A Comparative Study of Traditional Sun Drying and Hybrid Solar Drying on Quality, Safety, and Bioactive Compounds in “Pingo de Mel” Fig
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Moisture
2.4. Water Activity (aw)
2.5. Firmness
2.6. Size of the Fruits
2.7. Hardness
2.8. °Brix
2.9. Color Analysis
2.10. Sun, Drying Process
2.11. Hybrid Solar Drying Process
2.12. Microbiological Evaluation
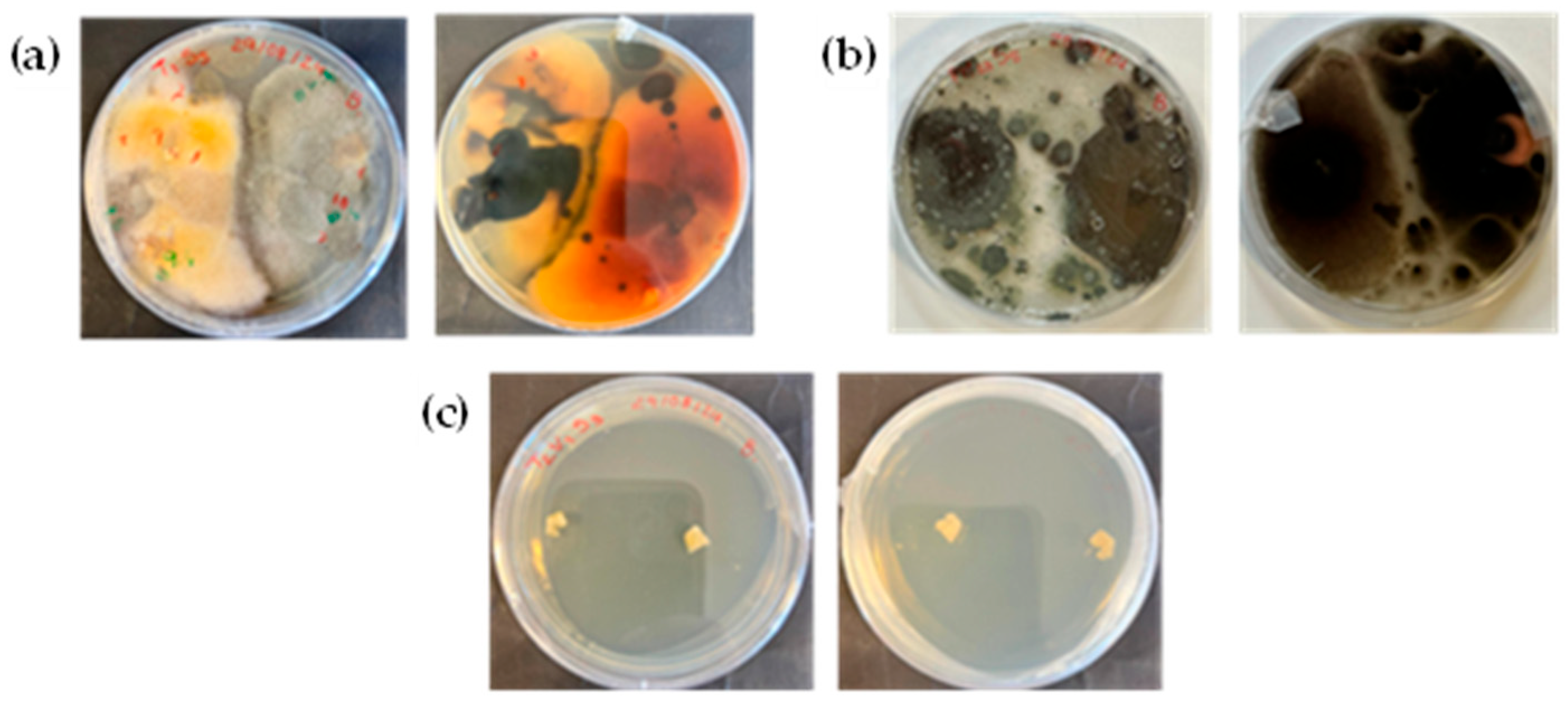
2.12.1. Fungi Isolation and Replication
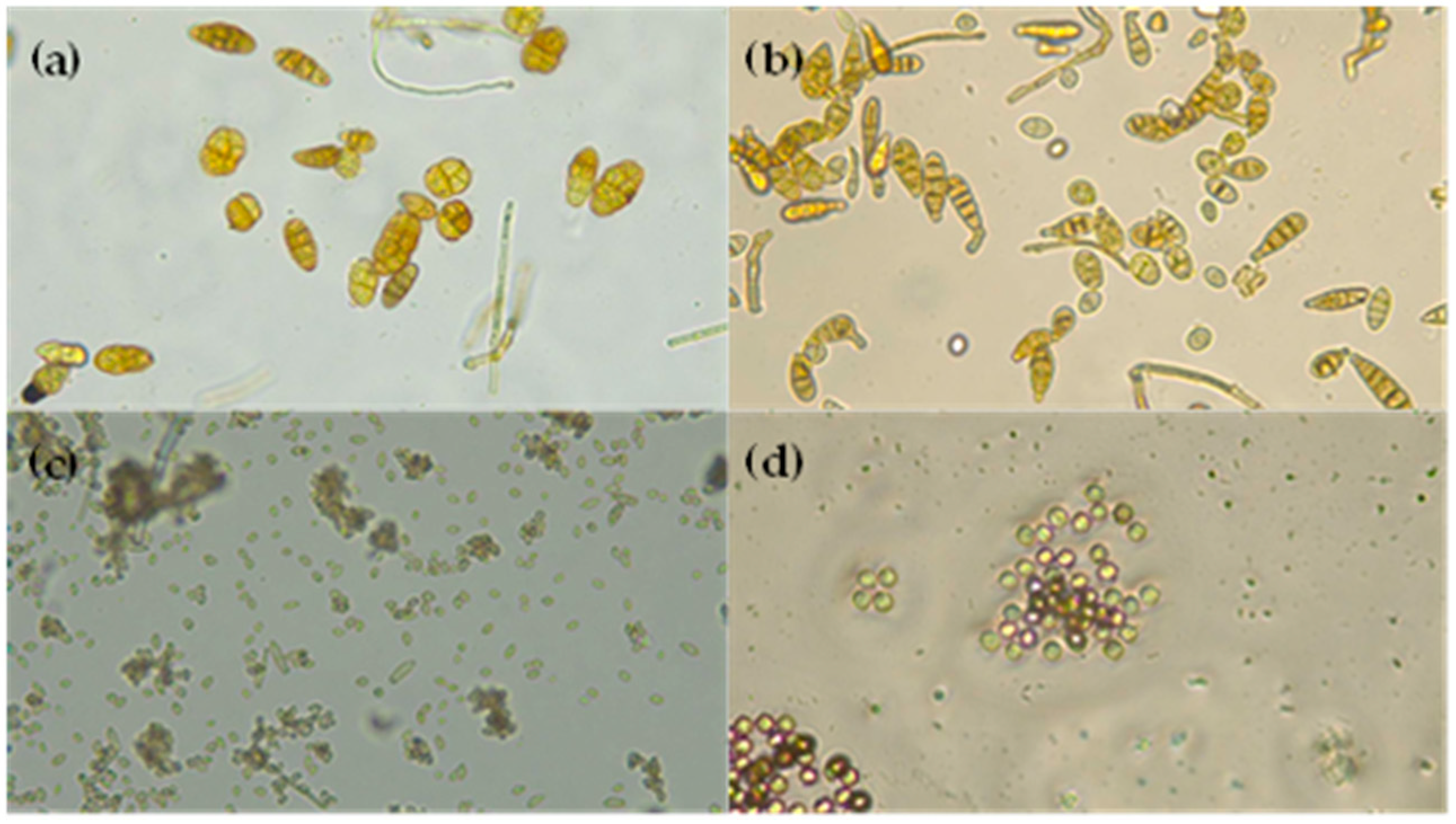
2.12.2. Morphological Identification
2.13. Biochemical Analysis
2.13.1. Extraction Procedure
2.13.2. Antioxidant Capacity
2.13.3. Analysis of Individual Compounds
2.14. Statistical Analysis
3. Results and Discussion
3.1. Quality Features of Fresh and Dried Figs
Physical Properties
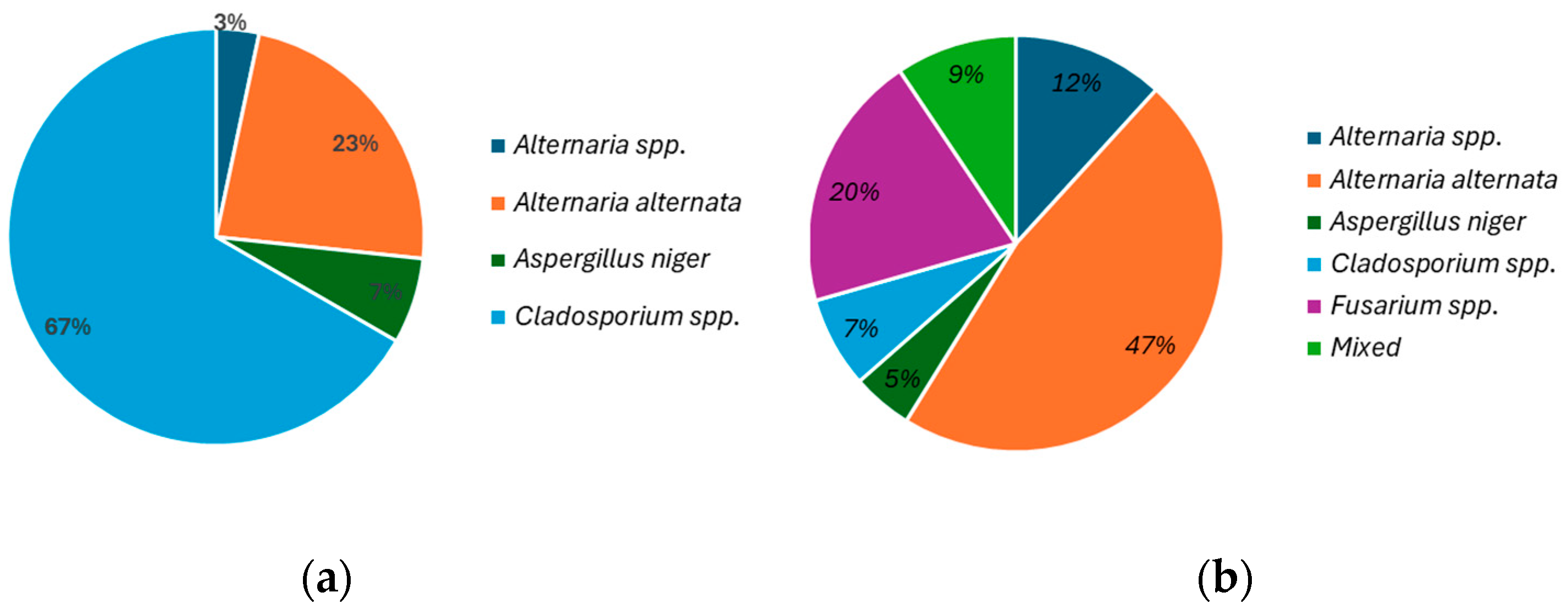
3.2. Microbiological Fungi Load
3.3. Phenolic Compound Profile and Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crisosto, H.; Ferguson, L.; Bremer, V.; Stover, E.; Colelli, G. Fig (Ficus carica L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing: Sawston, UK, 2011; pp. 134–160. [Google Scholar] [CrossRef]
- Kislev, M.E.; Hartmann, A.; Bar-Yosef, O. Early domesticated fig in the Jordan Valley. Science 2006, 312, 1372–1374. [Google Scholar] [CrossRef]
- Sousa, R.M. Manual de Boas Práticas de Fruticultura—Figueira—11o Fascículo; Frutas Legumes e Flores em parceria com INIAV, I.P.; (Estação Nacional de Fruticultura Vieira Natividade) e COTR: Alcobaça, Portugal, 2021. [Google Scholar]
- Food and Agriculture Organization of the United States. Crops and Livestock Products—Figs; Food and Agriculture Organization of the United States: Washington, DC, USA, 2013. [Google Scholar]
- Stover, E.; Aradhya, M.; Ferguson, L.; Crisosto, C.H. The Fig: Overview of an Ancient Fruit. HortScience 2007, 42, 1083–1087. [Google Scholar] [CrossRef]
