Sprouting Enhances Submergence Tolerance in Rice by Promoting Glutathione Biosynthesis and Turnover
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Treatments
2.2. Measurement of Rice Phenotypic and Biochemical Parameters
2.3. RNA Extraction, Library Construction, RNA Sequencing, and Data Analysis
2.4. Field Trial Verification
2.5. Statistical Analysis
3. Results
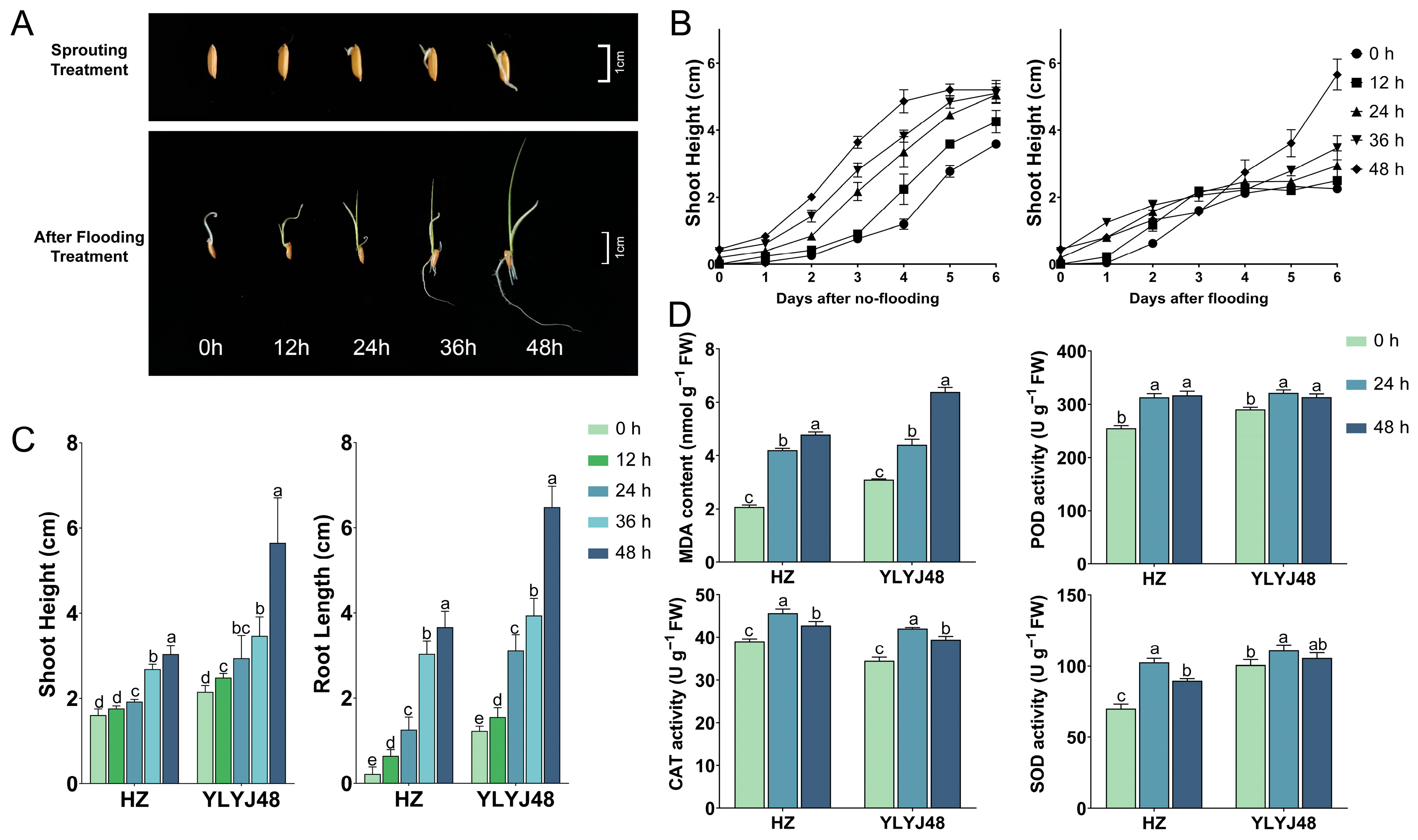
3.1. Phenotypic and Biochemical Changes of Sprouted Seedlings Under Submergence Stress
3.2. Transcriptome Analysis Reveals That Sprouting Regulates the Glutathione Metabolic Pathway
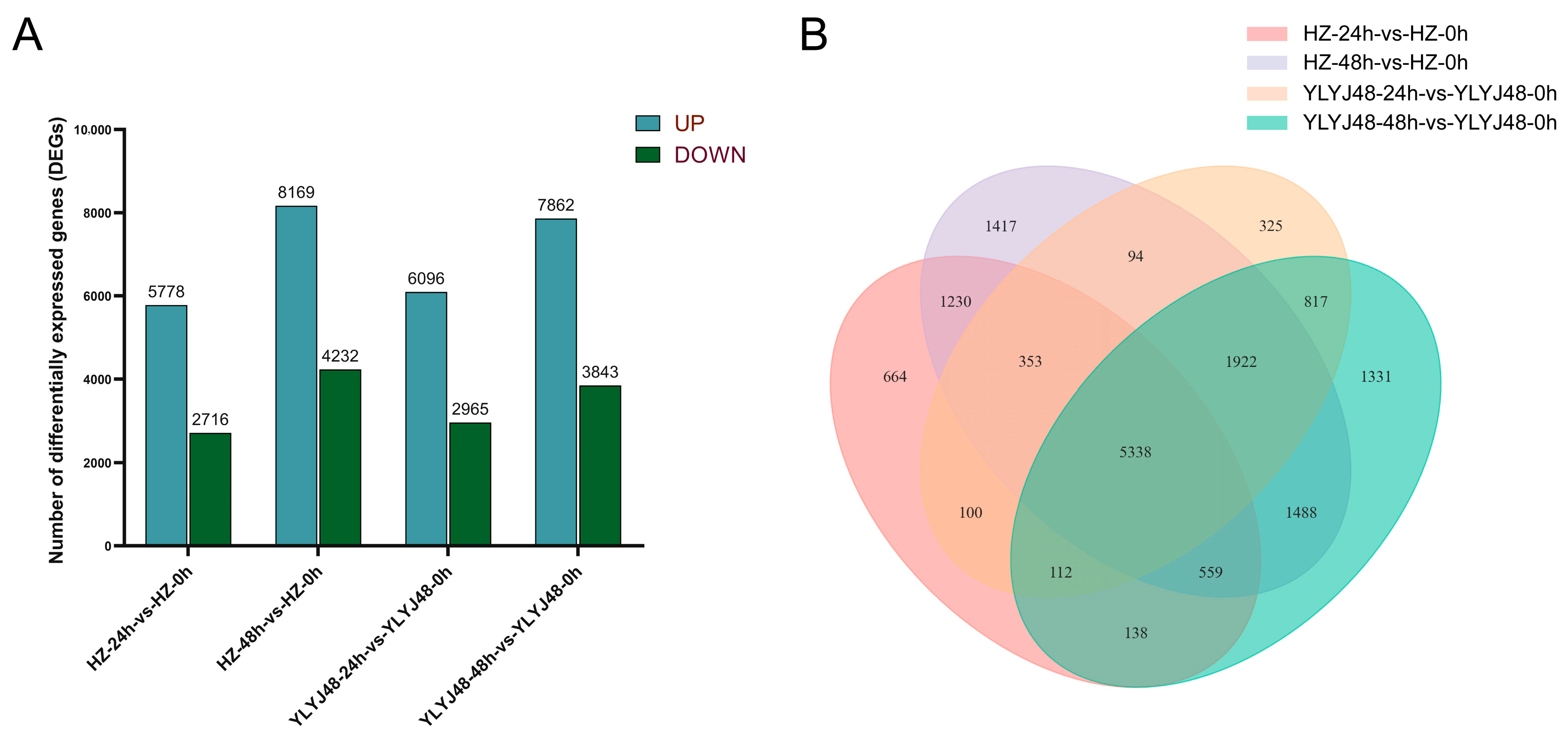
3.2.1. RNA Sequencing (RNA-Seq) Analysis and Identification of Differentially Expressed Genes (DEGs)
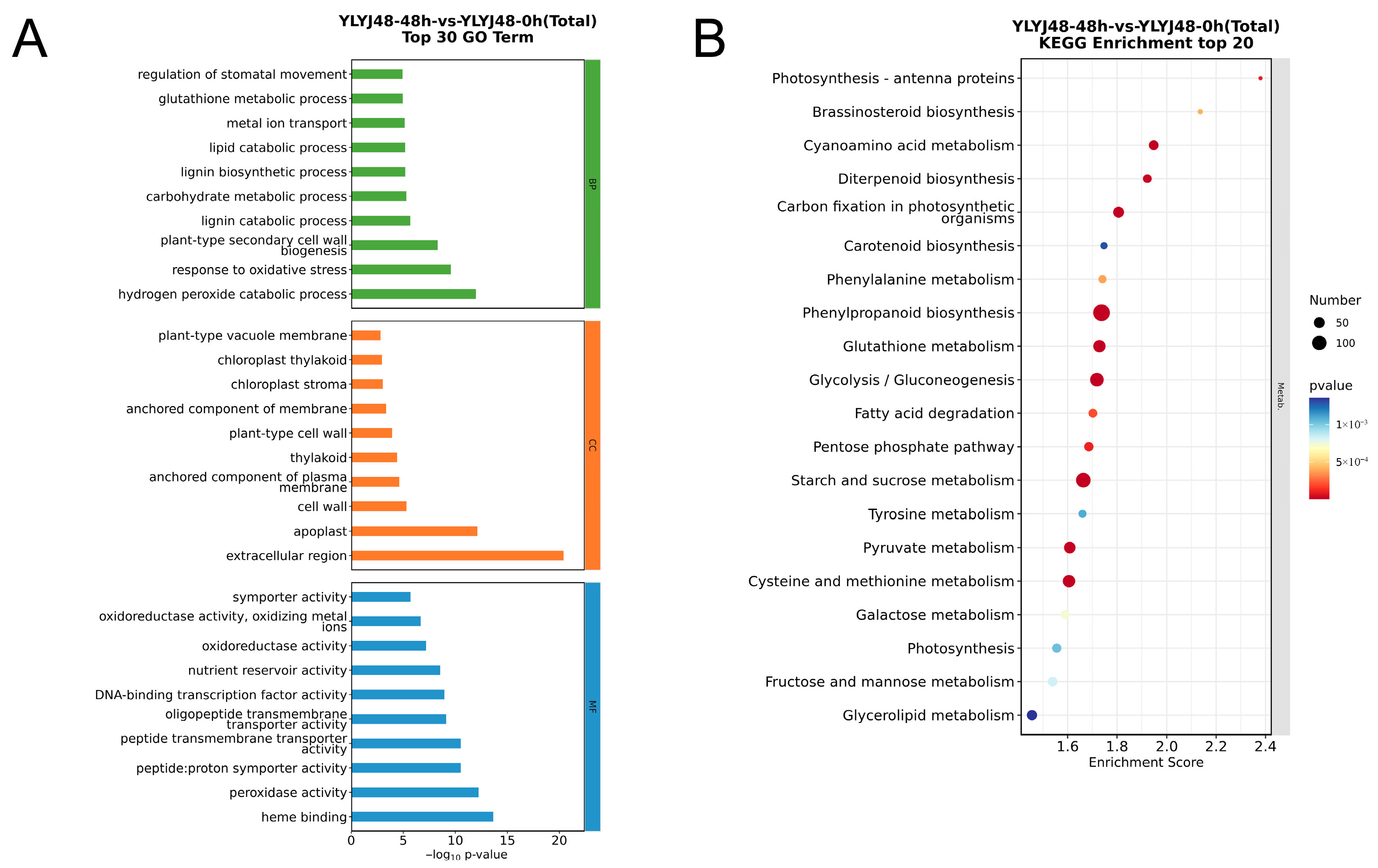
3.2.2. GO and KEGG Pathway Enrichment Analysis of DEGs from Different Sprouting Treatments
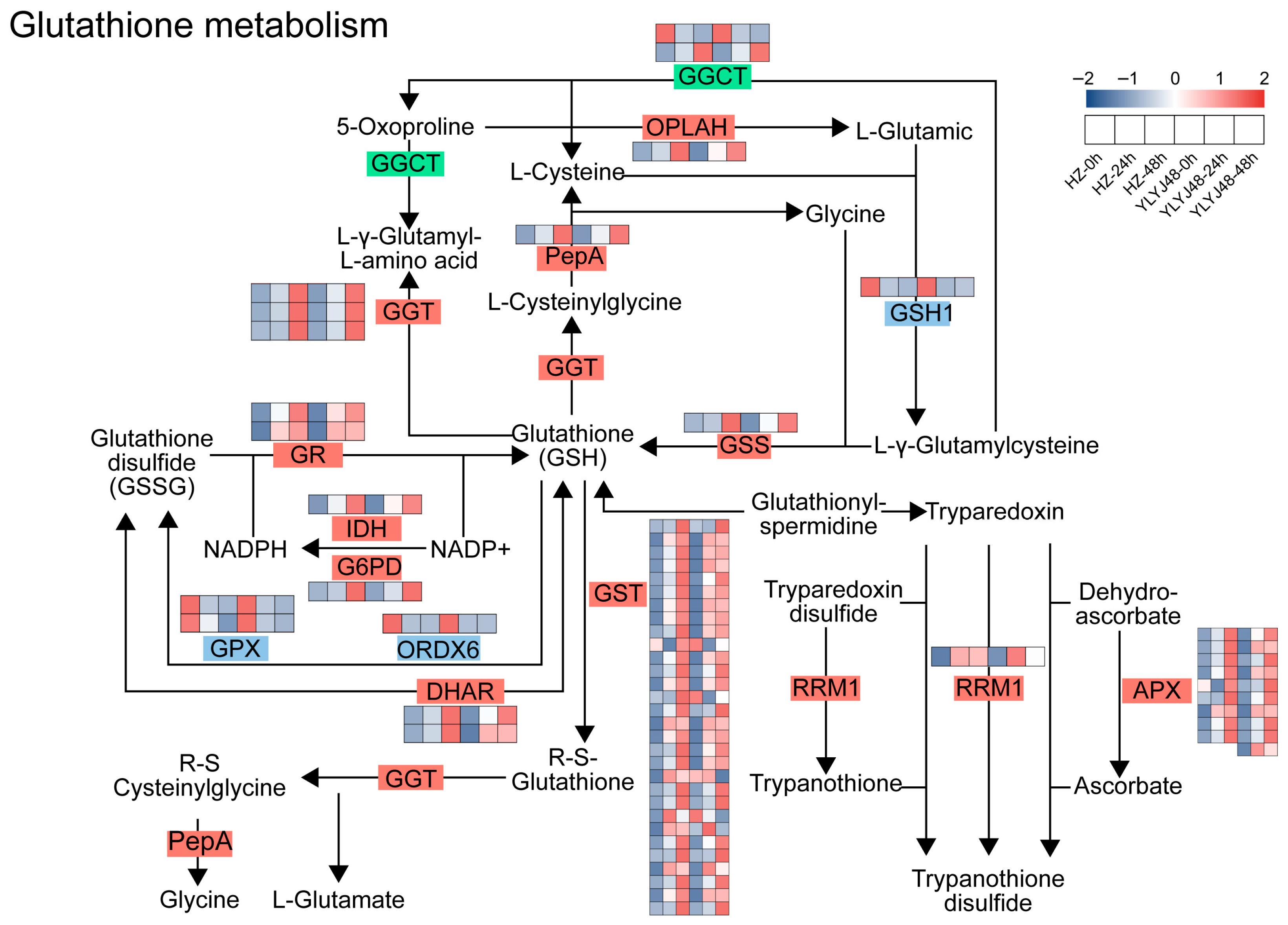
3.2.3. Sprouting Regulates Key Genes in the Glutathione Metabolism Pathway of Rice
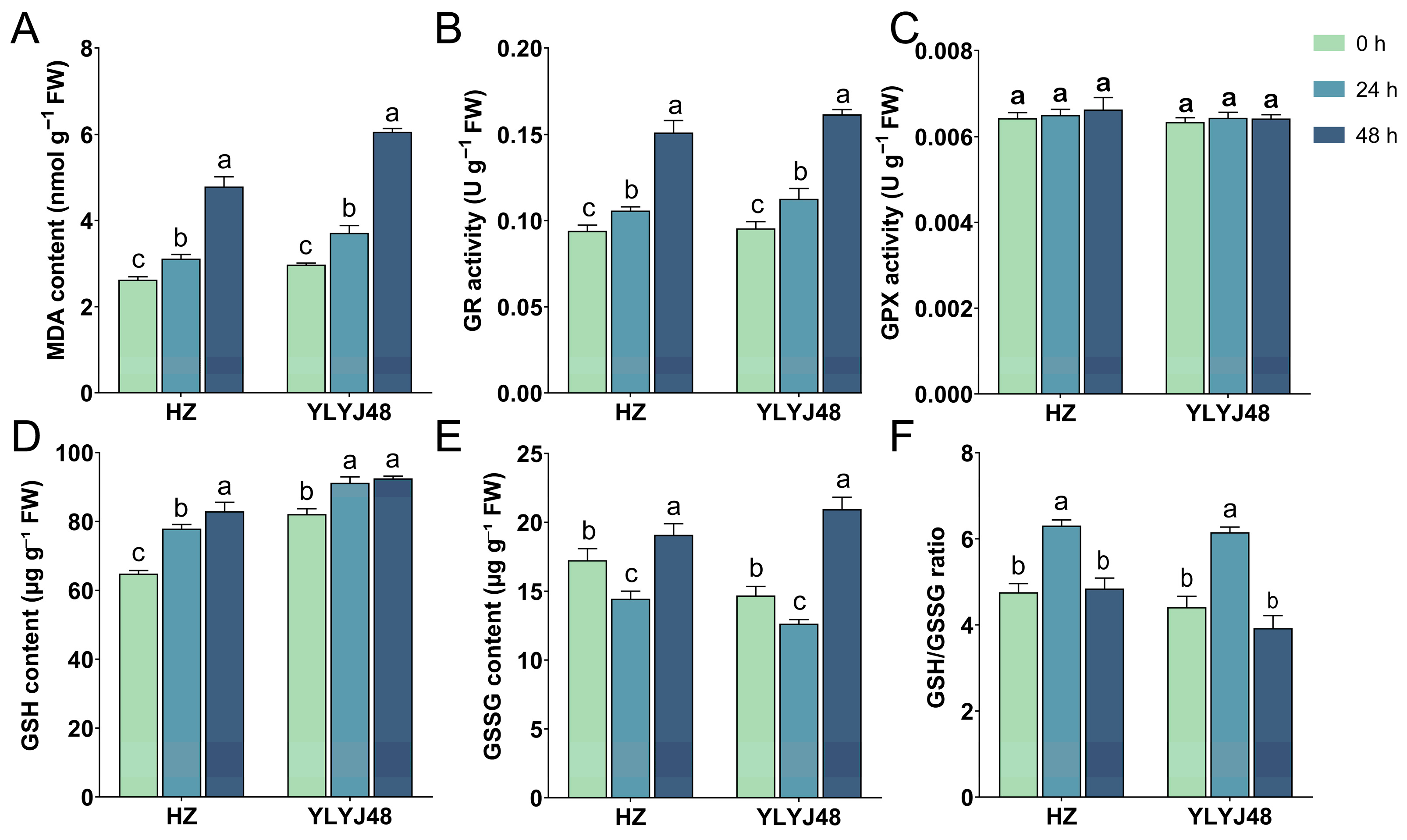
3.3. Effects of Sprouting Treatment on Basal Biochemical Indicators in Rice Seeds
3.4. Functional Verification and Field Application of Exogenous Glutathione in Enhancing Submergence Tolerance
4. Discussion
4.1. Sprouting Enhances Submergence Escape Through Improved Antioxidant Capacity
4.2. Regulation of Glutathione Metabolism by Sprouting Contributes to Submergence Tolerance in Rice
4.3. Practical Applications of Sprouting Treatment and Exogenous Glutathione
4.4. Implications for Breeding and Integrated Crop Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhan, P.; Zhu, W.; Zhang, T.; Li, N. Regional inequalities of future climate change impact on rice (Oryza sativa L.) yield in China. Sci. Total Environ. 2023, 898, 165495. [Google Scholar] [CrossRef]
