Does the Maternal Gut Microbiome Influence the Outcome of Perinatal Asphyxia?
Abstract
1. Introduction
2. Perinatal Asphyxia: Pathophysiology and Outcomes
3. The Maternal Gut Microbiome: Composition, Functions, and Impact on Foetal Development
3.1. Factors Influencing Microbiome Composition
3.2. Maternal Health Implications
3.3. Direct Effects on Foetal Development
3.3.1. Immune System Maturation
3.3.2. Neurodevelopmental Processes
4. Neuroprotective Mechanisms Mediated by the Maternal Microbiome
4.1. Anti-Inflammatory Mechanisms
4.2. Antioxidant Pathways: Maternal Gut Barrier, Placenta, Blood-Brain-Barrier, Glial Cells, and Neurons
4.3. Neurogenesis, Neuroplasticity, and Neural Repair
4.4. The Maternal Microbiome, Oxytocin Signaling, and Perinatal Asphyxia—Direct Neuroprotection?
5. The Maternal Gut Microbiome Dysbiosis and Perinatal Asphyxia Outcomes
5.1. Mechanisms of Dysbiosis-Induced Neuronal Vulnerability
5.2. Experimental Findings
| Intervention | Model | Primary Action | Microbiome Effect | Perinatal Asphyxia (PA) Outcomes | Key Markers | References |
|---|---|---|---|---|---|---|
| High-fat diet | Sprague-Dawley (SD) rats, PA P7 | Metabolic dysregulation | ↓ Bacteroidetes, ↑ Firmicutes/Bacteroidetes ratio | ↑ Hippocampal injury | ↑ TNF-α, IL-1β, S100B | [219] |
| Gestational antibiotics | SD rats, PA birth | Microbiome depletion # | ↓ Diversity, ↓ Lactobacillus/Bifidobacterium | ↑ Brain injury, altered reflexes | ↑ S100B | [9] |
| Neonatal antibiotics | Mice, PA | Microbiome disruption # | ↓ short-chain fatty acid (SCFA) producers | ↑ Neuronal damage, gliosis | ↑ GFAP, Iba1 | [7] |
| Omega-3 PUFA | Mice, HI P9 | Anti-inflammatory | ↑ Butyrate producers 1* | ↓ Injury volume (5 weeks) | ↓ NF-κB, apoptosis | [222] |
| Resveratrol | SD rats, PA P7 | SIRT1 activation | ↑ Lactobacillus and Bifidobacterium 2* | ↓ Hippocampal damage | ↓ IL-1β, TNF-α | [220] |
| Citicoline | SD rats, PA P7 | Membrane stabilization | Maintains homeostasis 3* | Preserved hippocampus | ↓ Inflammation | [221] |
| Lactoferrin | Rats, PA | ↑ Nrf2, ↓ ferroptosis | ↑ Bifidobacterium and Lactobacillus 4* | ↓ Neuronal death | ↓ Ferroptosis | [226,227] |
| SCFAs | SD rats, PA P7 | Metabolic dysregulation | Direct metabolite | ↓ 30% infarct, ↑ neurogenesis | ↓ IL-1β, COX-2 | [194] |
| Probiotics † | SD rats, PA birth | Microbiome depletion # | ↑ Lactobacillus and Bifidobacterium, ↑ SCFAs | Blood-brain barrier (BBB) protection | ↓ Microglial activation | [8] |
5.3. Vertical Transmission and Neonatal Gut-Brain Axis Disruption
6. Therapeutic Interventions Targeting the Maternal Microbiome
6.1. Dietary Modifications
6.2. Emerging Microbiome-Based Therapeutic Strategies
6.2.1. Prebiotics: Enhancing Endogenous Microbial Metabolism
6.2.2. Probiotics: Augmenting Beneficial Microbial Populations
6.2.3. Postbiotics: Delivering Microbial Metabolites Directly
6.2.4. Synbiotics and Other Advanced Strategies
6.2.5. Addressing Harmful Metabolites and Novel Pharmacological Targets
7. Current Challenges and Future Research Directions
7.1. Methodological and Scientific Gaps
7.2. Ethical and Safety Concerns
7.3. Clinical Evidence and Limitations
7.4. Future Research Recommendations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-CMBT | 4-Chloro-α-(1-methylethyl)-N-2-thiazolyl-benzeneacetamide |
| AhR | Aryl hydrocarbon receptor |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| BBB | Blood-brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BEVs | Bacterial extracellular vesicles |
| BMI | Body mass index |
| COX-2 | Cyclooxygenase-2 |
| CSF | Cerebrospinal fluid |
| DHA | Docosahexaenoic acid |
| DMB | 3,3-dimethyl-1-butanol |
| F/B | Firmicutes/Bacteroidetes ratio |
| FDA | U.S. Food and Drug Administration |
| FFAR | Free fatty acid receptor |
| FFAR2 | Free fatty acid receptor 2 |
| FFAR3 | Free fatty acid receptor 3 |
| FMT | Faecal microbiota transplantation |
| FOS | Fructo-oligosaccharides |
| GABA | Gamma-aminobutyric acid |
| GFAP | Glial fibrillary acidic protein |
| GOS | Galacto-oligosaccharides |
| GPCRs | G protein-coupled receptors |
| H3 | Histone 3 |
| HDAC | Histone deacetylase |
| HI | Hypoxia-ischaemia |
| HIE | Hypoxic-ischaemic encephalopathy |
| Iba1 | Ionised calcium-binding adapter molecule 1 |
| IBD | Inflammatory bowel disease |
| IDO | Indoleamine 2,3-dioxygenase |
| IgG | Immunoglobulin G |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-17 | Interleukin-17 |
| IPA | Indole-3-propionic acid |
| JAK-STAT | Janus kinase/Signal Transducer and Activator of Transcription |
| LPS | Lipopolysaccharide |
| LRRC19 | Leucine Rich Repeat Containing 19 |
| MCAO | Middle cerebral artery occlusion |
| MGM | Maternal gut microbiota |
| MIA | Maternal immune activation |
| NADH | Nicotinamide adenine dinucleotide |
| NEC | Necrotising enterocolitis |
| NF-κB | Nuclear factor kappa B |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OXT | Oxytocin |
| P7/P9 | Postnatal day 7/9 |
| PA | Perinatal asphyxia |
| PI3K/AKT | Phosphoinositide 3-kinase/Protein kinase B |
| PKC | Protein kinase C |
| PUFA | Polyunsaturated fatty acids |
| PVL | Periventricular leukomalacia |
| ROS | Reactive oxygen species |
| S100B | S100 calcium-binding protein B |
| SCFAs | Short-chain fatty acids |
| SD | Sprague-Dawley |
| SIRT1 | Sirtuin 1 |
| SOD | Superoxide dismutase |
| TECs | Thymic epithelial cells |
| TGF-β | Transforming growth factor-beta |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| TMG | Trimethylglycine |
| TNF-α | Tumour necrosis factor-alpha |
| Tregs | Regulatory T cells |
References
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, Regional, and National Causes of under-5 Mortality in 2000–15: An Updated Systematic Analysis with Implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035, Erratum in Lancet 2017, 389, 1884. [Google Scholar] [CrossRef]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of Neonatal Encephalopathy and Hypoxic-Ischaemic Encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef]
- Oza, S.; Lawn, J.E.; Hogan, D.R.; Mathers, C.; Cousens, S.N. Neonatal Cause-of-Death Estimates for the Early and Late Neonatal Periods for 194 Countries: 2000–2013. Bull. World Health Organ. 2015, 93, 19–28. [Google Scholar] [CrossRef]
- Cannavò, L.; Perrone, S.; Gitto, E. Brain-Oriented Strategies for Neuroprotection of Asphyxiated Newborns in the First Hours of Life. Pediatr. Neurol. 2023, 143, 44–49. [Google Scholar] [CrossRef]
- Lear, B.A.; Zhou, K.Q.; Dhillon, S.K.; Lear, C.A.; Bennet, L.; Gunn, A.J. Preventive, Rescue and Reparative Neuroprotective Strategies for the Fetus and Neonate. Semin. Fetal Neonatal Med. 2024, 29, 101542. [Google Scholar] [CrossRef]
- Wassink, G.; Davidson, J.O.; Dhillon, S.K.; Zhou, K.; Bennet, L.; Thoresen, M.; Gunn, A.J. Therapeutic Hypothermia in Neonatal Hypoxic-Ischemic Encephalopathy. Curr. Neurol. Neurosci. Rep. 2019, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Drobyshevsky, A.; Synowiec, S.; Goussakov, I.; Fabres, R.; Lu, J.; Caplan, M. Intestinal Microbiota Modulates Neuroinflammatory Response and Brain Injury after Neonatal Hypoxia-Ischemia. Gut Microbes 2024, 16, 2333808. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lu, L.; Yu, Y.; Baranowski, J.; Claud, E.C. Maternal Administration of Probiotics Promotes Brain Development and Protects Offspring’s Brain from Postnatal Inflammatory Insults in C57/BL6J Mice. Sci. Rep. 2020, 10, 8178. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.I.; Maria Catrina, A.; Dogaru, I.A.; Catalina Barbalata, D.; Ciotei, C.; Haidoiu, C.; Suhaianu, V.; Gradisteanu Pircalabioru, G.; O’Mahony, S.M.; Zagrean, A.-M. MICROBIOME: The Trials and Errors of Developing an Experimental Model to Study the Impact of Maternal Gut Microbiome Disruption on Perinatal Asphyxia. Reprod. Fertil. 2024, 5, e240050. [Google Scholar] [CrossRef]
- Husso, A.; Pessa-Morikawa, T.; Koistinen, V.M.; Kärkkäinen, O.; Kwon, H.N.; Lahti, L.; Iivanainen, A.; Hanhineva, K.; Niku, M. Impacts of Maternal Microbiota and Microbial Metabolites on Fetal Intestine, Brain, and Placenta. BMC Biol. 2023, 21, 207. [Google Scholar] [CrossRef]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Glass, C.K.; Mazmanian, S.K. Microbiome-Microglia Connections via the Gut-Brain Axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, M.; Chen, S.; Tang, Y.; Cui, J.; Ding, G. Short-Chain Fatty Acids in Fetal Development and Metabolism. Trends Mol. Med. 2024, 31, 625–639. [Google Scholar] [CrossRef]
- Lu, X.; Shi, Z.; Jiang, L.; Zhang, S. Maternal Gut Microbiota in the Health of Mothers and Offspring: From the Perspective of Immunology. Front. Immunol. 2024, 15, 1362784. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M. The Impact of Maternal Gut Microbiota during Pregnancy on Fetal Gut-Brain Axis Development and Life-Long Health Outcomes. Microorganisms 2023, 11, 2199. [Google Scholar] [CrossRef]
- Bennet, L. Sex, Drugs and Rock and Roll: Tales from Preterm Fetal Life. J. Physiol. 2017, 595, 1865–1881. [Google Scholar] [CrossRef]
- Giussani, D.A. The Fetal Brain Sparing Response to Hypoxia: Physiological Mechanisms: Fetal Brain Sparing. J. Physiol. 2016, 594, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Berger, R. Fetal Circulatory Responses to Oxygen Lack. J. Dev. Physiol. 1991, 16, 181–207. [Google Scholar]
- Okazaki, K.; Nakamura, S.; Koyano, K.; Konishi, Y.; Kondo, M.; Kusaka, T. Neonatal Asphyxia as an Inflammatory Disease: Reactive Oxygen Species and Cytokines. Front. Pediatr. 2023, 11, 1070743. [Google Scholar] [CrossRef]
- Szydlowska, K.; Tymianski, M. Calcium, Ischemia and Excitotoxicity. Cell Calcium 2010, 47, 122–129. [Google Scholar] [CrossRef]
- Hartings, J.A.; Shuttleworth, C.W.; Kirov, S.A.; Ayata, C.; Hinzman, J.M.; Foreman, B.; Andrew, R.D.; Boutelle, M.G.; Brennan, K.C.; Carlson, A.P.; et al. The Continuum of Spreading Depolarizations in Acute Cortical Lesion Development: Examining Leão’s Legacy. J. Cereb. Blood Flow Metab. 2017, 37, 1571–1594. [Google Scholar] [CrossRef] [PubMed]
- Wassink, G.; Gunn, E.R.; Drury, P.P.; Bennet, L.; Gunn, A.J. The Mechanisms and Treatment of Asphyxial Encephalopathy. Front. Neurosci. 2014, 8, 40. [Google Scholar] [CrossRef]
- Iwata, O.; Iwata, S.; Thornton, J.S.; De Vita, E.; Bainbridge, A.; Herbert, L.; Scaravilli, F.; Peebles, D.; Wyatt, J.S.; Cady, E.B.; et al. “Therapeutic Time Window” Duration Decreases with Increasing Severity of Cerebral Hypoxia-Ischaemia under Normothermia and Delayed Hypothermia in Newborn Piglets. Brain Res. 2007, 1154, 173–180. [Google Scholar] [PubMed]
