and Fermented Products on Production of NO in RAW264.7 Macrophages and BV2 Microglial Cells
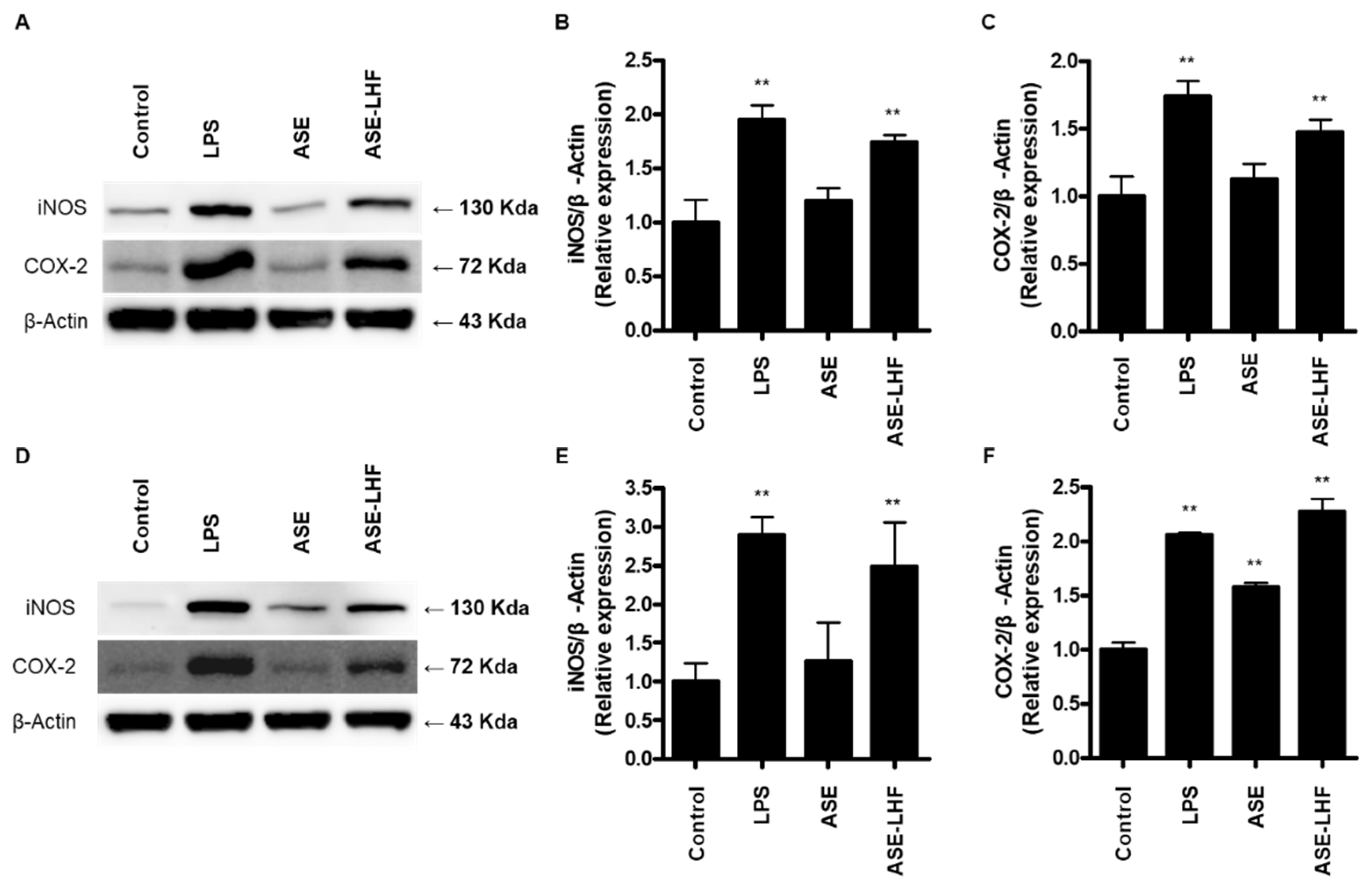
3.4. Effect of ASE and ASE-LHF on Expression of iNOS and COX-2 Proteins in RAW264.7 Macrophages and BV2 Microglial Cells
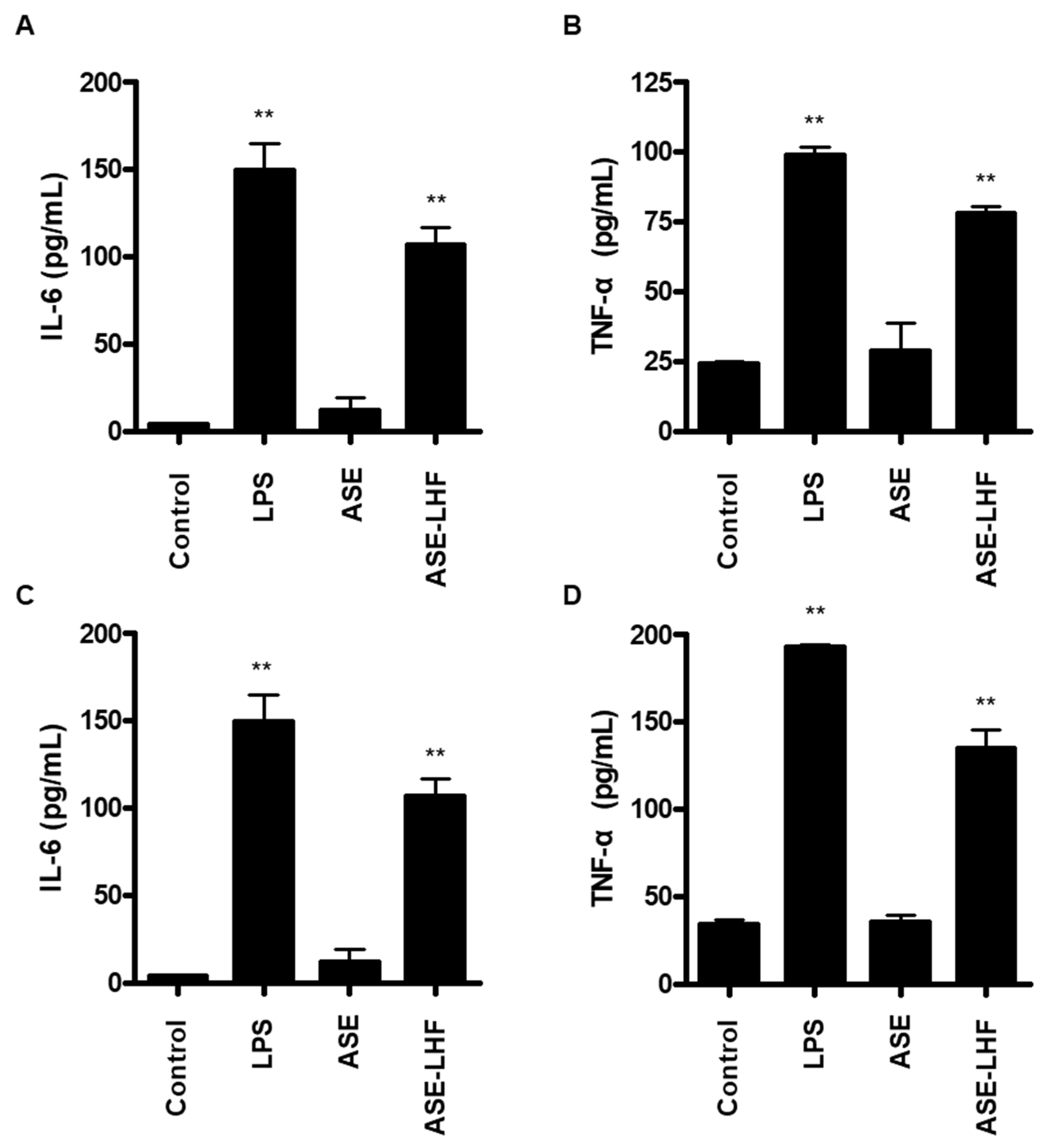
3.5. Effect of ASE and ASE-LHF on Production of Inflammatory Cytokines in RAW264.7 Macrophages and BV2 Microglial Cells
3.6. Effect of ASE-LHF on Activation of NF-κB Signaling Pathway in RAW264.7 Macrophages and BV2 Microglial Cells
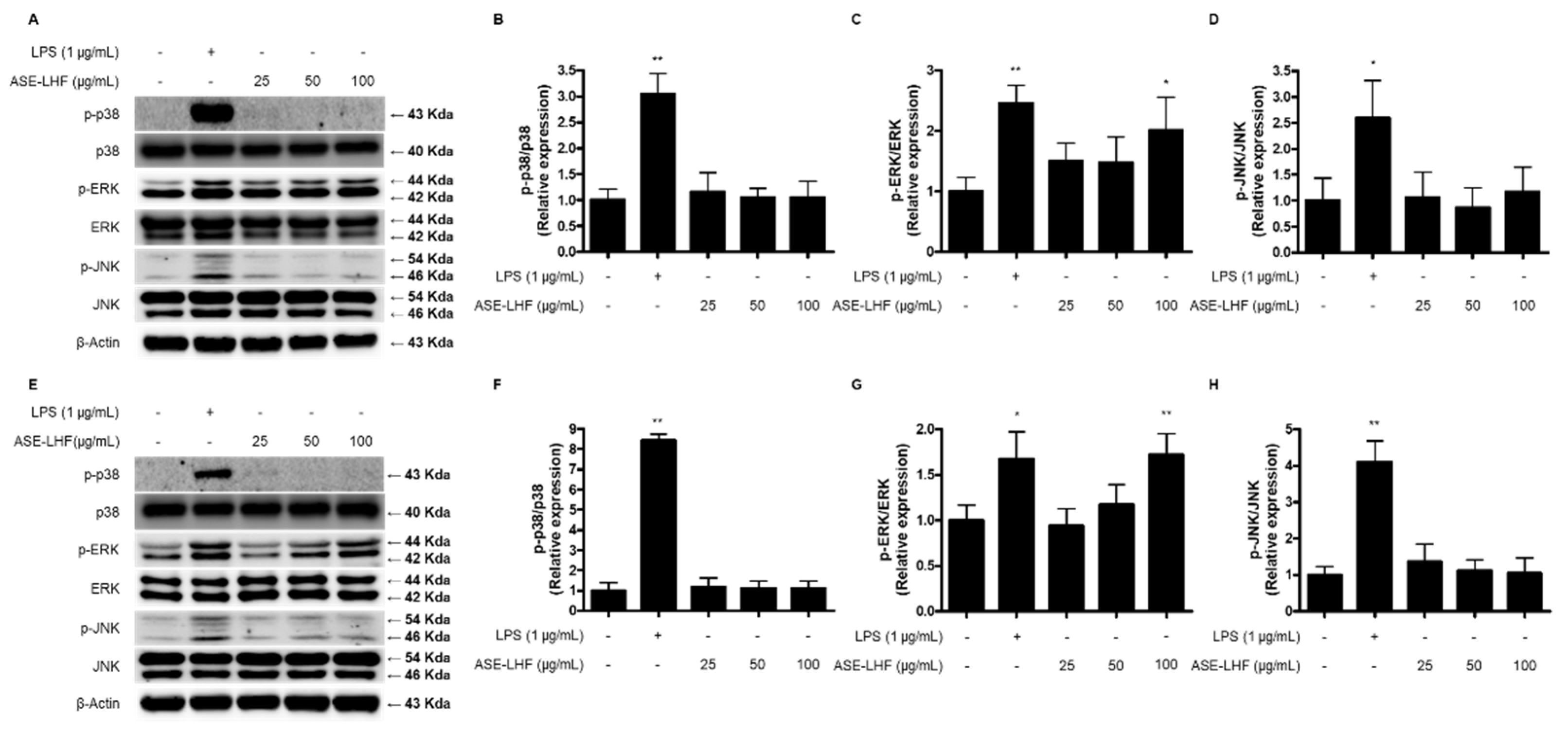
3.7. Effect of ASE-LHF on Activation of MAPK Signaling Pathway in RAW264.7 Macrophages and BV2 Microglial Cells
3.8. Secondary Metabolite Profiling of ASE and Its Fermented Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sompayrac, L.M. How the Immune System Works, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- Rich, R.; Fleisher, T.; Shearer, W.; Schroeder, H.; Frew, A.; Weyand, C. Clinical Immunology, Principles and Practice, 5th ed.; Elsevier: St. Louis, MO, USA, 2018. [Google Scholar]
- Li, R.J.; Gao, C.Y.; Guo, C.; Zhou, M.M.; Luo, J.; Kong, L.Y. The Anti-inflammatory Activities of Two Major Withanolides from Physalis minima Via Acting on NF-κB, STAT3, and HO-1 in LPS-Stimulated RAW264.7 Cells. Inflammation 2017, 40, 401–413. [Google Scholar] [CrossRef]
- Guo, Q.; Bi, D.; Wu, M.; Yu, B.; Hu, L.; Liu, C.; Gu, L.; Zhu, H.; Lei, A.; Xu, X.; et al. Immune activation of murine RAW264.7 macrophages by sonicated and alkalized paramylon from Euglena gracilis. BMC Microbiol. 2020, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, J.; Yang, Y.; Zhang, F.; Wang, S.; Wu, T.; Shen, M.; Xie, M. Sulfated Cyclocarya paliurus polysaccharides markedly attenuates inflammation and oxidative damage in lipopolysaccharide-treated macrophage cells and mice. Sci. Rep. 2017, 7, 40402. [Google Scholar]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar]
- Lawson, L.J.; Perry, V.H.; Gordon, S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 1992, 48, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Filiano, A.J.; Gadani, S.P.; Kipnis, J. Interactions of innate and adaptive immunity in brain development and function. Brain Res. 2015, 1617, 18–27. [Google Scholar]
