Functional and Metabolomic Analyses of Chamomile Jelly Derived from Gelatin Capsule Waste with Inulin and Polydextrose as Prebiotic Sugar Substitutes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chamomile Tea Jelly Preparation
2.3. Phase I: Study of Physicochemical and Functional Properties of Chamomile Jelly Products with Sugar Substituted by Prebiotics
2.3.1. Proximate Analysis
2.3.2. Water Activity and pH
2.3.3. Color Measurement
2.3.4. Texture Profile Analysis
2.3.5. Gel Strength Determination
2.3.6. Total Phenolic Content and Antioxidant Activity (ABTS•+ and DPPH• Assays)
2.3.7. Sensory Evaluation
2.3.8. Nutrition Profile
2.4. Phase II: Evaluation of Physicochemical and Functional Changes in Chamomile Jelly During Storage for 21 Days
2.4.1. Syneresis
2.4.2. Microbial Assessment
2.5. Phase III: Evaluation of Biological Properties of Chamomile Jelly Products
2.5.1. Analysis of In Vitro Prebiotic Activity
2.5.2. Metabolomic Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phase I: Physicochemical and Functional Properties of Chamomile Jelly Products with Sugar Substituted by Prebiotics
3.1.1. Proximate Analysis
3.1.2. Water Activity (aw)
3.1.3. pH Value
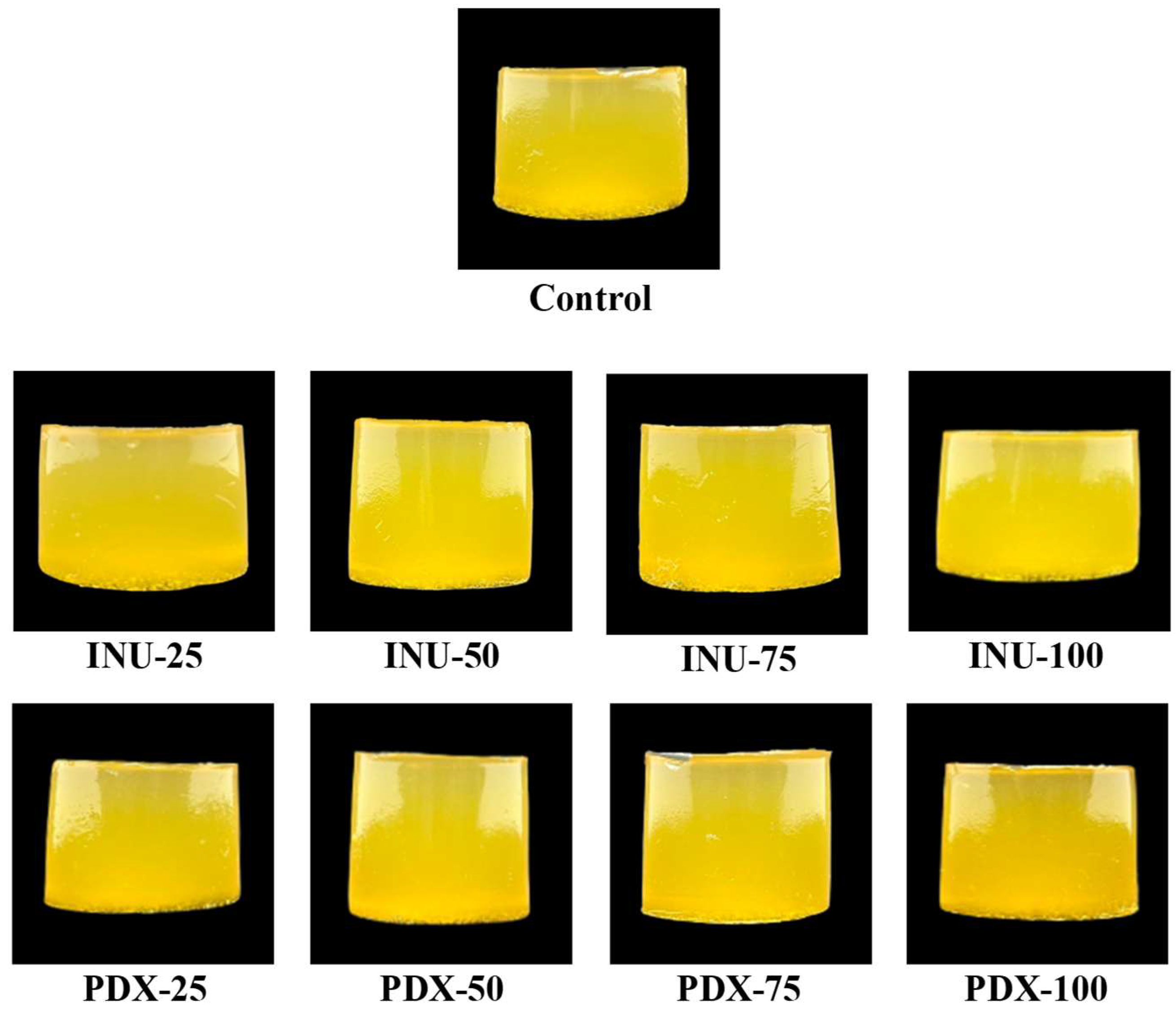
3.1.4. Appearance and Color
3.1.5. Texture Properties
3.1.6. Total Phenolic Content and Antioxidant Activity
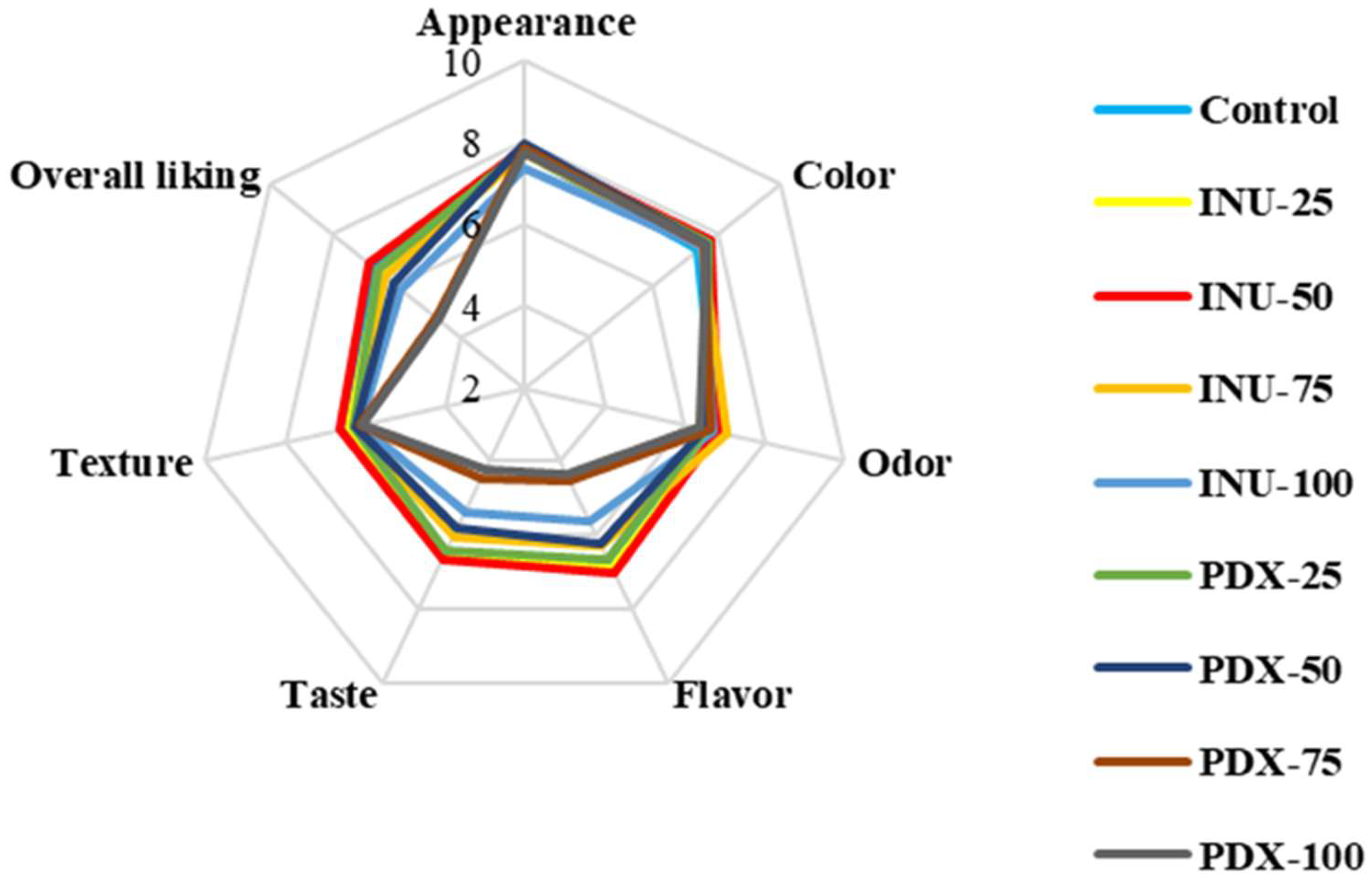
3.1.7. Consumer Acceptance
3.1.8. Nutrition Profile
3.2. Phase II: Evaluation of Physicochemical and Functional Changes in Chamomile Jelly During Storage for 21 Days
3.2.1. Chemical, Physical, and Physicochemical Characteristics of Selected Chamomile Jelly Sample
3.2.2. Texture Profile Parameters of Selected Chamomile Jelly Sample
