Dietary Soy Isoflavones Ameliorate Muscle Quality in High-Fat Diet-Fed Rice Field Eels (Monopterus albus) by Modulating Myogenesis, Collagen Synthesis, and Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Diets
2.3. Feeding Experiment and Sample Collection
2.4. Analytical Methods
2.4.1. Proximate Composition of Feed, Muscle and Whole Fish
2.4.2. Muscle Fatty Acid Composition
2.4.3. Muscle Water-Holding Capacity
2.4.4. Muscle Textural Properties
2.4.5. Muscle Histomorphology
2.4.6. Muscle Collagen Content
2.4.7. Muscle Antioxidant Indices
2.4.8. Real-Time Polymerase Chain Reaction
2.4.9. Statistical Analysis
3. Results
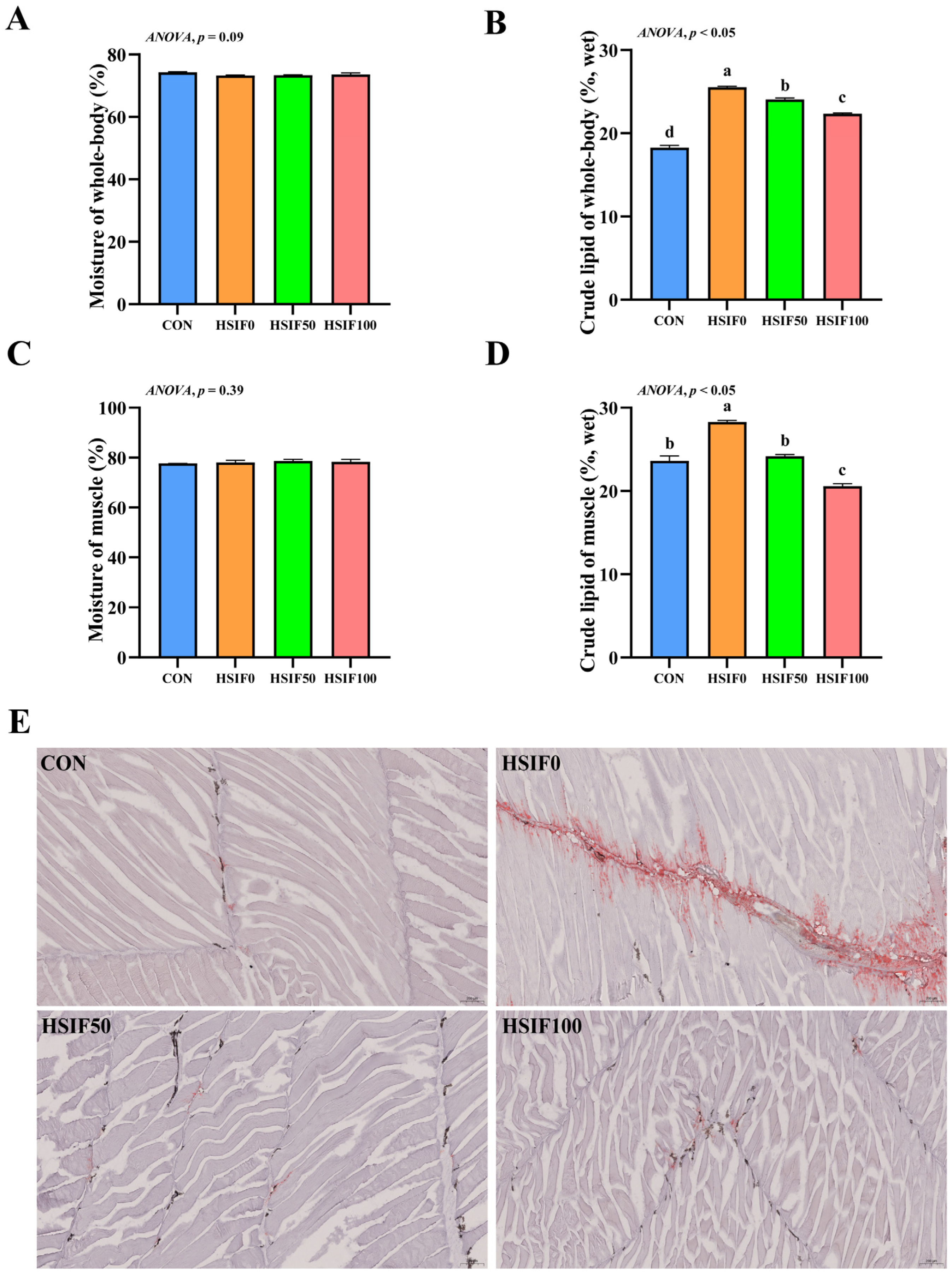
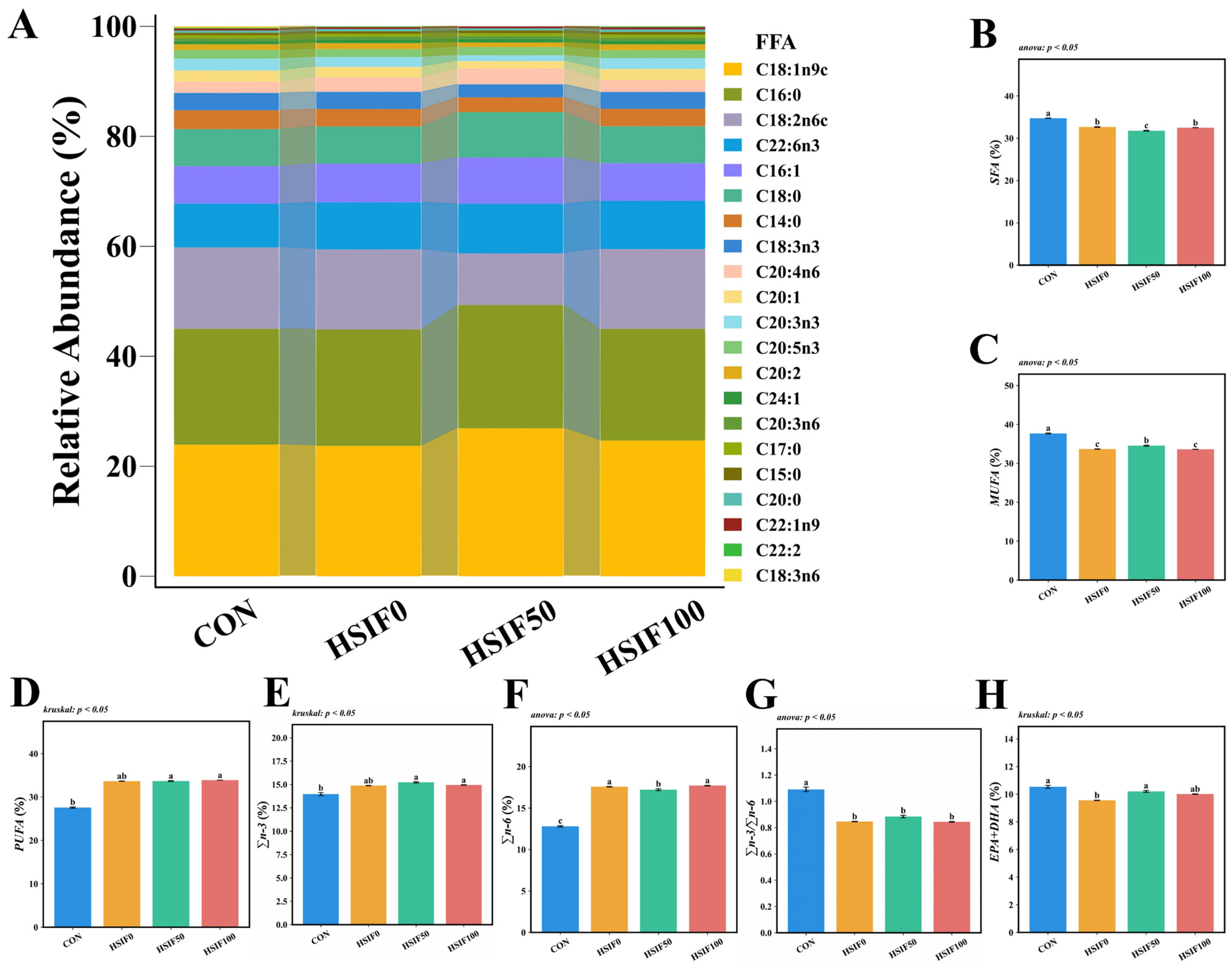
3.1. Proximate Composition, Muscle Oil Red O-Stained Sections, and Fatty Acid Profile
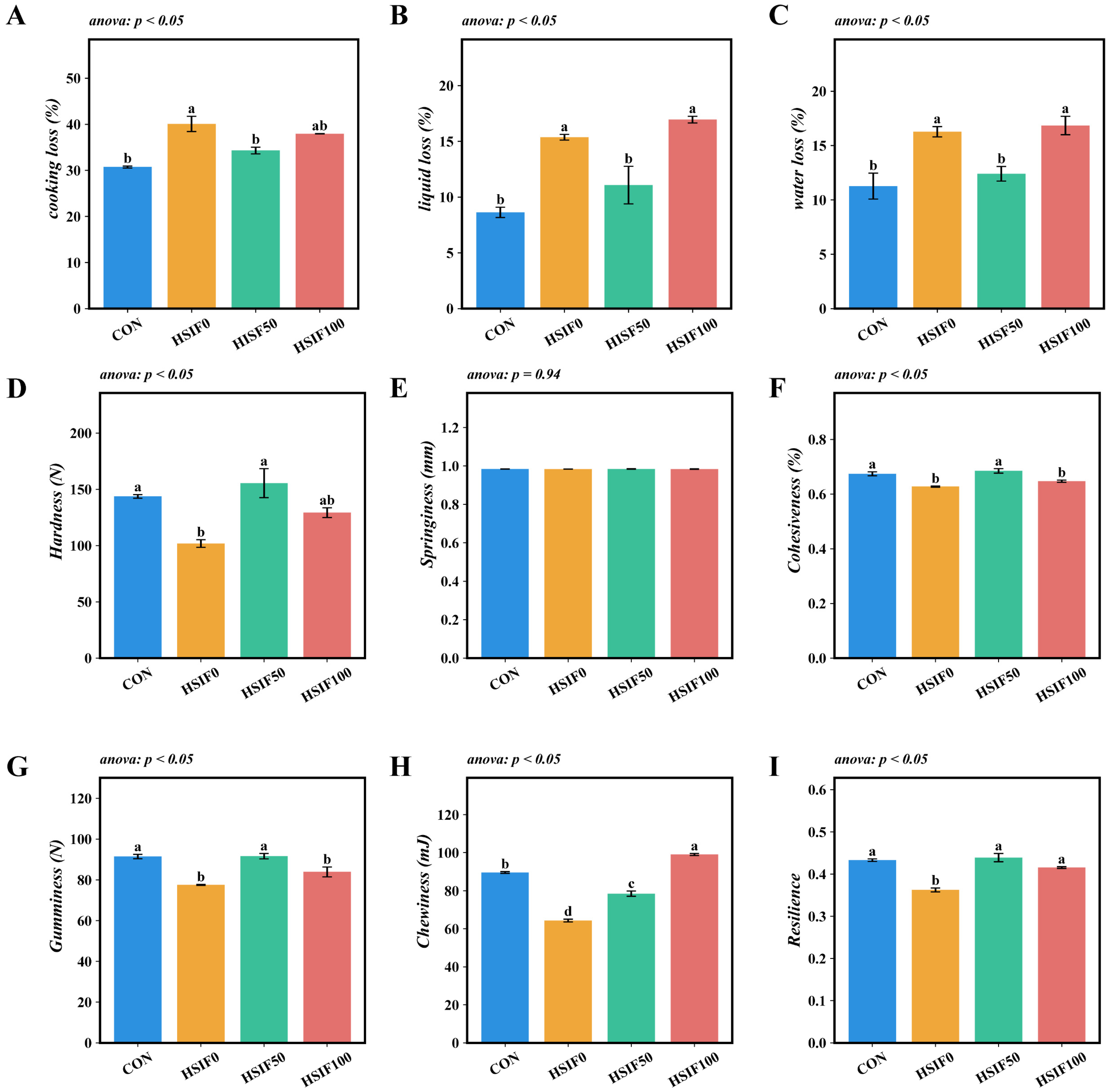
3.2. Muscle Water-Holding Capacity and Textural Properties
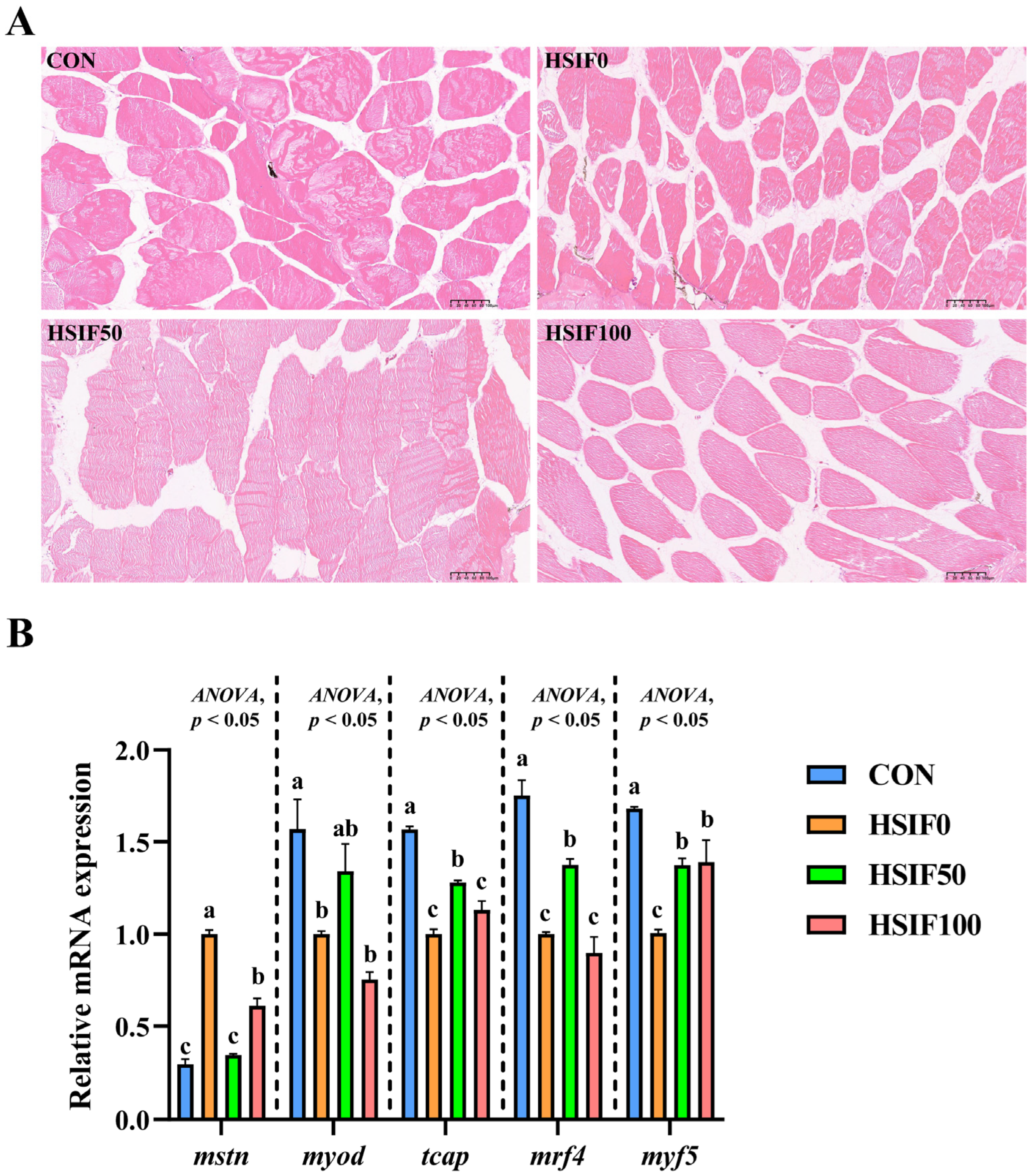
3.3. Muscle Histomorphology and Expression of Myogenesis-Related Genes
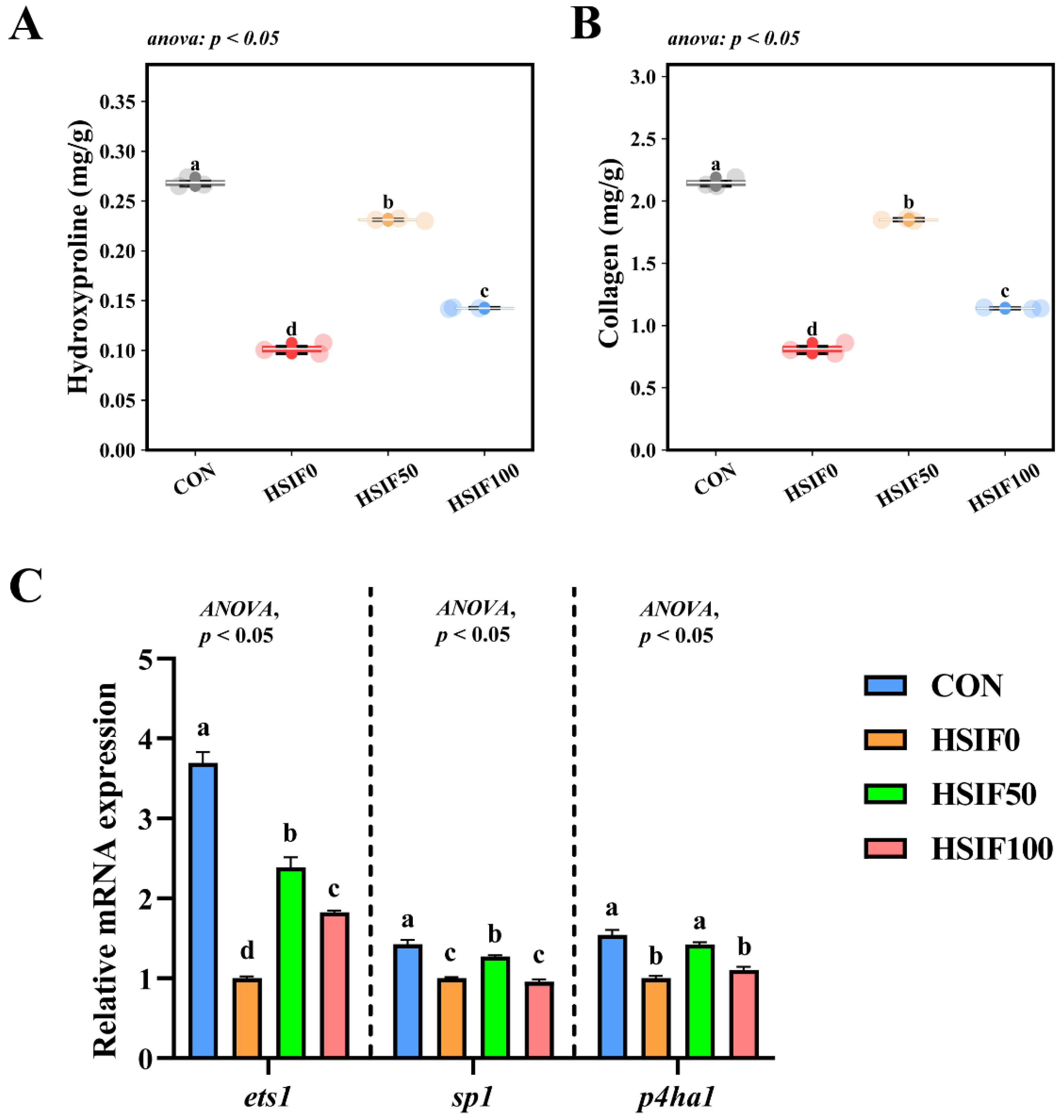
3.4. Muscle Collagen Content and Expression of Collagen Metabolism-Related Genes
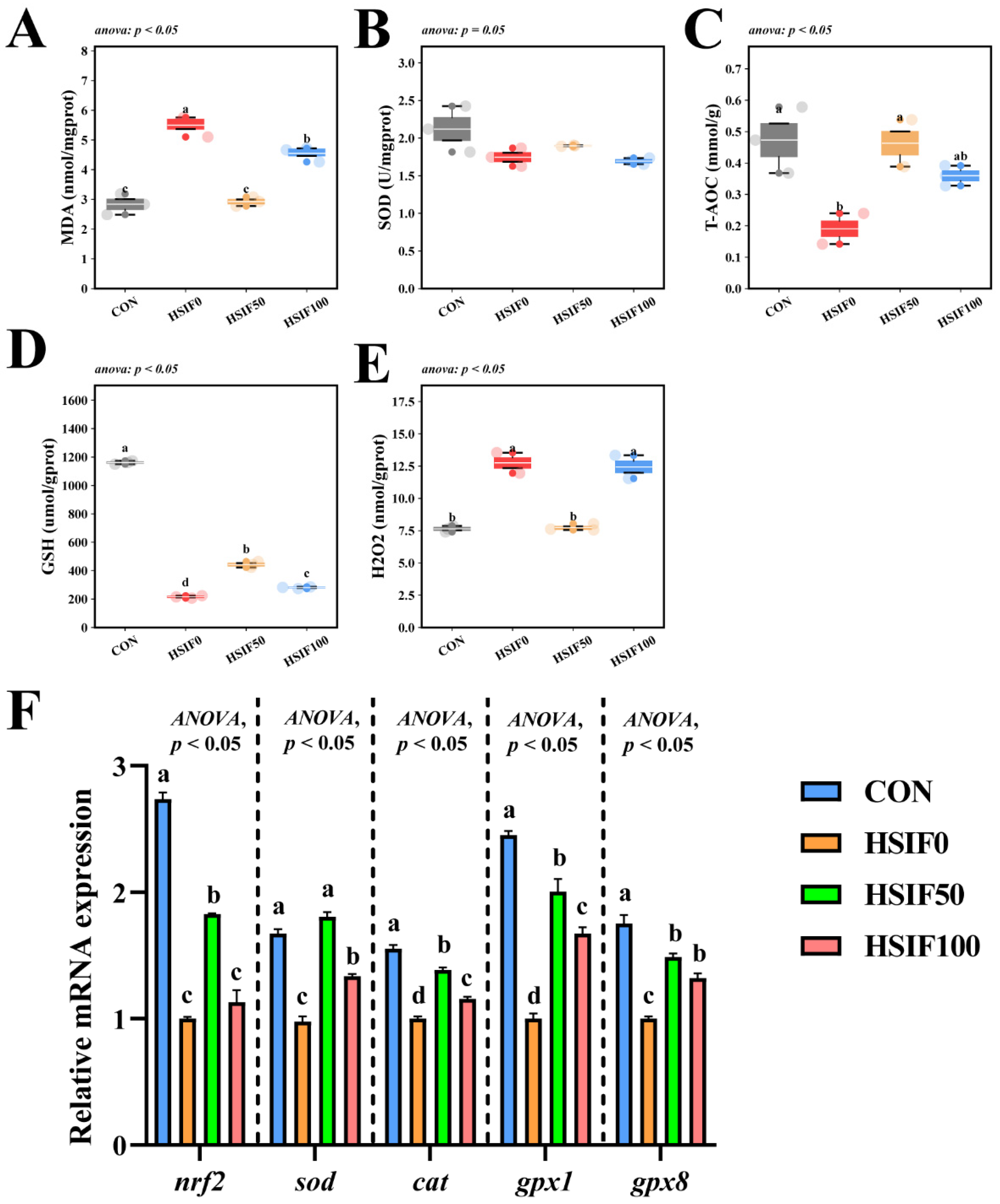
3.5. Antioxidant Capacity
4. Discussion
4.1. Proximate Composition, Muscle Oil Red O-Stained Sections, and Fatty Acid Profile
4.2. Muscle Water-Holding Capacity and Textural Properties
4.3. Muscle Histomorphology and Expression of Myogenesis-Related Genes
4.4. Muscle Collagen Content and Expression of Collagen Metabolism-Related Genes
4.5. Antioxidant Capacity
4.6. Economic and Regulatory Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naiel, M.A.E.; Negm, S.S.; Ghazanfar, S.; Shukry, M.; Abdelnour, S.A. The Risk Assessment of High-Fat Diet in Farmed Fish and Its Mitigation Approaches: A Review. J. Anim. Physiol. Anim. Nutr. 2023, 107, 948–969. [Google Scholar] [CrossRef]
