Effect of Long-Term Storage Temperature on the Quality of Extra-Virgin Olive Oil (Coratina cv.): A Multivariate Discriminant Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extra-Virgin Olive Oil Sample Collection
2.3. Storage Conditions of EVOO Samples
2.4. Qualitative Indices Determination
2.5. Extraction and HPLC-DAD Determination of Phenolic Compounds
2.6. Kinetic Data Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Evolution of Trade Quality Parameters of Coratina cv. EVOO During Storage
3.2. Behavior of Phenolic Compounds of Coratina cv. EVOO During Storage
3.3. Kinetics of Quality and Phenolic Indicators of Coratina cv. EVOO During Storage
3.4. LDA Discriminant Approach to Distinguish EVOO Samples Stored at Different Temperatures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zupo, R.; Castellana, F.; Crupi, P.; Desantis, A.; Rondanelli, M.; Corbo, F.; Clodoveo, M.L. Olive Oil Polyphenols Improve HDL Cholesterol and Promote Maintenance of Lipid Metabolism: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Metabolites 2023, 13, 1187. [Google Scholar] [CrossRef]
- De Santis, S.; Liso, M.; Verna, G.; Curci, F.; Milani, G.; Faienza, M.F.; Franchini, C.; Moschetta, A.; Chieppa, M.; Clodoveo, M.L.; et al. Extra Virgin Olive Oil Extracts Modulate the Inflammatory Ability of Murine Dendritic Cells Based on Their Polyphenols Pattern: Correlation between Chemical Composition and Biological Function. Antioxidants 2021, 10, 1016. [Google Scholar] [CrossRef]
- De Santis, S.; Crupi, P.; Piacente, L.; Mestice, A.; Colabufo, N.A.; Amodio, L.; Pontrelli, P.; Gesualdo, L.; Moschetta, A.; Clodoveo, M.L.; et al. Extra virgin olive oil extract rich in secoiridoids induces an anti-inflammatory profile in PBMCs from obese children. Front. Nutr. 2022, 9, 1017090. [Google Scholar] [CrossRef]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; García-González, D.L.; Moreda, W.; et al. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trends Food Sci. Technol. 2020, 105, 483–493. [Google Scholar] [CrossRef]
- Di Stefano, V.; Melilli, M.G. Effect of Storage on Quality Parameters and Phenolic Content of Italian Extra-Virgin Olive Oils. Nat. Prod. Res. 2020, 34, 78–86. [Google Scholar] [CrossRef]
- Calligaris, S.; Lucci, P.; Milani, A.; Rovellini, P.; Lagazio, C.; Conte, L.; Nicoli, M.C. Application of Accelerated Shelf-Life Test (ASLT) Procedure for the Estimation of the Shelf-Life of Extra Virgin Olive Oils: A Validation Study. Food Packag. Shelf Life 2022, 34, 100990. [Google Scholar] [CrossRef]
- Lobo-Prieto, A.; Tena, N.; Aparicio-Ruiz, R.; García-González, D.L.; Sikorska, E. Monitoring Virgin Olive Oil Shelf-Life by Fluorescence Spectroscopy and Sensory Characteristics: A Multidimensional Study Carried Out under Simulated Market Conditions. Foods 2020, 9, 1846. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.; Milani, A.; Calligaris, S.; Rovellini, P.; Lucci, P.; Nicoli, M.C. Temperature Dependence of Oxidation Kinetics of Extra Virgin Olive Oil (EVOO) and Shelf-Life Prediction. Foods 2020, 9, 295. [Google Scholar] [CrossRef]