3.2.3. Microbial Loads of Chamomile Jelly of Selected Chamomile Jelly Sample
3.3. Phase III: Evaluation of Biological Properties of Chamomile Jelly Products
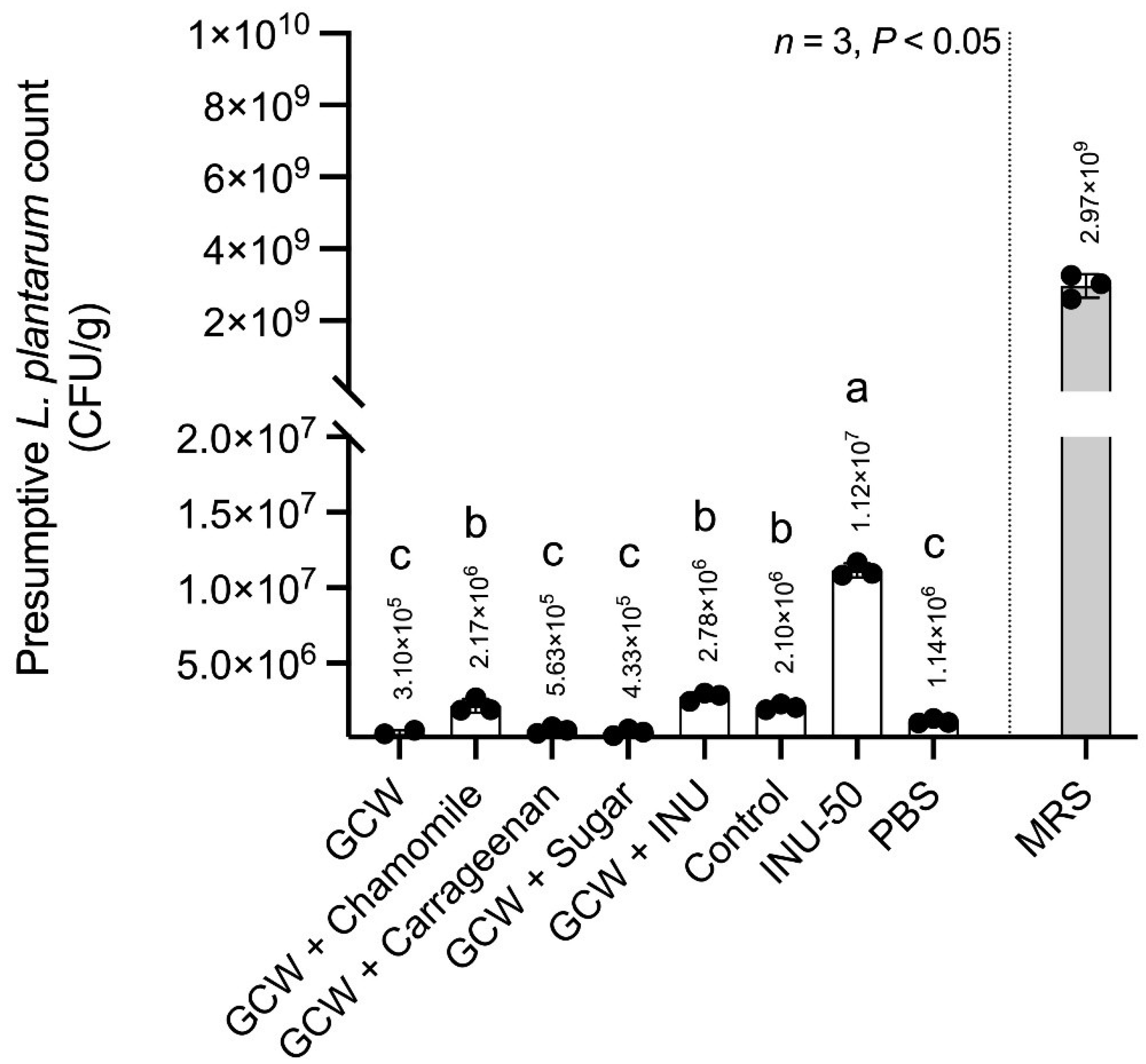
3.3.1. In Vitro Prebiotic Activity
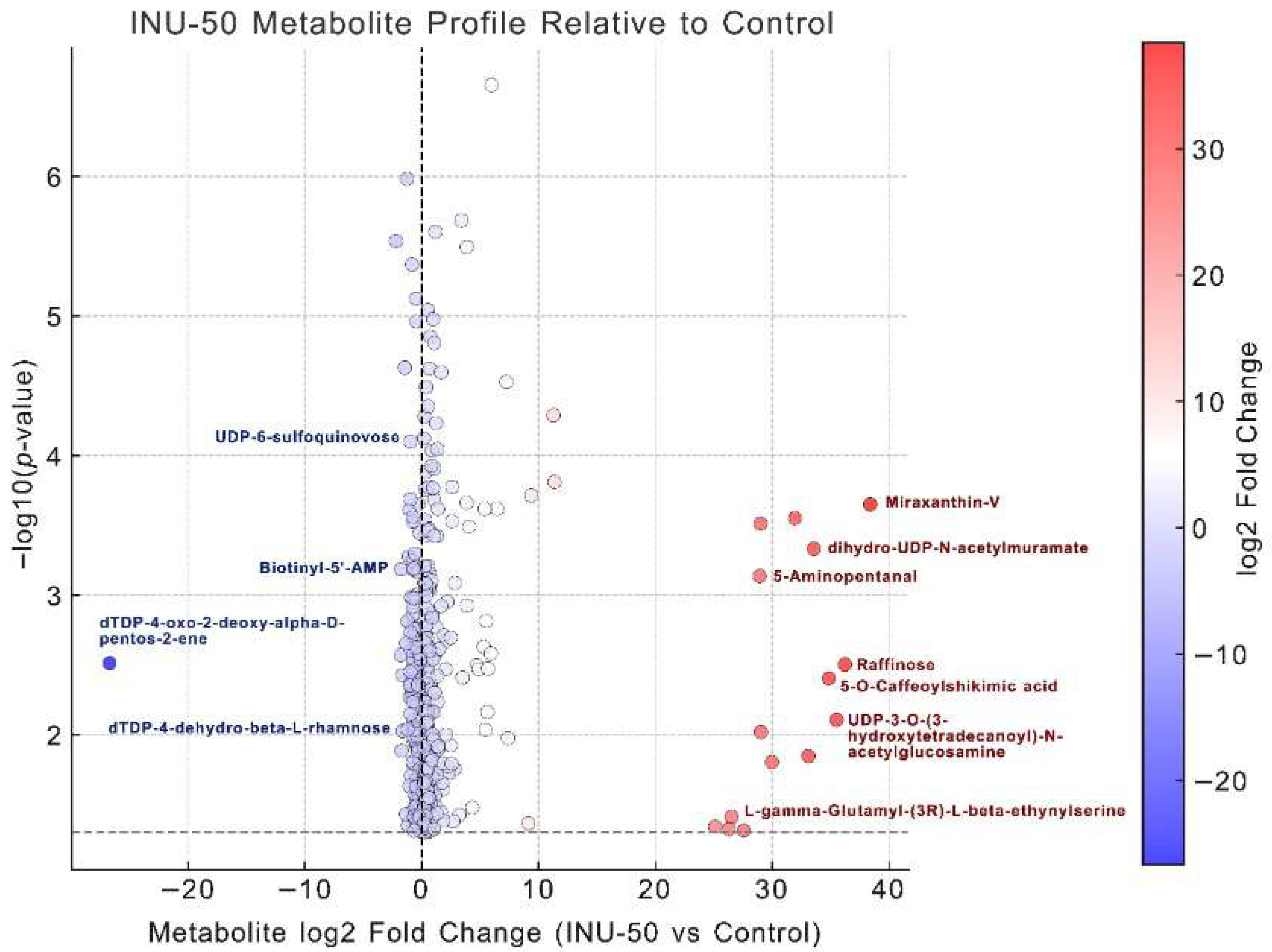
3.3.2. Metabolomic Analysis of Postbiotic Compounds Derived from Lactobacillus plantarum TISTR 1465 Grown on Chamomile Jelly Substituted with 50% Inulin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaewpetch, K.; Yolsuriyan, S.; Disayathanoowat, T.; Phokasem, P.; Jannu, T.; Renaldi, G.; Samakradhamrongthai, R.S. Influence of Gelatin and Propolis Extract on Honey Gummy Jelly Properties: Optimization Using D-Optimal Mixture Design. Gels 2024, 10, 282. [Google Scholar] [CrossRef]
- Sphericalinsights Global Jelly Market. Available online: https://www.sphericalinsights.com/reports/jelly-market (accessed on 11 November 2025).
