Growth-Associated Protein-43 Loss Promotes Ca2+ and ROS Imbalance in Cardiomyocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Animal Models
2.3. Isolation of Primary Cardiomyocytes from Neonatal Mouse Heart
2.4. Western Blotting Assays
2.5. Cytosolic and Mitochondrial Ca2+ Imaging
2.6. ROS and Mitochondrial Superoxide Anion Level Measurements
2.7. Mitochondrial Membrane Potential Measurements
2.8. Immunofluorescence Staining
2.9. Measurements of Glucose and Lactate Levels in Cell Culture Medium
2.10. Statistical Analysis
3. Results
3.1. GAP-43−/− Cardiomyocytes Express a Hypertrophic Phenotype
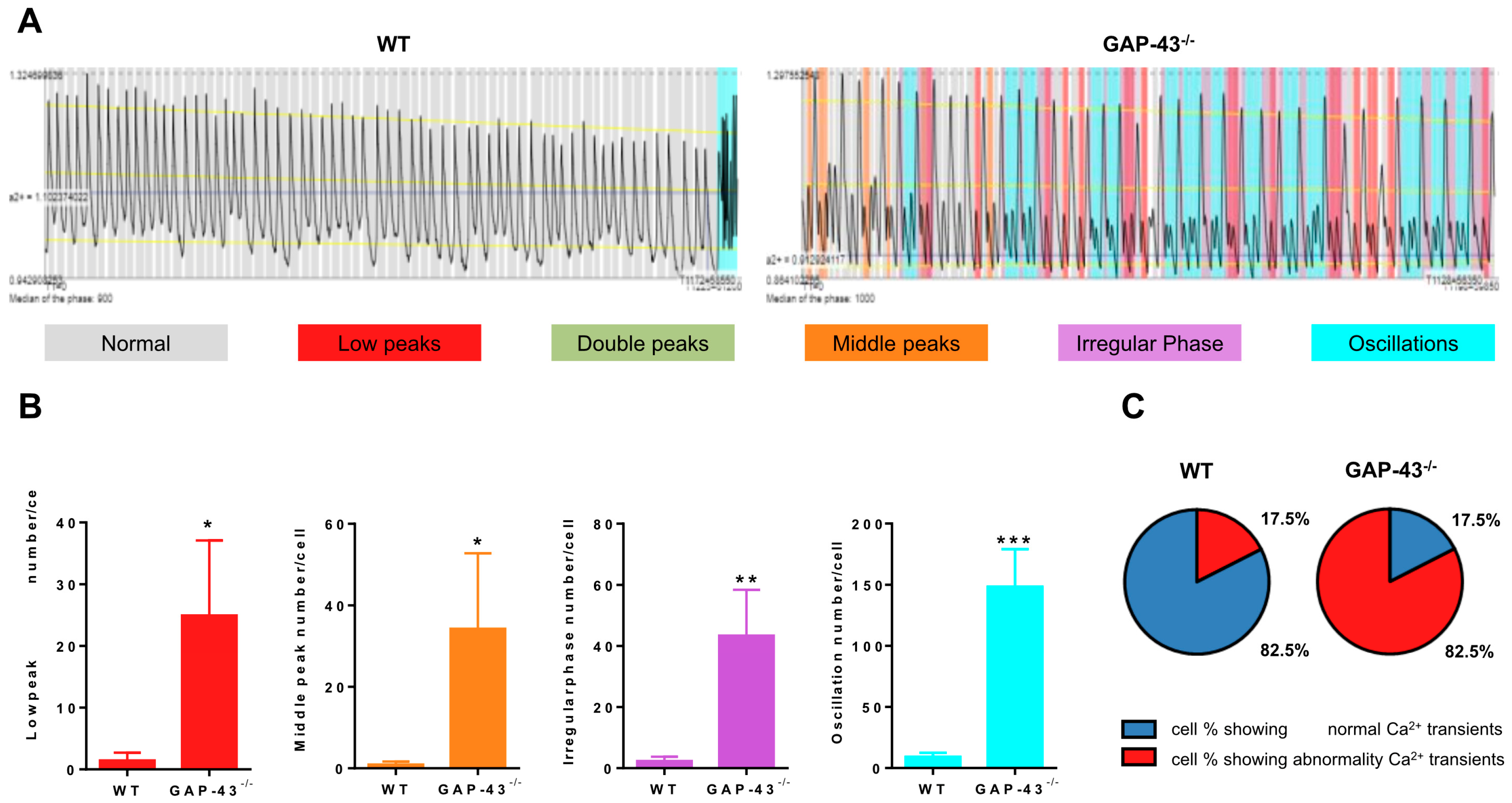
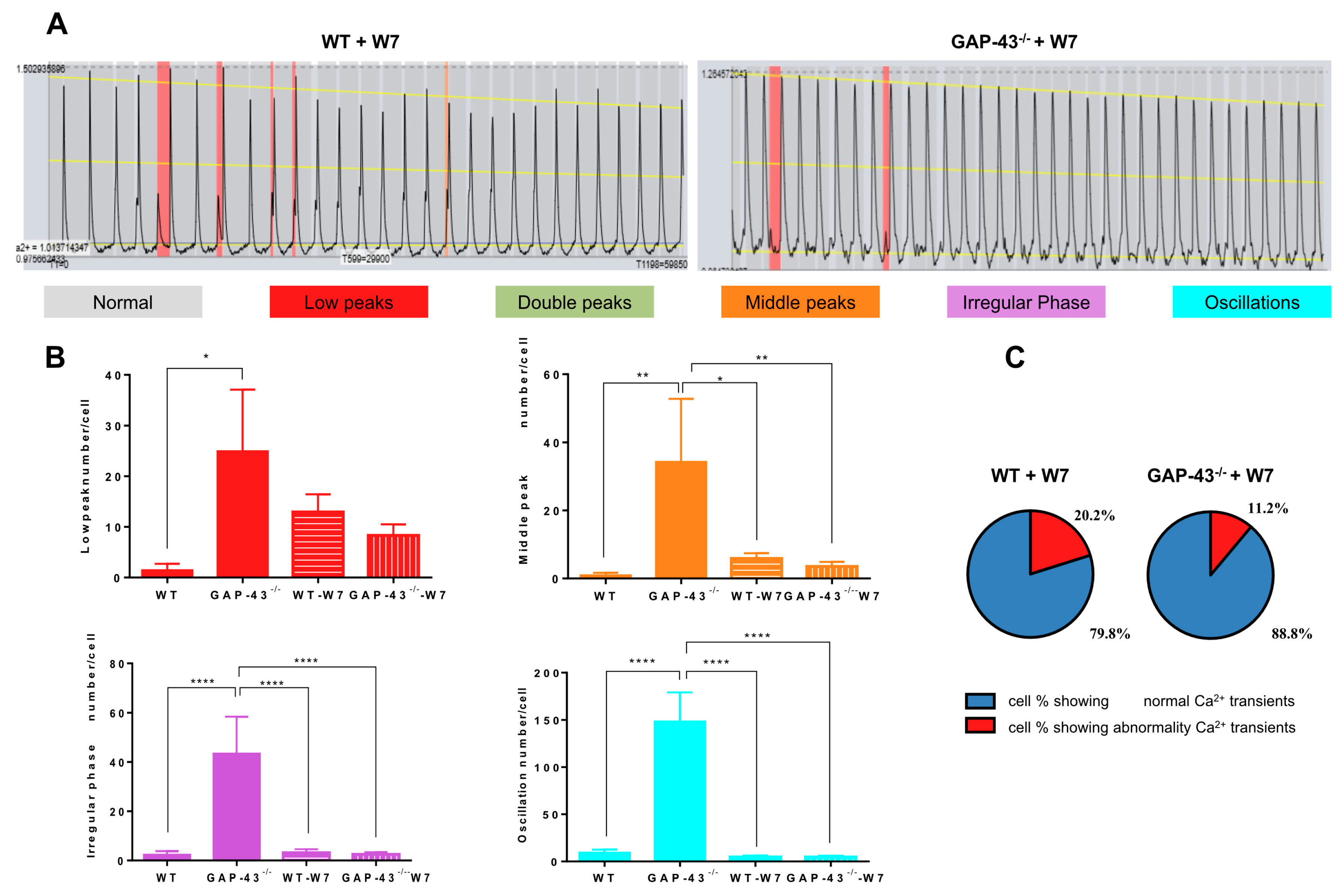
3.2. GAP-43−/− Cardiomyocytes Show Intracellular Ca2+ Dyshomeostasis
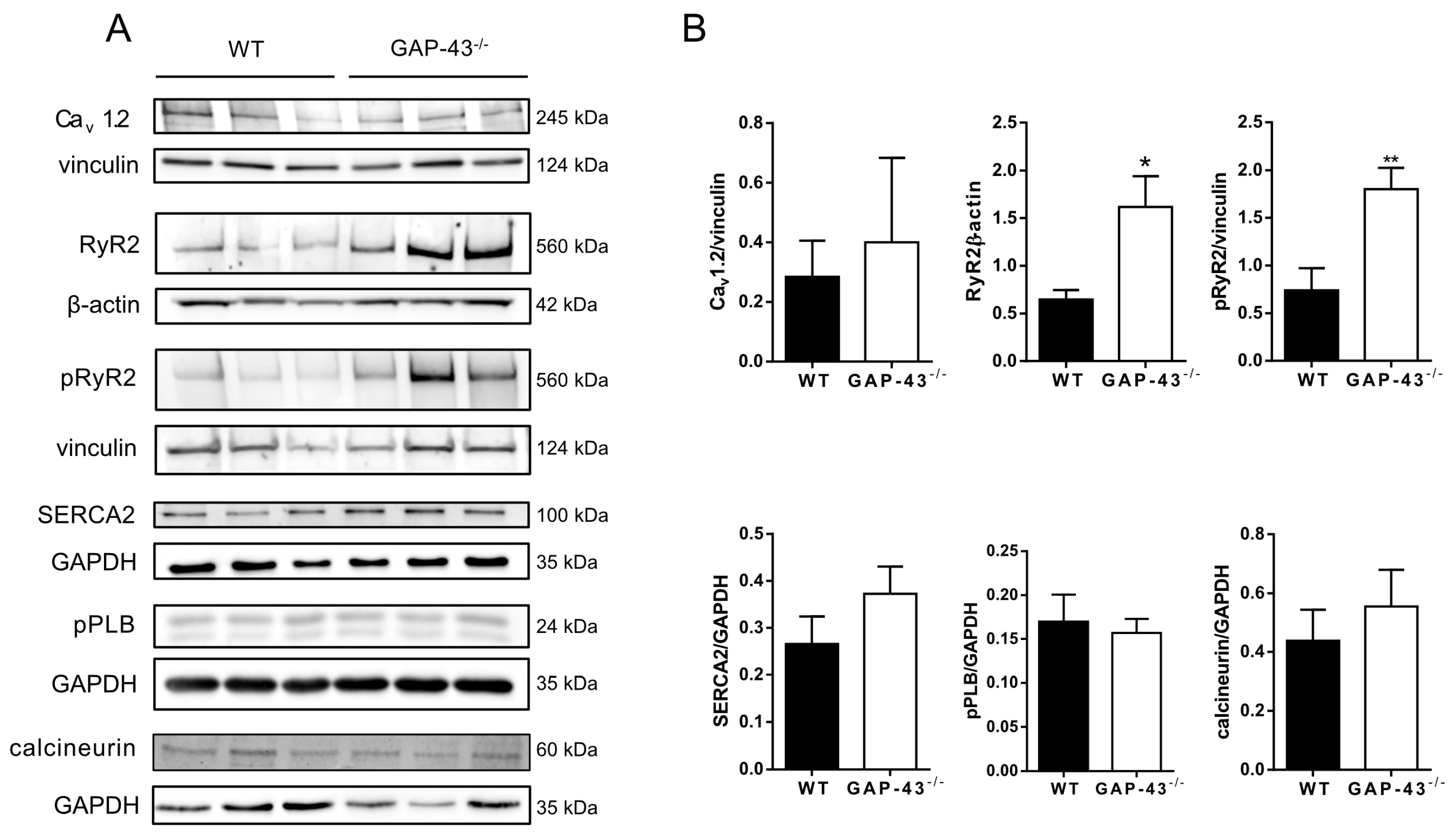
3.3. Expression Levels of Ca2+ Handling Proteins in GAP-43−/− Cardiomyocytes
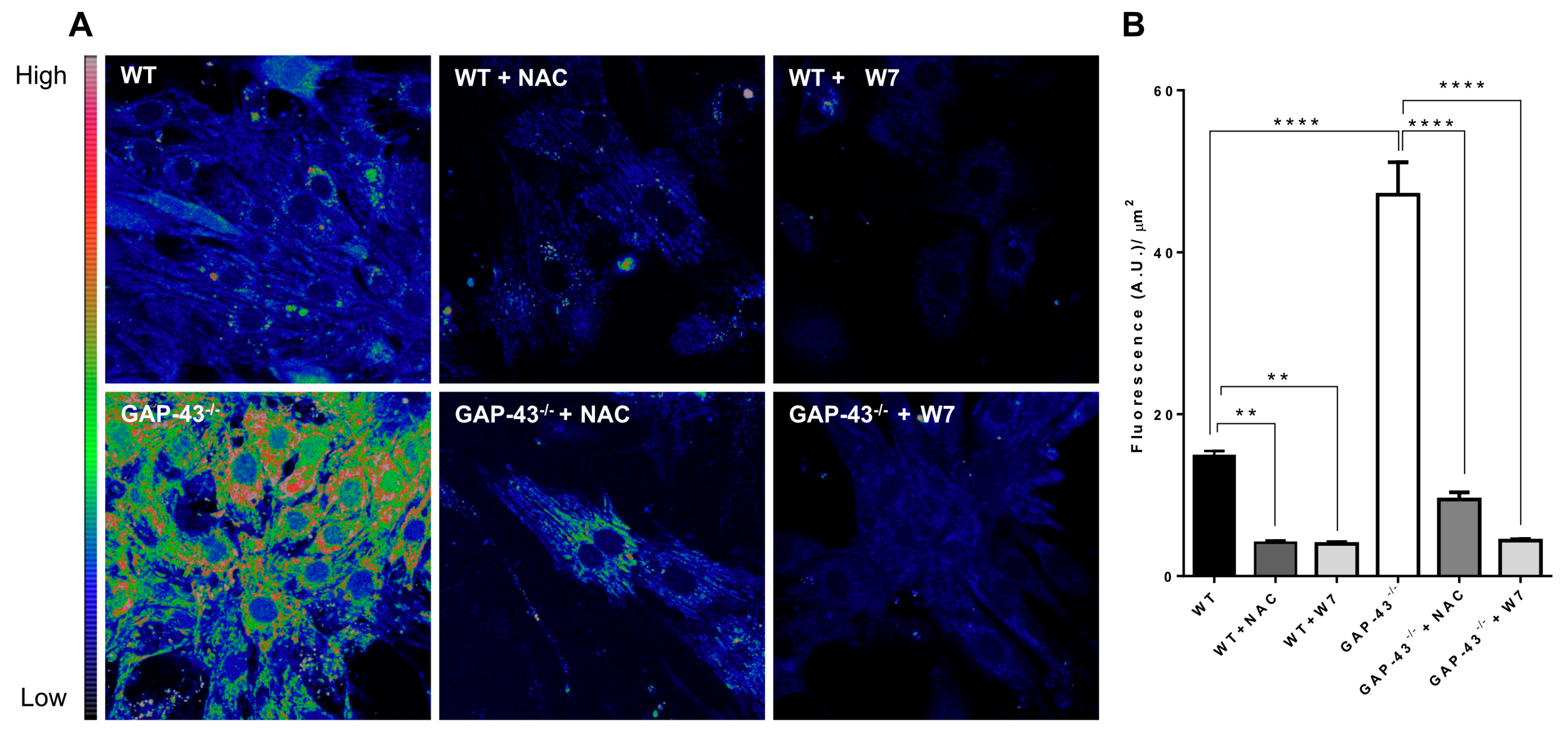
3.4. GAP-43−/− Cardiomyocytes Produce Increased Amounts of ROS
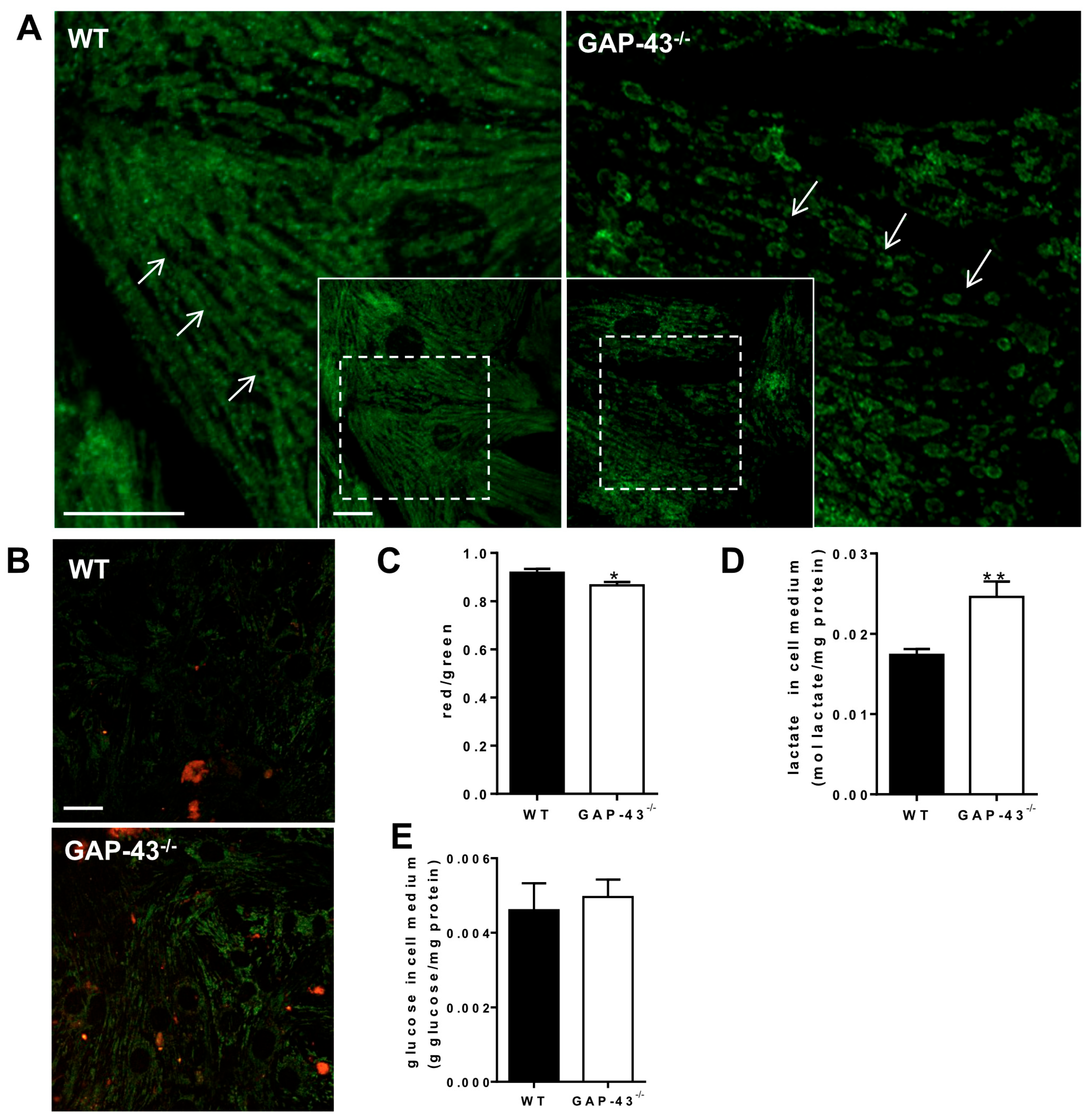
3.5. GAP-43−/− Mitochondria Show Altered Morphology and Metabolism
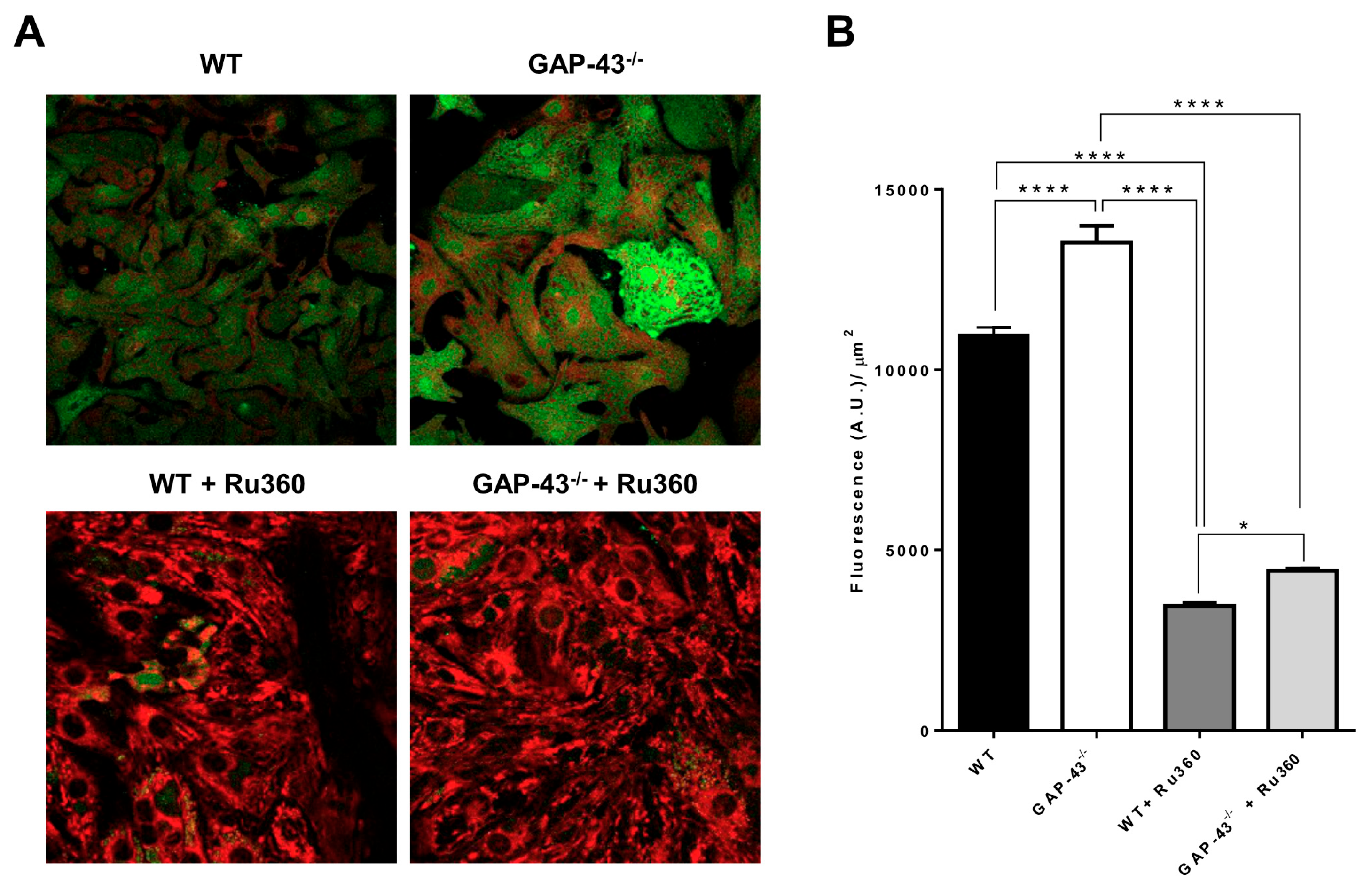
3.6. GAP-43−/− Mitochondria Show Ca2+ Overload and Higher Superoxide Production
4. Discussion
5. Limitations of the Study and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Strittmatter, S.M.; Fankhauser, C.; Huang, P.L.; Mashimo, H.; Fishman, M.C. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell 1995, 80, 445–452. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, A.; Vitkovic, L. Regulation of immunoreactive GAP-43 expression in rat cortical macroglia is cell type specific. J. Cell Biol. 1990, 111, 209–215. [Google Scholar] [CrossRef]
