Transylvanian Grape Pomaces as Sustainable Sources of Antioxidant Phenolics and Fatty Acids—A Study of White and Red Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. GP Generation and Conditioning
2.3. GP Polyphenols Extraction
2.4. Total Polyphenol Content (TPC) of GP Polyphenol Extracts
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.6. Liquid Chromatography–Diode Array Detection–Electro-Spray Ionization Mass Spectrometry (HPLC–DAD–ESI MS) Analysis
2.7. Gas Chromatography with Flame Ionization Detector (GC–FID Analysis)
2.8. Antioxidant Properties
2.8.1. Antiradical Assays
- •
- Measurement of Relative DPPH Radical Scavenging Capacity.
- •
- Measurement of ABTS Cation Radical Scavenging Capacity (ABTS).
2.8.2. Electron Transfer Assays
- •
- Measurement of Cupric Ion Reducing Antioxidant Capacity (CUPRAC).
- •
- Measurement of Ferric-Reducing Antioxidant Potential (FRAP).
- •
- Measurement of Reducing Power (RP).
2.8.3. Transition Metal Ion (Ferrous Fe2+ and Cupric Cu2+) Chelation Assays
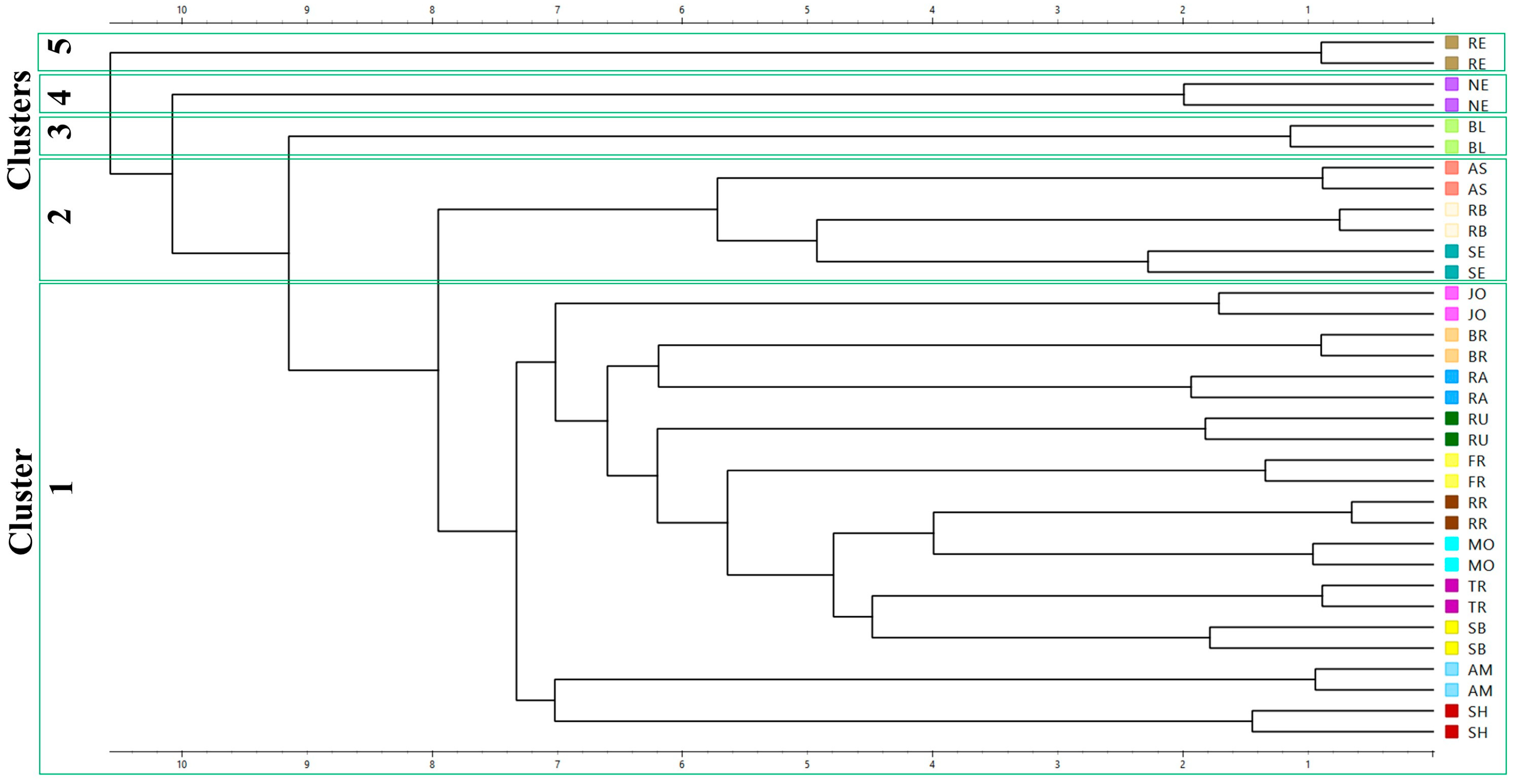
2.9. Statistical Analysis
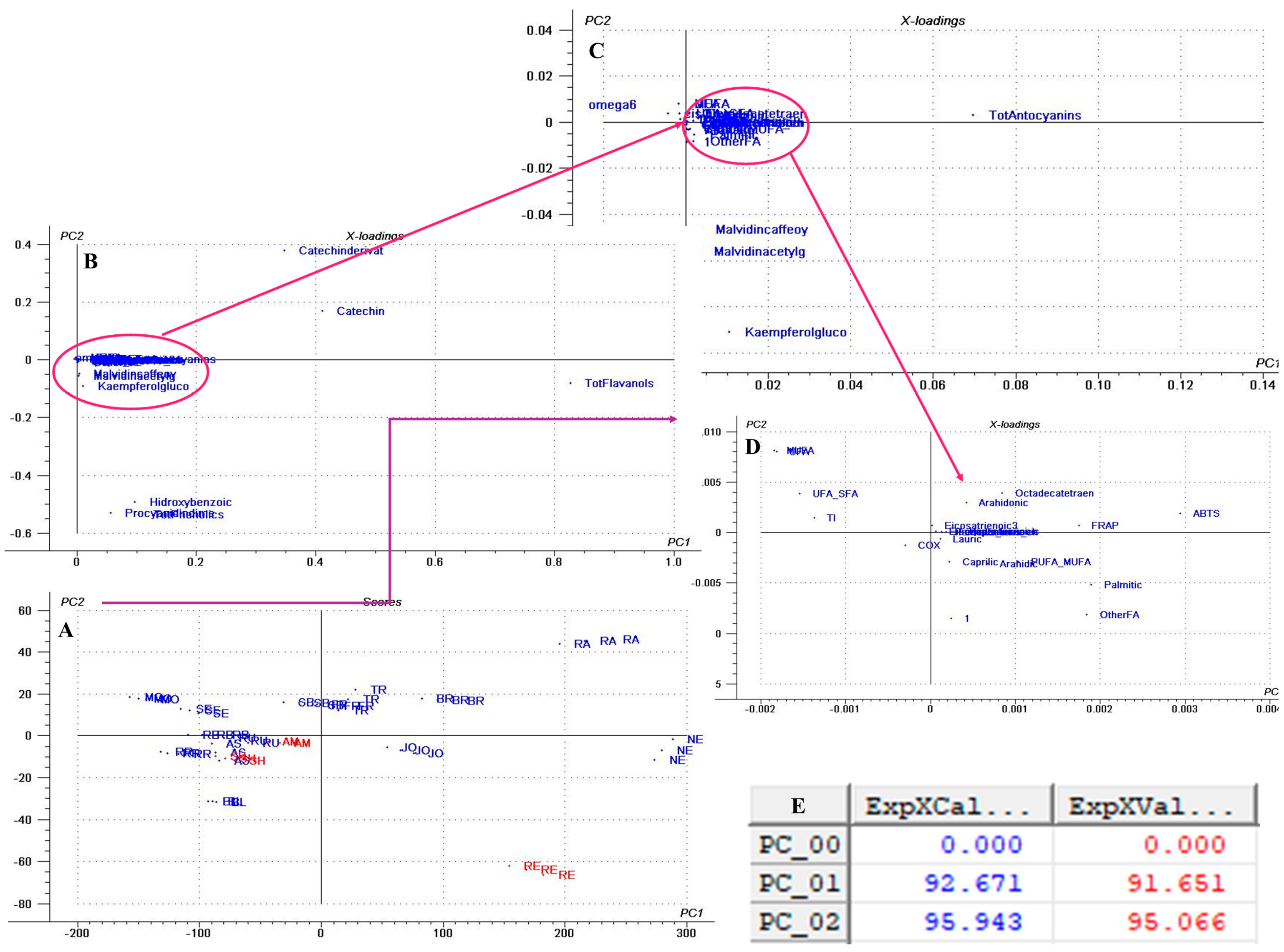
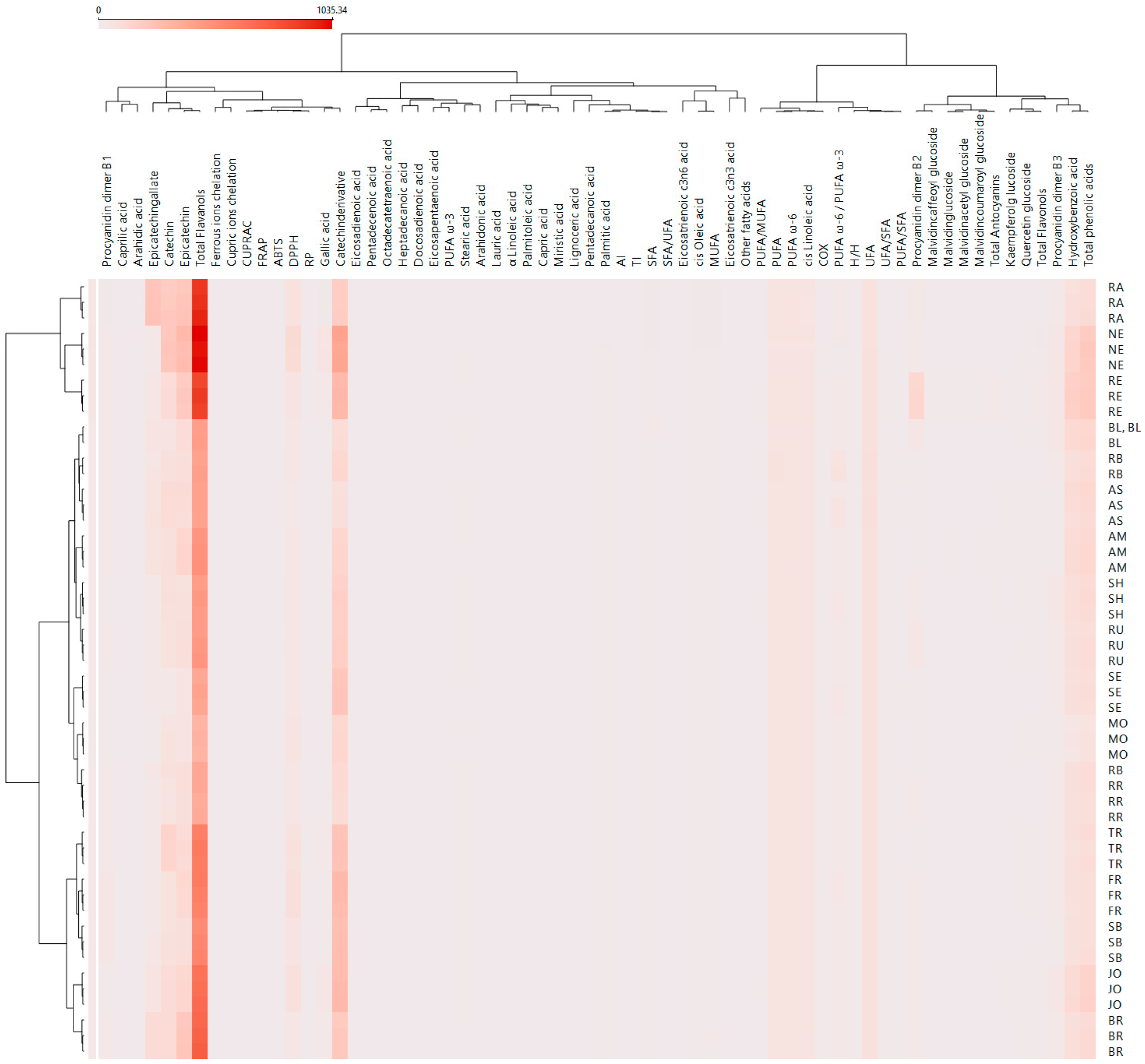
3. Results and Discussions
3.1. TPC of GP Polyphenol Extracts
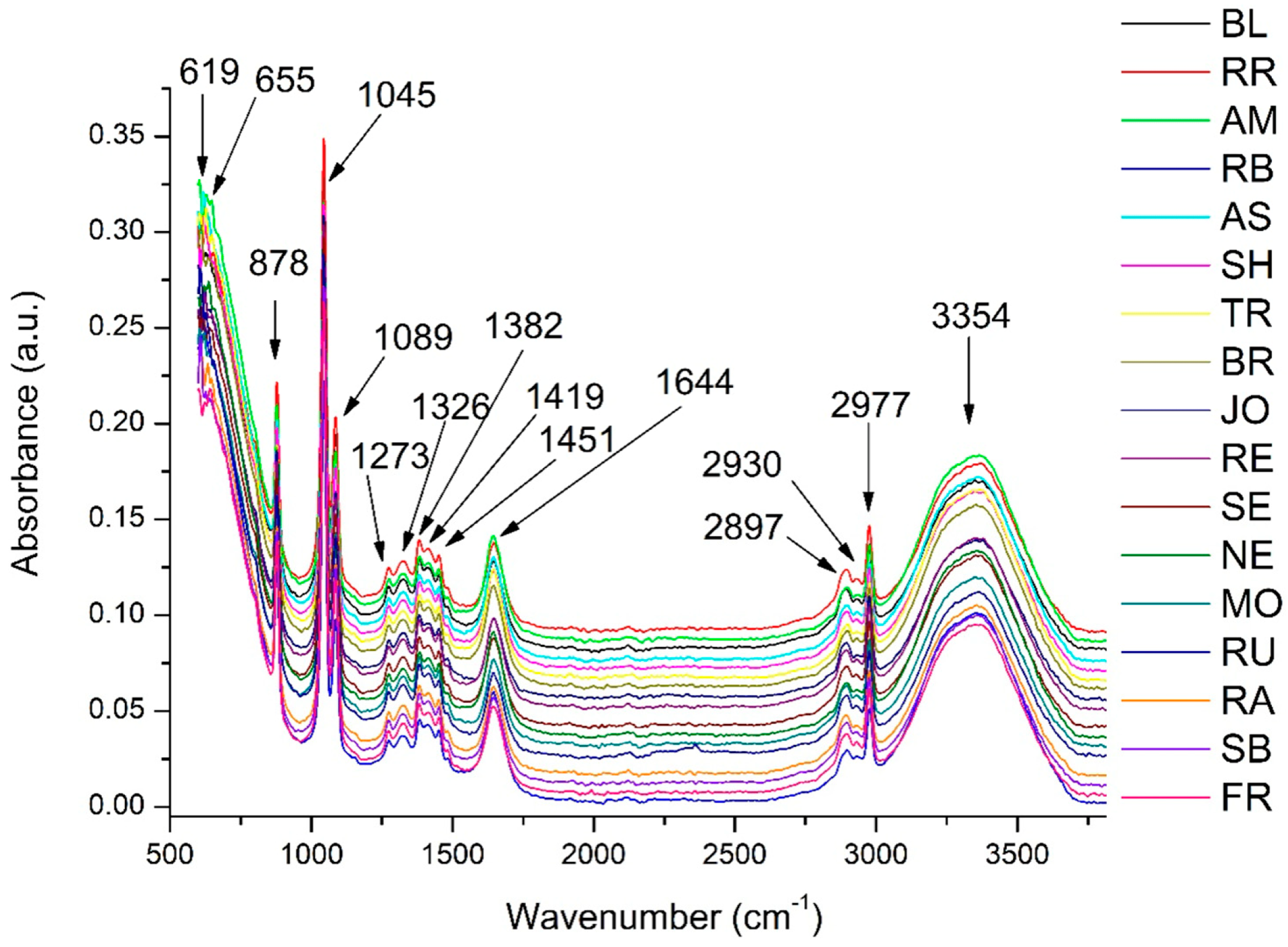
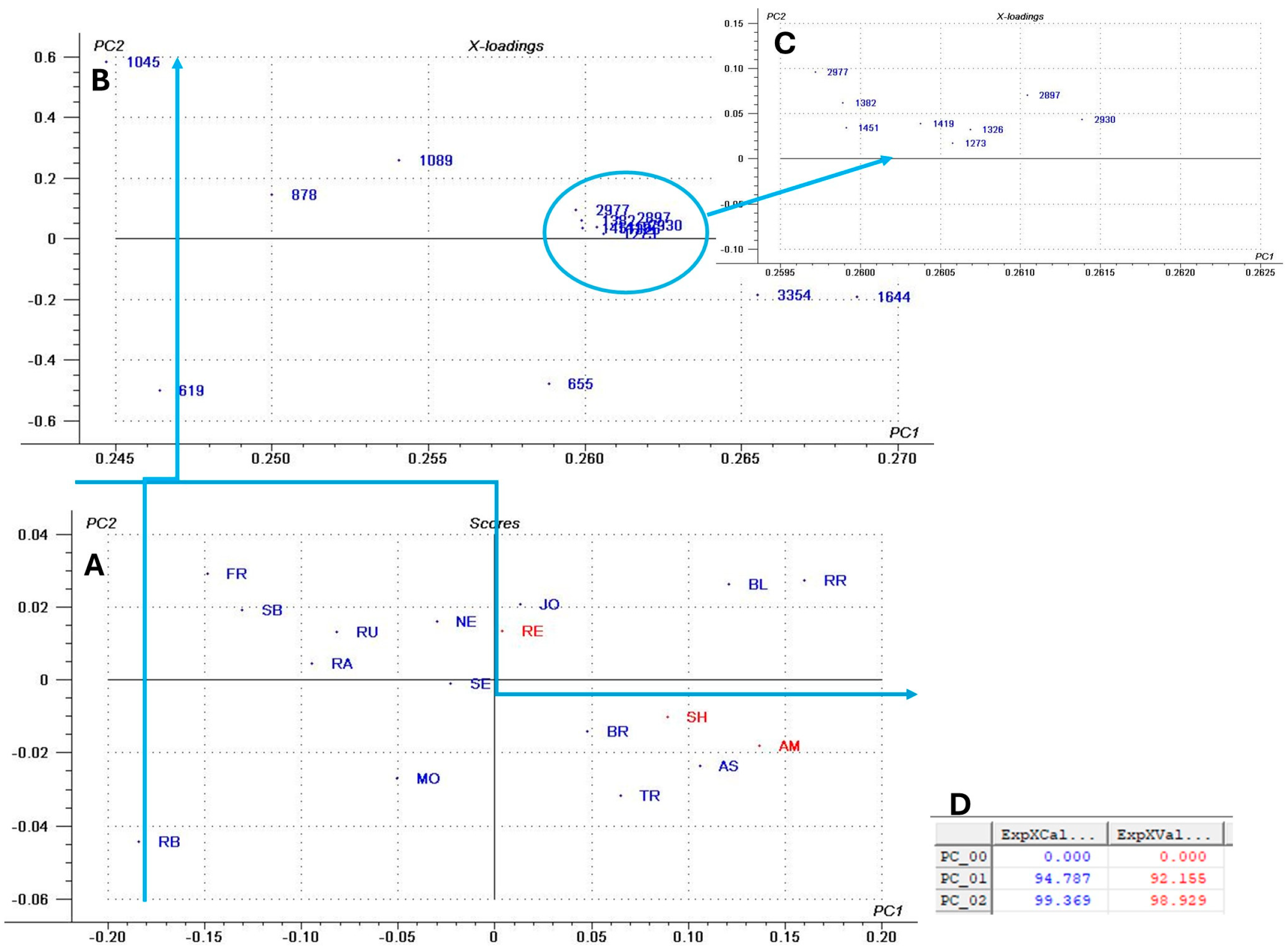
3.2. FTIR Analysis of GP Extracts
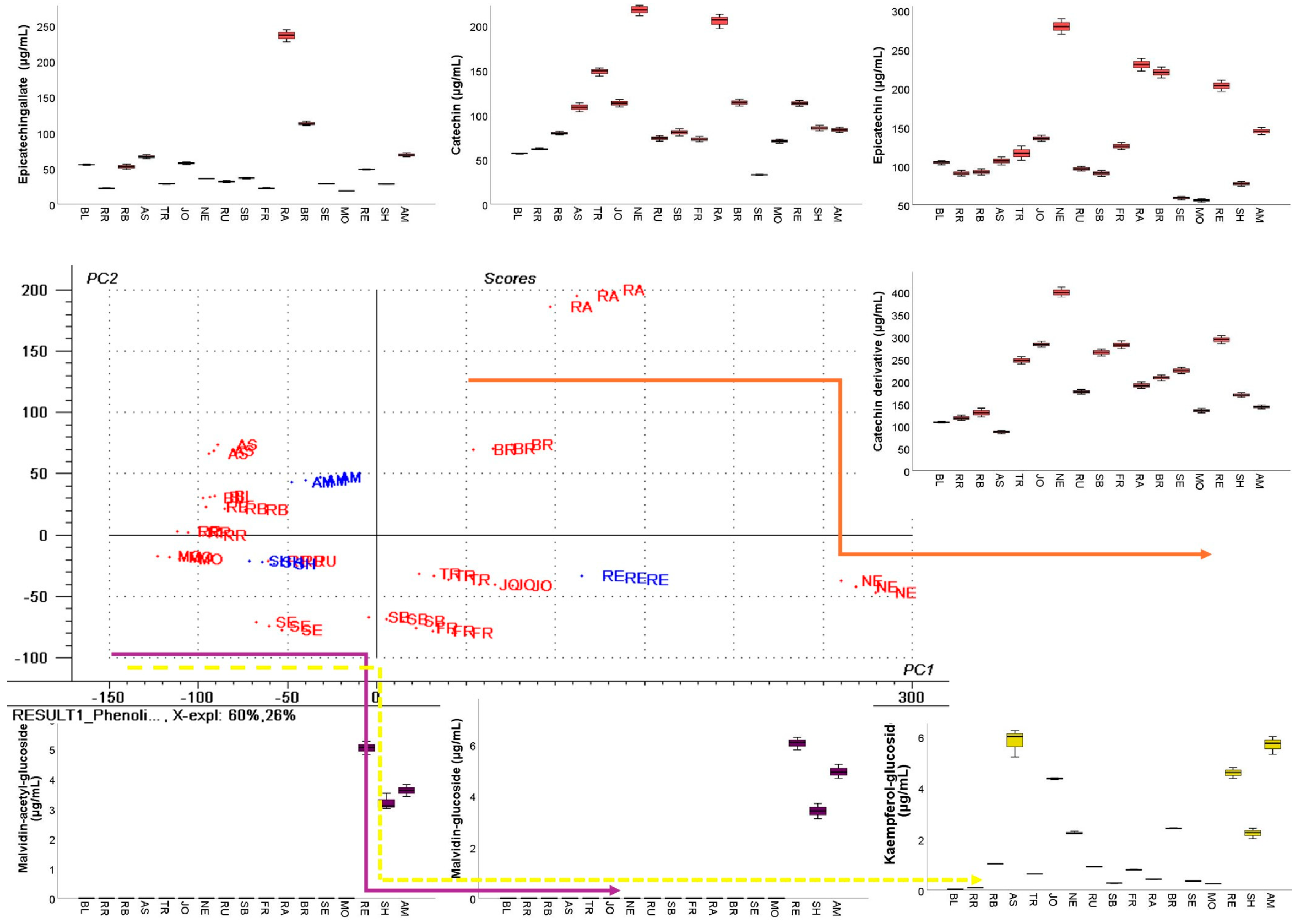
3.3. HPLC–DAD–ESI MS Analysis of GP Extracts
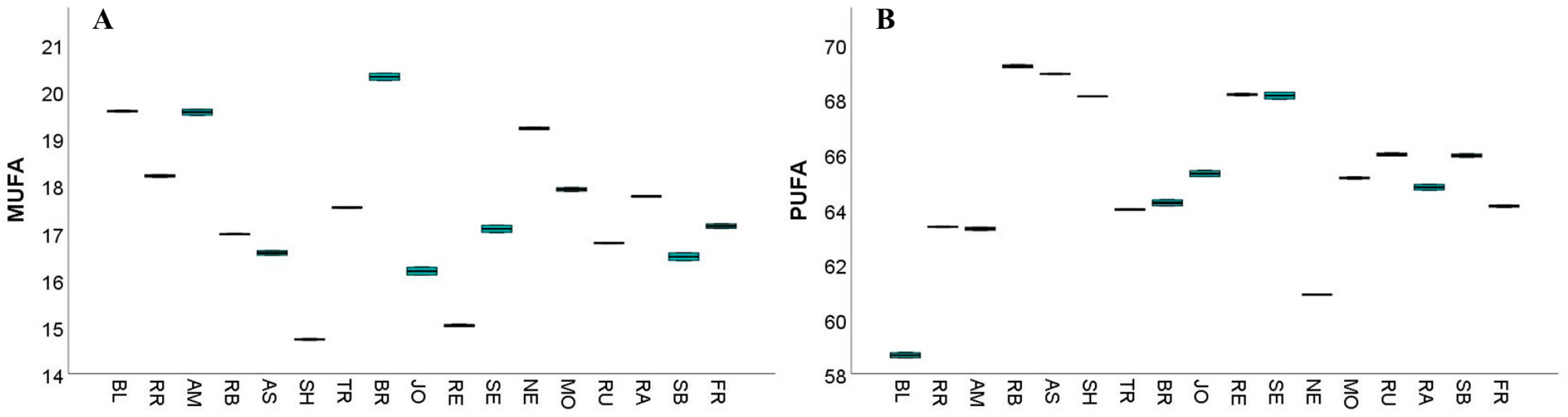
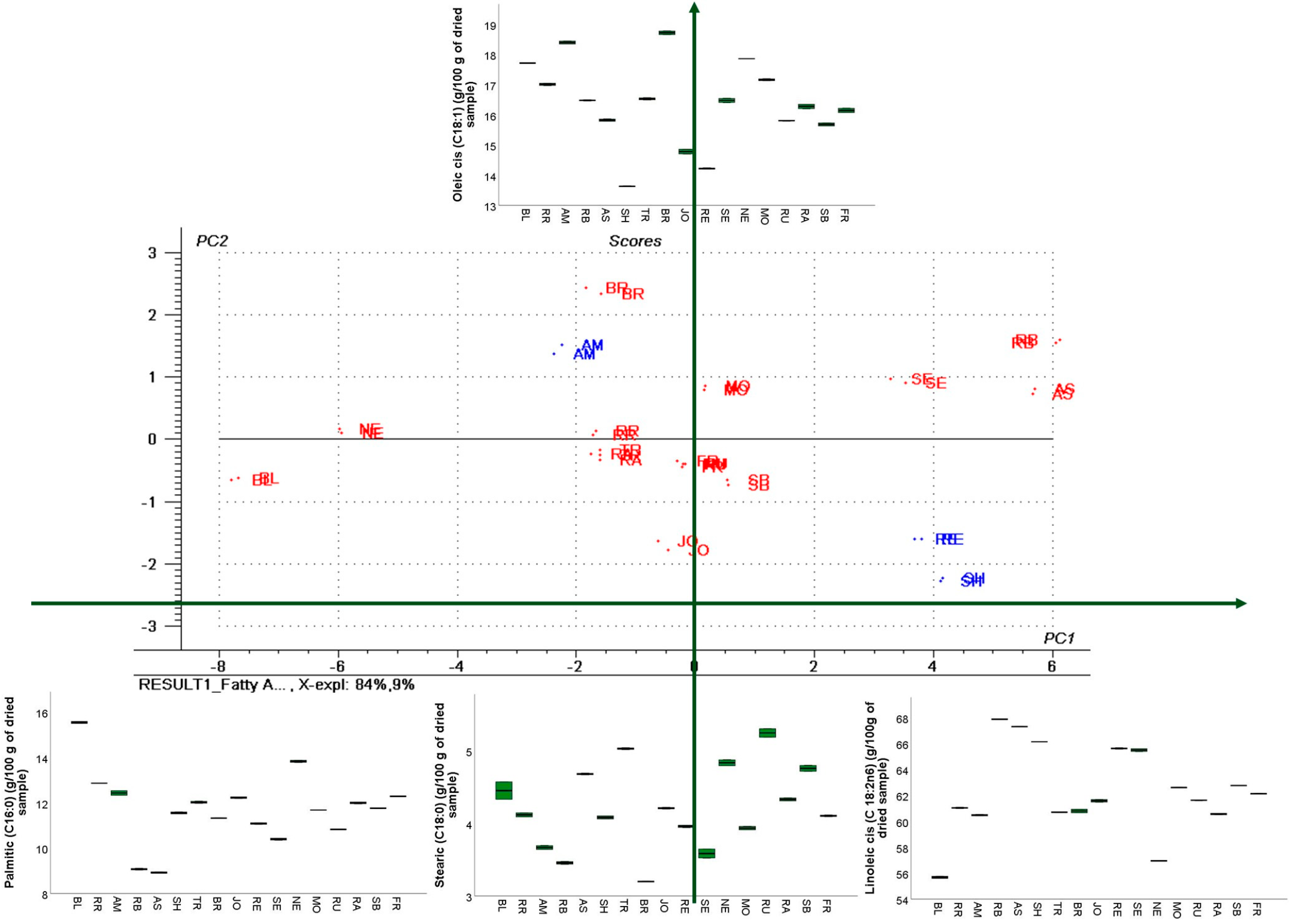
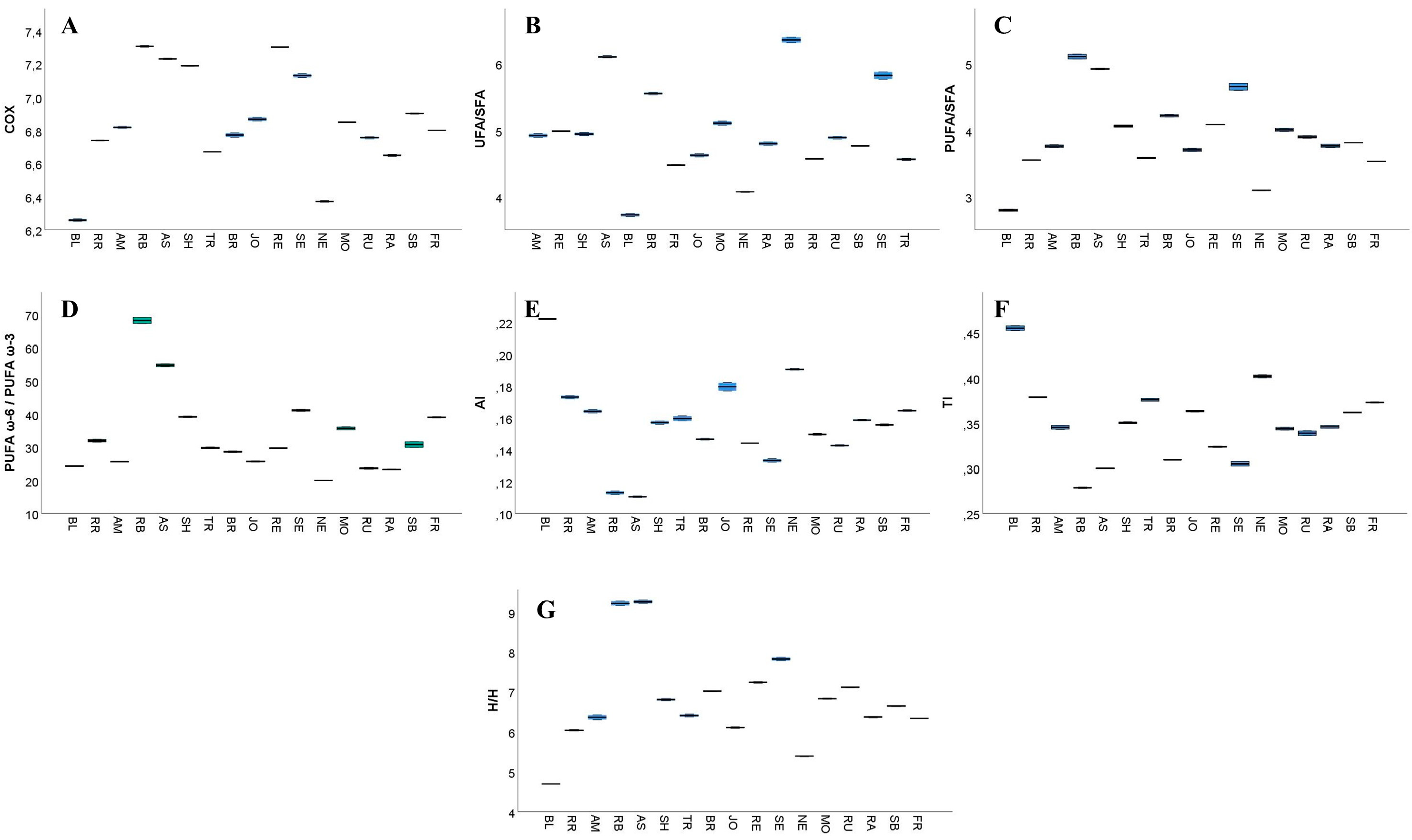
3.4. GC–FID Analysis of GP Extracts
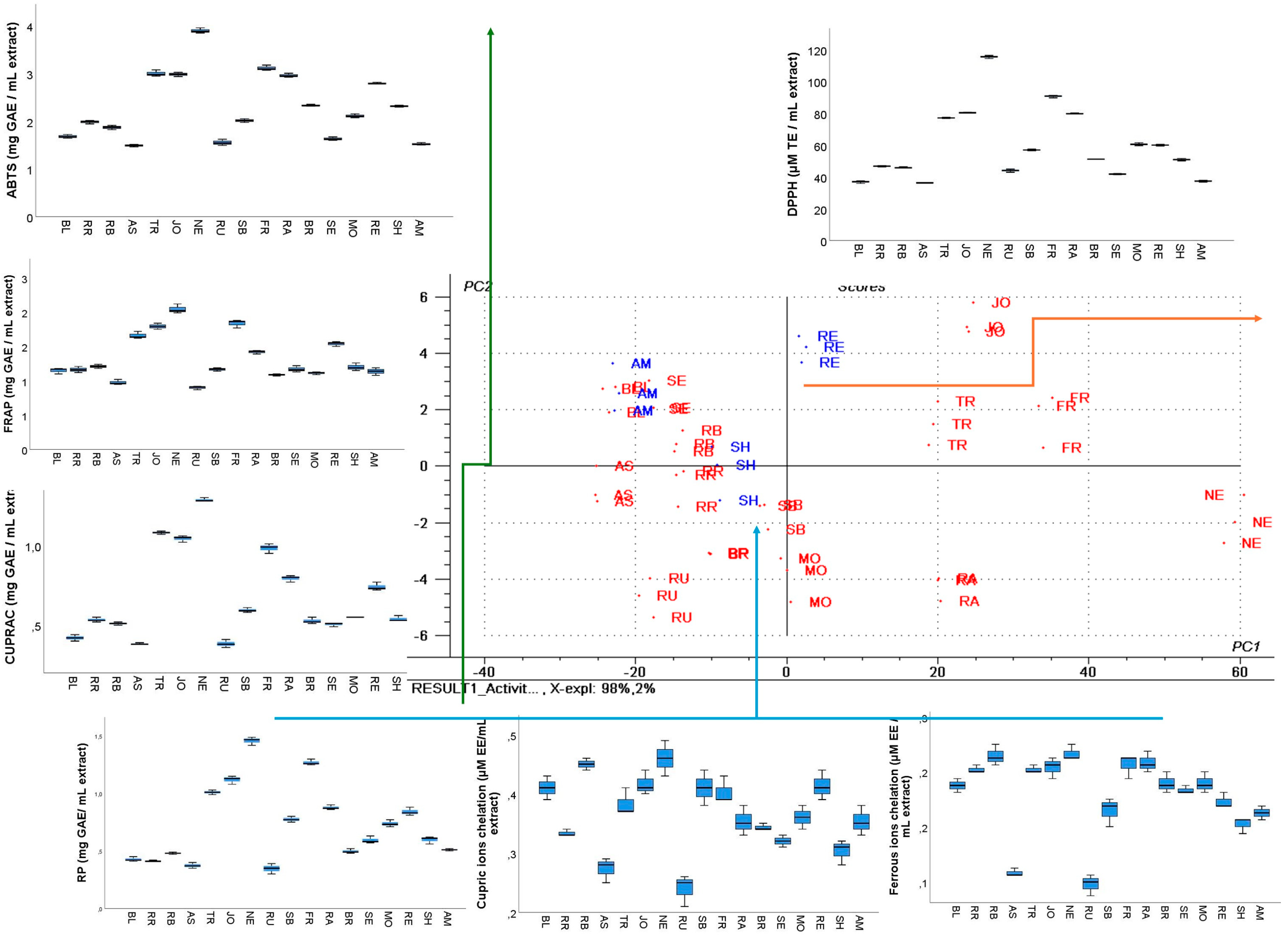
3.5. Antioxidant Capacity
3.5.1. Antiradical, Electron Transfer, and Chelation Assays
Antiradical Assays
Electron Transfer Assays
3.5.2. Metal Ion Chelation Assays
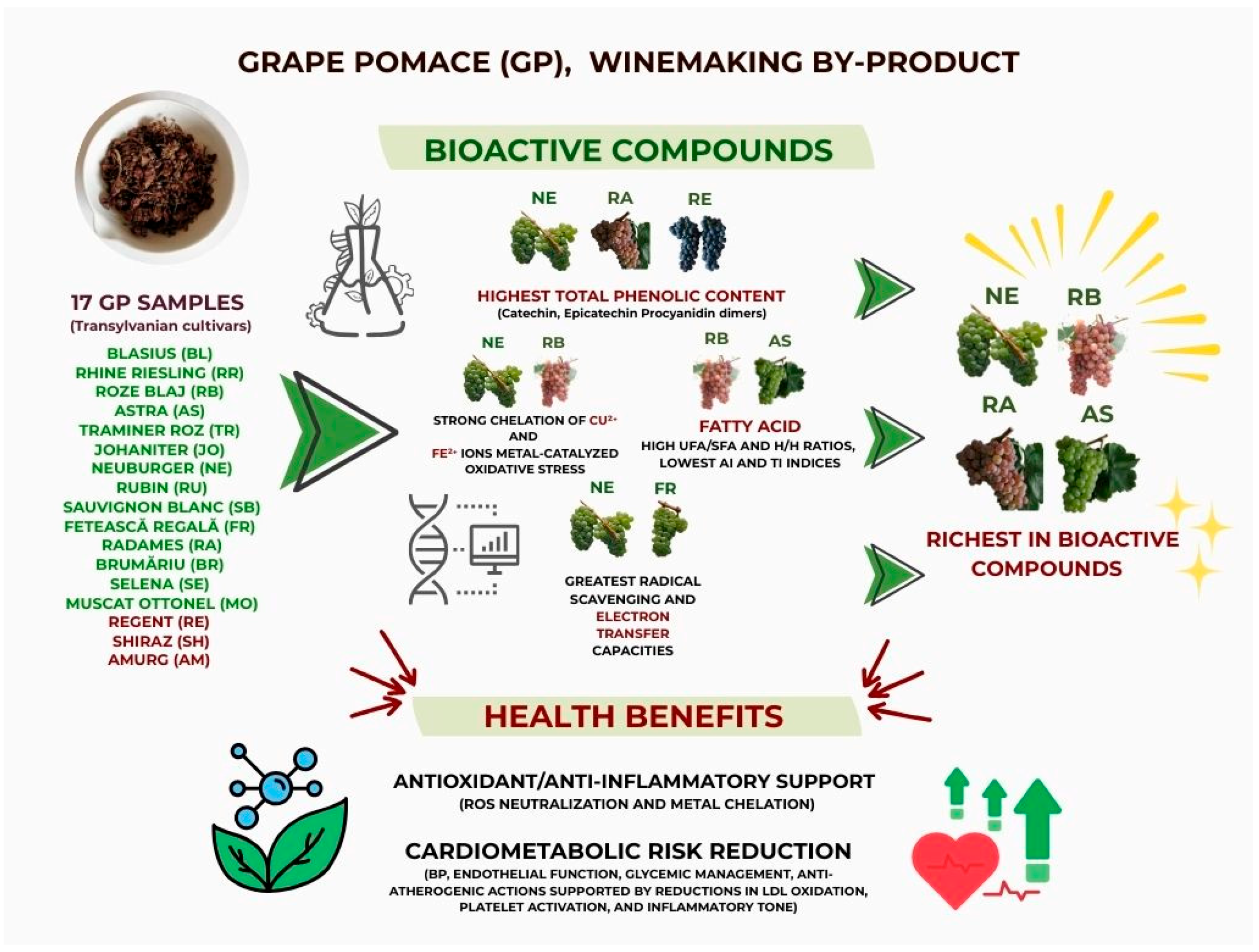
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GP | grape pomace |
| BL | GP from Blasius grapes |
| RR | GP from Rhine Riesling grapes |
| RB | GP from Roze Blaj grapes |
| AS | GP from Astra grapes |
| TR | GP from Traminer roz grapes |
| JO | GP from Johaniter grapes |
| NE | GP from Neuburger grapes |
| RU | GP from Rubin grapes |
| SB | GP from Sauvignon Blanc grapes |
| FR | GP from Fetească Regală grapes |
| RA | GP from Radames grapes |
| BR | GP from Brumăriu grapes |
| SE | GP from Selena grapes |
| MO | GP from Muscat Ottonel grapes |
| RE | GP from Regent grapes |
| SH | GP from Syrah grapes |
| AM | GP from Amurg grapes |
| FTIR | Fourier transform infrared spectroscopy |
