Oxidant Stress, Hyperoxia/Hypoxia and Neonatal Respiratory Disorders
Abstract
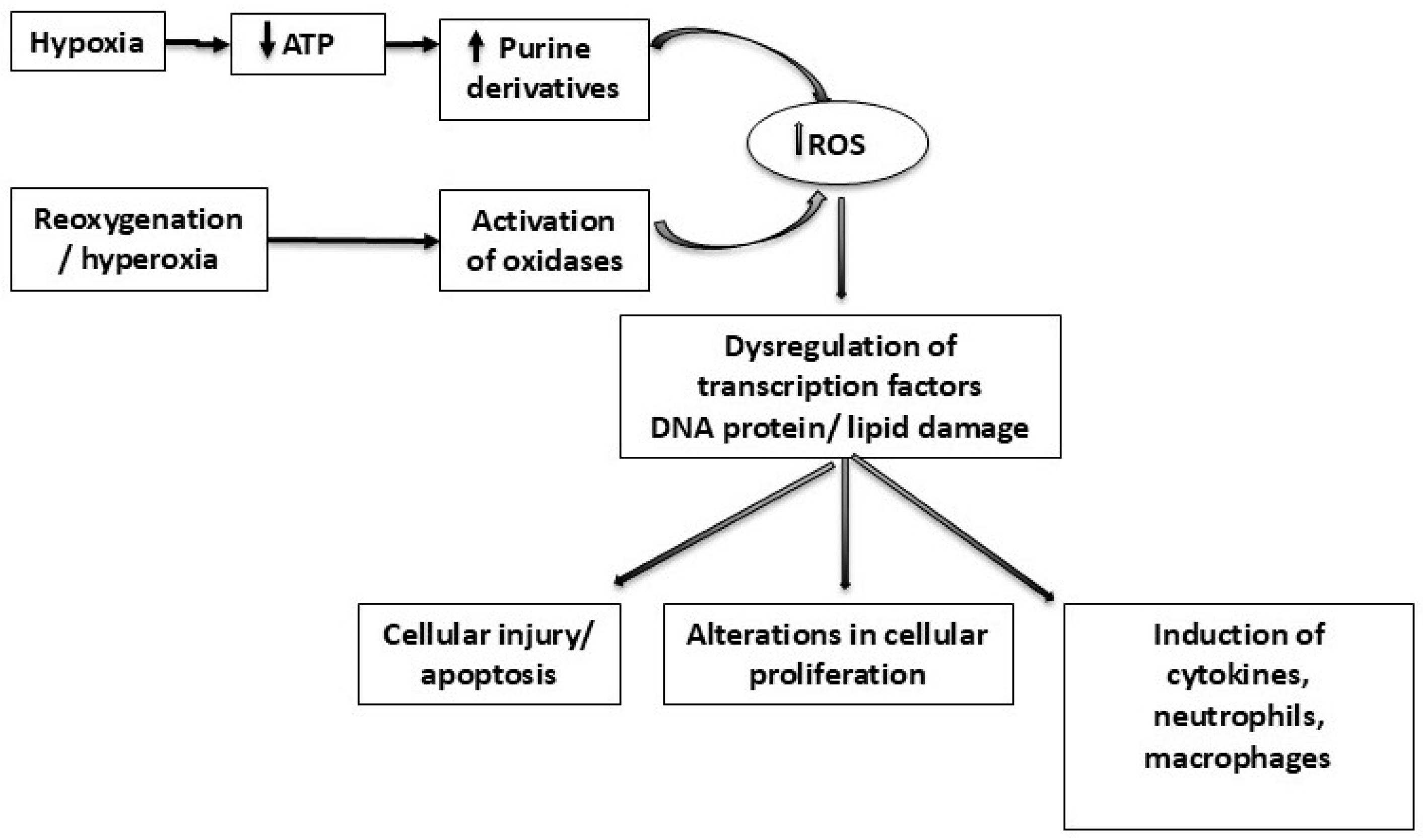
1. Introduction
2. Respiratory Distress Syndrome
3. Bronchopulmonary Dysplasia
4. Pulmonary Hypertension
5. Antioxidant Treatments
6. Closed-Loop Automated Oxygen Control Systems
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| BPD | Bronchopulmonary dysplasia |
| CAT | Catalase |
| CLAC | Closed-loop automated oxygen control |
| CNS | Central nervous system |
| COHb | Carboxyhaemoglobin |
| FiO2 | Fraction of inspired oxygen |
| FRC | Functional residual capacity |
| GSH | Reduced glutathione |
| GSSG | Oxidised glutathione |
| H2O2 | Hydrogen peroxide |
| IH | Intermittent hypoxia |
| IL | Interleukin |
| iNO | Inhaled nitric oxide |
| IUGR | Intrauterine growth restriction |
| MDA | Malondialdehyde |
| MV | Mechanical ventilation |
| NFE2L2 | Nuclear factor erythroid-2 related factor-2 |
| 8-OHdG | 8-hydroxy-20-deoxyguanosine |
| PH | Pulmonary hypertension |
| PMA | Postmenstrual age |
| PaO2 | Partial arterial oxygen pressure |
| PPHN | Persistent pulmonary hypertension of the newborn |
| RCT | Randomised controlled trial |
| RDS | Respiratory distress syndrome |
| ROP | Retinopathy of prematurity |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SpO2 | Peripheral oxygen saturation |
| TAC | Total antioxidant capacity |
| TNF | Tumour necrosis factor |
| VLBW | Very low birth weight |
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Zeida, A.; Babbush, R.; Lebrero, M.C.; Trujillo, M.; Radi, R.; Estrin, D.A. Molecular basis of the mechanism of thiol oxidation by hydrogen peroxide in aqueous solution: Challenging the SN2 paradigm. Chem. Res. Toxicol. 2012, 25, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Davies, K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 2000, 50, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Perrone, S.; Santacroce, A.; Picardi, A.; Buonocore, G. Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World J. Clin. Pediatr. 2016, 5, 172–181. [Google Scholar] [CrossRef]
- Longini, M.; Perrone, S.; Kenanidis, A.; Vezzosi, P.; Marzocchi, B.; Petraglia, F.; Centini, G.; Buonocore, G. Isoprostanes in amniotic fluid: A predictive marker for fetal growth restriction in pregnancy. Free Radic. Biol. Med. 2005, 38, 1537–1541. [Google Scholar] [CrossRef]
- Chiavaroli, V.; Giannini, C.; D’Adamo, E.; de Giorgis, T.; Chiarelli, F.; Mohn, A. Insulin resistance and oxidative stress in children born small and large for gestational age. Pediatrics 2009, 124, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, P.; Ramiro-Cortijo, D.; Reyes-Hernandez, C.G.; Lopez de Pablo, A.L.; Gonzalez, M.C.; Arribas, S.M. Implication of Oxidative Stress in Fetal Programming of Cardiovascular Disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef]
