Hyperosmolarity-Induced Oxidative Stress Leads to Senescence in Human Corneal Epithelial Cells (HCEPC) via DNA Damage, Metabolic Disturbance and Mitophagy Decline
Abstract
1. Introduction
2. Materials and Methods
2.1. HCEP Cell Culture and Experimental Design
2.2. Microscopy Observation
2.3. Senescence-Associated-β-Galactosidase (SA-β-Gal) Detection
2.4. Cell Viability and Proliferation
2.5. Immunofluorescence Staining
2.6. Western Blot
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. ROS Determination
2.9. JC-1 Staining
2.10. ADP/ATP Ratio Assay
2.11. Nicotinamide Adenine Dinucleotide (NAD+) Level and NAD+/NADH Ratio Assay
2.12. Glucose Uptake, Consumption and Lactic Acid Excretion
2.13. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
2.14. Monodansylcadaverine (MDC) Staining
2.15. Janus Green B Staining
2.16. Mitophagy Assay
2.17. Statistical Analysis
3. Results
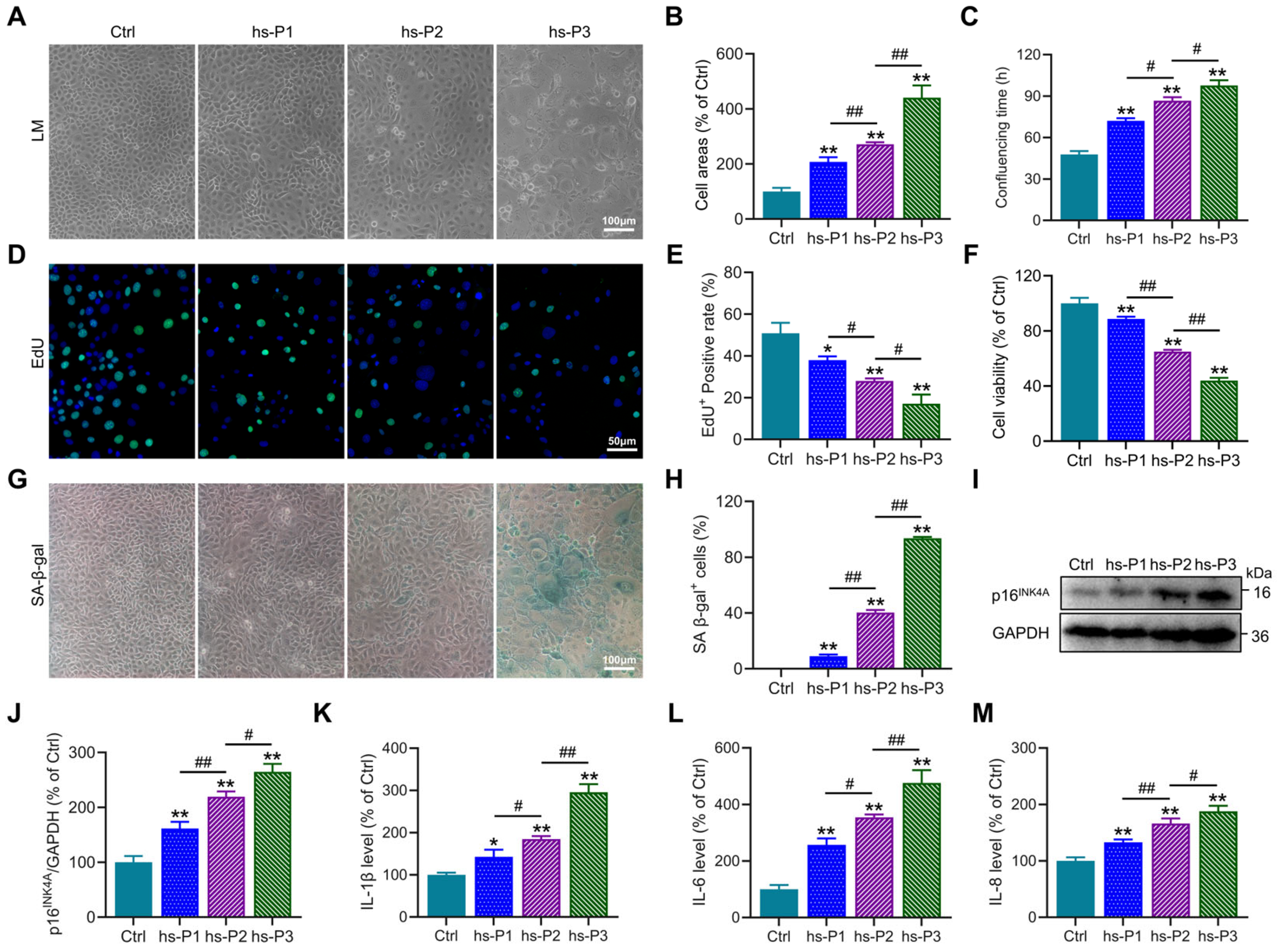
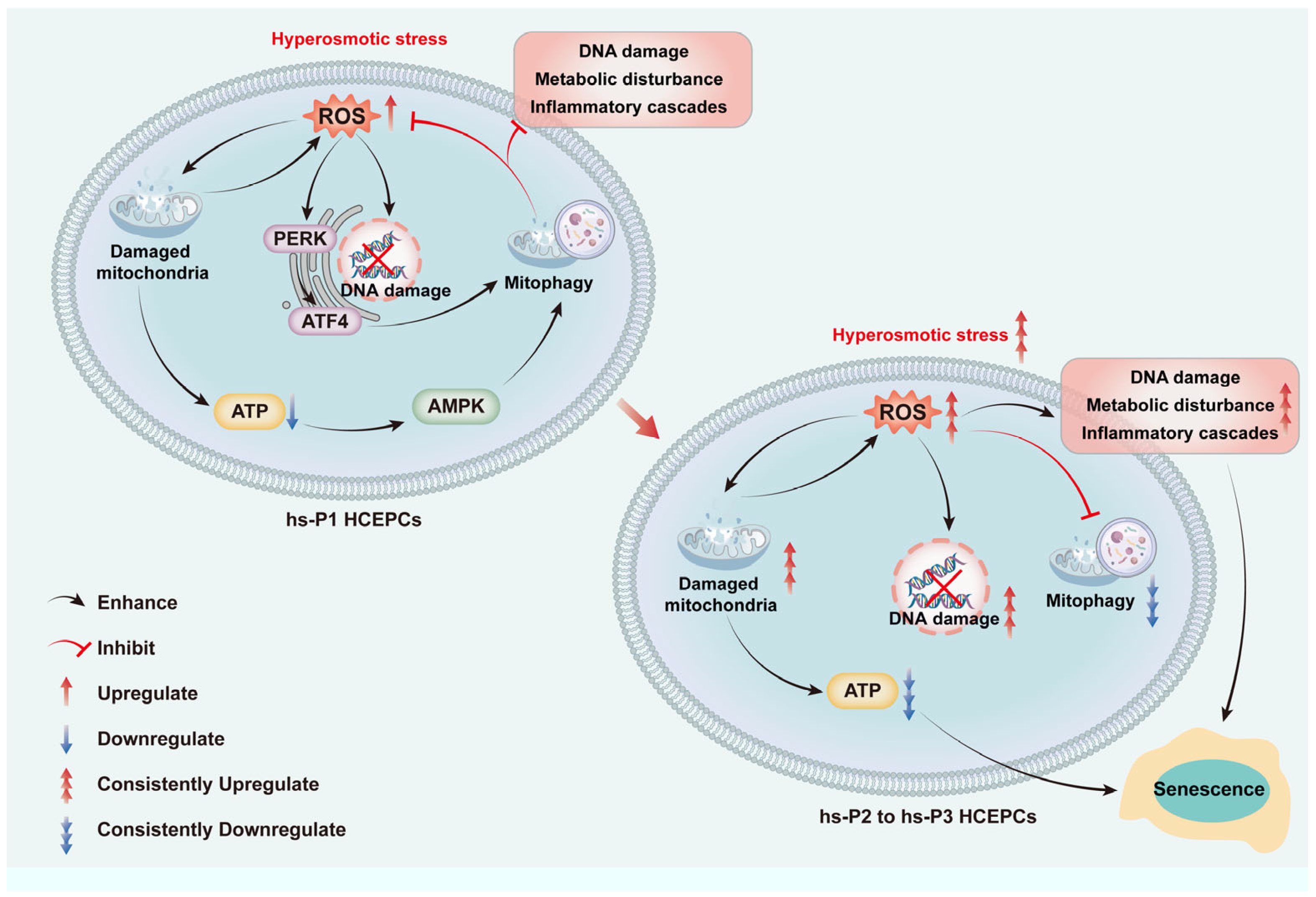
3.1. Establishment of a Dry Eye Disease Cellular Model Using Hyperosmotic Stress-Treated HCEPCs and Induction of HCEPC Senescence
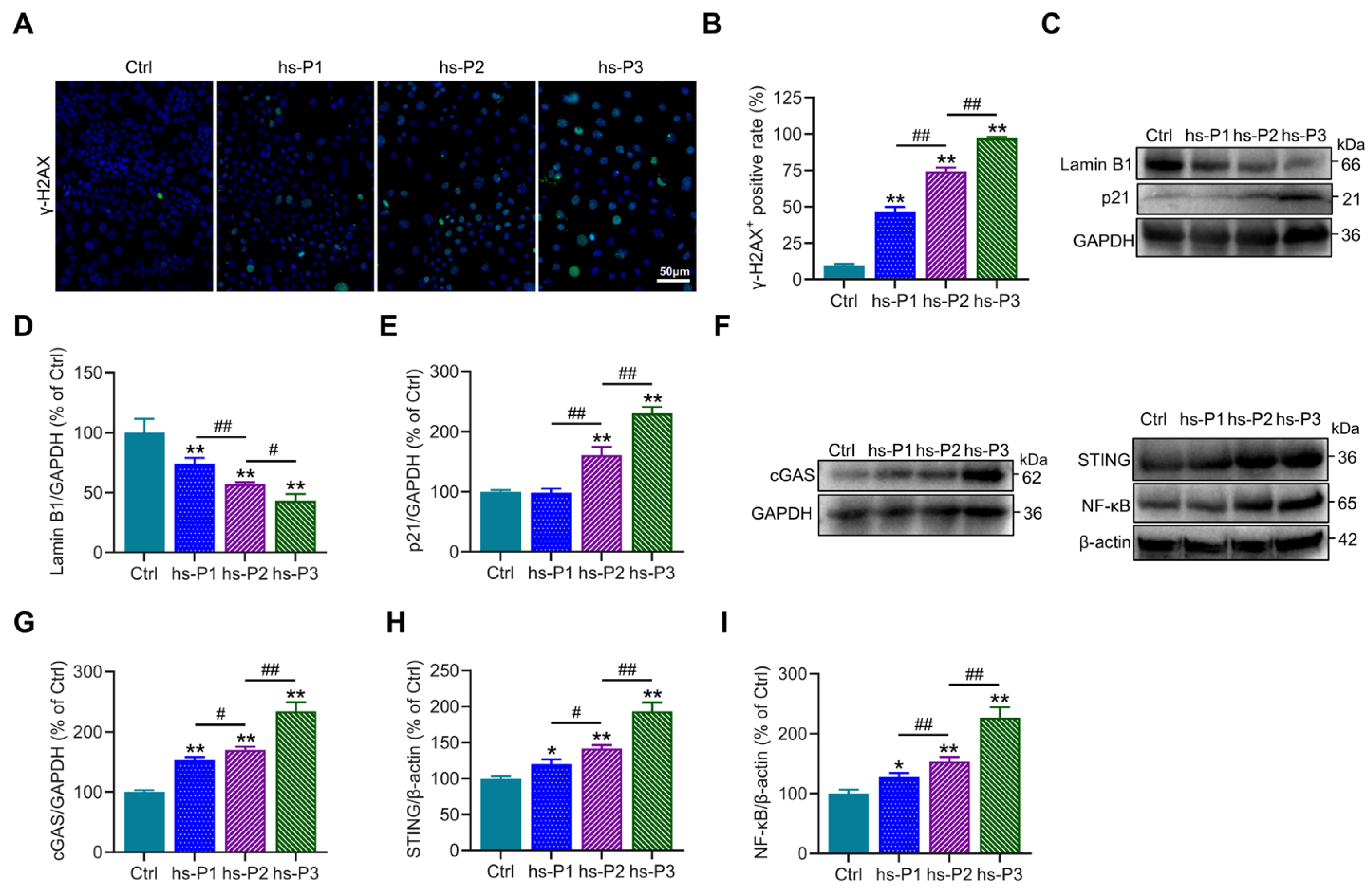
3.2. Hyperosmotic Stress Mediates the Induction of Chromatin Damage and Subsequent Inflammatory Activation in HCEPCs
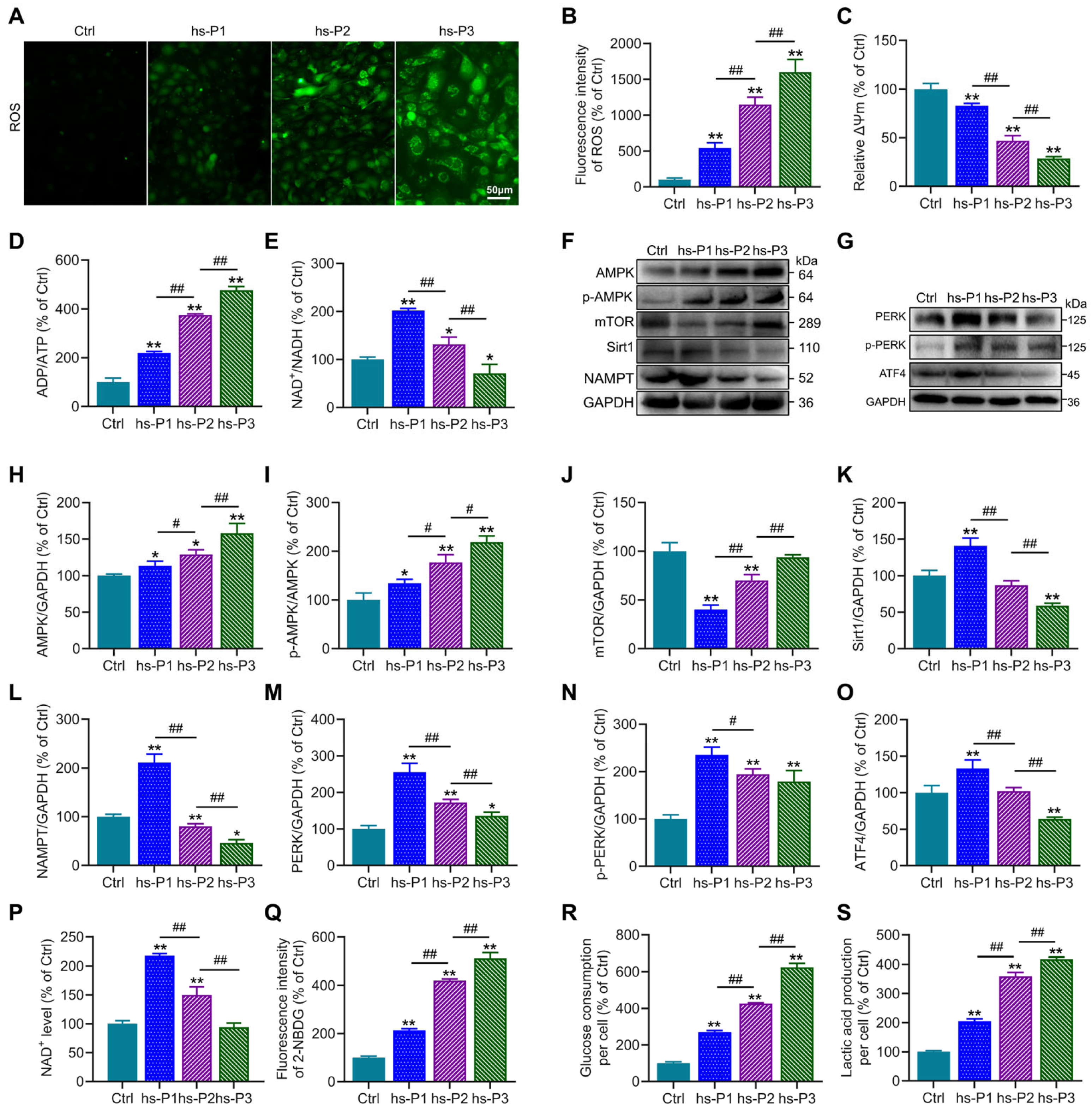
3.3. Hyperosmotic Stress Causes Oxidative Stress in HCEPCs, Leading to Energy Stress Response and Unfolded Protein Response (UPR)
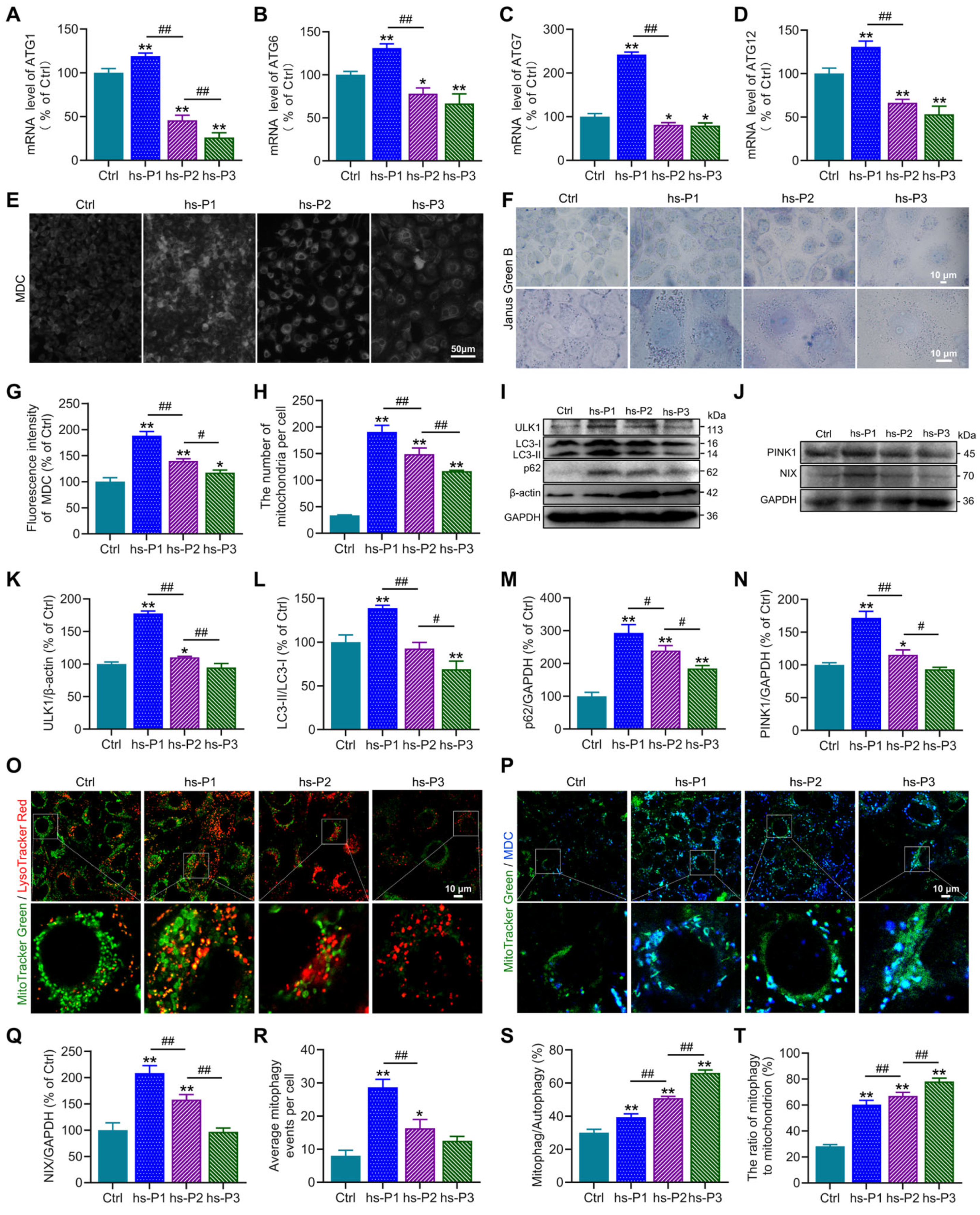
3.4. Autophagy, Particularly Mitophagy, in HCEPCs Are Activated by Oxidative Stress-Induced Energy Stress Response and UPR
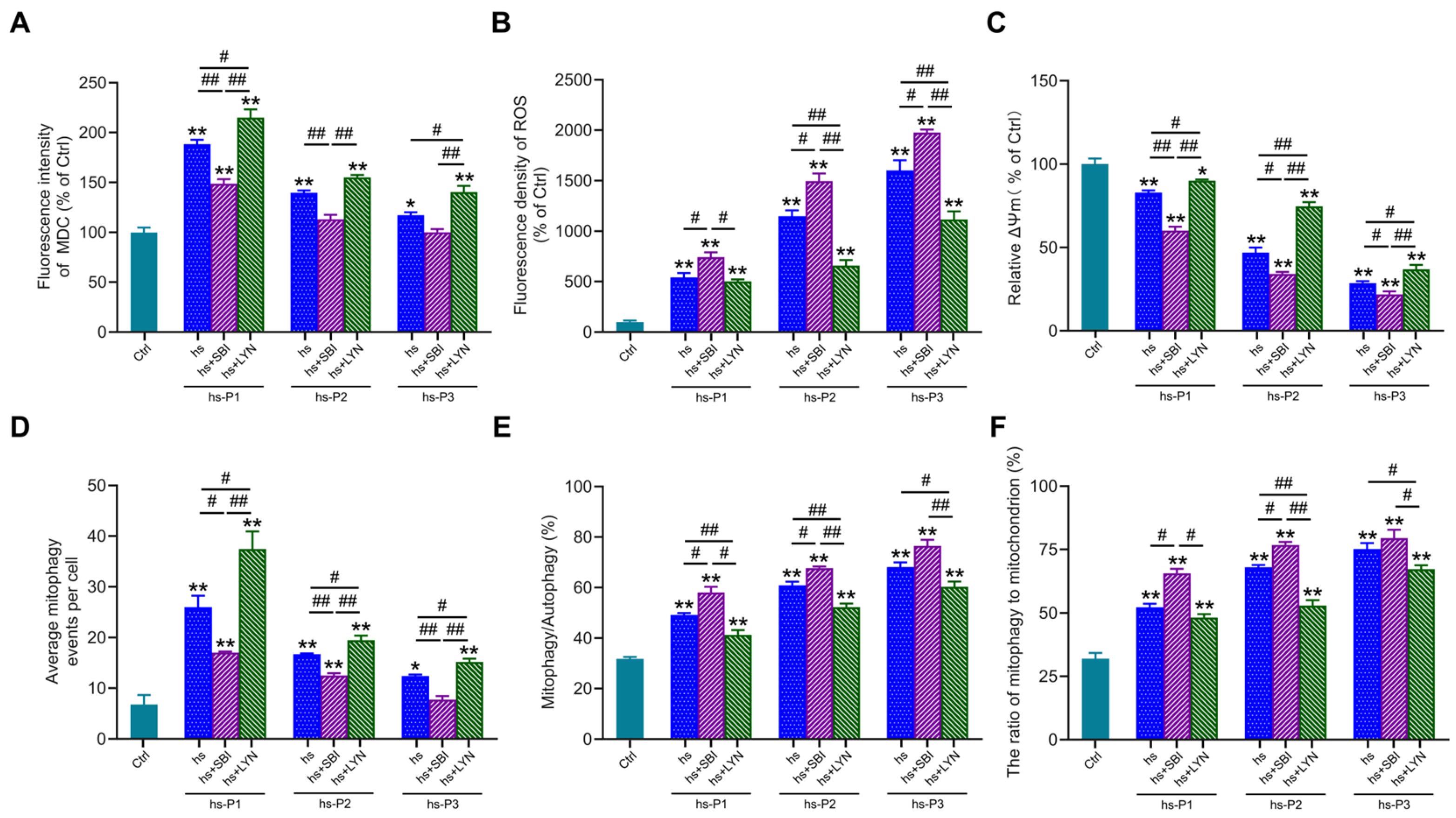
3.5. Activation of Autophagy Attenuates Oxidative Stress by Enhancing Mitophagy in Hyperosmotically Stressed HCEPCs
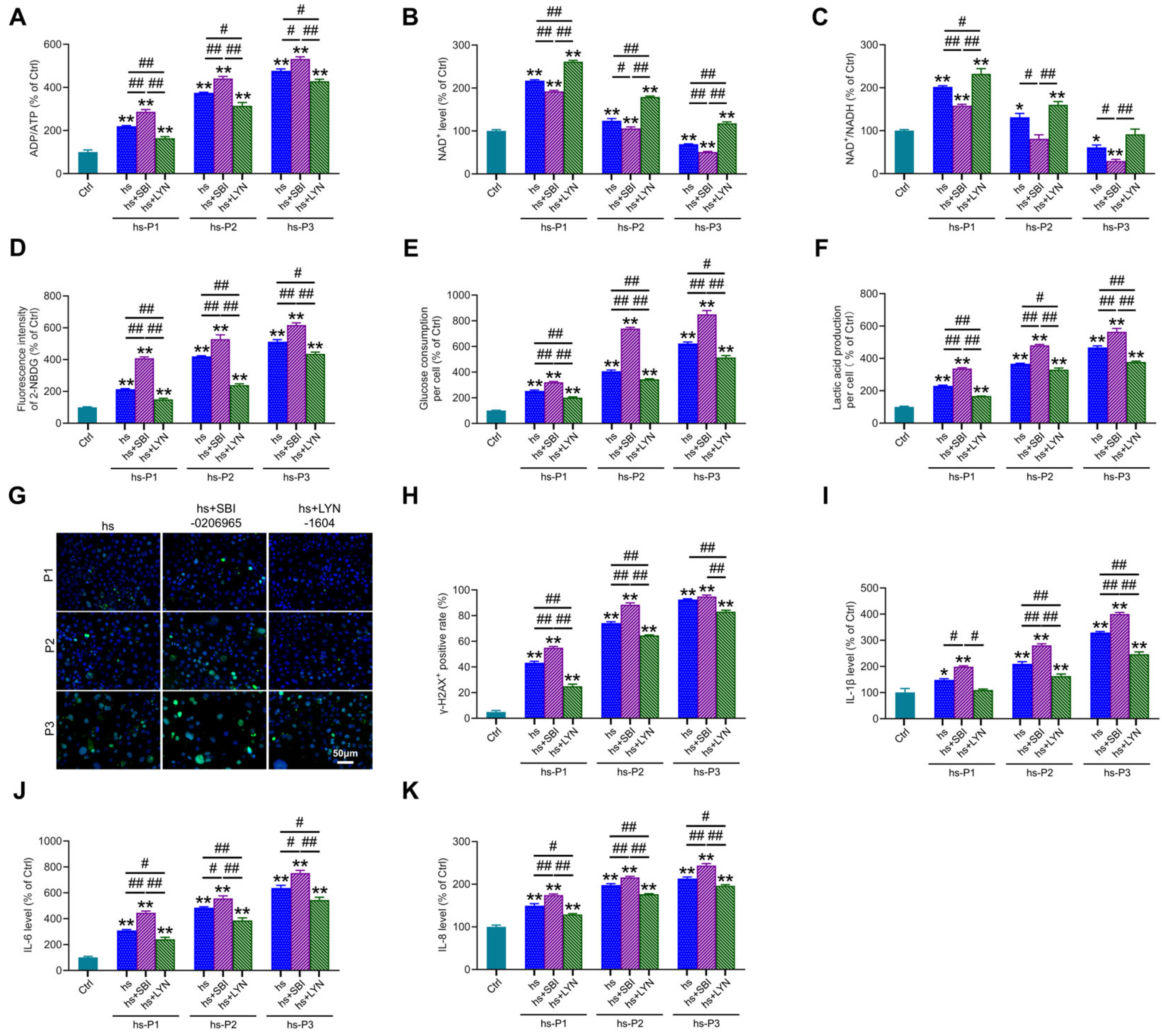
3.6. Mitophagy Activation Mitigates Energy Stress, DNA Damage, and Inflammation by Enhancing Mitophagy in HCEPCs Under Hyperosmotic Stress
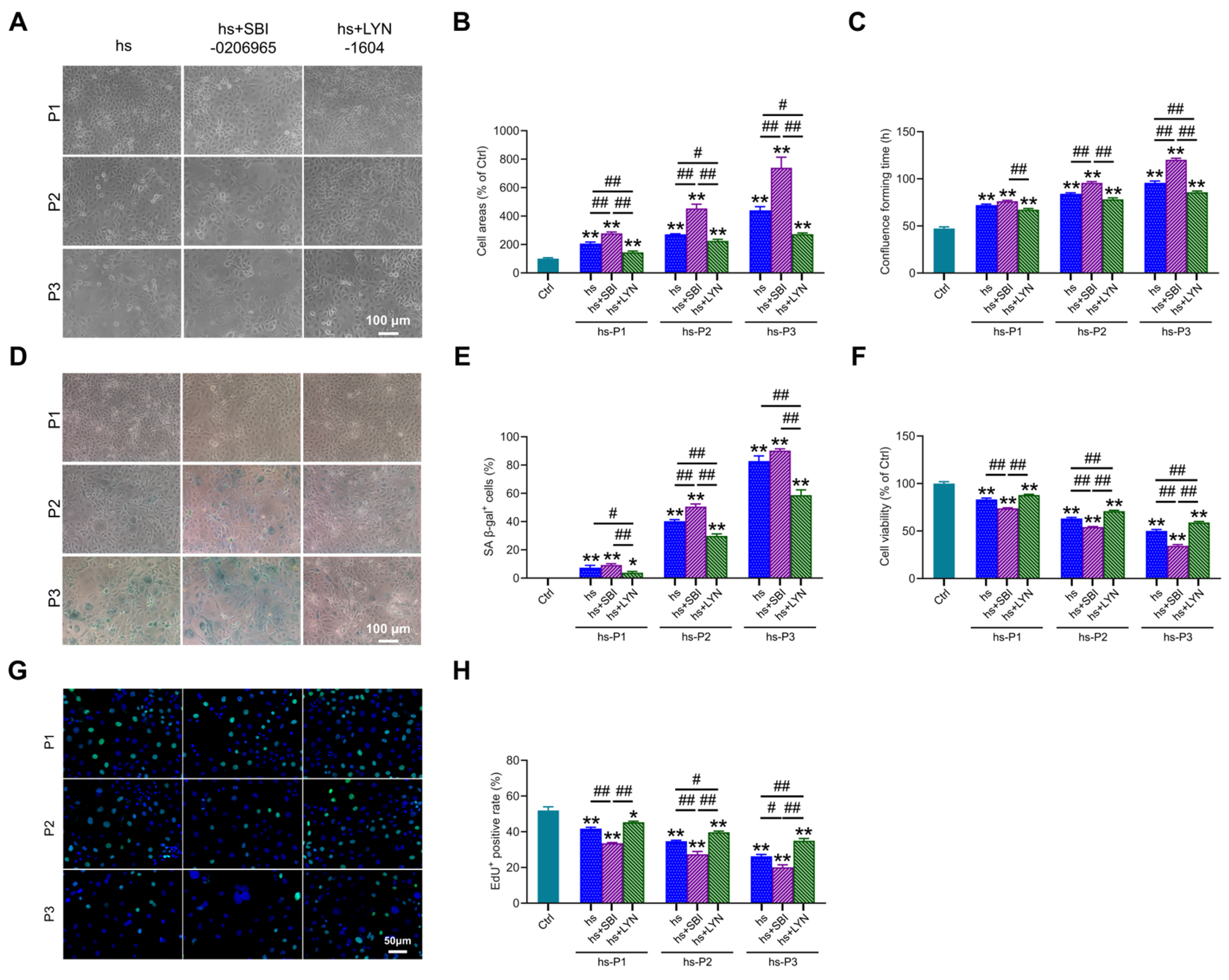
3.7. Mitophagy Activation Attenuates Cellular Senescence in Hyperosmotically Stressed HCEPCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-NBDG | 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose |
| AMPK | adenosine monophosphate-activated protein kinase |
| ATG | autophagy-related gene |
| CCK-8 | Cell Counting Kit-8 |
| cGAS | cyclic GMP-AMP synthase |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DED | Dry eye disease |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ECL | enhanced chemiluminescence |
| EdU | 5-ethynyl-2′-deoxyuridine |
| ELISA | enzyme-linked immunosorbent assay |
| ER | endoplasmic reticulum |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| HCEPC | human corneal epithelial cells |
| IL | Interleukin |