| HPLC-DAD-ESI MS | liquid chromatography–diode array detection–electrospray ionization mass spectrometry |
| GC-FID | gas chromatography with flame ionization detector |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical-scavenging capacity |
| ABTS | 2,2′-azinobis-(3-ethylbenzthiazolin-6-sulfonic acid) radical-scavenging capacity |
| CUPRAC | Cupric-reducing antioxidant capacity |
| FRAP | Ferric-reducing antioxidant potential |
| RP | reducing power |
| TI | thrombogenicity index |
| AI | atherogenicity index |
| H/H | ratio between hypo and hypercholesterolemic fatty acids |
| COX | calculated oxidizability |
| TPC | total polyphenol content |
| GAE | gallic acid equivalent |
| TE | Trolox equivalent |
| EE | EDTA equivalent |
| SFA | saturated fatty acid |
| UFA | unsaturated fatty acid |
| MUFA | monounsaturated fatty acid |
| PUFA | polyunsaturated fatty acid |
References
- FAOSTAT (Food and Agriculture Organization of the United Nation) Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 November 2022).
- World Wine Production Outlook OIV First Estimates; International Organisation of Vine and Wine: Dijon, France, 2024.
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Taşeri, L.; Gülcü, M.; Aktaş, T. Comparison of Grape Pomace Drying Using a Solar Dryer and Under Open Sun Conditions. Vitic. Stud. 2023, 2023, 31–40. [Google Scholar] [CrossRef]
- Tomoiagă, L.; Iliescu, M.L.; Răcoare, H.S.; Botea, V.; Sîrbu, A.D.; Puşcă, G.; Chedea, V.S. Grape Pomance Generation from Grape Cultivars Cultivated in Târnave Vineyards in the Framework of the Climate Change. Rom. J. Hortic. 2020, I, 81–88. [Google Scholar] [CrossRef]
- Mollica, A.; Scioli, G.; Della Valle, A.; Cichelli, A.; Novellino, E.; Bauer, M.; Kamysz, W.; Llorent-Martínez, E.J.; De Córdova, M.L.F.; Castillo-López, R.; et al. Phenolic Analysis and in Vitro Biological Activity of Red Wine, Pomace and Grape Seeds Oil Derived from Vitis Vinifera l. Cv. Montepulciano d’Abruzzo. Antioxidants 2021, 10, 1704. [Google Scholar] [CrossRef]
- Onache, P.A.; Geana, E.I.; Ciucure, C.T.; Florea, A.; Sumedrea, D.I.; Ionete, R.E.; Tița, O. Bioactive Phytochemical Composition of Grape Pomace Resulted from Different White and Red Grape Cultivars. Separations 2022, 9, 395. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef]