- Matyas, M.; Hasmasanu, M.G.; Zaharie, G. Antioxidant Capacity of Preterm Neonates Assessed by Hydrogen Donor Value. Medicina 2019, 55, 720. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, J.M.; Arko, M.K.; Miller, M.J.; Krauss, A.; Betkerur, A.; Zadell, A.; Kenney, S.R.; Martin, R.J. Cardiorespiratory events in preterm infants referred for apnea monitoring studies. Pediatrics 2001, 108, 1304–1308. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; MacFarlane, P.M.; Martin, R.J. Intermittent Hypoxemia in Preterm Infants. Clin. Perinatol. 2019, 46, 553–565. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Gerhardt, T.; Gonzalez, A.; Hummler, H.; Claure, N.; Everett, R.; Bancalari, E. Mechanisms for episodes of hypoxemia in preterm infants undergoing mechanical ventilation. J. Pediatr. 1995, 127, 767–773. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; Bloom, J.N.; Orge, F.; Schutt, A.; Schluchter, M.; Cheruvu, V.K.; Walsh, M.; Finer, N.; Martin, R.J. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 2010, 157, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.P.; Leick-Rude, M.K.; Meinert, K.A.; Anderson, B.; Sheehan, M.B.; Haney, B.M.; Leeks, S.R.; Simon, S.D.; Jackson, J.K. Overcoming barriers to oxygen saturation targeting. Pediatrics 2006, 118 (Suppl. 2), S177–S186. [Google Scholar] [CrossRef]
- Hagadorn, J.I.; Furey, A.M.; Nghiem, T.H.; Schmid, C.H.; Phelps, D.L.; Pillers, D.A.; Cole, C.H.; Group, A.V.S. Achieved versus intended pulse oximeter saturation in infants born less than 28 weeks’ gestation: The AVIOx study. Pediatrics 2006, 118, 1574–1582. [Google Scholar] [CrossRef]
- Mohamed, T.; Abdul-Hafez, A.; Gewolb, I.H.; Uhal, B.D. Oxygen injury in neonates: Which is worse? hyperoxia, hypoxia, or alternating hyperoxia/hypoxia. J. Lung Pulm. Respir. Res. 2020, 7, 4–13. [Google Scholar]
- Warburton, A.; Monga, R.; Sampath, V.; Kumar, N. Continuous pulse oximetry and respiratory rate trends predict short-term respiratory and growth outcomes in premature infants. Pediatr. Res. 2019, 85, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.L.; Larkin, E.K.; Kirchner, H.L.; Emancipator, J.L.; Bivins, S.F.; Surovec, S.A.; Martin, R.J.; Redline, S. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: Association with race and prematurity. J. Pediatr. 2003, 142, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Poets, C.F.; Roberts, R.S.; Schmidt, B.; Whyte, R.K.; Asztalos, E.V.; Bader, D.; Bairam, A.; Moddemann, D.; Peliowski, A.; Rabi, Y.; et al. Association Between Intermittent Hypoxemia or Bradycardia and Late Death or Disability in Extremely Preterm Infants. JAMA 2015, 314, 595–603. [Google Scholar] [CrossRef]
- Perrone, S.; Bracciali, C.; Di Virgilio, N.; Buonocore, G. Oxygen Use in Neonatal Care: A Two-edged Sword. Front. Pediatr. 2016, 4, 143. [Google Scholar] [CrossRef]
- Dylag, A.M.; Mayer, C.A.; Raffay, T.M.; Martin, R.J.; Jafri, A.; MacFarlane, P.M. Long-term effects of recurrent intermittent hypoxia and hyperoxia on respiratory system mechanics in neonatal mice. Pediatr. Res. 2017, 81, 565–571. [Google Scholar] [CrossRef]
