Ginseng Oligopeptides Promote Longevity and Enhance Stress Resistance in Caenorhabditis elegans via the DAF-16/FOXO Pathway
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials and Reagents
2.2. C. elegans Strains and Maintenance
2.3. Lifespan Assays
2.4. Locomotion Assays
2.5. Lipofuscin (Age Pigment) Accumulation Assay
2.6. ROS Measurement
2.7. DAF-16::GFP Nuclear Localization Assay
2.8. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Transcriptome Analysis
2.10. Statistical Analysis
3. Results
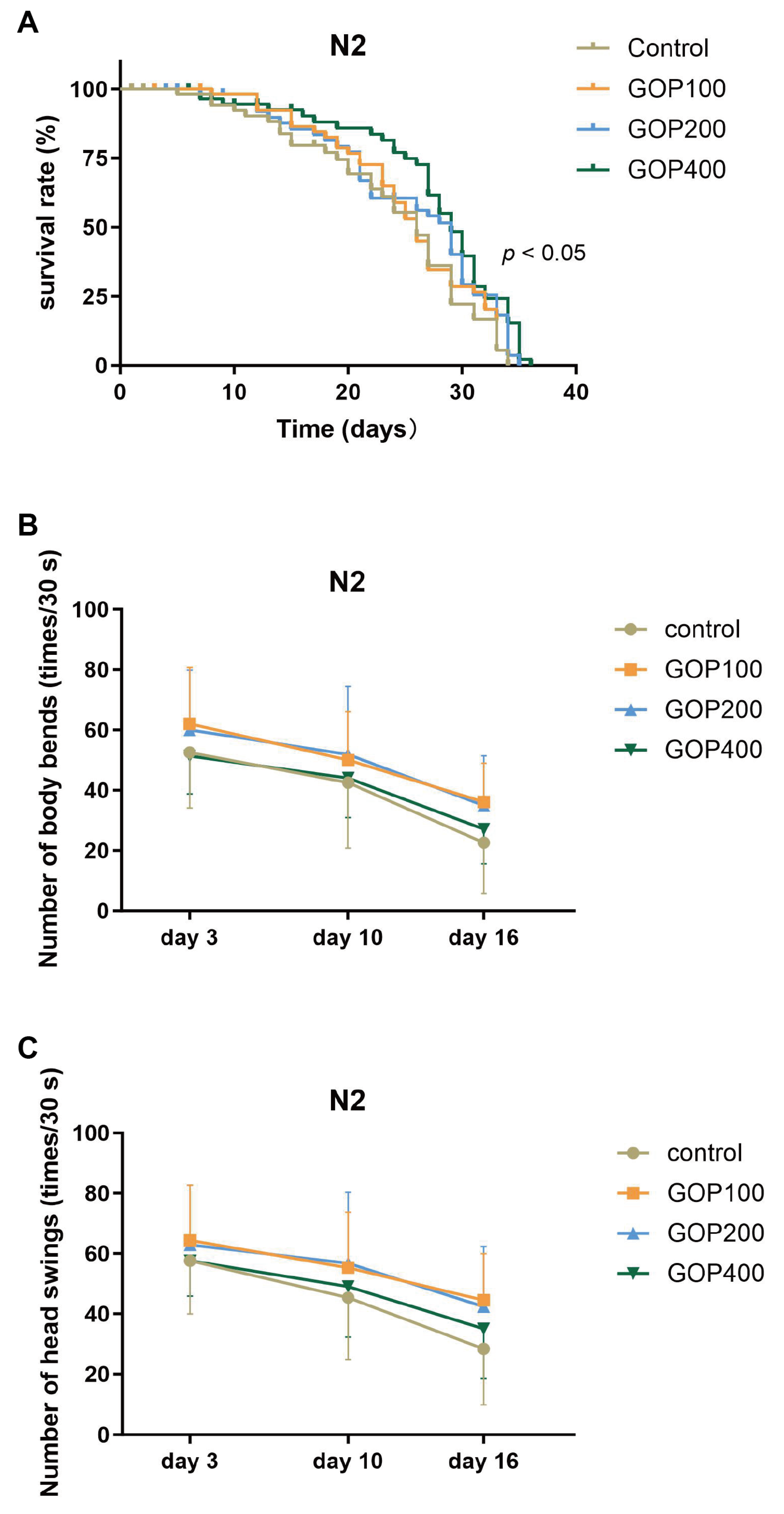
3.1. GOP Extends Lifespan and Attenuates Age-Associated Decline in Locomotion of C. elegans
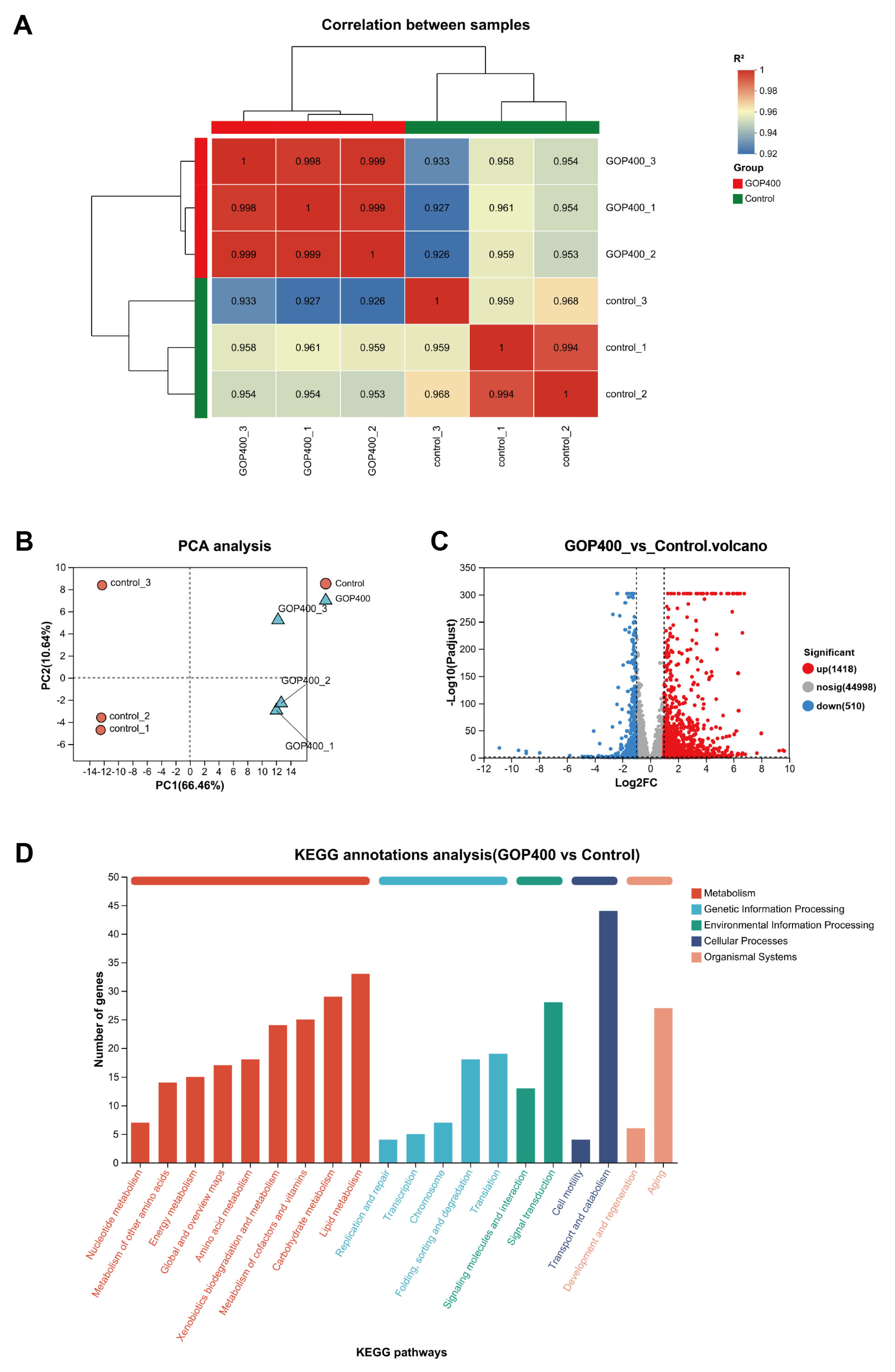
3.2. GOP Induces Global Transcriptomic Alterations and Activates Longevity-Related Pathways