- Abreu, T.; Luís, C.; Câmara, J.S.; Teixeira, J.; Perestrelo, R. Unveiling Potential Functional Applications of Grape Pomace Extracts Based on Their Phenolic Profiling, Bioactivities, and Circular Bioeconomy. Biomass Convers. Biorefin. 2025, in press. [Google Scholar] [CrossRef]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Stanišić, N.; Stajić, S.B.; Dmitrić, M.; Živković, S.; Kurćubić, L.V.; Živković, V.; Jakovljević, V.; Mašković, P.Z.; Mašković, J. Valorizing Grape Pomace: A Review of Applications, Nutritional Benefits, and Potential in Functional Food Development. Foods 2024, 13, 4169. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal Absorption and Antioxidant Activity of Grape Pomace Polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef]
- Chedea, V.S.; Palade, L.M.; Pelmus, R.S.; Dragomir, C.; Taranu, I. Red Grape Pomace Rich in Polyphenols Diet Increases the Antioxidant Status in Key Organs—Kidneys, Liver, and Spleen of Piglets. Animals 2019, 9, 149. [Google Scholar] [CrossRef]
- Blasi, F.; Trovarelli, V.; Mangiapelo, L.; Ianni, F.; Cossignani, L. Grape Pomace for Feed Enrichment to Improve the Quality of Animal-Based Foods. Foods 2024, 13, 3541. [Google Scholar] [CrossRef] [PubMed]
- Tsiapali, O.I.; Ayfantopoulou, E.; Tzourouni, A.; Ofrydopoulou, A.; Letsiou, S.; Tsoupras, A. Unveiling the Utilization of Grape and Winery By-Products in Cosmetics with Health Promoting Properties. Appl. Sci. 2025, 15, 1007. [Google Scholar] [CrossRef]
- Chedea, V.S.; Dragulinescu, A.M.; Tomoiaga, L.L.; Balaceanu, C.; Iliescu, M.L. Climate Change and Internet of Things Technologies—Sustainable Premises of Extending the Culture of the Amurg Cultivar in Transylvania—A Use Case for Târnave Vineyard. Sustainability 2021, 13, 8170. [Google Scholar] [CrossRef]
- Chedea, V.S.; Macovei, Ș.O.; Bocsan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape Pomace Polyphenols as a Source of Compounds for Management of Oxidative Stress and Inflammation—A Possible Alternative for Non-Steroidal Anti-Inflammatory Drugs? Molecules 2022, 27, 6826. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.d.C.; Madureira, J.; Margaça, F.M.A.; Cabo Verde, S. Grape Pomace: A Review of Its Bioactive Phenolic Compounds, Health Benefits, and Applications. Molecules 2025, 30, 362. [Google Scholar] [CrossRef]
- Fariña, E.; Daghero, H.; Bollati-Fogolín, M.; Boido, E.; Cantero, J.; Moncada-Basualto, M.; Olea-Azar, C.; Polticelli, F.; Paulino, M. Antioxidant Capacity and NF-KB-Mediated Anti-Inflammatory Activity of Six Red Uruguayan Grape Pomaces. Molecules 2023, 28, 3909. [Google Scholar] [CrossRef]