- Aloishi, F. Immune function of microglia. Glia 2001, 36, 165–179. [Google Scholar] [CrossRef]
- Galloway, D.A.; Pillips, A.E.M.; Owen, D.R.J.; Moore, C.S. Phagocytosis in the Brain: Homeostasis and Disease. Front. Immunol. 2019, 10, 790. [Google Scholar] [CrossRef]
- Ritter, M.R.; Banin, E.; Moreno, S.K.; Aguilar, E.; Dorrell, M.I.; Friedlander, M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J. Clin. Investig. 2006, 116, 3266–3276. [Google Scholar]
- Park, J.K.; Kim, C.K.; Gong, S.K.; Yu, A.R.; Lee, M.Y.; Park, S.K. Acanthopanax sessiliflorus stem confers increased resistance to environmental stresses and lifespan extension in Caenorhabditis elegans. Nutr. Res. Pract. 2014, 8, 526–532. [Google Scholar]
- Bian, X.B.; Wang, S.J.; Liu, J.P.; Zhao, Y.; Li, H.J.; Zhang, L.X.; Li, P.Y. Hepatoprotective effect of chiisanoside against acetaminophen-induced acute liver injury in mice. Nat. Prod. Res. 2019, 33, 2704–2707. [Google Scholar] [PubMed]
- Jo, B.S.; Cho, Y.J. Biological Activity of Extracts from Acanthopanax sessiliflorum Fruit. Korean J. Food Preserv. 2012, 19, 586–593. [Google Scholar]
- Yook, C.S.; Lee, D.H.; Seo, Y.K. A new forma of Acanthopanax species (I). Korean J. Pharmacogn. 1976, 7, 179–190. [Google Scholar]
- Kim, M.J.; Wang, H.S.; Lee, M.W. Anti-Inflammatory Effects of Fermented Bark of Acanthopanax sessiliflorus and Its Isolated Compounds on Lipopolysaccharide-Treated RAW 264.7 Macrophage Cells. eCAM 2020, 2020, 6749425. [Google Scholar]
- Song, Y.; Yang, C.J.; Wang, Z.B.; Zhao, N.; Feng, X.S.; Meng, F.H. Chemical constituents of Eleutherococcus sessiliflorus extract and its sedative-hypnotic effect. Nat. Prod. Res. 2017, 31, 1995–2000. [Google Scholar]
- Murthy, H.N.; Kim, Y.S.; Gerogiev, M.I.; Paek, K.Y. Biotechnological production of eleutherosides: Current state and perspectives. Appl. Microbiol. Biotechnol. 2014, 98, 7319–7329. [Google Scholar] [PubMed]
- Yang, C.; An, Q.; Xiong, Z.; Song, Y.; Yu, K.; Li, F. Triterpenes from Acanthopanax sessiliflorus Fruits and their Antiplatelet Aggregation Activities. Planta Med. 2009, 75, 656–659. [Google Scholar]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar]