- Cannavo, L.; Perrone, S.; Viola, V.; Marseglia, L.; Di Rosa, G.; Gitto, E. Oxidative Stress and Respiratory Diseases in Preterm Newborns. Int. J. Mol. Sci. 2021, 22, 12504. [Google Scholar] [CrossRef]
- Marseglia, L.; D’Angelo, G.; Granese, R.; Falsaperla, R.; Reiter, R.J.; Corsello, G.; Gitto, E. Role of oxidative stress in neonatal respiratory distress syndrome. Free Radic. Biol. Med. 2019, 142, 132–137. [Google Scholar] [CrossRef]
- Boda, D.; Nemeth, I.; Pinter, S. Surface tension, glutathione content and redox ratio of the tracheal aspirate fluid of premature infants with IRDS. Biol. Neonate 1998, 74, 281–288. [Google Scholar] [CrossRef]
- Dizdar, E.A.; Uras, N.; Oguz, S.; Erdeve, O.; Sari, F.N.; Aydemir, C.; Dilmen, U. Total antioxidant capacity and total oxidant status after surfactant treatment in preterm infants with respiratory distress syndrome. Ann. Clin. Biochem. 2011, 48 Pt 5, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.G.; Procianoy, R.S.; Neto, E.C.; Silveira, R.C. Preterm Neonates with Respiratory Distress Syndrome: Ventilator-Induced Lung Injury and Oxidative Stress. J. Immunol. Res. 2018, 2018, 6963754. [Google Scholar] [CrossRef] [PubMed]
- Hamid, E.R.A.; Ali, W.H.; Azmy, A.; Ahmed, H.H.; Sherif, L.S.; Saleh, M.T. Oxidative Stress and Anti-Oxidant Markers in Premature Infants with Respiratory Distress Syndrome. Open Access Maced. J. Med. Sci. 2019, 7, 2858–2863. [Google Scholar] [CrossRef]
- Mutlu, B.; Aksoy, N.; Cakir, H.; Celik, H.; Erel, O. The effects of the mode of delivery on oxidative-antioxidative balance. J. Matern. Fetal Neonatal Med. 2011, 24, 1367–1370. [Google Scholar] [CrossRef]
- Naples, R.; Ramaiah, S.; Rankin, J.; Berrington, J.; Harigopal, S. Life-threatening bronchopulmonary dysplasia: A British Paediatric Surveillance Unit Study. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.H. Mechanisms of Lung Injury and Bronchopulmonary Dysplasia. Am. J. Perinatol. 2016, 33, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Darnall, R.A.; Chen, X.; Nemani, K.V.; Sirieix, C.M.; Gimi, B.; Knoblach, S.; McEntire, B.L.; Hunt, C.E. Early postnatal exposure to intermittent hypoxia in rodents is proinflammatory, impairs white matter integrity, and alters brain metabolism. Pediatr. Res. 2017, 82, 164–172. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; Vento, M. Intermittent hypoxemia and oxidative stress in preterm infants. Respir. Physiol. Neurobiol. 2019, 266, 121–129. [Google Scholar] [CrossRef]
- Kotecha, S.; Chan, B.; Azam, N.; Silverman, M.; Shaw, R.J. Increase in interleukin-8 and soluble intercellular adhesion molecule-1 in bronchoalveolar lavage fluid from premature infants who develop chronic lung disease. Arch. Dis. Child. Fetal Neonatal Ed. 1995, 72, F90–F96. [Google Scholar] [CrossRef]
- Hsiao, C.C.; Chang, J.C.; Tsao, L.Y.; Yang, R.C.; Chen, H.N.; Lee, C.H.; Lin, C.Y.; Tsai, Y.G. Correlates of Elevated Interleukin-6 and 8-Hydroxy-2′-Deoxyguanosine Levels in Tracheal Aspirates from Very Low Birth Weight Infants Who Develop Bronchopulmonary Dysplasia. Pediatr. Neonatol. 2017, 58, 63–69. [Google Scholar] [CrossRef]