- Ünal, M.H.; Arslan, D. Single and combined use of isomalt, polydextrose, and inulin as sugar substitutes in production of pectin jelly. J. Food Process. Preserv. 2022, 46, e17174. [Google Scholar] [CrossRef]
- Sasiluksananukul, T.; Punya, N.; Sangpimpa, W.; Srichan, P.; Renaldi, G.; Samakradhamrongthai, R.S. Xylitol substitution in the development of reduced-sugar gummy jelly mixed with Gymnema inodorum (Lour.) Decne leaf powder. Appl. Food Res. 2025, 5, 101011. [Google Scholar] [CrossRef]
- Looi, W.T.; Yusri, A.S.; Sarbon, N.M.; Sarbon, N.M. Infused botanic herbs in collagen jelly with different turmeric concentrations: Effect on physicochemical properties, phytochemical composition, and sensory acceptability. Food Chem. Adv. 2025, 8, 101061. [Google Scholar] [CrossRef]
- Wong, K.Y.; Thoo, Y.Y.; Tan, C.P.; Siow, L.F. Effect of alternative sweetener and carbohydrate polymer mixtures on the physical properties, melting and crystallization behaviour of dark compound chocolate. Food Chem. 2024, 431, 137118. [Google Scholar] [CrossRef] [PubMed]
- Sarfarazi, M.; Mohebbi, M. An investigation into the crystalline structure, and the rheological, thermal, textural and sensory properties of sugar-free milk chocolate: Effect of inulin and maltodextrin. J. Food Meas. Charact. 2020, 14, 1568–1581. [Google Scholar] [CrossRef]
- Nikolić, I.; Šoronja-Simović, D.; Zahorec, J.; Dokić, L.; Lončarević, I.; Stožinić, M.; Petrović, J. Polysaccharide-Based Fat Replacers in the Functional Food Products. Processes 2024, 12, 2701. [Google Scholar] [CrossRef]
- Kazemi, A.; Shojaei-Zarghani, S.; Eskandarzadeh, P.; Hashempur, M.H. Effects of chamomile (Matricaria chamomilla L.) on sleep: A systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2024, 84, 103071. [Google Scholar] [CrossRef]
- Ostovar, M.; Rezaee, Z.; Najibi, S.M.; Hashempur, M.H. Chamomile: A systematic review of adverse events. Complement. Ther. Med. 2025, 91, 103192. [Google Scholar] [CrossRef]
- Cvetanović Kljakić, A.; Čizmić, M.; Zengin, G.; Leventić, M.; Mišković Špoljarić, K.; Glavaš-Obrovac, L.; Mašković, P.; Babić, S.; Zeković, Z. A Comprehensive Appraisal of Bioactive Potential of Spray-Dried Chamomile Extract for Designing Novel Industrial Applications. Chem. Biodivers. 2025, 22, e202403032. [Google Scholar] [CrossRef]
- Kumnerdsiri, P.; Sanprasert, S.; Praiboon, J.; Seubsai, A.; Sirisarn, W.; Pongsetkul, J.; Harnkarnsujarit, N.; Rawdkuen, S.; Karnjanapratum, S.; Sai-Ut, S.; et al. Characterization of Cha-Kram leaf extract powder using ultrasound-assisted extraction and its application in gelatin-based film as biodegradable active film. Future Foods 2024, 10, 100419. [Google Scholar] [CrossRef]
- Kumnerdsiri, P.; Sanprasert, S.; Seubsai, A.; Pongsetkul, J.; Harnkarnsujarit, N.; Rawdkuen, S.; Sai-ut, S.; Phongthai, S.; Lueangjaroenkit, P.; Onsaard, E.; et al. Properties of novel biodegradable film from gelatin capsule waste as influenced by various solvents and washing cycles. Future Foods 2024, 10, 100485. [Google Scholar] [CrossRef]
- Sanprasert, S.; Kumnerdsiri, P.; Seubsai, A.; Lueangjaroenkit, P.; Pongsetkul, J.; Indriani, S.; Petcharat, T.; Sai-ut, S.; Hunsakul, K.; Issara, U.; et al. Techno-Functional, Rheological, and Physico-Chemical Properties of Gelatin Capsule By-Product for Future Functional Food Ingredients. Foods. 2025, 14, 1279. [Google Scholar] [CrossRef]
- Sanprasert, S.; Kumnerdsiri, P.; Seubsai, A.; Lueangjaroenkit, P.; Pongsetkul, J.; Petcharat, T.; Kaewprachu, P.; Sai-ut, S.; Rawdkuen, S.; Teerapattarakan, N.; et al. Techno-Functional Gelling Mechanism and Rheological Properties of Gelatin Capsule-Waste Gel Modified with Kappa-Carrageenan for Future Functional Food Applications. Future Foods 2025, 12, 100723. [Google Scholar] [CrossRef]
- Anyiam, P.N.; Phongthai, S.; Sai-Ut, S.; Kingwascharapong, P.; Jung, Y.H.; Zhang, W.; Rawdkuen, S. Nutritional Components and Digestibility Profiles of Some Potential Plant-Based Protein Sources. Foods 2025, 14, 1769. [Google Scholar] [CrossRef] [PubMed]
- Petcharat, T.; Benjakul, S.; Hemar, Y. Improvement of gel properties of fish gelatin using gellan. Int. J. Food Eng. 2017, 13, 20160410. [Google Scholar] [CrossRef]
- Zainol, M.K.; Cheang, L.N.; Zuraidah, N.; Yahya, F.; Zin, Z.M. Storage stability of sweet corn (Zea mays var saccharata Bailey) jam: Effect of sugar to inulin ratios on physicochemical, ascorbic acid, β-carotene and sensory characteristics. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 3rd Asia Pacific Regional Conference on Food Security (ARCoFS 2021), Kelantan, Malaysia, 9 March 2021; IOP Publishing: Bristol, UK, 2021; Volume 756, p. 012074. [Google Scholar] [CrossRef]