| LC3 | microtubule-associated protein 1 light chain 3 |
| MDC | monodansylcadaverine |
| MMPs | matrix metalloproteinases |
| mTOR | mammalian target of rapamycin complex |
| NAD | nicotinamide adenine dinucleotide |
| NAMPT | nicotinamide phosphoribosyl transferase |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIX | Nip3-like protein X |
| PBS | Phosphate-buffered saline |
| PBST | phosphate-buffered saline with Tween 20 |
| PERK | protein kinase R like endoplasmic reticulum kinase |
| PINK1 | PTEN-induced kinase 1 |
| PVDF | polyvinylidene difluoride |
| RIPA | radio-immunoprecipitation assay lysis buffer |
| ROS | reactive oxygen species |
| RT-qPCR | real-time reverse transcription polymerase chain reaction |
| SASP | senescence-associated secretory phenotype |
| SA-β-gal | senescence-associated-β-galactosidase |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SEM | standard error of the mean |
| Sirt1 | silent information regulator factor 2-related enzyme 1 |
| STING | stimulator of interferon genes |
| TNF-α | tumor necrosis factor-α |
| ULK1 | Unc-51 like autophagy activating kinase 1 |
| UPR | unfolded protein response |
References
- Shetty, R.; Sethu, S. Newer Paradigms in Dry Eye Disease Research. Ind. J. Ophthalmol. 2023, 71, 1064. [Google Scholar] [CrossRef]
- Gupta, P.K.; Toyos, R.; Sheppard, J.D.; Toyos, M.; Mah, F.S.; Bird, B.; Theriot, P.E.; Higgins, D. Tolerability of Current Treatments for Dry Eye Disease: A Review of Approved and Investigational Therapies. Clin. Ophthalmol. 2024, 18, 2283–2302. [Google Scholar] [CrossRef]
- Verma, S.; Montoya, P.A.G.; Sun, M.; Puri, S.; Yuksel, S.; Wilkerson, A.; Gesteira, T.F.; Butovich, I.A.; Coulson-Thomas, V.J. Comparative Analysis of Age-Associated Changes in Meibum Composition, Distribution, and Function in Mice with Altered Hyaluronan Expression. Investig. Ophthalmol. Vis. Sci. 2025, 66, 72. [Google Scholar] [CrossRef]
- Ji, H.; Zhu, Y.; Zhang, Y.; Li, Z.; Ge, J.; Zhuo, Y. Dry Eye Disease in Patients with Functioning Filtering Blebs after Trabeculectomy. PLoS ONE 2016, 11, e0152696. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lin, S.; Gao, Y. Mesenchymal Stromal Cell-Based Therapy for Dry Eye: Current Status and Future Perspectives. Cell Transplant. 2022, 31, 9636897221133818. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G. [Emphasis on standardization and refinement in the diagnosis and treatment of dry eye]. Chin. J. Ophthalmol. 2017, 53, 641–644. [Google Scholar]
- Shan, H.; Liu, W.; Li, Y.; Pang, K. The Autoimmune Rheumatic Disease Related Dry Eye and Its Association with Retinopathy. Biomolecules 2023, 13, 724. [Google Scholar] [CrossRef]
