1. Introduction
Superoxide dismutases (SODs) are enzymes that catalyze the conversion of the highly reactive superoxide anion radical O
2•− into a more stable and less aggressive molecule, hydrogen peroxide H
2O
2. H
2O
2 has a number of signaling functions in the cell, including vasomotor effects [
1]. Notably, H
2O
2 may cause both relaxation or contraction of arterial smooth muscle, which depends on many factors, including the animal species, the artery type, the presence of endothelium, etc. [
2,
3,
4,
5,
6].
There are three isoforms of SODs: cytoplasmic Cu/ZnSOD (SOD1), mitochondrial MnSOD (SOD2), and extracellular Cu/ZnSOD (SOD3) [
1]. All three isoforms are expressed in blood vessels. However, among the isoforms of SOD, the functional roles of SOD1 and SOD3 in the regulation of arterial smooth muscle tone have been the most studied due to the existence of their inhibitor diethyldithiocarbamate (DETC) [
7], which chelates Cu
2+ ions in the active centers of the enzymes. Thus, in rat aorta, DETC did not affect phenylephrine-evoked contractile responses [
8,
9]. However, DETC caused an increase of norepinephrine-induced contractile responses of mouse aorta [
10] and rat mesenteric arteries [
11], as well as serotonin-induced contraction of mouse aorta [
12], which may indicate an anticontractile role of Cu/ZnSOD-produced H
2O
2 in several arteries. It is worth noting, however, that such effects were reported for arteries from mature animals.
Nevertheless, the mechanisms of smooth muscle tone regulation, including those involving reactive oxygen species (ROS) [
13], are considerably different between adulthood and the period of early postnatal ontogenesis. However, the functional contribution of SODs to the regulation of arterial tone in the early postnatal period has been poorly studied and the few existing studies focus mainly on the pulmonary circulation. For example, it was shown that DETC increased U46619-induced contractile responses of pulmonary arteries from newborn and two-week-old piglets [
14]. At the same time, SOD content/activity in lung tissue of newborn or early postnatal organisms (human, rabbit, and mouse) were lower compared to adults [
15,
16,
17] which might be associated with a smaller functional contribution of SODs in the pulmonary circulation at the early postnatal period compared to adulthood. However, the vasomotor impact of SODs on the regulation of smooth muscle contraction in systemic arteries, which play a key role in the regulation of systemic blood pressure and blood flow distribution during the early postnatal period, has not been studied yet, to the best of our knowledge. The need and significance to address this issue also follows from the widely used antioxidant therapy of newborns in the clinic [
18], which does not take into account the probable consequences of the vasomotor role of SODs.
Therefore, in this study we hypothesized that the functional impact of SODs (mainly SOD1 and SOD3) is different in systemic arteries of early postnatal rats and adult animals.
2. Materials and Methods
2.1. Animals and the Main Object of the Study
Animal studies are reported in compliance with the ARRIVE guidelines 2.0 [
19,
20]. The use of laboratory animals and of all procedures used in this study were approved by the Biomedical Ethics Committee of M.V. Lomonosov Moscow State University (Protocol number 149-g). Wistar rats (obtained from the Institute of General Pathology and Pathophysiology, Russia) were used. Rats were maintained at a controlled temperature and a 12/12 h light/dark cycle in the laboratory animal unit of the Biological Faculty, M.V. Lomonosov Moscow State University with access to food and water ad libitum. To obtain the offspring, sexually mature male and female rats were placed together for 4 days. Pregnant females were housed individually 3–4 days before the expected delivery. The next day after birth, the litters were reduced to 8 pups. Experiments were carried out on male rats aged 10 to 15 days (“young” in the following text) and 3 to 4 months (“adult” in the following text). In total, 30 adult and 75 young rats (offspring of 20 females) were used in the study. Rats were killed by decapitation, adult rats were first anesthetized by CO
2.
The main object of the study was the saphenous artery. There are several reasons for this choice. The rat saphenous artery is a muscular-type artery, is densely innervated, and develops strong contractile responses to the stimulation of sympathetic nerves [
21]. Therefore, along with its conduit function the saphenous artery participates in the control of cutaneous blood flow, which comprises up to 20% of the cardiac output in neonatal rats [
22]. Importantly, according to our data, developmental alterations of several regulatory mechanisms observed in the saphenous artery are associated with corresponding changes at the level of blood pressure control [
23]. Therefore, we consider the saphenous artery to be a representative artery from the peripheral circulation, i.e., the findings from the saphenous artery can be generalized to other vascular beds. In addition, the saphenous artery is large enough in 10–15-day-old rats to assess its responses by wire myography and to obtain a sufficient amount of tissue for qPCR, Western blot, and spectrophotometry analyses (described further).
2.2. Measurement of mRNA Expression Levels in Arterial Tissue by qPCR
Measurement of mRNA expression levels in arterial tissue by qPCR was performed as previously described [
13,
24]. Briefly, saphenous arteries were isolated, carefully cleaned from surrounding tissue and blood, and kept in RNAlater solution (Qiagen, Venlo, The Netherlands) at −20 °C pending further procedures. Each sample in the adult group included one artery and in the young group two arteries.