- Bednarczuk, N.; Williams, E.E.; Greenough, A.; Dassios, T. Carboxyhaemoglobin levels and free-radical-related diseases in prematurely born infants. Early Hum. Dev. 2022, 164, 105523. [Google Scholar] [CrossRef]
- Dani, C.; Remaschi, G.; Monti, N.; Pratesi, S. Carboxyhemoglobin as biomarker of late-onset sepsis in preterm infants. Eur. J. Pediatr. 2023, 182, 4523–4528. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, K.D.; Nagraj, V.P.; Sullivan, B.A.; Moorman, J.R.; Lake, D.E. Oxygen desaturations in the early neonatal period predict development of bronchopulmonary dysplasia. Pediatr. Res. 2019, 85, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Raffay, T.M.; Dylag, A.M.; Sattar, A.; Abu Jawdeh, E.G.; Cao, S.; Pax, B.M.; Loparo, K.A.; Martin, R.J.; Di Fiore, J.M. Neonatal intermittent hypoxemia events are associated with diagnosis of bronchopulmonary dysplasia at 36 weeks postmenstrual age. Pediatr. Res. 2019, 85, 318–323. [Google Scholar] [CrossRef]
- Jensen, E.A.; Whyte, R.K.; Schmidt, B.; Bassler, D.; Vain, N.E.; Roberts, R.S.; Canadian Oxygen Trial, I. Association between Intermittent Hypoxemia and Severe Bronchopulmonary Dysplasia in Preterm Infants. Am. J. Respir. Crit. Care Med. 2021, 204, 1192–1199. [Google Scholar] [CrossRef]
- DeMauro, S.B. Neurodevelopmental outcomes of infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 2021, 56, 3509–3517. [Google Scholar] [CrossRef]
- Ambalavanan, N.; Morty, R.E. Searching for better animal models of BPD: A perspective. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L924–L927. [Google Scholar] [CrossRef]
- Yee, M.; White, R.J.; Awad, H.A.; Bates, W.A.; McGrath-Morrow, S.A.; O’Reilly, M.A. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am. J. Pathol. 2011, 178, 2601–2610. [Google Scholar] [CrossRef]
- Davidson, L.M.; Berkelhamer, S.K. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J. Clin. Med. 2017, 6, 4. [Google Scholar] [CrossRef]
- Deulofeut, R.; Critz, A.; Adams-Chapman, I.; Sola, A. Avoiding hyperoxia in infants < or = 1250 g is associated with improved short- and long-term outcomes. J. Perinatol. 2006, 26, 700–705. [Google Scholar]
- Vento, M.; Moro, M.; Escrig, R.; Arruza, L.; Villar, G.; Izquierdo, I.; Roberts, L.J., 2nd; Arduini, A.; Escobar, J.J.; Sastre, J.; et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009, 124, e439–e449. [Google Scholar] [CrossRef] [PubMed]