- Hostetter, M.K. Society for Pediatric Research Presidential Address 1994: Yeast as Metaphor. Pediatr. Res. 1994, 36, 692–698. [Google Scholar] [CrossRef][Green Version]
- Thornton, C.; Leaw, B.; Mallard, C.; Nair, S.; Jinnai, M.; Hagberg, H. Cell Death in the Developing Brain after Hypoxia-Ischemia. Front. Cell. Neurosci. 2017, 11, 248. [Google Scholar] [CrossRef]
- Rodriguez, J.; Xie, C.; Li, T. Inhibiting the Interaction between Apoptosisinducing Factor and Cyclophilin A Prevents Brain Injury in Neonatal Mice after Hypoxia-Ischemia. Neuropharmacology 2020, 171, 108088. [Google Scholar] [CrossRef]
- Shaw, J.C.; Crombie, G.K.; Zakar, T.; Palliser, H.K.; Hirst, J.J. Perinatal Compromise Contributes to Programming of GABAergic and Glutamatergic Systems Leading to Long-Term Effects on Offspring Behaviour. J. Neuroendocrinol. 2020, 32, e12814. [Google Scholar] [CrossRef]
- Shaw, J.C.; Berry, M.J.; Dyson, R.M.; Crombie, G.K.; Hirst, J.J.; Palliser, H.K. Reduced Neurosteroid Exposure Following Preterm Birth and Its’ Contribution to Neurological Impairment: A Novel Avenue for Preventative Therapies. Front. Physiol. 2019, 10, 599. [Google Scholar] [CrossRef]
- Fleiss, B.; Gressens, P. Tertiary Mechanisms of Brain Damage: A New Hope for Treatment of Cerebral Palsy? Lancet Neurol. 2012, 11, 556–566. [Google Scholar] [CrossRef]
- Bennet, L.; Dhillon, S.; Lear, C.A.; van den Heuij, L.; King, V.; Dean, J.M.; Wassink, G.; Davidson, J.O.; Gunn, A.J. Chronic Inflammation and Impaired Development of the Preterm Brain. J. Reprod. Immunol. 2018, 125, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.K.; Gunn, E.R.; Lear, B.A.; King, V.J.; Lear, C.A.; Wassink, G.; Davidson, J.O.; Bennet, L.; Gunn, A.J. Cerebral Oxygenation and Metabolism after Hypoxia-Ischemia. Front. Pediatr. 2022, 10, 925951. [Google Scholar] [CrossRef] [PubMed]
- Buser, J.R.; Maire, J.; Riddle, A.; Gong, X.; Nguyen, T.; Nelson, K.; Luo, N.L.; Ren, J.; Struve, J.; Sherman, L.S.; et al. Arrested Preoligodendrocyte Maturation Contributes to Myelination Failure in Premature Infants. Ann. Neurol. 2012, 71, 93–109. [Google Scholar] [CrossRef]
- Dean, J.M.; Mcclendon, E.; Hansen, K.; Azimi-Zonooz, A.; Chen, K.; Riddle, A.; Gong, X.; Sharifnia, E.; Hagen, M.; Ahmad, T.; et al. Prenatal Cerebral Ischemia Disrupts MRIdefined Cortical Microstructure through Disturbances in Neuronal Arborization. Sci. Transl. Med. 2013, 5, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Villanueva-García, D.; Solimano, A.; Muns, R.; Ibarra-Ríos, D.; Mota-Reyes, A. Pathophysiology of Perinatal Asphyxia in Humans and Animal Models. Biomedicines 2022, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Rodríguez, M.; Harmony, T.; Carrillo-Prado, C.; Van Horn, J.D.; Irimia, A.; Torgerson, C.; Jacokes, Z. Clinical Neuroimaging in the Preterm Infant: Diagnosis and Prognosis. NeuroImage Clin. 2017, 16, 355–368. [Google Scholar] [CrossRef]
- Banker, B.Q.; Larroche, J.C. Periventricular Leukomalacia of Infancy. A Form of Neonatal Anoxic Encephalopathy. Arch. Neurol. 1962, 7, 386–410. [Google Scholar] [CrossRef]
- Schneider, J.; Miller, S.P. Preterm Brain Injury: White Matter Injury. Handb. Clin. Neurol. 2019, 162, 155–172. [Google Scholar]
- Volpe, J.J.; Kinney, H.C.; Jensen, F.E.; Rosenberg, P.A. The Developing Oligodendrocyte: Key Cellular Target in Brain Injury in the Premature Infant. Int. J. Dev. Neurosci. 2011, 29, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Nikas, I.; Dermentzoglou, V.; Theofanopoulou, M.; Theodoropoulos, V. Parasagittal Lesions and Ulegyria in Hypoxic-Ischemic Encephalopathy: Neuroimaging Findings and Review of the Pathogenesis. J. Child Neurol. 2008, 23, 51–58. [Google Scholar] [CrossRef]
- Rivkin, M.J. Hypoxic-Ischemic Brain Injury in the Term Newborn. Neuropathology, Clinical Aspects, and Neuroimaging. Clin. Perinatol. 1997, 24, 607–625. [Google Scholar] [CrossRef]
- Groenendaal, F.; de Vries, L.S. Fifty Years of Brain Imaging in Neonatal Encephalopathy Following Perinatal Asphyxia. Pediatr. Res. 2017, 81, 150–155. [Google Scholar] [CrossRef]
- Shah, P.; Anvekar, A.; McMichael, J.; Rao, S. Outcomes of Infants with Apgar Score of Zero at 10 Min: The West Australian Experience. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F492–F494. [Google Scholar] [CrossRef]
- Milner, K.M.; Neal, E.F.G.; Roberts, G.; Steer, A.C.; Duke, T. Long-Term Neurodevelopmental Outcome in High-Risk Newborns in Resource-Limited Settings: A Systematic Review of the Literature. Paediatr. Int. Child Health 2015, 35, 227–242. [Google Scholar] [CrossRef]
- Omizzolo, C.; Scratch, S.E.; Stargatt, R.; Kidokoro, H.; Thompson, D.K.; Lee, K.J.; Cheong, J.; Neil, J.; Inder, T.E.; Doyle, L.W.; et al. Neonatal Brain Abnormalities and Memory and Learning Outcomes at 7 Years in Children Born Very Preterm. Memory 2014, 22, 605–615. [Google Scholar] [CrossRef]
- Stipdonk, L.W.; Franken, M.-C.J.P.; Dudink, J. Language Outcome Related to Brain Structures in School-Aged Preterm Children: A Systematic Review. PLoS ONE 2018, 13, e0196607, Erratum in PLoS ONE 2018, 13, e0203298. [Google Scholar]
- Wheelock, M.D.; Austin, N.C.; Bora, S.; Eggebrecht, A.T.; Melzer, T.R.; Woodward, L.J.; Smyser, C.D. Altered Functional Network Connectivity Relates to Motor Development in Children Born Very Preterm. NeuroImage 2018, 183, 574–583. [Google Scholar] [CrossRef]
- Lee, B.L.; Gano, D.; Rogers, E.E.; Xu, D.; Cox, S.; James Barkovich, A.; Li, Y.; Ferriero, D.M.; Glass, H.C. Long-Term Cognitive Outcomes in Term Newborns with Watershed Injury Caused by Neonatal Encephalopathy. Pediatr. Res. 2022, 92, 505–512. [Google Scholar] [CrossRef]
- Ahearne, C.E.; Boylan, G.B.; Murray, D.M. Short and Long Term Prognosis in Perinatal Asphyxia: An Update. World J. Clin. Pediatr. 2016, 5, 67–74. [Google Scholar] [CrossRef] [PubMed]
- de Haan, M.; Wyatt, J.S.; Roth, S.; Vargha-Khadem, F.; Gadian, D.; Mishkin, M. Brain and Cognitive-Behavioural Development after Asphyxia at Term Birth. Dev. Sci. 2006, 9, 350–358. [Google Scholar] [CrossRef]
- Matara, D.-I.; Pouliakis, A.; Xanthos, T.; Sokou, R.; Kafalidis, G.; Iliodromiti, Z.; Boutsikou, T.; Iacovidou, N.; Salakos, C. Microbial Translocation and Perinatal Asphyxia/Hypoxia: A Systematic Review. Diagnostics 2022, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018, 24, 133–145.e5. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guo, R.; Li, S.; Liang, F.; Tian, C.; Zhao, X.; Long, Y.; Liu, F.; Jiang, M.; Zhang, Y.; et al. Systematic Analysis of Gut Microbiota in Pregnant Women and Its Correlations with Individual Heterogeneity. NPJ Biofilms Microbiomes 2020, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Smid, M.C.; Ricks, N.M.; Panzer, A.; Mccoy, A.N.; Azcarate-Peril, M.A.; Keku, T.O.; Boggess, K.A. Maternal Gut Microbiome Biodiversity in Pregnancy. Am. J. Perinatol. 2018, 35, 24–30. [Google Scholar]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Chaumeil, P.-A.; Rinke, C.; Mussig, A.J.; Hugenholtz, P. A Complete Domain-to-Species Taxonomy for Bacteria and Archaea. Nat. Biotechnol. 2020, 38, 1079–1086, Erratum in Nat. Biotechnol. 2020, 38, 1098. [Google Scholar] [CrossRef]
- Buttó, L.F.; Haller, D. Dysbiosis in Intestinal Inflammation: Cause or Consequence. Int. J. Med. Microbiol. 2016, 306, 302–309. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Vuong, H.E.; Pronovost, G.N.; Williams, D.W.; Coley, E.J.L.; Siegler, E.L.; Qiu, A.; Kazantsev, M.; Wilson, C.J.; Rendon, T.; Hsiao, E.Y. The Maternal Microbiome Modulates Fetal Neurodevelopment in Mice. Nature 2020, 586, 281–286. [Google Scholar] [CrossRef]
- Ruiz-Triviño, J.; Álvarez, D.; Cadavid J, Á.P.; Alvarez, A.M. From Gut to Placenta: Understanding How the Maternal Microbiome Models Life-Long Conditions. Front. Endocrinol. 2023, 14, 1304727. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Urbano, F.; Anaclerio, F.; Moscogiuri, L.A.; Konstantinidou, F.; Stuppia, L.; Gatta, V. Exploring Maternal Diet-Epigenetic-Gut Microbiome Crosstalk as an Intervention Strategy to Counter Early Obesity Programming. Curr. Issues Mol. Biol. 2024, 46, 4358–4378. [Google Scholar] [CrossRef] [PubMed]
- Ziętek, M.; Celewicz, Z.; Szczuko, M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients 2021, 13, 1244. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The Role of the Gut Microbiome and Its Metabolites in Metabolic Diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Beckers, K.F.; Sones, J.L. Maternal Microbiome and the Hypertensive Disorder of Pregnancy, Preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1–H10. [Google Scholar] [CrossRef] [PubMed]
- Deady, C.; FitzGerard, J.; Kara, N.; Mazzocchi, M.; O’Mahony, A.; Ionescu, M.I.; Shanahan, R.; Kelly, S.; Crispie, F.; Cotter, P.D.; et al. Maternal Immune Activation and Antibiotics Affect Offspring Neurodevelopment, Behaviour, and Microbiome. Brain Behav. Immun. Health 2025, 48, 101065. [Google Scholar] [CrossRef]
- Mann, P.E.; Huynh, K.; Widmer, G. Maternal High Fat Diet and Its Consequence on the Gut Microbiome: A Rat Model. Gut Microbes 2018, 9, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Gohir, W.; Whelan, F.J.; Surette, M.G.; Moore, C.; Schertzer, J.D.; Sloboda, D.M. Pregnancy-Related Changes in the Maternal Gut Microbiota Are Dependent upon the Mother’s Periconceptional Diet. Gut Microbes 2015, 6, 310–320. [Google Scholar] [CrossRef]
- Grant, E.T.; Boudaud, M.; Muller, A.; Macpherson, A.J.; Desai, M.S. Maternal Diet and Gut Microbiome Composition Modulate Early-Life Immune Development. EMBO Mol. Med. 2023, 15, e17241. [Google Scholar] [CrossRef]
- Barrientos, G.; Ronchi, F.; Conrad, M.L. Nutrition during Pregnancy: Influence on the Gut Microbiome and Fetal Development. Am. J. Reprod. Immunol. 2024, 91, e13802. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Selma-Royo, M.; González, S.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Distinct Maternal Microbiota Clusters Are Associated with Diet during Pregnancy: Impact on Neonatal Microbiota and Infant Growth during the First 18 Months of Life. Gut Microbes 2020, 11, 962–978. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, T.; Wu, Y.; Liu, Y.; Zou, Z.; Bai, J. Impacts of Maternal Diet and Alcohol Consumption during Pregnancy on Maternal and Infant Gut Microbiota. Biomolecules 2021, 11, 369. [Google Scholar] [CrossRef]