- Verma, V.; Vishal, B.; Kohli, A.; Kumar, P.P. Systems-based rice improvement approaches for sustainable food and nutritional security. Plant Cell Rep. 2021, 40, 2021–2036. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Wang, Z. Rice seed germination priming by salicylic acid and the emerging role of phytohormones in anaerobic germination. J. Integr. Plant Biol. 2024, 66, 1537–1539. [Google Scholar] [CrossRef]
- Chamara, B.S.; Marambe, B.; Kumar, V.; Ismail, A.M.; Septiningsih, E.M.; Chauhan, B.S. Optimizing Sowing and Flooding Depth for Anaerobic Germination-Tolerant Genotypes to Enhance Crop Establishment, Early Growth, and Weed Management in Dry-Seeded Rice (Oryza sativa L.). Front. Plant Sci. 2018, 9, 1654. [Google Scholar] [CrossRef]
- Lu, H.; Wang, M.; Zhou, S.; Chen, K.; Wang, L.; Yi, Z.; Bai, L.; Zhang, Y. Chitosan Oligosaccharides Mitigate Flooding Stress Damage in Rice by Affecting Antioxidants, Osmoregulation, and Hormones. Antioxidants 2024, 13, 521. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Cao, C.; Shen, T.; Zhong, L.; He, H.; Chen, X. Comprehensive metabolomic and proteomic analysis in biochemical metabolic pathways of rice spikes under drought and submergence stress. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2019, 1867, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Monika; Kumari, S.; Kumar, G. Sub1 QTL confers submergence tolerance in rice through nitro-oxidative regulation and phytohormonal signaling. Plant Physiol. Biochem. 2024, 211, 108682. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Cheng, X.-X.; Yu, M.; Zhang, N.; Zhou, Z.-Q.; Xu, Q.-T.; Mei, F.-Z.; Qu, L.-H. Reactive oxygen species regulate programmed cell death progress of endosperm in winter wheat (Triticum aestivum L.) under waterlogging. Protoplasma 2016, 253, 311–327. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, D.; Zhu, M.; Li, F. Exogenous 6-Benzyladenine Improves Waterlogging Tolerance in Maize Seedlings by Mitigating Oxidative Stress and Upregulating the Ascorbate-Glutathione Cycle. Front. Plant Sci. 2021, 12, 680376. [Google Scholar] [CrossRef]
- Rai, G.K.; Kumar, P.; Choudhary, S.M.; Singh, H.; Adab, K.; Kosser, R.; Magotra, I.; Kumar, R.R.; Singh, M.; Sharma, R.; et al. Antioxidant Potential of Glutathione and Crosstalk with Phytohormones in Enhancing Abiotic Stress Tolerance in Crop Plants. Plants 2023, 12, 1133. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Mostofa, M.G.; Rahman, M.M.; Tahjib-Ul-Arif, M.; Das, A.K.; Mohi-Ud-Din, M.; Rohman, M.M.; Hafiz, H.R.; Ansary, M.M.U.; Tran, L.-S.P. Glutathione improves rice tolerance to submergence: Insights into its physiological and biochemical mechanisms. J. Biotechnol. 2021, 325, 109–118. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef]
- Vento, M.; Della Croce, C.M.; Bellani, L.; Tassi, E.L.; Echeverria, M.C.; Giorgetti, L. Effect of Sprouting, Fermentation and Cooking on Antioxidant Content and Total Antioxidant Activity in Quinoa and Amaranth. Int. J. Mol. Sci. 2024, 25, 10972. [Google Scholar] [CrossRef]
- Dordevic, D.; Hrachovska, J.; Dordevic, S.; Kushkevych, I. Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds. Open Life Sci. 2025, 20, 20251125. [Google Scholar] [CrossRef] [PubMed]
- Handa, V.; Kumar, V.; Panghal, A.; Suri, S.; Kaur, J. Effect of soaking and germination on physicochemical and functional attributes of horsegram flour. J. Food Sci. Technol. 2017, 54, 4229–4239. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, M.; Li, W.; Chen, Z.; Li, S.; Yi, Z.; Zhang, Y. Superior Antioxidant Capacity and Auxin Production Promote Seedling Formation of Rice Seeds under Submergence Stress. Agronomy 2023, 13, 171. [Google Scholar] [CrossRef]
