Bilirubin Hepatic and Intestinal Transport and Catabolism: Physiology, Pathophysiology, and Benefits
Abstract
1. Introduction
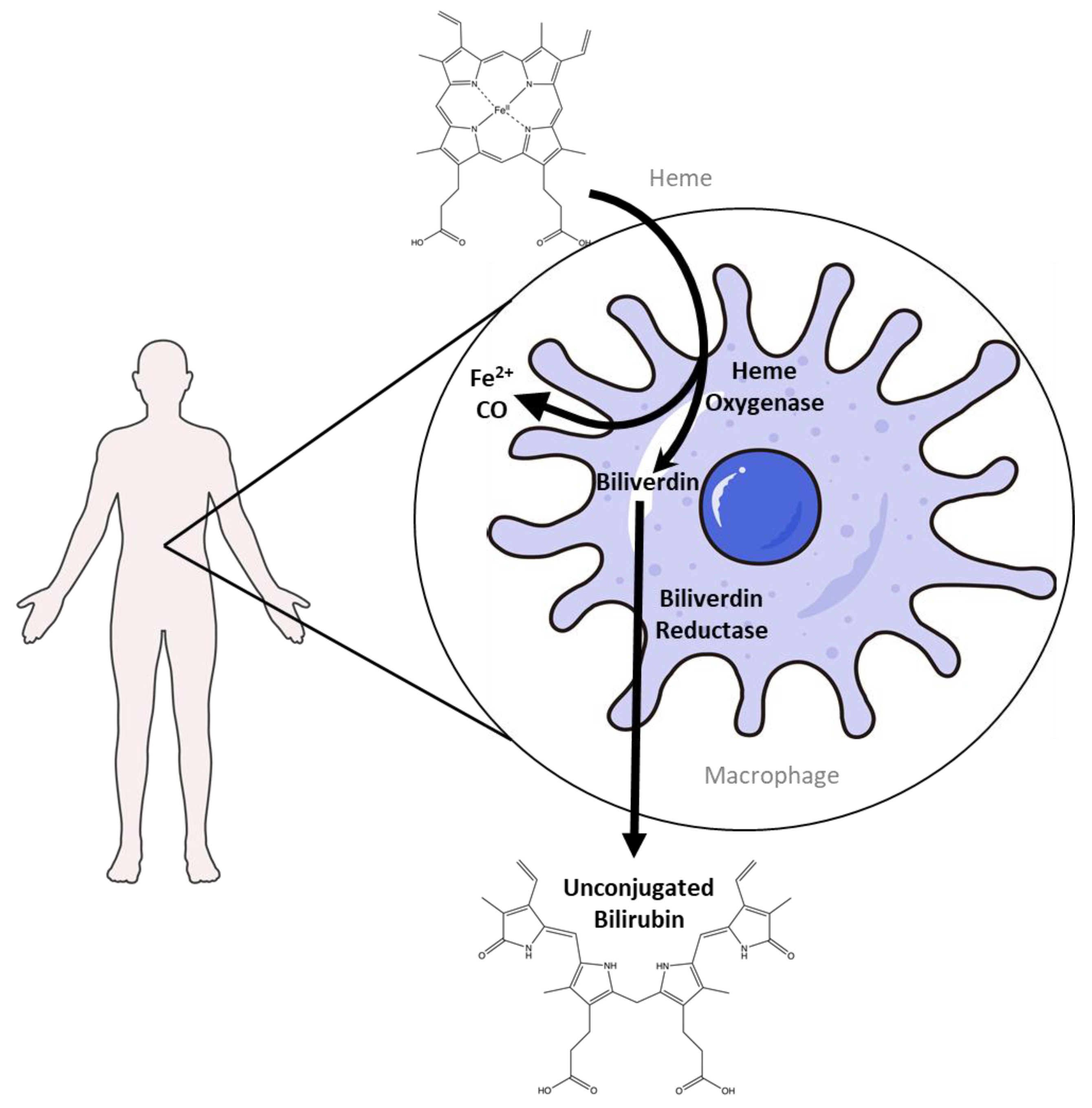
2. Bilirubin Production and Heme Degradation
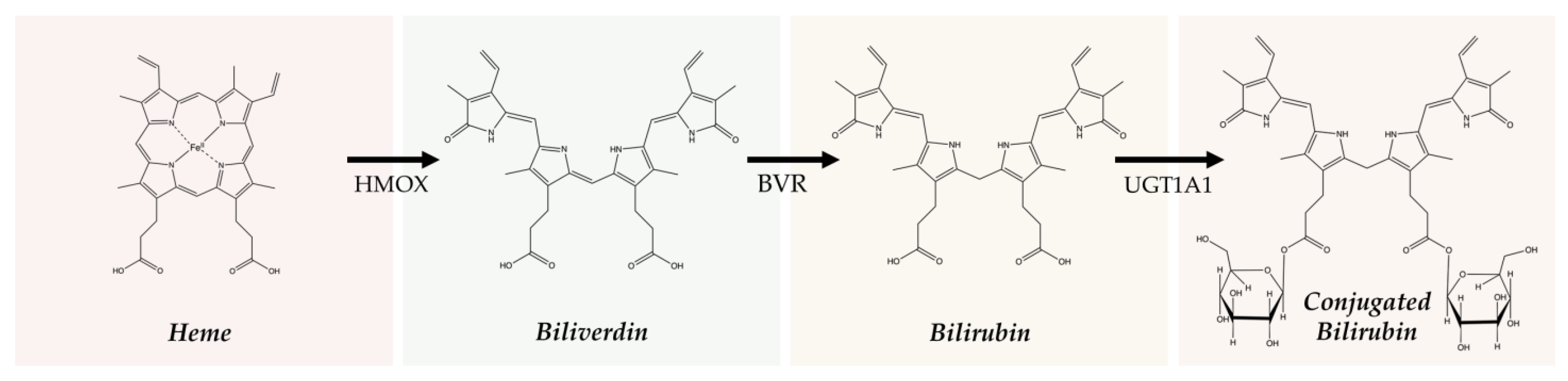
2.1. Heme Degradation Pathway
2.2. The Production of Bilirubin and Its Isoforms
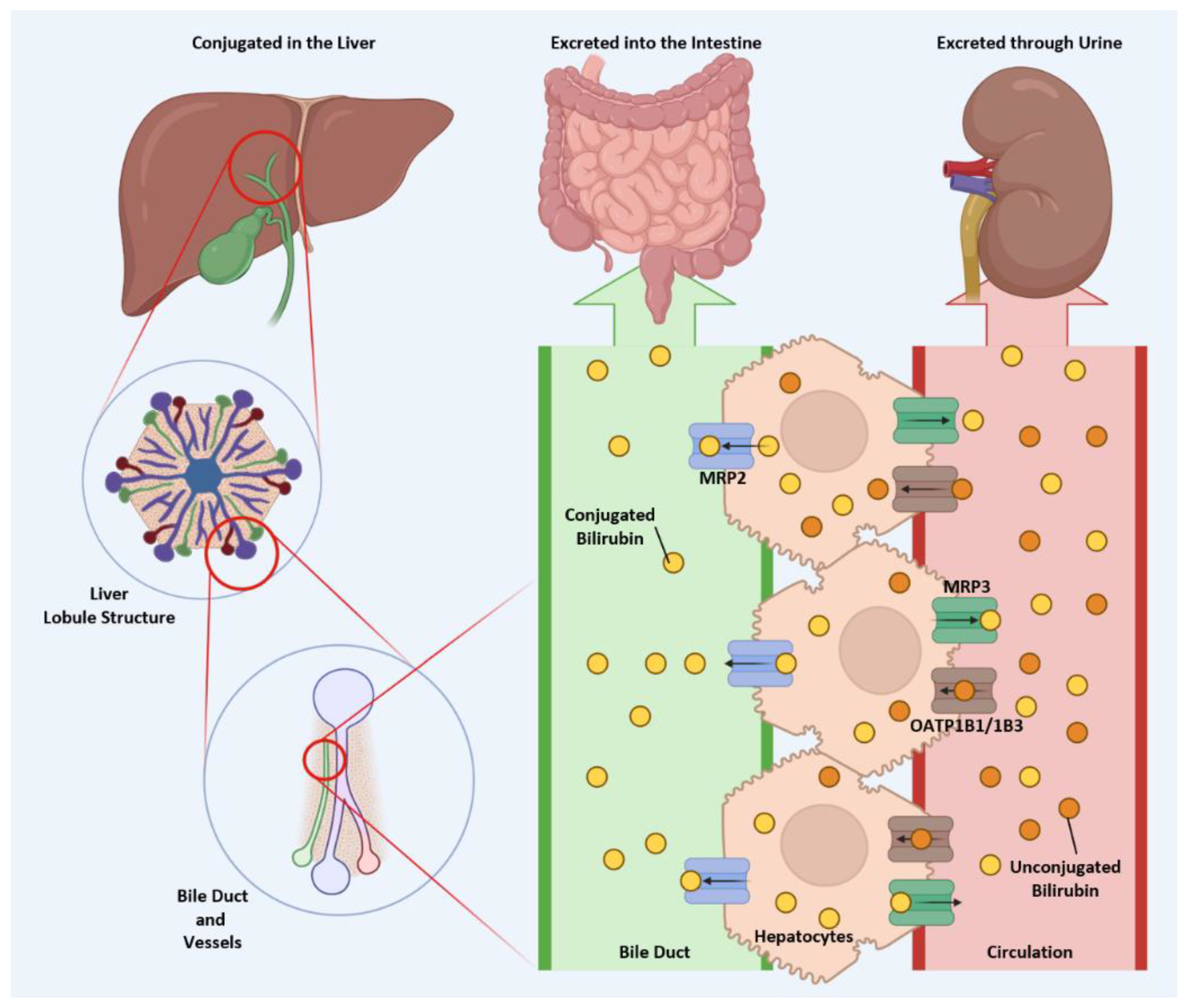
3. Hepatic Bilirubin Excretion
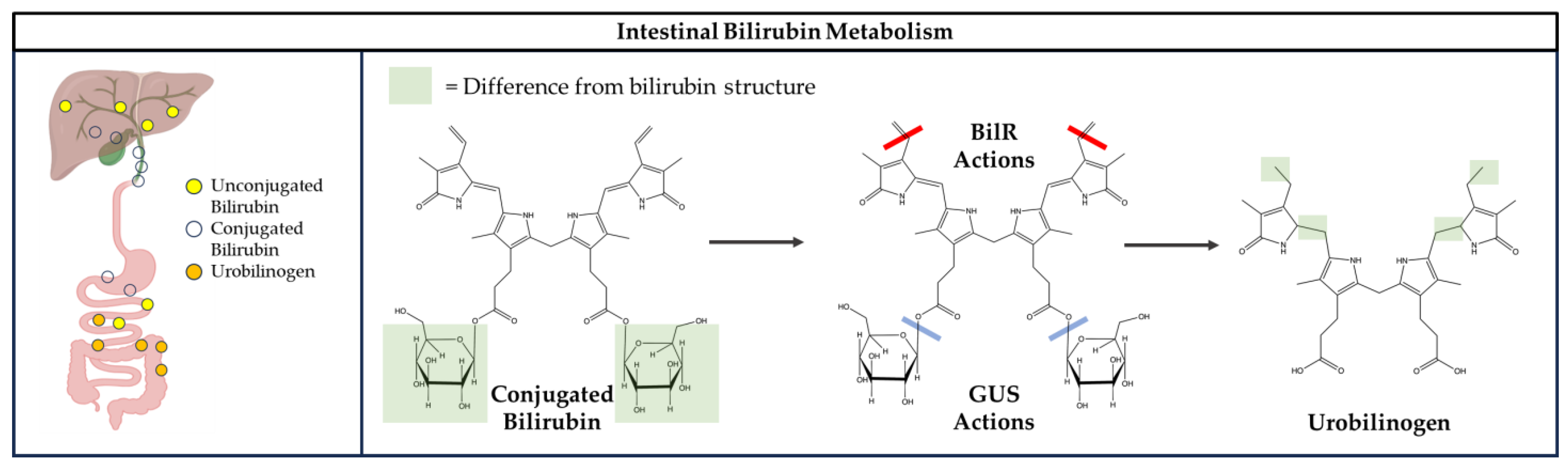
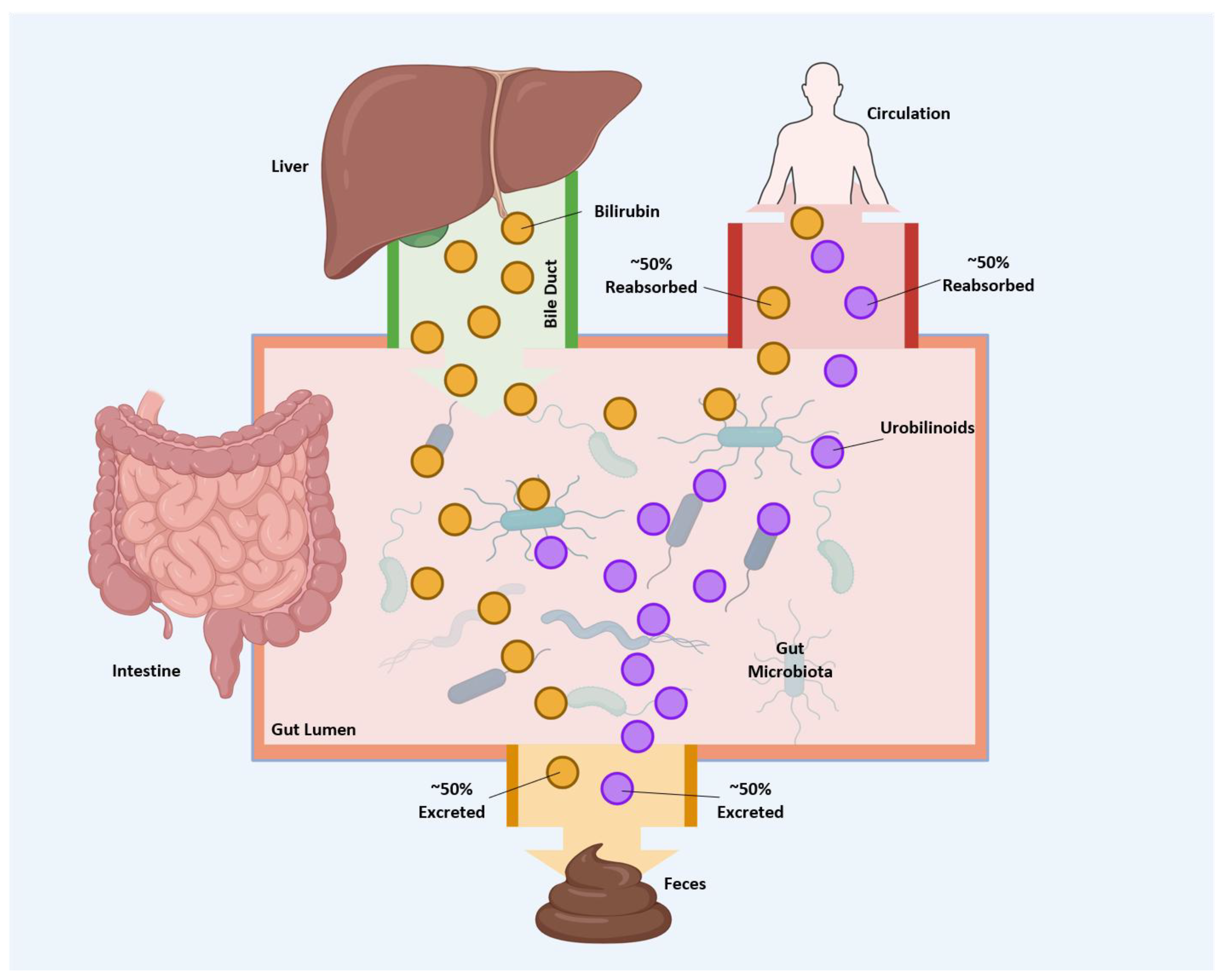
4. Gut Microbiome Metabolism of Bilirubin
5. Intestinal Reabsorption of Bilirubin and Its Metabolites
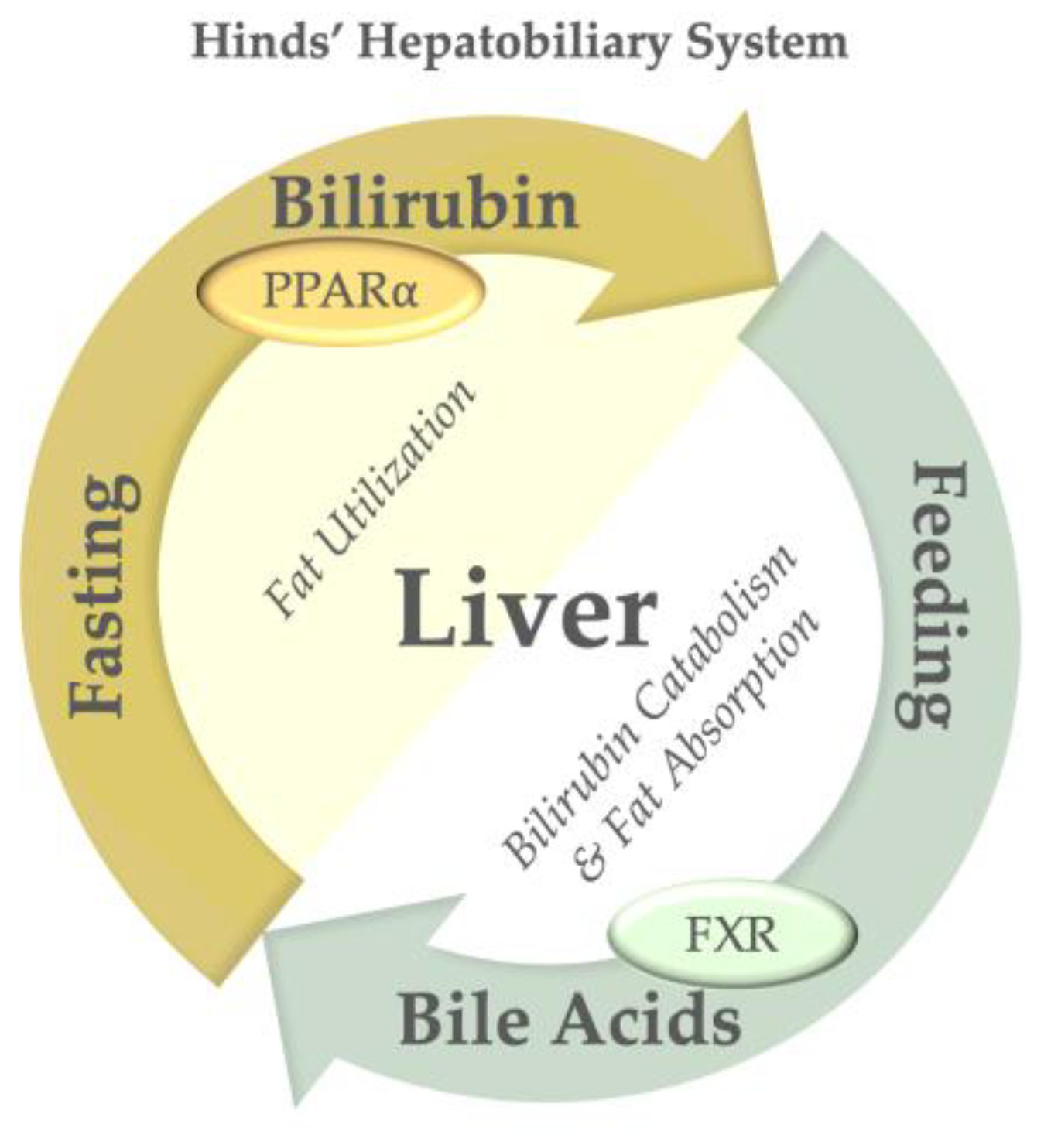
6. Physiological Consequences of Bilirubin Catabolism and Transport
7. Therapeutic Potential of Modulating Bilirubin Transport and Clearance
8. Bilirubin and Its Therapeutic Uses
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitek, L.; Hinds, T.D., Jr.; Stec, D.E.; Tiribelli, C. The physiology of bilirubin: Health and disease equilibrium. Trends Mol. Med. 2023, 29, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Creeden, J.F.; Gordon, D.M.; Stec, D.E.; Hinds, T.D., Jr. Bilirubin as a metabolic hormone: The physiological relevance of low levels. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E191–E207. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M.; Neifer, K.L.; Hamoud, A.A.; Hawk, C.F.; Nestor-Kalinoski, A.L.; Miruzzi, S.A.; Morran, M.P.; Adeosun, S.O.; Sarver, J.G.; Erhardt, P.W.; et al. Bilirubin remodels murine white adipose tissue by reshaping mitochondrial activity and the coregulator profile of peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 2020, 295, 9804–9822. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M.; Blomquist, T.M.; Miruzzi, S.A.; McCullumsmith, R.; Stec, D.E.; Hinds, T.D., Jr. RNA sequencing in human HepG2 hepatocytes reveals PPAR-alpha mediates transcriptome responsiveness of bilirubin. Physiol. Genom. 2019, 51, 234–240. [Google Scholar] [CrossRef]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds, T.D., Jr. Bilirubin Binding to PPARalpha Inhibits Lipid Accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef]
- Kipp, Z.A.; Badmus, O.O.; Stec, D.E.; Hall, B.; Hinds, T.D. Bilirubin bioconversion to urobilin in the gut-liver-kidney axis: A biomarker for insulin resistance in the Cardiovascular-Kidney-Metabolic (CKM) Syndrome. Metabolism 2025, 163, 156081. [Google Scholar] [CrossRef]
- Hamoud, A.R.; Weaver, L.; Stec, D.E.; Hinds, T.D., Jr. Bilirubin in the Liver-Gut Signaling Axis. Trends Endocrinol. Metab. 2018, 29, 140–150. [Google Scholar] [CrossRef]
- Sundararaghavan, V.L.; Sindhwani, P.; Hinds, T.D., Jr. Glucuronidation and UGT isozymes in bladder: New targets for the treatment of uroepithelial carcinomas? Oncotarget 2017, 8, 3640–3648. [Google Scholar] [CrossRef]
- Bosma, P.J. Inherited disorders of bilirubin metabolism. J. Hepatol. 2003, 38, 107–117. [Google Scholar] [CrossRef]
- Ambrosino, G.; Varotto, S.; Strom, S.C.; Guariso, G.; Franchin, E.; Miotto, D.; Caenazzo, L.; Basso, S.; Carraro, P.; Valente, M.L.; et al. Isolated hepatocyte transplantation for Crigler-Najjar syndrome type 1. Cell Transpl. 2005, 14, 151–157. [Google Scholar] [CrossRef]
- Kadakol, A.; Ghosh, S.S.; Sappal, B.S.; Sharma, G.; Chowdhury, J.R.; Chowdhury, N.R. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: Correlation of genotype to phenotype. Hum. Mutat. 2000, 16, 297–306. [Google Scholar] [CrossRef]
- Strassburg, C.P. Hyperbilirubinemia syndromes (Gilbert-Meulengracht, Crigler-Najjar, Dubin-Johnson, and Rotor syndrome). Best. Pract. Res. Clin. Gastroenterol. 2010, 24, 555–571. [Google Scholar] [CrossRef]
- Kimura, A.; Kagawa, T.; Takei, H.; Maruo, Y.; Sakugawa, H.; Sasaki, T.; Murai, T.; Naritaka, N.; Takikawa, H.; Nittono, H. Rotor Syndrome: Glucuronidated Bile Acidemia From Defective Reuptake by Hepatocytes. Hepatol. Commun. 2021, 5, 629–633. [Google Scholar] [CrossRef]
- Guerra-Ruiz, A.; Crespo, J.; Martínez, R.; Iruzubieta, P.; Mercadal, G.; Garcés, M.; Lavín Gómez, B.-A.; Morales-Ruiz, M. Measurement and clinical usefulness of bilirubin in liver disease. Adv. Lab. Med./Av. E. Med. D Lab. 2021, 2, 352–361. [Google Scholar] [CrossRef]
- Kartoun, U.; Fahed, A.C.; Kany, S.; Singh, P.; Khurshid, S.; Patel, A.P.; Batra, P.; Philippakis, A.; Khera, A.V.; Lubitz, S.A.; et al. Exploring the link between Gilbert’s syndrome and atherosclerotic cardiovascular disease: Insights from a subpopulation-based analysis of over one million individuals. Eur. Heart J. Open 2023, 3, oead059. [Google Scholar] [CrossRef]