Arteries were homogenized in RLT lysis buffer (Qiagen, Venlo, The Netherlands) containing 1% β-mercaptoethanol and proteinase K (20 mg/mL, MP Biomedicals, Irvine, CA, USA). RNA was extracted using the ExtractRNA kit (cat. number BC032, Evrogen, Moscow, Russia) according to the manufacturer’s instructions and then processed with DNase I (cat. number EN0525, Fermentas, Waltham, MA, USA, 1000 U/mL). The RNA concentration was measured by a NanoDrop 1000 (Thermo Scientific, Waltham, MA, USA), and then all samples were diluted to an equal concentration of 35 ng/μL. Reverse transcription was performed using the MMLV RT kit (Evrogen, Moscow, Russia) according to the manufacturer’s manual. qPCR was run in the Bio-Rad CFX 96 Real-Time PCR System (Bio-Rad, Hercules, CA, USA), using qPCRmix-HS SYBR (Evrogen, Moscow, Russia). Primers used in this study were synthesized by Evrogen; their sequences are listed in
Table 1. The mRNA expression level was calculated as E^(−Cq), where E is the primer efficiency, and Cq is the cycle number corresponding to the maximum of the second derivative of the fluorescence curve. Primer efficiency was identified using the LinRegPCR (version 2015.1) software [
25]. All E values were close to 2.0. mRNA expression levels of investigated genes were normalized to the mRNA expression level of the housekeeping gene beta-actin (
Actb), detected in the same sample. Importantly, mRNA expression values of
Actb did not differ between the two age groups and were 2.2 × 10
−6 ± 0.5 × 10
−6 in adult arteries (n = 9) and 1.6 × 10
−6 ± 0.3 × 10
−6 in young arteries (n = 9,
p > 0.05, unpaired Student’s
t-test).
2.3. Measurement of Superoxide Dismutase and Catalase Activity in Arterial Tissue
Two saphenous arteries from one adult rat or four saphenous arteries from two young rats were used for one sample. Arteries were homogenized with ice cold PBS and centrifuged at 1500× g over 10 min at +4 °C. After that, probes were incubated with ice for 15 min. Supernatants were collected and frozen at −80 °C till further analysis.
SOD activity was determined by a spectrophotometric method based on the inhibition of the superoxide anion radical production rate by SOD, formed in the reaction of quercetin autooxidation in a slightly alkaline medium [
26]. To determine the total SOD activity, 5 μL of the homogenate was mixed with 150 μL of a buffer containing 20 mM potassium phosphate buffer pH 7.8, 8 mM TEMED, and 0.08 mM EDTA in a 96-well plate. The reaction was started by adding 5 μL of 0.15 mg/mL quercetin in DMSO. To determine MnSOD activity, 3 mM KCN was added to the buffer. The change in optical density at 405 nm was recorded on a plate spectrophotometer INNO LTEK Co., Ltd. Instruments, Seongnam-si, Republic of Korea. One unit (U) of SOD activity was defined as the rate of 50% inhibition of the quercetin autooxidation reaction. Total SOD activity was expressed as U/mg total protein. Cu/ZnSOD activity was calculated as the difference between total SOD and MnSOD activities. Total protein content was determined by the modified Lowry method [
27].
Catalase activity was determined spectrophotometrically using a method based on the reaction of excess (unreacted) hydrogen peroxide with FOX reagent [
28]. The substrate–buffer mixture for catalase contained 50 mM potassium phosphate buffer (pH 7.4) and 0.2 mM hydrogen peroxide. In a 96-well plate, the supernatant of vascular homogenates was mixed with the substrate–buffer mixture at a ratio of 1:20 (
v/
v) and incubated for 10 min at room temperature. The reaction was stopped by the addition of FOX reagent and further incubated for 30 min at room temperature until a stable violet coloration developed. Optical density was recorded at 560 nm using a microplate spectrophotometer (INNO LTEK Co., Ltd. Instruments, Seongnam-si, Republic of Korea). The concentration of decomposed hydrogen peroxide was calculated using the millimolar extinction coefficient of 235 mM
−1·cm
−1. One unit (U) of catalase activity was defined as the amount of enzyme required to decompose 1 μmol of hydrogen peroxide per minute. Catalase activity in vascular homogenates was expressed as U/mg of total protein. Total protein concentration was determined using the modified Lowry method [
27].
2.4. Measurement of Protein Abundance in Arterial Tissue by Western Blotting
Measurement of protein abundance in arterial tissue was performed as described previously [
13,
29]. Four saphenous arteries from two young rats or two saphenous arteries from one adult rat were used for one sample. The endothelium was rapidly removed after isolation of the artery with the use of a custom-made wire myograph analogue [
30]. Thereafter, saphenous arteries were quickly frozen in liquid nitrogen and kept at −80 °C until further analysis. Samples were homogenized in ice-cold SDS buffer supplemented with protease inhibitor cocktail (Roche, Bazel, Switzerland) and phosphatase inhibitors (2 mg/mL NaF and 180 mg/mL Na
3VO
4), centrifuged at 16,900×
g for 5 min; the supernatant was kept at −20 °C till further analysis. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Santa Cruz, Dallas, TX, USA) using the Trans-Blot Turbo transfer system (Bio-Rad). The transfer was visualized with Ponceau S staining. Thereafter, membranes (each containing samples from adult and young groups) were cut into two pieces at the level of appr. 28 kDa based on the corresponding levels of protein markers (Abcam, Cambridge, UK, cat. number 116027). The membranes were blocked with 5% nonfat milk (AppliChem, Darmstadt, Germany) in TBS with 0.1% Tween 20 (TBSt). Then, two pieces of the first membrane were incubated overnight with antibodies against GAPDH (mouse, Abcam, cat. number 125247, 1:2000 in TBSt with 5% milk, molecular weight 37 kDa) or SOD1 (rabbit, Sigma, St. Louis, MO, USA, cat. number SAB5200083 1:4000 in TBSt with 5% milk, molecular weight 23 kDa), and one piece of the second membrane was incubated overnight with antibodies against SOD3 (rabbit, Enzo, New York, NY, USA, cat. number ADI-SOD-106-F 1:1000 in TBSt with 5% milk, molecular weight 35–40 kDa). The next day, the membranes were washed from the primary antibodies and incubated with appropriate secondary antibodies: anti-mouse (Cell Signaling, Boston, MA, USA, cat. number 7076, 1:5000 in 5% milk) or anti-rabbit (Cell Signaling, Boston, MA, USA, cat. number 7074, 1:10,000 in 5% milk) for 1 h and visualized with SuperSignal West Dura Substrate (Thermo Fisher Scientific, Waltham, MA, USA) using a ChemiDoc (Bio-Rad, Hercules, CA, USA). Of note, since SOD3 and GAPDH have similar molecular weights (close to 40 kDa), membrane stained with antibodies against SOD3 was stripped using stripping buffer (for composition see
Section 2.6), blocked again as described above, and incubated overnight with antibodies against GAPDH. Western blotting experiments were analyzed using the Image Lab software 6.0 (Bio-Rad, Hercules, CA, USA). SOD1 and SOD3 protein abundance was normalized to GAPDH in each sample—the rationale for using GAPDH as a reference protein for comparing arteries from adult and early postnatal animals was provided previously [
31])—and then the average ratio of the «Adult» group was taken as 100%.
