Evaluation of Dexamethasone and Swimming Exercise as Complementary Interventions in a Rat Sciatic Nerve Injury Model
Abstract
1. Introduction
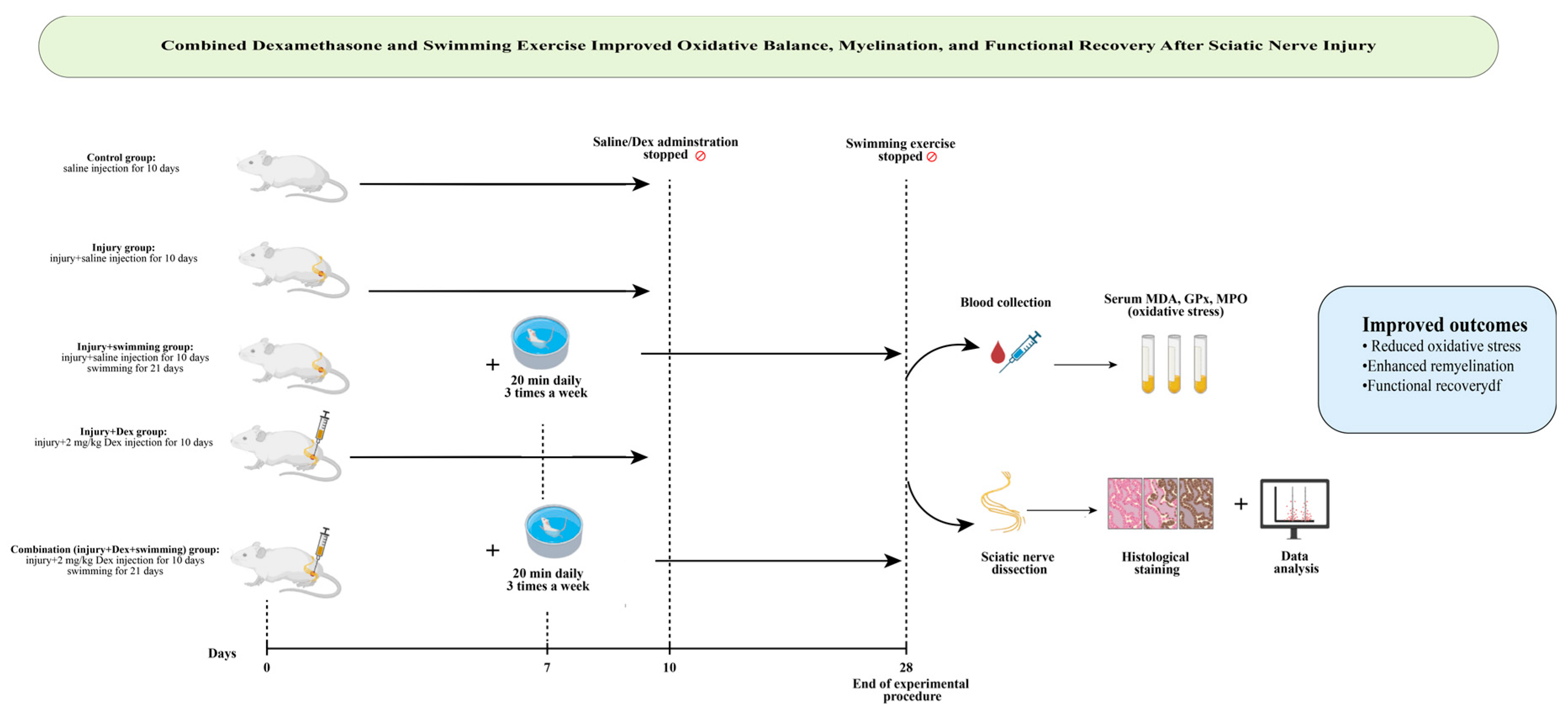
2. Materials and Methods
2.1. Animals and Ethical Approval
2.2. Experimental Sciatic Nerve Crush Model
- Control group: Sciatic nerve exposed but not crushed; saline (0.1 mL) injected perineurally for 10 days.
- Injury group: Nerve crushed; saline (0.1 mL) injected perineurally for 10 days.
- Injury + Swimming group: Nerve crushed, saline (0.1 mL) injected perineurally for 10 days, and then swimming protocol applied for 21 days.
- Injury + Dexamethasone group: Nerve crushed, only dexamethasone (2 mg/kg/day, local perineural injection at the injury site) administered for 10 days.
- Injury + Combination group: Nerve crushed, received dexamethasone (2 mg/kg/day, local perineural injection, 10 days) plus swimming exercise.