- Liao, H.-J.; Hsu, P.-N. Immunomodulatory Effects of Extracellular Vesicles from Mesenchymal Stromal Cells: Implication for Therapeutic Approach in Autoimmune Diseases. Kaohsiung J. Med. Sci. 2024, 40, 520–529. [Google Scholar] [CrossRef]
- Molero Senosiaín, M.; Burgos-Blasco, B.; Perez-García, P.; Sánchez-Ventosa, Á.; Villalba-González, M.; López Pérez, M.D.; Díaz, J.C.; Díaz-Mesa, V.; Villarrubia Cuadrado, A.; Artiaga Elordi, E.; et al. Performance and Safety of a Sodium Hyaluronate, Xanthan Gum, and Osmoprotectants Ophthalmic Solution in the Treatment of Dry Eye Disease: An Observational Clinical Investigation. Ophthalmol. Ther. 2025, 14, 675–692. [Google Scholar] [CrossRef]
- Martone, G.; Balestrazzi, A.; Ciprandi, G.; Balestrazzi, A. Alpha-Glycerylphosphorylcholine and D-Panthenol Eye Drops in Patients Undergoing Cataract Surgery. J. Ophthalmol. 2022, 2022, 1951014. [Google Scholar] [CrossRef]
- Gilbard, J.P.; Farris, R.L.; Santamaria, J. Osmolarity of Tear Microvolumes in Keratoconjunctivitis Sicca. Arch. Ophthalmol. 1978, 96, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Bunya, V.; Maguire, M.; Asbell, P.; Ying, G.-S. Dry Eye Assessment and Management Study Research Group Systemic Conditions Associated with Severity of Dry Eye Signs and Symptoms in the Dry Eye Assessment and Management Study. Ophthalmology 2021, 128, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Ding, D.; Yang, H.; Zou, W.; Yang, D.; Wang, K.; Zhang, C.; Chen, B.; Ji, D.; Hao, Y.; et al. Melatonin Protects Mitochondrial Function and Inhibits Oxidative Damage against the Decline of Human Oocytes Development Caused by Prolonged Cryopreservation. Cells 2022, 11, 4018. [Google Scholar] [CrossRef]
- Navel, V.; Sapin, V.; Henrioux, F.; Blanchon, L.; Labbé, A.; Chiambaretta, F.; Baudouin, C.; Dutheil, F. Oxidative and Antioxidative Stress Markers in Dry Eye Disease: A Systematic Review and Meta-Analysis. Acta Ophthalmol. 2022, 100, 45–57. [Google Scholar] [CrossRef]
- Uchino, Y.; Kawakita, T.; Ishii, T.; Ishii, N.; Tsubota, K. A New Mouse Model of Dry Eye Disease: Oxidative Stress Affects Functional Decline in the Lacrimal Gland. Cornea 2012, 31 (Suppl. S1), S63–S67. [Google Scholar] [CrossRef]
- Deng, R.; Hua, X.; Li, J.; Chi, W.; Zhang, Z.; Lu, F.; Zhang, L.; Pflugfelder, S.C.; Li, D.-Q. Oxidative Stress Markers Induced by Hyperosmolarity in Primary Human Corneal Epithelial Cells. PLoS ONE 2015, 10, e0126561. [Google Scholar] [CrossRef]
- Jiang, G.-J.; You, X.-G.; Fan, T.-J. Ultraviolet B Irradiation Induces Senescence of Human Corneal Endothelial Cells In Vitro by DNA Damage Response and Oxidative Stress. J. Photochem. Photobiol. B 2022, 235, 112568. [Google Scholar] [CrossRef]
- Zheng, X.; Jiang, G.-J.; Fan, T.-J. Blue Light Irradiation Elicits Senescence of Corneal Endothelial Cells In Vitro by Provoking Energy Crisis, Inflammasome Assembly and DNA Damage. Curr. Eye Res. 2025, 50, 791–802. [Google Scholar] [CrossRef]