- Miller, L.E.; Ouwehand, A.C.; Ibarra, A. Effects of probiotic-containing products on stool frequency and intestinal transit in constipated adults: Systematic review and meta-analysis of randomized controlled trials. Ann. Gastroenterol. 2017, 30, 629–639. [Google Scholar]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [PubMed]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey Jr, G.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [PubMed]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant Extracts Rich in Polyphenols as Potent Modulators in the Growth of Probiotic and Pathogenic Intestinal Microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar]
- Yu, A.R.; Park, H.Y.; Choi, I.W.; Park, Y.K.; Hong, H.D.; Choi, H.D. Immune Enhancing Effect of Medicinal Herb Extracts on a RAW 264.7 Macrophage Cell Line. J. Korean Soc. Food Sci. Nutr. 2012, 41, 1521–1527. [Google Scholar]
- Choi, B.R.; Yoon, D.; Kim, H.G.; Oh, S.M.; Yoo, Y.C.; Lee, Y.S.; Kim, K.W.; Lee, D.Y. NMR-Based Metabolomics Approach to Investigate the Effects of Fruits of Acanthopanax sessiliflorus in a High-Fat Diet Induced Mouse Model. Metabolites 2021, 11, 505. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar]
- Han, Y.; Zhang, A.; Sun, H.; Zhang, Y.; Meng, X.; Yan, G.; Liu, L.; Wang, X. High-throughput ultra high performance liquid chromatography combined with mass spectrometry approach for the rapid analysis and characterization of multiple constituents of the fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms. J. Sep. Sci. 2017, 40, 2178–2187. [Google Scholar]
- Li, Z.; Yao, N.; Liu, H.; Zhou, J.; Zhang, C.; Li, S.; Qian, S.; Zhang, C.; Yang, Z. Antiosteosarcoma effects of novel 23-nor-3,4-seco-3-acetallupane triterpenoids from Acanthopanax gracilistylus W.W. Smith var. gracilistylus in 143B cells. Fitoterapia 2022, 158, 105126. [Google Scholar]
- Wu, Z.Y.; Zhang, Y.B.; Zhu, K.K.; Luo, C.; Zhang, J.X.; Cheng, C.R.; Feng, R.H.; Yang, W.Z.; Zeng, F.; Wang, Y.; et al. Anti-inflammatory Diterpenoids from the Root Bark of Acanthopanax gracilistylus. J. Nat. Prod. 2014, 77, 2342–2351. [Google Scholar]
- Yang, J.; Yao, L.; Gong, K.; Li, K.; Sun, L.; Cai, W. Identification and Quantification of Chlorogenic Acids from the Root Bark of Acanthopanax gracilistylus by UHPLC-Q-Exactive Orbitrap Mass Spectrometry. ACS Omega 2022, 7, 25675–25685. [Google Scholar]