- Hajam, T.A.; Saleem, H. Phytochemistry, biological activities, industrial and traditional uses of fig (Ficus carica): A review. Chem. Interactions 2022, 368, 110237. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.Y.; Alshaikhi, A.I.; Hazzazi, J.S.; Kurdi, J.R.; Ramadan, M.F. Recent insight on nutritional value, active phytochemicals, and health-enhancing characteristics of fig (Ficus craica). Food Saf. Health 2024, 2, 179–195. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Valentão, P.; Pereira, J.A.; Silva, B.M.; Tavares, F.; Andrade, P.B. Ficus carica L.: Metabolic and biological screening. Food Chem. Toxicol. 2009, 47, 2841–2846. [Google Scholar] [CrossRef]
- Nuri, Z.; Uddin, S. A review on nutritional values and pharmacological importance of Ficus carica. Int. J. Hortic. Food Sci. 2020, 2, 55–59. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Tan, X.; Show, P.L.; Rambabu, K.; Banat, F.; Veeramuthu, A.; Lau, B.F.; Ng, E.P.; Ling, T.C. Incorporating biowaste into circular bioeconomy: A critical review of current trend and scaling up feasibility. Environ. Technol. Innov. 2020, 19, 101034. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 871, 416258. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kitabatake, M.; Kayano, S.-I.; Ito, T. Dietary Phenolic Compounds: Their Health Benefits and Association with the Gut Microbiota. Antioxidants 2023, 12, 880. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Islam, M.; Edirisinghe, I.; Burton-Freeman, B. Phytochemical Composition and Health Benefits of Figs (Fresh and Dried): A Review of Literature from 2000 to 2022. Nutrients 2023, 15, 2623. [Google Scholar] [CrossRef]
- Aljane, F.; Neily, M.; Msaddak, A. Phytochemical characteristics and antioxidant activity of several fig (Ficus carica L.) ecotypes. Ital. J. Food Sci. 2020, 32, 755–768. [Google Scholar] [CrossRef]
- Vallejo, F.; Marín, J.G.; Tomás-Barberán, F.A. Phenolic compound content of fresh and dried figs (Ficus carica L.). Food Chem. 2012, 130, 485–492. [Google Scholar] [CrossRef]
- Chimi, H.; Ouaouich, A.; Semmar, M.; Tayebi, S. Industrial processing of figs by solar drying in Morocco. Acta Hortic. 2008, 798, 331–334. [Google Scholar] [CrossRef]
