Therapeutic Potential of Lythrum salicaria L. Ethanol Extract in Experimental Rat Models of Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material Processing
2.3. Total Phenolic and Total Flavonoid Content Determination
2.4. HPLC-DAD-ESI MS
2.5. Evaluation of In Vitro Antioxidant Effects
2.6. Experimental Design
- CONTROL: negative control group.
- STZ: positive control group with DM induced with STZ (55 mg/kg b.w.) [9].
- STZ + TX: DM receiving TX (20 mg/kg b.w.) [10].
- STZ + M: DM receiving Metformin (100 mg/100 g b.w.) [11].
- STZ + LSEE 100%: DM receiving LSEE 100% (0.5 mL extract/100 g b.w./day)
- STZ + LSEE 50%: DM receiving LSEE 50% (0.5 mL extract/100 g b.w./day)
- STZ + LSEE 50%: DM receiving LSEE 50% (0.5 mL extract/100 g b.w./day)
- STZ + LSEE 25%: DM receiving LSEE 25% (0.5 mL extract/100 g b.w./day)
- CONTROL: negative control group;
- LET: positive control group with PCOS induced with LET (1 mg/kg b.w.) [12];
- LET + TX: PCOS receiving TX (20 mg/kg b.w., gavage);
- LET + M: PCOS receiving Metformin (100 mg/100 g b.w., gavage);
- LET + LSEE 100%: PCOS receiving LSEE 100% (0.5 mL extract/100 g b.w./day).
2.7. Pharmacological Studies
2.7.1. Serum Oxidative Stress Markers Evaluation
2.7.2. Serum Inflammatory Markers Evaluation
2.7.3. Blood Glucose, Lipid Profiles, Triglyceride-Glucose Index, Liver-Injury Makers, and Anthropometric Parameters Evaluation
2.7.4. Hormonal Assays
2.8. Histopathological Assessment of Reproductive Organs
2.9. Ultrasound Examination
2.10. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. The In Vitro Oxidative Stress Markers
3.3. Confirmation of Rodent DM and PCOS Models
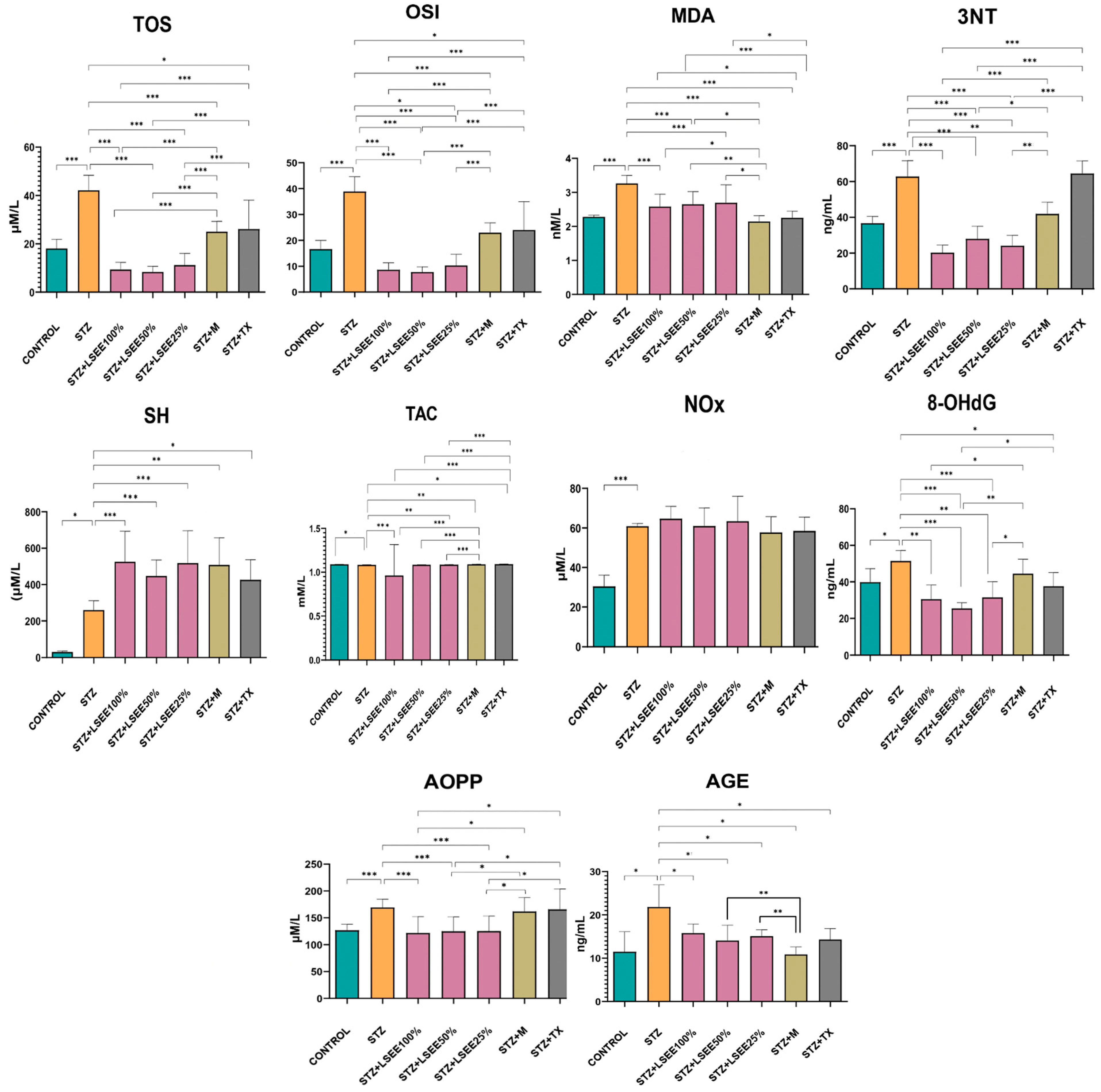
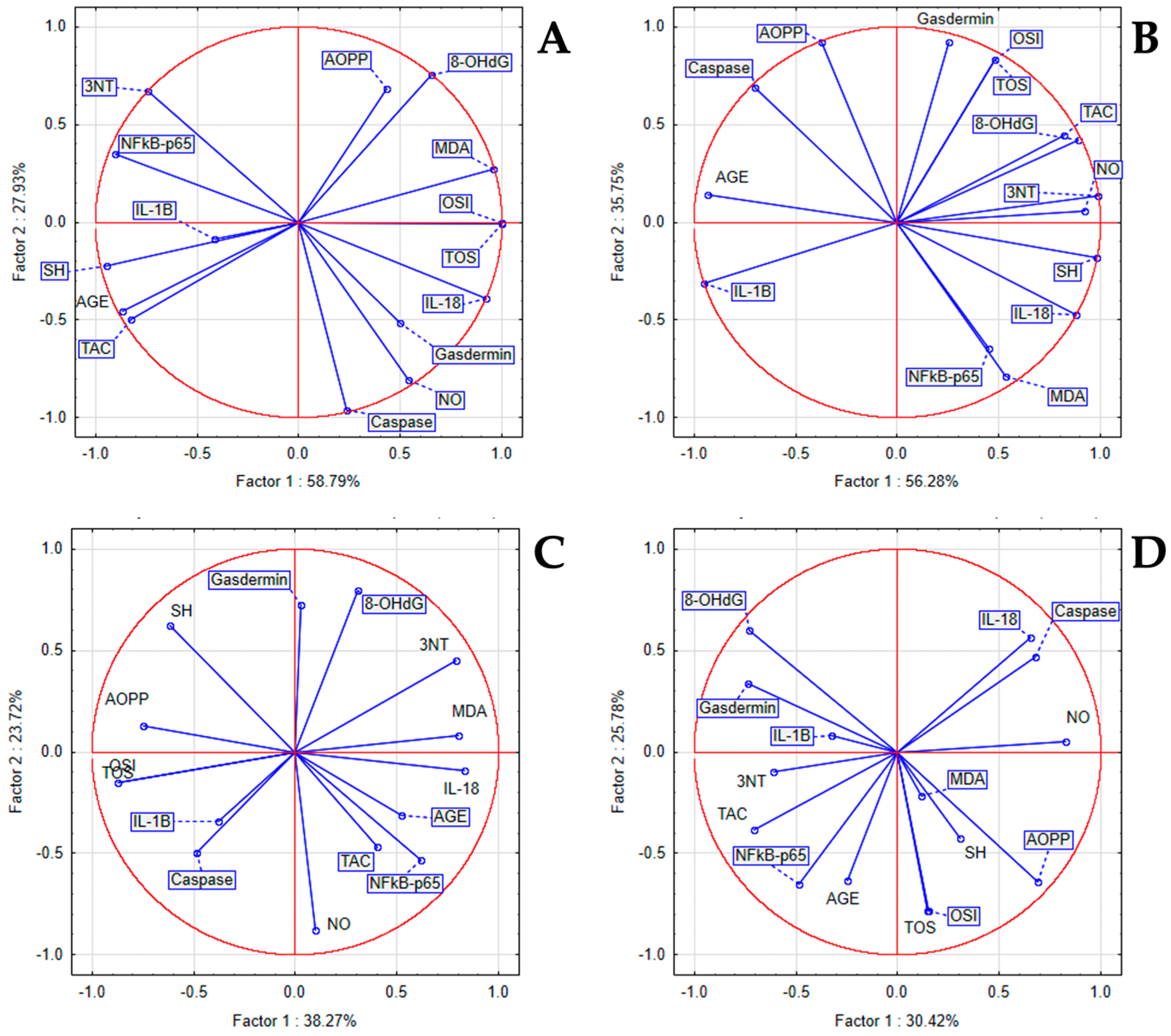
3.4. The In Vivo Oxidative Stress Markers
3.5. The In Vivo Inflammatory Markers
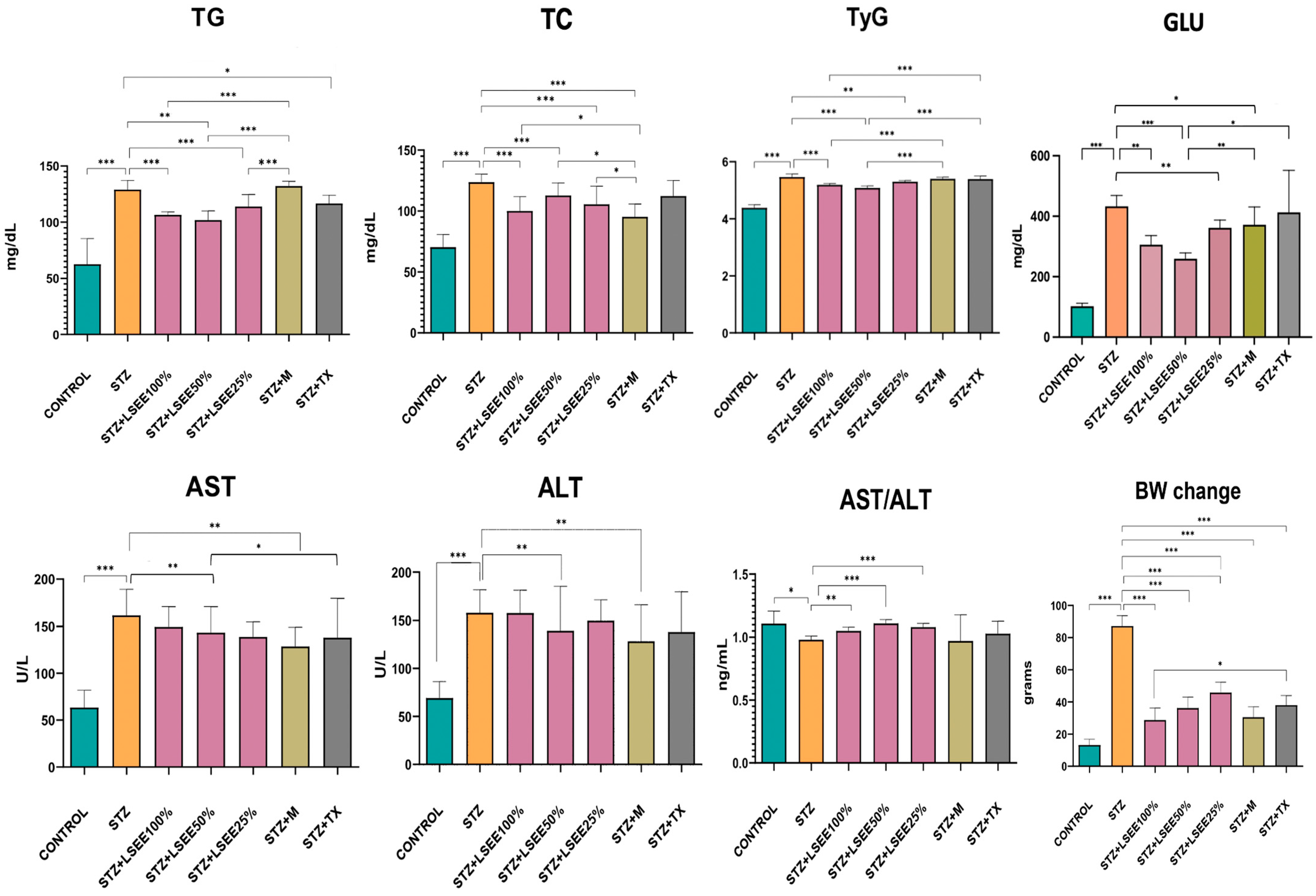
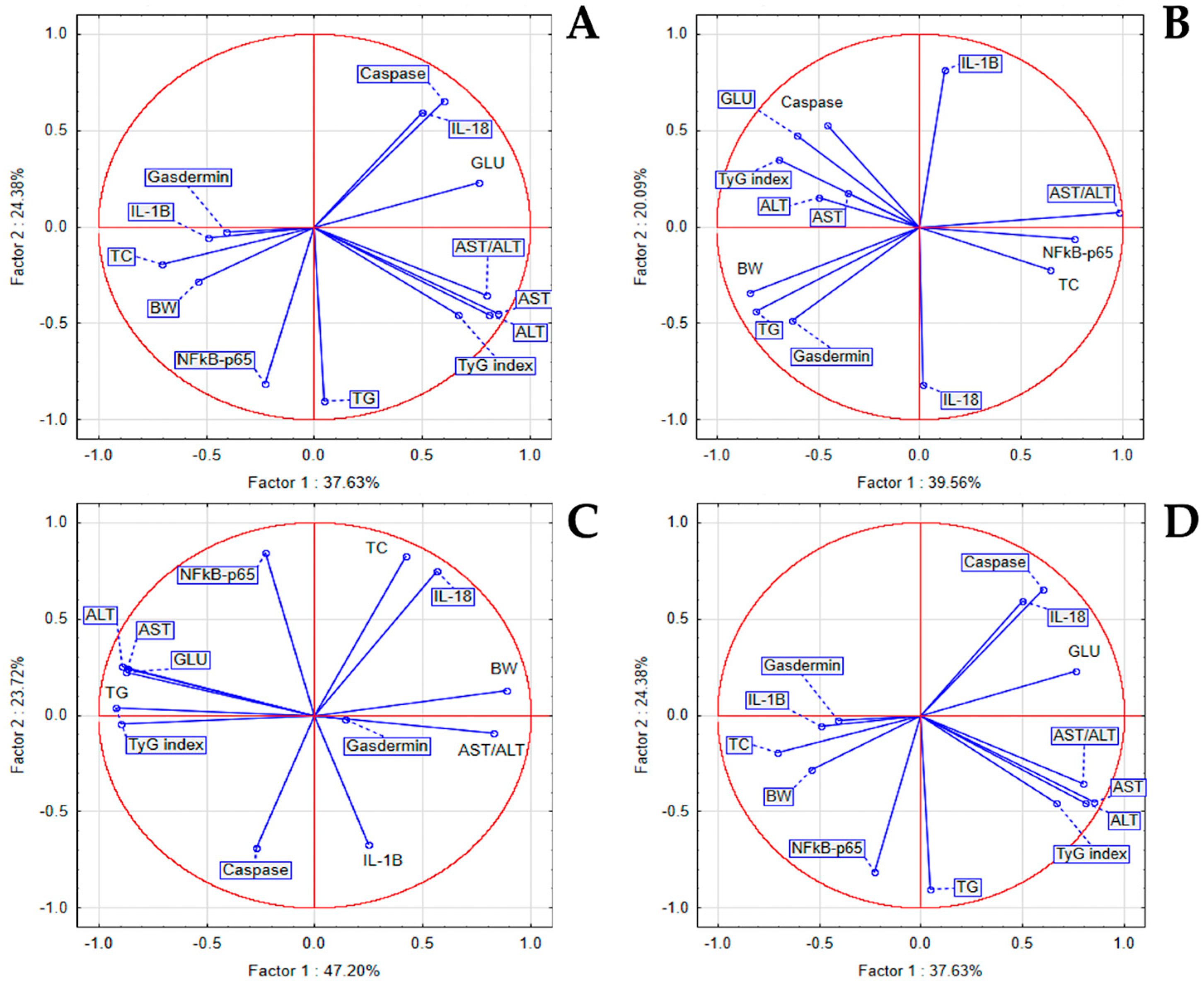
3.6. The In Vivo Hypoglycemic, Lipid-Regulating, and Liver-Protective Effects
3.7. Effects of L. salicaria L. Ethanolic Extract on Sex Hormones, Serum Insulin and HOMA-IR
3.8. Effect of L. salicaria L. Ethanolic Extract on Ovarian Histology in Letrozole-Induced PCOS
3.9. Ultrasonography Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piwowarski, J.P.; Granica, S.; Kiss, A.K. Lythrum salicaria L.-Underestimated medicinal plant from European traditional medicine. A review. J. Ethnopharmacol. 2015, 170, 226–250. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Savran, A.; Zengin, G.; Aktumsek, A.; Mocan, A.; Glamoćlija, J.; Ćirić, A.; Soković, M. Phenolic compounds and biological effects of edible Rumex scutatus and Pseudosempervivum sempervivum: Potential sources of natural agents with health benefits. Food Funct. 2016, 7, 3252–3262. [Google Scholar] [CrossRef] [PubMed]