- Degroote, S.; Hunting, D.J.; Baccarelli, A.A.; Takser, L. Maternal Gut and Fetal Brain Connection: Increased Anxiety and Reduced Social Interactions in Wistar Rat Offspring Following Peri-Conceptional Antibiotic Exposure. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 71, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Tye, K.D.; Luo, H.; Tang, X.; Liao, Y.; Wang, D.; Zhou, J.; Yang, P.; Li, Y.; et al. Probiotic Supplementation during Human Pregnancy Affects the Gut Microbiota and Immune Status. Front. Cell. Infect. Microbiol. 2019, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Dekker Nitert, M.; Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Anderson, G.J.; Frazer, D.M.; Callaway, L.K. Iron Supplementation Has Minor Effects on Gut Microbiota Composition in Overweight and Obese Women in Early Pregnancy. Br. J. Nutr. 2018, 120, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, G.; Cui, L.; Zhang, L.; Zhou, Q.; Mu, C.; Chi, R.; Zhang, N.; Ma, G. Dynamic Changes in Gut Microbiota during Pregnancy among Chinese Women and Influencing Factors: A Prospective Cohort Study. Front. Microbiol. 2023, 14, 1114228. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M.; SPRING Trial Group. Connections between the Gut Microbiome and Metabolic Hormones in Early Pregnancy in Overweight and Obese Women. Diabetes 2016, 65, 2214–2223. [Google Scholar] [CrossRef]
- Torres, J.; Hu, J.; Seki, A.; Eisele, C.; Nair, N.; Huang, R.; Tarassishin, L.; Jharap, B.; Cote-Daigneault, J.; Mao, Q.; et al. Infants Born to Mothers with IBD Present with Altered Gut Microbiome That Transfers Abnormalities of the Adaptive Immune System to Germ-Free Mice. Gut 2020, 69, 42–51. [Google Scholar] [CrossRef]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Maternal and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335.e4. [Google Scholar] [CrossRef]
- Peng, Y.; Tun, H.M.; Ng, S.C.; Wai, H.K.-F.; Zhang, X.; Parks, J.; Field, C.J.; Mandhane, P.; Moraes, T.J.; Simons, E.; et al. Maternal Smoking during Pregnancy Increases the Risk of Gut Microbiome-Associated Childhood Overweight and Obesity. Gut Microbes 2024, 16, 2323234. [Google Scholar] [CrossRef]
- Santarossa, S.; Sitarik, A.R.; Cassidy-Bushrow, A.E.; Comstock, S.S. Prenatal Physical Activity and the Gut Microbiota of Pregnant Women: Results from a Preliminary Investigation. Phys. Act. Nutr. 2023, 27, 1–7. [Google Scholar] [CrossRef]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in Gut Microbiota Profile between Women with Active Lifestyle and Sedentary Women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef]
- Ryan, N.; O’Mahony, S.; Leahy-Warren, P.; Philpott, L.; Mulcahy, H. The Impact of Perinatal Maternal Stress on the Maternal and Infant Gut and Human Milk Microbiomes: A Scoping Review. PLoS ONE 2025, 20, e0318237. [Google Scholar] [CrossRef]
- Baker, M. Pregnancy Alters Resident Gut Microbes. Nature 2012, 488, 123. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Plagemann, A.; Sommer, J.; Hofmann, M.; Henrich, W.; Barrett, J.F.R.; Surette, M.G.; Atkinson, S.; Braun, T.; Sloboda, D.M. Parity Modulates Impact of BMI and Gestational Weight Gain on Gut Microbiota in Human Pregnancy. Gut Microbes 2023, 15, 2259316. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The Impact of the Gut Microbiota on the Reproductive and Metabolic Endocrine System. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef]
- Amato, K.R.; Pradhan, P.; Mallott, E.K.; Shirola, W.; Lu, A. Host-Gut Microbiota Interactions during Pregnancy. Evol. Med. Public Health 2024, 12, 7–23. [Google Scholar] [CrossRef]
- DuPont, H.L.; Salge, M.M.H. The Importance of a Healthy Microbiome in Pregnancy and Infancy and Microbiota Treatment to Reverse Dysbiosis for Improved Health. Antibiotics 2023, 12, 1617. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.; Liu, M.; Zheng, H.; He, Y.; Chen, M.-X.; Tang, W.; Yue, X.; Huang, Y.; Zhuang, L.; et al. Gut Dysbiosis Induces the Development of Pre-Eclampsia through Bacterial Translocation. Gut 2020, 69, 513–522. [Google Scholar] [CrossRef]
- Dunlop, A.L.; Mulle, J.G.; Ferranti, E.P.; Edwards, S.; Dunn, A.B.; Corwin, E.J. Maternal Microbiome and Pregnancy Outcomes That Impact Infant Health: A Review: A Review. Adv. Neonatal Care 2015, 15, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Bauman, M.D.; Iosif, A.-M.; Smith, S.E.P.; Bregere, C.; Amaral, D.G.; Patterson, P.H. Activation of the Maternal Immune System during Pregnancy Alters Behavioral Development of Rhesus Monkey Offspring. Biol. Psychiatry 2014, 75, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Jarde, A.; Lewis-Mikhael, A.-M.; Moayyedi, P.; Stearns, J.C.; Collins, S.M.; Beyene, J.; McDonald, S.D. Pregnancy Outcomes in Women Taking Probiotics or Prebiotics: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth 2018, 18, 14. [Google Scholar] [CrossRef]
- Rao, S.C.; Athalye-Jape, G.K.; Deshpande, G.C.; Simmer, K.N.; Patole, S.K. Probiotic Supplementation and Late-Onset Sepsis in Preterm Infants: A Meta-Analysis. Pediatrics 2016, 137, e20153684. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Maggio, L.; Beghetti, I.; Gori, D.; Barone, G.; Callegari, M.L.; Fantini, M.P.; Indrio, F.; Meneghin, F.; Morelli, L.; et al. Probiotics Prevent Late-Onset Sepsis in Human Milk-Fed, Very Low Birth Weight Preterm Infants: Systematic Review and Meta-Analysis. Nutrients 2017, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Buggio, L.; Somigliana, E.; Borghi, A.; Vercellini, P. Probiotics and Vaginal Microecology: Fact or Fancy? BMC Womens Health 2019, 19, 25. [Google Scholar] [CrossRef]
- Pronovost, G.N.; Yu, K.B.; Coley-O’Rourke, E.J.L.; Telang, S.S.; Chen, A.S.; Vuong, H.E.; Williams, D.W.; Chandra, A.; Rendon, T.K.; Paramo, J.; et al. The Maternal Microbiome Promotes Placental Development in Mice. Sci. Adv. 2023, 9, eadk1887. [Google Scholar] [CrossRef]
- Lopez-Tello, J.; Schofield, Z.; Kiu, R.; Dalby, M.J.; van Sinderen, D.; Le Gall, G.; Sferruzzi-Perri, A.N.; Hall, L.J. Maternal Gut Microbiota Bifidobacterium Promotes Placental Morphogenesis, Nutrient Transport and Fetal Growth in Mice. Cell. Mol. Life Sci. 2022, 79, 386. [Google Scholar] [CrossRef]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A Critical Assessment of the “Sterile Womb” and “in Utero Colonization” Hypotheses: Implications for Research on the Pioneer Infant Microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Kennedy, K.M.; de Goffau, M.C.; Perez-Muñoz, M.E.; Arrieta, M.-C.; Bäckhed, F.; Bork, P.; Braun, T.; Bushman, F.D.; Dore, J.; de Vos, W.M.; et al. Questioning the Fetal Microbiome Illustrates Pitfalls of Low-Biomass Microbial Studies. Nature 2023, 613, 639–649. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, Z.; Chai, Y.; Yao, W.; Ma, G. Unveiling the Placental Bacterial Microbiota: Implications for Maternal and Infant Health. Front. Physiol. 2025, 16, 1544216. [Google Scholar] [CrossRef]
- Ygberg, S.; Nilsson, A. The Developing Immune System—From Foetus to Toddler. Acta Paediatr. 2012, 101, 120–127. [Google Scholar] [CrossRef]
- McDavid, A.; Laniewski, N.; Grier, A.; Gill, A.L.; Kessler, H.A.; Huyck, H.; Carbonell, E.; Holden-Wiltse, J.; Bandyopadhyay, S.; Carnahan, J.; et al. Aberrant Newborn T Cell and Microbiota Developmental Trajectories Predict Respiratory Compromise during Infancy. iScience 2022, 25, 104007. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; O’Hely, M.; Quinn, T.P.; Ponsonby, A.-L.; Harrison, L.C.; Frøkiær, H.; Tang, M.L.K.; Brix, S.; Kristiansen, K.; Burgner, D.; et al. Maternal Gut Microbiota during Pregnancy and the Composition of Immune Cells in Infancy. Front. Immunol. 2022, 13, 986340. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, G.; Hicks, A.L.; Tekieli, T.M.; Radens, C.M.; Williams, B.L.; Lamousé-Smith, E.S.N. Maternal Antibiotic Treatment Impacts Development of the Neonatal Intestinal Microbiome and Antiviral Immunity. J. Immunol. 2016, 196, 3768–3779. [Google Scholar] [CrossRef]
- Levan, S.R.; Stamnes, K.A.; Lin, D.L.; Panzer, A.R.; Fukui, E.; McCauley, K.; Fujimura, K.E.; McKean, M.; Ownby, D.R.; Zoratti, E.M.; et al. Elevated Faecal 12,13-DiHOME Concentration in Neonates at High Risk for Asthma Is Produced by Gut Bacteria and Impedes Immune Tolerance. Nat. Microbiol. 2019, 4, 1851–1861, Erratum in Nat. Microbiol. 2019, 4, 2020. [Google Scholar] [CrossRef]
- Gao, Y.; Nanan, R.; Macia, L.; Tan, J.; Sominsky, L.; Quinn, T.P.; O’Hely, M.; Ponsonby, A.-L.; Tang, M.L.K.; Collier, F.; et al. The Maternal Gut Microbiome during Pregnancy and Offspring Allergy and Asthma. J. Allergy Clin. Immunol. 2021, 148, 669–678. [Google Scholar] [CrossRef]
- de Moraes-Pinto, M.I.; Suano-Souza, F.; Aranda, C.S. Immune System: Development and Acquisition of Immunological Competence. J. Pediatr. (Rio J.) 2021, 97 (Suppl. 1), S59–S66. [Google Scholar] [CrossRef]
- Timm, S.; Schlünssen, V.; Olsen, J.; Ramlau-Hansen, C.H. Prenatal Antibiotics and Atopic Dermatitis among 18-Month-Old Children in the Danish National Birth Cohort. Clin. Exp. Allergy 2017, 47, 929–936. [Google Scholar] [CrossRef]
- Faas, M.M.; Smink, A.M. Shaping Immunity: The Influence of the Maternal Gut Bacteria on Fetal Immune Development. Semin. Immunopathol. 2025, 47, 13. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, S.; Li, M.; Yang, Y.; Ma, J.; Xie, R.; Wang, J.; Zhao, Q.; Qin, S.; He, L.; et al. Maternal High-Fat Diet Disrupts Intestinal Mucus Barrier of Offspring by Regulating Gut Immune Receptor LRRC19. Commun. Biol. 2025, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.T.; Niu, X.; Raetz, M.; Savani, R.C.; Hooper, L.V.; Mirpuri, J. Maternal High-Fat Diet Results in Microbiota-Dependent Expansion of ILC3s in Mice Offspring. JCI Insight 2018, 3, e99223. [Google Scholar] [CrossRef]
- Wallace, J.G.; Bellissimo, C.J.; Yeo, E.; Fei Xia, Y.; Petrik, J.J.; Surette, M.G.; Bowdish, D.M.E.; Sloboda, D.M. Obesity during Pregnancy Results in Maternal Intestinal Inflammation, Placental Hypoxia, and Alters Fetal Glucose Metabolism at Mid-Gestation. Sci. Rep. 2019, 9, 17621. [Google Scholar] [CrossRef]
- Liang, W.; Feng, Y.; Yang, D.; Qin, J.; Zhi, X.; Wu, W.; Jie, Q. Oral Probiotics Increased the Proportion of Treg, Tfr, and Breg Cells to Inhibit the Inflammatory Response and Impede Gestational Diabetes Mellitus. Mol. Med. 2023, 29, 122. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, C.; Selle, A.; Duval, A.; Misme-Aucouturier, B.; Chesneau, M.; Brouard, S.; Cherbuy, C.; Cariou, V.; Bouchaud, G.; Mincham, K.T.; et al. Prebiotic Supplementation during Pregnancy Modifies the Gut Microbiota and Increases Metabolites in Amniotic Fluid, Driving a Tolerogenic Environment in Utero. Front. Immunol. 2021, 12, 712614. [Google Scholar] [CrossRef]