- Jafari, S.; Akhavan-Bahabadi, M.; Shekarabi, S.P.H.; Mehrgan, M.S.; Lowen, E.; Lotfi, K.; Azari, A. Astaxanthin-Rich Nannochloropsis Oculata Mitigates High-Fat Diet-Induced Liver Dysfunction in Zebrafish by Modulating Lipid Metabolism and Oxidative Stress. Sci. Rep. 2025, 15, 28862. [Google Scholar] [CrossRef]
- Lv, H.-B.; Ma, Y.; Hu, C.-T.; Lin, Q.-Y.; Yue, J.; Chen, L.-Q.; Zhang, M.-L.; Du, Z.-Y.; Qiao, F. The Individual and Combined Effects of Hypoxia and High-Fat Diet Feeding on Nutrient Composition and Flesh Quality in Nile Tilapia (Oreochromis niloticus). Food Chem. 2021, 343, 128479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xia, J.; Zhang, X.; He, X.; Li, L.; Tang, R.; Chi, W.; Li, D. Diet Affects Muscle Quality and Growth Traits of Grass Carp (Ctenopharyngodon idellus): A Comparison Between Grass and Artificial Feed. Front. Physiol. 2018, 9, 283. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, X.; Guan, L.; Bao, S.; Zhuo, L.; Tian, H.; Li, C.; Ma, R. Does Dietary Lipid Level Affect the Quality of Triploid Rainbow Trout and How Should It Be Assessed? Foods 2023, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhuo, M.; Jiang, J.; Yu, A.; Yu, D.; Huang, F. Effects of Dietary Lipid Levels on Fiber Quality, Lipidomic Profiles, Antioxidant and Inflammation Responses in Muscle of Yellow Catfish Pelteobagrus fulvidraco. Aquac. Rep. 2023, 33, 101855. [Google Scholar] [CrossRef]
- Grgic, D.; Varga, E.; Novak, B.; Müller, A.; Marko, D. Isoflavones in Animals: Metabolism and Effects in Livestock and Occurrence in Feed. Toxins 2021, 13, 836. [Google Scholar] [CrossRef]
- Kim, I.-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.L.; Bidner, T.D.; Southern, L.L.; Geaghan, J.P. Effects of Dietary Soy Isoflavones on Growth, Carcass Traits, and Meat Quality in Growing-Finishing Pigs2. J. Anim. Sci. 2001, 79, 1230–1239. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Jiang, S.Q.; Lin, Y.C.; Xi, P.B.; Yu, D.Q.; Wu, T.X. Effects of Soybean Isoflavone on Growth Performance, Meat Quality, and Antioxidation in Male Broilers. Poult. Sci. 2007, 86, 1356–1362. [Google Scholar] [CrossRef]