2.3. Swimming Exercise Protocol
2.4. Functional Assessment of Sciatic Nerve
2.5. Biochemical Analysis
2.6. Histological and Immunohistochemical Analyses
2.7. Ultrastructural Analysis
2.8. Randomization and Blinding
2.9. In Silico Analysis
2.10. Molecular Docking Analysis
2.11. Statistical Analysis
3. Results
3.1. Dexamethasone and Swimming Markedly Reduce Oxidative Stress Following Sciatic Nerve Injury
3.2. Combined Therapy Restores Motor Function to Near-Normal Levels by Day 28
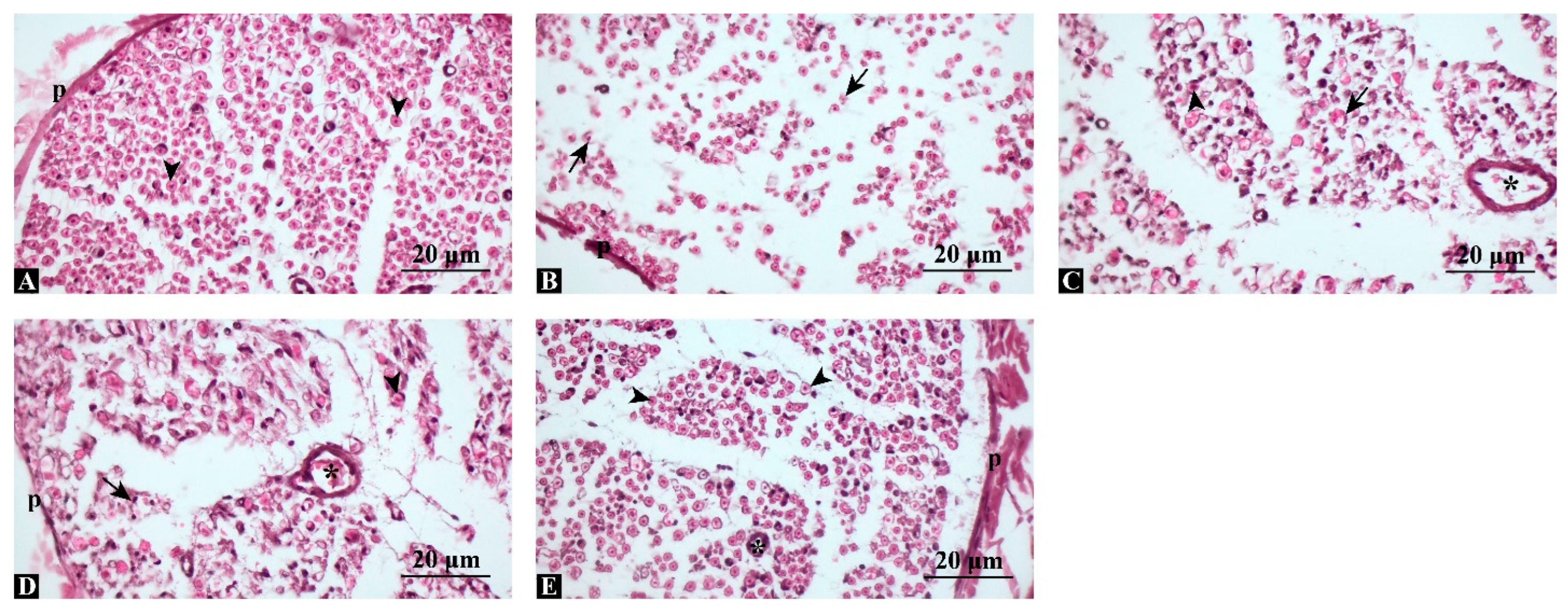
3.3. Combined Treatment Preserves Axonal Integrity and Minimizes Structural Degeneration
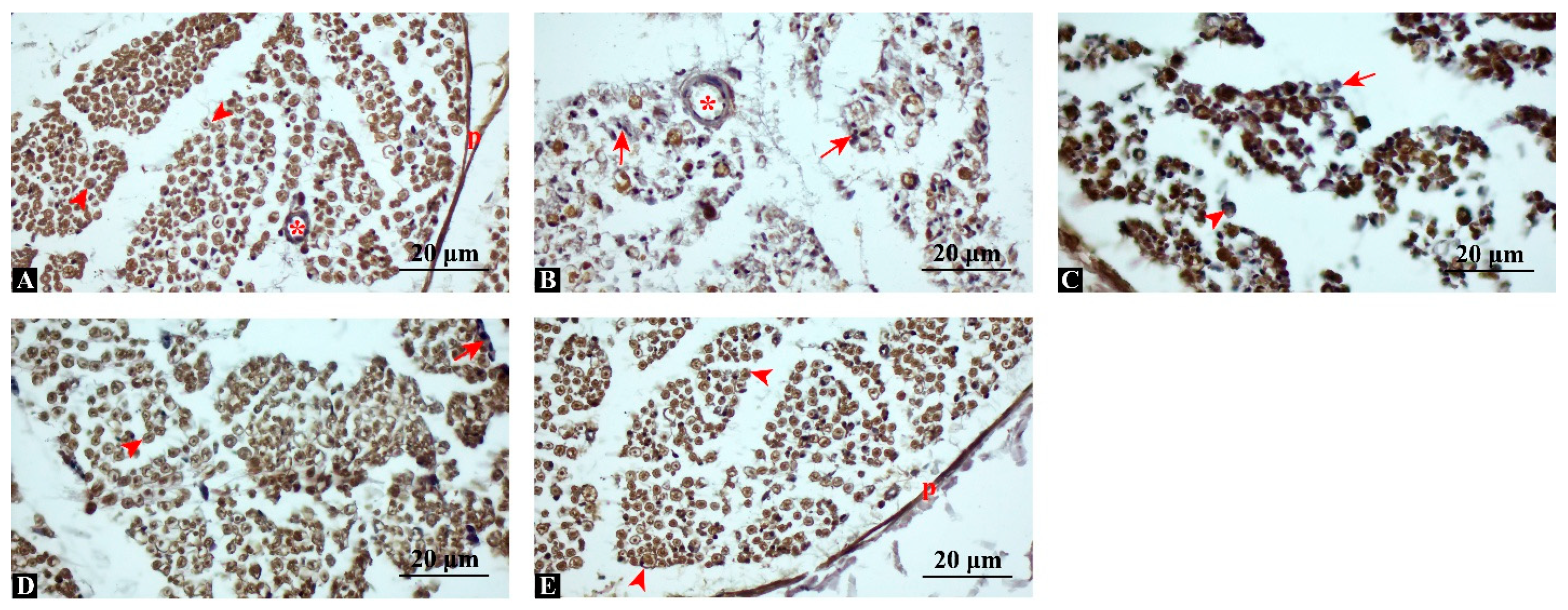
3.4. MBP Expression Is Significantly Enhanced, Indicating Improved Remyelination
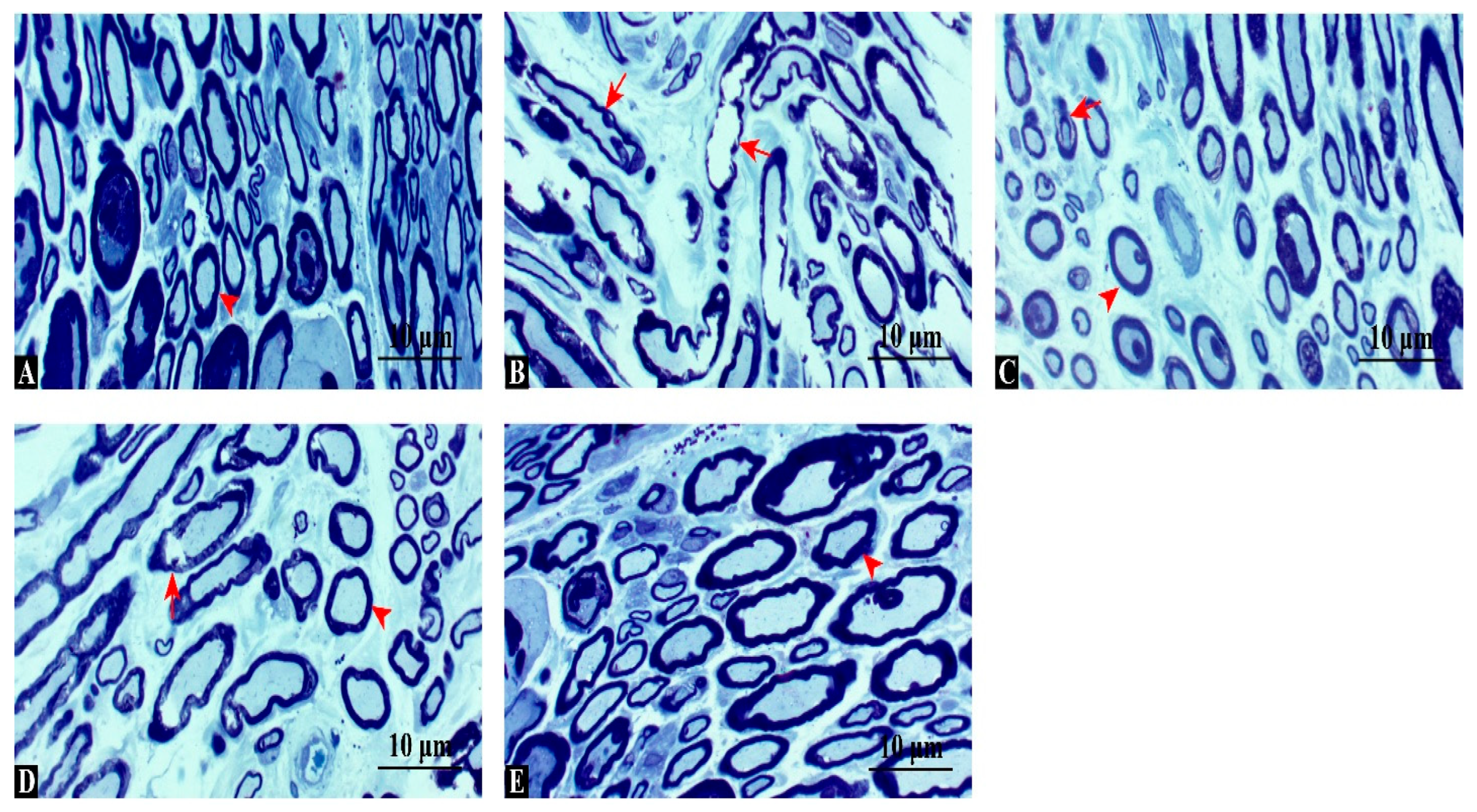
3.5. TEM Confirms Thicker Myelin and Lower G-Ratio Values with Combination Therapy
3.6. Bioinformatic Analysis Links Dexamethasone Targets to NF-κB, HIF-1, and Oxidative Stress Pathways
3.7. Dexamethasone Shows Strong Binding Affinity to NF-κB and CASP3 in Silico
3.8. Dexamethasone Interacts with MBP, Suggesting Structural Myelin Stabilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, J.; Yang, X.; Wang, L.; Zhang, Q.; Ma, W.; Huang, Z.; Bao, Y.; Zhong, L.; Sun, H.; Ding, F. Isoquercitrin promotes peripheral nerve regeneration through inhibiting oxidative stress following sciatic crush injury in mice. Ann. Transl. Med. 2019, 7, 680. [Google Scholar] [CrossRef]
- Murphy, R.N.A.; de Schoulepnikoff, C.; Chen, J.H.C.; Columb, M.O.; Bedford, J.; Wong, J.K.; Reid, A.J. The incidence and management of peripheral nerve injury in England (2005–2020). J. Plast. Reconstr. Aesthet. Surg. 2023, 80, 75–85. [Google Scholar] [CrossRef]
- Magnéli, M.; Axenhus, M. Epidemiology and regional variance of traumatic peripheral nerve injuries in Sweden: A 15-year observational study. PLoS ONE 2024, 19, e0310988. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, M.; Novak, C.B.; Midha, R.; Dengler, J. Epidemiology of peripheral nerve and brachial plexus injuries in a trauma population. Can. J. Surg. 2024, 67, E261–E268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, R.; Xian, L.; Ning, L.; Lu, P.; Liu, Q.; Liu, M. Selection of sciatic nerve injury models: Implications for pathogenesis and treatment. Front. Neurol. 2025, 16, 1521941. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Kuss, M.; Shi, Y.; Fang, F.; Xue, W.; Shi, W.; Liu, Y.; Zhang, C.; Zhong, P.; Duan, B. Exercise facilitates regeneration after severe nerve transection and further modulates neural plasticity. Brain Behav. Immun. Health 2022, 26, 100556. [Google Scholar] [CrossRef] [PubMed]
- Moattari, M.; Moattari, F.; Kaka, G.; Mohseni Kouchesfehani, H.; Sadraie, S.H.; Naghdi, M.; Mansouri, K. Evaluation of dexamethasone treated mesenchymal stem cells for recovery in neurotmesis model of peripheral nerve injury. Neurol. Res. 2018, 40, 1060–1070. [Google Scholar] [CrossRef]
- Lee, J.M.; Byun, S.W.; Kim, S.S.; Park, K.S.; Ryoo, J.-H.; Yeo, S.G. The role of antioxidants in facial nerve injury. Front. Mol. Biosci. 2025, 12, 1663998. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yuan, W. Dexamethasone Enhanced Functional Recovery after Sciatic Nerve Crush Injury in Rats. BioMed Res. Int. 2015, 2015, 627923. [Google Scholar] [CrossRef] [PubMed]
- Işik, Ü.G.; Ensari, N.; Sarikçioğlu, L.; Sonbay Yilmaz, N.D.; Yüksel, Y.; Senirli, R.T.; Yildiz, M.; Selçuk, Ö.T.; Çetinkaya, E.A.; Eyigör, H.; et al. Effects of platelet-rich fibrin and dexamethasone on nerve regeneration after acute facial-nerve injury. Acta Oto-Laryngol. 2023, 143, 623–629. [Google Scholar] [CrossRef]
- Suslu, H.; Altun, M.; Erdivanli, B.; Turan Suslu, H. Comparison of the effects of local and systemic dexamethasone on the rat traumatic sciatic nerve model. Turk. Neurosurg. 