- Birenbaum, H.J.; Dentry, A.; Cirelli, J.; Helou, S.; Pane, M.A.; Starr, K.; Melick, C.F.; Updegraff, L.; Arnold, C.; Tamayo, A.; et al. Reduction in the incidence of chronic lung disease in very low birth weight infants: Results of a quality improvement process in a tertiary level neonatal intensive care unit. Pediatrics 2009, 123, 44–50. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Konduri, G.G.; Steinhorn, R.H. Considerations in the management of hypoxemic respiratory failure and persistent pulmonary hypertension in term and late preterm neonates. J. Perinatol. 2016, 36 (Suppl. 2), S12–S19. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Russell, J.A.; Steinhorn, R.H.; Ryan, R.M.; Gugino, S.F.; Morin, F.C., 3rd; Swartz, D.D.; Kumar, V.H. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr. Res. 2006, 59, 137–141. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Swartz, D.D.; Gugino, S.F.; Ma, C.X.; Wynn, K.A.; Ryan, R.M.; Russell, J.A.; Steinhorn, R.H. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr. Res. 2009, 66, 539–544. [Google Scholar] [CrossRef]
- Sheth, S.; Goto, L.; Bhandari, V.; Abraham, B.; Mowes, A. Factors associated with development of early and late pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. J. Perinatol. 2020, 40, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Suh, M.; Park, J.Y.; Park, J.K.; Kim, Y.I.; Kim, H.; Cho, Y.S.; Kang, H.; Kim, K.; Choi, J.H.; et al. Assessment of Inflammation in Pulmonary Artery Hypertension by (68)Ga-Mannosylated Human Serum Albumin. Am. J. Respir. Crit. Care Med. 2020, 201, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Mankouski, A.; Kantores, C.; Wong, M.J.; Ivanovska, J.; Jain, A.; Benner, E.J.; Mason, S.N.; Tanswell, A.K.; Auten, R.L.; Jankov, R.P. Intermittent hypoxia during recovery from neonatal hyperoxic lung injury causes long-term impairment of alveolar development: A new rat model of BPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L208–L216. [Google Scholar] [CrossRef]
- Damianos, A.; Kulandavelu, S.; Chen, P.; Nwajei, P.; Batlahally, S.; Sharma, M.; Alvarez-Cubela, S.; Dominguez-Bendala, J.; Zambrano, R.; Huang, J.; et al. Neonatal intermittent hypoxia persistently impairs lung vascular development and induces long-term lung mitochondrial DNA damage. J. Appl. Physiol. 2022, 133, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Gentle, S.J.; Travers, C.P.; Nakhmani, A.; Indic, P.; Carlo, W.A.; Ambalavanan, N. Intermittent Hypoxemia and Bronchopulmonary Dysplasia with Pulmonary Hypertension in Preterm Infants. Am. J. Respir. Crit. Care Med. 2023, 207, 899–907. [Google Scholar] [CrossRef]
- Darlow, B.A.; Graham, P.J. Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants. Cochrane Database Syst. Rev. 2000, 4, CD000501. [Google Scholar]
- Rakshasbhuvankar, A.A.; Pillow, J.J.; Simmer, K.N.; Patole, S.K. Vitamin A supplementation in very-preterm or very-low-birth-weight infants to prevent morbidity and mortality: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2021, 114, 2084–2096. [Google Scholar] [CrossRef]
- Farahbakhsh, N.; Sharma, D.; Fatahi, S.; Fathi, M.; Vakili, K.; Deravi, N.; Tutunchian, Z.; Ahsan, E.; Yaghoobpoor, S.; Tabatabaii, S.A. Effects of Vitamin D and E Supplementation on Prevention of Bronchopulmonary Dysplasia (BPD) in Premature Neonates: A Systematic Review and Meta-Analysis. Curr. Pediatr. Rev. 2024, 21, 362–373. [Google Scholar] [CrossRef]