- Gu, M.; Jia, Q.; Zhang, Z.; Bai, N.; Xu, X.; Xu, B. Soya-Saponins Induce Intestinal Inflammation and Barrier Dysfunction in Juvenile Turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2018, 77, 264–272. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Ge, X.; Niu, J.; Wang, J.; Wang, Y.; Chen, L.; Huang, Z.; Yu, W.; Tan, X. The Effects of Dietary Soybean Isoflavones on Growth, Innate Immune Responses, Hepatic Antioxidant Abilities and Disease Resistance of Juvenile Golden Pompano Trachinotus ovatus. Fish Shellfish Immunol. 2015, 43, 158–166. [Google Scholar] [CrossRef]
- Cao, S.; Xiong, D.; Luo, W.; Tang, J.; Qu, F.; Zhou, Y.; He, Z.; Xie, S.; Liu, Z. Effects of Dietary Soy Isoflavones on Growth, Antioxidant Status, Immune Response and Resistance of Juvenile Grass Carp (Ctenopharyngodon idella) to Aeromonas Hydrophila Challenge. Aquac. Res. 2020, 51, 2472–2482. [Google Scholar] [CrossRef]
- Mai, K.; Zhang, Y.; Chen, W.; Xu, W.; Ai, Q.; Zhang, W. Effects of Dietary Soy Isoflavones on Feed Intake, Growth Performance and Digestibility in Juvenile Japanese Flounder (Paralichthys olivaceus). J. Ocean Univ. China 2012, 11, 511–516. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Xue, J.; Chu, W.; Hu, Y. Effects of Dietary Soy Isoflavone and Soy Saponin on Growth Performance, Intestinal Structure, Intestinal Immunity and Gut Microbiota Community on Rice Field Eel (Monopterus albus). Aquaculture 2021, 537, 736506. [Google Scholar] [CrossRef]
- He, L.; Wang, H.; Li, E.; Huang, Q.; Wang, X.; Qiao, F.; Qin, C.; Qin, J.; Chen, L. Effects of Soy Isoflavones on Growth Performance, Antioxidant Capacity, Non-Specific Immunity and Lipid Metabolism of Juvenile Chinese Mitten Crab, Eriocheir sinensis. Aquaculture 2024, 581, 740470. [Google Scholar] [CrossRef]