3.3. GOP Modulates Pathways Related to Structural Maintenance, Immune Defense, and Cell Cycle Regulation
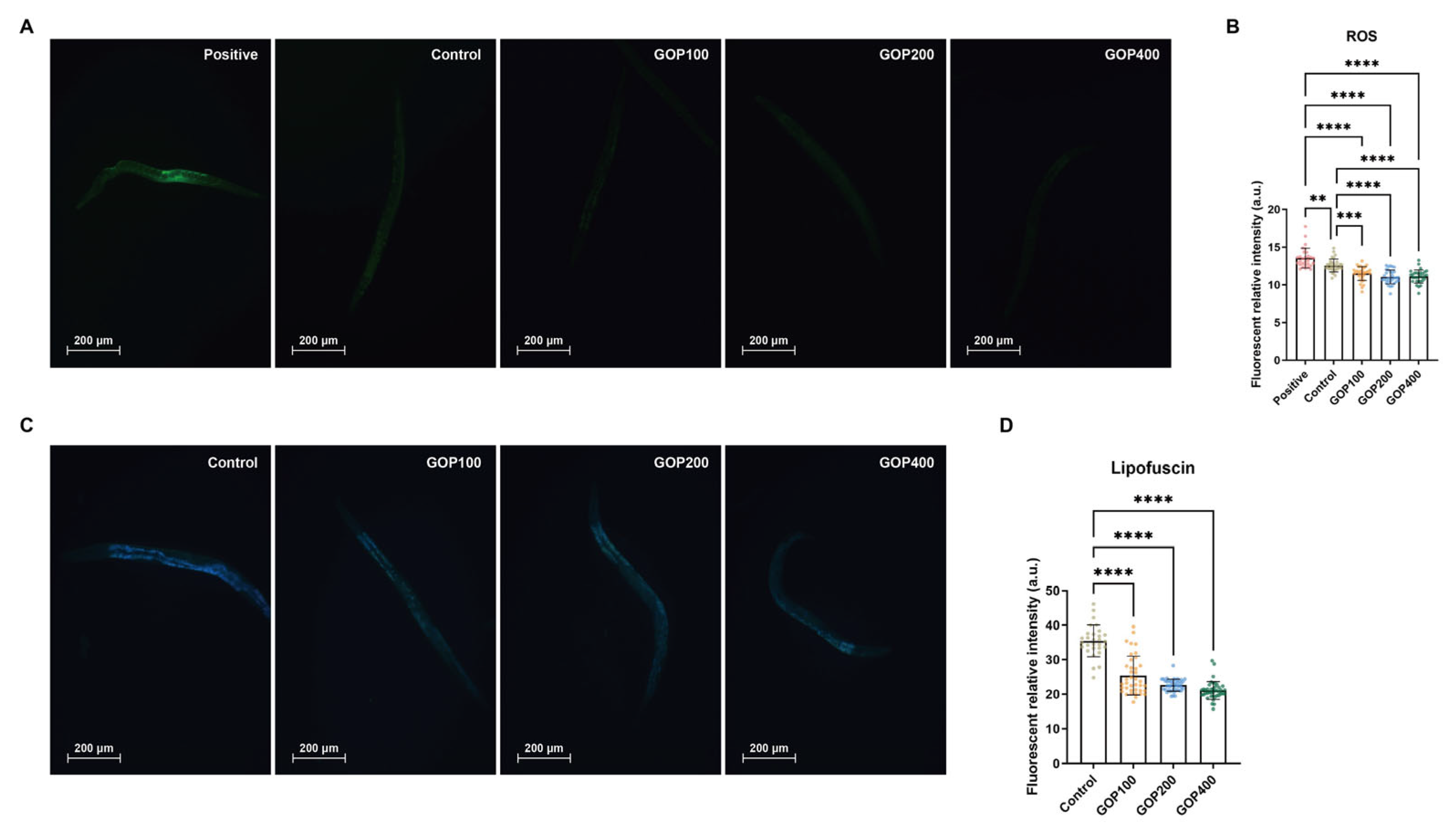
3.4. GOP Reduces Intracellular ROS Levels and Lipofuscin Accumulation in C. elegans
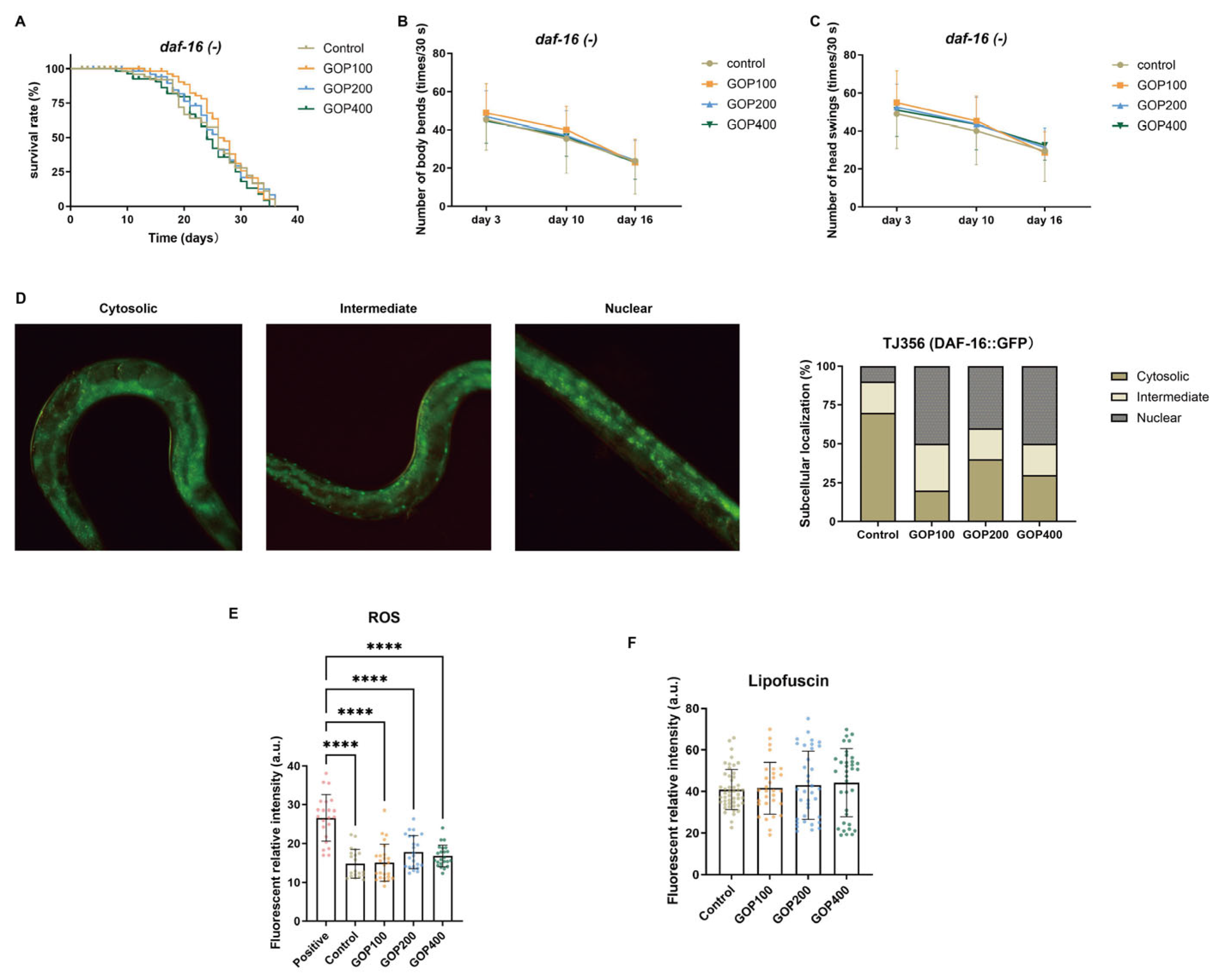
3.5. The Anti-Aging Effects of GOP Depend on the DAF-16/FOXO Pathway
4. Discussion
4.1. GOPs Exhibit Anti-Aging Effects in C. elegans