- Brown, D. Cardioprotection from a Silent Syndrome: Effect of Gilbert’s Syndrome on Cardiovascular Disease in Patients with Familial Hypercholesteremia. J. Clin. Lipidol. 2023, 17, e29. [Google Scholar] [CrossRef]
- Vítek, L.; Jirsa, M.; Brodanová, M.; Kalab, M.; Marecek, Z.; Danzig, V.; Novotný, L.; Kotal, P. Gilbert syndrome and ischemic heart disease: A protective effect of elevated bilirubin levels. Atherosclerosis 2002, 160, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Kundur, A.R.; Singh, I.; Bulmer, A.C. Bilirubin, platelet activation and heart disease: A missing link to cardiovascular protection in Gilbert’s syndrome? Atherosclerosis 2015, 239, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, H.A.; Vítek, L. Gilbert syndrome, UGT1A1*28 allele, and cardiovascular disease risk: Possible protective effects and therapeutic applications of bilirubin. Atherosclerosis 2008, 198, 1–11. [Google Scholar] [CrossRef]
- Bulmer, A.C.; Bakrania, B.; Du Toit, E.F.; Boon, A.-C.; Clark, P.J.; Powell, L.W.; Wagner, K.-H.; Headrick, J.P. Bilirubin acts as a multipotent guardian of cardiovascular integrity: More than just a radical idea. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H429–H447. [Google Scholar] [CrossRef]
- Lee, W.H.; Kipp, Z.A.; Pauss, S.N.; Martinez, G.J.; Bates, E.A.; Badmus, O.O.; Stec, D.E.; Hinds, T.D., Jr. Heme oxygenase, biliverdin reductase, and bilirubin pathways regulate oxidative stress and insulin resistance: A focus on diabetes and therapeutics. Clin Sci. 2025, 139, 171–198. [Google Scholar] [CrossRef]
- Kipp, Z.A.; Martinez, G.J.; Bates, E.A.; Maharramov, A.B.; Flight, R.M.; Moseley, H.N.B.; Morris, A.J.; Stec, D.E.; Hinds, T.D., Jr. Bilirubin Nanoparticle Treatment in Obese Mice Inhibits Hepatic Ceramide Production and Remodels Liver Fat Content. Metabolites 2023, 13, 215. [Google Scholar] [CrossRef]
- Kipp, Z.A.; Xu, M.; Bates, E.A.; Lee, W.H.; Kern, P.A.; Hinds, T.D., Jr. Bilirubin Levels Are Negatively Correlated with Adiposity in Obese Men and Women, and Its Catabolized Product, Urobilin, Is Positively Associated with Insulin Resistance. Antioxidants 2023, 12, 170. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Q.; Zhang, L.; Liu, J.; Wang, G. Serum Bilirubin Level Is Increased in Metabolically Healthy Obesity. Front. Endocrinol. 2022, 12, 792795. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, C.; Lai, F.; Dong, S.; Chen, H.; Chen, L.; Shi, L.; Zhu, F.; Zhang, C.; Lv, X.; et al. Associations between serum total bilirubin, obesity and type 2 diabetes. Diabetol. Metab. Syndr. 2021, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Takei, R.; Inoue, T.; Sonoda, N.; Kohjima, M.; Okamoto, M.; Sakamoto, R.; Inoguchi, T.; Ogawa, Y. Bilirubin reduces visceral obesity and insulin resistance by suppression of inflammatory cytokines. PLoS ONE 2019, 14, e0223302. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, H.; Zhang, Y.; Cao, M.; Song, L.; Pan, Q.; Bulmer, A.; Adams, D.B.; Dong, X.; Wang, H. Corrigendum: Bilirubin Increases Insulin Sensitivity by Regulating Cholesterol Metabolism, Adipokines and PPARγ Levels. Sci. Rep. 2016, 6, 19170. [Google Scholar] [CrossRef]
- Seyed Khoei, N.; Grindel, A.; Wallner, M.; Molzer, C.; Doberer, D.; Marculescu, R.; Bulmer, A.; Wagner, K.H. Mild hyperbilirubinaemia as an endogenous mitigator of overweight and obesity: Implications for improved metabolic health. Atherosclerosis 2018, 269, 306–311. [Google Scholar] [CrossRef]
- White, C.; Yuan, X.; Schmidt, P.J.; Bresciani, E.; Samuel, T.K.; Campagna, D.; Hall, C.; Bishop, K.; Calicchio, M.L.; Lapierre, A.; et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 2013, 17, 261–270. [Google Scholar] [CrossRef]
- Chiabrando, D.; Vinchi, F.; Fiorito, V.; Mercurio, S.; Tolosano, E. Heme in pathophysiology: A matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharmacol. 2014, 5, 61. [Google Scholar] [CrossRef]
- Wilks, A.; Heinzl, G. Heme oxygenation and the widening paradigm of heme degradation. Arch. Biochem. Biophys. 2014, 544, 87–95. [Google Scholar] [CrossRef]
- Marton, L.S.; Wang, X.; Kowalczuk, A.; Zhang, Z.D.; Windmeyer, E.; Macdonald, R.L. Effects of hemoglobin on heme oxygenase gene expression and viability of cultured smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2405–H2413. [Google Scholar] [CrossRef]
- Hirota, K. An intimate crosstalk between iron homeostasis and oxygen metabolism regulated by the hypoxia-inducible factors (HIFs). Free Radic. Biol. Med. 2019, 133, 118–129. [Google Scholar] [CrossRef]