- Redondo-Cuevas, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the Relationship between Vegetable Oil Composition and Oxidative Stability: A Multifactorial Approach. J. Food Compost. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef]
- Shendi, G.E.; Sivri Ozay, D.; Ozkaya, M.T.; Ustunel, N.F. Changes Occurring in Chemical Composition and Oxidative Stability of Virgin Olive Oil during Storage. OCL 2018, 25, A602. [Google Scholar] [CrossRef]
- Caipo, L.; Sandoval, A.; Sepúlveda, B.; Fuentes, E.; Valenzuela, R.; Metherel, A.H.; Romero, N. Effect of Storage Conditions on the Quality of Arbequina Extra Virgin Olive Oil and the Impact on the Composition of Flavor-Related Compounds (Phenols and Volatiles). Foods 2021, 10, 2161. [Google Scholar] [CrossRef]
- Fregapane, G.; Salvador, M.D. Oxidative Stability and the Role of Minor and Functional Components of Olive Oil. In Olives and Olive Oil as Functional Foods; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 249–265. ISBN 9781119135340. [Google Scholar]
- Bosque-Sendra, J.M.; de la Mata-Espinosa, P.; Cuadros-Rodríguez, L.; González-Casado, A.; Rodríguez-García, F.P.; García-Toledo, H. Stability for Olive Oil Control Materials. Food Chem. 2011, 125, 1418–1422. [Google Scholar] [CrossRef]
- Mulinacci, N.; Ieri, F.; Ignesti, G.; Romani, A.; Michelozzi, M.; Creti, D.; Innocenti, M.; Calamai, L. The Freezing Process Helps to Preserve the Quality of Extra Virgin Olive Oil over Time: A Case Study up to 18months. Food Res. Int. 2013, 54, 2008–2015. [Google Scholar] [CrossRef]
- Romani, A.; Lapucci, C.; Cantini, C.; Ieri, F.; Mulinacci, N.; Visioli, F. Evolution of minor polar compounds and antioxidant capacity during storage of bottled extra virgin olive oil. J. Agric. Food Chem. 2007, 55, 1315–1320. [Google Scholar] [CrossRef]
- Mousavi, S.; Mariotti, R.; Stanzione, V.; Pandolfi, S.; Mastio, V.; Baldoni, L.; Cultrera, N.G.M. Evolution of Extra Virgin Olive Oil Quality under Different Storage Conditions. Foods 2021, 10, 1945. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Viggiani, I.; del Nobile, M.A. Changes Produced in Extra-Virgin Olive Oils from Cv. Coratina during a Prolonged Storage Treatment. Czech J. Food Sci. 2014, 32, 1–9. [Google Scholar] [CrossRef]
- Irigaray, B.A.; Martínez, N.I.; Feller, C.; Amarillo, M.; Grompone, M.A. Shelf life of monovarietal extra virgin olive oils cv. Arbequina and Coratina from Uruguay. J. Food Res. 2016, 5, 88–94. [Google Scholar] [CrossRef]
- Macciola, V.; De Leonardis, A. Exceptional long-term durability of Coratina monovarietal extra virgin olive oil evaluated through chemical parameters and oxidative stability test. OCL 2022, 29, 24. [Google Scholar] [CrossRef]
