Effect of pH on the Formation, Disintegration and Antioxidant Activity of Mung Bean Protein Fibrils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Optical Microscopy
2.4. Transmission Electron Microscopy (TEM)
2.5. Atomic Force Microscopy (AFM) Measurements
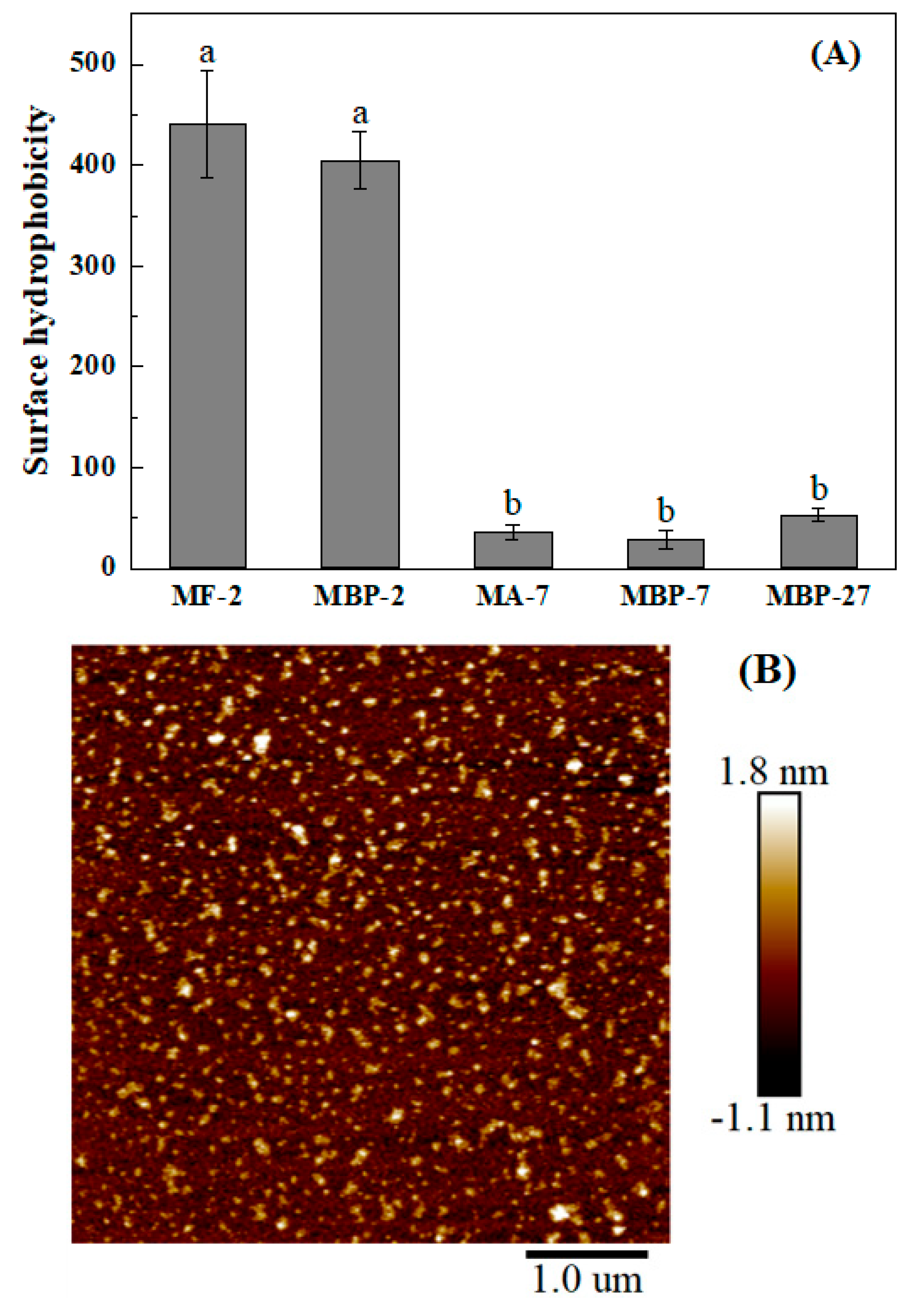
2.6. Surface Hydrophobicity
2.7. ζ-Potential
2.8. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.9. Circular Dichroism
2.10. ABTS and DPPH Radical Scavenging Activity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Formation of Mung Bean Protein Fibrils
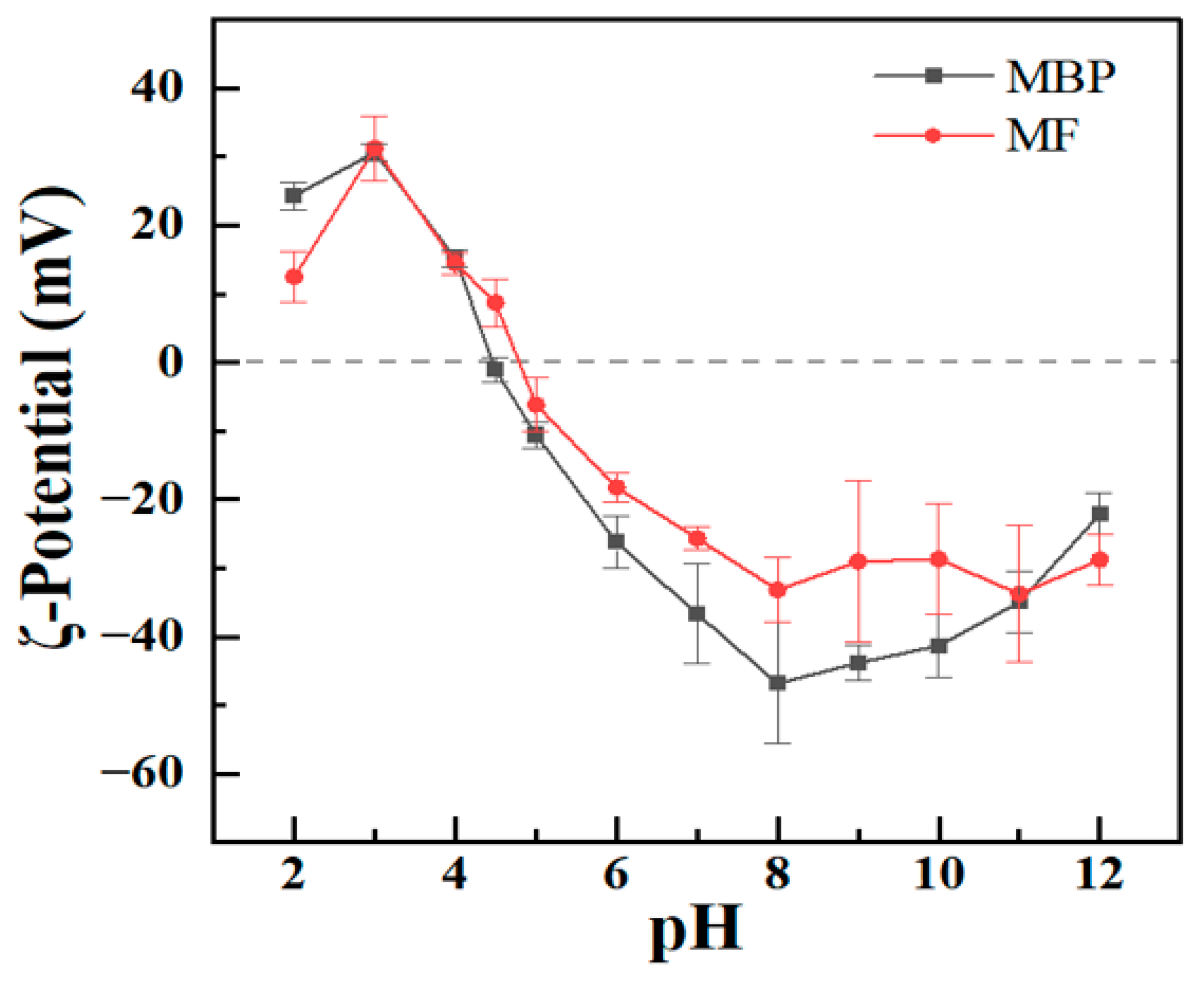
3.2. Characterization of Mung Bean Protein and Its Fibrils at Various pH
3.3. Formation and Disintegration Mechanism of Mung Bean Protein Fibrils
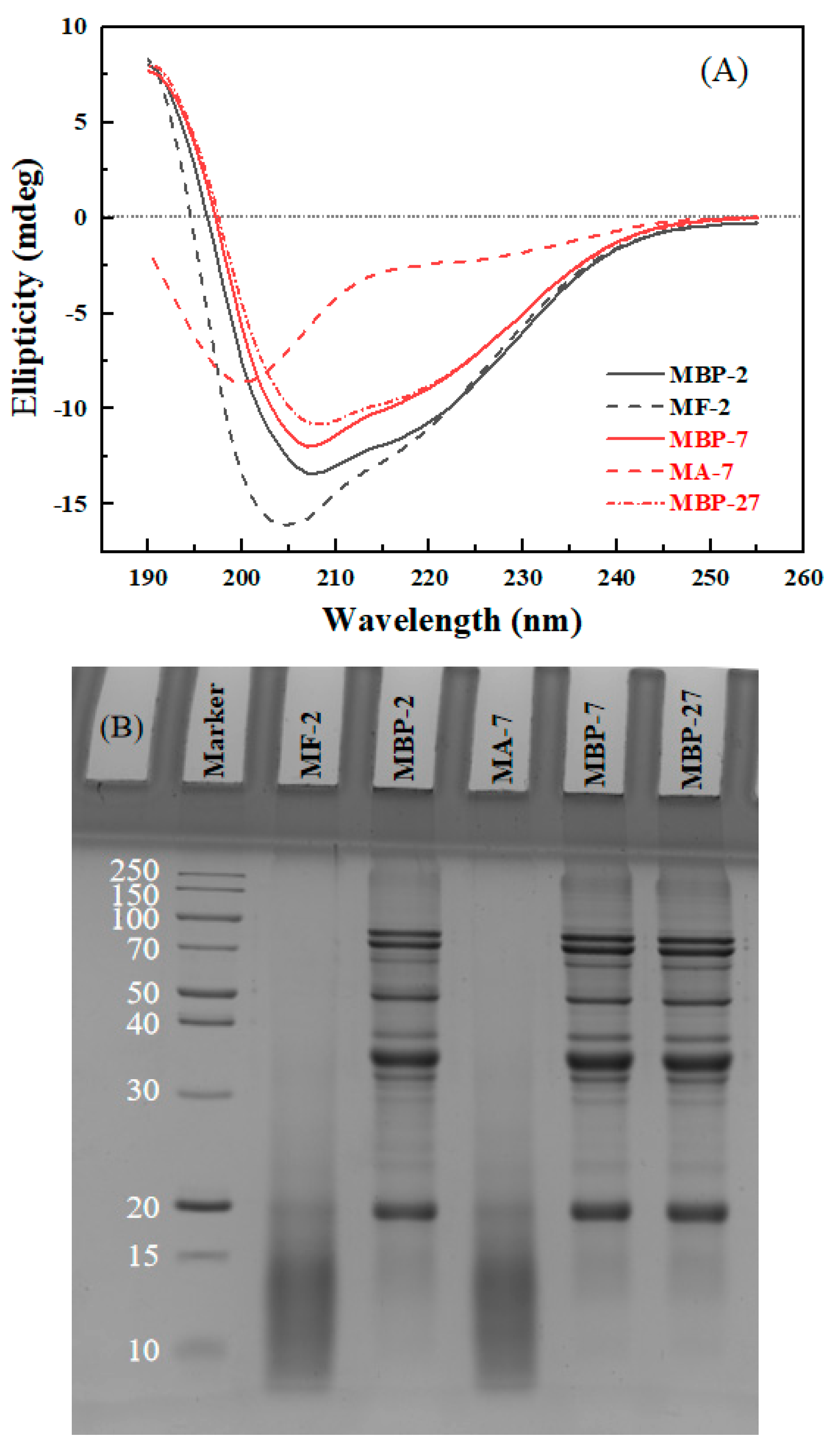
3.3.1. Secondary Structure and Hydrolysis
3.3.2. Fibrillation of Enzyme-Hydrolyzed Mung Bean Protein
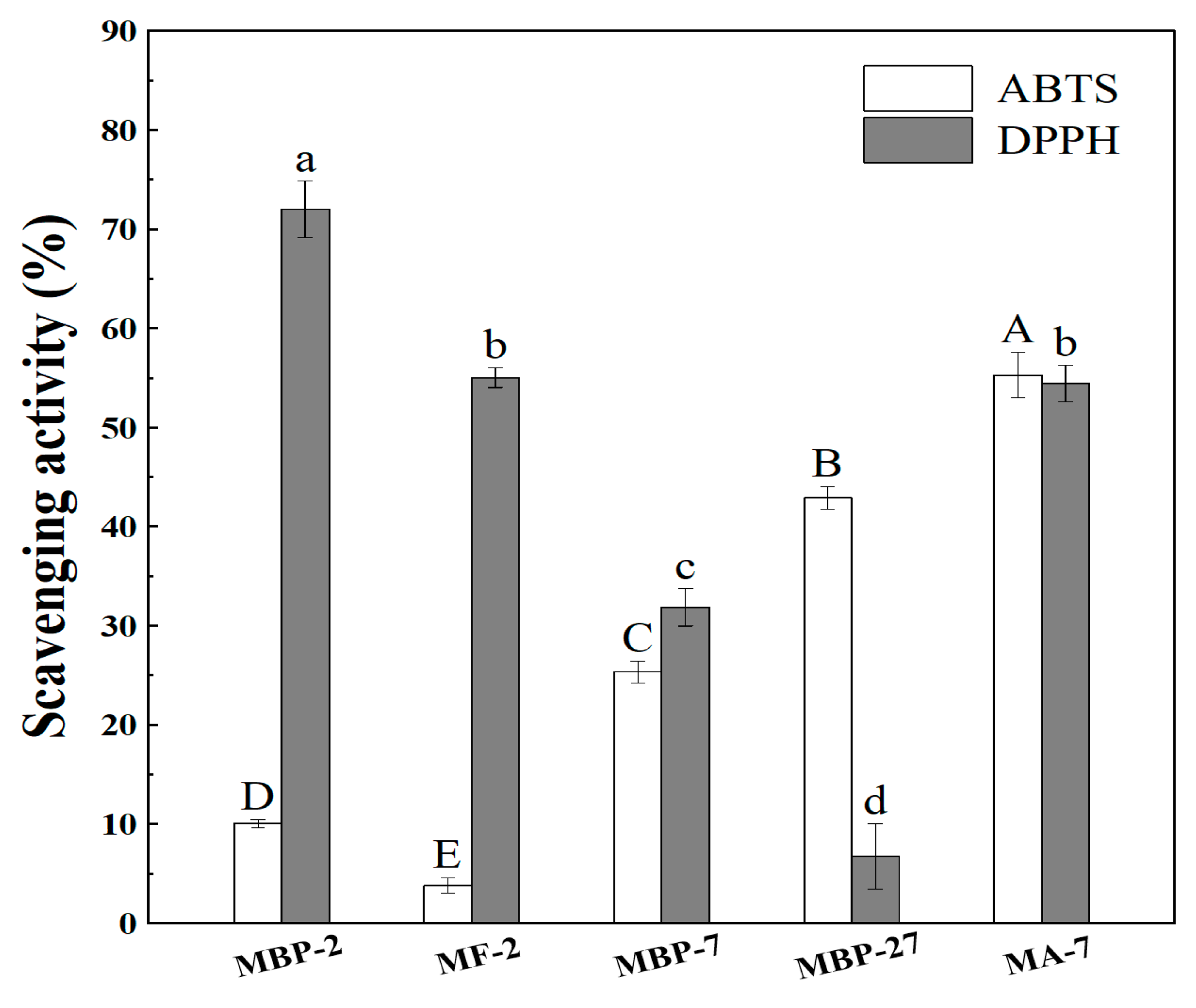
3.4. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Wang, C.; Lu, W.; Sun, C.; Zhu, X.; Fang, Y. Evolution of Physicochemical and Antioxidant Properties of Whey Protein Isolate during Fibrillization Process. Food Chem. 2021, 357, 129751. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, L.; Wu, S.; Chen, P.; Jiang, Q.; Huang, W. Recent Progress of Plant Protein-Based Amyloid-like Nanofibrils. Food Hydrocoll. 2025, 160, 110749. [Google Scholar] [CrossRef]
- Yu, Z.; Li, N.; Liu, Y.; Zhang, B.; Zhang, M.; Wang, X.; Wang, X. Formation, Structure and Functional Characteristics of Amyloid Fibrils Formed Based on Soy Protein Isolates. Int. J. Biol. Macromol. 2024, 254, 127956. [Google Scholar] [CrossRef] [PubMed]