- Yang, T.; Fan, T.-J.; Xu, B. Norfloxacin Induces Apoptosis and Necroptosis in Human Corneal Epithelial Cells. Toxicol. In Vitro 2020, 66, 104868. [Google Scholar] [CrossRef]
- Li, H.; Fan, T.-J.; Zou, P.; Xu, B. Diclofenac Sodium Triggers P53-Dependent Apoptosis in Human Corneal Epithelial Cells via ROS-Mediated Crosstalk. Chem. Res. Toxicol. 2021, 34, 70–79. [Google Scholar] [CrossRef]
- Fan, T.-J.; Xu, B.; Zhao, J.; Yang, H.-S.; Wang, R.-X.; Hu, X.-Z. Establishment of an Untransfected Human Corneal Epithelial Cell Line and Its Biocompatibility with Denuded Amniotic Membrane. Int. J. Ophthalmol. 2011, 4, 228–234. [Google Scholar]
- Lu, J.; Zhu, L.; Zheng, L.; Cui, Q.; Zhu, H.; Zhao, H.; Shen, Z.; Dong, H.; Chen, S.; Wu, W.; et al. Overexpression of ULK1 Represents a Potential Diagnostic Marker for Clear Cell Renal Carcinoma and the Antitumor Effects of SBI-0206965. eBioMedicine 2018, 34, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fu, L.; Zhang, S.; Zhang, J.; Zhao, Y.; Zheng, Y.; He, G.; Yang, S.; Ouyang, L.; Liu, B. Discovery of a Small Molecule Targeting ULK1-Modulated Cell Death of Triple Negative Breast Cancer In Vitro and In Vivo. Chem. Sci. 2017, 8, 2687–2701. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhang, X.; Xiao, D.; Chang, W. Decreased in N-3 DHA Enriched Triacylglycerol in Small Extracellular Vesicles of Diabetic Patients with Cardiac Dysfunction. J. Diabetes 2023, 15, 1070. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Li, C.; Liu, W.; Sun, M.; Deng, S.; Cao, J.; Yang, H.; Chen, P. Scutellarin Activates IDH1 to Exert Antitumor Effects in Hepatocellular Carcinoma Progression. Cell Death Dis. 2024, 15, 267. [Google Scholar] [CrossRef]
- Zhao, L.; Kang, M.; Liu, X.; Wang, Z.; Wang, Y.; Chen, H.; Liu, W.; Liu, S.; Li, B.; Li, C.; et al. UBR7 Inhibits HCC Tumorigenesis by Targeting Keap1/Nrf2/Bach1/HK2 and Glycolysis. J. Exp. Clin. Cancer Res. 2022, 41, 330. [Google Scholar] [CrossRef]
- Aguayo-Mazzucato, C.; van Haaren, M.; Mruk, M.; Lee, T.B.; Crawford, C.; Hollister-Lock, J.; Sullivan, B.A.; Johnson, J.W.; Ebrahimi, A.; Dreyfuss, J.M.; et al. β-Cell Aging Markers Have Heterogeneous Distribution and Are Induced by Insulin Resistance. Cell Metab. 2017, 25, 898–910.e5. [Google Scholar] [CrossRef]
- Voskamp, C.; Koevoet, W.J.L.M.; Van Osch, G.J.V.M.; Narcisi, R. Senescence during Early Differentiation Reduced the Chondrogenic Differentiation Capacity of Mesenchymal Progenitor Cells. Front. Bioeng. Biotechnol. 2023, 11, 1241338. [Google Scholar] [CrossRef]
- Al-Azab, M.; Safi, M.; Idiiatullina, E.; Al-Shaebi, F.; Zaky, M.Y. Aging of Mesenchymal Stem Cell: Machinery, Markers, and Strategies of Fighting. Cell. Mol. Biol. Lett. 2022, 27, 69. [Google Scholar] [CrossRef]