- Martin-Torres, S.; Ruiz-Castro, L.; Jimenez-Carvelo, A.M.; Cuadros-Rodriguez, L. Applications of Multivariate Data Analysis in Shelf Life Studies of Edible Vegetal Oils—A Review of the Few Past Years. Food Packag. Shelf Life 2022, 31, 100790. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Dipalmo, T.; Crupi, P.; Durante, V.; Pesce, V.; Maiellaro, I.; Lovece, A.; Mercurio, A.; Laghezza, A.; Corbo, F.; et al. Comparison between different flavored olive oil production techniques: Healthy value and process efficiency. Plant Foods Hum. Nutr. 2016, 71, 81–87. [Google Scholar] [CrossRef]
- Esposto, S.; Selvaggini, R.; Taticchi, A.; Veneziani, G.; Sordini, B.; Servili, M. Quality Evolution of Extra-Virgin Olive Oils according to Their Chemical Composition during 22 Months of Storage under Dark Conditions. Food Chem. 2020, 311, 126044. [Google Scholar] [CrossRef]
- Morales, M.T.; Przybylski, R. Olive Oil Oxidation. In Handbook of Olive Oil: Analysis and Properties, 1st ed.; Harwood, J., Aparicio, R., Eds.; Springer: New York, NY, USA, 2010; ISBN 9781441951946. [Google Scholar]
- Díez-Betriu, A.; Romero, A.; Ninot, A.; Tres, A.; Vichi, S.; Guardiola, F. Effect of Freezing, Fast-Freezing by Liquid Nitrogen or Refrigeration to Preserve Premium Extra Virgin Olive Oil during Storage. Eur. Food Res. Technol. 2022, 248, 2651–2663. [Google Scholar] [CrossRef]
- Allouche, Y.; Jiménez, A.; Gaforio, J.J.; Uceda, M.; Beltrán, G. How Heating Affects Extra Virgin Olive Oil Quality Indexes and Chemical Composition. J. Agric. Food Chem. 2007, 55, 9646–9654. [Google Scholar] [CrossRef]
- Pastrana, M.L. Análisis de la Calidad del Aceite de Oliva Virgen: Relación Entre la Estabilidad Oxidativa y la Composición Fenólica. Master’s Thesis, Degree in Agricultural Engineering, Agricultural Operations, U. of Sevilla and Instituto de la Grasa, CSIC, Seville, Spain, 2016. [Google Scholar]
- Kalogeropoulos, N.; Tsimidou, M.Z. Antioxidants in Greek Virgin Olive Oils. Antioxidants 2014, 3, 387–413. [Google Scholar] [CrossRef]
- Clodoveo, M.; Delcuratolo, D.; Gomes, T.; Colelli, G. Effect of Different Temperatures and Storage Atmospheres on Coratina Olive Oil Quality. Food Chem. 2007, 102, 571–576. [Google Scholar] [CrossRef]
- Molinu, M.G.; Deiana, P.; Dettori, S.; Mercenaro, L.; Nieddu, G.; Dore, A.; Culeddu, N.; Santona, M. Looking for Typical Traits in Monovarietal VOOs According to Their Phenolic Composition. Foods 2024, 13, 3425. [Google Scholar] [CrossRef]
- Fuentes, E.; Paucar, F.; Tapia, F.; Ortiz, J.; Jimenez, P.; Romero, N. Effect of the composition of extra virgin olive oils on the differentiation and antioxidant capacities of twelve monovarietals. Food Chem. 2018, 243, 285–294. [Google Scholar] [CrossRef]
- Abbattista, R.; Losito, I.; Castellaneta, A.; De Ceglie, C.; Calvano, C.D.; Cataldi, T.R.I. Insight into the Storage-Related Oxidative/Hydrolytic Degradation of Olive Oil Secoiridoids by Liquid Chromatography and High-Resolution Fourier Transform Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 12310–12325. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Modelling Virgin Olive Oil Potential Shelf-Life from Antioxidants and Lipid Oxidation Progress. Antioxidants 2022, 11, 539. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: A Critical Review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Lavelli, V.; Fregapane, G.; Salvador, M.D. Effect of storage on secoiridoid and tocopherol contents and antioxidant activity of monovarietal extra virgin olive oils. J. Agric. Food Chem. 2006, 54, 3002–3007. [Google Scholar] [CrossRef]