2.5. Wire Myograph Experiments
Saphenous arteries were carefully cleaned from surrounding tissue in PSS for vessel dissection (PSS I), cut into 2-mm-long segments, and mounted in a wire myograph (410 A or 620 M, DMT A/S, Aarhus, Denmark) to measure the isometric force. Experiments were carried out both on arteries with an intact endothelium and on arteries with the endothelium removed. The endothelium was removed mechanically using a rat whisker by performing rotational movements inside the artery.
During the subsequent experiments, arterial segments were kept in PSS II. The myograph chambers were heated to 37 °C and continuously bubbled with 5% CO
2 in O
2 to maintain pH at 7.4. Data were recorded at 10 Hz using an analogue-to-digital converter (E14-140M, L-CARD, Moscow, Russia) and the PowerGraph 3.3 software (DISoft, Moscow, Russia). Each arterial segment was stretched to 0.9 × d100 (90% of the inner diameter it would have at a transmural pressure of 100 mmHg), corresponding to maximum active force development [
13,
32].
At the beginning of each experiment, all vessels were exposed to a standard start-up procedure. This included the following: (a) application of noradrenaline (10 μM); (b) application of acetylcholine (10 μM) on the top of methoxamine-induced contraction (1 μM) to confirm the integrity or removal of the endothelium (by the presence or the absence of a relaxation response, respectively); (c) application of methoxamine (10 μM).
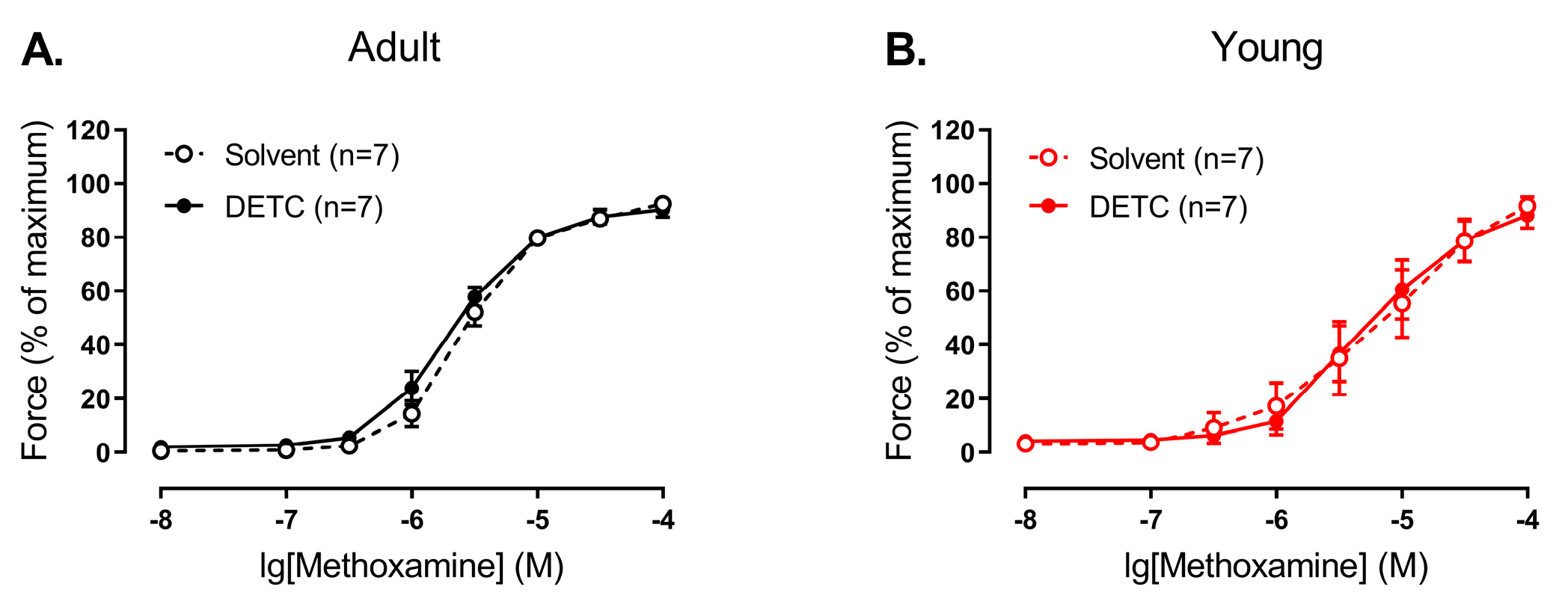
Two experimental protocols were used in the study. The first experimental protocol included two sequential concentration–response relationships to methoxamine (concentration range from 0.01 to 100 μM, for three minutes each). The first relationship was started 20 min after the end of the activation procedure. After washout, one arterial segment was incubated for 20 min with one of the following substances or their combinations: inhibitor of Cu/ZnSOD DETC (1 mM), NO-synthase inhibitor L-NNA (0.1 mM), soluble guanylate cyclase inhibitor ODQ (1 µM), NO-donor sodium nitroprusside SNP (0.1 µM), superoxide dismutase SOD (300 U/mL), catalase (1500 U/mL). The other segment was incubated with an equivalent volume of the solvent (DMSO or H2O or their combination). Then, the second concentration–response relationship was obtained. The second experimental protocol also included sequential concentration–response relationships. The first one was to methoxamine (concentration range from 0.01 to 100 μM), which allowed us to determine the concentration of methoxamine that causes a contraction of approximately 60% of the maximum. After washout, contractions at the level of approximately 60% of the maximum in two arterial segments were induced, and then H2O2 (concentration range from 1 μM to 0.3 mM, for 2 min each) was added sequentially to one of the segments and an equivalent volume of H2O was added to another segment (Time-control).