- Pastore, M.R.; Negrato, E.; Poltronieri, C.; Barion, G.; Messina, M.; Tulli, F.; Ballarin, C.; Maccatrozzo, L.; Radaelli, G.; Bertotto, D. Effects of Dietary Soy Isoflavones on Estrogenic Activity, Cortisol Level, Health and Growth in Rainbow Trout, Oncorhynchus mykiss. Aquac. Res. 2018, 49, 1469–1479. [Google Scholar] [CrossRef]
- Xie, K.; Tang, Z.; Shi, Y.; Deng, Z.; Cai, M.; Dai, J.; Shao, C.; Zhang, J.; Hu, Y.; Li, D. Effects of Corbicula Fluminea Meal in High-Fat Diet on Growth, Lipid Metabolism and Intestinal Microbiota of Juvenile Rice Field Eel Monopterus albus. Aquaculture 2024, 590, 741064. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of Official Analytical Chemists International, 16th ed.; AOAC Int.: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Honikel, K.O. Reference Methods for the Assessment of Physical Characteristics of Meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Jiang, X.; Song, Z.; Li, C.; Hu, X.; Ge, Y.; Cheng, L.; Shi, X.; Jia, Z. Effects of Dietary Lipid Levels on the Growth, Muscle Fatty Acid and Amino Acid Composition, Antioxidant Capacity, and Lipid Deposition in Mirror Carp (Cyprinus carpio). Animals 2024, 14, 2583. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological Effect of Soy Isoflavones in the Prevention of Civilization Diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Ide, T. Effects of Soy Protein and Isoflavone on Hepatic Fatty Acid Synthesis and Oxidation and mRNA Expression of Uncoupling Proteins and Peroxisome Proliferator-Activated Receptor γ in Adipose Tissues of Rats. J. Nutr. Biochem. 2008, 19, 682–693. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, M.; Liu, M.; Zhang, W.; Zhi, S.; Qu, L.; Xiong, J.; Wang, L.; Qin, C.; Nie, G. Effects of Genistein on Lipid Metabolism, Antioxidant Activity, and Immunity of Common Carp (Cyprinus carpio L.) Fed with High-Carbohydrate and High-Fat Diets. Aquac. Nutr. 2023, 2023, 9555855. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Y.; Wang, L.; Song, K.; Zhang, C.; Lu, K.; Rahimnejad, S. Dietary N-3/n-6 Polyunsaturated Fatty Acid Ratio Modulates Growth Performance in Spotted Seabass (Lateolabrax maculatus) through Regulating Lipid Metabolism, Hepatic Antioxidant Capacity and Intestinal Health. Anim. Nutr. 2023, 14, 20–31. [Google Scholar] [CrossRef]