- Deloulme, J.C.; Janet, T.; Au, D.; Storm, D.R.; Sensenbrenner, M.; Baudier, J. Neuromodulin (GAP43): A neuronal protein kinase C substrate is also present in 0-2A glial cell lineage. Characterization of neuromodulin in secondary cultures of oligodendrocytes and comparison with the neuronal antigen. J. Cell Biol. 1990, 111, 1559–1569. [Google Scholar] [CrossRef]
- Stocker, K.M.; Ciment, G.; Baizer, L. GAP-43 in non-neuronal cells of the embryonic chick limb: Clues to function. Perspect. Dev. Neurobiol. 1992, 1, 53–62. [Google Scholar]
- Heuss, D.; Schlotzer-Schrehardt, U. Subcellular localization of phosphoprotein B-50 in regenerating muscle. An immuno-electron microscopic study. Neurol. Res. 1998, 20, 360–364. [Google Scholar] [CrossRef]
- Guarnieri, S.; Morabito, C.; Paolini, C.; Boncompagni, S.; Pilla, R.; Fano-Illic, G.; Mariggio, M.A. Growth associated protein 43 is expressed in skeletal muscle fibers and is localized in proximity of mitochondria and calcium release units. PLoS ONE 2013, 8, e53267. [Google Scholar] [CrossRef]
- Caprara, G.A.; Perni, S.; Morabito, C.; Mariggio, M.A.; Guarnieri, S. Specific association of growth-associated protein 43 with calcium release units in skeletal muscles of lower vertebrates. Eur. J. Histochem. 2014, 58, 2453. [Google Scholar] [CrossRef]
- Mosevitsky, M.I. Nerve ending “signal” proteins GAP-43, MARCKS, and BASP1. Int. Rev. Cytol. 2005, 245, 245–325. [Google Scholar]
- Caprara, G.A.; Morabito, C.; Perni, S.; Navarra, R.; Guarnieri, S.; Mariggio, M.A. Evidence for Altered Ca2+ Handling in Growth Associated Protein 43-Knockout Skeletal Muscle. Front. Physiol. 2016, 7, 493. [Google Scholar] [CrossRef]