To calculate active force values at each methoxamine concentration, the force value at the fully relaxed state was subtracted from all recorded values. All active force values obtained during the second concentration–response relationship to methoxamine were expressed as the percentage of the maximum active force developed during the respective first concentration–response relationship or as a percentage of the precontraction level (for H
2O
2). Areas under the curves (AUCs) were calculated in several experimental series as an additional way to evaluate the effects of inhibitors (
Table S1). The internal diameter (d100), maximal force and values of acetylcholine-induced relaxation of the arteries in different series of wire-myography experiments are presented in
Table S2.
2.6. Solutions
1. PSS for vessel isolation (PSS I), in mM: 145 NaCl, 4.5 KCl, 1.2 NaH2PO4, 1 MgSO4, 0.1 CaCl2, 0.025 EDTA, 5 HEPES (pH 7.4).
2. PSS for myograph experiments (PSS II), in mM: 120 NaCl, 26 NaHCO3, 4.5 KCl, 1.2 NaH2PO4, 1.0 MgSO4, 1.6 CaCl2, 5.5 D-glucose, 0.025 EDTA, 5 HEPES; equilibrated with 5% CO2 in 95% O2 (pH 7.4).
3. SDS-buffer: 0.0625 M Tris-HCl (pH 6.8), 2.5% SDS, 10% water-free glycerin, 2.47% dithiothreitol, 0.002% bromophenol blue.
4. TBS: 50 mM Tris-HCl, 150 mM NaCl, pH 7.6.
5. TBSt: TBS with 0.1% Tween.
6. Stripping buffer: 0.2 M glycine, 0.1% SDS, 1% Tween (pH 2.2).
2.7. Drugs
Noradrenaline, acetylcholine, methoxamine, DETC, catalase (all dissolved in H2O) and ODQ (dissolved in DMSO) were obtained from Sigma (St. Louis, MO, USA). L-NNA (dissolved in H2O) was obtained from Alexis Biochemicals (San Diego, CA, USA). SOD (dissolved in H2O) was obtained from BiosynthCarbosynth (Staad, Switzerland).
2.8. Statistics
Statistical analysis was performed using GraphPad Prism 8.0. The normality of data distribution was checked using the Shapiro–Wilk test; data are presented as the mean and standard error of the mean or as the median and interquartile range, depending on the type of data distribution. Statistical analysis was performed using two-way repeated measures ANOVA, one-way ANOVA, the parametric Student’s t-test, and the Mann–Whitney test. Differences were accepted as statistically significant if the p value was less than 0.05; n represents the number of animals, i.e., biological replicates.
4. Discussion
In the present study, for the first time, age-related changes of SOD1 and SOD3 vasomotor functional contribution in systemic arteries were evaluated. We showed that activities of Cu/ZnSODs (SOD1 and SOD3) as well as SOD3 protein content were considerably increased in arterial tissue from early postnatal rats in comparison to adult animals. In accordance with these results, functional experiments indicate that SOD1 and SOD3 functioning promotes contraction of arterial smooth muscle of young, but not adult rats. In addition, endothelial NO (or its derivatives) considerably counteracts the SOD1 and SOD3 influence in arterial smooth muscle of early postnatal rats.