- Babotă, M.; Mocan, A.; Vlase, L.; Crișan, O.; Ielciu, I.; Gheldiu, A.M.; Vodnar, D.C.; Crișan, G.; Păltinean, R. Phytochemical Analysis, Antioxidant and Antimicrobial Activities of Helichrysum arenarium (L.) Moench. and Antennaria dioica (L.) Gaertn. Flowers. Molecules 2018, 23, 409. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A.; Patel, A.; Patel, N.M. Determination of polyphenols and free radical scavenging activity of Tephrosia purpurea linn leaves (Leguminosae). Pharmacognosy Res. 2010, 2, 152–158. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.; Mohamad, A.B. Hydrogen Peroxide Scavenging Activity of Novel Coumarins Synthesized Using Different Approaches. PLoS ONE 2015, 10, e0132175. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S. Streptozotocin as a tool for induction of rat models of diabetes: A practical guide. Excli J. 2023, 22, 274–294. [Google Scholar] [CrossRef]
- But, A.E.; Pop, R.M.; Binsfeld, G.F.; Ranga, F.; Orăsan, M.S.; Cecan, A.D.; Morar, I.I.; Chera, E.I.; Bonci, T.I.; Usatiuc, L.O.; et al. The Phytochemical Composition and Antioxidant Activity of Matricaria recutita Blossoms and Zingiber officinale Rhizome Ethanol Extracts. Nutrients 2024, 17, 5. [Google Scholar] [CrossRef]
- Calco, G.N.; Proskocil, B.J.; Jacoby, D.B.; Fryer, A.D.; Nie, Z. Metformin prevents airway hyperreactivity in rats with dietary obesity. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 321, L1105–L1118. [Google Scholar] [CrossRef]
- Ragy, M.M.; Abdel-Hamid, H.A.; Toni, N.D.M. Pathophysiological changes in experimental polycystic ovary syndrome in female albino rats: Using either hemin or L-arginine. J. Cell Physiol. 2019, 234, 8426–8435. [Google Scholar] [CrossRef] [PubMed]
- Harma, M.; Harma, M.; Erel, O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med. Wkly. 2003, 133, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Essel, L.B.; Obiri, D.D.; Osafo, N.; Antwi, A.O.; Duduyemi, B.M. The Ethanolic Stem-Bark Extract of Antrocaryon micraster Inhibits Carrageenan-Induced Pleurisy and Pedal Oedema in Murine Models of Inflammation. Int. Sch. Res. Notices 2017, 2017, 6859230. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Laragione, T.; Gianazza, E.; Tonelli, R.; Bigini, P.; Mennini, T.; Casoni, F.; Massignan, T.; Bonetto, V.; Ghezzi, P. Regulation of redox-sensitive exofacial protein thiols in CHO cells. Biol. Chem. 2006, 387, 1371–1376. [Google Scholar] [CrossRef]
- Nabipoorashrafi, S.A.; Seyedi, S.A.; Rabizadeh, S.; Ebrahimi, M.; Ranjbar, S.A.; Reyhan, S.K.; Meysamie, A.; Nakhjavani, M.; Esteghamati, A. The accuracy of triglyceride-glucose (TyG) index for the screening of metabolic syndrome in adults: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2677–2688. [Google Scholar] [CrossRef]