- Rahmati, M.; Taherabadi, S.J. The effects of exercise training on Kinesin and GAP-43 expression in skeletal muscle fibers of STZ-induced diabetic rats. Sci. Rep. 2021, 11, 9535. [Google Scholar] [CrossRef]
- Bevere, M.; Morabito, C.; Guarnieri, S.; Mariggio, M.A. Mice lacking growth-associated protein 43 develop cardiac remodeling and hypertrophy. Histochem. Cell Biol. 2022, 157, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Helms, A.S.; Alvarado, F.J.; Yob, J.; Tang, V.T.; Pagani, F.; Russell, M.W.; Valdivia, H.H.; Day, S.M. Genotype-Dependent and -Independent Calcium Signaling Dysregulation in Human Hypertrophic Cardiomyopathy. Circulation 2016, 134, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Cortassa, S.; Marban, E.; O’Rourke, B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003, 278, 44735–44744. [Google Scholar] [CrossRef]
- Penttinen, K.; Siirtola, H.; Avalos-Salguero, J.; Vainio, T.; Juhola, M.; Aalto-Setala, K. Novel Analysis Software for Detecting and Classifying Ca2+ Transient Abnormalities in Stem Cell-Derived Cardiomyocytes. PLoS ONE 2015, 10, e0135806. [Google Scholar] [CrossRef]
- Morabito, C.; Rovetta, F.; Bizzarri, M.; Mazzoleni, G.; Fano, G.; Mariggio, M.A. Modulation of redox status and calcium handling by extremely low frequency electromagnetic fields in C2C12 muscle cells: A real-time, single-cell approach. Free Radic. Biol. Med. 2010, 48, 579–589. [Google Scholar] [CrossRef]
- Berardini, M.; Gesualdi, L.; Morabito, C.; Ferranti, F.; Reale, A.; Zampieri, M.; Karpach, K.; Tinari, A.; Bertuccini, L.; Guarnieri, S.; et al. Simulated Microgravity Exposure Induces Antioxidant Barrier Deregulation and Mitochondria Enlargement in TCam-2 Cell Spheroids. Cells 2023, 12, 335–344. [Google Scholar] [CrossRef]