- Liu, P.; Gao, C.; Li, S.; Wang, X.; Dong, Y.; Wang, C.; Jiao, Z.; Sun, J. Comparative Transcriptome Analysis of Gene Responses of Salt-Tolerant and Salt-Sensitive Watermelon Cultivars’ Roots to Salt Stress. Plants 2025, 14, 1013. [Google Scholar] [CrossRef]
- Liu, P.; Li, Q.; Gao, Y.; Wang, H.; Chai, L.; Yu, H.; Jiang, W. A New Perspective on the Effect of UV-B on l-Ascorbic Acid Metabolism in Cucumber Seedlings. J. Agric. Food Chem. 2019, 67, 4444–4452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, S.; Yang, X.; He, L.; Hu, L.; Tang, R.; Li, J.; Liu, Z. Physiological and Multi-Omics Integrative Analysis Provides New Insights into Tolerance to Waterlogging Stress in Sesame (Sesamum indicum L.). Int. J. Mol. Sci. 2025, 26, 351. [Google Scholar] [CrossRef]
- He, Y.; Sun, S.; Zhao, J.; Huang, Z.; Peng, L.; Huang, C.; Tang, Z.; Huang, Q.; Wang, Z. UDP-glucosyltransferase OsUGT75A promotes submergence tolerance during rice seed germination. Nat. Commun. 2023, 14, 2296. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, L.; Shen, L.; Zhan, C.; Dai, L.; Huang, L.; Zhang, Q.; Fang, Y.; Ren, D.; Zhu, L.; et al. Genome-wide association study uncovers a novel gene responsible for rice seedling submergence tolerance. Plant Biotechnol. J. 2025, 23, 4092–4108. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, W.-C.; Han, C.; Wang, S.; Bai, M.-Y.; Song, C.-P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Meng, X.; Xu, Y.; Hao, R.; Fu, X.; Ding, Y.; Zhao, R.; Dai, L.; Zhang, H.; Zhou, Y.; Zhang, L.; et al. The WRKY76-miR528-SOD2 module: Regulating submergence tolerance through ROS scavenging in sorghum. New Phytol. 2025, 248, 1841–1856. [Google Scholar] [CrossRef]