- Pop, R.M.; Vassilopoulou, E.; Jianu, M.E.; Roșian, Ș.H.; Taulescu, M.; Negru, M.; Bercian, C.; Boarescu, P.M.; Bocsan, I.C.; Feketea, G.; et al. Nigella sativa oil attenuates inflammation and oxidative stress in experimental myocardial infarction. BMC Complement. Med. Ther. 2024, 24, 362. [Google Scholar] [CrossRef]
- Sumitra, M.; Manikandan, P.; Rao, K.V.; Nayeem, M.; Manohar, B.M.; Puvanakrishnan, R. Cardiorespiratory effects of diazepam-ketamine, xylazine-ketamine and thiopentone anesthesia in male Wistar rats—A comparative analysis. Life Sci. 2004, 75, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Khanavi, M.; Saeidnia, S.; Azizi, E.; Mahmoodpour, M.R.; Vafi, F.; Malmir, M.; Siavashi, F.; Hadjiakhoondi, A. Biological activity and microscopic characterization of Lythrum salicaria L. Daru 2013, 21, 61. [Google Scholar] [CrossRef]
- Lamela, M.; Cadavid, I.; Calleja, J.M. Effects of Lythrum salicaria extracts on hyperglycemic rats and mice. J. Ethnopharmacol. 1986, 15, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bencsik, T.; Horváth, G.; Papp, N. Variability of total flavonoid, polyphenol and tannin contents in some Lythrum salicaria populations. Nat. Prod. Commun. 2011, 6, 1417–1420. [Google Scholar] [CrossRef]
- Granica, S.; Vahjen, W.; Zentek, J.; Melzig, M.F.; Pawłowska, K.A.; Piwowarski, J.P. Lythrum salicaria Ellagitannins Stimulate IPEC-J2 Cells Monolayer Formation and Inhibit Enteropathogenic Escherichia coli Growth and Adhesion. J. Nat. Prod. 2020, 83, 3614–3622. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Ren, X.; He, K.; Chen, X.; Zhang, S.; Roller, M.; Zheng, B.; Zheng, Q.; Ho, C.T.; Bai, N. The anti-diabetic effect of eight Lagerstroemia speciosa leaf extracts based on the contents of ellagitannins and ellagic acid derivatives. Food Funct. 2020, 11, 1560–1571. [Google Scholar] [CrossRef]
- Tang, B.; Chen, G.X.; Liang, M.Y.; Yao, J.P.; Wu, Z.K. Ellagic acid prevents monocrotaline-induced pulmonary artery hypertension via inhibiting NLRP3 inflammasome activation in rats. Int. J. Cardiol. 2015, 180, 134–141. [Google Scholar] [CrossRef]
- Shen, S.C.; Chang, W.C. Hypotriglyceridemic and hypoglycemic effects of vescalagin from Pink wax apple [Syzygium samarangense (Blume) Merrill and Perry cv. Pink] in high-fructose diet-induced diabetic rats. Food Chem. 2013, 136, 858–863. [Google Scholar] [CrossRef]
- Polce, S.A.; Burke, C.; França, L.M.; Kramer, B.; de Andrade Paes, A.M.; Carrillo-Sepulveda, M.A. Ellagic Acid Alleviates Hepatic Oxidative Stress and Insulin Resistance in Diabetic Female Rats. Nutrients 2018, 10, 531. [Google Scholar] [CrossRef]