- Alavi, F.; Emam-Djomeh, Z.; Mohammadian, M.; Salami, M.; Moosavi-Movahedi, A.A. Physico-Chemical and Foaming Properties of Nanofibrillated Egg White Protein and Its Functionality in Meringue Batter. Food Hydrocoll. 2020, 101, 105554. [Google Scholar] [CrossRef]
- Xu, J.; Tang, M.; Wang, D.; Xie, Q.; Xu, X. Exploring the Self-Assembly Journey of Oat Globulin Fibrils: From Structural Evolution to Modified Functionality. Food Hydrocoll. 2024, 149, 109587. [Google Scholar] [CrossRef]
- Meng, Y.; Wei, Z.; Xue, C. Protein Fibrils from Different Food Sources: A Review of Fibrillation Conditions, Properties, Applications and Research Trends. Trends Food Sci. Technol. 2022, 121, 59–75. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Characterization of Fibrillated Antioxidant Whey Protein Hydrolysate and Comparison with Fibrillated Protein Solution. Food Hydrocoll. 2016, 52, 221–230. [Google Scholar] [CrossRef]
- Pang, L.; Liu, M.; Gao, Y.; Chen, C.; Zhao, Q.; Yang, X.; Zhang, W.; Jiang, Y. Induction of Beta-Lactoglobulin Amyloid Fibril Formation by Acid Heating to Reduce Allergenicity and Improve Functional Properties: Insights from Structural Changes and Protein Hydrolysis. Food Hydrocoll. 2025, 169, 111592. [Google Scholar] [CrossRef]
- Qi, X.; Li, Y.; Shen, M.; Yu, Q.; Chen, Y.; Xie, J. Formation of Rice Protein Fibrils Is Highly Sensitive to the Different Types of Metal Ions: Aggregation Behavior and Possible Mechanisms. Food Chem. 2024, 431, 137101. [Google Scholar] [CrossRef]
- Kroes-Nijboer, A.; Sawalha, H.; Venema, P.; Bot, A.; Flöter, E.; Den Adel, R.; Bouwman, W.G.; Van Der Linden, E. Stability of Aqueous Food Grade Fibrillar Systems against pH Change. Faraday Discuss. 2012, 158, 125. [Google Scholar] [CrossRef]