- Gouhie, F.A.; Cezar, A.C.N.; Silva, D.A.; Parreira, B.R.; Machado, L.F.; de Freitas, L.C.F.; da Silva Lucena Patriota, P.R. Vitamin E for intraventricular hemorrhage prevention in preterm neonates: A systematic review and meta-analysis. Neuroprotection 2025, 3, 165–171. [Google Scholar] [CrossRef]
- Brion, L.P.; Bell, E.F.; Raghuveer, T.S. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2003, 4, CD003665. [Google Scholar] [CrossRef] [PubMed]
- Streeter, M.L.; Rosso, P. Transport mechanisms for ascorbic acid in the human placenta. Am. J. Clin. Nutr. 1981, 34, 1706–1711. [Google Scholar] [CrossRef]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef]
- Silvers, K.M.; Gibson, A.T.; Russell, J.M.; Powers, H.J. Antioxidant activity, packed cell transfusions, and outcome in premature infants. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 78, F214–F219. [Google Scholar] [CrossRef]
- Darlow, B.A.; Buss, H.; McGill, F.; Fletcher, L.; Graham, P.; Winterbourn, C.C. Vitamin C supplementation in very preterm infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F117–F122. [Google Scholar] [CrossRef] [PubMed]
- Bronnert, A.; Bloomfield, P.M.; Paramo, L.D.; Lin, L.; Bloomfield, F.H.; Cormack, B.E. The effect of vitamin supplementation on neurodevelopmental and clinical outcomes in very low birth weight and very preterm infants: A systematic review and meta-analysis. PLoS ONE 2025, 20, e0327628. [Google Scholar] [CrossRef] [PubMed]
- Murphy, V.E.; Jensen, M.E.; Harvey, S.; Beyene, T.; Gregson, J.; Islam, F.; Huang, W.; Aistrope, K.; Collison, A. Vitamin A, C and/or E Intake During Pregnancy and Offspring Respiratory Health: A Systematic Review and Meta-Analysis. J. Hum. Nutr. Diet. 2025, 38, e70086. [Google Scholar] [CrossRef]
- Roberts, K.; Stepanovich, G.; Bhatt-Mehta, V.; Donn, S.M. New Pharmacologic Approaches to Bronchopulmonary Dysplasia. J. Exp. Pharmacol. 2021, 13, 377–396. [Google Scholar] [CrossRef]
- Farrow, K.N.; Lakshminrusimha, S.; Czech, L.; Groh, B.S.; Gugino, S.F.; Davis, J.M.; Russell, J.A.; Steinhorn, R.H. SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L109–L116. [Google Scholar] [CrossRef]
- Farrow, K.N.; Lakshminrusimha, S.; Reda, W.J.; Wedgwood, S.; Czech, L.; Gugino, S.F.; Davis, J.M.; Russell, J.A.; Steinhorn, R.H. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L979–L987. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Parad, R.B.; Michele, T.; Allred, E.; Price, A.; Rosenfeld, W.; North American Recombinant Human CuZn SODSG. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics 2003, 111, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, S.; Deng, X.; Luo, Z.; Chen, A.; Yu, R. Effects of Antioxidants in Human Milk on Bronchopulmonary Dysplasia Prevention and Treatment: A Review. Front. Nutr. 2022, 9, 924036. [Google Scholar] [CrossRef]