- Rodríguez-Morgado, B.; Candiracci, M.; Santa-María, C.; Revilla, E.; Gordillo, B.; Parrado, J.; Castaño, A. Obtaining from Grape Pomace an Enzymatic Extract with Anti-Inflammatory Properties. Plant Foods Hum. Nutr. 2015, 70, 42–49. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Chirilə, F.; Ogola, H.J.O.; Pelmuş, R.Ş.; Cəlin, L.G.; Socaciu, C. Antioxidant/Prooxidant and Antibacterial/Probacterial Effects of a Grape Seed Extract in Complex with Lipoxygenase. Biomed. Res. Int. 2014, 2014, 313684. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Zheng, L.; Li, J. Advance on the Bioactivity and Potential Applications of Dietary Fibre from Grape Pomace. Food Chem. 2015, 186, 207–212. [Google Scholar] [CrossRef]
- Karastergiou, A.; Gancel, A.L.; Jourdes, M.; Teissedre, P.L. Valorization of Grape Pomace: A Review of Phenolic Composition, Bioactivity, and Therapeutic Potential. Antioxidants 2024, 13, 1131. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagă, L.L.; Ropota, M.; Marc, G.; Ranga, F.; Comșa, M.; Muntean, M.D.; Sîrbu, A.D.; Giurca, I.S.; Răcoare, H.S.; et al. Phenolic Profile, Fatty Acid Composition, and Antioxidant Activity of Italian Riesling Grape Pomace from Two Transylvanian Microclimates. Plants 2025, 14, 1809. [Google Scholar] [CrossRef]
- Carmona-Jiménez, Y.; Igartuburu, J.M.; Guillén-Sánchez, D.A.; García-Moreno, M.V. Fatty Acid and Tocopherol Composition of Pomace and Seed Oil from Five Grape Varieties Southern Spain. Molecules 2022, 27, 6980. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, Q.; Xu, Z.; Iniguez, A.B.; Du, M.; Zhu, M.-J. Metabolomic Insights Into the Preventive Effects of Grape Pomace Against Colorectal Cancer. Curr. Dev. Nutr. 2021, 5, 612. [Google Scholar] [CrossRef]
- Yu, J.; Bansode, R.R.; Smith, I.N.; Hurley, S.L. Impact of Grape Pomace Consumption on the Blood Lipid Profile and Liver Genes Associated with Lipid Metabolism of Young Rats. Food Funct. 2017, 8, 2731–2738. [Google Scholar] [CrossRef]
- Iliescu, M.; Tomoiaga, L.; Farago, M.; Comsa, M. The Nutrition of Grapevine in Tarnave Vineyard [Nutriția la Vița de vie în Podgoria Târnave-Ro]; Academic Press: Cluj-Napoca, Romania, 2010; ISBN 978-973-744-225-3. [Google Scholar]
- Florina, C.; Maria, I.; Maria, C.; Cristian, C. Soil Type Influence on Yield Quantity and Quality at Grape Varieties for White Wines Obtained in the Viticultural Centre Blaj. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Hortic. 2014, 71, 21–28. [Google Scholar]
- Călugăr, A.; Babeş, A.C.; Bunea, C.I.; Pop, T.I.; Tomoiagă, L.; Iliescu, M. Oenological Characterization of Wines from Grape Clones Created at Research Station for Viticulture and Enology Blaj, Romania. CZU 2018, 663, 50–56. [Google Scholar]