- Wan, Y.; Guo, S. The Formation and Disaggregation of Soy Protein Isolate Fibril: Effects of pH. Food Biophys. 2019, 14, 164–172. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Zhang, X.; Geng, H.; Xue, W.; Chen, Z. Assembly Behavior, Structural Characterization and Rheological Properties of Legume Proteins Based Amyloid Fibrils. Food Hydrocoll. 2021, 111, 106396. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.; Chang, Y.; Li, S.; Zhang, Y.; Fang, Y. Understanding the Differences in Heat-Induced Gel Properties of Twelve Legume Proteins: A Comparative Study. Food Res. Int. 2023, 163, 112134. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, L.; Tao, N.; Wang, X. Ultrasound and Enzymolysis Pretreatment Induce Pea Protein to Form New Fibrils for Efficient Delivery of Astaxanthin. Food Hydrocoll. 2026, 170, 111691. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Fan, M.; Qian, H.; Wang, L. Recent Advances in Mung Bean Protein: From Structure, Function to Application. Int. J. Biol. Macromol. 2024, 273, 133210. [Google Scholar] [CrossRef]
- Carlos, M.G.A.; Walter, M.; Jonh, J.M.A. Antioxidant Potential Use of Bioactive Peptides Derived from Mung Bean Hydrolysates (Vigna radiata). Afr. J. Food Sci. 2017, 11, 67–73. [Google Scholar] [CrossRef]
- Xie, J.; Du, M.; Shen, M.; Wu, T.; Lin, L. Physico-Chemical Properties, Antioxidant Activities and Angiotensin-I Converting Enzyme Inhibitory of Protein Hydrolysates from Mung Bean (Vigna radiate). Food Chem. 2019, 270, 243–250. [Google Scholar] [CrossRef]
- Liu, F.-F.; Li, Y.-Q.; Wang, C.-Y.; Liang, Y.; Zhao, X.-Z.; He, J.-X.; Mo, H.-Z. Physicochemical, Functional and Antioxidant Properties of Mung Bean Protein Enzymatic Hydrolysates. Food Chem. 2022, 393, 133397. [Google Scholar] [CrossRef]