- Zakhary, R.; Gaine, S.P.; Dinerman, J.L.; Ruat, M.; Flavahan, N.A.; Snyder, S.H. Heme oxygenase 2: Endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc. Natl. Acad. Sci. USA 1996, 93, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Bakken, A.F.; Thaler, M.M.; Schmid, R. Metabolic regulation of heme catabolism and bilirubin production. I. Hormonal control of hepatic heme oxygenase activity. J. Clin. Investig. 1972, 51, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Braggins, P.E.; Trakshel, G.M.; Kutty, R.K.; Maines, M.D. Characterization of two heme oxygenase isoforms in rat spleen: Comparison with the hematin-induced and constitutive isoforms of the liver. Biochem. Biophys. Res. Commun. 1986, 141, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Wanner, G.A.; Rensing, H.; Alte, C.; Miescher, E.A.; Wolf, B.; Pannen, B.H.; Clemens, M.G.; Bauer, M. Expression pattern of heme oxygenase isoenzymes 1 and 2 in normal and stress-exposed rat liver. Hepatology 1998, 27, 829–838. [Google Scholar] [CrossRef]
- Li, S.; Fujino, M.; Takahara, T.; Li, X.K. Protective role of heme oxygenase-1 in fatty liver ischemia-reperfusion injury. Med. Mol. Morphol. 2019, 52, 61–72. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Soares, M.P.; Yamashita, K.; Bach, F.H. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol. 2003, 24, 449–455. [Google Scholar] [CrossRef]
- Bernardini, C.; Grilli, E.; Duvigneau, J.C.; Zannoni, A.; Tugnoli, B.; Gentilini, F.; Bertuzzi, T.; Spinozzi, S.; Camborata, C.; Bacci, M.L.; et al. Cellular stress marker alteration and inflammatory response in pigs fed with an ochratoxin contaminated diet. Res. Vet. Sci. 2014, 97, 244–250. [Google Scholar] [CrossRef]
- Duvigneau, J.C.; Piskernik, C.; Haindl, S.; Kloesch, B.; Hartl, R.T.; Huttemann, M.; Lee, I.; Ebel, T.; Moldzio, R.; Gemeiner, M.; et al. A novel endotoxin-induced pathway: Upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab. Investig. 2008, 88, 70–77. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. CMLS 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Vanella, L.; Barbagallo, I.; Tibullo, D.; Forte, S.; Zappalà, A.; Li Volti, G. The non-canonical functions of the heme oxygenases. Oncotarget 2016, 7, 69075–69086. [Google Scholar] [CrossRef]
- Pittala, V.; Vanella, L.; Salerno, L.; Romeo, G.; Marrazzo, A.; Di Giacomo, C.; Sorrenti, V. Effects of Polyphenolic Derivatives on Heme Oxygenase-System in Metabolic Dysfunctions. Curr. Med. Chem. 2018, 25, 1577–1595. [Google Scholar] [CrossRef] [PubMed]
- Pittalà, V.; Vanella, L.; Maria Platania, C.B.; Salerno, L.; Raffaele, M.; Amata, E.; Marrazzo, A.; Floresta, G.; Romeo, G.; Greish, K.; et al. Synthesis, in vitro and in silico studies of HO-1 inducers and lung antifibrotic agents. Future Med. Chem. 2019, 11, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.; Volk, H.D.; Willis, D.; Abraham, N.G.; Soares, M.P.; Adema, G.J.; Figdor, C.G. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol. Rev. 2003, 55, 551–571. [Google Scholar] [CrossRef] [PubMed]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 Signaling and Redox Homeostasis in Physiopathological Conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef]
- Reichard, J.F.; Motz, G.T.; Puga, A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007, 35, 7074–7086. [Google Scholar] [CrossRef]
- Tulsulkar, J.; Glueck, B.; Hinds, T.D., Jr.; Shah, Z.A. Ginkgo biloba Extract Prevents Female Mice from Ischemic Brain Damage and the Mechanism Is Independent of the HO1/Wnt Pathway. Transl. Stroke Res. 2016, 7, 120–131. [Google Scholar] [CrossRef]
- Ryter, S.W. Significance of Heme and Heme Degradation in the Pathogenesis of Acute Lung and Inflammatory Disorders. Int. J. Mol. Sci. 2021, 22, 5509. [Google Scholar] [CrossRef]
- Williams, K.I.; Suryadevara, P.; Zhan, C.G.; Hinds, T.D., Jr.; Kipp, Z.A. Urobilin Derived from Bilirubin Bioconversion Binds Albumin and May Interfere with Bilirubin Interacting with Albumin: Implications for Disease Pathology. Biomedicines 2025, 13, 302. [Google Scholar] [CrossRef]
- Gordon, D.M.; Hong, S.H.; Kipp, Z.A.; Hinds, T.D., Jr. Identification of Binding Regions of Bilirubin in the Ligand-Binding Pocket of the Peroxisome Proliferator-Activated Receptor-A (PPARalpha). Molecules 2021, 26, 2975. [Google Scholar] [CrossRef]
- Gu, R.; Qin, F.-Y.; Wang, L.; Zhang, J.; Emerson, J.; Ma, Q.; Lu, J.; Anderson, K.E.; Wang, J.; Ma, X. OATP1B1/1B3 deficiency exacerbates hyperbilirubinemia in erythropoietic protoporphyria. Drug Metab. Dispos. 2025, 53, 100105. [Google Scholar] [CrossRef] [PubMed]
- Adeosun, S.O.; Moore, K.H.; Lang, D.M.; Nwaneri, A.C.; Hinds, T.D., Jr.; Stec, D.E. A Novel Fluorescence-Based Assay for the Measurement of Biliverdin Reductase Activity. React. Oxyg. Species 2018, 5, 35–45. [Google Scholar] [CrossRef][Green Version]