2013, 23, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Badr, N.S.; El-Nabi, S.H.; El-Gerbed, M.S.A. Impact of Corn Silk Extract on paclitaxel-induced spinal cord and sciatic nerve injuries in rats: Biochemical and histological evaluation. Neurosci. Lett. 2025, 862, 138282. [Google Scholar] [CrossRef] [PubMed]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Kavlak, E.; Belge, F.; Unsal, C.; Uner, A.G.; Cavlak, U.; Cömlekçi, S. Effects of pulsed electromagnetic field and swimming exercise on rats with experimental sciatic nerve injury. J. Phys. Ther. Sci. 2014, 26, 1355–1361. [Google Scholar] [CrossRef]
- Park, J.S.; Höke, A. Treadmill exercise induced functional recovery after peripheral nerve repair is associated with increased levels of neurotrophic factors. PLoS ONE 2014, 9, e90245. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.D.F.; Gonçalves, N.P.; Gomes, C.P.; Saraiva, M.J.; Pêgo, A.P. BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials 2017, 121, 83–96. [Google Scholar] [CrossRef]
- Seo, T.B.; Han, I.S.; Yoon, J.H.; Hong, K.E.; Yoon, S.J.; Namgung, U. Involvement of Cdc2 in axonal regeneration enhanced by exercise training in rats. Med. Sci. Sports Exerc. 2006, 38, 1267–1276. [Google Scholar] [CrossRef]
- Willand, M.P.; Nguyen, M.-A.; Borschel, G.H.; Gordon, T. Electrical Stimulation to Promote Peripheral Nerve Regeneration. Neurorehabilit. Neural Repair 2016, 30, 490–496. [Google Scholar] [CrossRef]
- Börzsei, D.; Szabó, R.; Hoffmann, A.; Harmath, A.; Sebestyén, J.; Osman, J.; Juhász, B.; Priksz, D.; Varga, C.; Pósa, A. Multiple Applications of Different Exercise Modalities with Rodents. Oxid. Med. Cell. Longev. 2021, 2021, 3898710. [Google Scholar] [CrossRef]
- Yu, S.; Liu, L.; Li, M.; He, S.; Hu, Y.; Sun, S.; Yan, Y.; Zhao, F.; Cheng, X.; Li, J.; et al. Swimming behavior indicates stress and adaptations to exercise. Front. Physiol. 2024, 15, 1357120. [Google Scholar] [CrossRef]
- Liao, C.F.; Yang, T.Y.; Chen, Y.H.; Yao, C.H.; Way, T.D.; Chen, Y.S. Effects of swimming exercise on nerve regeneration in a rat sciatic nerve transection model. Biomedicine 2017, 7, 3. [Google Scholar] [CrossRef]
- Mansouri, N.; Fattahian, H.; Jahandideh, A.; Akbarein, H. The effects of dexamethasone and erythropoietin on mice sciatic nerve crush injury: Histopathologic and functional outcomes. Arch. Vet. Sci. 2023, 28, e0238208. [Google Scholar] [CrossRef]