- Liu, J.; Tang, C.-H. Heat-Induced Fibril Assembly of Vicilin at pH2.0: Reaction Kinetics, Influence of Ionic Strength and Protein Concentration, and Molecular Mechanism. Food Res. Int. 2013, 51, 621–632. [Google Scholar] [CrossRef]
- Du, X.; Chen, Z.; Zhao, R.; Hu, B. Salt-Promoted Fibrillation of Legume Proteins Enhanced Interfacial Modulus for Stabilization of HIPEs Encapsulating Carotenoids with Improved Nutritional Performance. J. Agric. Food Chem. 2024, 72, 690–703. [Google Scholar] [CrossRef]
- Herneke, A.; Lendel, C.; Johansson, D.; Newson, W.; Hedenqvist, M.; Karkehabadi, S.; Jonsson, D.; Langton, M. Protein Nanofibrils for Sustainable Food-Characterization and Comparison of Fibrils from a Broad Range of Plant Protein Isolates. ACS Food Sci. Technol. 2021, 1, 854–864. [Google Scholar] [CrossRef]
- Herneke, A.; Karkehabadi, S.; Lu, J.; Lendel, C.; Langton, M. Protein Nanofibrils from Mung Bean: The Effect of pH on Morphology and the Ability to Form and Stabilise Foams. Food Hydrocoll. 2023, 136, 108315. [Google Scholar] [CrossRef]
- Zhao, J.; Chang, B.; Hu, Y.; Wang, X.; Cao, Z.; Zhang, Y.; Xu, Z.; Sui, X. Adsorption Mechanism of Soy Protein Amyloid Fibrils with Different Morphological Structures at the Interface of Oil-in-Water Emulsion. Food Hydrocoll. 2025, 162, 110899. [Google Scholar] [CrossRef]
- Jiao, Q.; Liu, Z.; Li, B.; Tian, B.; Zhang, N.; Liu, C.; Feng, Z.; Jiang, B. Development of Antioxidant and Stable Conjugated Linoleic Acid Pickering Emulsion with Protein Nanofibers by Microwave-Assisted Self-Assembly. Foods 2021, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhai, M.; Nian, Y.; Hu, B. Mung Bean Protein Pre-Fibrils Are Superior than the Mature Fibrils in Stabilizing HIPEs and Controlling the Lipid Digestion to Inhibit In Vivo Adipose Accumulation. Food Hydrocoll. 2025, 158, 110522. [Google Scholar] [CrossRef]