- Hanwell, H.E.C.; Kay, C.D.; Lampe, J.W.; Holub, B.J.; Duncan, A.M. Acute Fish Oil and Soy Isoflavone Supplementation Increase Postprandial Serum (n-3) Polyunsaturated Fatty Acids and Isoflavones but Do Not Affect Triacylglycerols or Biomarkers of Oxidative Stress in Overweight and Obese Hypertriglyceridemic Men. J. Nutr. 2009, 139, 1128–1134. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, W.-D.; Wu, P.; Liu, Y.; Zeng, Y.-Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Wang, S.-W.; et al. Soybean Isoflavones Improve the Health Benefits, Flavour Quality Indicators and Physical Properties of Grass Carp (Ctenopharygodon idella). PLoS ONE 2019, 14, e0209570. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xue, R.; Zhou, L.; Sun, J.; Ji, H. The Individual and Combined Effect of DHA and High-Fat Diet on Flesh Quality, Antioxidant Capacity and Myofiber Characteristics of Grass Carp (Ctenopharyngodon idellus). Aquaculture 2025, 595, 741487. [Google Scholar] [CrossRef]
- Li, H.; Pan, Y.; Liu, L.; Li, Y.; Huang, X.; Zhong, Y.; Tang, T.; Zhang, J.; Chu, W.; Shen, Y. Effects of High-Fat Diet on Muscle Textural Properties, Antioxidant Status and Autophagy of Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Aquaculture 2019, 511, 734228. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Tang, R.; He, X.; Li, L.; Takagi, Y.; Li, D. Improvement of Muscle Quality of Grass Carp (Ctenopharyngodon Idellus) with a Bio-Floating Bed in Culture Ponds. Front. Physiol. 2019, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; He, Y.; Zheng, J.; Xu, Y.; Tan, Y.; Jia, L.; Chen, L.; Ye, J.; Qi, C. Effects of Soybean Isoflavones on the Growth Performance and Lipid Metabolism of the Juvenile Chinese Mitten Crab Eriocheir sinensis. Fishes 2024, 9, 335. [Google Scholar] [CrossRef]