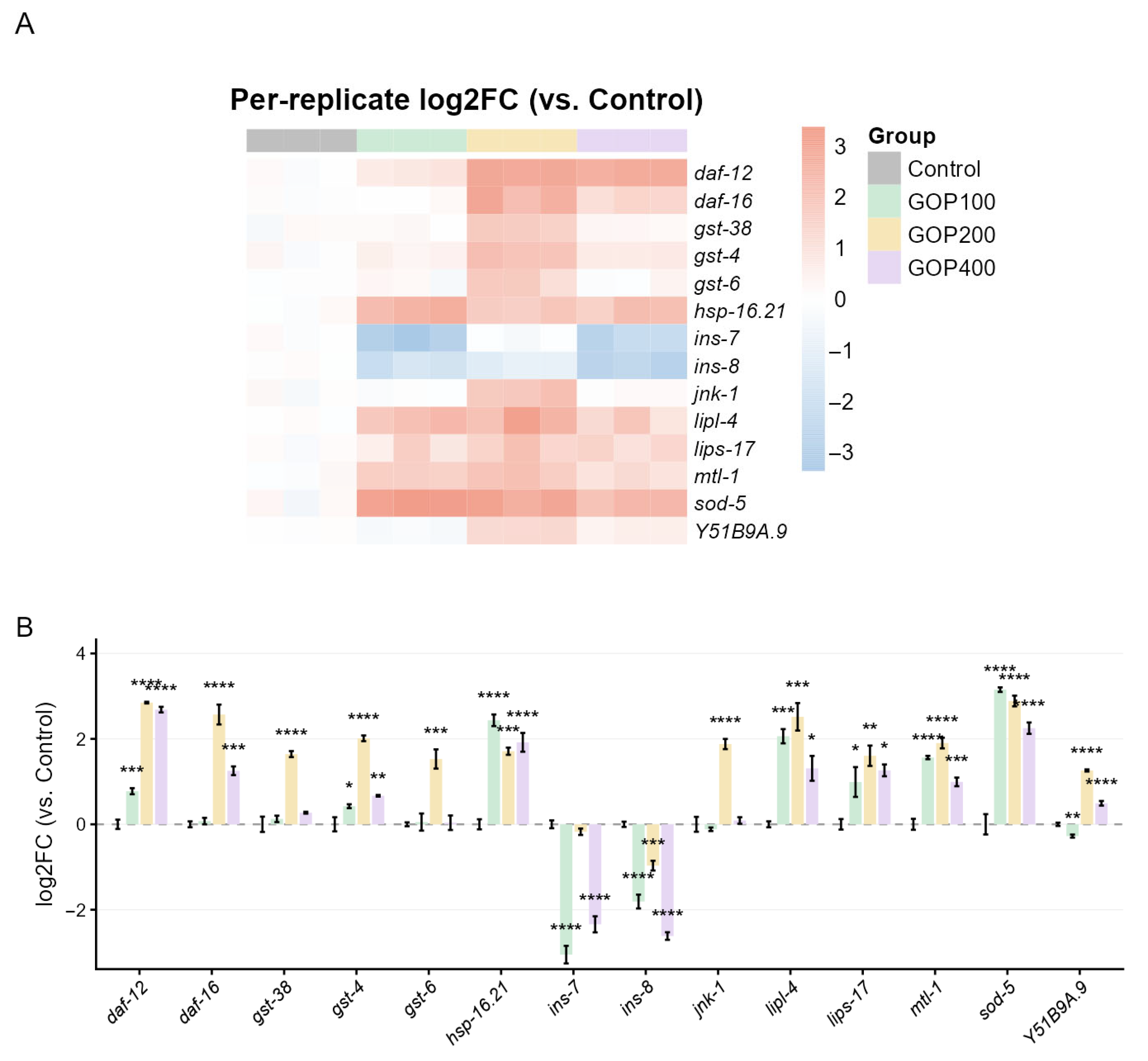
4.2. Transcriptomic and qPCR Evidence Reveals GOP-Mediated Activation of Antioxidant, Metabolic, and Longevity Pathways
4.3. GOPs Attenuate Oxidative Stress and Reduce Aging-Associated Biomarkers
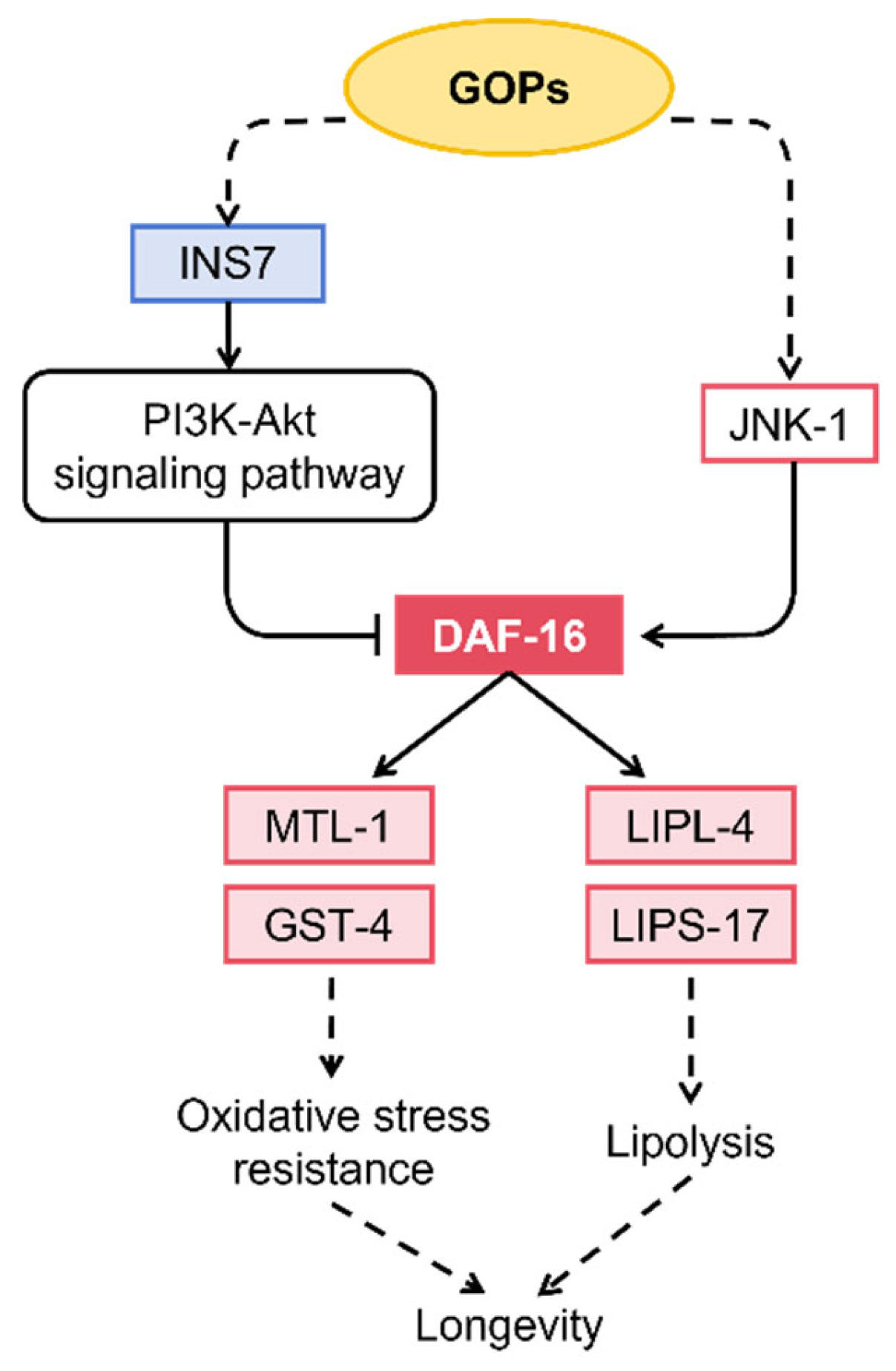
4.4. DAF-16/FOXO Signaling Mediates the Longevity Effects of GOPs
4.5. Limitations and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular Mechanisms of Aging and Anti-Aging Strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The Senescence-Associated Secretory Phenotype and Its Physiological and Pathological Implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2024: Key Messages [EB/OL]; United Nations: New York, NY, USA, 2024; Available online: https://www.un.org/development/desa/pd/ (accessed on 21 September 2025).