- Umićević, S.; Kukavica, B.; Maksimović, I.; Gašić, U.; Milutinović, M.; Antić, M.; Mišić, D. Stress response in tomato as influenced by repeated waterlogging. Front. Plant Sci. 2024, 15, 1331281. [Google Scholar] [CrossRef]
- Madhu; Kaur, A.; Tyagi, S.; Shumayla; Singh, K.; Upadhyay, S.K. Exploration of glutathione reductase for abiotic stress response in bread wheat (Triticum aestivum L.). Plant Cell Rep. 2022, 41, 639–654. [Google Scholar] [CrossRef]
- Roach, T.; Neuner, G.; Kranner, I.; Buchner, O. Heat Acclimation under Drought Stress Induces Antioxidant Enzyme Activity in the Alpine Plant Primula minima. Antioxidants 2023, 12, 1093. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Y.; Ruan, M.; Ye, Q.; Yao, Z.; Wang, R.; Zhou, G.; Liu, D.; Wan, H. Comprehensive identification of glutathione peroxidase (GPX) gene family in response to abiotic stress in pepper (Capsicum annuum L.). Gene 2023, 881, 147625. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Siddika, A.; Rahman, K.; Nahar, K. Supplementation with Ascophyllum nodosum extracts mitigates arsenic toxicity by modulating reactive oxygen species metabolism and reducing oxidative stress in rice. Ecotoxicol. Environ. Saf. 2023, 255, 114819. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, F.; Zhai, F.; Su, Y.; Zhou, Y.; Ge, Z.; Tilak, P.; Eirich, J.; Finkemeier, I.; Fu, L. Rice GLUTATHIONE PEROXIDASE1-mediated oxidation of bZIP68 positively regulates ABA-independent osmotic stress signaling. Mol. Plant 2022, 15, 651–670. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huai, X.; Li, P.; Raza, A.; Mubarik, M.S.; Habib, M.; Fiaz, S.; Zhang, B.; Pan, J.; Khan, R.S.A. Genome-Wide Characterization of Glutathione Peroxidase (GPX) Gene Family in Rapeseed (Brassica napus L.) Revealed Their Role in Multiple Abiotic Stress Response and Hormone Signaling. Antioxidants 2021, 10, 1481. [Google Scholar] [CrossRef]