- Heperkan, D.; Moretti, A.; Dikmen, C.D.; Logrieco, A.F. Toxigenic fungi and mycotoxin associated with figs in the Mediterranean area. Phytopathol. Mediterr. 2012, 51, 119–130. [Google Scholar]
- Slatnar, A.; Klancar, U.; Stampar, F.; Veberic, R. Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J. Agric. Food Chem. 2011, 59, 11696–11702. [Google Scholar] [CrossRef]
- Ferreira, D.; Silva, J.A.L.D.; Pinto, G.; Santos, C.; Delgadillo, I.; Coimbra, M.A. Effect of sun-drying on microstructure and texture of S. Bartolomeu pears (Pyrus communis L.). Eur. Food Res. Technol. 2008, 226, 1545–1552. [Google Scholar] [CrossRef]
- Catorze, C.; Tavares, A.; Cardão, P.; Castro, A.; Silva, M.; Ferreira, D.; Lopes, S.; Brás, I. Study of a solar energy drying system—Energy savings and effect in dried food quality. Energy Rep. 2022, 8, 392–398. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Cardoso, S.M. Salvia species as nutraceuticals: Focus on antioxidant, antidiabetic and anti-obesity properties. Appl. Sci. 2021, 11, 9365. [Google Scholar] [CrossRef]
- Mohammadi, X.; Deng, Y.; Matinfar, G.; Singh, A.; Mandal, R.; Pratap-Singh, A. Impact of Three Different Dehydration Methods on Nutritional Values and Sensory Quality of Dried Broccoli, Oranges, and Carrots. Foods 2020, 9, 1464. [Google Scholar] [CrossRef]
- Sandman. Water Activity (aw) and Food Safety. The Food Untold, 22 July 2021. [Google Scholar]
- Rahman, M.S.; Labuza, T.P. Water activity and food preservation. In Handbook of Food Preservation; Shafiur, M., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 339–382. [Google Scholar] [CrossRef]
- Kępińska-Pacelik, J.; Biel, W. Mycotoxins—Prevention, Detection, Impact on Animal Health. Processes 2021, 9, 2035. [Google Scholar] [CrossRef]
- Galván, A.I.; Córdoba, M.d.G.; Rodríguez, A.; Martín, A.; López-Corrales, M.; Ruiz-Moyano, S.; Serradilla, M.J. Evaluation of fungal hazards associated with dried fig processing. Int. J. Food Microbiol. 2022, 365, 109541. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria Fungi and Their Bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef] [PubMed]