- Kwon, R.H.; Na, H.; Kim, J.H.; Kim, S.A.; Kim, S.Y.; Jung, H.A.; Lee, S.H.; Wee, C.D.; Lee, K.S.; Kim, H.W. Comprehensive profiling of phenolic compounds and triterpenoid saponins from Acanthopanax senticosus and their antioxidant, α-glucosidase inhibitory activities. Sci. Rep. 2024, 14, 26330. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Kim, H.G.; Ko, W.; Dong, L.; Yoon, D.; Oh, S.M.; Lee, Y.S.; Lee, D.Y. Noble 3,4-Seco-triterpenoid Glycosides from the Fruits of Acanthopanax sessiliflorus and Their Anti-Neuroinflammatory Effects. Antioxidants 2021, 10, 1334. [Google Scholar] [CrossRef]
- Sun, H.; Han, Y.; Zhang, A.H.; Meng, X.C.; Wang, Z.Y.; Sun, W.J.; Sun, H.F.; Wang, X.J. UPLC-MS based metabolic profiling of the phenotypes of Acanthopanax senticosus reveals the changes in active metabolites distinguishing the diversities of the plant grown in northeast area of China. Chin. J. Med. 2012, 10, 196–206. [Google Scholar]
- Sun, H.; Liu, J.; Zhang, A.; Zhang, Y.; Meng, X.; Han, Y.; Zhang, Y.; Wang, X. Characterization of the multiple components of Acanthopanax senticosus stem by ultra high performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J. Sep. Sci. 2016, 39, 496–502. [Google Scholar] [CrossRef]
- Nhien, N.X.; Kim, K.C.; Kim, A.D.; Hyun, J.W.; Kang, H.K.; Kiem, P.V.; Ming, C.V.; Thu, V.K.; Tai, B.H.; Kim, J.A.; et al. Phenylpropanoids from the leaves of Acanthopanax koreanum and their antioxidant activity. J. Asian Nat. Prod. Res. 2011, 13, 56–61. [Google Scholar] [CrossRef]
- Wang, Z.B.; Jiang, H.; Xia, Y.G.; Yang, B.Y.; Kuang, H.X. α-Glucosidase Inhibitory Constituents from Acanthopanax senticosus Harm Leaves. Molecules 2012, 17, 6269–6276. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Hamed, Y.S.; Abdin, M.; Chen, G.; Akhtar, H.M.S.; Zeng, X. Effects of impregnate temperature on extraction of caffeoylquinic acid derivatives from Moringa oleifera leaves and evaluation of inhibitory activity on digestive enzyme, antioxidant, anti-proliferative and antibacterial activities of the extract. Int. J. Food Sci. Technol. 2020, 55, 3082–3090. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- Sasaki, K.; Davies, J.; Doldan, N.G.; Arao, S.; Ferdousi, F.; Szele, F.G.; Isoda, H. 3,4,5-Tricaffeoylquinic acid induces adult neurogenesis and improves deficit of learning and memory in aging model senescence-accelerated prone 8 mice. Aging 2019, 11, 401. [Google Scholar] [CrossRef]