- Krichene, D.; Salvador, M.D.; Fregapane, G. Stability of Virgin Olive Oil Phenolic Compounds during Long-Term Storage (18 Months) at Temperatures of 5–50 °C. J. Agric. Food Chem. 2015, 63, 6779–6786. [Google Scholar] [CrossRef]


| Seasons | 2020 | 2021 | 2022 | ||
|---|---|---|---|---|---|
| Samples/ Parameters | 3COL, CICO, P18, C33, D02, D03 | 6-C, 11-C, 12-C, 13-C, 14-C, 16-C | 1C/22, 2C/22, 3C/22, 4C/22, 5C/22, 7C/22, 8C/22, 10C/22, 16C/22 | ||
| FA | m ± σ | m ± σ | m ± σ | ||
| RT | t0 | 0.22 ± 0.02 | 0.18 ± 0.03 | 0.26 ± 0.05 | |
| t1 | 0.218 ± 0.018 | 0.18 ± 0.03 | 0.27 ± 0.05 | ||
| t2 | 0.222 ± 0.019 | 0.18 ± 0.03 | 0.27 ± 0.05 | ||
| t3 | 0.23 ± 0.02 | 0.17 ± 0.03 | 0.28 ± 0.06 | ||
| LT | t0 | 0.22 ± 0.02 | 0.18 ± 0.03 | 0.26 ± 0.05 | |
| t1 | 0.22 ± 0.02 | 0.18 ± 0.03 | 0.27 ± 0.05 | ||
| t2 | 0.22 ± 0.02 | 0.18 ± 0.03 | 0.27 ± 0.05 | ||
| t3 | 0.22 ± 0.02 | 0.18 ± 0.03 | 0.27 ± 0.06 | ||
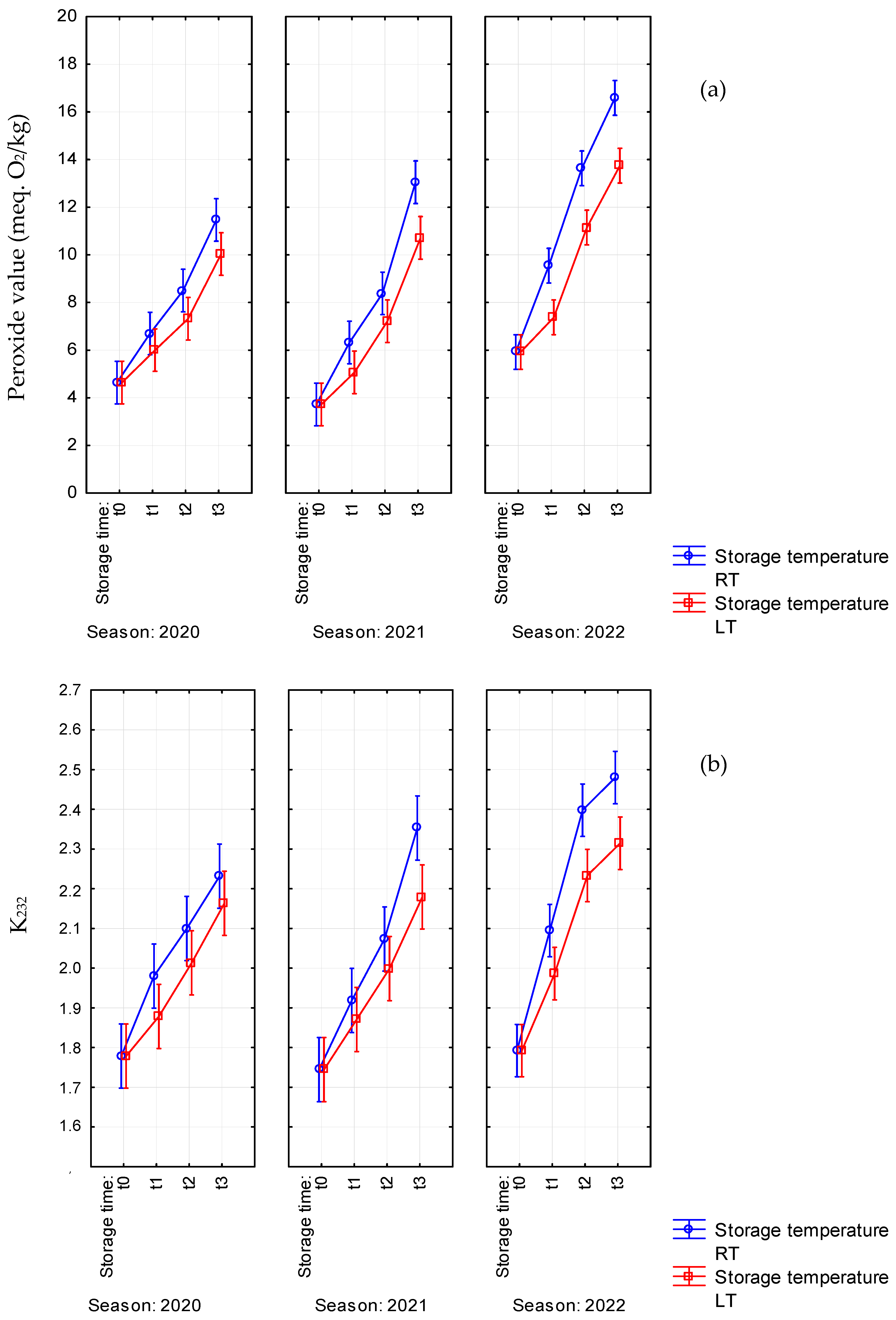
| PV | RT | t0 | 4.6 ± 0.8 e | 3.7 ± 0.7 e | 5.9 ± 1.0 e |
| t1 | 6.7 ± 1.1 cde | 6.3 ± 0.5 cd | 9.5 ± 0.8 c | ||
| t2 | 8.5 ± 1.3 bc | 8.4 ± 0.6 c | 13.6 ± 1.6 b | ||
| t3 | 11.5 ± 1.7 a | 13.1 ± 0.5 a | 16.6 ± 1.1 a | ||
| LT | t0 | 4.6 ± 0.8 e | 3.7 ± 0.7 e | 5.9 ± 1.0 e | |
| t1 | 6.0 ± 1.3 de | 5.1 ± 0.3 de | 7.4 ± 1.0 de | ||
| t2 | 7.3 ± 1.4 cd | 7.2 ± 0.7 cd | 11.1 ± 1.0 c | ||
| t3 | 10 ± 2 ab | 10.7 ± 1.4 b | 13.7 ± 0.8 b | ||
| K232 | RT | t0 | 1.78 ± 0.12 d | 1.74 ± 0.11 d | 1.79 ± 0.09 e |
| t1 | 1.98 ± 0.17 bcd | 1.92 ± 0.06 cd | 2.09 ± 0.06 cd | ||
| t2 | 2.10 ± 0.17 ab | 2.07 ± 0.05 bc | 2.40 ± 0.08 ab | ||
| t3 | 2.23 ± 0.18 a | 2.35 ± 0.12 a | 2.48 ± 0.07 a | ||
| LT | t0 | 1.78 ± 0.12 d | 1.74 ± 0.11d | 1.79 ± 0.09 e | |
| t1 | 1.88 ± 0.10 cd | 1.87 ± 0.09 cd | 1.99 ± 0.05 d | ||
| t2 | 2.01 ± 0.12 bc | 2.00 ± 0.05 bc | 2.23 ± 0.05bc | ||
| t3 | 2.16 ± 0.16 ab | 2.18 ± 0.05 ab | 2.31 ± 0.03 ab | ||
| K270 | RT | t0 | 0.134 ± 0.017 | 0.153 ± 0.008 | 0.143 ± 0.010 |
| t1 | 0.16 ± 0.03 | 0.163 ± 0.009 | 0.175 ± 0.008 | ||
| t2 | 0.17 ± 0.03 | 0.180 ± 0.011 | 0.211 ± 0.011 | ||
| t3 | 0.19 ± 0.03 | 0.21 ± 0.03 | 0.222 ± 0.008 | ||
| LT | t0 | 0.134 ± 0.017 | 0.153 ± 0.008 | 0.143 ± 0.010 | |
| t1 | 0.15 ± 0.02 | 0.158 ± 0.007 | 0.159 ± 0.007 | ||
| t2 | 0.17 ± 0.02 | 0.169 ± 0.008 | 0.192 ± 0.016 | ||
| t3 | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.214 ± 0.010 | ||
| ΔK | RT | t0 | −0.001 ± 0.003 | −0.002 ± 0.001 | −0.002 ± 0.002 |
| t1 | 0.001 ± 0.001 | 0.000 ± 0.001 | 0.000 ± 0.001 | ||
| t2 | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.001 ± 0.001 | ||
| t3 | 0.002 ± 0.000 | 0.002 ± 0.001 | 0.001 ± 0.001 | ||