- Ghadimi, M.; Foroughi, F.; Hashemipour, S.; Nooshabadi, M.R.; Ahmadi, M.H.; Yari, M.G.; Kavianpour, M.; Haghighian, H.K. Decreased insulin resistance in diabetic patients by influencing Sirtuin1 and Fetuin-A following supplementation with ellagic acid: A randomized controlled trial. Diabetol. Metab. Syndr. 2021, 13, 16. [Google Scholar] [CrossRef]
- Kilic, I.; Yeşiloğlu, Y.; Bayrak, Y. Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Mehrzadi, S.; Mehrabani, M.; Malayeri, A.R.; Bakhshayesh, M.; Kalantari, H.; Goudarzi, M. Ellagic acid as a potential antioxidant, alleviates methotrexate-induced hepatotoxicity in male rats. Acta Chir. Belg. 2019, 119, 69–77. [Google Scholar] [CrossRef]
- Gil, T.Y.; Hong, C.H.; An, H.J. Anti-Inflammatory Effects of Ellagic Acid on Keratinocytes via MAPK and STAT Pathways. Int. J. Mol. Sci. 2021, 22, 1277. [Google Scholar] [CrossRef] [PubMed]
- Bains, M.; Kaur, J.; Akhtar, A.; Kuhad, A.; Sah, S.P. Anti-inflammatory effects of ellagic acid and vanillic acid against quinolinic acid-induced rat model of Huntington’s disease by targeting IKK-NF-κB pathway. Eur. J. Pharmacol. 2022, 934, 175316. [Google Scholar] [CrossRef]
- Kahksha; Alam, O.; Al-Keridis, L.A.; Khan, J.; Naaz, S.; Alam, A.; Ashraf, S.A.; Alshammari, N.; Adnan, M.; Beg, M.A. Evaluation of Antidiabetic Effect of Luteolin in STZ Induced Diabetic Rats: Molecular Docking, Molecular Dynamics, In Vitro and In Vivo Studies. J. Funct. Biomater. 2023, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Li, X.; He, L.; Zheng, Y.; Lu, H.; Li, J.; Zhong, L.; Tong, R.; Jiang, Z.; Shi, J.; et al. Antidiabetic Potential of Flavonoids from Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2019, 47, 933–957. [Google Scholar] [CrossRef]
- Liu, H.; Huang, P.; Wang, X.; Ma, Y.; Tong, J.; Li, J.; Ding, H. Apigenin analogs as α-glucosidase inhibitors with antidiabetic activity. Bioorg Chem. 2024, 143, 107059. [Google Scholar] [CrossRef]
- Chagas, M.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Zhao, J.; Sun, W.; Yang, Q.; Chen, C.; Xia, P.; Zhu, J.; Zhou, Y.; Huang, G.; et al. Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation. Biomed. Pharmacother. 2021, 137, 111308. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; Liu, C.; Tang, S.; Gu, D.; Tian, J.; Huang, D.; He, F. Ellagic acid from pomegranate peel: Consecutive countercurrent chromatographic separation and antioxidant effect. Biomed. Chromatogr. 2023, 37, e5662. [Google Scholar] [CrossRef]