- Xing, R.-X.; Sun, X.-D.; Wang, Y.; Xie, X.-M.; Tan, M.-M.; Xu, M.-X.; Liu, X.-Y.; Jiang, Y.-Q.; Liu, M.-Y.; Duan, J.-L.; et al. Seed Priming with Dynamically Transformed Selenium Nanoparticles to Enhance Salt Tolerance in Rice. Environ. Sci. Technol. 2024, 58, 19725–19735. [Google Scholar] [CrossRef]
- García-Cristobal, J.; García-Villaraco, A.; Ramos, B.; Gutierrez-Mañero, J.; Lucas, J.A. Priming of pathogenesis related-proteins and enzymes related to oxidative stress by plant growth promoting rhizobacteria on rice plants upon abiotic and biotic stress challenge. J. Plant Physiol. 2015, 188, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Luo, K.; Tang, T.; Ma, G.; Peng, Y.; Zhang, Y.; Liu, Y.; Pan, L.; Li, S. Characterization of a Topramezone-Resistant Rice Mutant TZR1: Insights into GST-Mediated Detoxification and Antioxidant Responses. Plants 2025, 14, 425. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic Stress Tolerance in Plants: Myriad Roles of Ascorbate Peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef]
- Tiwari, M.; Kidwai, M.; Dutta, P.; Narayan, S.; Gautam, N.; Chawda, K.; Shirke, P.A.; Mishra, A.K.; Chakrabarty, D. A tau class glutathione-S-transferase (OsGSTU5) confers tolerance against arsenic toxicity in rice by accumulating more arsenic in root. J. Hazard. Mater. 2022, 426, 128100. [Google Scholar] [CrossRef] [PubMed]
- Sheela, H.S.; Vennapusa, A.R.; Melmaiee, K.; Prasad, T.G.; Reddy, C.P. Pyramiding of transcription factor, PgHSF4, and stress-responsive genes of p68, Pg47, and PsAKR1 impart multiple abiotic stress tolerance in rice (Oryza sativa L.). Front. Plant Sci. 2023, 14, 1233248. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Yang, S.; He, D.; Khan, A.R.; Salam, A.; Azhar, W.; Muhammad, S.; Ali, S.; Hamid, Y.; Khan, I.; et al. Seed priming with nano-silica effectively ameliorates chromium toxicity in Brassica napus. J. Hazard. Mater. 2023, 458, 131906. [Google Scholar] [CrossRef]