- Guo, C.; Li, X.; Fan, Z.; Zhang, J.; Chen, M. Progress in the Study of the Role of C5a-Induced Tubular Cell Senescence in the Progression of Diabetic Kidney Disease. Ann. Med. 2025, 57, 2561232. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Jiang, Y.; Chen, Z.B.; Rhee, J.-W.; Deng, Y.; Wang, Z.V. Mitochondrial Dysfunction in Cardiac Arrhythmias. Cells 2023, 12, 679. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, X.; Xu, W.; Li, J.; Sun, Y.; Cui, S.; Xu, R.; Li, W.; Jiao, L.; Wang, T. ROS-Induced Endothelial Dysfunction in the Pathogenesis of Atherosclerosis. Aging Dis. 2024, 16, 250–268. [Google Scholar] [CrossRef]
- Wang, R.; Liang, L.; Matsumoto, M.; Iwata, K.; Umemura, A.; He, F. Reactive Oxygen Species and NRF2 Signaling, Friends or Foes in Cancer? Biomolecules 2023, 13, 353. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, Y.; Sun, J.; Geng, S.; Pan, Z.; Prinz, R.A.; Wang, C.; Sun, J.; Jiao, X.; Xu, X. Activation of TGF-β-Activated Kinase 1 (TAK1) Restricts Salmonella Typhimurium Growth by Inducing AMPK Activation and Autophagy. Cell Death Dis. 2018, 9, 570. [Google Scholar] [CrossRef]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial Ischemia/Reperfusion Injury: Mechanisms of Injury and Implications for Management (Review). Exp. Ther. Med. 2022, 23, 430. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between Mitochondrial Biogenesis and Mitophagy to Maintain Mitochondrial Homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, T.; Liang, Q.; Feng, X.; Jiang, J.; Chen, Z.; Tang, Y.; Chu, Y.; Wang, B.; Hu, K. A First-in-Human, Prospective Pilot Trial of Umbilical Cord-Derived Mesenchymal Stem Cell Eye Drops Therapy for Patients with Refractory Non-Sjögren’s and Sjögren’s Syndrome Dry Eye Disease. Stem Cell Res. Ther. 2025, 16, 202. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Feng, J.; Wang, Y.; Li, K.; Chen, Y.; Wang, W.; Zhao, W.; Ge, S.; Li, J. Adoptive Cell Transfer of Piezo-Activated Macrophage Rescues Immunosuppressed Rodents from Life-Threating Bacterial Infections. Nat. Commun. 2025, 16, 1363. [Google Scholar] [CrossRef]
- Kim, M.; Chun, Y.S.; Kim, K.W. Different Perception of Dry Eye Symptoms between Patients with and without Primary Sjogren’s Syndrome. Sci. Rep. 2022, 12, 2172. [Google Scholar] [CrossRef]
- Huo, J.; Xie, H.; Li, J.; Zhou, D. [A primary study of L-carnitine protective effect on corneal and conjunctival epithelium of mouse dry eye model induced by hyperosmolar saline]. Chin. J. Ophthalmol. 2012, 48, 330–336. [Google Scholar]