- Alcazar Magana, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar]
- Collins, S.L.; McMillan, A.; Seney, S.; van der Veer, C.; Kort, R.; Sumarah, M.W.; Reid, G. Promising Prebiotic Candidate Established by Evaluation of Lactitol, Lactulose, Raffinose, and Oligofructose for Maintenance of a Lactobacillus-Dominated Vaginal Microbiota. Appl. Environ. Microbiol. 2018, 84, e02200-17. [Google Scholar] [PubMed]
- Gibson, G.R.; Rastall, R.A. Prebiotics: Development and Application; John Wiley & Sons, Ltd.: West Sussex, UK, 2006. [Google Scholar]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar]
- DeGroote, M.A.; Fang, F.C. Antimicrobial properties of nitric oxide. In Nitric Oxide and Infection; Fang, F.C., Ed.; Kluwer Academic Publishers: New York, NY, USA, 1999; pp. 231–261. [Google Scholar]
- Xie, K.; Dong, Z.; Fidler, I.J. Activation of nitric oxide synthase gene for inhibition of cancer metastasis. J. Leukoc. Biol. 1996, 59, 797–803. [Google Scholar]
- Taylor-Robinson, A.W.; Liew, F.Y.; Severn, A.; Xu, D.; McSorley, S.J.; Garside, P.; Padron, J.; Phillips, R.S. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur. J. Immunol. 1994, 24, 980–984. [Google Scholar]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [PubMed]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on immune cells and its role in diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Seo, H.J.; Jeong, J.B. Immune-enhancing effects of green lettuce (Lactuca sativa L.) extracts through the TLR4-MAPK/NF-κB signaling pathways in RAW264. 7 macrophage cells. Korean J. Plant Res. 2020, 33, 183–193. [Google Scholar]
- Monmai, C.; Rod-In, W.; Jang, A.Y.; Lee, S.M.; Jung, S.K.; You, S.; Park, W.J. Immune-enhancing effects of anionic macromolecules extracted from Codium fragile coupled with arachidonic acid in RAW264. 7 cells. PLoS ONE 2020, 15, e0239422. [Google Scholar]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immun. 2012, 188, 21–28. [Google Scholar]