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef]
- Liang, Q.; De Windt, L.J.; Witt, S.A.; Kimball, T.R.; Markham, B.E.; Molkentin, J.D. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J. Biol. Chem. 2001, 276, 30245–30253. [Google Scholar] [CrossRef]
- Akazawa, H.; Komuro, I. Roles of cardiac transcription factors in cardiac hypertrophy. Circ. Res. 2003, 92, 1079–1088. [Google Scholar] [CrossRef]
- Ridinger, H.; Rutenberg, C.; Lutz, D.; Buness, A.; Petersen, I.; Amann, K.; Maercker, C. Expression and tissue localization of beta-catenin, alpha-actinin and chondroitin sulfate proteoglycan 6 is modulated during rat and human left ventricular hypertrophy. Exp. Mol. Pathol. 2009, 86, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.B.; Sondergaard, M.T.; Overgaard, M.T. Calmodulin in a heartbeat. FEBS J. 2013, 280, 5511–5532. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Altered cardiac myocyte Ca regulation in heart failure. Physiology 2006, 21, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Mazeto, I.F.S.; Okoshi, K.; Silveira, C.; Sant’Ana, P.G.; Silva, V.L.D.; Mota, G.A.F.; Souza, S.L.B.; Vileigas, D.F.; Padovani, C.R.; Cicogna, A.C. Calcium homeostasis behavior and cardiac function on left ventricular remodeling by pressure overload. Braz. J. Med. Biol. Res. 2021, 54, e10138. [Google Scholar] [CrossRef]
- Wehrens, X.H.; Lehnart, S.E.; Reiken, S.R.; Marks, A.R. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 2004, 94, e61–e70. [Google Scholar] [CrossRef]
- Asghari, P.; Scriven, D.R.; Ng, M.; Panwar, P.; Chou, K.C.; van Petegem, F.; Moore, E.D. Cardiac ryanodine receptor distribution is dynamic and changed by auxiliary proteins and post-translational modification. eLife 2020, 9, e51602. [Google Scholar] [CrossRef]