- Chrustek, A.; Dombrowska-Pali, A.; Olszewska-Slonina, D. Analysis of the composition and antioxidant status of breast milk in women giving birth prematurely and on time. PLoS ONE 2021, 16, e0255252. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Villamor, E. Mother’s Own Milk and Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2019, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Mathias, M.; Chang, J.L.; Perez, M.; Saugstad, O. Supplemental Oxygen in the Newborn: Historical Perspective and Current Trends. Antioxidants 2021, 10, 1879. [Google Scholar] [CrossRef]
- Gitto, E.; Reiter, R.J.; Sabatino, G.; Buonocore, G.; Romeo, C.; Gitto, P.; Bugge, C.; Trimarchi, G.; Barberi, I. Correlation among cytokines, bronchopulmonary dysplasia and modality of ventilation in preterm newborns: Improvement with melatonin treatment. J. Pineal Res. 2005, 39, 287–293. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Dani, C. Automated control of inspired oxygen (FiO2) in preterm infants: Literature review. Pediatr. Pulmonol. 2019, 54, 358–363. [Google Scholar] [CrossRef]
- Sturrock, S.; Ambulkar, H.; Williams, E.E.; Sweeney, S.; Bednarczuk, N.F.; Dassios, T.; Greenough, A. A randomised crossover trial of closed loop automated oxygen control in preterm, ventilated infants. Acta Paediatr. 2021, 110, 833–837. [Google Scholar] [CrossRef]
- Abdo, M.; Hanbal, A.; Asla, M.M.; Ishqair, A.; Alfar, M.; Elnaiem, W.; Ragab, K.M.; Nourelden, A.Z.; Zaazouee, M.S. Automated versus manual oxygen control in preterm infants receiving respiratory support: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2022, 35, 6069–6076. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Singh, B.; El-Naggar, W.; McMillan, D.D. Automated versus manual control of inspired oxygen to target oxygen saturation in preterm infants: A systematic review and meta-analysis. J. Perinatol. 2018, 38, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Stafford, I.G.; Lai, N.M.; Tan, K. Automated oxygen delivery for preterm infants with respiratory dysfunction. Cochrane Database Syst. Rev. 2023, 11, CD013294. [Google Scholar] [CrossRef]
- Sturrock, S.; Williams, E.; Dassios, T.; Greenough, A. Closed loop automated oxygen control in neonates—A review. Acta Paediatr. 2020, 109, 914–922. [Google Scholar] [CrossRef]
- van Kaam, A.H.; Hummler, H.D.; Wilinska, M.; Swietlinski, J.; Lal, M.K.; te Pas, A.B.; Lista, G.; Gupta, S.; Fajardo, C.A.; Onland, W.; et al. Automated versus Manual Oxygen Control with Different Saturation Targets and Modes of Respiratory Support in Preterm Infants. J. Pediatr. 2015, 167, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.R.; Miller, T.L.; Volakis, L.I.; Holland, N.; Dungan, G.C.; Roehr, C.C.; Ives, K. Randomised cross-over study of automated oxygen control for preterm infants receiving nasal high flow. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F366–F371. [Google Scholar] [CrossRef]
- Claure, N.; Bancalari, E.; D’Ugard, C.; Nelin, L.; Stein, M.; Ramanathan, R.; Hernandez, R.; Donn, S.M.; Becker, M.; Bachman, T. Multicenter crossover study of automated control of inspired oxygen in ventilated preterm infants. Pediatrics 2011, 127, e76–e83. [Google Scholar] [CrossRef]