- Pastore, M.R. Effects of Dietary Soy Isoflavones on Rainbow Trout, Oncorhynchus mykiss. Ph.D. Thesis, Università di Padova, Padua, Italy, 2018. [Google Scholar]
- Dong, M.; Zhang, L.; Wu, P.; Feng, L.; Jiang, W.; Liu, Y.; Kuang, S.; Li, S.; Mi, H.; Tang, L.; et al. Dietary Protein Levels Changed the Hardness of Muscle by Acting on Muscle Fiber Growth and the Metabolism of Collagen in Sub-Adult Grass Carp (Ctenopharyngodon idella). J. Anim. Sci. Biotechnol. 2022, 13, 109. [Google Scholar] [CrossRef]
- Zou, Y.-Y.; Chen, Z.-L.; Sun, C.-C.; Yang, D.; Zhou, Z.-Q.; Xiao, Q.; Peng, X.-Y.; Tang, C.-F. A High-Fat Diet Induces Muscle Mitochondrial Dysfunction and Impairs Swimming Capacity in Zebrafish: A New Model of Sarcopenic Obesity. Nutrients 2022, 14, 1975. [Google Scholar] [CrossRef] [PubMed]
- Piekarz, A.V.; Ward, W.E. Effect of Neonatal Exposure to Genistein on Bone Metabolism in Mice at Adulthood. Pediatr. Res. 2007, 61, 48–53. [Google Scholar] [CrossRef]
- He, L.; Huang, K.; Wang, X.; Qin, J.; Li, E.; Chen, L. Optimizing Gonadal Development and Muscle Flavor Quality in the Male Chinese Mitten Crabs (Eriocheir sinensis): Utilization of Soy Isoflavones in Crustaceans. Food Chem. Mol. Sci. 2025, 11, 100284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mai, K.; Zhang, W. mTOR Signaling in Aquaculture Animals: A Central Nutrient-Sensing Hub Orchestrating Growth, Metabolism, Health and Quality. Rev. Aquac. 2025, 17, e70068. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Liu, B.; Gao, Q.; Sun, C.; Zhou, Q.; Zheng, X.; Liu, B. Effects of High Fat in the Diet on Growth, Antioxidant, Immunity and Fat Deposition of Macrobrachium Rosenbergii Post-Larvae. Fish Shellfish Immunol. 2022, 129, 13–21. [Google Scholar] [CrossRef]