- Tunalier, Z.; Koşar, M.; Küpeli, E.; Çaliş, İ.; Başer, K.H.C. Antioxidant, anti-inflammatory, anti-nociceptive activities and composition of Lythrum salicaria L. extracts. J. Ethnopharmacol. 2007, 110, 539–547. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Usatiuc, L.O.; Pârvu, M.; Pop, R.M.; Uifălean, A.; Vălean, D.; Szabo, C.E.; Țicolea, M.; Cătoi, F.A.; Ranga, F.; Pârvu, A.E. Phytochemical Profile and Antidiabetic, Antioxidant, and Anti-Inflammatory Activities of Gypsophila paniculata Ethanol Extract in Rat Streptozotocin-Induced Diabetes Mellitus. Antioxidants 2024, 13, 1029. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Zhong, Q.; Benite-Ribeiro, S.A.; Heck, T.G. New Insights into the Role of Oxidative Stress in the Development of Diabetes Mellitus and Its Complications. J. Diabetes Res. 2023, 2023, 9824864. [Google Scholar] [CrossRef]
- Dong, H.; Sun, Y.; Nie, L.; Cui, A.; Zhao, P.; Leung, W.K.; Wang, Q. Metabolic memory: Mechanisms and diseases. Signal Transduct. Target. Ther. 2024, 9, 38. [Google Scholar] [CrossRef]
- Brunelli, E.; La Russa, D.; Pellegrino, D. Impaired Oxidative Status Is Strongly Associated with Cardiovascular Risk Factors. Oxid. Med. Cell Longev. 2017, 2017, 6480145. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk-Sedlak, I.; Folwarczna, J.; Sedlak, L.; Zych, M.; Wojnar, W.; Szumińska, I.; Wyględowska-Promieńska, D.; Mrukwa-Kominek, E. Effect of caffeine on biomarkers of oxidative stress in lenses of rats with streptozotocin-induced diabetes. Arch. Med. Sci. 2019, 15, 1073–1080. [Google Scholar] [CrossRef]
- Urbaniak, S.K.; Boguszewska, K.; Szewczuk, M.; Kaźmierczak-Barańska, J.; Karwowski, B.T. 8-Oxo-7,8-Dihydro-2′-Deoxyguanosine (8-oxodG) and 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) as a Potential Biomarker for Gestational Diabetes Mellitus (GDM) Development. Molecules 2020, 25, 202. [Google Scholar] [CrossRef]
- Tessari, P.; Cecchet, D.; Cosma, A.; Vettore, M.; Coracina, A.; Millioni, R.; Iori, E.; Puricelli, L.; Avogaro, A.; Vedovato, M. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes 2010, 59, 2152–2159. [Google Scholar] [CrossRef]
- Gutiérrez-Camacho, L.R.; Kormanovski, A.; Del Carmen Castillo-Hernández, M.; Guevara-Balcázar, G.; Lara-Padilla, E. Alterations in glutathione, nitric oxide and 3-nitrotyrosine levels following exercise and/or hyperbaric oxygen treatment in mice with diet-induced diabetes. Biomed. Rep. 2020, 12, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Balcerczyk, A.; Grzelak, A.; Janaszewska, A.; Jakubowski, W.; Koziol, S.; Marszalek, M.; Rychlik, B.; Soszynski, M.; Bilinski, T.; Bartosz, G. Thiols as major determinants of the total antioxidant capacity. Biofactors 2003, 17, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Zhu, L.; Han, J.; Yuan, R.; Xue, L.; Pang, W. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway. Biol. Res. 2018, 51, 9. [Google Scholar] [CrossRef]