- López-Cano, J.J.; González-Cela-Casamayor, M.A.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Benítez-Del-Castillo, J.M.; Molina-Martínez, I.T. Combined Hyperosmolarity and Inflammatory Conditions in Stressed Human Corneal Epithelial Cells and Macrophages to Evaluate Osmoprotective Agents as Potential DED Treatments. Exp. Eye Res. 2021, 211, 108723. [Google Scholar] [CrossRef]
- Ren, Y.; Lu, H.; Reinach, P.S.; Zheng, Q.; Li, J.; Tan, Q.; Zhu, H.; Chen, W. Hyperosmolarity-Induced AQP5 Upregulation Promotes Inflammation and Cell Death via JNK1/2 Activation in Human Corneal Epithelial Cells. Sci. Rep. 2017, 7, 4727. [Google Scholar] [CrossRef] [PubMed]
- Ngoenkam, J.; Pejchang, D.; Nuamchit, T.; Wichai, U.; Pongcharoen, S.; Laorob, T.; Paensuwan, P. Nitro Dihydrocapsaicin Attenuates Hyperosmotic Stress-Induced Inflammation in the Corneal Epithelial Cells via SIRT1/Nrf2/HO-1 Pathway. Exp. Eye Res. 2025, 261, 110680. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, Y.; Du, Y.; Wang, Z.; Cheng, L.; Du, Z. Comprehensive Dry Eye Therapy: Overcoming Ocular Surface Barrier and Combating Inflammation, Oxidation, and Mitochondrial Damage. J. Nanobiotechnol. 2024, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhao, H.; He, Y.; Zhang, M. BMSC Alleviates Dry Eye by Inhibiting the ROS-NLRP3-IL-1β Signaling Axis by Reducing Inflammation Levels. Curr. Eye Res. 2024, 49, 698–707. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Zheng, Y.; Xu, Y. Ameliorative Potential of Resveratrol in Dry Eye Disease by Restoring Mitochondrial Function. Evid.-Based Complement. Altern. Med. 2022, 2022, 1013444. [Google Scholar] [CrossRef]
- Hu, R.; Shi, J.; Xie, C.-M.; Yao, X.-L. Dry Eye Disease: Oxidative Stress on Ocular Surface and Cutting-Edge Antioxidants. Glob. Chall. 2025, 9, e00068. [Google Scholar] [CrossRef]
- Zou, H.; Hong, Y.; Xu, B.; Wang, M.; Xie, H.; Lin, Q. Multifunctional Cerium Oxide Nanozymes with High Ocular Surface Retention for Dry Eye Disease Treatment Achieved by Restoring Redox Balance. Acta Biomater. 2024, 185, 441–455. [Google Scholar] [CrossRef]
- Guo, X.-X.; Chang, X.-J.; Pu, Q.; Li, A.-L.; Li, J.; Li, X.-Y. Urolithin A Alleviates Cell Senescence by Inhibiting Ferroptosis and Enhances Corneal Epithelial Wound Healing. Front. Med. 2024, 11, 1441196. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, M.; Yu, H.; Wang, L.; Li, X.; Rak, J.; Wang, S.; Zhao, R.C. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Mitigate Oxidative Stress-Induced Senescence in Endothelial Cells via Regulation of miR-146a/Src. Signal Transduct. Target. Ther. 2021, 6, 354. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, D.; Xiao, H. Methods of Cellular Senescence Induction Using Oxidative Stress. Methods Mol. Biol. 2013, 1048, 135–144. [Google Scholar]