- Martínez-Colón, G.J.; Moore, B.B. Prostaglandin E2 as a regulator of immunity to pathogens. Pharmacol. Ther. 2018, 185, 135–146. [Google Scholar]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1851, 414–421. [Google Scholar]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Peters, M. Actions of cytokines on the immune response and viral interactions: An overview. Hepatology 1996, 23, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Nie, M.; Fan, M.W.; Bian, Z. Anti-inflammatory activity of curcumin in macrophages stimulated by lipopolysaccharides from Porphyromonas gingivalis. Pharmacology 2008, 82, 264–269. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, Y.S.; Kim, S.M.; Jung, A.J.; Yoo, J.H.; Lee, S.H.; Jeong, H.C.; Lee, H.J. Immune-enhancing effects of red Platycodon grandiflorus root extract via p38 MAPK-mediated NF-κB activation. Appl. Sci. 2020, 10, 5457. [Google Scholar] [CrossRef]
- Lappas, M.; Permezel, M.; Georgiou, H.M.; Rice, G.E. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol. Reprod. 2002, 67, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases—Regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, L.; Liu, Y.; Qian, W.; Liu, J.; Zhou, J.; Gao, R.; Xiao, H.; Wang, J. MAPK and NF-κB pathways are involved in bisphenol A-induced TNF-α and IL-6 production in BV2 microglial cells. Inflammation 2015, 38, 637–648. [Google Scholar] [CrossRef]
- Cai, N.; Luo, W.; Yao, L.; Li, X.; Wang, Z.; Xu, H.; Li, H.; Hu, Z.; Bao, W.; Xu, X. Activation of murine RAW264. 7 macrophages by oligopeptides from sea cucumber (Apostichopus japonicus) and its molecular mechanisms. J. Funct. Foods 2020, 75, 104229. [Google Scholar] [CrossRef]
- Su, J.; Fu, X.; Zhang, R.; Li, X.; Li, Y.; Chu, X. Exploring the Effects of Solid-State Fermentation on Polyphenols in Acanthopanax senticosus Based on Response Surface Methodology and Nontargeted Metabolomics Techniques. J. Food Biochem. 2023, 2023, 6711132. [Google Scholar] [CrossRef]