Age-related changes in SOD expression and/or activity were addressed previously, but only in the pulmonary circulation, not in the systemic arteries. For example, the activity and protein amount of Cu/ZnSOD were significantly decreased in the lung tissue of newborn rabbits compared to adults [
15]. Similarly, the expression of Cu/ZnSOD mRNA in human lung tissue increased from newborns to adults [
16]. In addition, the protein content of SOD1 and SOD3 in the lung tissue of adult mice was higher than in 7-day-old mice [
17]. Of note, lung tissue, used in the cited studies, includes many types of cells, not just vascular ones. Nevertheless, taken together, these data suggest that the activity and expression of SOD1 and SOD3 in the pulmonary circulation at the early stages of postnatal development is decreased, making newborn organisms more susceptible to persistent pulmonary hypertension development [
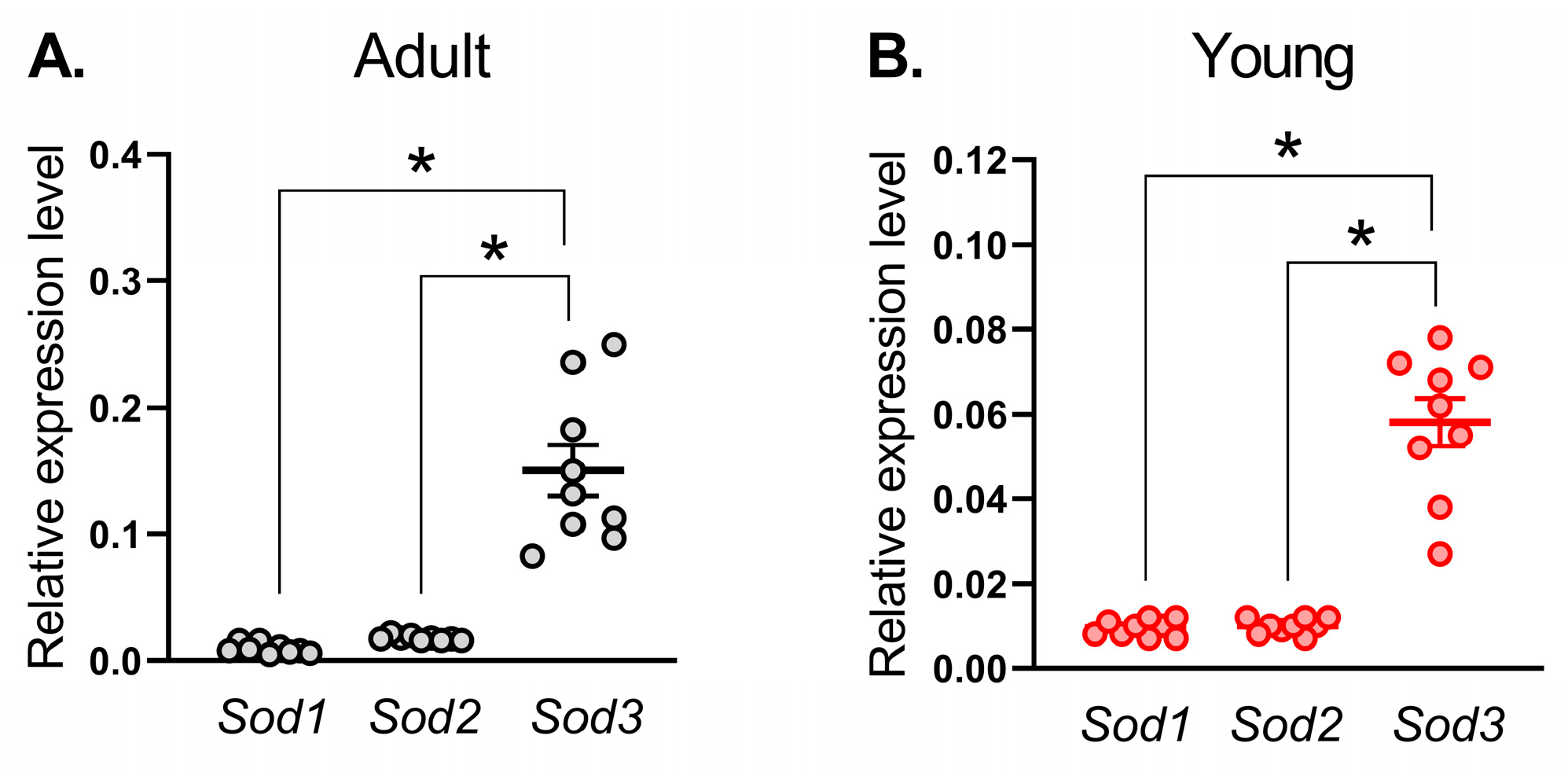
18]. Our study, for the first time, addresses the expression pattern and age-related changes in SOD activity/protein content in systemic arteries. We found that the mRNA expression level of
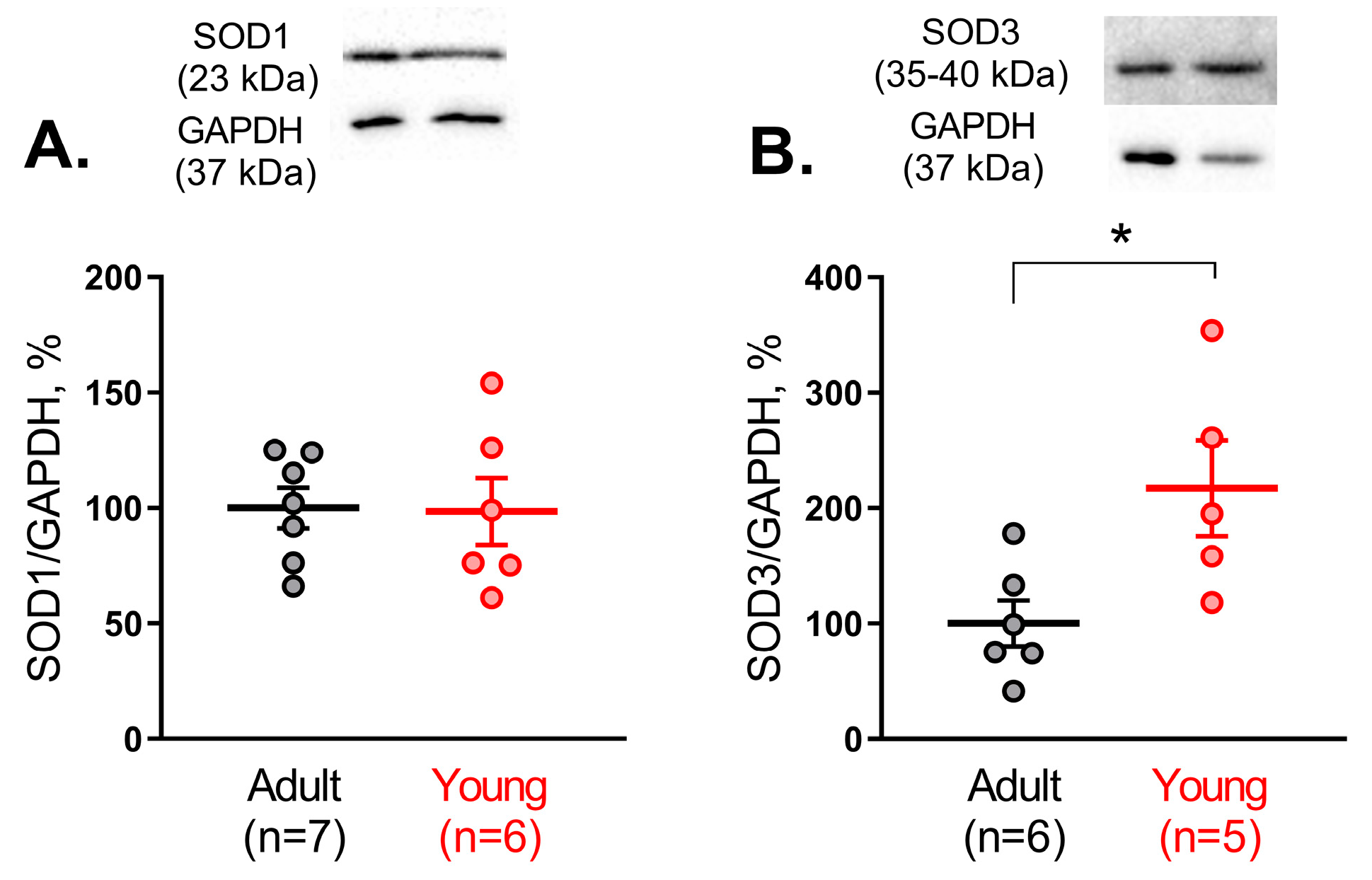
Sod3 was the highest in both adult and young rats. At the same time, total SOD activity as well as Cu/ZnSOD activity was considerably increased in arterial tissue from early postnatal rats in comparison to adult. Importantly, although SOD1 protein content did not differ between arterial smooth muscle of