| LT | t0 | −0.001 ± 0.003 | −0.002 ± 0.001 | −0.002 ± 0.002 | |
| t1 | 0.001 ± 0.002 | −0.001 ± 0.001 | −0.001 ± 0.002 | ||
| t2 | 0.001 ± 0.001 | −0.002 ± 0.004 | 0.000 ± 0.001 | ||
| t3 | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.001 ± 0.001 |
| Season | 2020 | 2021 | 2022 | ||
|---|---|---|---|---|---|
| Samples/ Compounds | 3COL, CICO, P18, C33, D02, D03 | 6-C, 11-C, 12-C, 13-C, 14-C, 16-C | 1C/22, 2C/22, 3C/22, 4C/22, 5C/22, 7C/22, 8C/22, 10C/22, 16C/22 | ||
| TPP | RT | t0 | 860 ± 140 a | 720 ± 80 a | 530 ± 70 a |
| t1 | 580 ± 30 bc | 550 ± 60 b | 410 ± 70 abc | ||
| t2 | 390 ± 70 d | 500 ± 70 b | 360 ± 60 bc | ||
| t3 | 270 ± 30 d | 430 ± 80 b | 300 ± 40 c | ||
| LT | t0 | 860 ± 140 a | 720 ± 80 a | 530 ± 70 a | |
| t1 | 730 ± 50 ab | 580 ± 70 b | 460 ± 80 ab | ||
| t2 | 620 ± 80 bc | 540 ± 70 b | 430 ± 80 abc | ||
| t3 | 470 ± 50 c | 480 ± 80 b | 380 ± 60 bc | ||
| 3,4-DHPEA | RT | t0 | 4 ± 2 c | 2.0 ± 0.9 c | 5 ± 2 |
| t1 | 13 ± 3 bc | 11 ± 10 bc | 10 ± 5 | ||
| t2 | 22 ± 4 ab | 21 ± 16 ab | 12 ± 6 | ||
| t3 | 32 ± 5 a | 26 ± 14 a | 14 ± 5 | ||
| LT | t0 | 4 ± 2 c | 2.0 ± 0.9 c | 5 ± 2 | |
| t1 | 9 ± 3 bc | 9 ± 7 bc | 7 ± 4 | ||
| t2 | 12 ± 4 bc | 14 ± 9 abc | 11 ± 5 | ||
| t3 | 16 ± 3 bc | 18 ± 9 ab | 13 ± 5 | ||
| p-HPEA | RT | t0 | 5 ± 2 c | 2.7 ± 1.8 c | 7 ± 3 |
| t1 | 12.6 ± 1.8 bc | 7 ± 5 bc | 8 ± 3 | ||
| t2 | 16 ± 3 ab | 18 ± 14 a | 10 ± 6 | ||
| t3 | 26 ± 4 a | 18 ± 10 a | 12 ± 4 | ||
| LT | t0 | 5 ± 2 c | 2.7 ± 1.8 c | 7 ± 3 | |
| t1 | 9 ± 4 bc | 6 ± 3 bc | 8 ± 2 | ||
| t2 | 10 ± 4 bc | 13 ± 9 abc | 9 ± 2 | ||
| t3 | 13 ± 3 bc | 15 ± 6 ab | 10 ± 3 | ||
| 3,4-DHPEA-EDA | RT | t0 | 180 ± 70 | 150 ± 70 | 130 ± 40 |
| t1 | 100 ± 40 | 90 ± 60 | 90 ± 20 | ||
| t2 | 60 ± 30 | 70 ± 40 | 80 ± 30 | ||
| t3 | 39 ± 19 | 60 ± 40 | 60 ± 20 | ||
| LT | t0 | 180 ± 70 | 150 ± 70 | 130 ± 40 | |
| t1 | 170 ± 60 | 100 ± 60 | 110 ± 30 | ||
| t2 | 110 ± 50 | 90 ± 50 | 110 ± 30 | ||
| t3 | 90 ± 40 | 70 ± 40 | 80 ± 20 | ||
| p-HPEA-EDA | RT | t0 | 180 ± 40 | 120 ± 60 | 150 ± 20 |
| t1 | 110 ± 40 | 80 ± 30 | 100 ± 17 | ||
| t2 | 58 ± 14 | 60 ± 30 | 90 ± 20 | ||
| t3 | 40 ± 11 | 50 ± 30 | 67 ± 15 | ||
| LT | t0 | 180 ± 40 | 120 ± 60 | 150 ± 20 | |
| t1 | 140 ± 30 | 90 ± 40 | 130 ± 20 | ||
| t2 | 100 ± 40 | 70 ± 40 | 118 ± 18 | ||
| t3 | 90 ± 30 | 70 ± 40 | 89 ± 13 | ||
| Lignans | RT | t0 | 110 ± 30 | 100 ± 40 | 70 ± 30 |
| t1 | 83 ± 19 | 60 ± 18 | 40 ± 20 | ||
| t2 | 52 ± 10 | 42 ± 13 | 33 ± 14 | ||
| t3 | 39 ± 10 | 36 ± 10 | 28 ± 10 | ||
| LT | t0 | 110 ± 30 | 100 ± 40 | 70 ± 30 | |
| t1 | 100 ± 30 | 70 ± 20 | 50 ± 30 | ||
| t2 | 70 ± 40 | 60 ± 20 | 50 ± 20 | ||
| t3 | 60 ± 40 | 50 ± 20 | 38 ± 18 | ||
| 3,4-DHPEA-EA | RT | t0 | 110 ± 50 | 83 ± 19 | 74 ± 12 |
| t1 | 68 ± 16 | 140 ± 60 | 70 ± 30 | ||
| t2 | 48 ± 14 | 170 ± 70 | 70 ± 30 | ||
| t3 | 30 ± 17 | 160 ± 60 | 70 ± 20 | ||
| LT | t0 | 110 ± 50 | 83 ± 19 | 74 ± 12 | |
| t1 | 110 ± 40 | 140 ± 70 | 70 ± 30 | ||
| t2 | 110 ± 30 | 170 ± 70 | 80 ± 30 | ||