- von Zglinicki, T. Oxidative Stress and Cell Senescence as Drivers of Ageing: Chicken and Egg. Ageing Res. Rev. 2024, 102, 102558. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kawakami, Y.; Tsubota, K. Cascade of Inflammatory, Fibrotic Processes, and Stress-Induced Senescence in Chronic GVHD-Related Dry Eye Disease. Int. J. Mol. Sci. 2021, 22, 6114. [Google Scholar] [CrossRef]
- Zhou, P.; Wu, W.; Wei, J.; Yang, Y.; Jongkaewwattana, A.; Xiao, Y.; Jin, H.; Zhou, H.; Luo, R. MARCH6 Suppresses Tembusu Virus Replication by Targeting Viral NS5 Protein for TOLLIP-Mediated Selective Autophagic Degradation. J. Virol. 2025, 99, 7. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Huang, K.; Le, W. Why Should Autophagic Flux Be Assessed? Acta Pharmacol. Sin. 2013, 34, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Di Caprio, G.; Santangelo, L.; Fimia, G.M.; Cozzolino, A.M.; Komatsu, M.; Ippolito, G.; Tripodi, M.; Alonzi, T. Autophagy Regulates Hepatocyte Identity and Epithelial-to-Mesenchymal and Mesenchymal-to-Epithelial Transitions Promoting Snail Degradation. Cell Death Dis. 2015, 6, e1880. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.B. EGFR-Signaling and Autophagy: How They Fit in the Cancer Landscape. J. Adenocarcinoma 2016, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Goenka, L.; Rajappa, M.; Gochhait, D.; Manivannan, P.; Chaturvedula, L.; L, C.; Charanraj Goud, A.; Dubashi, B.; Kayal, S.; Ganesan, P. Assessing Autophagy Activation in Advanced Ovarian Cancer Using Ascitic Fluid: A Feasibility Study. Cureus 2025, 17, e79371. [Google Scholar] [CrossRef]
- Byun, Y.-S.; Lee, H.J.; Shin, S.; Chung, S.-H. Elevation of Autophagy Markers in Sjögren Syndrome Dry Eye. Sci. Rep. 2017, 7, 17280. [Google Scholar] [CrossRef]
- Jeyabalan, N.; Pillai, A.M.; Khamar, P.; Shetty, R.; Mohan, R.R.; Ghosh, A. Autophagy in Dry Eye Disease: Therapeutic Implications of Autophagy Modulators on the Ocular Surface. Indian J. Ophthalmol. 2023, 71, 1285–1291. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, D.; Chen, X.; Bian, F.; Gao, N.; Li, J.; Pflugfelder, S.C.; Li, D.-Q. Autophagy Activation Protects Ocular Surface from Inflammation in a Dry Eye Model In Vitro. Int. J. Mol. Sci. 2020, 21, 8966. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.; Taisne, C.; Lussignol, M.; Esclatine, A.; Labetoulle, M. Commercially Available Eye Drops Containing Trehalose Protect Against Dry Conditions via Autophagy Induction. J. Ocul. Pharmacol. Ther. 2021, 37, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zuo, X.; Peng, L.; Wang, X.; Zeng, H.; Zhong, J.; Li, S.; Xiao, Y.; Wang, L.; Ouyang, H.; et al. Melatonin Ameliorates Oxidative Stress-Mediated Injuries through Induction of HO-1 and Restores Autophagic Flux in Dry Eye. Exp. Eye Res. 2021, 205, 108491. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Peng, L.; Ouyang, H.; Wang, L.; He, D.; Zhong, J.; Xiao, Y.; Deng, Y.; Li, M.; Li, S.; et al. Induction of DDIT4 Impairs Autophagy Through Oxidative Stress in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2836–2847. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.; Zhang, D.; Chu, Y.; Chen, Z.; Tsao, J.; Chen, T.; Jiang, J.; Hu, K. Evodiamine Promotes Autophagy and Alleviates Oxidative Stress in Dry Eye Disease Through the P53/mTOR Pathway. Investig. Ophthalmol. Vis. Sci. 2025, 66, 44. [Google Scholar] [CrossRef]
- Liang, Q.; Guo, R.; Tsao, J.-R.; He, Y.; Wang, C.; Jiang, J.; Zhang, D.; Chen, T.; Yue, T.; Hu, K. Salidroside Alleviates Oxidative Stress in Dry Eye Disease by Activating Autophagy through AMPK-Sirt1 Pathway. Int. Immunopharmacol. 2023, 121, 110397. [Google Scholar] [CrossRef]
- Chmielewski, P.P.; Data, K.; Strzelec, B.; Farzaneh, M.; Anbiyaiee, A.; Zaheer, U.; Uddin, S.; Sheykhi-Sabzehpoush, M.; Mozdziak, P.; Zabel, M.; et al. Human Aging and Age-Related Diseases: From Underlying Mechanisms to Pro-Longevity Interventions. Aging Dis. 2024, 16, 1853–1877. [Google Scholar] [CrossRef]
- Hansel, C.; Jendrossek, V.; Klein, D. Cellular Senescence in the Lung: The Central Role of Senescent Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 3279. [Google Scholar] [CrossRef]
- Messmer, E.M. The Pathophysiology, Diagnosis, and Treatment of Dry Eye Disease. Dtsch. Ärztebl. Int. 2015, 112, 71–82. [Google Scholar] [CrossRef]
- Sheppard, J.; Shen Lee, B.; Periman, L.M. Dry Eye Disease: Identification and Therapeutic Strategies for Primary Care Clinicians and Clinical Specialists. Ann. Med. 2023, 55, 241–252. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Fan, T. Hyperosmolarity-Induced Oxidative Stress Leads to Senescence in Human Corneal Epithelial Cells (HCEPC) via DNA Damage, Metabolic Disturbance and Mitophagy Decline. Antioxidants 2025, 14, 1381. https://doi.org/10.3390/antiox14111381
Zhang Y, Fan T. Hyperosmolarity-Induced Oxidative Stress Leads to Senescence in Human Corneal Epithelial Cells (HCEPC) via DNA Damage, Metabolic Disturbance and Mitophagy Decline. Antioxidants. 2025; 14(11):1381. https://doi.org/10.3390/antiox14111381
Chicago/Turabian StyleZhang, Yongjie, and Tingjun Fan. 2025. "Hyperosmolarity-Induced Oxidative Stress Leads to Senescence in Human Corneal Epithelial Cells (HCEPC) via DNA Damage, Metabolic Disturbance and Mitophagy Decline" Antioxidants 14, no. 11: 1381. https://doi.org/10.3390/antiox14111381
APA StyleZhang, Y., & Fan, T. (2025). Hyperosmolarity-Induced Oxidative Stress Leads to Senescence in Human Corneal Epithelial Cells (HCEPC) via DNA Damage, Metabolic Disturbance and Mitophagy Decline. Antioxidants, 14(11), 1381. https://doi.org/10.3390/antiox14111381