- Galván, A.; Córdoba, M.; Ruiz-Moyano, S.; López-Corrales, M.; Aranda, E.; Rodríguez, A.; Serradilla, M. Impact of water management and geographic location on the physicochemical traits and fungal population of ‘Calabacita’ dried figs in Extremadura (Spain). Sci. Hortic. 2023, 308, 111543. [Google Scholar] [CrossRef]
- Woo, S.Y.; Lee, S.Y.; Jeong, T.K.; Park, S.M.; Auh, J.H.; Shin, H.-S.; Chun, H.S. Natural Occurrence of Alternaria Toxins in Agricultural Products and Processed Foods Marketed in South Korea by LC–MS/MS. Toxins 2022, 14, 824. [Google Scholar] [CrossRef]
- López, P.; Venema, D.; de Rijk, T.; de Kok, A.; Scholten, J.M.; Mol, H.G.; de Nijs, M. Occurrence of Alternaria toxins in food products in The Netherlands. Food Control. 2016, 60, 196–204. [Google Scholar] [CrossRef]
- Navale, V.; Vamkudoth, K.R.; Ajmera, S.; Dhuri, V. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicol. Rep. 2021, 8, 1008–1030. [Google Scholar] [CrossRef]
- Baker, S.E. Aspergillus niger genomics: Past, present and into the future. Med. Mycol. 2006, 44, S17–S21. [Google Scholar] [CrossRef]
- Iamanaka, B.T.; Taniwaki, M.H.; Menezes, H.C.; Vicente, E.; Fungaro, M.H.P. Incidence of toxigenic fungi and ochratoxin A in dried fruits sold in Brazil. Food Addit. Contam. 2005, 22, 1258–1263. [Google Scholar] [CrossRef]
- Heperkan, D. The Importance of Mycotoxins and a Brief History of Mycotoxin Studies in Turkey. ARI Bull. Instanbul Tech. Univ. 2006, 54, 18–54. [Google Scholar]
- Şenyuva, H.; Gilbert, J.; Samson, R.; Özcan, S.; Öztürkoğlu, Ş.; Önal, D. Occurrence of fungi and their mycotoxins in individual Turkish dried figs. World Mycotoxin J. 2008, 1, 79–86. [Google Scholar] [CrossRef]
- Senyuva, H.Z.; Gilbert, J.; Ozcan, S.; Ulken, U. Survey for Co-occurrence of Ochratoxin A and Aflatoxin B1 in Dried Figs in Turkey by Using a Single Laboratory-Validated Alkaline Extraction Method for Ochratoxin A. J. Food Prot. 2005, 68, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Alwatban, M.A.; Hadi, S.; Moslem, M.A. Mycotoxin production in Cladosporium species influenced by temperature regimes. J. Pure Appl. Microbiol. 2014, 8, 4061–4069. [Google Scholar]