| t3 | 90 ± 20 | 150 ± 50 | 80 ± 30 | ||
| p-HPEA-EA | RT | t0 | 38 ± 15 | 33 ± 14 | 27 ± 5 |
| t1 | 35 ± 14 | 40 ± 30 | 36 ± 10 | ||
| t2 | 27 ± 20 | 70 ± 50 | 40 ± 12 | ||
| t3 | 14 ± 3 | 70 ± 40 | 45 ± 11 | ||
| LT | t0 | 38 ± 15 | 33 ± 14 | 27 ± 5 | |
| t1 | 35 ± 13 | 40 ± 30 | 36 ± 13 | ||
| t2 | 42 ± 20 | 80 ± 30 | 43 ± 16 | ||
| t3 | 26 ± 12 | 80 ± 30 | 52 ± 14 |
| EVOOs | Year | T | k0(PV) (meqO2kg−1day−1) | k0(K232) (D.O. day−1) | k0(K270) (D.O. day−1) | k2(TPP) (kg mg−1 day−1) | k0(3,4 DHPEA) (mg kg−1 day−1) | k0(p-HPEA) (mg kg−1 day−1) | k2(3,4 DHPEA-EDA) (kg mg−1 day−1) | k2(p-HPEA-EDA) (kg mg−1 day−1) | k2(lignans) (kg mg−1 day−1) | k2(3,4 DHPEA-EA) (kg mg−1 day−1) | k2(p-HPEA-EA) (kg mg−1 day−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3COL | 2020 | RT | 0.0102 | 7.1 × 10−4 | 1.0 × 10−4 | 4.8 × 10−6 | 0.0577 | 0.0438 | 4.0 × 10−5 | 5.0 × 10−5 | 1.8 × 10−5 | −3.1 × 10−3 | −2.1 × 10−3 |
| CICO | 2020 | RT | 0.0098 | 5.7 × 10−4 | 9.2 × 10−5 | 4.5 × 10−6 | 0.0344 | 0.0213 | 6.4 × 10−5 | 3.7 × 10−5 | 2.5 × 10−5 | −1.7 × 10−3 | −2.4 × 10−3 |
| P18 | 2020 | RT | 0.0132 | 7.2 × 10−4 | 1.1 × 10−4 | 4.4 × 10−6 | 0.0512 | 0.0402 | 2.0 × 10−5 | 2.5 × 10−5 | 4.8 × 10−5 | −2.4 × 10−3 | −1.9 × 10−3 |
| C33 | 2020 | RT | 0.0149 | 11 × 10−4 | 8.2 × 10−5 | 3.9 × 10−6 | 0.0537 | 0.0379 | 2.3 × 10−5 | 3.0 × 10−5 | 3.4 × 10−5 | −3.2 × 10−3 | −2.3 × 10−3 |
| D02 | 2020 | RT | 0.0169 | 12 × 10−4 | 1.5 × 10−4 | 5.8 × 10−6 | 0.052 | 0.0356 | 5.8 × 10−5 | 4.7 × 10−5 | 2.9 × 10−5 | −1.6 × 10−3 | −3.0 × 10−4 |
| D03 | 2020 | RT | 0.0092 | 6.5 × 10−4 | 8.5 × 10−5 | 5.6 × 10−6 | 0.0567 | 0.0418 | 6.8 × 10−5 | 4.1 × 10−5 | 3.7 × 10−5 | −2.8 × 10−3 | −1.4 × 10−3 |
| 3COL | 2020 | LT | 0.0071 | 4.1 × 10−4 | 9.1 × 10−5 | 1.9 × 10−6 | 0.0289 | 0.0145 | 1.2 × 10−5 | 1.8 × 10−5 | 5.7 × 10−6 | −4.0 × 10−4 | −5.0 × 10−4 |
| CICO | 2020 | LT | 0.0062 | 4.7 × 10−4 | 8.2 × 10−5 | 1.5 × 10−6 | 0.0212 | 0.0108 | 4.2 × 10−5 | 4.0 × 10−5 | -7.0 × 10−7 | −6.0 × 10−4 | −1.0 × 10−4 |
| P18 | 2020 | LT | 0.009 | 7.8 × 10−4 | 1.2 × 10−4 | 2.5 × 10−6 | 0.016 | 0.0114 | 1.2 × 10−5 | 1.3 × 10−5 | 3.0 × 10−5 | −5.0 × 10−5 | −6.0 × 10−4 |
| C33 | 2020 | LT | 0.015 | 9.6 × 10−4 | 9.4 × 10−5 | 1.2 × 10−6 | 0.0128 | 0.0054 | 7.0 × 10−6 | 7.0 × 10−6 | 7.2 × 10−5 | −7.0 × 10−4 | −2.0 × 10−3 |
| D02 | 2020 | LT | 0.012 | 10 × 10−4 | 1.2 × 10−4 | 1.6 × 10−6 | 0.0248 | 0.0216 | 6.0 × 10−6 | 4.0 × 10−6 | 2.7 × 10−5 | 7.0 × 10−4 | −4.0 × 10−4 |
| D03 | 2020 | LT | 0.0089 | 7.0 × 10−4 | 9.1 × 10−5 | 1.8 × 10−6 | 0.0223 | 0.0237 | 9.0 × 10−6 | 3.0 × 10−6 | 3.4 × 10−5 | −4.0 × 10−4 | −7.0 × 10−4 |
| 6C | 2021 | RT | 0.0138 | 13 × 10−4 | 8.0 × 10−5 | 8.6 × 10−7 | 0.034 | 0.021 | 6.3 × 10−5 | 6.5 × 10−5 | 3.3 × 10−5 | 2.4 × 10−3 | 3.6 × 10−3 |
| 11C | 2021 | RT | 0.0216 | 13 × 10−4 | 2.0 × 10−4 | 2.4 × 10−6 | 0.0205 | 0.0161 | 4.4 × 10−5 | 7.7 × 10−5 | 7.9 × 10−5 | 1.6 × 10−3 | 2.2 × 10−3 |
| 12C | 2021 | RT | 0.0174 | 12 × 10−4 | 8.0 × 10−5 | 2.0 × 10−6 | 0.0885 | 0.0633 | 2.7 × 10−5 | 1.3 × 10−5 | 4.7 × 10−5 | 5.0 × 10−4 | −3.0 × 10−5 |