- Zhao, H. Dynamic Changes in Interfacial and Emulsifying Properties of Soy Protein during Fibrillation. Food Hydrocoll. 2025, 161, 110893. [Google Scholar] [CrossRef]
- Tong, X.; Cao, J.; Tian, T.; Lyu, B.; Miao, L.; Lian, Z.; Cui, W.; Liu, S.; Wang, H.; Jiang, L. Changes in Structure, Rheological Property and Antioxidant Activity of Soy Protein Isolate Fibrils by Ultrasound Pretreatment and EGCG. Food Hydrocoll. 2022, 122, 107084. [Google Scholar] [CrossRef]
- Kutzli, I.; Zhou, J.; Li, T.; Baier, S.K.; Mezzenga, R. Formation and Characterization of Plant-Based Amyloid Fibrils from Hemp Seed Protein. Food Hydrocoll. 2023, 137, 108307. [Google Scholar] [CrossRef]
- Tang, C.-H.; Sun, X. Physicochemical and Structural Properties of 8S and/or 11S Globulins from Mungbean [Vigna radiata (L.) Wilczek] with Various Polypeptide Constituents. J. Agric. Food Chem. 2010, 58, 6395–6402. [Google Scholar] [CrossRef]
- Heyn, T.R.; Garamus, V.M.; Neumann, H.R.; Uttinger, M.J.; Guckeisen, T.; Heuer, M.; Selhuber-Unkel, C.; Peukert, W.; Keppler, J.K. Influence of the Polydispersity of pH 2 and pH 3.5 Beta-Lactoglobulin Amyloid Fibril Solutions on Analytical Methods. Eur. Polym. J. 2019, 120, 109211. [Google Scholar] [CrossRef]
- Ni, Y.; Yan, T.; Fu, K.; Xu, C.; Zhang, L.; Liu, D.; Wang, W. Enhancement of Physicochemical and Techno-Functional Properties of Soy Protein Isolate Amyloid Fibrils by Moderate Ultrasonic Pretreatment. Ultrason. Sonochem. 2025, 112, 107157. [Google Scholar] [CrossRef]
- Sulatsky, M.I.; Sulatskaya, A.I.; Povarova, O.I.; Antifeeva, I.A.; Kuznetsova, I.M.; Turoverov, K.K. Effect of the Fluorescent Probes ThT and ANS on the Mature Amyloid Fibrils. Prion 2020, 14, 67–75. [Google Scholar] [CrossRef]