- Donici, A.; Bunea, C.I.; Călugăr, A.; Harsan, E.; Bora, F.D. Investigation of the Copper Content in Vineyard Soil, Grape, Must and Wine in the Main Vineyards of Romania: A Preliminary Study. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2019, 76, 31. [Google Scholar] [CrossRef]
- Stroe, M. Ampelografie; Ceres Publishing House: Bucharest, Hungary, 2012. [Google Scholar]
- ISTIS—Institutului de Stat Pentru Testarea Si Inregistrarea Soiurilor. Available online: https://istis.ro/ (accessed on 2 September 2025).
- Röckel M Vitis International Variety Catalogue (VIVC). Available online: https://www.vivc.de/ (accessed on 28 July 2025).
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Levai, A.M.; Bocsan, L.C.; Buzoianu, A.D. Pot Aloe Vera Gel—A Natural Source of Antioxidants. Not. Bot. Horti Agrobot. Cluj. Napoca 2022, 50, 12732. [Google Scholar] [CrossRef]
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Leopold, N.; Bocsan, I.C.; Buzoianu, A.D. Characterization of Trametes Versicolor: Medicinal Mushroom with Important Health Benefits. Not. Bot. Horti Agrobot. Cluj. Napoca 2018, 46, 343–349. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagă, L.L.; Ranga, F.; Muntean, M.-D.; Sîrbu, A.; Comșa, M.; Cruceru, J.; Răcoare, H.-S.; Pop, R.M. From Grape to Wine-Muscat Ottonel from Blaj-Târnave Vineyard Chemical and Sensory Analysis. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2023, 80, 25–36. [Google Scholar] [CrossRef]
- Habeanu, M.; Lefter, N.; Gheorghe, A.; Nagy, A.; Marin, D.; Ropota, M. Effects of Dietary Flaxseed Oil on the Muscle Fatty Acid Composition in Mangalitsa Pigs in an Extensive Rearing System. S. Afr. J. Anim. Sci. 2014, 44, 240–244. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Marc, G.; Stana, A.; Franchini, A.H.; Vodnar, D.C.; Barta, G.; Tertiş, M.; Şanta, I.; Cristea, C.; Pîrnau, A.; Ciorîta, A.; et al. Phenolic Thiazoles with Antioxidant and Antiradical Activity. Synthesis, in Vitro Evaluation, Toxicity, Electrochemical Behavior, Quantum Studies and Antimicrobial Screening. Antioxidants 2021, 10, 1707. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Mic, M.; Pîrnău, A.; Floare, C.G.; Marc, G.; Franchini, A.H.; Oniga, O.; Vlase, L.; Bogdan, M. Synthesis and Molecular Interaction Study of a Diphenolic Hidrazinyl-Thiazole Compound with Strong Antioxidant and Antiradical Activity with HSA. J. Mol. Struct. 2021, 1244, 131278. [Google Scholar] [CrossRef]