- Fei, S.; Xia, Y.; Chen, Z.; Liu, C.; Liu, H.; Han, D.; Jin, J.; Yang, Y.; Zhu, X.; Xie, S. A High-Fat Diet Alters Lipid Accumulation and Oxidative Stress and Reduces the Disease Resistance of Overwintering Hybrid Yellow Catfish (Pelteobagrus fulvidraco ♀ × P. Vachelli ♂). Aquac. Rep. 2022, 23, 101043. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Fan, Z.; Wang, L.; Zheng, X. Resveratrol Ameliorates Oxidative Stress, Inflammatory Response and Lipid Metabolism in Common Carp (Cyprinus carpio) Fed with High-Fat Diet. Front. Immunol. 2022, 13, 965954. [Google Scholar] [CrossRef]
| Ingredient | CON | HSIF0 | HSIF50 | HSIF100 |
|---|---|---|---|---|
| Fish meal | 40.00 | 40.00 | 40.00 | 40.00 |
| Soy protein concentrate | 16.00 | 16.00 | 16.00 | 16.00 |
| Poultry by-product meal | 3.00 | 3.00 | 3.00 | 3.00 |
| Beer yeast | 5.00 | 5.00 | 5.00 | 5.00 |
| Fish oil | 2.20 | 8.20 | 8.20 | 8.20 |
| Microcrystalline cellulose | 10.26 | 4.26 | 4.26 | 4.26 |
| Corn starch | 20.00 | 20.00 | 20.00 | 20.00 |
| Choline | 0.50 | 0.50 | 0.50 | 0.50 |
| Ca(H2PO4)2 | 2.00 | 2.00 | 2.00 | 2.00 |
| Premix 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Antioxidants 2 | 0.01 | 0.01 | 0.01 | 0.01 |
| Mold inhibitor 3 | 0.03 | 0.03 | 0.03 | 0.03 |
| Soy isoflavone 4, mg/kg | 0.00 | 0.00 | 50.00 | 100.00 |
| Nutrition levels 5 | ||||
| Moisture | 7.45 | 7.52 | 7.56 | 7.49 |
| Crude protein | 41.36 | 41.26 | 40.96 | 41.51 |
| Crude fat | 6.16 | 11.98 | 12.11 | 12.08 |
| Ash | 10.23 | 10.35 | 10.17 | 10.31 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Accession No. |
|---|---|---|---|
| rpl17 | CGAGAACCCGACTAAATCA | GTTGTAGCGACGGAAAGG | XM_020587712.1 |
| mstn | TTGGCTGGGACTGGATTATTG | TTGGTGACATCTTGGTGGGG | KM103284.1 |
| myod | ACATCCAGCTCCTTTCCTCC | TGCTCAATCCCAAACACACC | XM_020593504.1 |
| tcap | TGGAGAAGTGTAATGGTATGGTGG | GCCTTTCCTGAGGTTGGGTT | XM_020607417.1 |
| mrf4 | CAAGGTGGAGATTTTACGCAGC | GTTTGGGTTTTCTCCTGTTCGT | KM103283.1 |
| myf5 | ATGTCTTCTCATCATCCCAGGTC | CCTGACGTGCTCATCTTCCTCT | KM103285.1 |