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Kroemer, G.; Maier, A.B.; Cuervo, A.M.; Gladyshev, V.N.; Ferrucci, L.; Gorbunova, V.; Kennedy, B.K.; Rando, T.A.; Seluanov, A.; Sierra, F.; et al. From Geroscience to Precision Geromedicine: Understanding and Managing Aging. Cell 2025, 188, 2043–2062. [Google Scholar] [CrossRef]
- Duan, H.; Pan, J.; Guo, M.; Li, J.; Yu, L.; Fan, L. Dietary Strategies with Anti-Aging Potential: Dietary Patterns and Supplements. Food Res. Int. 2022, 158, 111501. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Hooten, N.N.; O’Connell, J.F.; Lee, B.-Y.; Kim, Y. The Role of Ginseng and Its Bioactive Compounds in Aging: Cells and Animal Studies. Annu. Rev. Food Sci. Technol. 2025, 16, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Xu, M.H.; Li, Y. Bioactive Oligopeptides from Ginseng (Panax ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts via NAD+/SIRT1/PGC-1α Signaling Pathway. Nutrients 2022, 14, 5289. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Lin, D.; Guo, W.; Xu, Z.; Wang, L.; Guan, S. Ginsenoside Prolongs the Lifespan of C. Elegans via Lipid Metabolism and Activating the Stress Response Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 9668. [Google Scholar] [CrossRef]
- Sun, J.; Zhong, X.; Sun, D.; Xu, L.; Shi, L.; Sui, J.; Liu, Y. Anti-Aging Effects of Polysaccharides from Ginseng Extract Residues in Caenorhabditis elegans. Int. J. Biol. Macromol. 2023, 225, 1072–1084. [Google Scholar] [CrossRef]
- Patil, P.J.; Usman, M.; Zhang, C.; Mehmood, A.; Zhou, M.; Teng, C.; Li, X. An Updated Review on Food-Derived Bioactive Peptides: Focus on the Regulatory Requirements, Safety, and Bioavailability. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1732–1776. [Google Scholar] [CrossRef]
- You, M.; Xu, M. Ginseng Oligopeptides Improve the Intestinal Physiology and Promote the Antioxidant Capacity of the Gut-on-a-Chip Model. Nutrients 2024, 16, 845. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Sun, B.; Li, D.; Mao, R.; Li, H.; Li, Y.; Wang, J. Beneficial Effects of Small Molecule Oligopeptides Isolated from Panax ginseng Meyer on Pancreatic Beta-Cell Dysfunction and Death in Diabetic Rats. Nutrients 2017, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, Q.; Fan, R.; Wang, J.; Li, Y. Anti-Inflammation Effect of Small Molecule Oligopeptides Prepared from Panax ginseng C. A. Meyer in Rats. Molecules 2019, 24, 858. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Wang, J.; Zhang, Y.; Sun, B.; Li, Y. Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer in Mice. Nutrients 2016, 8, 807. [Google Scholar] [CrossRef]
- Ye, Y.; Gu, Q.; Sun, X. Potential of Caenorhabditis elegans as an Antiaging Evaluation Model for Dietary Phytochemicals: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3084–3105. [Google Scholar] [CrossRef]
- Rani, N.; Alam, M.M.; Jamal, A.; Bin Ghaffar, U.; Parvez, S. Caenorhabditis elegans: A Transgenic Model for Studying Age-Associated Neurodegenerative Diseases. Ageing Res. Rev. 2023, 91, 102036. [Google Scholar] [CrossRef]
- Conover, C.A.; Oxvig, C. The IGF System and Aging. Endocr. Rev. 2025, 46, 214–223. [Google Scholar] [CrossRef]
- Zečić, A.; Braeckman, B.P. DAF-16/FoxO in Caenorhabditis elegans and Its Role in Metabolic Remodeling. Cells 2020, 9, E109. [Google Scholar] [CrossRef]
- Xu, Q.; Zheng, B.; Li, T.; Liu, R.H. Black Goji Berry Anthocyanins Extend Lifespan and Enhance the Antioxidant Defenses in Caenorhabditis elegans via the JNK-1 and DAF-16/FOXO Pathways. J. Sci. Food Agric. 2025, 105, 2282–2293. [Google Scholar] [CrossRef]