- Lightowler, H.; Thondre, S.; Holz, A.; Theis, S. Replacement of glycaemic carbohydrates by inulin-type fructans from chicory (oligofructose, inulin) reduces the postprandial blood glucose and insulin response to foods: Report of two double-blind, randomized, controlled trials. Eur. J. Nutr. 2018, 57, 1259–1268. [Google Scholar] [CrossRef]
- Do Carmo, M.M.; Walker, J.C.; Novello, D.; Caselato, V.M.; Sgarbieri, V.C.; Ouwehand, A.C.; Andreollo, N.A.; Hiane, P.A.; Dos Santos, E.F. Polydextrose: Physiological Function, and Effects on Health. Nutrients 2016, 8, 553. [Google Scholar] [CrossRef]
- Mudannayake, D.C.; Meegahawaththa, W.K.; Illippangama, A.U.; Pitawala, H.M.J.C.; Wimalasiri, K.M.S.; Silva, K.F.S.T. Extraction, purification and structural characterization of inulin-type fructans from different selected Asparagus species. Bioact. Carbohydr. Diet. Fibre 2025, 34, 100486. [Google Scholar] [CrossRef]
- Han, Y.J.; Tra Tran, T.T.; Man Le, V.V. Corn snack with high fiber content: Effects of different fiber types on the product quality. LWT 2018, 96, 1–6. [Google Scholar] [CrossRef]
- Mudannayake, D.C.; Wimalasiri, K.M.S.; Silva, K.F.S.T.; Ajlouni, S. Comparison of Properties of New Sources of Partially Purified Inulin to Those of Commercially Pure Chicory Inulin. J. Food Sci. 2015, 80, C950–C960. [Google Scholar] [CrossRef]
- Rodriguez-Huezo, M.E.; Valeriano-Garcia, N.; Totosaus-Sanchez, A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. The effect of the addition of soluble fibers (polydextrose, corn, pea) on the color, texture, structural features and protein digestibility of semolina pasta. Appl. Food Res. 2022, 2, 100187. [Google Scholar] [CrossRef]
- Zamora-Gasga, V.M.; Bello-Pérez, L.A.; Ortíz-Basurto, R.I.; Tovar, J.; Sáyago-Ayerdi, S.G. Granola bars prepared with Agave tequilana ingredients: Chemical composition and in vitro starch hydrolysis. LWT 2014, 56, 309–314. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Capriles, V.D.; Conti-Silva, A.C. Inulin as an ingredient for improvement of glycemic response and sensory acceptance of breakfast cereals. Food Hydrocoll. 2021, 114, 106582. [Google Scholar] [CrossRef]
- Yang, J.H.; Tran, T.T.T.; Le, V.V.M. Use of corn flour and polydextrose in fried extrudate making: Effects of polydextrose content in the blend and extrusion temperature on the product quality. J. Food Process Eng. 2020, 43, e13438. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Barajas-Álvarez, P.; Morales-Hernández, N.; Camacho-Ruíz, R.M.; Espinosa-Andrews, H. Physical Properties and Prebiotic Activities (Lactobacillus spp.) of Gelatine-Based Gels Formulated with Agave Fructans and Agave Syrups as Sucrose and Glucose Substitutes. Molecules 2022, 27, 4902. [Google Scholar] [CrossRef]
- Linggo, G.; Phothisoot, T.; Kongpichitchoke, T.; Lo, D. Utilization of Fructose-olygosaccharides as Sugar Substitute in Marshmallow and its Effect on the Physicochemical and Consumer Acceptance. In IOP Conference Series: Earth and Environmental Science, Proceedings of the International Collaborative Conference of Modern Agricultural Technologies, Ramadi, Iraq, 9–10 October 2024; IOP Publishing: Bristol, UK, 2025; Volume 1449, p. 012164. [Google Scholar] [CrossRef]
- Ergun, R.; Lietha, R.; Hartel, R.W. Moisture and Shelf Life in Sugar Confections. Crit. Rev. Food Sci. Nutr. 2010, 50, 162–192. [Google Scholar] [CrossRef]
- Siriwattanasilp, A.; Thirathumthavorn, D.; Siriwongwilaichat, P. Effects of sugar alcohol and rebaudioside A substitution on the physicochemical, thermal and microstructure properties of coconut milk agar-based jelly. J. Food Meas. Charact. 2025, 19, 7787–7795. [Google Scholar] [CrossRef]
- Chheng, S.; Jafari, S.; Mishra, D.; Assatarakul, K. Sesban flower extract as a natural functional ingredient: Effects on texture, antioxidant activity, and shelf- life stability of jelly formulation. Sustain. Food Technol. 2025, 3, 1865–1879. [Google Scholar] [CrossRef]
- Mieszkowska, A.; Marzec, A. Effect of polydextrose and inulin on texture and consumer preference of short-dough biscuits with chickpea flour. LWT 2016, 73, 60–66. [Google Scholar] [CrossRef]
- Pekdogan Goztok, S.; Habibzadeh Khiabani, A.; Toker, O.S.; Palabiyik, I.; Konar, N. Development of healthier gummy candy by substituting glucose syrup with various fruit juice concentrates. Food Sci. Nutr. 2024, 12, 7864–7876. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.; Bañón, S. Effects of replacing starch by inulin on the physicochemical, texture and sensory characteristics of gummy jellies. CyTA-J. Food. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Keskin Kuzey, F.; Karimidastjerd, A.; Toker, O.S.; Arici, M.; Palabiyik, I.; Tasan, M.; Konar, N. Composition of Commercial Licorice Candies and Development of Sugar-Reduced Licorice-Type Extruded Soft Candy Using Resistant Dextrin. ACS Omega 2025, 10, 30616–30626. [Google Scholar] [CrossRef]