- Hu, M.; Eviston, D.; Hsu, P.; Mariño, E.; Chidgey, A.; Santner-Nanan, B.; Wong, K.; Richards, J.L.; Yap, Y.A.; Collier, F.; et al. Decreased Maternal Serum Acetate and Impaired Fetal Thymic and Regulatory T Cell Development in Preeclampsia. Nat. Commun. 2019, 10, 3031. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence That Asthma Is a Developmental Origin Disease Influenced by Maternal Diet and Bacterial Metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450, Erratum in Nature 2014, 506, 254. [Google Scholar] [CrossRef]
- Jain, N. The Early Life Education of the Immune System: Moms, Microbes and (Missed) Opportunities. Gut Microbes 2020, 12, 1824564. [Google Scholar] [CrossRef]
- Humann, J.; Mann, B.; Gao, G.; Moresco, P.; Ramahi, J.; Loh, L.N.; Farr, A.; Hu, Y.; Durick-Eder, K.; Fillon, S.A.; et al. Bacterial Peptidoglycan Traverses the Placenta to Induce Fetal Neuroproliferation and Aberrant Postnatal Behavior. Cell Host Microbe 2016, 19, 388–399. [Google Scholar] [CrossRef]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The Maternal Microbiota Drives Early Postnatal Innate Immune Development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Kaisanlahti, A.; Turunen, J.; Byts, N.; Samoylenko, A.; Bart, G.; Virtanen, N.; Tejesvi, M.V.; Zhyvolozhnyi, A.; Sarfraz, S.; Kumpula, S.; et al. Maternal Microbiota Communicates with the Fetus through Microbiota-Derived Extracellular Vesicles. Microbiome 2023, 11, 249. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and Macrophages in Brain Homeostasis and Disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Zengeler, K.E.; Lukens, J.R. Innate Immunity at the Crossroads of Healthy Brain Maturation and Neurodevelopmental Disorders. Nat. Rev. Immunol. 2021, 21, 454–468. [Google Scholar] [CrossRef]
- Yeo, X.Y.; Choi, Y.; Hong, Y.; Kwon, H.N.; Jung, S. Contemporary Insights into Neuroimmune Interactions across Development and Aging. Front. Neurol. 2025, 16, 1611124. [Google Scholar] [CrossRef] [PubMed]
- Gohlke, J.M.; Stockton, P.S.; Sieber, S.; Foley, J.; Portier, C.J. AhR-Mediated Gene Expression in the Developing Mouse Telencephalon. Reprod. Toxicol. 2009, 28, 321–328. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal Gut Bacteria Promote Neurodevelopmental Abnormalities in Mouse Offspring. Nature 2017, 549, 528–532. [Google Scholar] [CrossRef]
- Kim, E.; Paik, D.; Ramirez, R.N.; Biggs, D.G.; Park, Y.; Kwon, H.-K.; Choi, G.B.; Huh, J.R. Maternal Gut Bacteria Drive Intestinal Inflammation in Offspring with Neurodevelopmental Disorders by Altering the Chromatin Landscape of CD4+ T Cells. Immunity 2022, 55, 145–158.e7. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The Maternal Interleukin-17a Pathway in Mice Promotes Autism-like Phenotypes in Offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef]
- Sun, Z.; Lee-Sarwar, K.; Kelly, R.S.; Lasky-Su, J.A.; Litonjua, A.A.; Weiss, S.T.; Liu, Y.-Y. Revealing the Importance of Prenatal Gut Microbiome in Offspring Neurodevelopment in Humans. EBioMedicine 2023, 90, 104491. [Google Scholar] [CrossRef]
- Surzenko, N.; Pjetri, E.; Munson, C.A.; Friday, W.B.; Hauser, J.; Mitchell, E.S. Prenatal Exposure to the Probiotic Lactococcus Lactis Decreases Anxiety-like Behavior and Modulates Cortical Cytoarchitecture in a Sex Specific Manner. PLoS ONE 2020, 15, e0223395. [Google Scholar] [CrossRef]
- Sarker, G.; Peleg-Raibstein, D. Maternal Overnutrition Induces Long-Term Cognitive Deficits across Several Generations. Nutrients 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Xia, B.; Jin, X.; Zou, Q.; Zeng, Z.; Zhao, W.; Yan, S.; Li, L.; Yuan, S.; et al. High-Fiber Diet Mitigates Maternal Obesity-Induced Cognitive and Social Dysfunction in the Offspring via Gut-Brain Axis. Cell Metab. 2021, 33, 923–938.e6. [Google Scholar] [CrossRef]
- Falsaperla, R.; Sciuto, S.; Gioè, D.; Sciuto, L.; Pisani, F.; Pavone, P.; Ruggieri, M. Mild Hypoxic-Ischemic Encephalopathy: Can Neurophysiological Monitoring Predict Unfavorable Neurological Outcome? A Systematic Review and Meta-Analysis. Am. J. Perinatol. 2023, 40, 833–838. [Google Scholar] [CrossRef]
- Hayes, B.C.; Ryan, S.; McGarvey, C.; Mulvany, S.; Doherty, E.; Grehan, A.; Madigan, C.; Matthews, T.; King, M.D. Brain Magnetic Resonance Imaging and Outcome after Hypoxic Ischaemic Encephalopathy. J. Matern. Fetal. Neonatal Med. 2016, 29, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Törn, A.E.; Hesselman, S.; Johansen, K.; Ågren, J.; Wikström, A.-K.; Jonsson, M. Outcomes in Children after Mild Neonatal Hypoxic Ischaemic Encephalopathy: A Population-Based Cohort Study. BJOG 2023, 130, 1602–1609. [Google Scholar] [CrossRef]
- DuPont, T.L.; Chalak, L.F.; Morriss, M.C.; Burchfield, P.J.; Christie, L.; Sánchez, P.J. Short-Term Outcomes of Newborns with Perinatal Acidemia Who Are Not Eligible for Systemic Hypothermia Therapy. J. Pediatr. 2013, 162, 35–41. [Google Scholar] [CrossRef]
- Chen, A.; Teng, C.; Wei, J.; Wu, X.; Zhang, H.; Chen, P.; Cai, D.; Qian, H.; Zhu, H.; Zheng, X.; et al. Gut Microbial Dysbiosis Exacerbates Long-Term Cognitive Impairments by Promoting Intestinal Dysfunction and Neuroinflammation Following Neonatal Hypoxia-Ischemia. Gut Microbes 2025, 17, 2471015. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Khachatryan, L.G.; Younis, N.K.; Mustafa, M.A.; Ahmad, N.; Athab, Z.H.; Polyanskaya, A.V.; Kasanave, E.V.; Mirzaei, R.; Karampoor, S. Microbiota-Derived Short Chain Fatty Acids in Pediatric Health and Diseases: From Gut Development to Neuroprotection. Front. Microbiol. 2024, 15, 1456793. [Google Scholar] [CrossRef]
- Chakkarapani, A.A.; Aly, H.; Benders, M.; Cotten, C.M.; El-Dib, M.; Gressens, P.; Hagberg, H.; Sabir, H.; Wintermark, P.; Robertson, N.J.; et al. Therapies for Neonatal Encephalopathy: Targeting the Latent, Secondary and Tertiary Phases of Evolving Brain Injury. Semin. Fetal Neonatal Med. 2021, 26, 101256. [Google Scholar] [CrossRef] [PubMed]
- Perlman, J.M. Intervention Strategies for Neonatal Hypoxic-Ischemic Cerebral Injury. Clin. Ther. 2006, 28, 1353–1365. [Google Scholar] [CrossRef]
- Aly, H.; Khashaba, M.T.; El-Ayouty, M.; El-Sayed, O.; Hasanein, B.M. IL-1beta, IL-6 and TNF-Alpha and Outcomes of Neonatal Hypoxic Ischemic Encephalopathy. Brain Dev. 2006, 28, 178–182. [Google Scholar] [CrossRef]
- O’Dea, M.I.; Kelly, L.A.; McKenna, E.; Strickland, T.; Hurley, T.P.; Butler, J.; Vavasseur, C.; El-Khuffash, A.F.; Miletin, J.; Fallah, L.; et al. Altered Cytokine Endotoxin Responses in Neonatal Encephalopathy Predict MRI Outcomes. Front. Pediatr. 2021, 9, 734540. [Google Scholar] [CrossRef]
- Ziemka-Nalecz, M.; Jaworska, J.; Sypecka, J.; Polowy, R.; Filipkowski, R.K.; Zalewska, T. Sodium Butyrate, a Histone Deacetylase Inhibitor, Exhibits Neuroprotective/Neurogenic Effects in a Rat Model of Neonatal Hypoxia-Ischemia. Mol. Neurobiol. 2017, 54, 5300–5318. [Google Scholar] [CrossRef]
- Lewis, K.; Lutgendorff, F.; Phan, V.; Söderholm, J.D.; Sherman, P.M.; McKay, D.M. Enhanced Translocation of Bacteria across Metabolically Stressed Epithelia Is Reduced by Butyrate. Inflamm. Bowel Dis. 2010, 16, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Aburto, M.R.; Tessier, C.; Nagpal, J.; Clarke, G.; O’Driscoll, C.M.; Cryan, J.F. Microbial-Derived Metabolites Induce Actin Cytoskeletal Rearrangement and Protect Blood-Brain Barrier Function. iScience 2022, 25, 105648. [Google Scholar] [CrossRef]
- Wojciech, L.; Tan, K.S.W.; Gascoigne, N.R.J. Taming the Sentinels: Microbiome-Derived Metabolites and Polarization of T Cells. Int. J. Mol. Sci. 2020, 21, 7740. [Google Scholar] [CrossRef]
- Mancino, S.; Boraso, M.; Galmozzi, A.; Serafini, M.M.; De Fabiani, E.; Crestani, M.; Viviani, B. Dose-Dependent Dual Effects of HDAC Inhibitors on Glial Inflammatory Response. Sci. Rep. 2025, 15, 12262. [Google Scholar] [CrossRef] [PubMed]
- Leus, N.G.; Zwinderman, M.R.; Dekker, F.J. Histone Deacetylase 3 (HDAC 3) as Emerging Drug Target in NF-ΚB-Mediated Inflammation. Curr. Opin. Chem. Biol. 2016, 33, 160–168. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Rund, L.; Hutchinson, N.T.; Woods, J.A.; Steelman, A.J.; Johnson, R.W. Inhibition of Inflammatory Microglia by Dietary Fiber and Short-Chain Fatty Acids. Sci. Rep. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, N.; Matei, N.; McBride, D.W.; Ding, Y.; Liang, H.; Tang, J.; Zhang, J.H. Sodium Butyrate Attenuated Neuronal Apoptosis via GPR41/Gβγ/PI3K/Akt Pathway after MCAO in Rats. J. Cereb. Blood Flow Metab. 2021, 41, 267–281. [Google Scholar] [CrossRef]
- Sun, J.; Lu, L.; Lian, Y.; Xu, S.; Zhu, Y.; Wu, Y.; Lin, Q.; Hou, J.; Li, Y.; Yu, Z. Sodium Butyrate Attenuates Microglia-Mediated Neuroinflammation by Modulating the TLR4/MyD88/NF-ΚB Pathway and Microbiome-Gut-Brain Axis in Cardiac Arrest Mice. Mol. Brain 2025, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, X.; Shen, Q.; Liu, L.; Hou, X.; Liu, N. Sodium Butyrate Improves the Effects of Brain Injury in a Small-for-Gestational-Age Rat Model by Activating the JAK1/STAT3 Pathway. J. Neuropathol. Exp. Neurol. 2025, nlaf085. [Google Scholar] [CrossRef]
- Dietz, R.M.; Wright, C.J. Oxidative Stress Diseases Unique to the Perinatal Period: A Window into the Developing Innate Immune Response. Am. J. Reprod. Immunol. 2018, 79, e12787. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-Host Systems Interactions: Protective Effects of Propionate upon the Blood-Brain Barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, A.; Huang, D.; Teng, C.; Cai, D.; Wu, X.; Wang, T.; Hu, W.; Huang, Z.; Wang, P.; et al. Gut Microbiome-Derived Lipopolysaccharides Aggravate Cognitive Impairment via TLR4-Mediated Inflammatory Signaling in Neonatal Rats Following Hypoxic-Ischemic Brain Damage. Brain Behav. Immun. 2025, 127, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Kaisar, M.M.M.; Pelgrom, L.R.; van der Ham, A.J.; Yazdanbakhsh, M.; Everts, B. Butyrate Conditions Human Dendritic Cells to Prime Type 1 Regulatory T Cells via Both Histone Deacetylase Inhibition and G Protein-Coupled Receptor 109A Signaling. Front. Immunol. 2017, 8, 1429. [Google Scholar] [CrossRef]
- Tao, R.; de Zoeten, E.F.; Ozkaynak, E.; Chen, C.; Wang, L.; Porrett, P.M.; Li, B.; Turka, L.A.; Olson, E.N.; Greene, M.I.; et al. Deacetylase Inhibition Promotes the Generation and Function of Regulatory T Cells. Nat. Med. 2007, 13, 1299–1307. [Google Scholar] [CrossRef]
- Pessa-Morikawa, T.; Husso, A.; Kärkkäinen, O.; Koistinen, V.; Hanhineva, K.; Iivanainen, A.; Niku, M. Maternal Microbiota-Derived Metabolic Profile in Fetal Murine Intestine, Brain and Placenta. BMC Microbiol. 2022, 22, 46. [Google Scholar] [CrossRef]