| 13C | 2021 | RT | 0.0183 | 12 × 10−4 | 1.0 × 10−4 | 2.3 × 10−6 | 0.0598 | 0.0204 | 1.7 × 10−5 | 2.4 × 10−5 | 2.4 × 10−5 | 4.0 × 10−4 | 1.6 × 10−3 |
| 14C | 2021 | RT | 0.0168 | 8.0 × 10−4 | 9.0 × 10−5 | 1.1 × 10−6 | 0.0644 | 0.0642 | 7.7 × 10−6 | 2.4 × 10−5 | 2.0 × 10−5 | 3.0 × 10−4 | 7.0 × 10−4 |
| 16C | 2021 | RT | 0.0189 | 12 × 10−4 | 1.0 × 10−4 | 2.1 × 10−6 | 0.0153 | 0.0172 | 1.7 × 10−5 | 2.5 × 10−5 | 2.6 × 10−5 | 1.7 × 10−3 | 1.4 × 10−3 |
| 6C | 2021 | LT | 0.0131 | 10 × 10−4 | 7.0 × 10−5 | 7.1 × 10−7 | 0.024 | 0.0247 | 6.3 × 10−5 | 1.0 × 10−5 | 1.6 × 10−5 | 2.2 × 10−3 | 3.2 × 10−3 |
| 11C | 2021 | LT | 0.0173 | 10 × 10−4 | 1.0 × 10−4 | 1.6 × 10−6 | 0.0156 | 0.0143 | 3.8 × 10−5 | 2.4 × 10−5 | 5.7 × 10−5 | 1.5 × 10−3 | 3.1 × 10−3 |
| 12C | 2021 | LT | 0.0116 | 11 × 10−4 | 9.0 × 10−5 | 2.0 × 10−6 | 0.0552 | 0.0399 | 1.4 × 10−5 | 1.0 × 10−5 | 3.5 × 10−5 | 5.0 × 10−5 | −9.0 × 10−5 |
| 13C | 2021 | LT | 0.0167 | 8.0 × 10−4 | 8.0 × 10−5 | 1.9 × 10−6 | 0.0345 | 0.0176 | 1.0 × 10−5 | 1.0 × 10−5 | 1.6 × 10−5 | 2.0 × 10−4 | 7.0 × 10−4 |
| 14C | 2021 | LT | 0.0083 | 5.0 × 10−4 | 8.0 × 10−5 | 8.3 × 10−7 | 0.046 | 0.0435 | 5.7 × 10−6 | 1.0 × 10−5 | 1.4 × 10−5 | 7.0 × 10−4 | 1.9 × 10−3 |
| 16C | 2021 | LT | 0.0156 | 6.0 × 10−4 | 8.0 × 10−5 | 1.1 × 10−6 | 0.0118 | 0.013 | 8.9 × 10−6 | 1.4 × 10−5 | 8.0 × 10−6 | 1.8 × 10−3 | 2.3 × 10−3 |
| 1C/22 | 2022 | RT | 0.0242 | 13 × 10−4 | 1.4 × 10−4 | 2.1 × 10−6 | 0.0098 | 0.0056 | 4.9 × 10−6 | 6.0 × 10−6 | 1.7 × 10−5 | −3.2 × 10−4 | −1.1 × 10−4 |
| 2C/22 | 2022 | RT | 0.0253 | 17 × 10−4 | 2.3 × 10−4 | 3.4 × 10−6 | 0.0111 | 0.0158 | 1.0 × 10−5 | 8.9 × 10−6 | 4.0 × 10−5 | −1.4 × 10−4 | 8.0 × 10−5 |
| 3C/22 | 2022 | RT | 0.0229 | 16 × 10−4 | 1.4 × 10−4 | 3.6 × 10−6 | 0.0203 | 0.0071 | 3.9 × 10−5 | 2.2 × 10−5 | 3.1 × 10−5 | −3.7 × 10−4 | 1.6 × 10−3 |
| 4C/22 | 2022 | RT | 0.0216 | 16 × 10−4 | 1.4 × 10−4 | 2.3 × 10−6 | 0.0066 | −0.0086 | 1.5 × 10−5 | 1.0 × 10−5 | 4.9 × 10−6 | −3.3 × 10−4 | 7.7 × 10−4 |
| 5C/22 | 2022 | RT | 0.0254 | 16 × 10−4 | 1.9 × 10−4 | 2.7 × 10−6 | 0.0134 | 0.0056 | 1.2 × 10−5 | 1.5 × 10−5 | 7.1 × 10−5 | 1.3 × 10−4 | 6.5 × 10−4 |
| 7C/22 | 2022 | RT | 0.0265 | 17 × 10−4 | 2.0 × 10−4 | 3.4 × 10−6 | 0.022 | 0.0017 | 5.6 × 10−5 | 2.2 × 10−5 | 6.4 × 10−5 | 2.4 × 10−4 | 1.2 × 10−3 |
| 8C/22 | 2022 | RT | 0.0243 | 14 × 10−4 | 1.8 × 10−4 | 3.1 × 10−6 | 0.0184 | 0.0064 | 1.4 × 10−5 | 2.2 × 10−5 | 6.2 × 10−5 | 1.0 × 10−4 | 8.9 × 10−4 |
| 10C/22 | 2022 | RT | 0.0256 | 14 × 10−4 | 1.9 × 10−4 | 3.3 × 10−6 | 0.0241 | 0.0218 | 2.3 × 10−5 | 2.6 × 10−5 | 3.0 × 10−5 | 4.3 × 10−4 | 1.8 × 10−3 |
| 16C/22 | 2022 | RT | 0.0152 | 14 × 10−4 | 1.6 × 10−4 | 1.8 × 10−6 | 0.0405 | 0.0388 | 1.9 × 10−5 | 1.7 × 10−5 | 7.9 × 10−5 | 9.0 × 10−5 | 1.1 × 10−3 |
| 1C/22 | 2022 | LT | 0.0183 | 11 × 10−4 | 1.1 × 10−4 | 9.0 × 10−7 | 0.0268 | 0.018 | 5.9 × 10−6 | 9.1 × 10−6 | 2.1 × 10−5 | 6.6 × 10−5 | −9.8 × 10−5 |
| 2C/22 | 2022 | LT | 0.0163 | 10 × 10−4 | 1.7 × 10−4 | 2.3 × 10−6 | 0.0091 | 0.0066 | 9.6 × 10−6 | 7.5 × 10−6 | 3.9 × 10−5 | −2.3 × 10−4 | 5.3 × 10−4 |