- Li, A.-P.; Li, D.; Tan, X.; Xu, R.; Mao, L.-X.; Kang, J.-J.; Li, S.-H.; Liu, Y. Crocin Extends Lifespan by Mitigating Oxidative Stress and Regulating Lipid Metabolism through the DAF-16/FOXO Pathway. Food Funct. 2025, 16, 3369–3383. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. In WormBook: The Online Review of C. elegans Biology [Internet]; WormBook: Pasadena, CA, USA, 2006. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, F.; Wang, T.; Luo, X.; Li, B.; You, Y.; Wu, C.; Liu, X. Jujubae Fructus Extract Prolongs Lifespan and Improves Stress Tolerance in Caenorhabditis elegans Dependent on DAF-16/SOD-3. Sci. Rep. 2024, 14, 13713. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Sun, Y.; Wang, Z.; Su, Y.; Wang, Y.; Wang, X. Exendin-4 Alleviates β-Amyloid Peptide Toxicity via DAF-16 in a Caenorhabditis elegans Model of Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 955113. [Google Scholar] [CrossRef] [PubMed]
- Shibato, J.; Takenoya, F.; Kimura, A.; Yamashita, M.; Rakwal, R.; Shioda, S. Lifespan Extension and Motor Function Improvement Effects of Whale Meat Extract in Caenorhabditis elegans. Int. J. Mol. Sci. 2024, 25, 12833. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, J.; Zhang, Y.; Hacariz, O.; Xia, J.; Simpson, B.K.; Wang, Z. Oxidative Stress Suppression in C. elegans by Peptides from Dogfish Skin via Regulation of Transcription Factors DAF-16 and HSF-1. Food Funct. 2022, 13, 716–724. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Zheng, F.; Zhu, Z.; Bai, F.; Gao, R. Collagen Peptides from Sturgeon Swim Bladder Prolong the Lifespan and Healthspan in Caenorhabditis elegans. J. Sci. Food Agric. 2024, 104, 5244–5251. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, J.; Xu, C.; Li, Q.; Ma, Y.; Zhao, S.; Zhuang, J.; Shen, F.; Wang, Q.; Feng, F.; et al. Sea Cucumber (Acaudina leucoprocta) Peptides Extended the Lifespan and Enhanced Antioxidant Capacity via DAF-16/DAF-2/SOD-3/OLD-1/PEPT-1 in Caenorhabditis elegans. Front. Nutr. 2022, 9, 1065145. [Google Scholar] [CrossRef]
- Cai, J.; Chen, Z.; Wu, Y.; Chen, Y.; Wang, J.; Lin, Q.; Liang, Y. The Rice Bran Peptide KF-8 Extends the Lifespan and Improves the Healthspan of Caenorhabditis elegans via Skn-1 and Daf-16. Food Funct. 2022, 13, 2427–2440. [Google Scholar] [CrossRef]
- Zavagno, G.; Raimundo, A.; Kirby, A.; Saunter, C.; Weinkove, D. Rapid Measurement of Ageing by Automated Monitoring of Movement of C. elegans Populations. GeroScience 2023, 46, 2281–2293. [Google Scholar] [CrossRef]
- Rodriguez-Colman, M.J.; Dansen, T.B.; Burgering, B.M.T. FOXO Transcription Factors as Mediators of Stress Adaptation. Nat. Rev. Mol. Cell Biol. 2024, 25, 46–64. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin a Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Vitamin C: A Comprehensive Review of Its Role in Health, Disease Prevention, and Therapeutic Potential. Molecules 2025, 30, 748. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef] [PubMed]
- Bonkovsky, H.L.; Guo, J.-T.; Hou, W.; Li, T.; Narang, T.; Thapar, M. Porphyrin and Heme Metabolism and the Porphyrias. Compr. Physiol. 2013, 3, 365–401. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Nasreen, A.; Lenchyk, L.; Lysiuk, R.; Peana, M.; Shapovalova, N.; Piscopo, S.; Komisarenko, M.; Shanaida, M.; Smetanina, K.; et al. An Update on Glutathione’s Biosynthesis, Metabolism, Functions, and Medicinal Purposes. Curr. Med. Chem. 2024, 31, 4579–4601. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D. Glutathione and Glutathione-Dependent Enzymes: From Biochemistry to Gerontology and Successful Aging. Ageing Res. Rev. 2023, 92, 102066. [Google Scholar] [CrossRef]