- O’Brien, L.; Hosick, P.A.; John, K.; Stec, D.E.; Hinds, T.D., Jr. Biliverdin reductase isozymes in metabolism. Trends Endocrinol. Metab. 2015, 26, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Nakajima, H. Changes in the composition of bilirubin-IX isomers during human prenatal development. Eur. J. Biochem.S 1995, 233, 467–472. [Google Scholar]
- Berk, P.D.; Howe, R.B.; Bloomer, J.R.; Berlin, N.I. Studies of bilirubin kinetics in normal adults. J. Clin. Investig. 1969, 48, 2176–2190. [Google Scholar] [CrossRef]
- Defoinstraatmann, R.; Defoin, A.; Kuhn, H.J.; Schaffner, K. Chromatographic-Separation and Spectroscopic Characterization of the Bilirubin Isomer-Iii-Alpha, Isomer-Ix-Alpha, Isomer-Xiii-Alpha, and Their Dimethyl Esters. Liebigs Ann. Chem. 1982, 1982, 1759–1765. [Google Scholar]
- McDonagh, A.F.; Assisi, F. Commercial bilirubin: A trinity of isomers. FEBS Lett. 1971, 18, 315–317. [Google Scholar] [CrossRef]
- Fevery, J. Bilirubin in clinical practice: A review. Liver Int. 2008, 28, 592–605. [Google Scholar] [CrossRef]
- Blanckaert, N.; Gollan, J.; Schmid, R. Mechanism of bilirubin diglucuronide formation in intact rats: Bilirubin diglucuronide formation in vivo. J. Clin. Investig. 1980, 65, 1332–1342. [Google Scholar] [CrossRef]
- Hinds, T.D., Jr.; Creeden, J.F.; Gordon, D.M.; Spegele, A.C.; Britton, S.L.; Koch, L.G.; Stec, D.E. Rats Genetically Selected for High Aerobic Exercise Capacity Have Elevated Plasma Bilirubin by Upregulation of Hepatic Biliverdin Reductase-A (BVRA) and Suppression of UGT1A1. Antioxidants 2020, 9, 889. [Google Scholar] [CrossRef]
- Swift, D.L.; Johannsen, N.M.; Earnest, C.P.; Blair, S.N.; Church, T.S. Effect of different doses of aerobic exercise training on total bilirubin levels. Med. Sci. Sports Exerc. 2012, 44, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Flack, K.D.; Vitek, L.; Fry, C.S.; Stec, D.E.; Hinds, T.D., Jr. Cutting edge concepts: Does bilirubin enhance exercise performance? Front. Sports Act. Living 2022, 4, 1040687. [Google Scholar] [CrossRef] [PubMed]
- Goluch, Z.; Wierzbicka-Rucinska, A.; Ksiazek, E. Nutrition in Gilbert’s Syndrome-A Systematic Review of Clinical Trials According to the PRISMA Statement. Nutrients 2024, 16, 2247. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Ottosson, F.; Hellstrand, S.; Ericson, U.; Orho-Melander, M.; Fernandez, C.; Melander, O. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart 2020, 106, 691–697. [Google Scholar] [CrossRef]
- Kullak-Ublick, G.A.; Baretton, G.B.; Oswald, M.; Renner, E.L.; Paumgartner, G.; Beuers, U. Expression of the hepatocyte canalicular multidrug resistance protein (MRP2) in primary biliary cirrhosis. Hepatol. Res. 2002, 23, 78–82. [Google Scholar] [CrossRef]
- Beer, A.J.; Hertz, D.; Seemann, E.; Beretta, M.; Westermann, M.; Bauer, R.; Bauer, M.; Kessels, M.M.; Qualmann, B. Reduced Mrp2 surface availability as PI3Kγ-mediated hepatocytic dysfunction reflecting a hallmark of cholestasis in sepsis. Sci. Rep. 2020, 10, 13110. [Google Scholar] [CrossRef]
- Hall, B.; Levy, S.; Dufault-Thompson, K.; Arp, G.; Zhong, A.; Ndjite, G.M.; Weiss, A.; Braccia, D.; Jenkins, C.; Grant, M.R.; et al. BilR is a gut microbial enzyme that reduces bilirubin to urobilinogen. Nat. Microbiol. 2024, 9, 173–184. [Google Scholar] [CrossRef]
- Vitek, L.; Tiribelli, C. Gut microbiota and bilirubin metabolism: Unveiling new pathways in health and disease. Trends Mol. Med. 2025, 31, 591–594. [Google Scholar] [CrossRef]
- Vitek, L.; Zelenka, J.; Zadinova, M.; Malina, J. The impact of intestinal microflora on serum bilirubin levels. J. Hepatol. 2005, 42, 238–243. [Google Scholar] [CrossRef]
- Takashi Nakamura, K.S.; Mitsuo, A.; Masao, O. Urobilinogen, as a Bile Pigment Metabolite, Has an Antioxidant Function. J. Oleo Sci. 2006, 55, 191–197. [Google Scholar] [CrossRef]
- Sundararaghavan, V.L.; Binepal, S.; Stec, D.E.; Sindhwani, P.; Hinds, T.D., Jr. Bilirubin, a new therapeutic for kidney transplant? Transpl. Rev. 2018, 32, 234–240. [Google Scholar] [CrossRef]
- Seyed Khoei, N.; Wagner, K.H.; Sedlmeier, A.M.; Gunter, M.J.; Murphy, N.; Freisling, H. Bilirubin as an indicator of cardiometabolic health: A cross-sectional analysis in the UK Biobank. Cardiovasc. Diabetol. 2022, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Bakrania, B.; Du Toit, E.F.; Ashton, K.J.; Wagner, K.H.; Headrick, J.P.; Bulmer, A.C. Chronically elevated bilirubin protects from cardiac reperfusion injury in the male Gunn rat. Acta Physiol. 2017, 220, 461–470. [Google Scholar] [CrossRef]
- Tiribelli, C.; Ostrow, J.D. Intestinal flora and bilirubin. J. Hepatol. 2005, 42, 170–172. [Google Scholar] [CrossRef]