- Figueroa, L.E.; Hughes, M.H.; Tarifa, M.C.; Brugnoni, L.I.; Genovese, D.B. Development and stability of low-sugar, plant-based gummy candies prepared via an innovative ionotropic gelation technique. Food Humanit. 2025, 5, 100708. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Calín-Sánchez, Á.; Clemente-Villalba, J.; Hernández, F.; Carbonell-Barrachina, Á.A.; Sendra, E.; Wojdyło, A. Quality Parameters and Consumer Acceptance of Jelly Candies Based on Pomegranate Juice “Mollar de Elche”. Foods 2020, 9, 516. [Google Scholar] [CrossRef]
- Luo, Y.; Tu, Y.; Ren, F.; Zhang, H. Characterization and functional properties of Maillard reaction products of β-lactoglobulin and polydextrose. Food Chem. 2022, 377, 131749. [Google Scholar] [CrossRef]
- Khan, H.; Mudgil, P.; Alkaabi, S.A.S.; AlRashdi, Y.H.S.; Maqsood, S. Maillard reaction-based conjugation of pea protein and prebiotic (polydextrose): Optimization, characterization, and functional properties enhancement. Front. Sustain. Food Syst. 2024, 8, 1463058. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Pereira, G.A.; Meireles, M.A.A.; Pastore, G.M. Inulin thermal stability in prebiotic carbohydrate-enriched araticum whey beverage. LWT 2020, 128, 109418. [Google Scholar] [CrossRef]
- Maringka, C.T.; Putra, A.B.N.; Lo, D. Development of gummy candy with polydextrose, isomalto-oligosaccharides, fructo-oligosaccharides, and xylitol as sugar replacers. Int. J. Gastron. Food Sci. 2024, 35, 100881. [Google Scholar] [CrossRef]
- Aamir, M.; Abid, A.; Azam, I.; Ikram, A.; Saeed, F.; Afzaal, M.; Ateeq, H.; Akram, N.; Hussain, S.; Khan, M.R. Characterization of carbonated beverage fortified with chamomile herbal extract. Food Sci. Nutr. 2024, 12, 4353–4361. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Saucedo, M.C.; Ramírez-Anaya, J.D.P.; Tapia-Campos, E.; Diaz-Ochoa, E.G. Comparison of total phenol content and antioxidant activity of herbal infusions with added Stevia reabaudiana Bertoni. Food Sci. Technol. 2019, 40, 117–123. [Google Scholar] [CrossRef]
- Jung, J.; Kim, S.; Park, S.; Hong, J.-H. Sweetness profiles of glycosylated rebaudioside A and its binary mixtures with allulose and maltitol. Food Sci. Biotechnol. 2021, 30, 423–432. [Google Scholar] [CrossRef]
- Bolshakova, E.I.; Kruchinin, A.G.; Turovskaya, S.N.; Illarionova, E.E.; Yurova, E.A.; Barkovskaya, I.A.; Galstyan, A.G. Effects provided by sugar substitutes upon the quality indicators of model systems of sweetened condensed milk in storage. J. Dairy Sci. 2024, 107, 9110–9123. [Google Scholar] [CrossRef] [PubMed]
- Suechawakornkul, J. Consumer Need Analysis and Development of Low Calories Vegan Jelly Enhanced Immunity from Fingerroot. Master’s Thesis, Faculty of Science and Technology, Thammasat University, Bangkok, Thailand, 2021. [Google Scholar]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus plantarum ATCC14917. Molecules 2019, 24, 51. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Qin, Y.; Luo, Y. The impact of different lactobacilli fermentations on secondary metabolites of red raspberry juice and their biotransformation pathways via metabolomics based on UHPLC-MS/MS. Int. J. Food Microbiol. 2025, 427, 110974. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Song, X.; Cui, L.; Bai, J.; Lu, H.; Wang, S. The lactate dehydrogenase gene is involved in the growth and metabolism of Lacticaseibacillus paracasei and the production of fermented milk flavor substances. Front. Microbiol. 2023, 14, 1195360. [Google Scholar] [CrossRef]
| Ingredient (%) | Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | INU- 25 | INU- 50 | INU- 75 | INU- 100 | PDX- 25 | PDX- 50 | PDX- 75 | PDX- 100 | |
| Chamomile tea | 85.71 | 85.71 | 85.71 | 85.71 | 85.71 | 85.71 | 85.71 | 85.71 | 85.71 |
| GCW * | 5.15 | 5.15 | 5.15 | 5.15 | 5.15 | 5.15 | 5.15 | 5.15 | 5.15 |
| Carrageenan | 0.57 | 0.57 | 0.57 | 0.57 | 0.57 | 0.57 | 0.57 | 0.57 | 0.57 |
| Sugar | 8.57 | 6.43 | 4.29 | 2.14 | 0.00 | 6.43 | 4.29 | 2.14 | 0.00 |
| Inulin (INU) | - | 2.14 | 4.29 | 6.43 | 8.57 | - | - | - | - |
| Polydextrose (PDX) | - | - | - | - | - | 2.14 | 4.29 | 6.43 | 8.57 |
| Samples | Moisture (%) | Fat (%) | Ash (%) | Protein (%) | Carbohydrate (%) |
|---|---|---|---|---|---|
| Control | 85.14 ± 0.04 a | 0.01 ± 0.01 c | 0.19 ± 0.01 a | 1.67 ± 1.45 b | 13.50 ± 1.70 a |
| INU-25 | 85.13 ± 0.16 a | 0.05 ± 0.03 bc | 0.21 ± 0.04 a | 2.28 ± 0.03 ab | 12.34 ± 0.08 bc |
| INU-50 | 85.11 ± 0.04 a | 0.08 ± 0.06 abc | 0.24 ± 0.06 a | 2.10 ± 0.06 ab | 12.47 ± 0.15 b |
| INU-75 | 85.00 ± 0.36 a | 0.04 ± 0.05 bc | 0.28 ± 0.09 a | 2.28 ± 0.04 ab | 12.40 ± 0.34 bc |
| INU-100 | 85.17 ± 0.38 a | 0.15 ± 0.01 a | 0.43 ± 0.35 a | 2.45 ± 0.00 ab | 11.80 ± 0.57 bc |
| PDX-25 | 85.29 ± 0.12 a | 0.13 ± 0.03 ab | 0.20 ± 0.06 a | 2.46 ± 0.03 ab | 11.92 ± 0.21 bc |