- Goulart, C.O.; Jürgensen, S.; Souto, A.; Oliveira, J.T.; de Lima, S.; Tonda-Turo, C.; Marques, S.A.; de Almeida, F.M.; Martinez, A.M. A combination of Schwann-cell grafts and aerobic exercise enhances sciatic nerve regeneration. PLoS ONE 2014, 9, e110090. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Behera, S.; Basu, A.; Dey, S.; Ghosh-Roy, A. Swimming Exercise Promotes Post-injury Axon Regeneration and Functional Restoration through AMPK. eNeuro 2021, 8, ENEURO.0414-20.2021. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Türck, D.; Roth, W.; Busch, U. A review of the clinical pharmacokinetics of meloxicam. Br. J. Rheumatol. 1996, 35 (Suppl. S1), 13–16. [Google Scholar] [CrossRef]
- Teodori, R.M.; Betini, J.; de Oliveira, L.S.; Sobral, L.L.; Takeda, S.Y.M.; Montebelo, M.I.d.L. Swimming Exercise in the Acute or Late Phase after Sciatic Nerve Crush Accelerates Nerve Regeneration. Neural Plast. 2011, 2011, 783901. [Google Scholar] [CrossRef] [PubMed]
- Debastiani, J.C.; Santana, A.J.; Ribeiro, L.d.F.C.; Brancalhão, R.M.C.; Bertolini, G.R.F. Sericin silk protein in peripheral nervous repair associated with the physical exercise of swimming in Wistar rats. Neurol. Res. 2019, 41, 326–334. [Google Scholar] [CrossRef]
- Onu, A.; Trofin, D.-M.; Tutu, A.; Onu, I.; Galaction, A.-I.; Sardaru, D.-P.; Trofin, D.; Onita, C.A.; Iordan, D.-A.; Matei, D.-V. Integrative Strategies for Preventing and Managing Metabolic Syndrome: The Impact of Exercise and Diet on Oxidative Stress Reduction—A Review. Life 2025, 15, 757. [Google Scholar] [CrossRef] [PubMed]
- Varejão, A.S.; Meek, M.F.; Ferreira, A.J.; Patrício, J.A.; Cabrita, A.M. Functional evaluation of peripheral nerve regeneration in the rat: Walking track analysis. J. Neurosci. Methods 2001, 108, 1–9. [Google Scholar] [CrossRef]
- Aşır, F.; Özalp, Z.; Yülek, Ö.U.; Erdemci, F.; Korak, T.; Taş, F. CITED1 expression in odontogenic cysts. BMC Oral. Health 2024, 24, 782. [Google Scholar] [CrossRef] [PubMed]