- Lester, R.; Schmid, R. Intestinal absorption of bile pigments. II. Bilirubin absorption in man. N. Engl. J. Med. 1963, 269, 178–182. [Google Scholar] [CrossRef]
- Lester, R.; Schmid, R. Intestinal Absorption of Bile Pigments. 3. The Enterohepatic Circulation of Urobilinogen in the Rat. J. Clin. Investig. 1965, 44, 722–730. [Google Scholar] [CrossRef]
- Lester, R.; Schmid, R. Enterohepatic Circulation of Urobilinogen. Nature 1964, 201, 711–712. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, M.; Kim, M.; Kang, M.; Yoo, H.J.; Lee, N.H.; Kim, Y.H.; Song, M.; Lee, J.H. Metabolites distinguishing visceral fat obesity and atherogenic traits in individuals with overweight. Obes. Silver Spring 2017, 25, 323–331. [Google Scholar] [CrossRef]
- Ottosson, F.; Smith, E.; Fernandez, C.; Melander, O. Plasma Metabolites Associate with All-Cause Mortality in Individuals with Type 2 Diabetes. Metabolites 2020, 10, 315. [Google Scholar] [CrossRef]
- Stenemo, M.; Ganna, A.; Salihovic, S.; Nowak, C.; Sundstrom, J.; Giedraitis, V.; Broeckling, C.D.; Prenni, J.E.; Svensson, P.; Magnusson, P.K.E.; et al. The metabolites urobilin and sphingomyelin (30:1) are associated with incident heart failure in the general population. ESC Heart Fail. 2019, 6, 764–773. [Google Scholar] [CrossRef]
- Bloomer, J.R.; Barrett, P.V.; Rodkey, F.L.; Berlin, N.I. Studies on the mechanism of fasting hyperbilirubinemia. Gastroenterology 1971, 61, 479–487. [Google Scholar] [CrossRef]
- Barrett, P.V. The effect of diet and fasting on the serum bilirubin concentration in the rat. Gastroenterology 1971, 60, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.V. Hyperbilirubinemia of fasting. JAMA 1971, 217, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Kipp, Z.A.; Bates, E.A.; Pauss, S.N.; Martinez, G.J.; Hinds, T.D., Jr. The physiology of MASLD: Molecular pathways between liver and adipose tissues. Clin. Sci. 2025, 139, 1046. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, T.C.; Kruger, R.; Ferrario, P.G.; Molzer, C.; Wallner, M.; Marculescu, R.; Doberer, D.; Bulmer, A.C.; Wagner, K.H. Bilirubin Metabolism Does Not Influence Serum Bile Acid Profiles According to LC-MS: A Human Case-Control Study. Int. J. Mol. Sci. 2025, 26, 2475. [Google Scholar] [CrossRef]
- ElTatawy, S.S.; Elmazzahy, E.A.; El Shennawy, A.M.; Madani, H.A.; Abou Youssef, H.; Iskander, I.F. The spectrum of bilirubin neurotoxicity in term and near-term babies with hyperbilirubinemia: Does outcome improve with time? Early Hum. Dev. 2020, 140, 104909. [Google Scholar] [CrossRef]
- Reddy, D.K.; Pandey, S. Kernicterus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Gazzin, S.; Bellarosa, C.; Tiribelli, C. Molecular events in brain bilirubin toxicity revisited. Pediatr. Res. 2024, 95, 1734–1740. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Badmus, O.O.; Kipp, Z.A.; Bates, E.A.; da Silva, A.A.; Taylor, L.C.; Martinez, G.J.; Lee, W.H.; Creeden, J.F.; Hinds, T.D., Jr.; Stec, D.E. Loss of hepatic PPARalpha in mice causes hypertension and cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 325, R81–R95. [Google Scholar] [CrossRef]
- Yao, M.E.; Su, M.Y.; Huang, Y.; Chen, W. Physiologically increased total bilirubin is associated with reduced risk of first myocardial infarction: A meta-analysis and dose-response analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qiao, L.; Tang, Z.; Zhou, S.; Min, J.; Li, M. Association between bilirubin levels and risk of stroke: A systematic review and meta-analysis. BMJ Open 2023, 13, e064433. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, L. Bilirubin as a predictor of severity and adverse clinical outcomes of acute ischemic stroke: A systematic review and meta-analysis. BMC Neurol. 2025, 25, 159. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Mangoni, A.A. The role of bilirubin as a biomarker of rheumatic diseases: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1369284. [Google Scholar] [CrossRef]
- Horsfall, L.J.; Hardy, R.; Wong, A.; Kuh, D.; Swallow, D.M. Genetic variation underlying common hereditary hyperbilirubinaemia (Gilbert’s syndrome) and respiratory health in the 1946 British birth cohort. J. Hepatol. 2014, 61, 1344–1351. [Google Scholar] [CrossRef]
- de Vries, H.S.; Te Morsche, R.H.; Jenniskens, K.; Peters, W.H.; de Jong, D.J. A functional polymorphism in UGT1A1 related to hyperbilirubinemia is associated with a decreased risk for Crohn’s disease. J. Crohns Colitis 2012, 6, 597–602. [Google Scholar] [CrossRef]
- Peng, F.; Deng, X.; Yu, Y.; Chen, X.; Shen, L.; Zhong, X.; Qiu, W.; Jiang, Y.; Zhang, J.; Hu, X. Serum bilirubin concentrations and multiple sclerosis. J. Clin. Neurosci. 2011, 18, 1355–1359. [Google Scholar] [CrossRef]