- Ding, S.; Xu, S.; Ma, Y.; Liu, G.; Jang, H.; Fang, J. Modulatory Mechanisms of the NLRP3 Inflammasomes in Diabetes. Biomolecules 2019, 9, 850. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Chillarón, J.J.; Sales, M.P.; Flores Le-Roux, J.A.; Castells, I.; Benaiges, D.; Sagarra, E.; Pedro-Botet, J. Atherogenic dyslipidemia in patients with type 1 diabetes mellitus. Med. Clin. 2013, 141, 465–470. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, L. Non-linear association of triglyceride-glucose index with prevalence of prediabetes and diabetes: A cross-sectional study. Front. Endocrinol. 2023, 14, 1295641. [Google Scholar] [CrossRef]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Teck, J. Diabetes-Associated Comorbidities. Prim. Care 2022, 49, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Roldán-Martín, M.B. Type 1 Diabetes and Polycystic Ovary Syndrome: Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 639–648. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Cacciapuoti, N.; Lonardo, M.S.; Nasti, G.; Gautiero, C.; Belfiore, A.; Guida, B.; Chiurazzi, M. Pathophysiology and Nutritional Approaches in Polycystic Ovary Syndrome (PCOS): A Comprehensive Review. Curr. Nutr. Rep. 2023, 12, 527–544. [Google Scholar] [CrossRef]
- Liao, B.; Qiao, J.; Pang, Y. Central Regulation of PCOS: Abnormal Neuronal-Reproductive-Metabolic Circuits in PCOS Pathophysiology. Front. Endocrinol. 2021, 12, 667422. [Google Scholar] [CrossRef]
- Malik, S.; Saeed, S.; Saleem, A.; Khan, M.I.; Khan, A.; Akhtar, M.F. Alternative treatment of polycystic ovary syndrome: Pre-clinical and clinical basis for using plant-based drugs. Front. Endocrinol. 2023, 14, 1294406. [Google Scholar] [CrossRef]
- Alesi, S.; Ee, C.; Moran, L.J.; Rao, V.; Mousa, A. Nutritional Supplements and Complementary Therapies in Polycystic Ovary Syndrome. Adv. Nutr. 2022, 13, 1243–1266. [Google Scholar] [CrossRef]
- Manouchehri, A.; Abbaszadeh, S.; Ahmadi, M.; Nejad, F.K.; Bahmani, M.; Dastyar, N. Polycystic ovaries and herbal remedies: A systematic review. JBRA Assist. Reprod. 2023, 27, 85–91. [Google Scholar] [CrossRef]
- Cappelli, V.; Musacchio, M.C.; Bulfoni, A.; Morgante, G.; De Leo, V. Natural molecules for the therapy of hyperandrogenism and metabolic disorders in PCOS. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 15–29. [Google Scholar]
- Macut, D.; Bjekić-Macut, J.; Savić-Radojević, A. Dyslipidemia and oxidative stress in PCOS. Front. Horm. Res. 2013, 40, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Mizutani, T.; Miyazaki, Y.; Shirafuji, A.; Tamamura, C.; Fujita, M.; Tsuyoshi, H.; Yoshida, Y. Chronic low-grade inflammation and ovarian dysfunction in women with polycystic ovarian syndrome, endometriosis, and aging. Front. Endocrinol. 2023, 14, 1324429. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Pang, Y. Systemic and ovarian inflammation in women with polycystic ovary syndrome. J. Reprod. Immunol. 2022, 151, 103628. [Google Scholar] [CrossRef]