adult and young rats, the protein amount of SOD3 in the arterial smooth muscle of 10–15-day old rats was increased in comparison to adults. Thus, in contrast to the pulmonary circulation, arteries of the systemic circulation demonstrate a higher activity and protein amount of Cu/ZnSODs during the early postnatal period.
As mentioned, SODs catalyze the conversion of O
2•− into H
2O
2, which participates in cell signaling. H
2O
2 may cause both relaxation and contraction of arteries, as it was shown for different arteries from adult animals [
2,
3,
4,
5,
6]. However, much less is known about vasomotor effects of H
2O
2 in arteries of newborn/early postnatal organisms. A few studies demonstrated transient H
2O
2-induced contractions of piglets’ pial arterioles [
34] as well as small pulmonary arteries of 1–2-week-old piglets [
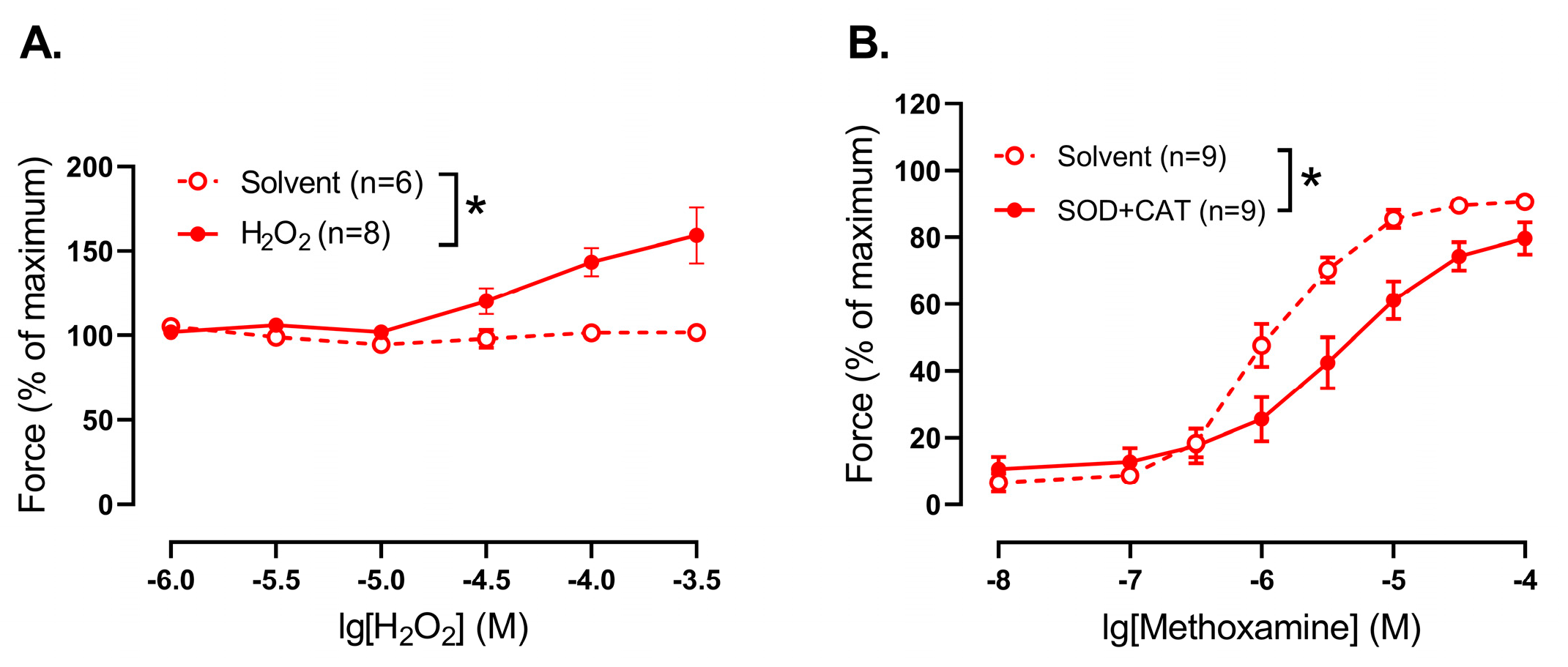
35]. In accordance with this, our data demonstrate that H
2O
2 addition promotes contraction, while H
2O
2 elimination, on the contrary, results in the weakening of contractile responses of endothelium-denuded saphenous arteries from 10–15 day-old rats, indicating a pro-contractile influence of H
2O
2 in arterial smooth muscle in the early postnatal period. Of note, previously we demonstrated that H
2O
2 elimination had no effect on methoxamine-induced contractile responses of saphenous arteries of adult rats [
33], indicating that H
2O
2 does not have a pronounced vasomotor effect in this artery at adulthood.