- Ege, S.; Akduman, H.; Aşır, A.; Korak, T. Excessive Weight Gain During Pregnancy Increased Ponoxarase 1 Level in Neonatal Cord Blood. Antioxidants 2025, 14, 105. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2019, 36, 2628–2629. [Google Scholar] [CrossRef]
- Korak, T.; Bal Albayrak, M.G.; Kasap, M.; Akpinar, G. Thymoquinone and Metabolic Reprogramming in Breast Cancer: A New Dimension From Proteomic Analysis. J. Biochem. Mol. Toxicol. 2025, 39, e70124. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Rolta, R.; Mehta, B.B.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Role of Dexamethasone and Methylprednisolone Corticosteroids in Coronavirus Disease 2019 Hospitalized Patients: A Review. Front. Microbiol. 2022, 13, 813358. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Azad-Tirgan, M.; Amini, K. Dexamethasone topically accelerates peripheral nerve repair and target organ reinnervation: A transected sciatic nerve model in rat. Injury 2013, 44, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Ghayour, M.B.; Abdolmaleki, A.; Behnamrasouli, M.; Moghimi, A.; Mahdavishahri, N. Effects of Dexamethasone on Regeneration Process after Sciatic Nerve Transect and Acellular Scaffold Graft in a Rat Animal Model. J. Ilam Univ. Med. Sci. 2018, 26, 104–115. [Google Scholar] [CrossRef]
- Cefis, M.; Chaney, R.; Wirtz, J.; Méloux, A.; Quirié, A.; Leger, C.; Prigent-Tessier, A.; Garnier, P. Molecular mechanisms underlying physical exercise-induced brain BDNF overproduction. Front. Mol. Neurosci. 2023, 16, 1275924. [Google Scholar] [CrossRef]
- de Sousa Fernandes, M.S.; Ordônio, T.F.; Santos, G.C.J.; Santos, L.E.R.; Calazans, C.T.; Gomes, D.A.; Santos, T.M. Effects of Physical Exercise on Neuroplasticity and Brain Function: A Systematic Review in Human and Animal Studies. Neural Plast. 2020, 2020, 8856621. [Google Scholar] [CrossRef]