| ets1 | TCAGCTCAGAGGAACTGCTGAC | AGTCCTGGCCACCAAGTTTACC | XM_020600359.1 |
| sp1 | ACAGCCAGTGTCTTCCAACAG | AAGGATCTGCTGTGACTGCTG | XM_020600700.1 |
| p4ha3 | AAAGACTCTGCCAGACCCAAG | ATGTCTTCAGCCTCTGTGTCAG | XM_020588625.1 |
| nrf2 | TCACAGACGAGAATGATGCC | CTGCTACTGGGAACTGAAACTG | XM_020596408.1 |
| sod | GTTGCCAAGATAGACATCACGG | TCATTGCCTCCTTTTCCCAG | XM_020598412.1 |
| cat | CATTGGGAAGACTACACCTATCGC | GATGAAGAAGATGGGGGTGTTG | XM_020624985.1 |
| gpx1 | AGATGTGAATGGGAAGGATGCC | AAACTTCGGGTCAGTCATCAGG | XM_020607739.1 |
| gpx8 | ATCCTGCCTTCAGATTCCTCAC | TCATTTCTCGCACCAGCACT | XM_020593975.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, K.; Li, Q.; Zheng, S.; Wei, H.; Zhou, T.; Hu, Y.; Zhang, J. Dietary Soy Isoflavones Ameliorate Muscle Quality in High-Fat Diet-Fed Rice Field Eels (Monopterus albus) by Modulating Myogenesis, Collagen Synthesis, and Antioxidant Capacity. Antioxidants 2025, 14, 1195. https://doi.org/10.3390/antiox14101195
Xie K, Li Q, Zheng S, Wei H, Zhou T, Hu Y, Zhang J. Dietary Soy Isoflavones Ameliorate Muscle Quality in High-Fat Diet-Fed Rice Field Eels (Monopterus albus) by Modulating Myogenesis, Collagen Synthesis, and Antioxidant Capacity. Antioxidants. 2025; 14(10):1195. https://doi.org/10.3390/antiox14101195
Chicago/Turabian StyleXie, Kai, Quan Li, Shuang Zheng, Huahong Wei, Tao Zhou, Yi Hu, and Junzhi Zhang. 2025. "Dietary Soy Isoflavones Ameliorate Muscle Quality in High-Fat Diet-Fed Rice Field Eels (Monopterus albus) by Modulating Myogenesis, Collagen Synthesis, and Antioxidant Capacity" Antioxidants 14, no. 10: 1195. https://doi.org/10.3390/antiox14101195
APA StyleXie, K., Li, Q., Zheng, S., Wei, H., Zhou, T., Hu, Y., & Zhang, J. (2025). Dietary Soy Isoflavones Ameliorate Muscle Quality in High-Fat Diet-Fed Rice Field Eels (Monopterus albus) by Modulating Myogenesis, Collagen Synthesis, and Antioxidant Capacity. Antioxidants, 14(10), 1195. https://doi.org/10.3390/antiox14101195