- Hinds, T.D., Jr.; Burns, K.A.; Hosick, P.A.; McBeth, L.; Nestor-Kalinoski, A.; Drummond, H.A.; AlAmodi, A.A.; Hankins, M.W.; Vanden Heuvel, J.P.; Stec, D.E. Biliverdin Reductase A Attenuates Hepatic Steatosis by Inhibition of Glycogen Synthase Kinase (GSK) 3beta Phosphorylation of Serine 73 of Peroxisome Proliferator-activated Receptor (PPAR) alpha. J. Biol. Chem. 2016, 291, 25179–25191. [Google Scholar] [CrossRef]
- Tramutola, A.; Di Domenico, F.; Perluigi, M.; Barone, E. Biliverdin reductase-A is a key modulator in insulin signaling and metabolism. Trends Endocrinol. Metab. 2025, S1043z–S2760. [Google Scholar] [CrossRef]
- Vasavda, C.; Kothari, R.; Ammal Kaidery, N.; Chakraborty, S.; Jamuna Tripathi, S.; Dhindsa, R.S.; Ricco, C.; Shanmukha, S.; Saberi, S.; Lefler, J.E.; et al. Biliverdin reductase A is a major determinant of protective NRF2 signaling. Proc. Natl. Acad. Sci. USA 2025, 122, e2513120122. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tumanov, S.; Fazakerley, D.J.; Cantley, J.; James, D.E.; Dunn, L.L.; Shaik, T.; Suarna, C.; Stocker, R. Bilirubin deficiency renders mice susceptible to hepatic steatosis in the absence of insulin resistance. Redox Biol. 2021, 47, 102152. [Google Scholar] [CrossRef]
- Lanzillotta, C.; Tramutola, A.; Lanzillotta, S.; Greco, V.; Pagnotta, S.; Sanchini, C.; Di Angelantonio, S.; Forte, E.; Rinaldo, S.; Paone, A.; et al. Biliverdin Reductase-A integrates insulin signaling with mitochondrial metabolism through phosphorylation of GSK3beta. Redox Biol. 2024, 73, 103221. [Google Scholar] [CrossRef] [PubMed]
- Adeosun, S.O.; Gordon, D.M.; Weeks, M.F.; Moore, K.H.; Hall, J.E.; Hinds, T.D., Jr.; Stec, D.E. Loss of biliverdin reductase-A promotes lipid accumulation and lipotoxicity in mouse proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2018, 315, F323–F331. [Google Scholar] [CrossRef]
- Gordon, D.M.; Adeosun, S.O.; Ngwudike, S.I.; Anderson, C.D.; Hall, J.E.; Hinds, T.D., Jr.; Stec, D.E. CRISPR Cas9-mediated deletion of biliverdin reductase A (BVRA) in mouse liver cells induces oxidative stress and lipid accumulation. Arch. Biochem. Biophys. 2019, 672, 108072. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.E.; Gordon, D.M.; Nestor-Kalinoski, A.L.; Donald, M.C.; Mitchell, Z.L.; Creeden, J.F.; Hinds, T.D., Jr. Biliverdin Reductase A (BVRA) Knockout in Adipocytes Induces Hypertrophy and Reduces Mitochondria in White Fat of Obese Mice. Biomolecules 2020, 10, 387. [Google Scholar] [CrossRef]
- Cimini, F.A.; Barchetta, I.; Zuliani, I.; Pagnotta, S.; Bertoccini, L.; Dule, S.; Zampieri, M.; Reale, A.; Baroni, M.G.; Cavallo, M.G.; et al. Biliverdin reductase-A protein levels are reduced in type 2 diabetes and are associated with poor glycometabolic control. Life Sci. 2021, 284, 119913. [Google Scholar] [CrossRef]
- Ceccarelli, V.; Barchetta, I.; Cimini, F.A.; Bertoccini, L.; Chiappetta, C.; Capoccia, D.; Carletti, R.; Di Cristofano, C.; Silecchia, G.; Fontana, M.; et al. Reduced Biliverdin Reductase-A Expression in Visceral Adipose Tissue is Associated with Adipocyte Dysfunction and NAFLD in Human Obesity. Int. J. Mol. Sci. 2020, 21, 9091. [Google Scholar] [CrossRef]
- Sharma, N.; Tramutola, A.; Lanzillotta, C.; Arena, A.; Blarzino, C.; Cassano, T.; Butterfield, D.A.; Di Domenico, F.; Perluigi, M.; Barone, E. Loss of biliverdin reductase-A favors Tau hyper-phosphorylation in Alzheimer’s disease. Neurobiol. Dis. 2019, 125, 176–189. [Google Scholar] [CrossRef]
- Cimini, F.A.; Arena, A.; Barchetta, I.; Tramutola, A.; Ceccarelli, V.; Lanzillotta, C.; Fontana, M.; Bertoccini, L.; Leonetti, F.; Capoccia, D.; et al. Reduced biliverdin reductase-A levels are associated with early alterations of insulin signaling in obesity. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1490–1501. [Google Scholar] [CrossRef]
- Canesin, G.; Hejazi, S.M.; Swanson, K.D.; Wegiel, B. Heme-Derived Metabolic Signals Dictate Immune Responses. Front. Immunol. 2020, 11, 66. [Google Scholar] [CrossRef]
- Bisht, K.; Canesin, G.; Cheytan, T.; Li, M.; Nemeth, Z.; Csizmadia, E.; Woodruff, T.M.; Stec, D.E.; Bulmer, A.C.; Otterbein, L.E.; et al. Deletion of Biliverdin Reductase A in Myeloid Cells Promotes Chemokine Expression and Chemotaxis in Part via a Complement C5a—C5aR1 Pathway. J. Immunol. 2019, 202, 2982–2990. [Google Scholar] [CrossRef]
- Nikouei, M.; Cheraghi, M.; Ghaempanah, F.; Kohneposhi, P.; Saniee, N.; Hemmatpour, S.; Moradi, Y. The association between bilirubin levels, and the incidence of metabolic syndrome and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Clin. Diabetes Endocrinol. 2024, 10, 1. [Google Scholar] [CrossRef]