- Fu, L.; Feng, X.; Wu, C.; Wei, J.; Chen, L.; Yu, X.; Liu, Q.; Tang, X. Bromelain Hydrolysis and CaCl2 Coordination Promote the Fibrillation of Quinoa Protein at pH 7. Food Hydrocoll. 2025, 159, 110659. [Google Scholar] [CrossRef]
- Lan, H.; Liu, H.; Ye, Y.; Yin, Z. The Role of Surface Properties on Protein Aggregation Behavior in Aqueous Solution of Different pH Values. AAPS PharmSciTech 2020, 21, 122. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Zhao, J.; Cao, Z.; Zhang, Y.; Jiang, L.; Xu, Z.; Sui, X. Acceleration of Soy Protein Amyloid Fibrils Formation: Homologous Seeding Mechanism. Food Chem. 2025, 465, 142063. [Google Scholar] [CrossRef]
- Mendoza, E.M.T.; Adachi, M.; Bernardo, A.E.N.; Utsumi, S. Mungbean [Vigna radiata (L.) Wilczek] Globulins: Purification and Characterization. J. Agric. Food Chem. 2001, 49, 1552–1558. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Gao, Y.; Zhang, Y.; Jiang, L.; Sui, X. Structural Insights into Acidic Heating-Induced Amyloid Fibrils Derived from Soy Protein as a Function of Protein Concentration. Food Hydrocoll. 2023, 145, 109085. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Xu, X.; Kong, B.; Xia, X.; Chen, Q.; Liu, H.; Qin, L. Food-Derived Protein Fibril-Based Multiscale Structured Systems: Construction, Potential Applications, and Future Prospects. Nano Today 2025, 61, 102615. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Zhang, Y.; Li, X.; Liu, C.; Jiang, B.; Xu, J.; Sun, Z. Formation of Whey Protein Isolate Nanofibrils by Endoproteinase GluC and Their Emulsifying Properties. Food Hydrocoll. 2019, 94, 71–79. [Google Scholar] [CrossRef]
- Qi, X.; Li, Y.; Zhang, W.; Shen, M.; Chen, Y.; Yu, Q.; Xie, J. Proteolysis Improves the Foaming Properties of Rice Protein Fibrils: Structure, Physicochemical Properties Changes, and Application in Angel Food Cake. Food Chem. 2024, 437, 137765. [Google Scholar] [CrossRef]