| PDX-50 | 85.15 ± 0.06 a | 0.10 ± 0.01 abc | 0.35 ± 0.26 a | 2.57 ± 0.00 ab | 11.82 ± 0.20 bc |
| PDX-75 | 85.07 ± 0.05 a | 0.10 ± 0.07 abc | 0.28 ± 0.11 a | 2.49 ± 0.02 ab | 12.07 ± 0.05 bc |
| PDX-100 | 85.35 ± 0.32 a | 0.12 ± 0.09 ab | 0.47 ± 0.49 a | 2.72 ± 0.02 a | 11.35 ± 0.80 c |
| Samples | pH | Color | |||
|---|---|---|---|---|---|
| L* | a* | b* | ΔE* | ||
| Control | 7.59 ± 0.17 a | 29.01 ± 0.35 a | 6.69 ± 0.23 cd | 37.29 ± 1.87 a | - |
| INU-25 | 7.24 ± 0.02 bc | 28.90 ± 0.68 a | 6.88 ± 0.42 bcd | 35.82 ± 1.49 ab | 2.36 ± 1.46 b |
| INU-50 | 7.25 ± 0.01 bc | 28.74 ± 0.47 ab | 6.81 ± 0.37 cd | 35.10 ± 2.04 b | 2.12 ± 1.42 b |
| INU-75 | 7.24 ± 0.03 bc | 28.50 ± 0.43 abc | 7.50 ± 0.21 a | 35.38 ± 2.42 b | 2.23 ± 1.15 b |
| INU-100 | 7.29 ± 0.01 b | 28.16 ± 0.40 bc | 6.90 ± 0.22 bc | 35.18 ± 1.12 b | 2.27 ± 0.72 b |
| PDX-25 | 7.18 ± 0.02 bcd | 28.00 ± 0.68 c | 6.58 ± 0.30 de | 31.51 ± 0.70 c | 4.59 ± 1.13 a |
| PDX-50 | 7.20 ± 0.02 bcd | 28.47 ± 1.04 abc | 6.64 ± 0.20 cde | 31.66 ± 1.50 c | 4.87 ± 1.09 a |
| PDX-75 | 7.09 ± 0.01 d | 28.14 ± 0.94 bc | 7.13 ± 0.19 b | 32.29 ± 0.96 c | 4.82 ± 1.04 a |
| PDX-100 | 7.13 ± 0.07 cd | 28.77 ± 0.54 ab | 6.35 ± 0.41 e | 32.77 ± 1.21 c | 4.77 ± 0.94 a |
| Samples | Texture Profile Analysis | Gel Strength/ Bloom Value (g) | ||||
|---|---|---|---|---|---|---|
| Hardness (N) | Adhesiveness (Nxsec) | Springiness | Cohesiveness | Gumminess (N) | ||
| Control | 9.61 ± 0.17 c | −0.83 ± 0.36 c | 0.91 ± 0.03 bc | 0.76 ± 0.02 a | 7.30 ± 0.15 c | 54.35 ± 3.24 e |
| INU-25 | 9.77 ± 0.35 c | −0.41 ± 0.43 ab | 0.94 ± 0.04 ab | 0.74 ± 0.02 bc | 8.17 ± 1.09 bc | 68.15 ± 2.98 c |
| INU-50 | 9.79 ± 0.88 c | −0.44 ± 0.20 ab | 0.93 ± 0.03 bc | 0.71 ± 0.02 de | 8.02 ± 1.42 bc | 56.59 ± 2.77 e |
| INU-75 | 10.08 ± 0.60 c | −0.42 ± 0.27 ab | 0.93 ± 0.03 bc | 0.72 ± 0.03 de | 7.87 ± 0.84 bc | 55.97 ± 3.13 e |
| INU-100 | 11.08 ± 0.77 b | −0.74 ± 0.38 bc | 0.91 ± 0.03 c | 0.70 ± 0.04 e | 8.03 ± 0.65 bc | 63.01 ± 4.38 d |
| PDX-25 | 13.46 ± 0.81 a | −0.32 ± 0.40 a | 0.97 ± 0.02 a | 0.73 ± 0.02 bcd | 9.46 ± 0.53 a | 81.45 ± 4.56 a |
| PDX-50 | 9.83 ± 0.85 c | −0.35 ± 0.17 a | 0.94 ± 0.02 ab | 0.74 ± 0.02 ab | 7.72 ± 1.15 bc | 57.34 ± 1.86 e |
| PDX-75 | 11.31 ± 0.93 b | −0.48 ± 0.35 ab | 0.94 ± 0.03 bc | 0.74 ± 0.02 bc | 8.37 ± 0.85 b | 61.06 ± 2.94 d |
| PDX-100 | 10.20 ± 0.46 c | −0.50 ± 0.28 ab | 0.93 ± 0.03 bc | 0.71 ± 0.02 e | 7.61 ± 0.42 bc | 72.81 ± 2.37 b |
| Composition | Content per 100 g | Serving Size (90 g) |
|---|---|---|
| Total energy (Kcal) | 61.30 | 60 |
| Total fat (g) | 0.06 | - |
| Saturated fatty acids (g) | 0.02 | - |
| Cholesterol (mg) | - | - |
| Protein (g) | 2.95 | 3 |
| Total carbohydrate (g) | 12.24 | 11 |
| Total sugar (g) | 6.31 | 6 |
| Sodium (mg) | 48.33 | 45 |
| Potassium (mg) | 56.73 | 45 |
| Ash (g) | 0.20 | - |
| Moisture (g) | 84.55 | - |
| Sample | Day | pH | Syneresis | Color | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| Control | 1 | 7.44 ± 0.02 c | 1.70 ± 2.54 a | 30.84 ± 2.64 bc | 31.68 ± 2.20 a | 3.85 ± 0.34 a |
| 7 | 7.60 ± 0.05 a | 0.57 ± 0.18 a | 31.88 ± 3.79 ab | 31.26 ± 3.44 ab | 3.64 ± 0.53 ab | |
| 14 | 7.53 ± 0.04 ab | 0.13 ± 0.02 a | 32.16 ± 1.19 ab | 31.01 ± 0.81 ab | 3.25 ± 0.80 b | |
| 21 | 7.50 ± 0.07 bc | 0.12 ± 0.26 a | 34.45 ± 3.43 a | 30.76 ± 1.16 ab | 3.25 ± 0.53 b | |
| INU-50 | 1 | 7.36 ± 0.05 d | 0.28 ± 0.03 a | 32.39 ± 1.82 ab | 31.68 ± 2.20 a | 3.85 ± 0.34 a |
| 7 | 7.53 ± 0.01 ab | 0.48 ± 0.03 a | 29.04 ± 3.65 c | 31.26 ± 3.44 ab | 3.64 ± 0.53 ab | |
| 14 | 7.48 ± 0.03 bc | 0.22 ± 0.13 a | 23.93 ± 1.15 e | 31.01 ± 0.81 ab | 3.25 ± 0.80 b | |
| 21 | 7.46 ± 0.01 bc | 0.21 ± 0.10 a | 26.53 ± 2.41 d | 30.76 ± 1.16 ab | 3.25 ± 0.53 b | |
| Samples | Day | Texture Profile Analysis | Gel Strength/ Bloom Value (g) | ||||
|---|---|---|---|---|---|---|---|
| Hardness (N) | Adhesiveness (Nxsec) | Springiness | Cohesiveness | Gumminess (N) | |||
| Control | 1 | 3.90 ± 0.70 d | −0.56 ± 0.23 bc | 0.81 ± 0.05 b | 0.27 ± 0.04 e | 1.04 ± 0.13 f | 64.80 ± 7.04 d |
| 7 | 4.37 ± 0.43 d | −0.62 ± 0.30 d | 0.85 ± 0.03 b | 0.26 ± 0.02 e | 1.15 ± 0.13 f | 65.80 ± 7.58 d | |
| 14 | 8.80 ± 0.74 b | −0.27 ± 0.22 b | 0.94 ± 0.03 ab | 0.56 ± 0.09 b | 4.99 ± 1.09 c | 69.79 ± 6.43 bcd | |
| 21 | 9.47 ± 0.89 b | −0.33 ± 0.37 bc | 1.09 ± 0.47 a | 0.70 ± 0.04 a | 6.66 ± 0.93 b | 88.89 ± 6.42 a | |
| INU-50 | 1 | 4.32 ± 0.50 d | −0.48 ± 0.37 bc | 0.87 ± 0.05 b | 0.37 ± 0.05 d | 1.58 ± 0.31 f | 67.16 ± 4.58 cd |
| 7 | 6.93 ± 0.84 c | 0.07 ± 0.01 a | 0.89 ± 0.04 b | 0.34 ± 0.07 d | 2.38 ± 0.60 e | 71.87 ± 4.00 bc | |
| 14 | 8.99 ± 1.11 b | −0.46 ± 0.30 bc | 0.94 ± 0.02 ab | 0.45 ± 0.15 c | 4.15 ± 1.70 d | 73.24 ± 3.87 b | |
| 21 | 10.25 ± 0.70 a | −0.49 ± 0.36 bc | 0.95 ± 0.05 ab | 0.75 ± 0.02 a | 7.73 ± 0.54 a | 88.78 ± 6.39 a | |
| Sample | Day | Parameter | |||