- Yano, M.; Ikeda, Y.; Matsuzaki, M. Altered intracellular Ca2+ handling in heart failure. J. Clin. Investig. 2005, 115, 556–564. [Google Scholar] [CrossRef]
- Muth, J.N.; Bodi, I.; Lewis, W.; Varadi, G.; Schwartz, A. A Ca2+-dependent transgenic model of cardiac hypertrophy: A role for protein kinase Calpha. Circulation 2001, 103, 140–147. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Takahashi, N.; Xu, L.; Smithies, O.; Meissner, G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca release channel. J. Clin. Investig. 2007, 117, 1344–1353. [Google Scholar] [CrossRef]
- Xu, X.; Yano, M.; Uchinoumi, H.; Hino, A.; Suetomi, T.; Ono, M.; Tateishi, H.; Oda, T.; Okuda, S.; Doi, M.; et al. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem. Biophys. Res. Commun. 2010, 394, 660–666. [Google Scholar] [CrossRef]
- Ono, M.; Yano, M.; Hino, A.; Suetomi, T.; Xu, X.; Susa, T.; Uchinoumi, H.; Tateishi, H.; Oda, T.; Okuda, S.; et al. Dissociation of calmodulin from cardiac ryanodine receptor causes aberrant Ca2+ release in heart failure. Cardiovasc. Res. 2010, 87, 609–617. [Google Scholar] [CrossRef] [PubMed]
- De Koninck, P.; Schulman, H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 1998, 279, 227–230. [Google Scholar] [CrossRef]
- Bers, D.M. Ca2+-calmodulin-dependent protein kinase II regulation of cardiac excitation-transcription coupling. Heart Rhythm. 2011, 8, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M.; Grandi, E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J. Cardiovasc. Pharmacol. 2009, 54, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, E.; Kass, D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2007, 49, 241–248. [Google Scholar] [CrossRef]