- Soltani, A.; Chugaeva, U.Y.; Ramadan, M.F.; Saleh, E.A.M.; Al-Hasnawi, S.S.; Romero-Parra, R.M.; Alsaalamy, A.; Mustafa, Y.F.; Zamanian, M.Y.; Golmohammadi, M. A narrative review of the effects of dexamethasone on traumatic brain injury in clinical and animal studies: Focusing on inflammation. Inflammopharmacology 2023, 31, 2955–2971. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- López-Álvarez, V.M.; Modol, L.; Navarro, X.; Cobianchi, S. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain 2015, 156, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.R.; Pansani, A.; Machado, F.; Andrade, M.; Silva, A.C.; Scorza, F.A.; Cavalheiro, E.A.; Arida, R.M. Acute strength exercise and the involvement of small or large muscle mass on plasma brain-derived neurotrophic factor levels. Clinics 2010, 65, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.S.; Donoghue, K.M.; Ghosh, U.; Nelson, C.M.; Roth, T.L. Early Life Stress Affects Bdnf Regulation: A Role for Exercise Interventions. Int. J. Mol. Sci. 2022, 23, 11729. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Wang, Y.; Zhang, Z.; Zhang, H.; Wang, Z.; Tao, Z.; Wang, J.; Zhang, P. Dexamethasone improves thymoma-associated myasthenia gravis via the AKT-mTOR pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 817–828. [Google Scholar] [CrossRef]
- Zhang, Z.; Ran, Y.; Xu, L.; Pan, Z.; Xie, Y. High-dose dexamethasone injection disordered metabolism and multiple protein kinases expression in the mouse kidney. Biosci. Rep. 2021, 41, BSR20211847. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, Q.; Gao, J.; Li, C.; Hou, P.; Xu, J.; Cao, K.; Hu, M.; Cheng, L.; Wang, X.; et al. Novel mechanisms underlying inhibition of inflammation-induced angiogenesis by dexamethasone and gentamicin via PI3K/AKT/NF-κB/VEGF pathways in acute radiation proctitis. Sci. Rep. 2022, 12, 14116. [Google Scholar] [CrossRef] [PubMed]
- Kurkinen, S.T.; Lätti, S.; Pentikäinen, O.T.; Postila, P.A. Getting Docking into Shape Using Negative Image-Based Rescoring. J. Chem. Inf. Model. 2019, 59, 3584–3599. [Google Scholar] [CrossRef] [PubMed]
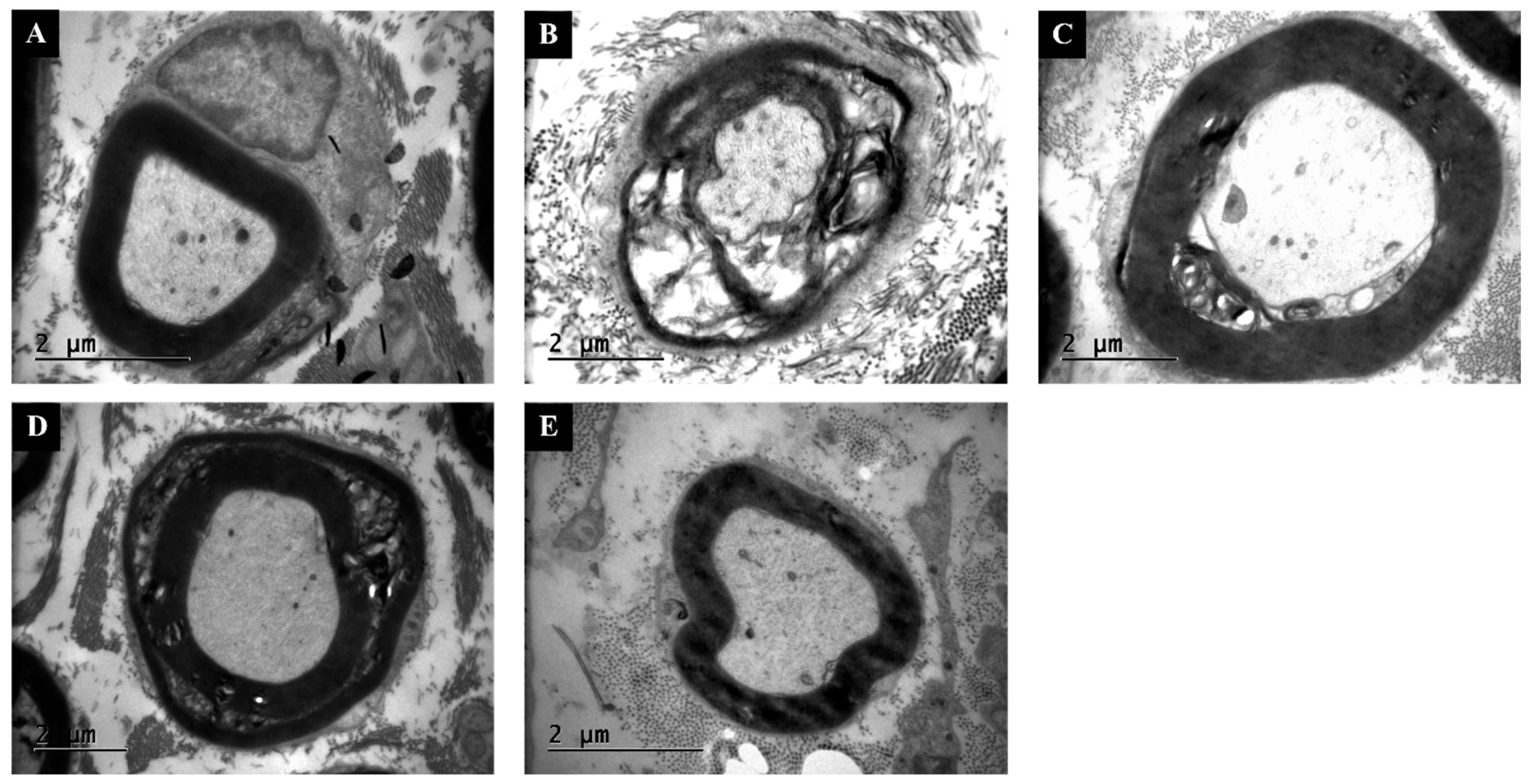
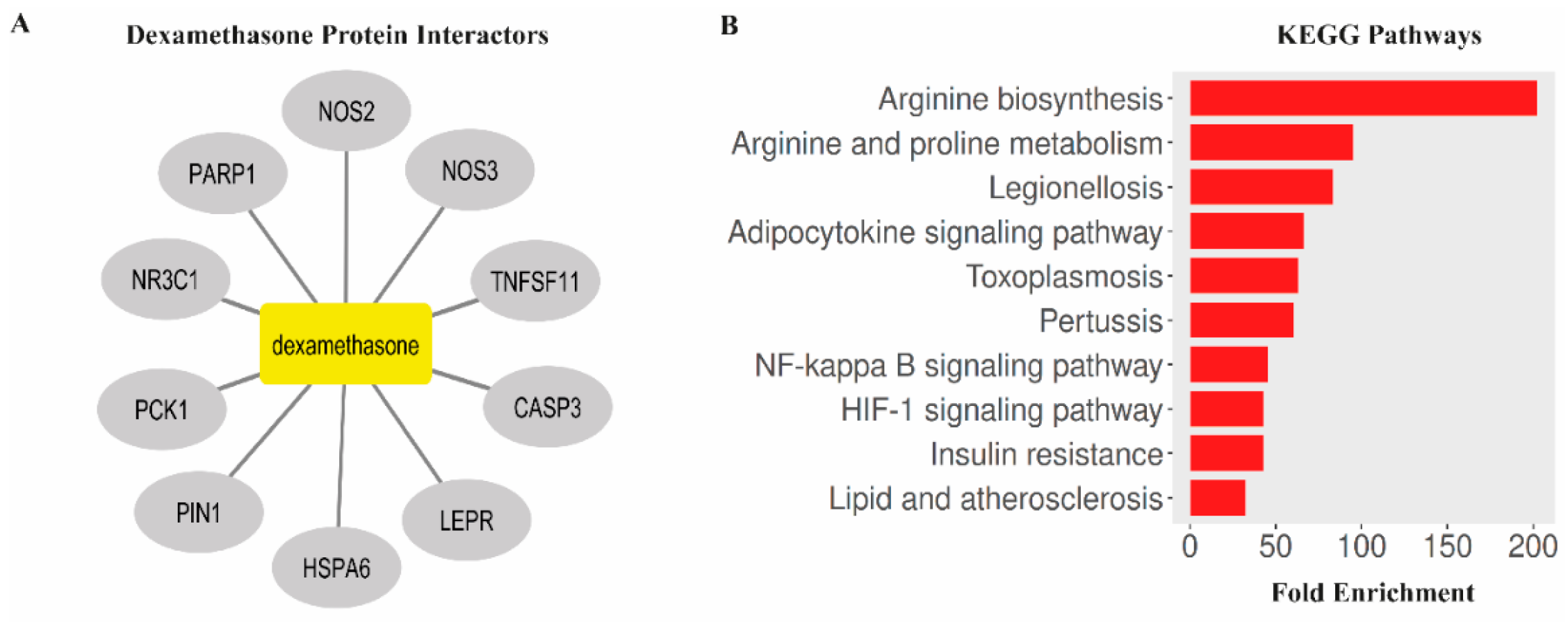
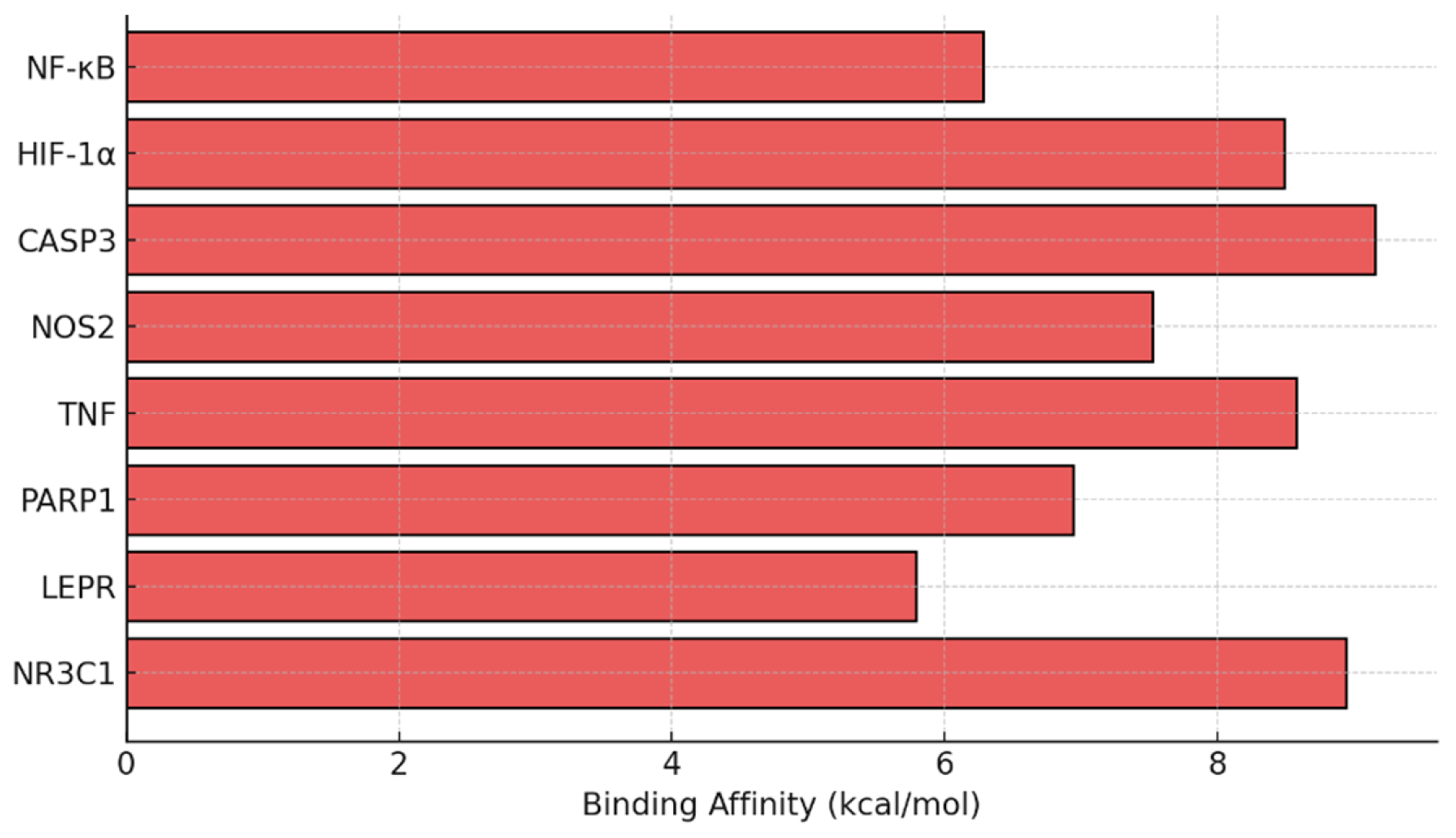