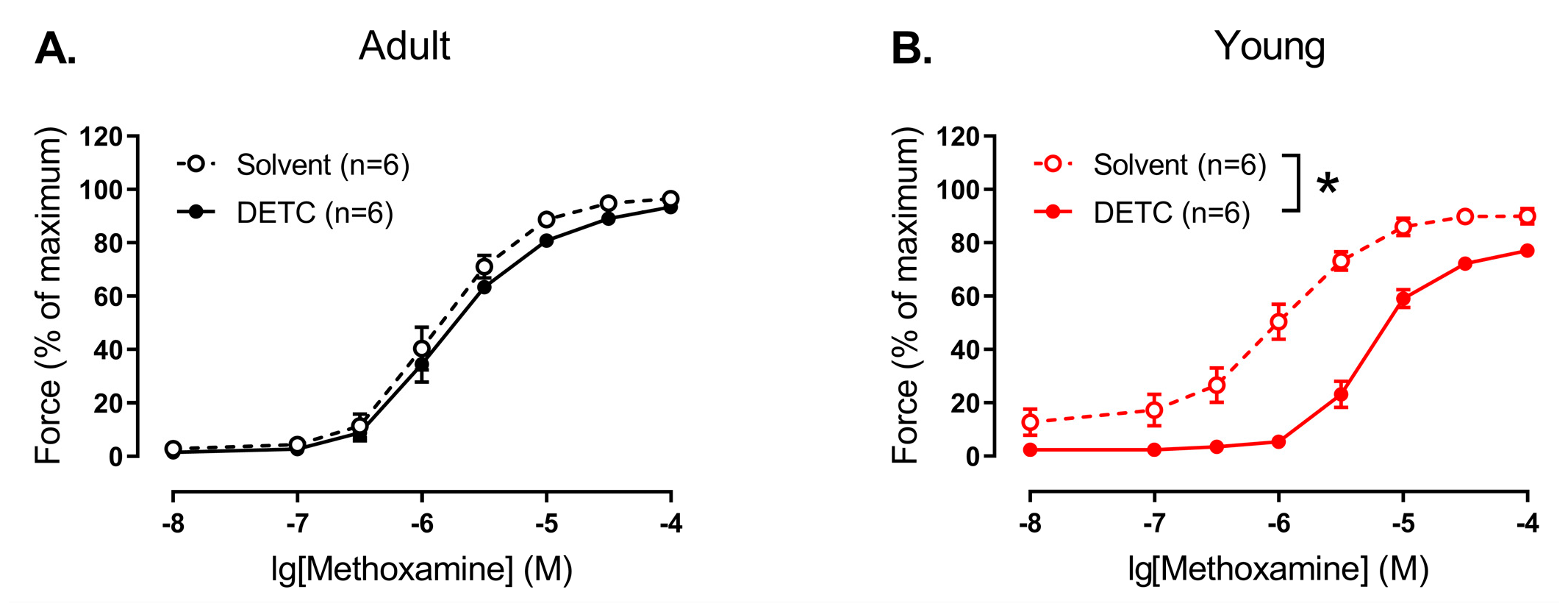
Our next step was to evaluate the functional impact of H
2O
2, produced by Cu/ZnSODs, on the regulation of smooth muscle contraction in saphenous arteries from two age groups of rats. To do so, we used DETC, which chelates Cu
2+, leading to a decrease of enzyme activity [
7] and, consequently, H
2O
2 content in the arterial wall [
36]. In accordance with our molecular experiments, which demonstrated low activity and protein amount of Cu/ZnSODs in the arteries of adult rats, DETC had no influence on methoxamine-induced contractions of endothelium-denuded arteries in adult animals. Similarly, in previous studies, DETC had no effect on phenylephrine-induced contractile responses of rat aorta [
8,
9] and contractile responses to PGF2alpha of mouse aorta [
12]. Along with that, DETC augmented vasocontraction of mouse aorta in response to serotonin [
12] and norepinephrine [
10] as well as induced further vasoconstriction of precontracted by norepinephrine rat mesenteric arteries [
11], pointing to an anti-contractile influence of H
2O
2, produced by Cu/ZnSODs. Thus, the vasomotor contribution of SODs may depend on the artery type and/or the agonist used. Importantly, our data demonstrate that the vasomotor influence of SODs may also depend on the stage of the organism’s development: DETC led to pronounced weakening of methoxamine-induced contraction of endothelium-denuded saphenous arteries of young rats. In accordance with the described above pro-contractile influence of H
2O
2 in endothelium-denuded saphenous arteries of young rats, the weakening of arterial contraction after Cu/ZnSOD inhibition indicates a pro-contractile role of H
2O
2, produced by these enzymes, in the smooth muscle of early postnatal saphenous arteries. Of note, the mechanism of such pro-contractile influence remains unclear. Previously we demonstrated, that ROS, produced by NADPH-oxidase, promote vasocontraction of saphenous artery in early postnatal rats by activation of LTCC [
24]. Taking into account (1) the high production of O
2•− (demonstrated previously [
13]) and (2) high activity of Cu/ZnSODs in saphenous arteries of early postnatal rats (demonstrated in the present study), it is possible that H
2O
2 formed from O
2•− activates LTCC. However, such a hypothesis should be tested in future studies.