- Pele, R.; Marc, G.; Stana, A.; Ionuț, I.; Nastasă, C.; Tiperciuc, B.; Oniga, I.; Pîrnău, A.; Vlase, L.; Oniga, O. Synthesis of New Phenolic Derivatives of Quinazolin-4(3H)-One as Potential Antioxidant Agents—In Vitro Evaluation and Quantum Studies. Molecules 2022, 27, 2599. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar]
- Wu, H.C.; Shiau, C.Y.; Chen, H.M.; Chiou, T.K.; Wu, H.-C.; Shiau, C.-Y.; Chen, H.-M.; Chiou, T.-K. Antioxidant Activities of Carnosine, Anserine, Some Free Amino Acids and Their Combination. J. Food Drug Anal. 2020, 11, 13. [Google Scholar] [CrossRef]
- Cesari, L.; Mutelet, F.; Canabady-Rochelle, L. Antioxidant Properties of Phenolic Surrogates of Lignin Depolymerisation. Ind. Crops Prod. 2019, 129, 480–487. [Google Scholar] [CrossRef]
- Pollini, L.; Blasi, F.; Ianni, F.; Grispoldi, L.; Moretti, S.; Di Veroli, A.; Cossignani, L.; Cenci-goga, B.T. Ultrasound-Assisted Extraction and Characterization of Polyphenols from Apple Pomace, Functional Ingredients for Beef Burger Fortification. Molecules 2022, 27, 1933. [Google Scholar] [CrossRef] [PubMed]
- Grispoldi, L.; Ianni, F.; Blasi, F.; Pollini, L.; Crotti, S.; Cruciani, D.; Cenci-Goga, B.T.; Cossignani, L. Apple Pomace as Valuable Food Ingredient for Enhancing Nutritional and Antioxidant Properties of Italian Salami. Antioxidants 2022, 11, 1221. [Google Scholar] [CrossRef]
- Arnold, M.; Gramza-Michalowska, A. Recent Development on the Chemical Composition and Phenolic Extraction Methods of Apple (Malus Domestica)—A Review. Food Bioproc. Tech. 2023, 17, 2519–2560. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and Analysis of Biophenols Recovered from Olive Mill Waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Ribeiro, T.B.; Lopes, A.I.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Comparison among Different Green Extraction Methods of Polyphenolic Compounds from Exhausted Olive Oil Pomace and the Bioactivity of the Extracts. Molecules 2024, 29, 1935. [Google Scholar] [CrossRef]
- Tejeda-Miramontes, J.P.; González-Frías, S.E.; Padlon-Manjarrez, S.; García-Cayuela, T.; Tejada-Ortigoza, V.; Garcia-Amezquita, L.E. Obtaining a Fiber-Rich Ingredient from Blueberry Pomace through Convective Drying: Process Modeling and Its Impact on Techno-Functional and Bioactive Properties. LWT 2024, 210, 116862. [Google Scholar] [CrossRef]
- Cîrstea, N.; Nour, V.; Corbu, A.R.; Codină, G.G. Blackcurrant Pomace Extract as a Natural Antioxidant in Vienna Sausages Reformulated by Replacement of Pork Backfat with Emulsion Gels Based on High Oleic Sunflower and Flaxseed Oils. Gels 2024, 10, 534. [Google Scholar] [CrossRef]