- Xia, F.; Cheng, H.; Chen, L.; Zhu, H.; Mao, P.; Wang, M. Influence of exogenous ascorbic acid and glutathione priming on mitochondrial structural and functional systems to alleviate aging damage in oat seeds. BMC Plant Biol. 2020, 20, 104. [Google Scholar] [CrossRef]
- Mi, C.; Hong, L.; Sun, S.; Zhao, S.; Dou, L.; Mao, P. Ascorbic acid priming restores the seed vigor by enhancing the mitochondrial AsA-GSH cycle and related gene expression in the aged oat seeds. Physiol. Plant. 2025, 177, e70190. [Google Scholar] [CrossRef]
- Wu, M.; Cong, X.; Li, M.; Rao, S.; Liu, Y.; Guo, J.; Zhu, S.; Chen, S.; Xu, F.; Cheng, S.; et al. Effects of different exogenous selenium on Se accumulation, nutrition quality, elements uptake, and antioxidant response in the hyperaccumulation plant Cardamine violifolia. Ecotoxicol. Environ. Saf. 2020, 204, 111045. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, H.; Sun, Z.; Li, J.; Hong, G.; Zhu, Q.; Zhou, X.; MacFarlane, S.; Yan, F.; Chen, J. Jasmonic acid-mediated defense suppresses brassinosteroid-mediated susceptibility to Rice black streaked dwarf virus infection in rice. New Phytol. 2017, 214, 388–399. [Google Scholar] [CrossRef]
- Liu, D.; Ma, M.; Yu, F.; Li, Q.; Li, M.; Li, R.; Fu, J.; Wang, Y.; Wang, F.; Yin, H.; et al. Identification of submergence tolerance QTLs/genes during seed germination in 432 rice varieties by GWAS. BMC Plant Biol. 2025, 25, 1172. [Google Scholar] [CrossRef]
- Jiang, D.; Du, S.; Shi, J.; Xu, H.; Liu, S.; Han, H.; Xu, Y.; Wang, H.; Yan, M.; Huang, X.; et al. Glutathione mitigates aluminum toxicity in root-apex transition zone of rice through reducing aluminum absorption and maintaining redox balance. Plant Physiol. Biochem. 2025, 219, 109366. [Google Scholar] [CrossRef] [PubMed]