| Peak No. | Rt (min) | UV λmax (nm) | [M+H]+ (m/z) | Phenolic Compound | Subclass | LSEE |
|---|---|---|---|---|---|---|
| 1 | 3.19 | 270 | 139 | 2-Hydroxybenzoic acid | Hydroxybenzoic acid | 253.16 ± 9.32 |
| 2 | 9.03 | 260, 360 | 935 | Castalagin | Ellagitannin | 301.23 ± 15.26 |
| 3 | 10.56 | 260, 360 | 935 | Vescalagin | Ellagitannin | 537.53 ± 30.62 |
| 4 | 11.99 | 277 | 485 | Digalloyl-glucoside | Gallotanin | 231.69 ± 5.78 |
| 5 | 13.54 | 260 | 465, 303 | Ellagic acid-glucoside | Hydroxybenzoic acid | 288.59 ± 6.44 |
| 6 | 14.65 | 350, 260 | 449, 287 | Luteolin-glucoside | Flavone | 208.94 ± 5.03 |
| 7 | 15.03 | 350, 260 | 449, 287 | Luteolin-glucoside isomer | Flavone | 133.21 ± 2.38 |
| 8 | 15.93 | 340, 270 | 433, 271 | Apigenin-glucoside | Flavone | 140.85 ± 4.55 |
| 9 | 16.37 | 260 | 303 | Ellagic acid | Hydroxybenzoic acid | 326.17 ± 11.92 |
| 10 | 18.38 | 260 | 792, 303 | Ellagic acid derivative | Hydroxybenzoic acid | 249.406 ± 12.51 |
| 11 | 23.37 | 350, 260 | 287 | Luteolin | Flavone | 58.39 ± 1.22 |
| Total phenolics | 2729.168 ± 139.48 |
| Sample | DPPH (μgTE/mL) | H2O2 Scavenging Activity (μgTE/mL) | NO Scavenging Activity (μgQE/mL) | FRAP (mgTE/mL) |
|---|---|---|---|---|
| L. salicaria IC50 | 79.84 ± 8.22 | 107.05 ± 12.03 | 27.51 ± 2.56 | 313.82 ± 39.82 |
| Trolox IC50 | 11.2 ± 1.7 | 24.23 ± 3.12 | - | 12.07 ± 2.04 |
| Quercetin IC50 | - | - | 20.58 ± 3.67 | - |
| p-value | <0.001 | <0.001 | <0.01 | <0.001 |
| Parameters | Control | STZ | STZ + LSEE 100% | STZ + LSEE 50% | STZ + LSEE 25% | STZ + M | STZ + TX |
|---|---|---|---|---|---|---|---|
| Gasdermin (ng/mL) | 5.87 ± 1.05 | 9.50 ± 1.65 aa | 0.28 ± 0.05 bbb,ccc,ddd | 0.93 ± 0.05 bbb,ccc,ddd | 0.34 ± 0.05 bbb,ccc,ddd | 9.38 ± 1.11 | 6.52 ± 1.35 b |
| IL-18 (pg/mL) | 16.30 ± 9.86 | 59.45 ± 8.49 aa | 16.19 ± 3.70 bbb,ccc | 12.69 ± 5.18 bbb,ccc, | 14.30 ± 2.33 bbb,ccc | 37.29 ± 6.97 bb | 11.74 ± 4.59 bbb |
| Caspase (pg/mL) | 37.03 ± 7.11 | 39.49 ± 2.14 | 35.18 ± 5.42 | 30.53 ± 5.39 b,c | 20.83 ± 7.13 bbb,cc | 51.63 ± 21.77 | 42.19 ± 8.81 |
| NFkB-p65 (pg/mL) | 165.11 ± 16.21 | 306.33 ± 28.07 aaa | 255.24 ± 83.59 | 487.87 ± 69.34 bbb,ccc,ddd | 509.93 ± 98.29 bb,ccc,ddd | 230.55 ± 18.46 bb | 222.58 ± 21.94 bb |
| IL-1β (pg/mL) | 23.75 ± 3.15 | 132.50 ± 21.48 aaa | 33.71 ± 5.99 bbb,cc | 23.88 ± 2.46 bbb,c | 32.42 ± 5.63 bbb,cc | 19.58 ± 3.99 bbb | 27.92 ± 3.15 bbb |
| Parameters | Control | LET | LET + LSEE100% | LET + M | LET + TX |
|---|---|---|---|---|---|
| NFkB-p65 (pg/mL) | 258.95 ± 48.74 | 521.69 ± 75.02 aa | 341.65 ± 58.01 bb,cc | 541.67 ± 94.76 | 269.08 ± 90.45 b |