- Kaltsogianni, O.; Dassios, T.; Jenkinson, A.; Jeffreys, E.; Ikeda, K.; Sugino, M.; Greenough, A. Closed-loop automated oxygen control in preterm ventilated infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2025; in press. [Google Scholar]
- Ofman, G.; Tipple, T.E. Antioxidants & bronchopulmonary dysplasia: Beating the system or beating a dead horse? Free Radic. Biol. Med. 2019, 142, 138–145. [Google Scholar] [PubMed]
- Sampath, V.; Garland, J.S.; Helbling, D.; Dimmock, D.; Mulrooney, N.P.; Simpson, P.M.; Murray, J.C.; Dagle, J.M. Antioxidant response genes sequence variants and BPD susceptibility in VLBW infants. Pediatr. Res. 2015, 77, 477–483. [Google Scholar] [CrossRef] [PubMed]
| Oxidants | |
| Reactive oxygen species | |
| Superoxide (O2˙─) | |
| Hydrogen peroxide (H2O2) | |
| Hydroxyl radical (HO˙) | |
| Hypochlorite (HOCl) | |
| Reactive nitrogen species | |
| Nitric oxide (NO) | |
| Nitric dioxide (NO2˙) | |
| Nitric trioxide (NO3˙) | |
| Nitrate (NO3─) | |
| Peroxynitrite (ONOO˙) | |
| Reactive sulfur species | |
| Radicals | Thiyl radical (RS˙) |
| Glutathionyl radical (GSSG˙) | |
| Reactive sulfane species (RSR˙) | |
| Non-radicals | Reactive sulfane species (RSR) |
| Reducing sulfur species (hydrogen sulfide (H2S), reduced glutathione (GSH)) | |
| Reactive sulfur substances (sulfur dioxide (SO2), sulfur trioxide (SO3)) | |
| Sulfur secondary metabolites (Allicin) | |
| Antioxidants | |
| Enzymatic | Primary enzymes: superoxide dismutase, catalase, glutathione peroxidase |
| Secondary enzymes: glutathione reductase, glucose-6-phosphate-dehydrogenase | |
| Non- enzymatic | Flavonoids |
| Cofactors: coenzyme Q10 | |
| Minerals: zinc, selenium | |
| Organosulfur compounds: glutathione | |
| Vitamins and derivatives: A (retinol), C (Ascorbic acid), E (tocopherol), K | |
| Carotenoids: β-carotene, lycopene, lutein, zeaxanthin | |
| Nitrogen non-protein compounds: uric acid | |
| Phenolic acids: hydroxycinnamic acids, hydroxybenzoic acids | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsogianni, O.; Dassios, T.; Greenough, A. Oxidant Stress, Hyperoxia/Hypoxia and Neonatal Respiratory Disorders. Antioxidants 2025, 14, 1389. https://doi.org/10.3390/antiox14121389
Kaltsogianni O, Dassios T, Greenough A. Oxidant Stress, Hyperoxia/Hypoxia and Neonatal Respiratory Disorders. Antioxidants. 2025; 14(12):1389. https://doi.org/10.3390/antiox14121389
Chicago/Turabian StyleKaltsogianni, Ourania, Theodore Dassios, and Anne Greenough. 2025. "Oxidant Stress, Hyperoxia/Hypoxia and Neonatal Respiratory Disorders" Antioxidants 14, no. 12: 1389. https://doi.org/10.3390/antiox14121389
APA StyleKaltsogianni, O., Dassios, T., & Greenough, A. (2025). Oxidant Stress, Hyperoxia/Hypoxia and Neonatal Respiratory Disorders. Antioxidants, 14(12), 1389. https://doi.org/10.3390/antiox14121389