- Sabri, A.; Hughie, H.H.; Lucchesi, P.A. Regulation of hypertrophic and apoptotic signaling pathways by reactive oxygen species in cardiac myocytes. Antioxid. Redox Signal 2003, 5, 731–740. [Google Scholar] [CrossRef]
- Pitter, J.G.; Maechler, P.; Wollheim, C.B.; Spat, A. Mitochondria respond to Ca2+ already in the submicromolar range: Correlation with redox state. Cell Calcium 2002, 31, 97–104. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Margreiter, R.; Ausserlechner, M.J.; Hagenbuchner, J. The Complex Interplay between Mitochondria, ROS and Entire Cellular Metabolism. Antioxidants 2022, 11, 1995. [Google Scholar] [CrossRef]
- Alves-Figueiredo, H.; Silva-Platas, C.; Estrada, M.; Oropeza-Almazan, Y.; Ramos-Gonzalez, M.; Bernal-Ramirez, J.; Vazquez-Garza, E.; Tellez, A.; Salazar-Ramirez, F.; Mendez-Fernandez, A.; et al. Mitochondrial Ca2+ Uniporter-Dependent Energetic Dysfunction Drives Hypertrophy in Heart Failure. JACC Basic Transl. Sci. 2024, 9, 496–518. [Google Scholar] [CrossRef]
- Hamilton, S.; Terentyeva, R.; Kim, T.Y.; Bronk, P.; Clements, R.T.; O-Uchi, J.; Csordas, G.; Choi, B.R.; Terentyev, D. Pharmacological Modulation of Mitochondrial Ca2+ Content Regulates Sarcoplasmic Reticulum Ca2+ Release via Oxidation of the Ryanodine Receptor by Mitochondria-Derived Reactive Oxygen Species. Front. Physiol. 2018, 9, 1831. [Google Scholar] [CrossRef]
- Zaglia, T.; Ceriotti, P.; Campo, A.; Borile, G.; Armani, A.; Carullo, P.; Prando, V.; Coppini, R.; Vida, V.; Stolen, T.O.; et al. Content of mitochondrial calcium uniporter (MCU) in cardiomyocytes is regulated by microRNA-1 in physiologic and pathologic hypertrophy. Proc. Natl. Acad. Sci. USA 2017, 114, E9006–E9015. [Google Scholar] [CrossRef]