| IL-1β (pg/mL) | 24.75 ± 1.73 | 44.82 ± 11.83 aa | 18.68 ± 2.63 bb | 21.92 ± 3.21 bb | 26.45 ± 1.30 bb |
| IL-18 (pg/mL) | 19.50 ± 1.80 | 34.16 ± 7.30 aa | 24.16 ± 8.95 | 15.39 ± 2.22 bbb | 21.35 ± 6.50 b |
| IL-10 (ng/mL) | 15.27 ± 2.15 | 29.48 ± 5.45 aaa | 23.35 ± 2.45 b,cc | 14.90 ± 1.73 bbb | 22.67 ± 1.74 b |
| Parameters | Control | LET | LET + LSEE 100% | LET + M | LET + TX |
|---|---|---|---|---|---|
| Insulin (pg/mL) | 30.55 ± 5.70 | 98.72 ± 11.32 aaa | 88.91 ± 9.87 ccc,ddd | 25.91 ± 4.80 bb | 27.90 ± 8.5 bb |
| HOMA-IR | 6.64 ± 0.53 | 31.93 ± 4.50 | 20.78 ± 1.98 bb,ccc.ddd | 5.08 ± 0.56 bbb | 7.16 ± 0.74 bbb |
| GLU (mg/dL) | 90.43 ± 15.56 | 131.52 ± 14.09 | 95.25 ± 11.35 bb | 80.4 ± 11.31 bb | 111.73 ± 35.21 |
| FSH (pg/mL) | 39.80 ± 5.74 | 99.96 ± 31.89 aa | 51.08 ± 3.05 b,ccc,ddd | 99.39 ± 3.61 | 101.15 ± 34.59 |
| LH (pg/mL) | 9.81 ± 4.37 | 25.08 ± 8.22 aa | 17.51 ± 2.91 bbb | 20.62 ± 4.04 bbb | 13.11 ± 2.95 bbb |
| Estrogen (pg/mL) | 596.40 ± 66.99 | 329.78 ± 55.13 aaa | 629.57 ± 25.87 bbb,cc | 774.88 ± 55.77 bbb | 622.02 ± 166.26 bbb |
| Testosterone (ng/mL) | 0.94 ± 0.28 | 1.60 ± 0.86 a | 0.82 ± 0.07 b,dd | 1.51 ± 0.99 | 2.36 ± 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usatiuc, L.O.; Pârvu, M.; Pop, R.M.; Uifălean, A.; Vălean, D.; Surd, A.; Țicolea, M.; Hîruța, A.; Ranga, F.; Cătoi, F.A.; et al. Therapeutic Potential of Lythrum salicaria L. Ethanol Extract in Experimental Rat Models of Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome. Antioxidants 2025, 14, 573. https://doi.org/10.3390/antiox14050573
Usatiuc LO, Pârvu M, Pop RM, Uifălean A, Vălean D, Surd A, Țicolea M, Hîruța A, Ranga F, Cătoi FA, et al. Therapeutic Potential of Lythrum salicaria L. Ethanol Extract in Experimental Rat Models of Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome. Antioxidants. 2025; 14(5):573. https://doi.org/10.3390/antiox14050573
Chicago/Turabian StyleUsatiuc, Lia Oxana, Marcel Pârvu, Raluca Maria Pop, Ana Uifălean, Dan Vălean, Adrian Surd, Mădălina Țicolea, Ana Hîruța, Floricuța Ranga, Florinela Adriana Cătoi, and et al. 2025. "Therapeutic Potential of Lythrum salicaria L. Ethanol Extract in Experimental Rat Models of Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome" Antioxidants 14, no. 5: 573. https://doi.org/10.3390/antiox14050573
APA StyleUsatiuc, L. O., Pârvu, M., Pop, R. M., Uifălean, A., Vălean, D., Surd, A., Țicolea, M., Hîruța, A., Ranga, F., Cătoi, F. A., Cătană, C., & Pârvu, A. E. (2025). Therapeutic Potential of Lythrum salicaria L. Ethanol Extract in Experimental Rat Models of Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome. Antioxidants, 14(5), 573. https://doi.org/10.3390/antiox14050573