It should be also noted that the activity of one of the H
2O
2 scavengers, catalase, was higher in arteries of young compared to adult rats. As already mentioned, we demonstrated previously increased O
2•− production [
13] in saphenous arteries of early postnatal rats. Therefore, apparently, saphenous arteries of early postnatal rats exhibit both higher levels of ROS production and ROS degradation. Such an augmentation of catalase activity in young arteries may serve as an adjustment for increased ROS levels, where initially increased production leads to a rise in ROS levels and a secondary increase in ROS degradation then establishes a new, higher ROS steady state with balanced production and degradation. However, we do not know the kinetics of these processes and cannot say whether such a compensation occurs to the same extent as in adults. Determination of the kinetics of the production and degradation processes requires a large number of experiments that were beyond the scope of the present study. In addition, a set of data demonstrating (1) higher ROS production (O
2•− [
13]), (2) higher Cu/ZnSODs activity, (3) higher SOD3 protein content in saphenous arteries of early postnatal rats allows the suggestion that the differences in functional impact of Cu/ZnSODs between young and adult arteries are mainly associated with ROS generation than with their downstream signaling.
Importantly, vasomotor effects of many molecules, including H
2O
2, on smooth muscle tone may depend on the endothelium. For example, endothelium denudation eliminated contraction of pressurized gracilis muscle arterioles of adult rats in response to 0.1 mM of H
2O
2 [
3]. In addition, incubation with SOD weakened phenylephrine-induced contraction of endothelium-intact pulmonary arteries of newborn rabbits, which was not observed after endothelium denudation [
37]. Therefore, we evaluated the effects of the Cu/ZnSOD inhibitor DETC in endothelium-intact saphenous arteries. The presence of endothelium did not affect the effect of DETC in adult arteries: contractile responses to methoxamine were not affected by DETC. However, DETC did not weaken anymore the contraction of young arteries when the endothelium was left intact. Thus, endothelium is able to counteract the vasomotor impact of Cu/ZnSODs in the arteries of young rats. Since the anti-contractile effect of endothelial NO is particularly pronounced during early postnatal ontogenesis [
33], we hypothesized that the lack of DETC effect in endothelium-intact arteries of young rats is primarily associated with the NO pathway. Indeed, DETC decreased contractile responses of young arteries in the presence of the NO-synthase inhibitor L-NNA, as we previously observed in vessels without endothelium (
Figure 5B). This indicates that endothelial NO counteracts the vasomotor impact of Cu/ZnSODs in the arterial smooth muscle of young rats.
Further experiments were aimed to answer the question of how NO counteracts the effect of DETC in the arteries of young rats. According to the literature, the activity of Cu/ZnSODs can be altered by post-translational modifications via phosphorylation [
38]. Therefore, we suggested that the signaling cascade activated by NO in the smooth muscle cell (activation of soluble guanylate cyclase followed by activation of PKG) may be involved. To answer this question, we evaluated the effect of DETC in the presence of the soluble guanylate cyclase inhibitor ODQ. Importantly, the effectiveness of using 1 µM of ODQ in the saphenous artery of rats was previously demonstrated [
33]. Our data show that DETC had no effect in the presence of ODQ, indicating that the neutralizing effect of NO on the effects of DETC in endothelium-intact arteries of young rats is not linked with soluble guanylate cyclase activation, and, probably, its main target in smooth muscle cell—PKG. Of note, Cu/ZnSODs may be nitrosylated, which may also change their activity [
38]. Therefore, we suggested that NO (or its derivatives, such as peroxynitrite (ONOO
−)) may neutralize SODs directly. To check this, we assessed the effects of DETC in endothelium-denuded arteries in the presence of the NO donor sodium nitroprusside (SNP), which mimics best the effect of naturally produced endothelial NO [
39]. Importantly, incubation with DETC did not further attenuate the contractile response in the presence of SNP, similar to that which we observed in endothelium-intact arteries. Thus, NO (or its derivatives, such as peroxynitrite (ONOO
−)) counteracts the SOD1 and SOD3 functional impact in the arterial smooth muscle of young rats regardless of the classical signaling cascade of NO, in particular soluble guanylate cyclase activation.
Limitations
The present study has a number of limitations. First, the effect of exogenous H2O2 was not studied in the arteries of adult rats. Second, sex-dependent differences were not assessed, since only males were used. Third, H2O2 downstream signaling mechanisms were not explored.