- Vreeke, G.J.C.; Vincken, J.-P.; Wierenga, P.A. The Path of Proteolysis by Bovine Chymotrypsin. Food Res. Int. 2023, 165, 112485. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Sun-Waterhouse, D.; Ivan Neil Waterhouse, G.; Zhao, M.; Zhang, J.; Wang, F.; Su, G. Two-Stage Selective Enzymatic Hydrolysis Generates Protein Hydrolysates Rich in Asn-Pro and Ala-His for Enhancing Taste Attributes of Soy Sauce. Food Chem. 2021, 345, 128803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Zhong, H.; Wang, S.; Sun, W.; Zhou, Q. Enzymatic Hydrolysis of Rice Dreg Protein: Effects of Enzyme Type on the Functional Properties and Antioxidant Activities of Recovered Proteins. Food Chem. 2012, 134, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Fan, L.; Liu, D.; Wei, C.; Yang, N.; Fu, X.; Tang, R.; Xu, J.; Yin, S.; Chen, X. A Peptidomics-Based Investigation of the Synergistic Effects of Neutral and Alcalase Proteases on Release of Antioxidant Peptides from Soybean Protein Isolates. Food Chem. 2025, 496, 146696. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhang, H.; Chen, J.; Hu, H.; Rasulov, F.; Bi, D.; Huang, X.; Pan, S. Formation of Amyloid Fibrils from Soy Protein Hydrolysate: Effects of Selective Proteolysis on β-Conglycinin. Food Res. Int. 2017, 100, 268–276. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Technological Functionality and Biological Properties of Food Protein Nanofibrils Formed by Heating at Acidic Condition. Trends Food Sci. Technol. 2018, 75, 115–128. [Google Scholar] [CrossRef]