- Chapman, M.D. Challenges associated with indoor moulds: Health effects, immune response and exposure assessment. Med. Mycol. 2006, 44, S29–S32. [Google Scholar] [CrossRef] [PubMed]
- Cambaza, E.; Koseki, S.; Kawamura, S. The Use of Colors as an Alternative to Size in Fusarium graminearum Growth Studies. Foods 2018, 7, 100. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Mwanza, M. Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture 2023, 13, 1810. [Google Scholar] [CrossRef]
- Kumar, D.; Ladaniya, M.S.; Gurjar, M.; Kumar, S.; Mendke, S. Quantification of Flavonoids, Phenols and Antioxidant Potential from Dropped Citrus reticulata Blanco Fruits Influenced by Drying Techniques. Molecules 2021, 26, 4159. [Google Scholar] [CrossRef]
- Chen, M.; Yang, D.; Liu, S. Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. Int. J. Food Sci. Technol. 2011, 46, 1179–1185. [Google Scholar] [CrossRef]
- Mondolot, L.; LA Fisca, P.; Buatois, B.; Talansier, E.; DE Kochko, A.; Campa, C. Evolution in Caffeoylquinic Acid Content and Histolocalization During Coffea canephora Leaf Development. Ann. Bot. 2006, 98, 33–40. [Google Scholar] [CrossRef]
- Konak, R.; Kösoğlu, I.; Yemenıcıoğlu, A. Effects of different drying methods on phenolic content, antioxidant capacity and general characteristics of selected dark colored Turkish fig cultivars. Acta Hortic. 2017, 335–340. [Google Scholar] [CrossRef]


| Fresh | Sun-Dried | Hybrid Solar-Dried | ||
|---|---|---|---|---|
| Moisture (%) | 75.66 ± 0.20 a | 29.43 ± 0.06 b | 28.14 ± 0.08 b | |
| Brix | 26.87 ± 3.63 | ND | ND | |
| aw | ND | 0.68 ± 0.01 a | 0.63 ± 0.02 b | |
| Firmness (N) | 2.83 ± 0.85 | ND | ND | |
| Hardness (N) | ND | 2.36 ± 0.48 a | 2.61 ± 0.51 a | |
| a* | −8.07 ± 3.59 a | 11.54 ± 1.03 b | 10.34 ± 3.11 b | |
| Color | b* | 56.72 ± 4.22 a | 31.39 ± 2.77 b | 38.06 ± 7.86 b |
| L* | 47.77 ± 8.77 a | 60.89 ± 3.48 b | 69.08 ± 3.29 c | |
| [M-H]− | MS2 | UV Max | Compound | Fresh | Sun-Dried | Hybrid Solar-Dried |
|---|---|---|---|---|---|---|
| 515 | 353, 191 | 317 | Dicaffeoylquinic acid | 5.04 ± 0.11 a | 1.81 ± 0.13 b | 0.80 ± 0.22 c |