- Nawawi, N.I.M.; Khushairi, N.A.A.A.; Ijod, G.; Azman, E.M. Extraction of Anthocyanins and Other Phenolics from Dried Blackcurrant (Ribes Nigrum L.) Pomace via Ultrasonication. Sustain. Chem. Environ. 2025, 9, 100208. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Gao, W.; Zhang, L.; Li, J.; Li, J.; Zang, J. Pomegranate Peel as a Source of Bioactive Compounds: A Mini Review on Their Physiological Functions. Front. Nutr. 2022, 9, 887113. [Google Scholar] [CrossRef]
- de la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic Composition and Antioxidant Capacity of Pomaces from Four Grape Varieties (Vitis Vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonalves, A.E.D.S.S.; Fett, R. Phenolic Compounds Content and Antioxidant Activity in Pomace from Selected Red Grapes (Vitis Vinifera L. and Vitis Labrusca L.) Widely Produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, M.I. Quantification of Catechins and Proanthocyanidins in Several Portuguese Grapevine Varieties and Red Wines. Ciência Téc. Vitiv. 2001, 16, 23–34. [Google Scholar]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.L. Characterization of Polyphenols and Antioxidant Potential of White Grape Pomace Byproducts (Vitis Vinifera L.). J. Agric. Food Chem. 2013, 61, 11579–11587. [Google Scholar] [CrossRef]
- Alvarez-Casas, M.; Pájaro, M.; Lores, M.; Garcia-Jares, C. Characterization of Grape Marcs from Native and Foreign White Varieties Grown in Northwestern Spain by Their Polyphenolic Composition and Antioxidant Activity. Eur. Food Res. Technol. 2016, 242, 655–665. [Google Scholar] [CrossRef]
- Vojáčkova, K.; Mlček, J.; Škrovankova, S.; Adamkova, A.; Adamek, M.; Orsavova, J.; Bučkova, M.; Fic, V.; Kouřimska, L.; Buran, M. Biologically Active Compounds Contained in Grape Pomace. Potravin. Slovak. J. Food Sci. 2020, 14, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Schweiggert-Weisz, U.; Carle, R. In Vitro-Study of Antioxidant Extracts from Garcinia Mangostana Pericarp and Riesling Grape Pomace-a Contribution to by-Products Valorization as Cosmetic Ingredients. J. Appl. Bot. Food Qual. 2016, 89, 249–257. [Google Scholar] [CrossRef]
- Winkler, A.; Weber, F.; Ringseis, R.; Eder, K.; Dusel, G. Determination of Polyphenol and Crude Nutrient Content and Nutrient Digestibility of Dried and Ensiled White and Red Grape Pomace Cultivars. Arch. Anim. Nutr. 2015, 69, 187–200. [Google Scholar] [CrossRef]