| 3C/22 | 2022 | LT | 0.0198 | 13 × 10−4 | 1.5 × 10−4 | 2.4 × 10−6 | 0.013 | 0.0061 | 5.7 × 10−6 | 1.2 × 10−5 | 2.1 × 10−5 | −3.8 × 10−4 | 1.1 × 10−3 |
| 4C/22 | 2022 | LT | 0.0208 | 15 × 10−4 | 1.6 × 10−4 | 1.6 × 10−6 | 0.014 | −0.0029 | 6.9 × 10−6 | 7.5 × 10−6 | -5.6 × 10−6 | 5.9 × 10−4 | 1.1 × 10−3 |
| 5C/22 | 2022 | LT | 0.019 | 11 × 10−4 | 1.5 × 10−4 | 1.2 × 10−6 | 0.0114 | 0.0106 | 9.0 × 10−6 | 8.3 × 10−6 | 4.5 × 10−6 | 2.6 × 10−4 | 9.6 × 10−4 |
| 7C/22 | 2022 | LT | 0.0197 | 13 × 10−4 | 1.6 × 10−4 | 2.0 × 10−6 | 0.0148 | −0.0037 | 2.1 × 10−5 | 1.5 × 10−5 | 4.7 × 10−5 | 3.6 × 10−4 | 1.6 × 10−3 |
| 8C/22 | 2022 | LT | 0.0166 | 12 × 10−4 | 1.7 × 10−4 | 1.2 × 10−6 | 0.0103 | 0.0026 | 1.0 × 10−5 | 7.5 × 10−6 | 2.4 × 10−5 | 3.4 × 10−4 | 1.4 × 10−3 |
| 10C/22 | 2022 | LT | 0.0192 | 12 × 10−4 | 2.5 × 10−4 | 1.3 × 10−6 | 0.0282 | 0.013 | 1.3 × 10−5 | 4.6 × 10−6 | 8.4 × 10−6 | 8.5 × 10−4 | 2.2 × 10−3 |
| 16C/22 | 2022 | LT | 0.011 | 9.0 × 10−4 | 1.2 × 10−4 | 1.7 × 10−6 | 0.0337 | 0.0169 | 2.3 × 10−5 | 1.0 × 10−5 | 5.7 × 10−5 | 5.0 × 10−4 | 2.1 × 10−3 |
| Observed Classification | Predicted Classification | ||
|---|---|---|---|
| RT p = 0.50 | LT p = 0.50 | ||
| 1C/22 | RT | 26.34 a | 26.24 a |
| 2C/22 | RT | 29.87 | 28.44 |
| 3C/22 | RT | 27.96 | 26.68 |
| 4C/22 | RT | 17.83 | 20.81 |
| 5C/22 | RT | 31.43 | 29.28 |
| 7C/22 | RT | 38.19 | 33.24 |
| 8C/22 | RT | 32.74 | 29.71 |
| 10C/22 | RT | 36.35 | 31.97 |
| 16C/22 | RT | 17.45 | 19.14 |
| 1C/22 | LT | 20.54 | 21.66 |
| 2C/22 | LT | 6.44 | 13.27 |
| 3C/22 | LT | 15.63 | 19.16 |
| 4C/22 | LT | 16.38 | 19.84 |
| 5C/22 | LT | 12.19 | 17.08 |
| 7C/22 | LT | 15.69 | 19.13 |
| 8C/22 | LT | 4.37 | 12.14 |
| 10C/22 | LT | 12.72 | 17.43 |
| 16C/22 | LT | −0.99 | 7.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crupi, P.; Clodoveo, M.L.; Desantis, A.; Zupo, R.; Corbo, F. Effect of Long-Term Storage Temperature on the Quality of Extra-Virgin Olive Oil (Coratina cv.): A Multivariate Discriminant Approach. Antioxidants 2025, 14, 1379. https://doi.org/10.3390/antiox14111379
Crupi P, Clodoveo ML, Desantis A, Zupo R, Corbo F. Effect of Long-Term Storage Temperature on the Quality of Extra-Virgin Olive Oil (Coratina cv.): A Multivariate Discriminant Approach. Antioxidants. 2025; 14(11):1379. https://doi.org/10.3390/antiox14111379
Chicago/Turabian StyleCrupi, Pasquale, Maria Lisa Clodoveo, Addolorata Desantis, Roberta Zupo, and Filomena Corbo. 2025. "Effect of Long-Term Storage Temperature on the Quality of Extra-Virgin Olive Oil (Coratina cv.): A Multivariate Discriminant Approach" Antioxidants 14, no. 11: 1379. https://doi.org/10.3390/antiox14111379
APA StyleCrupi, P., Clodoveo, M. L., Desantis, A., Zupo, R., & Corbo, F. (2025). Effect of Long-Term Storage Temperature on the Quality of Extra-Virgin Olive Oil (Coratina cv.): A Multivariate Discriminant Approach. Antioxidants, 14(11), 1379. https://doi.org/10.3390/antiox14111379