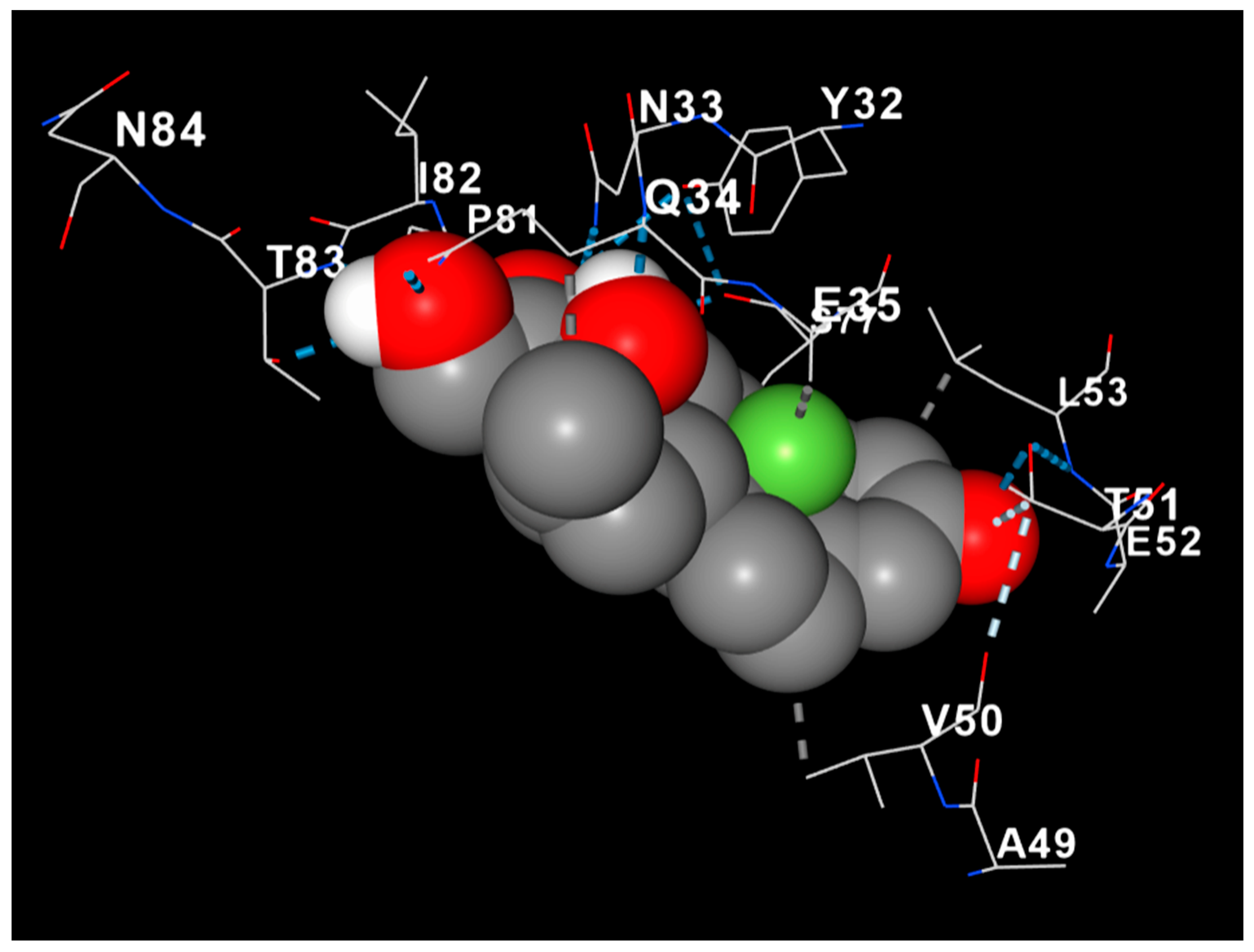
| Group (n:8) | MDA | GPx | MPO | p-Value (vs. Injury) |
|---|---|---|---|---|
| Control | 0.19 ± 0.05 | 115.16 ± 12.48 | 8.41 ± 1.63 | p < 0.001 |
| Injury | 2.13 ± 0.05 | 68.1 ± 6.19 | 25.84 ± 2.47 | - |
| Injury + Swimming | 0.98 ± 0.10 | 94.12 ± 3.72 | 14.37 ± 2.33 | p < 0.001 |
| Injury + Dexamethasone | 0.67 ± 0.04 | 81.87 ± 3.99 | 18.99 ± 1.5 | p < 0.001 |
| Combination | 0.37 ± 0.05 | 101.91 ± 4.31 | 10.41 ± 1.97 | p < 0.001 |
| Day | Control | Injury | Swimming | Dexamethasone | Combination |
|---|---|---|---|---|---|
| 1 | −5.58 ± 0.52 | −86.16 ± 1.66 | −85.39 ± 1.35 | −85.69 ± 1.11 | −85.62 ± 0.96 |
| 7 | −5.50 ± 0.35 | −74.93 ± 2.41 | −67.85 ± 0.82 | −64.65 ± 2.05 | −54.96 ± 2.72 |
| 14 | −5.50 ± 0.57 | −66.31 ± 2.98 | −56.07 ± 3.16 | −46.65 ± 1.24 | −30.53 ± 1.58 |
| 21 | −5.86 ± 0.39 | −57.08 ± 2.97 | −41.23 ± 1.73 | −34.11 ± 1.21 | −14.05 ± 1.98 |
| 28 | −5.74 ± 0.39 | −45.85 ± 2.48 | −27.36 ± 0.99 | −20.75 ± 1.23 | −7.25 ± 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakoç, M.; Ayaz, H.; Çelik, F.; Aşır, F. Evaluation of Dexamethasone and Swimming Exercise as Complementary Interventions in a Rat Sciatic Nerve Injury Model. Antioxidants 2025, 14, 1382. https://doi.org/10.3390/antiox14111382
Karakoç M, Ayaz H, Çelik F, Aşır F. Evaluation of Dexamethasone and Swimming Exercise as Complementary Interventions in a Rat Sciatic Nerve Injury Model. Antioxidants. 2025; 14(11):1382. https://doi.org/10.3390/antiox14111382
Chicago/Turabian StyleKarakoç, Meral, Hayat Ayaz, Ferhat Çelik, and Fırat Aşır. 2025. "Evaluation of Dexamethasone and Swimming Exercise as Complementary Interventions in a Rat Sciatic Nerve Injury Model" Antioxidants 14, no. 11: 1382. https://doi.org/10.3390/antiox14111382
APA StyleKarakoç, M., Ayaz, H., Çelik, F., & Aşır, F. (2025). Evaluation of Dexamethasone and Swimming Exercise as Complementary Interventions in a Rat Sciatic Nerve Injury Model. Antioxidants, 14(11), 1382. https://doi.org/10.3390/antiox14111382