- Malyala, S.; Zhang, Y.; Strubbe, J.O.; Bazil, J.N. Calcium phosphate precipitation inhibits mitochondrial energy metabolism. PLoS Comput. Biol. 2019, 15, e1006719. [Google Scholar] [CrossRef]
- Walkon, L.L.; Strubbe-Rivera, J.O.; Bazil, J.N. Calcium Overload and Mitochondrial Metabolism. Biomolecules 2022, 12, 1891. [Google Scholar] [CrossRef]
- Ordono, J.; Perez-Amodio, S.; Ball, K.; Aguirre, A.; Engel, E. The generation of a lactate-rich environment stimulates cell cycle progression and modulates gene expression on neonatal and hiPSC-derived cardiomyocytes. Biomater. Adv. 2022, 139, 213035. [Google Scholar] [CrossRef]
- Luti, S.; Militello, R.; Pinto, G.; Illiano, A.; Marzocchini, R.; Santi, A.; Becatti, M.; Amoresano, A.; Gamberi, T.; Pellegrino, A.; et al. Chronic lactate exposure promotes cardiomyocyte cytoskeleton remodelling. Heliyon 2024, 10, e24719. [Google Scholar] [CrossRef]
- Erickson, J.R.; Joiner, M.L.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Wang, S.Q.; Chakir, K.; Yang, D.; Zhang, T.; Brown, J.H.; Devic, E.; Kobilka, B.K.; Cheng, H.; Xiao, R.P. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J. Clin. Investig. 2003, 111, 617–625. [Google Scholar] [CrossRef]
- Zhu, W.; Woo, A.Y.; Yang, D.; Cheng, H.; Crow, M.T.; Xiao, R.P. Activation of CaMKIIdeltaC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J. Biol. Chem. 2007, 282, 10833–10839. [Google Scholar] [CrossRef]
- Peng, W.; Zhang, Y.; Zheng, M.; Cheng, H.; Zhu, W.; Cao, C.M.; Xiao, R.P. Cardioprotection by CaMKII-deltaB is mediated by phosphorylation of heat shock factor 1 and subsequent expression of inducible heat shock protein 70. Circ. Res. 2010, 106, 102–110. [Google Scholar] [CrossRef]