- Peesh, P.; Blasco-Conesa, M.P.; El Hamamy, A.; Khan, R.; Guzman, G.U.; Honarpisheh, P.; Mohan, E.C.; Goodman, G.W.; Nguyen, J.N.; Banerjee, A.; et al. Benefits of Equilibrium between Microbiota- and Host-Derived Ligands of the Aryl Hydrocarbon Receptor after Stroke in Aged Male Mice. Nat. Commun. 2025, 16, 1767. [Google Scholar] [CrossRef]
- Miyamoto, K.; Sujino, T.; Kanai, T. The Tryptophan Metabolic Pathway of the Microbiome and Host Cells in Health and Disease. Int. Immunol. 2024, 36, 601–616. [Google Scholar] [CrossRef]
- Honarpisheh, P.; Bryan, R.M.; McCullough, L.D. Aging Microbiota-Gut-Brain Axis in Stroke Risk and Outcome. Circ. Res. 2022, 130, 1112–1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, B.; Luo, M.; Xie, L.; Lu, M.; Lu, X.; Zhang, S.; Wei, L.; Zhou, X.; Yao, B.; et al. Microbiota-Indole 3-Propionic Acid-Brain Axis Mediates Abnormal Synaptic Pruning of Hippocampal Microglia and Susceptibility to ASD in IUGR Offspring. Microbiome 2023, 11, 245. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Sun, P.; Zhu, W.; Chen, Y.; Chen, M.; Yang, X.; Du, X.; Zhao, Y. Farrerol Alleviates Hypoxic-Ischemic Encephalopathy by Inhibiting Ferroptosis in Neonatal Rats via the Nrf2 Pathway. Physiol. Res. 2023, 72, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Peeples, E.S.; Genaro-Mattos, T.C. Ferroptosis: A Promising Therapeutic Target for Neonatal Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 2022, 23, 7420. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zheng, Y.; Gao, Q.; Pang, M.; Wu, Y.; Feng, X.; Tao, X.; Hu, Y.; Lin, Z.; Lin, W. Activation of the Nrf2/Keap1 Signaling Pathway Mediates the Neuroprotective Effect of Perillyl Alcohol against Cerebral Hypoxic-Ischemic Damage in Neonatal Rats. Redox Rep. 2024, 29, 2394714. [Google Scholar] [CrossRef]
- Liu, J.-X.; Zheng, D.; Chen, L.; Chen, S.; Min, J.-W. Nuclear Factor Erythroid 2-Related Factor 2 as a Potential Therapeutic Target in Neonatal Hypoxic-Ischemic Encephalopathy. J. Integr. Neurosci. 2024, 23, 103. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zhang, L.; Yang, Y.; Lin, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Feng, B.; Xu, S.; et al. Maternal Dietary Fiber Composition during Gestation Induces Changes in Offspring Antioxidative Capacity, Inflammatory Response, and Gut Microbiota in a Sow Model. Int. J. Mol. Sci. 2019, 21, 31. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Ma, Z.; Fu, Z.; Zhao, Y.; Zeng, X.; Lin, G.; Zhang, S.; Guan, W.; Chen, F. Enhanced Antioxidative Capacity Transfer between Sow and Fetus via the Gut-Placenta Axis with Dietary Selenium Yeast and Glycerol Monolaurate Supplementation during Pregnancy. Antioxidants 2024, 13, 141. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the Gut Barrier Integrity by a Microbial Metabolite through the Nrf2 Pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Uchiyama, J.; Akiyama, M.; Hase, K.; Kumagai, Y.; Kim, Y.-G. Gut Microbiota Reinforce Host Antioxidant Capacity via the Generation of Reactive Sulfur Species. Cell Rep. 2022, 38, 110479. [Google Scholar] [CrossRef]
- Xu, K.; Liu, G.; Fu, C. The Tryptophan Pathway Targeting Antioxidant Capacity in the Placenta. Oxidative Medicine and Cellular Longevity 2018, 2018, 1054797. [Google Scholar] [CrossRef]
- Cömert, T.K.; Akpinar, F.; Erkaya, S.; Durmaz, B.; Durmaz, R. The Effect of Gestational Weight Gain on Serum Total Oxidative Stress, Total Antioxidant Capacity and Gut Microbiota. Biosci. Microbiota Food Health 2022, 41, 160–167. [Google Scholar] [CrossRef]
- Du, X.; Elsabagh, M.; He, F.; Wu, H.; Zhang, B.; Fan, K.; Wang, M.; Zhang, H. Gut Microbiota and Its Metabolites Modulate Pregnancy Outcomes by Regulating Placental Autophagy and Ferroptosis. Antioxidants 2025, 14, 970. [Google Scholar] [CrossRef]
- Jithoo, A.; Penny, T.R.; Pham, Y.; Sutherland, A.E.; Smith, M.J.; Petraki, M.; Fahey, M.C.; Jenkin, G.; Malhotra, A.; Miller, S.L.; et al. The Temporal Relationship between Blood-Brain Barrier Integrity and Microglial Response Following Neonatal Hypoxia Ischemia. Cells 2024, 13, 660. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014, 6, 263ra158, Erratum in Sci. Transl. Med. 2014, 6, 266er7. [Google Scholar] [CrossRef]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-Chain Fatty Acids as Modulators of Redox Signaling in Health and Disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Li, H.; Zhang, H.; Jin, J.; Chen, W.; Pang, M.; Yu, J.; He, Y.; Liu, J.; et al. Neuroprotective Effect of Sodium Butyrate against Cerebral Ischemia/Reperfusion Injury in Mice. BioMed Res. Int. 2015, 2015, 395895. [Google Scholar] [CrossRef]
- Sun, J.; Ling, Z.; Wang, F.; Chen, W.; Li, H.; Jin, J.; Zhang, H.; Pang, M.; Yu, J.; Liu, J. Clostridium Butyricum Pretreatment Attenuates Cerebral Ischemia/Reperfusion Injury in Mice via Anti-Oxidation and Anti-Apoptosis. Neurosci. Lett. 2016, 613, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Sohrabji, F. The Histone Deacetylase Inhibitor, Sodium Butyrate, Exhibits Neuroprotective Effects for Ischemic Stroke in Middle-Aged Female Rats. J. Neuroinflamm. 2016, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tao, X.; Xiong, Z.; Wang, H.; Zeng, L. Mechanism of HDAC2 Regulating Nrf2 Acetylation Level in Neuronal Ferroptosis of Neonatal Rats with Hypoxic-Ischemic Brain Injury. Brain Inj. 2025, 39, 635–645. [Google Scholar] [CrossRef]
- Hwang, I.K.; Yoo, K.-Y.; Li, H.; Park, O.K.; Lee, C.H.; Choi, J.H.; Jeong, Y.-G.; Lee, Y.L.; Kim, Y.-M.; Kwon, Y.-G.; et al. Indole-3-Propionic Acid Attenuates Neuronal Damage and Oxidative Stress in the Ischemic Hippocampus. J. Neurosci. Res. 2009, 87, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Maternal Obesity and Gut Microbiota Are Associated with Fetal Brain Development. Nutrients 2022, 14, 4515. [Google Scholar] [CrossRef]
- Radford-Smith, D.E.; Probert, F.; Burnet, P.W.J.; Anthony, D.C. Modifying the Maternal Microbiota Alters the Gut-Brain Metabolome and Prevents Emotional Dysfunction in the Adult Offspring of Obese Dams. Proc. Natl. Acad. Sci. USA 2022, 119, e2108581119. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, T.; Zeng, Y.; Pei, P.; Liu, Y.; Jia, W.; Zhao, H.; Bi, M.; Wang, S. Sodium Butyrate Mediates Histone Crotonylation and Alleviated Neonatal Rats Hypoxic-Ischemic Brain Injury through Gut-Brain Axis. Front. Microbiol. 2022, 13, 993146. [Google Scholar] [CrossRef]
- Chen, A.; Xiong, L.-J.; Tong, Y.; Mao, M. The Neuroprotective Roles of BDNF in Hypoxic Ischemic Brain Injury. Biomed. Rep. 2013, 1, 167–176. [Google Scholar] [CrossRef]
- Urbonaite, G.; Knyzeliene, A.; Bunn, F.S.; Smalskys, A.; Neniskyte, U. The Impact of Maternal High-Fat Diet on Offspring Neurodevelopment. Front. Neurosci. 2022, 16, 909762. [Google Scholar] [CrossRef]
- Fusco, S.; Spinelli, M.; Cocco, S.; Ripoli, C.; Mastrodonato, A.; Natale, F.; Rinaudo, M.; Livrizzi, G.; Grassi, C. Maternal Insulin Resistance Multigenerationally Impairs Synaptic Plasticity and Memory via Gametic Mechanisms. Nat. Commun. 2019, 10, 4799. [Google Scholar] [CrossRef] [PubMed]
- Page, K.C.; Jones, E.K.; Anday, E.K. Maternal and Postweaning High-Fat Diets Disturb Hippocampal Gene Expression, Learning, and Memory Function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R527–R537. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Lupu, D.S. High Fat Diet-Induced Maternal Obesity Alters Fetal Hippocampal Development. Int. J. Dev. Neurosci. 2009, 27, 627–633. [Google Scholar] [CrossRef]
- Mastenbroek, L.J.M.; Kooistra, S.M.; Eggen, B.J.L.; Prins, J.R. The Role of Microglia in Early Neurodevelopment and the Effects of Maternal Immune Activation. Semin. Immunopathol. 2024, 46, 1. [Google Scholar] [CrossRef]
- Cavaliere, G.; Catapano, A.; Trinchese, G.; Cimmino, F.; Penna, E.; Pizzella, A.; Cristiano, C.; Lama, A.; Crispino, M.; Mollica, M.P. Butyrate Improves Neuroinflammation and Mitochondrial Impairment in Cerebral Cortex and Synaptic Fraction in an Animal Model of Diet-Induced Obesity. Antioxidants 2022, 12, 4. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, K.; Li, X.; Xu, L.; Yang, Z. Sodium Butyrate Ameliorates the Impairment of Synaptic Plasticity by Inhibiting the Neuroinflammation in 5XFAD Mice. Chem. Biol. Interact. 2021, 341, 109452. [Google Scholar] [CrossRef]
- Jaworska, J.; Ziemka-Nalecz, M.; Sypecka, J.; Zalewska, T. The Potential Neuroprotective Role of a Histone Deacetylase Inhibitor, Sodium Butyrate, after Neonatal Hypoxia-Ischemia. J. Neuroinflamm. 2017, 14, 34. [Google Scholar] [CrossRef]
- Jaworska, J.; Zalewska, T.; Sypecka, J.; Ziemka-Nalecz, M. Effect of the HDAC Inhibitor, Sodium Butyrate, on Neurogenesis in a Rat Model of Neonatal Hypoxia-Ischemia: Potential Mechanism of Action. Mol. Neurobiol. 2019, 56, 6341–6370. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Zou, N.; Zhang, L.; Wang, Y.; Zhang, M.; Wang, C.; Yang, L. Pathogenesis from the Microbial-Gut-Brain Axis in White Matter Injury in Preterm Infants: A Review. Front. Integr. Neurosci. 2023, 17, 1051689. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef]
- Poutahidis, T.; Kearney, S.M.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Microbial Symbionts Accelerate Wound Healing via the Neuropeptide Hormone Oxytocin. PLoS ONE 2013, 8, e78898. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef]
- Ibrahim, Y.M.; Kearney, S.M.; Levkovich, T.; Springer, A.; Mirabal, S.; Poutahidis, T.; Varian, B.J.; Lakritz, J.R.; Alm, E.J.; Erdman, S.E. Maternal Gut Microbes Control Offspring Sex and Survival. J. Probiotics Health 2014, 2, 1. [Google Scholar]
- Tyzio, R.; Cossart, R.; Khalilov, I.; Minlebaev, M.; Hübner, C.A.; Represa, A.; Ben-Ari, Y.; Khazipov, R. Maternal Oxytocin Triggers a Transient Inhibitory Switch in GABA Signaling in the Fetal Brain during Delivery. Science 2006, 314, 1788–1792. [Google Scholar] [CrossRef]
- Ceanga, M.; Spataru, A.; Zagrean, A.-M. Oxytocin Is Neuroprotective against Oxygen-Glucose Deprivation and Reoxygenation in Immature Hippocampal Cultures. Neurosci. Lett. 2010, 477, 15–18. [Google Scholar] [CrossRef]
- Panaitescu, A.M.; Isac, S.; Pavel, B.; Ilie, A.S.; Ceanga, M.; Totan, A.; Zagrean, L.; Peltecu, G.; Zagrean, A.M. Oxytocin Reduces Seizure Burden and Hippocampal Injury in a Rat Model of Perinatal Asphyxia. Acta Endocrinol. 2018, 14, 315–319. [Google Scholar] [CrossRef]
- Pâslaru, A.-C.; Călin, A.; Morozan, V.-P.; Stancu, M.; Tofan, L.; Panaitescu, A.M.; Zăgrean, A.-M.; Zăgrean, L.; Moldovan, M. Burst-Suppression EEG Reactivity to Photic Stimulation—A Translational Biomarker in Hypoxic–Ischemic Brain Injury. Biomolecules 2024, 14, 953. [Google Scholar] [CrossRef]