| 353 | 191 | 325 | 5-O-caffeoylquinic acid | 32.49 ± 2.09 a | 5.30 ± 0.36 b | 11.33 ± 0.23 c |
| 609 | 301 | 256, 354 | Rutin | 191.45 ± 12.07 a | 48.00 ± 10.91 b | 68.48 ± 1.57 c |
| 463 | 301 | 256, 353 | Isoquercitrin | 17.89 ± 1.71 a | 4.09 ± 0.91 b | 3.43 ± 0.43 b |
| 505 | 301, 463 | 256, 353 | Quercetin acetyl hexoside | 36.87 ± 2.63 a | 7.38 ± 1.68 b | 4.41 ± 0.10 c |
| Total Phenolic Compounds | 283.74 ± 18.62 a | 66.58 ± 13.98 b | 88.46 ± 2.56 c | |||
| Fresh | Sun-Dried | Hybrid Solar-Dried | |
|---|---|---|---|
| ABTS (mg TE/100 g DM) | 137.7 ± 10.8 a | 44.1 ± 2.0 b | 98.2 ± 6.7 c |
| DPPH (mg TE/100 g DM) | 50.0 ± 12.1 a | 9.6 ± 2.7 b | 25.5 ± 4.0 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriques, B.R.; Neves, C.M.B.; Moumni, M.; Romanazzi, G.; Le Bourvellec, C.; Cardoso, S.M.; Wessel, D.F. A Comparative Study of Traditional Sun Drying and Hybrid Solar Drying on Quality, Safety, and Bioactive Compounds in “Pingo de Mel” Fig. Antioxidants 2025, 14, 362. https://doi.org/10.3390/antiox14030362
Henriques BR, Neves CMB, Moumni M, Romanazzi G, Le Bourvellec C, Cardoso SM, Wessel DF. A Comparative Study of Traditional Sun Drying and Hybrid Solar Drying on Quality, Safety, and Bioactive Compounds in “Pingo de Mel” Fig. Antioxidants. 2025; 14(3):362. https://doi.org/10.3390/antiox14030362
Chicago/Turabian StyleHenriques, Bárbara R., Cláudia M. B. Neves, Marwa Moumni, Gianfranco Romanazzi, Carine Le Bourvellec, Susana M. Cardoso, and Dulcineia F. Wessel. 2025. "A Comparative Study of Traditional Sun Drying and Hybrid Solar Drying on Quality, Safety, and Bioactive Compounds in “Pingo de Mel” Fig" Antioxidants 14, no. 3: 362. https://doi.org/10.3390/antiox14030362
APA StyleHenriques, B. R., Neves, C. M. B., Moumni, M., Romanazzi, G., Le Bourvellec, C., Cardoso, S. M., & Wessel, D. F. (2025). A Comparative Study of Traditional Sun Drying and Hybrid Solar Drying on Quality, Safety, and Bioactive Compounds in “Pingo de Mel” Fig. Antioxidants, 14(3), 362. https://doi.org/10.3390/antiox14030362