- Little, G.H.; Saw, A.; Bai, Y.; Dow, J.; Marjoram, P.; Simkhovich, B.; Leeka, J.; Kedes, L.; Kloner, R.A.; Poizat, C. Critical role of nuclear calcium/calmodulin-dependent protein kinase IIdeltaB in cardiomyocyte survival in cardiomyopathy. J. Biol. Chem. 2009, 284, 24857–24868. [Google Scholar] [CrossRef]
- Purcell, N.H.; Tang, G.; Yu, C.; Mercurio, F.; DiDonato, J.A.; Lin, A. Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 6668–6673. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.A.; Siddiqui, M.A. The JAK-STAT pathway in hypertrophic stress signaling and genomic stress response. JAKSTAT 2012, 1, 131–141. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bevere, M.; Morabito, C.; Verucci, D.; Di Sinno, N.; Mariggiò, M.A.; Guarnieri, S. Growth-Associated Protein-43 Loss Promotes Ca2+ and ROS Imbalance in Cardiomyocytes. Antioxidants 2025, 14, 361. https://doi.org/10.3390/antiox14030361
Bevere M, Morabito C, Verucci D, Di Sinno N, Mariggiò MA, Guarnieri S. Growth-Associated Protein-43 Loss Promotes Ca2+ and ROS Imbalance in Cardiomyocytes. Antioxidants. 2025; 14(3):361. https://doi.org/10.3390/antiox14030361
Chicago/Turabian StyleBevere, Michele, Caterina Morabito, Delia Verucci, Noemi Di Sinno, Maria A. Mariggiò, and Simone Guarnieri. 2025. "Growth-Associated Protein-43 Loss Promotes Ca2+ and ROS Imbalance in Cardiomyocytes" Antioxidants 14, no. 3: 361. https://doi.org/10.3390/antiox14030361
APA StyleBevere, M., Morabito, C., Verucci, D., Di Sinno, N., Mariggiò, M. A., & Guarnieri, S. (2025). Growth-Associated Protein-43 Loss Promotes Ca2+ and ROS Imbalance in Cardiomyocytes. Antioxidants, 14(3), 361. https://doi.org/10.3390/antiox14030361