- Ionescu, M.I.; Grigoras, I.-F.; Ionescu, R.-B.; Chitimus, D.M.; Haret, R.M.; Ianosi, B.; Ceanga, M.; Zagrean, A.-M. Oxytocin Exhibits Neuroprotective Effects on Hippocampal Cultures under Severe Oxygen-Glucose Deprivation Conditions. Curr. Issues Mol. Biol. 2024, 46, 6223–6236. [Google Scholar] [CrossRef]
- Spoljaric, A.; Seja, P.; Spoljaric, I.; Virtanen, M.A.; Lindfors, J.; Uvarov, P.; Summanen, M.; Crow, A.K.; Hsueh, B.; Puskarjov, M.; et al. Vasopressin Excites Interneurons to Suppress Hippocampal Network Activity across a Broad Span of Brain Maturity at Birth. Proc. Natl. Acad. Sci. USA 2017, 114, E10819–E10828. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Aguilera, G. Vasopressin Protects Hippocampal Neurones in Culture against Nutrient Deprivation or Glutamate-Induced Apoptosis: Vasopressin Is Anti-Apoptotic in Hippocampal Neurone Cultures. J. Neuroendocrinol. 2010, 22, 1072–1081. [Google Scholar] [CrossRef]
- Wellmann, S.; Benzing, J.; Cippà, G.; Admaty, D.; Creutzfeldt, R.; Mieth, R.A.; Beinder, E.; Lapaire, O.; Morgenthaler, N.G.; Haagen, U.; et al. High Copeptin Concentrations in Umbilical Cord Blood after Vaginal Delivery and Birth Acidosis. J. Clin. Endocrinol. Metab. 2010, 95, 5091–5096. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut Microbiota Depletion from Early Adolescence in Mice: Implications for Brain and Behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar]
- Stigger, F.; Lovatel, G.; Marques, M.; Bertoldi, K.; Moysés, F.; Elsner, V.; Siqueira, I.R.; Achaval, M.; Marcuzzo, S. Inflammatory Response and Oxidative Stress in Developing Rat Brain and Its Consequences on Motor Behavior Following Maternal Administration of LPS and Perinatal Anoxia. Int. J. Dev. Neurosci. 2013, 31, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Eklind, S.; Mallard, C.; Arvidsson, P.; Hagberg, H. Lipopolysaccharide Induces Both a Primary and a Secondary Phase of Sensitization in the Developing Rat Brain. Pediatr. Res. 2005, 58, 112–116. [Google Scholar] [CrossRef]
- Osredkar, D.; Thoresen, M.; Maes, E.; Flatebø, T.; Elstad, M.; Sabir, H. Hypothermia Is Not Neuroprotective after Infection-Sensitized Neonatal Hypoxic-Ischemic Brain Injury. Resuscitation 2014, 85, 567–572. [Google Scholar] [CrossRef]
- Beckers, K.F.; Flanagan, J.P.; Sones, J.L. Microbiome and Pregnancy: Focus on Microbial Dysbiosis Coupled with Maternal Obesity. Int. J. Obes. 2024, 48, 439–448, Erratum in Int. J. Obes. 2025, 49, 1421. [Google Scholar] [CrossRef] [PubMed]
- Kalyan, M.; Tousif, A.H.; Sonali, S.; Vichitra, C.; Sunanda, T.; Praveenraj, S.S.; Ray, B.; Gorantla, V.R.; Rungratanawanich, W.; Mahalakshmi, A.M.; et al. Role of Endogenous Lipopolysaccharides in Neurological Disorders. Cells 2022, 11, 4038. [Google Scholar] [CrossRef]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G.; et al. Lipopolysaccharide-Induced Blood-Brain Barrier Disruption: Roles of Cyclooxygenase, Oxidative Stress, Neuroinflammation, and Elements of the Neurovascular Unit. J. Neuroinflamm. 2015, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation Induced by Lipopolysaccharide Causes Cognitive Impairment in Mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [PubMed]
- Ostrem, B.E.L.; Domínguez-Iturza, N.; Stogsdill, J.A.; Faits, T.; Kim, K.; Levin, J.Z.; Arlotta, P. Fetal Brain Response to Maternal Inflammation Requires Microglia. Development 2024, 151, dev.202252. [Google Scholar] [CrossRef]
- Yeh, H.; Ikezu, T. Transcriptional and Epigenetic Regulation of Microglia in Health and Disease. Trends Mol. Med. 2019, 25, 96–111. [Google Scholar] [CrossRef]
- Isac, S.; Panaitescu, A.M.; Iesanu, M.; Grigoras, I.F.; Totan, A.; Udriste, A.; Cucu, N.; Peltecu, G.; Zagrean, L.; Zagrean, A.-M. Maternal High-Fat Diet Modifies the Immature Hippocampus Vulnerability to Perinatal Asphyxia in Rats. Neonatology 2018, 114, 355–361. [Google Scholar] [CrossRef]
- Isac, S.; Panaitescu, A.M.; Spataru, A.; Iesanu, M.; Totan, A.; Udriste, A.; Cucu, N.; Peltecu, G.; Zagrean, L.; Zagrean, A.-M. Trans-Resveratrol Enriched Maternal Diet Protects the Immature Hippocampus from Perinatal Asphyxia in Rats. Neurosci. Lett. 2017, 653, 308–313. [Google Scholar] [CrossRef]
- Isac, S.; Panaitescu, A.M.; Iesanu, M.I.; Zeca, V.; Cucu, N.; Zagrean, L.; Peltecu, G.; Zagrean, A.-M. Maternal Citicoline-Supplemented Diet Improves the Response of the Immature Hippocampus to Perinatal Asphyxia in Rats. Neonatology 2020, 117, 729–735. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Yang, W.; Gao, Y.; Chen, J. Omega-3 Polyunsaturated Fatty Acid Supplementation Confers Long-Term Neuroprotection against Neonatal Hypoxic-Ischemic Brain Injury through Anti-Inflammatory Actions. Stroke 2010, 41, 2341–2347. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Q.; Gao, Y.; Jiang, H.; Zhou, F.; Zhang, F.; Xu, M. CDP-Choline Modulates Cholinergic Signaling and Gut Microbiota to Alleviate DSS-Induced Inflammatory Bowel Disease. Biochem. Pharmacol. 2023, 217, 115845. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Bose, C.; Sunilkumar, D.; Cherian, R.M.; Thomas, S.S.; Nair, B.G. Resveratrol as a Promising Nutraceutical: Implications in Gut Microbiota Modulation, Inflammatory Disorders, and Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 3370. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Kaliannan, K.; Strain, C.R.; Ross, R.P.; Stanton, C.; Kang, J.X. Maternal Omega-3 Fatty Acids Regulate Offspring Obesity through Persistent Modulation of Gut Microbiota. Microbiome 2018, 6, 95. [Google Scholar] [CrossRef]
- Carvalho, A.V.S.; Sanches, E.F.; Ribeiro, R.T.; Durán-Carabali, L.E.; Júnior, O.R.; Muniz, B.D.; Wajner, M.; Wyse, A.T.; Netto, C.A.; Sizonenko, S.V. Maternal Lactoferrin Supplementation Prevents Mitochondrial and Redox Homeostasis Dysfunction, and Improves Antioxidant Defenses through Nrf2 and UCP2 Signaling after Neonatal Hypoxia-Ischemia. Free Radic. Biol. Med. 2025, 231, 68–79. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, N.; Ashaolu, T.J. Prebiotic and Modulatory Evidence of Lactoferrin on Gut Health and Function. J. Funct. Foods 2023, 108, 105741. [Google Scholar] [CrossRef]
- Coscia, A.; Bardanzellu, F.; Caboni, E.; Fanos, V.; Peroni, D.G. When a Neonate Is Born, so Is a Microbiota. Life 2021, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Martínez, C.; Santaella-Pascual, M.; Yagüe-Guirao, G.; Martínez-Graciá, C. Infant Gut Microbiota Colonization: Influence of Prenatal and Postnatal Factors, Focusing on Diet. Front. Microbiol. 2023, 14, 1236254. [Google Scholar] [CrossRef]
- Iribarren, I.; Hilario, E.; Álvarez, A.; Alonso-Alconada, D. Neonatal Multiple Organ Failure after Perinatal Asphyxia. An. Pediatr. (Engl. Ed.) 2022, 97, 280.e1–280.e8. [Google Scholar] [CrossRef]
- Gamsu, H.R.; Kempley, S.T. Enteral Hypoxia/Ischaemia and Necrotizing Enterocolitis. Semin. Neonatol. 1997, 2, 245–254. [Google Scholar] [CrossRef]
- Nikiforou, M.; Willburger, C.; de Jong, A.E.; Kloosterboer, N.; Jellema, R.K.; Ophelders, D.R.M.G.; Steinbusch, H.W.M.; Kramer, B.W.; Wolfs, T. Global Hypoxia-Ischemia Induced Inflammation and Structural Changes in the Preterm Ovine Gut Which Were Not Ameliorated by Mesenchymal Stem Cell Treatment. Mol. Med. 2016, 22, 244–257. [Google Scholar] [CrossRef]
- Guzmán-De La Garza, F.J.; Cámara-Lemarroy, C.R.; Ballesteros-Elizondo, R.G.; Alarcón-Galván, G.; Cordero-Pérez, P.; Fernández-Garza, N.E. Ketamine Reduces Intestinal Injury and Inflammatory Cell Infiltration after Ischemia/Reperfusion in Rats. Surg. Today 2010, 40, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Torres, M.; Sanchez-Alcoholado, L.; Cardona, F.; Almendros, I.; Gozal, D.; Montserrat, J.M.; Queipo-Ortuño, M.I.; Farré, R. Normoxic Recovery Mimicking Treatment of Sleep Apnea Does Not Reverse Intermittent Hypoxia-Induced Bacterial Dysbiosis and Low-Grade Endotoxemia in Mice. Sleep 2016, 39, 1891–1897. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Torres, M.; Montserrat, J.M.; Sanchez-Alcoholado, L.; Cardona, F.; Tinahones, F.J.; Gozal, D.; Poroyko, V.A.; Navajas, D.; Queipo-Ortuño, M.I.; et al. Intermittent Hypoxia Alters Gut Microbiota Diversity in a Mouse Model of Sleep Apnoea. Eur. Respir. J. 2015, 45, 1055–1065. [Google Scholar] [CrossRef]
- Chen, G.; Li, F.; Du, J. Change of Gut Microbiome Structure in Preterm Infants with Hypoxic Ischemic Encephalopathy Induced by Apnea. Pediatr. Neonatol. 2023, 64, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.N.; Madan, J.C.; Emond, J.A.; Morrison, H.G.; Christensen, B.C.; Karagas, M.R.; Hoen, A.G. Maternal Diet during Pregnancy Is Related with the Infant Stool Microbiome in a Delivery Mode-Dependent Manner. Microbiome 2018, 6, 109. [Google Scholar] [CrossRef]
- Ionescu, M.I.; Zahiu, C.D.M.; Vlad, A.; Galos, F.; Gradisteanu Pircalabioru, G.; Zagrean, A.-M.; O’Mahony, S.M. Nurturing Development: How a Mother’s Nutrition Shapes Offspring’s Brain through the Gut. Nutr. Neurosci. 2025, 28, 50–72. [Google Scholar] [CrossRef]
- Nakajima, A.; Kaga, N.; Nakanishi, Y.; Ohno, H.; Miyamoto, J.; Kimura, I.; Hori, S.; Sasaki, T.; Hiramatsu, K.; Okumura, K.; et al. Maternal High Fiber Diet during Pregnancy and Lactation Influences Regulatory T Cell Differentiation in Offspring in Mice. J. Immunol. 2017, 199, 3516–3524. [Google Scholar] [CrossRef]
- Nakajima, A.; Habu, S.; Kasai, M.; Okumura, K.; Ishikawa, D.; Shibuya, T.; Kobayashi, O.; Osada, T.; Ohkusa, T.; Watanabe, S.; et al. Impact of Maternal Dietary Gut Microbial Metabolites on an Offspring’s Systemic Immune Response in Mouse Models. Biosci. Microbiota Food Health 2020, 39, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Strobel, K.M.; Juul, S.E.; Hendrixson, D.T. Maternal Nutritional Status and the Microbiome across the Pregnancy and the Post-Partum Period. Microorganisms 2023, 11, 1569. [Google Scholar] [CrossRef]
- Miller, C.B.; Benny, P.; Riel, J.; Boushey, C.; Perez, R.; Khadka, V.; Qin, Y.; Maunakea, A.K.; Lee, M.-J. Adherence to Mediterranean Diet Impacts Gastrointestinal Microbial Diversity throughout Pregnancy. BMC Pregnancy Childbirth 2021, 21, 558. [Google Scholar] [CrossRef]
- Crovetto, F.; Nakaki, A.; Arranz, A.; Borras, R.; Vellvé, K.; Paules, C.; Boutet, M.L.; Castro-Barquero, S.; Freitas, T.; Casas, R.; et al. Effect of a Mediterranean Diet or Mindfulness-Based Stress Reduction during Pregnancy on Child Neurodevelopment: A Prespecified Analysis of the IMPACT BCN Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2330255. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of Dietary Compounds, Especially Polyphenols, with the Intestinal Microbiota: A Review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Roumes, H.; Sanchez, S.; Benkhaled, I.; Fernandez, V.; Goudeneche, P.; Perrin, F.; Pellerin, L.; Guillard, J.; Bouzier-Sore, A.-K. Neuroprotective Effect of Eco-Sustainably Extracted Grape Polyphenols in Neonatal Hypoxia-Ischemia. Nutrients 2022, 14, 773. [Google Scholar] [CrossRef]