|---|---|---|---|---|---|
| Staphylococcus aureus (CFU/g) | E. coli (MPN/g) | Yeasts (CFU/g) | Molds (CFU/g) | ||
| Control | 1 | ND | <3 | <10 | <10 |
| 7 | ND | <3 | <10 | <10 | |
| 14 | ND | <3 | <10 | <10 | |
| 21 | ND | <3 | <10 | <10 | |
| INU-50 | 1 | ND | <3 | <10 | <10 |
| 7 | ND | <3 | <10 | <10 | |
| 14 | ND | <3 | <10 | <10 | |
| 21 | ND | <3 | <10 | <10 | |
| No. | Name | Fold-Change (FC) | log2FC | p-Value |
|---|---|---|---|---|
| Up Regulation | ||||
| 1 | Miraxanthin-V | 358,675,324,476.240 | 38.3839 | 0.0002 |
| 2 | Raffinose ** | 80,710,592,233.243 | 36.2320 | 0.0031 |
| 3 | dihydro-UDP-N-acetylmuramate ** | 48,596,078,146.530 | 35.5001 | 0.0078 |
| 4 | 5-O-Caffeoylshikimic acid ** | 30,955,125,405.091 | 34.8495 | 0.0039 |
| 5 | (S)-3-Hydroxyhexadecanoyl-CoA | 12,674,769,966.638 | 33.5612 | 0.0005 |
| 6 | UDP-3-O-(3-hydroxytetradecanoyl)-N-acetylglucosamine ** | 9,241,193,149.530 | 33.1054 | 0.0142 |
| 7 | L-Rhamnono-1,4-lactone | 4,126,665,535.711 | 31.9423 | 0.0003 |
| 8 | Cobalt-precorrin 7 | 1,058,583,229.174 | 29.9795 | 0.0157 |
| 9 | gabaculine | 545,654,526.282 | 29.0234 | 0.0096 |
| 10 | 5-Aminopentanal ** | 537,965,022.664 | 29.0029 | 0.0003 |
| 11 | 3-Methoxytyramine | 508,790,935.368 | 28.9225 | 0.0007 |
| 12 | L-gamma-Glutamyl-(3R)-L-beta-ethynylserine ** | 198,930,782.304 | 27.5677 | 0.0485 |
| 13 | 2-Amino-5-oxohexanoate | 96,834,576.078 | 26.5290 | 0.0386 |
| 14 | 2-Phenyl-1,3-propanediol monocarbamate | 83,061,174.704 | 26.3077 | 0.0474 |
| 15 | Aminoadipic acid | 36,331,460.343 | 25.1147 | 0.0455 |
| 16 | Maltotetraose | 2621.107 | 11.3560 | 0.0002 |
| 17 | Maltohexaose | 2500.159 | 11.2878 | 0.0001 |
| 18 | N2-Citryl-N6-acetyl-N6-hydroxy-L-lysine | 661.468 | 9.3695 | 0.0002 |
| 19 | Isonocardicin A | 559.952 | 9.1292 | 0.0432 |
| 20 | N-amino DAP | 168.248 | 7.3944 | 0.0106 |
| No. | Name | Fold-Change | log2FC | p-Value |
|---|---|---|---|---|
| Down Regulation | ||||
| 1 | dTDP-4-oxo-2-deoxy-alpha-D-pentos-2-ene ** | 0.000 | −26.6906 | 0.0031 |
| 2 | 1-OH-Nogalamycinone | 0.220 | −2.1837 | 0.0000 |
| 3 | dTDP-4-dehydro-beta-L-rhamnose ** | 0.293 | −1.7728 | 0.0027 |
| 4 | (+)-Gallocatechin | 0.293 | −1.7708 | 0.0007 |
| 5 | 7,8-Dihydromethanopterin | 0.303 | −1.7215 | 0.0130 |
| 6 | Arachidonyl-CoA | 0.315 | −1.6650 | 0.0037 |
| 7 | Baicalin | 0.321 | −1.6388 | 0.0094 |
| 8 | Tetrahydrosarcinapterin | 0.370 | −1.4353 | 0.0000 |
| 9 | UDP-6-sulfoquinovose ** | 0.395 | −1.3417 | 0.0372 |
| 10 | Sucrose | 0.396 | −1.3365 | 0.0022 |
| 11 | Ethyl-D-glucuronide | 0.424 | −1.2392 | 0.0000 |
| 12 | Hygromycin B | 0.430 | −1.2189 | 0.0092 |
| 13 | N1-Amidinostreptamine 6-phosphate | 0.435 | −1.2001 | 0.0015 |
| 14 | CDP-DG (16:0/20:4(8Z,11Z,14Z,17Z)) | 0.447 | −1.1625 | 0.0446 |
| 15 | Biotinyl-5′-AMP ** | 0.466 | −1.1019 | 0.0005 |
| 16 | Acarbose 7IV-phosphate | 0.480 | −1.0598 | 0.0234 |
| 17 | Glycolate | 0.481 | −1.0548 | 0.0002 |
| 18 | Ampicillin | 0.490 | −1.0282 | 0.0054 |
| 19 | N-Methylethanolamine phosphate | 0.505 | −0.9856 | 0.0045 |
| 20 | 4-Amino-4-deoxychorismate | 0.511 | −0.9695 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanprasert, S.; Uchuwittayakul, A.; Kumnerdsiri, P.; Kitsanayanyong, L.; Seubsai, A.; Pongsetkul, J.; Petsong, K.; Karnjanapratum, S.; Jaisan, C.; Sai-ut, S.; et al. Functional and Metabolomic Analyses of Chamomile Jelly Derived from Gelatin Capsule Waste with Inulin and Polydextrose as Prebiotic Sugar Substitutes. Antioxidants 2025, 14, 1380. https://doi.org/10.3390/antiox14111380
Sanprasert S, Uchuwittayakul A, Kumnerdsiri P, Kitsanayanyong L, Seubsai A, Pongsetkul J, Petsong K, Karnjanapratum S, Jaisan C, Sai-ut S, et al. Functional and Metabolomic Analyses of Chamomile Jelly Derived from Gelatin Capsule Waste with Inulin and Polydextrose as Prebiotic Sugar Substitutes. Antioxidants. 2025; 14(11):1380. https://doi.org/10.3390/antiox14111380
Chicago/Turabian StyleSanprasert, Sasina, Anurak Uchuwittayakul, Pudthaya Kumnerdsiri, Lalitphan Kitsanayanyong, Anusorn Seubsai, Jaksuma Pongsetkul, Kantiya Petsong, Supatra Karnjanapratum, Chalalai Jaisan, Samart Sai-ut, and et al. 2025. "Functional and Metabolomic Analyses of Chamomile Jelly Derived from Gelatin Capsule Waste with Inulin and Polydextrose as Prebiotic Sugar Substitutes" Antioxidants 14, no. 11: 1380. https://doi.org/10.3390/antiox14111380
APA StyleSanprasert, S., Uchuwittayakul, A., Kumnerdsiri, P., Kitsanayanyong, L., Seubsai, A., Pongsetkul, J., Petsong, K., Karnjanapratum, S., Jaisan, C., Sai-ut, S., Rawdkuen, S., & Kingwascharapong, P. (2025). Functional and Metabolomic Analyses of Chamomile Jelly Derived from Gelatin Capsule Waste with Inulin and Polydextrose as Prebiotic Sugar Substitutes. Antioxidants, 14(11), 1380. https://doi.org/10.3390/antiox14111380