- Vlocskó, R.B.; Mastyugin, M.; Török, B.; Török, M. Correlation of Physicochemical Properties with Antioxidant Activity in Phenol and Thiophenol Analogues. Sci. Rep. 2025, 15, 73. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and Pitfalls in Measuring Antioxidant Efficacy: A Critical Evaluation of ABTS, DPPH, and ORAC Assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Fragou, F.; Theofanous, A.; Deligiannakis, Y.; Louloudi, M. Nanoantioxidant Materials: Nanoengineering Inspired by Nature. Micromachines 2023, 14, 383. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Qiu, S.; Deng, F. Mechanism in pH Effects of Electrochemical Reactions: A Mini-Review. Carbon Lett. 2024, 34, 1269–1286. [Google Scholar] [CrossRef]
- Musialik, M.; Kuzmicz, R.; Pawłowski, T.S.; Litwinienko, G. Acidity of Hydroxyl Groups: An Overlooked Influence on Antiradical Properties of Flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Zhang, S.; Huang, W.; Li, N.; Liang, L. Effect of pH on the Formation, Disintegration and Antioxidant Activity of Mung Bean Protein Fibrils. Antioxidants 2025, 14, 1399. https://doi.org/10.3390/antiox14121399
Tian Y, Zhang S, Huang W, Li N, Liang L. Effect of pH on the Formation, Disintegration and Antioxidant Activity of Mung Bean Protein Fibrils. Antioxidants. 2025; 14(12):1399. https://doi.org/10.3390/antiox14121399
Chicago/Turabian StyleTian, Yike, Shuning Zhang, Weining Huang, Ning Li, and Li Liang. 2025. "Effect of pH on the Formation, Disintegration and Antioxidant Activity of Mung Bean Protein Fibrils" Antioxidants 14, no. 12: 1399. https://doi.org/10.3390/antiox14121399
APA StyleTian, Y., Zhang, S., Huang, W., Li, N., & Liang, L. (2025). Effect of pH on the Formation, Disintegration and Antioxidant Activity of Mung Bean Protein Fibrils. Antioxidants, 14(12), 1399. https://doi.org/10.3390/antiox14121399