- Pontes, P.B.; Toscano, A.E.; Lacerda, D.C.; da Silva Araújo, E.R.; da Costa, P.C.T.; Alves, S.M.; de Brito Alves, J.L.; Manhães-de-Castro, R. Effectiveness of Polyphenols on Perinatal Brain Damage: A Systematic Review of Preclinical Studies. Foods 2023, 12, 2278. [Google Scholar] [CrossRef] [PubMed]
- Loren, D.J.; Seeram, N.P.; Schulman, R.N.; Holtzman, D.M. Maternal Dietary Supplementation with Pomegranate Juice Is Neuroprotective in an Animal Model of Neonatal Hypoxic-Ischemic Brain Injury. Pediatr. Res. 2005, 57, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Dumont, U.; Sanchez, S.; Olivier, B.; Chateil, J.-F.; Deffieux, D.; Quideau, S.; Pellerin, L.; Beauvieux, M.-C.; Bouzier-Sore, A.-K.; Roumes, H. Maternal Alcoholism and Neonatal Hypoxia-Ischemia: Neuroprotection by Stilbenoid Polyphenols. Brain Res. 2020, 1738, 146798. [Google Scholar] [CrossRef]
- Ray, S.K.; Mukherjee, S. Evolving Interplay between Dietary Polyphenols and Gut Microbiota-an Emerging Importance in Healthcare. Front. Nutr. 2021, 8, 634944. [Google Scholar] [CrossRef]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary Polyphenols to Combat the Metabolic Diseases via Altering Gut Microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Leng, P.; Wang, Y.; Xie, M. Ellagic Acid and Gut Microbiota: Interactions, and Implications for Health. Food Sci. Nutr. 2025, 13, e70133. [Google Scholar] [CrossRef]
- Wang, P.; Wang, R.; Zhao, W.; Zhao, Y.; Wang, D.; Zhao, S.; Ge, Z.; Ma, Y.; Zhao, X. Gut Microbiota-Derived 4-Hydroxyphenylacetic Acid from Resveratrol Supplementation Prevents Obesity through SIRT1 Signaling Activation. Gut Microbes 2025, 17, 2446391. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, H.; Arai, Y.; Kitamura, Y.; Hayashi, M.; Okumura, A.; Shimizu, T. Maternal Docosahexaenoic Acid-Enriched Diet Prevents Neonatal Brain Injury: Effect of Docosahexaenoic Acid Diet. Neuropathology 2010, 30, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 Fatty Acid Addition during Pregnancy. Cochrane Libr. 2018, 11, CD003402. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Wang, C.; Wei, S.; Liu, B.; Wang, F.; Lu, Z.; Jin, M.; Wang, Y. Maternal Consumption of a Fermented Diet Protects Offspring against Intestinal Inflammation by Regulating the Gut Microbiota. Gut Microbes 2022, 14, 2057779. [Google Scholar] [CrossRef]
- Erçelik, H.C.; Kaya, V. The Effects of Fermented Food Consumption in Pregnancy on Neonatal and Infant Health: An Integrative Review. J. Pediatr. Nurs. 2024, 75, 173–179. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Pandiyan, A.; Gurung, M.; Mulakala, B.K.; Ponniah, S.K.; Yeruva, L. The Role of Fermented Foods in Maternal Health during Pregnancy and Infant Health during the First 1,000 Days of Life. Front. Nutr. 2025, 12, 1581723. [Google Scholar] [CrossRef]
- Jones, J.M.; Reinke, S.N.; Mousavi-Derazmahalleh, M.; Garssen, J.; Jenmalm, M.C.; Srinivasjois, R.; Silva, D.; Keelan, J.; Prescott, S.L.; Palmer, D.J.; et al. Maternal Prebiotic Supplementation during Pregnancy and Lactation Modifies the Microbiome and Short Chain Fatty Acid Profile of Both Mother and Infant. Clin. Nutr. 2024, 43, 969–980. [Google Scholar] [CrossRef]
- Hogenkamp, A.; Thijssen, S.; van Vlies, N.; Garssen, J. Supplementing Pregnant Mice with a Specific Mixture of Nondigestible Oligosaccharides Reduces Symptoms of Allergic Asthma in Male Offspring. J. Nutr. 2015, 145, 640–646. [Google Scholar] [CrossRef]
- Cuinat, C.; Stinson, S.E.; Ward, W.E.; Comelli, E.M. Maternal Intake of Probiotics to Program Offspring Health. Curr. Nutr. Rep. 2022, 11, 537–562. [Google Scholar] [CrossRef]
- Musazadeh, V.; Faghfouri, A.H.; Zarezadeh, M.; Pakmehr, A.; Moghaddam, P.T.; Hamedi-Kalajahi, F.; Jahandideh, A.; Ghoreishi, Z. Remarkable Impacts of Probiotics Supplementation in Enhancing of the Antioxidant Status: Results of an Umbrella Meta-Analysis. Front. Nutr. 2023, 10, 1117387, Erratum in Front. Nutr. 2024, 11, 1371746. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus Reuteri Biofilms Produce Antimicrobial and Anti-Inflammatory Factors. BMC Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674, Erratum in Front. Microbiol. 2024, 15, 1378225. [Google Scholar] [CrossRef]
- Grev, J.; Berg, M.; Soll, R. Maternal Probiotic Supplementation for Prevention of Morbidity and Mortality in Preterm Infants. Cochrane Database Syst. Rev. 2018, 12, CD012519. [Google Scholar] [CrossRef] [PubMed]
- Keunen, K.; van Elburg, R.M.; van Bel, F.; Benders, M.J.N.L. Impact of Nutrition on Brain Development and Its Neuroprotective Implications Following Preterm Birth. Pediatr. Res. 2015, 77, 148–155. [Google Scholar] [CrossRef]
- Sha, C.; Jin, Z.; Ku, S.Y.; Kogosov, A.S.; Yu, S.; Bergese, S.D.; Hsieh, H. Necrotizing Enterocolitis and Neurodevelopmental Impairments: Microbiome, Gut, and Brain Entanglements. Biomolecules 2024, 14, 1254. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kong, X.; Azad, M.A.K.; Zhu, Q.; Xiong, L.; Zheng, Y.; Hu, Z.; Yin, Y.; He, Q. Maternal Probiotic or Synbiotic Supplementation Modulates Jejunal and Colonic Antioxidant Capacity, Mitochondrial Function, and Microbial Abundance in Bama Mini-Piglets. Oxid. Med. Cell. Longev. 2021, 2021, 6618874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, W.; Zhan, L.; Hou, S.; Zhao, C.; Bi, T.; Lu, X. Fecal Microbiota Transplantation Alters the Susceptibility of Obese Rats to Type 2 Diabetes Mellitus. Aging 2020, 12, 17480–17502. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7, Erratum in Gastroenterology 2013, 144, 250. [Google Scholar] [CrossRef]
- Proença, I.M.; Allegretti, J.R.; Bernardo, W.M.; de Moura, D.T.H.; Ponte Neto, A.M.; Matsubayashi, C.O.; Flor, M.M.; Kotinda, A.P.S.T.; de Moura, E.G.H. Fecal Microbiota Transplantation Improves Metabolic Syndrome Parameters: Systematic Review with Meta-Analysis Based on Randomized Clinical Trials. Nutr. Res. 2020, 83, 1–14. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Tain, Y.-L.; Hsu, C.-N. Maternal Supplementation of Probiotics, Prebiotics or Postbiotics to Prevent Offspring Metabolic Syndrome: The Gap between Preclinical Results and Clinical Translation. Int. J. Mol. Sci. 2022, 23, 10173. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-Lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Chang-Chien, G.-P.; Lin, S.; Hou, C.-Y.; Tain, Y.-L. Targeting on Gut Microbial Metabolite Trimethylamine-N-Oxide and Short-Chain Fatty Acid to Prevent Maternal High-Fructose-Diet-Induced Developmental Programming of Hypertension in Adult Male Offspring. Mol. Nutr. Food Res. 2019, 63, e1900073. [Google Scholar] [CrossRef]
- Milligan, G.; Barki, N.; Tobin, A.B. Chemogenetic Approaches to Explore the Functions of Free Fatty Acid Receptor 2. Trends Pharmacol. Sci. 2021, 42, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, T.; Ni, C.; Hu, Y.; Nan, Y.; Lin, W.; Liu, Y.; Zheng, F.; Shi, X.; Lin, Z.; et al. Indole-3-Propionic Acid Attenuates HI-Related Blood-Brain Barrier Injury in Neonatal Rats by Modulating the PXR Signaling Pathway. ACS Chem. Neurosci. 2022, 13, 2897–2912. [Google Scholar] [CrossRef]
- Amat, S.; Dahlen, C.R.; Swanson, K.C.; Ward, A.K.; Reynolds, L.P.; Caton, J.S. Bovine Animal Model for Studying the Maternal Microbiome, in Utero Microbial Colonization and Their Role in Offspring Development and Fetal Programming. Front. Microbiol. 2022, 13, 854453. [Google Scholar] [CrossRef] [PubMed]
- Daiy, K.; Wiley, K.; Allen, J.; Bailey, M.T.; Dettmer, A.M. Associations among Rearing Environment and the Infant Gut Microbiome with Early-Life Neurodevelopment and Cognitive Development in a Nonhuman Primate Model (Macaca Mulatta). J. Dev. Orig. Health Dis. 2025, 16, e1. [Google Scholar] [CrossRef]
- Ma, J.; Prince, A.L.; Bader, D.; Hu, M.; Ganu, R.; Baquero, K.; Blundell, P.; Alan Harris, R.; Frias, A.E.; Grove, K.L.; et al. High-Fat Maternal Diet during Pregnancy Persistently Alters the Offspring Microbiome in a Primate Model. Nat. Commun. 2014, 5, 3889. [Google Scholar] [CrossRef]
- Li, M.; Brokaw, A.; Furuta, A.M.; Coler, B.; Obregon-Perko, V.; Chahroudi, A.; Wang, H.-Y.; Permar, S.R.; Hotchkiss, C.E.; Golos, T.G.; et al. Non-Human Primate Models to Investigate Mechanisms of Infection-Associated Fetal and Pediatric Injury, Teratogenesis and Stillbirth. Front. Genet. 2021, 12, 680342. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.; Lemanski, E.A.; Wright-Jin, E. The Importance of Including Maternal Immune Activation in Animal Models of Hypoxic-Ischemic Encephalopathy. Biomedicines 2024, 12, 2559. [Google Scholar] [CrossRef]
- Koehler, R.C.; Yang, Z.-J.; Lee, J.K.; Martin, L.J. Perinatal Hypoxic-Ischemic Brain Injury in Large Animal Models: Relevance to Human Neonatal Encephalopathy. J. Cereb. Blood Flow Metab. 2018, 38, 2092–2111. [Google Scholar] [CrossRef]
- Jacobson Misbe, E.N.; Richards, T.L.; McPherson, R.J.; Burbacher, T.M.; Juul, S.E. Perinatal Asphyxia in a Nonhuman Primate Model. Dev. Neurosci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S129–S134. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Pillonel, T.; Torregrossa, A.; Prod’hom, G.; Fischer, C.J.; Greub, G.; Giannoni, E. Bifidobacterium Longum Bacteremia in Preterm Infants Receiving Probiotics. Clin. Infect. Dis. 2015, 60, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.; Tan, J.; Paquette, V.; Panczuk, J. Does Probiotic Bacteremia in Premature Infants Impact Clinically Relevant Outcomes? A Case Report and Updated Review of Literature. Clin. Nutr. ESPEN 2020, 39, 255–259. [Google Scholar] [CrossRef]
- Roy, U.; Jessani, L.G.; Rudramurthy, S.M.; Gopalakrishnan, R.; Dutta, S.; Chakravarty, C.; Jillwin, J.; Chakrabarti, A. Seven Cases of Saccharomyces Fungaemia Related to Use of Probiotics. Mycoses 2017, 60, 375–380. [Google Scholar] [CrossRef]
- Farella, I.; Fortunato, M.; Martinelli, D.; De Carlo, C.; Sparapano, E.; Stolfa, S.; Romanelli, F.; De Laurentiis, V.; Martinotti, S.; Capozzi, L.; et al. Lactobacillus Rhamnosus Sepsis in a Preterm Infant Following Probiotic Administration: Challenges in Diagnosis. Microorganisms 2025, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Umberger, E.; Patel, R.M. Safety and Efficacy of Probiotic Administration to Preterm Infants: Ten Common Questions. Pediatr. Res. 2020, 88, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/media/172606/download?attachment (accessed on 3 May 2025).