| Experiment | Soaking Treatment | Sprouting Duration | Subsequent Stress Treatment |
|---|---|---|---|
| Effect of Sprouting on Submergence Tolerance | Pure water | 0, 12, 24, 36, 48 h | Normal hydroponics and submergence (9 cm, 6 d) |
| GSH Concentration Screening | 0 (Pure water), 0.001%, 0.01%, 0.1%, 1% GSH | 0 h | Submergence (9 cm, 6 d) |
| GSH Functional Verification | 0 (Pure water), 0.1% GSH | 0 h | Submergence (9 cm, 6 d) |
| Field Trial Verification | 0 (Pure water), 0.1% GSH | 0 h | Artificially created 5 cm water layer (6 d) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Kuang, N.; Mao, Z.; Zhou, S.; Liu, Z.; Chen, K.; Liu, L.; Xu, J.; Wang, L.; Lu, H. Sprouting Enhances Submergence Tolerance in Rice by Promoting Glutathione Biosynthesis and Turnover. Antioxidants 2025, 14, 1387. https://doi.org/10.3390/antiox14121387
Wang M, Kuang N, Mao Z, Zhou S, Liu Z, Chen K, Liu L, Xu J, Wang L, Lu H. Sprouting Enhances Submergence Tolerance in Rice by Promoting Glutathione Biosynthesis and Turnover. Antioxidants. 2025; 14(12):1387. https://doi.org/10.3390/antiox14121387
Chicago/Turabian StyleWang, Mei, Na Kuang, Ziyi Mao, Shangfeng Zhou, Zhixuan Liu, Ke Chen, Licheng Liu, Jingbo Xu, Lifeng Wang, and Haoyu Lu. 2025. "Sprouting Enhances Submergence Tolerance in Rice by Promoting Glutathione Biosynthesis and Turnover" Antioxidants 14, no. 12: 1387. https://doi.org/10.3390/antiox14121387
APA StyleWang, M., Kuang, N., Mao, Z., Zhou, S., Liu, Z., Chen, K., Liu, L., Xu, J., Wang, L., & Lu, H. (2025). Sprouting Enhances Submergence Tolerance in Rice by Promoting Glutathione Biosynthesis and Turnover. Antioxidants, 14(12), 1387. https://doi.org/10.3390/antiox14121387