- Hinds, T.D., Jr.; Hosick, P.A.; Chen, S.; Tukey, R.H.; Hankins, M.W.; Nestor-Kalinoski, A.; Stec, D.E. Mice with hyperbilirubinemia due to Gilbert’s syndrome polymorphism are resistant to hepatic steatosis by decreased serine 73 phosphorylation of PPARalpha. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E244–E252. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Qu, H.; Gu, R.; Chen, D.; Chen, X.; Yin, B.; Huang, Y.; Xi, W.; Wang, C.; Huang, Y. Proteomics study of the effect of high-fat diet on rat liver. Br. J. Nutr. 2019, 122, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shao, X.; Bao, Y.; Zhu, J.; Chen, L.; Zhang, L.; Ma, X.; Zhong, X.B. Impact of obese levels on the hepatic expression of nuclear receptors and drug-metabolizing enzymes in adult and offspring mice. Acta Pharm. Sin. B 2020, 10, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kulkarni, S.R.; Li, L.; Slitt, A.L. UDP-glucuronosyltransferase expression in mouse liver is increased in obesity- and fasting-induced steatosis. Drug Metab. Dispos. 2012, 40, 259–266. [Google Scholar] [CrossRef]
- Bates, E.A.; Kipp, Z.A.; Martinez, G.J.; Badmus, O.O.; Soundarapandian, M.M.; Foster, D.; Xu, M.; Creeden, J.F.; Greer, J.R.; Morris, A.J.; et al. Suppressing Hepatic UGT1A1 Increases Plasma Bilirubin, Lowers Plasma Urobilin, Reorganizes Kinase Signaling Pathways and Lipid Species and Improves Fatty Liver Disease. Biomolecules 2023, 13, 252. [Google Scholar] [CrossRef]
- Chang, J.H.; Plise, E.; Cheong, J.; Ho, Q.; Lin, M. Evaluating the in vitro inhibition of UGT1A1, OATP1B1, OATP1B3, MRP2, and BSEP in predicting drug-induced hyperbilirubinemia. Mol. Pharm. 2013, 10, 3067–3075. [Google Scholar] [CrossRef]
- Gammal, R.S.; Court, M.H.; Haidar, C.E.; Iwuchukwu, O.F.; Gaur, A.H.; Alvarellos, M.; Guillemette, C.; Lennox, J.L.; Whirl-Carrillo, M.; Brummel, S.S.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing. Clin. Pharmacol. Ther. 2016, 99, 363–369. [Google Scholar] [CrossRef]
- Alvarellos, M.; Guillemette, C.; Altman, R.B.; Klein, T.E. PharmGKB summary: Atazanavir pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet Genom. 2018, 28, 127–137. [Google Scholar] [CrossRef]
- Zucker, S.D.; Qin, X.; Rouster, S.D.; Yu, F.; Green, R.M.; Keshavan, P.; Feinberg, J.; Sherman, K.E. Mechanism of indinavir-induced hyperbilirubinemia. Proc. Natl. Acad. Sci. USA 2001, 98, 12671–12676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chando, T.J.; Everett, D.W.; Patten, C.J.; Dehal, S.S.; Humphreys, W.G. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab. Dispos. 2005, 33, 1729–1739. [Google Scholar] [CrossRef]
- Kimyai-Asadi, A.; Jih, M.H. Indirect hyperbilirubinemia caused by cyclosporine. J. Am. Acad. Dermatol. 2002, 47, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, M.; Kato, Y.; Tyson, C.A.; Sugiyama, Y. The Potential for an Interaction between MRP2 (ABCC2) and Various Therapeutic Agents: Probenecid as a Candidate Inhibitor of the Biliary Excretion of Irinotecan Metabolites. Drug Metab. Pharmacokinet. 2002, 17, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, E.-M.; Kim, I.-W.; Kim, D.-D.; Chung, S.-J.; Shim, C.-K. Effect of probenecid on the biliary excretion of belotecan. Arch. Pharmacal Res. 2007, 30, 1482–1488. [Google Scholar] [CrossRef]
- Hesselink, D.A.; van Hest, R.M.; Mathot, R.A.A.; Bonthuis, F.; Weimar, W.; de Bruin, R.W.F.; van Gelder, T. Cyclosporine Interacts with Mycophenolic Acid by Inhibiting the Multidrug Resistance-Associated Protein 2. Am. J. Transplant. 2005, 5, 987–994. [Google Scholar] [CrossRef]
- Westley, I.S.; Brogan, L.R.; Morris, R.G.; Evans, A.M.; Sallustio, B.C. Role of MRP2 in the Hepatic Disposition of Mycophenolic Acid and Its Glucuronide Metabolites: Effect of Cyclosporine. Drug Metab. Dispos. 2006, 34, 261–266. [Google Scholar] [CrossRef]
- Huang, C.; Xia, F.; Xue, L.; Liu, L.; Bian, Y.; Jin, Z.; Miao, L. Coadministration of vindesine with high-dose methotrexate therapy increases acute kidney injury via BCRP, MRP2, and OAT1/OAT3. Cancer Chemother. Pharmacol. 2020, 85, 433–441. [Google Scholar] [CrossRef]
- Barrington, R.D.; Needs, P.W.; Williamson, G.; Kroon, P.A. MK571 inhibits phase-2 conjugation of flavonols by Caco-2/TC7 cells, but does not specifically inhibit their apical efflux. Biochem. Pharmacol. 2015, 95, 193–200. [Google Scholar] [CrossRef]
- König, J.; Nies, A.T.; Cui, Y.; Leier, I.; Keppler, D. Conjugate export pumps of the multidrug resistance protein (MRP) family: Localization, substrate specificity, and MRP2-mediated drug resistance. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1999, 1461, 377–394. [Google Scholar] [CrossRef]