- O’Doherty, K.C.; Virani, A.; Wilcox, E.S. The Human Microbiome and Public Health: Social and Ethical Considerations. Am. J. Public Health 2016, 106, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.H.; Hawkins, C.C.; Rodrigues, B.V.; Cornet, M.-C.; Gonzalez, F.F.; Wu, Y.W. Neuroprotection for Neonatal Hypoxic-Ischemic Encephalopathy: A Review of Novel Therapies Evaluated in Clinical Studies. Dev. Med. Child Neurol. 2025, 67, 591–599. [Google Scholar] [CrossRef]

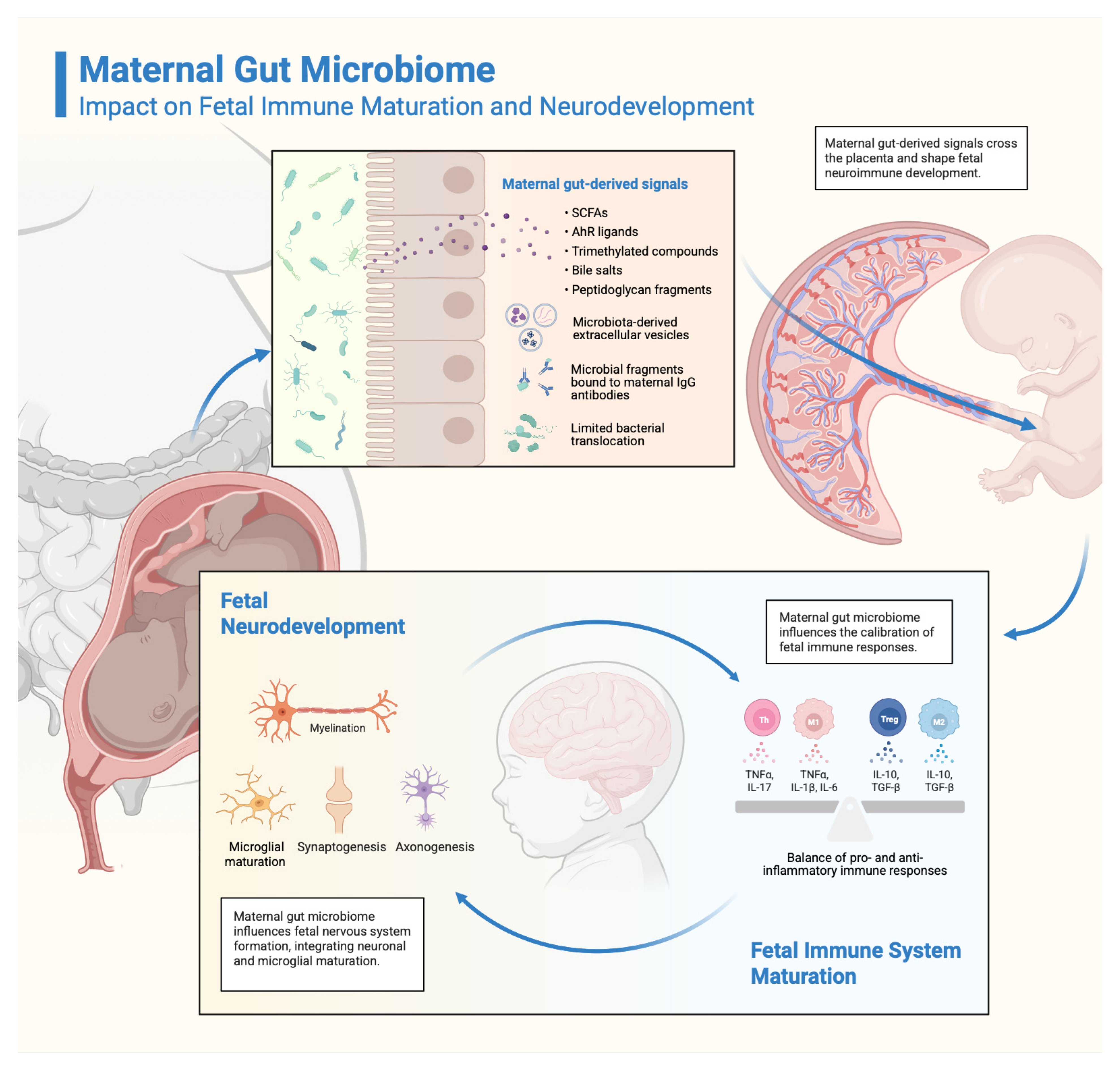
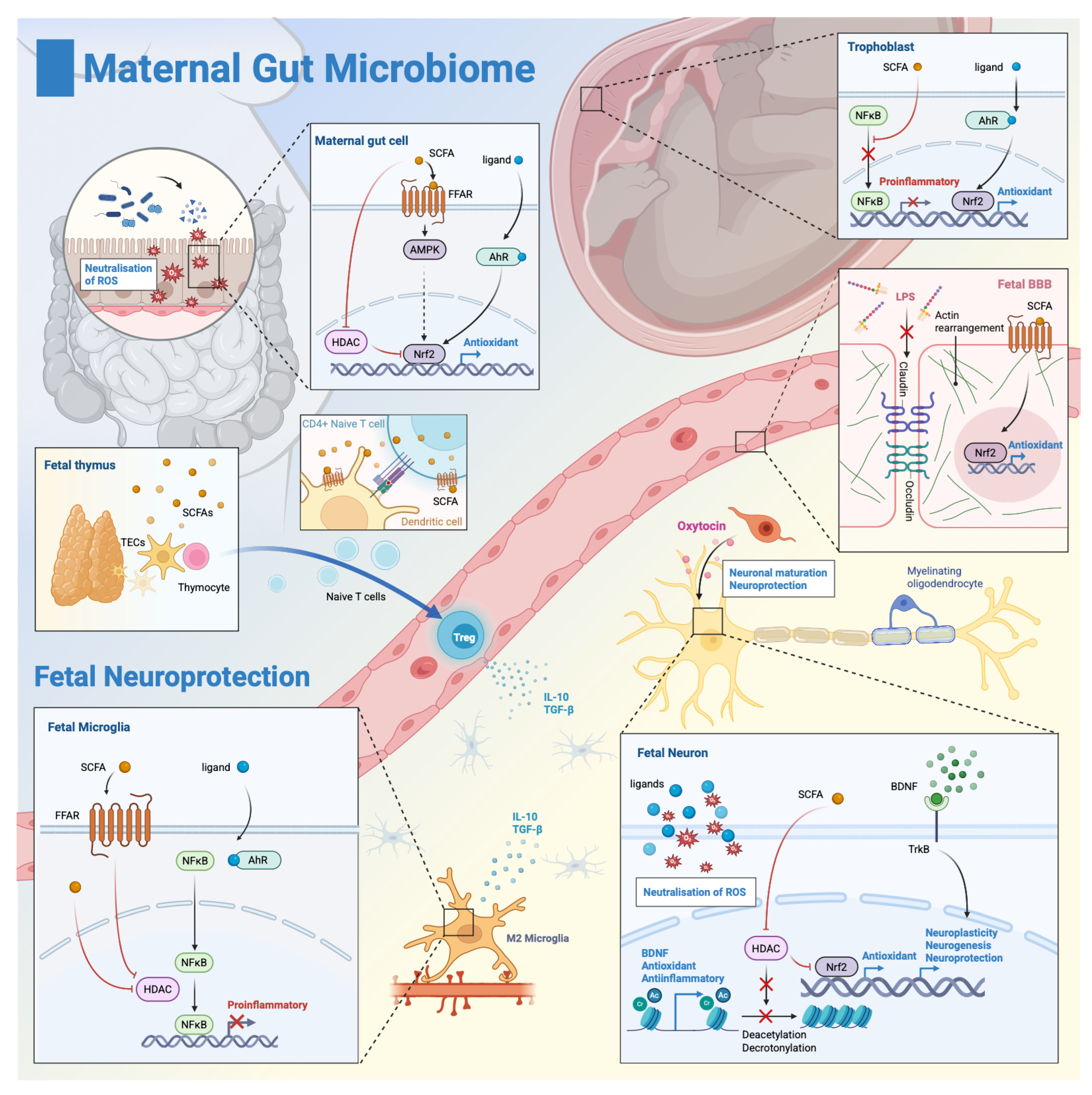
| Factor | Effect on Microbiome | Impact Type | References |
|---|---|---|---|
| High-fat diet | ↓ Bacteroidetes, ↑ Firmicutes, ↑ pro-inflammatory taxa | Dysbiotic | [64,65] |
| High-fiber diet | ↑ short-chain fatty acids (SCFA)-producers, ↑ Bifidobacterium, ↑ diversity | Beneficial | [66,67,68] |
| Alcohol consumption | ↓ beneficial bacteria, altered metabolic pathways | Dysbiotic | [69] |
| Antibiotic use | ↓ diversity, loss of key commensals, ↑ resistant strains | Dysbiotic | [63,70] |
| Probiotic supplementation | ↑ specific beneficial strains, ↑ barrier function | Beneficial | [71] |
| Iron supplementation | Altered Bacteroidetes to Firmicutes ratio, ↑ pathobionts | Variable | [72] |
| Elevated body mass index (BMI)/Obesity | ↓ diversity, ↑ Firmicutes, altered SCFA production | Dysbiotic | [73,74] |
| Insulin resistance | Altered glucose metabolism pathways, ↓ butyrate producers | Dysbiotic | [75] |
| Pre-existing gastrointestinalconditions: inflammatory bowel disease (IBD) | ↓ diversity, ↑ Proteobacteria, ↑ inflammatory markers | Dysbiotic | [76] |
| Smoking | ↓ beneficial anaerobes, ↑ opportunistic pathogens | Dysbiotic | [77,78] |
| Regular exercise | ↑ diversity, ↑ SCFA production, ↑ Akkermansia | Beneficial | [79,80] |
| Psychological stress | ↑ Proteobacteria, ↓ Lactobacillus, altered barrier function | Dysbiotic | [13,81] |
| Pregnancy progression | ↑ Proteobacteria (with pregnancy progression), ↓ diversity (adaptive) | Adaptive | [51,52,82] |
| Previous pregnancies | Enhanced microbial stability, faster adaptation | Beneficial | [83] |
| Maternal age | Age-dependent diversity changes, altered metabolic capacity | Variable | [51] |
| Residential environment | Urban vs. rural differences in diversity and composition | Variable | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozan, V.-P.; Ionescu, M.I.; Zahiu, C.M.D.; Catrina, A.M.; Racoviță, A.; Chirilă, A.-T.; Dogaru, I.-A.; Ciotei, C.; Pircalabioru, G.G.; Zăgrean, A.-M. Does the Maternal Gut Microbiome Influence the Outcome of Perinatal Asphyxia? Antioxidants 2025, 14, 1134. https://doi.org/10.3390/antiox14091134
Morozan V-P, Ionescu MI, Zahiu CMD, Catrina AM, Racoviță A, Chirilă A-T, Dogaru I-A, Ciotei C, Pircalabioru GG, Zăgrean A-M. Does the Maternal Gut Microbiome Influence the Outcome of Perinatal Asphyxia? Antioxidants. 2025; 14(9):1134. https://doi.org/10.3390/antiox14091134
Chicago/Turabian StyleMorozan, Vlad-Petru, Mara I. Ionescu, Carmen M. D. Zahiu, Ana Maria Catrina, Andreea Racoviță, Ana-Teodora Chirilă, Ioana-Alexandra Dogaru, Cristian Ciotei, Gratiela Gradisteanu Pircalabioru, and Ana-Maria Zăgrean. 2025. "Does the Maternal Gut Microbiome Influence the Outcome of Perinatal Asphyxia?" Antioxidants 14, no. 9: 1134. https://doi.org/10.3390/antiox14091134
APA StyleMorozan, V.-P., Ionescu, M. I., Zahiu, C. M. D., Catrina, A. M., Racoviță, A., Chirilă, A.-T., Dogaru, I.-A., Ciotei, C., Pircalabioru, G. G., & Zăgrean, A.-M. (2025). Does the Maternal Gut Microbiome Influence the Outcome of Perinatal Asphyxia? Antioxidants, 14(9), 1134. https://doi.org/10.3390/antiox14091134