- Acquah, C.; Dzuvor, C.K.O.; Tosh, S.; Agyei, D. Anti-Diabetic Effects of Bioactive Peptides: Recent Advances and Clinical Implications. Crit. Rev. Food Sci. Nutr. 2022, 62, 2158–2171. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.-J.V. Recent Progress in Regulation of Aging by Insulin/IGF-1 Signaling in Caenorhabditis elegans. Mol. Cells 2022, 45, 763–770. [Google Scholar] [CrossRef]
- Baldensperger, T.; Jung, T.; Heinze, T.; Schwerdtle, T.; Höhn, A.; Grune, T. The Age Pigment Lipofuscin Causes Oxidative Stress, Lysosomal Dysfunction, and Pyroptotic Cell Death. Free Radic. Biol. Med. 2024, 225, 871–880. [Google Scholar] [CrossRef]
- Yu, X.; Su, Q.; Shen, T.; Chen, Q.; Wang, Y.; Jia, W. Antioxidant Peptides from Sepia Esculenta Hydrolyzate Attenuate Oxidative Stress and Fat Accumulation in Caenorhabditis elegans. Mar. Drugs 2020, 18, 490. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Nie, X.; Huang, W.; Wang, C.; Lai, R.; Lu, Q.; He, Q.; Yu, X. Unlocking the Potential of Chicken Liver Byproducts: Identification of Antioxidant Peptides through in Silico Approaches and Anti-Aging Effects of a Selected Peptide in Caenorhabditis elegans. Int. J. Biol. Macromol. 2024, 272, 132833. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J. The Genetics of Ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.-D.; Wang, Y.-D. DAF-16/FOXO Transcription Factor in Aging and Longevity. Front. Pharmacol. 2017, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, J.; Wang, J.; Hu, X.; Wang, W.; Lu, S.; Sheng, Y. Anoectochilus roxburghii Extract Extends the Lifespan of Caenorhabditis elegans through Activating the Daf-16/FoxO Pathway. Antioxidants 2024, 13, 945. [Google Scholar] [CrossRef]


| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| tba-1 | TCAACACTGCCATCGCCGCC | TCCAAGCGAGACCAGGCTTCAG |
| gst-4 | ATGCTCGTGCTCTTGCTGAG | CCAAATGGAGTCGTTGGCTT |
| gst-6 | GCACGCAAAAATAACACTCCA | CGGTGTCATTTTGTCCAGCA |
| gst-38 | TTCAAAGCGGCCGGAAAAAC | AAGATAACGAGCCATCGCGT |
| daf-16 | AATTCCTCTCAACAGCAGCAGACC | ACCACCGCCTTGTGACAGATTAAG |
| daf-12 | CCTATCAACAAACGTGCGGC | TGTGCACTATTGCCAGATGATG |
| mtl-1 | CATGGCTTGCAAGTGTGACTG | TCTCACTGGCCTCCTCACAG |
| sod-5 | ATGCCGTTCTTCCACAGGAC | TTCACCTTCGGCTTTCTGGG |
| lipl-4 | TGTGTGCAACATGAGAGAATCAA | TGATTTTATTAATTCCGGCGTATCT |
| lips-17 | GGGATGGAGTGAAGAATTGGTGT | GGGATGGAGTGAAGAATTGGTGT |
| jnk-1 | CGTATCCGTCACATCCAGGTAG | GTCCACGGGTTCCTCGAATG |
| Y51B9A.9 | TCGAGTTGGTCACAGAGACTT | TCTTCTCGATTTGCATGTGCG |
| ins-7 | GCATGCGAATCGAATACTGAA | GGACAGCACTGTTTTCGAATGA |
| ins-8 | ATTGTGCGGAAAGCAAGTCT | TCGCAATATCGACTTTTGTATTTGA |
| hsp-16.21 | AGATATGGGCGGAATGCAAC | GACTTTCAGCTCTTCTGGCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Q.; Zhang, Y.; Guo, X.; Cai, M.; Li, Y.; Xu, M. Ginseng Oligopeptides Promote Longevity and Enhance Stress Resistance in Caenorhabditis elegans via the DAF-16/FOXO Pathway. Antioxidants 2025, 14, 1390. https://doi.org/10.3390/antiox14121390
Du Q, Zhang Y, Guo X, Cai M, Li Y, Xu M. Ginseng Oligopeptides Promote Longevity and Enhance Stress Resistance in Caenorhabditis elegans via the DAF-16/FOXO Pathway. Antioxidants. 2025; 14(12):1390. https://doi.org/10.3390/antiox14121390
Chicago/Turabian StyleDu, Qian, Yiping Zhang, Xiaoyu Guo, Meng Cai, Yong Li, and Meihong Xu. 2025. "Ginseng Oligopeptides Promote Longevity and Enhance Stress Resistance in Caenorhabditis elegans via the DAF-16/FOXO Pathway" Antioxidants 14, no. 12: 1390. https://doi.org/10.3390/antiox14121390
APA StyleDu, Q., Zhang, Y., Guo, X., Cai, M., Li, Y., & Xu, M. (2025). Ginseng Oligopeptides Promote Longevity and Enhance Stress Resistance in Caenorhabditis elegans via the DAF-16/FOXO Pathway. Antioxidants, 14(12), 1390. https://doi.org/10.3390/antiox14121390