- Katina, K.; Liukkonen, K.H.; Kaukovirta-Norja, A.; Adlercreutz, H.; Heinonen, S.M.; Lampi, A.M. Fermentation-induced changes in the nutritional value of native or germinated rye. J. Cereal Sci. 2007, 46, 348–355. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, W.; Yang, X.; Li, X.; Song, Z.; Meng, Y.; Liu, H.; Guo, L.; Zhang, T. Study on screening of fermentation agents and optimization of the fermentation process for pharyngitis tablet residue. Front. Vet. Sci. 2022, 9, 981388. [Google Scholar] [CrossRef] [PubMed]




| No. | Compound Name | Observed RT (min) | Formula | Neutral Mass (Da) | Observed m/z | Adducts | Average of Relative Percentage (%) | |
|---|---|---|---|---|---|---|---|---|
| ASE | Fermented ASE | |||||||
| 1 | 22α-hydrochiisanoside | 4.32 | C48H74O20 | 971.1 | 1015.41 | +COOH | 0.61 | 0.14 |
| 2 | Chlorogenic acid | 5.13 | C16H18O9 | 354.31 | 353.16 | -H | 0.44 | 0.05 |
| 3 | Chiisanoside | 5.48 | C48H74O19 | 955.1 | 999.42 | +COOH | 1.19 | N.D |
| 4 | 3-O-β-D-Glucuronopyranosyl-gypsogenin-28-O-β-D-glucopyranoside | 5.78 | C42H64O15 | 808.9 | 853.38 | +COOH | 0.38 | 0.03 |
| 5 | Acanthosessilioside H | 6.27 | C48H76O19 | 957.1 | 955.44 | -H | 1.6 | - |
| 6 | Gracilistylacid B | 7.9 | C31H48O5 | 500.7 | 499.31 | -H | 6.01 | 1.96 |
| 7 | Acanthopanaxoside E | 8.38 | C42H66O15 | 811 | 809.40 | -H | 5.08 | 1.8 |
| 8 | hederagenin-3-O-glucuronopyraoside | 9.39 | C36H56O10 | 648.8 | 647.32 | -H | 6.14 | 1.34 |
| 9 | Silphioside G | 10.07 | C42H66O14 | 795 | 793.41 | -H | 7.12 | 1.06 |
| 10 | 28-Hydroxy-28-oxoolean-12-en-3-yl-3-O-xylopyranosyl-glucopyranosiduronic acid | 10.65 | C41H64O13 | 764.9 | 763.40 | -H | 5.8 | 0.73 |
| 11 | Laciniatoside V | 11.81 | C27H38O14 | 586.6 | 631.38 | +COOH | 6.25 | 0.97 |
| 12 | Chiisanogenin | 12.08 | C30H44O5 | 484.31 | 483.32 | -H | 3.94 | 3.67 |
| 13 | [R-(E)]-1-[8-(β-D-Glucopyranosyloxy)-2,6-dimethyl-2-octenoate] β-D-glucopyranose | 1.25 | C22H38O13 | 510.5 | 555.28 | +COOH | 0.07 | 2.13 |
| 14 | Ficusequilignan A | 1.65 | C31H36O11 | 584.6 | 584.30 | -H | 0.09 | 3.05 |
| 15 | 4-(1,3-Dihydroxy-2-{4-[(1E)-3-hydroxy-1-propen-1-yl]-2,6-dimethoxyphenoxy}propyl)-2-methoxyphenyl-β-D-allopyranoside | 2.15 | C27H36O13 | 568.6 | 612.33 | +COOH | 0.23 | 2.18 |
| 16 | 3-O-[(α-L-Rhamnopyranosyl)(1→2)]-[β-D-glucuronopyranosyl-6-O-methyl ester]-olean-12-ene-28-olic acid | 3.52 | C43H68O12 | 776.9 | 819.39 | +COOH | N.D | 1.12 |