- Hinds, T.D., Jr.; Creeden, J.F.; Gordon, D.M.; Stec, D.F.; Donald, M.C.; Stec, D.E. Bilirubin Nanoparticles Reduce Diet-Induced Hepatic Steatosis, Improve Fat Utilization, and Increase Plasma beta-Hydroxybutyrate. Front. Pharmacol. 2020, 11, 594574. [Google Scholar] [CrossRef] [PubMed]
- Shinn, J.; Park, S.; Lee, S.; Park, N.; Kim, S.; Hwang, S.; Moon, J.J.; Kwon, Y.; Lee, Y. Antioxidative Hyaluronic Acid-Bilirubin Nanomedicine Targeting Activated Hepatic Stellate Cells for Anti-Hepatic-Fibrosis Therapy. ACS Nano 2024, 18, 4704–4716. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, Q.; Lee, W.H.; Funcke, J.B.; Zhang, Z.; Wang, M.Y.; Lin, Q.; Field, B.; Sun, X.N.; Li, G.; et al. The adiponectin-PPARgamma axis in hepatic stellate cells regulates liver fibrosis. Cell Rep. 2025, 44, 115165. [Google Scholar] [CrossRef]
- Lee, W.H.; Bates, E.A.; Kipp, Z.A.; Pauss, S.N.; Martinez, G.J.; Blair, C.A.; Hinds, T.D., Jr. Insulin receptor responsiveness governs TGFbeta-induced hepatic stellate cell activation: Insulin resistance instigates liver fibrosis. FASEB J. 2025, 39, e70427. [Google Scholar] [CrossRef]
- Bates, E.A.; Kipp, Z.A.; Lee, W.H.; Martinez, G.J.; Weaver, L.; Becker, K.N.; Pauss, S.N.; Creeden, J.F.; Anspach, G.B.; Helsley, R.N.; et al. FOXS1 is increased in liver fibrosis and regulates TGFbeta responsiveness and proliferation pathways in human hepatic stellate cells. J. Biol. Chem. 2024, 300, 105691. [Google Scholar] [CrossRef]
- Creeden, J.F.; Kipp, Z.A.; Xu, M.; Flight, R.M.; Moseley, H.N.B.; Martinez, G.J.; Lee, W.H.; Alganem, K.; Imami, A.S.; McMullen, M.R.; et al. Hepatic kinome atlas: An in-depth identification of kinase pathways in liver fibrosis of humans and rodents. Hepatology 2022, 76, 1376–1388. [Google Scholar] [CrossRef]
- Ai, W.; Bae, S.; Ke, Q.; Su, S.; Li, R.; Chen, Y.; Yoo, D.; Lee, E.; Jon, S.; Kang, P.M. Bilirubin Nanoparticles Protect Against Cardiac Ischemia/Reperfusion Injury in Mice. J. Am. Heart Assoc. 2021, 10, e021212. [Google Scholar] [CrossRef]
- Xia, X.; Sun, T.; Zhao, Y.; Sheng, H.; Dong, X.; Cheng, Y.; Wu, F.; Kou, L.; Chen, R.; Yao, Q.; et al. Bilirubin Nanoparticles Alleviate Sepsis-Induced Acute Lung Injury by Protecting Pulmonary Endothelia Glycocalyx and Reducing Inflammation. ACS Appl. Nano Mater. 2024, 7, 18566–18578. [Google Scholar] [CrossRef]
- Pareek, S.; Flegle, A.S.; Boagni, D.; Kim, J.Y.; Yoo, D.; Trujillo-Ocampo, A.; Lee, S.E.; Zhang, M.; Jon, S.; Im, J.S. Post Transplantation Bilirubin Nanoparticles Ameliorate Murine Graft Versus Host Disease via a Reduction of Systemic and Local Inflammation. Front. Immunol. 2022, 13, 893659. [Google Scholar] [CrossRef]

| Name | Solubility and Transport | Model | Ref. |
|---|---|---|---|
| Bilirubin IXα |
| 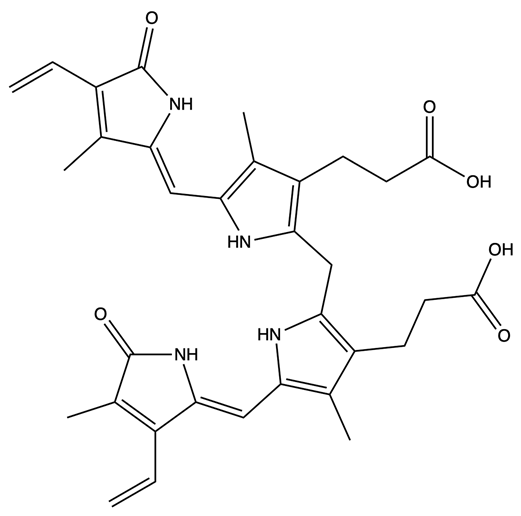 | [59] |
| Bilirubin IXα (ZE) |
|  | [60] |
| Bilirubin IXα (EZ) |
| 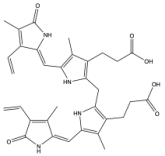 | [60] |
| Bilirubin IXα (EE) |
| 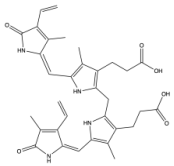 | [60] |
| Bilirubin XIIIα |
| 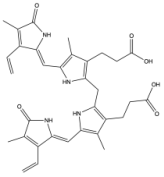 | [59,61] |
| Bilirubin IIIα |
| 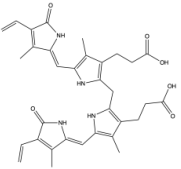 | [59] |
| Phylum | Produce BilR | Produce GUS |
|---|---|---|
| Bacteroidetes | ✗ | ✓ |
| Firmicutes | ✓ | ✓ |
| Proteobacteria | Some (Flavobacteria) | ✓ |
| Actinobacteria | Some (Bifidobacterium) | ✓ |
| Name | Properties | Clinical Relevance |
|---|---|---|
| Unconjugated Bilirubin |
|
|
| Conjugated Bilirubin |
|
|
| GUS Enzymes |
|
|
| BilR Enzyme |
|
|
| Urobilinogen |
|
|
| Urobilin |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kipp, Z.A.; Pauss, S.N.; Martinez, G.J.; Hinds, T.D., Jr.; Lee, W.-H. Bilirubin Hepatic and Intestinal Transport and Catabolism: Physiology, Pathophysiology, and Benefits. Antioxidants 2025, 14, 1326. https://doi.org/10.3390/antiox14111326
Kipp ZA, Pauss SN, Martinez GJ, Hinds TD Jr., Lee W-H. Bilirubin Hepatic and Intestinal Transport and Catabolism: Physiology, Pathophysiology, and Benefits. Antioxidants. 2025; 14(11):1326. https://doi.org/10.3390/antiox14111326
Chicago/Turabian StyleKipp, Zachary A., Sally N. Pauss, Genesee J. Martinez, Terry D. Hinds, Jr., and Wang-Hsin Lee. 2025. "Bilirubin Hepatic and Intestinal Transport and Catabolism: Physiology, Pathophysiology, and Benefits" Antioxidants 14, no. 11: 1326. https://doi.org/10.3390/antiox14111326
APA StyleKipp, Z. A., Pauss, S. N., Martinez, G. J., Hinds, T. D., Jr., & Lee, W.-H. (2025). Bilirubin Hepatic and Intestinal Transport and Catabolism: Physiology, Pathophysiology, and Benefits. Antioxidants, 14(11), 1326. https://doi.org/10.3390/antiox14111326