| 17 | Feruloylquinic acid-hexoside | 3.7 | C23H30O14 | 530.5 | 528.28 | -H | 0.02 | 0.32 |
| 18 | a-terpineol 8-O-β-D-glucopyranoside | 4.68 | C23H33O10 | 468.5 | 512.27 | +COOH | 0.03 | 0.4 |
| 19 | Caffeoylquinic acid hexoside | 5.54 | C22H28O14 | 516.4 | 515.29 | -H | 0.75 | 7.8 |
| 20 | 16α-Hydroxy-17-methylbutyryloxy-ent-kaur-19-oic acid | 6.69 | C25H39O5 | 419.6 | 464.31 | +COOH | 0.24 | 6.83 |
| 21 | 5-O-Caffeoyl-3-O-pcoumaroylquinic acid | 7.03 | C25H24O11 | 500.4 | 498.29 | -H | 0.08 | 1.64 |
| 22 | 3-O-Caffeoyl-4-O-pcoumaroylquinic acid | 7.37 | C25H24O11 | 500.4 | 498.29 | -H | 0.46 | 5.28 |
| 23 | 1-O-p-hydroxybenzoyl-β-D-apiofuranosyl-(1→6)-β-D-glucopyranoside | 9.11 | C18H24O13 | 448.4 | 448.31 | -H | 0.02 | 3.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-W.; Choi, B.-R.; Shin, W.-C.; Jang, J.-K.; Lee, Y.-S.; Yoon, D.; Lee, D.Y. Metabolic Profiling of Fermented Products of the Ethanolic Extract of Acanthopanax sessiliflorus Fruit and Evaluation of Its Immune Enhancement Effect in RAW 264.7 Macrophages and BV2 Microglia. Antioxidants 2025, 14, 397. https://doi.org/10.3390/antiox14040397
Kim K-W, Choi B-R, Shin W-C, Jang J-K, Lee Y-S, Yoon D, Lee DY. Metabolic Profiling of Fermented Products of the Ethanolic Extract of Acanthopanax sessiliflorus Fruit and Evaluation of Its Immune Enhancement Effect in RAW 264.7 Macrophages and BV2 Microglia. Antioxidants. 2025; 14(4):397. https://doi.org/10.3390/antiox14040397
Chicago/Turabian StyleKim, Kwan-Woo, Bo-Ram Choi, Woo-Cheol Shin, Jin-Kyu Jang, Young-Seob Lee, Dahye Yoon, and Dae Young Lee. 2025. "Metabolic Profiling of Fermented Products of the Ethanolic Extract of Acanthopanax sessiliflorus Fruit and Evaluation of Its Immune Enhancement Effect in RAW 264.7 Macrophages and BV2 Microglia" Antioxidants 14, no. 4: 397. https://doi.org/10.3390/antiox14040397
APA StyleKim, K.-W., Choi, B.-R., Shin, W.-C., Jang, J.-K., Lee, Y.-S., Yoon, D., & Lee, D. Y. (2025). Metabolic Profiling of Fermented Products of the Ethanolic Extract of Acanthopanax sessiliflorus Fruit and Evaluation of Its Immune Enhancement Effect in RAW 264.7 Macrophages and BV2 Microglia. Antioxidants, 14(4), 397. https://doi.org/10.3390/antiox14040397