- Gaita, C.; Alexa, E.; Popescu, I.; Popescu, S.; Negrea, M.; Poiana, M.-A. Grape Pomace: A Potential Sustainable Resource for Natural Bioactive Compounds Recovery. J. Agroaliment. Process. Technol. 2017, 23, 141–147. [Google Scholar]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent Selection for Efficient Extraction of Bioactive Compounds from Grape Pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Bran, E.P.; Nicuţă, D.; Grosu, L.; Patriciu, O.-I.; Alexa, I.-C. Investigation Regarding the Potential Application of Grape Pomace Extracts on in Vitro Plant Growth and Development. Ovidius Univ. Ann. Chem. 2022, 33, 135–142. [Google Scholar] [CrossRef]
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljević, D.; Pejin, B. The Lignicolous Fungus Trametes Versicolor (L.) Lloyd (1920): A Promising Natural Source of Antiradical and AChE Inhibitory Agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef]
- Ping, Y.; Zhang, J.; Xing, T.; Chen, G.; Tao, R.; Choo, K.H. Green Synthesis of Silver Nanoparticles Using Grape Seed Extract and Their Application for Reductive Catalysis of Direct Orange 26. J. Ind. Eng. Chem. 2018, 58, 74–79. [Google Scholar] [CrossRef]
- Gowman, A.C.; Picard, M.; Rodriguez, A.; Misra, M.; Khalil, H.; Thimmanagari, M.; Mohanty, A.K. Physicochemical Analysis of Apple and Grape Pomaces. Bioresources 2019, 14, 3210–3230. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Wilkanowicz, S.; Melendez-Rodriguez, B.; Lagaron, J.M. Nanoencapsulation of Aloe Vera in Synthetic and Naturally Occurring Polymers by Electrohydrodynamic Processing of Interest in Food Technology and Bioactive Packaging. J. Agric. Food Chem. 2017, 65, 4439–4448. [Google Scholar] [CrossRef]
- Zarrinbakhsh, N.; Wang, T.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A.K. Characterization of Wastes and Coproducts from the Coffee Industry for Composite Material Production. Bioresources 2016, 11, 7637–7653. [Google Scholar] [CrossRef]
- Lim, Z.X.; Cheong, K.Y. Effects of Drying Temperature and Ethanol Concentration on Bipolar Switching Characteristics of Natural Aloe Vera-Based Memory Devices. Phys. Chem. Chem. Phys. 2015, 17, 26833–26853. [Google Scholar] [CrossRef]
- Bele, A.A.; Khale, A. Comparison of Constituents in Aloe Vera Gel Collected in Different Seasons by Chromatography and Spectroscopy Techniques. World J. Pharm. Res. 2016, 5, 1028–1040. [Google Scholar] [CrossRef]
- Xu, W.; Reddy, N.; Yang, Y. Extraction, Characterization and Potential Applications of Cellulose in Corn Kernels and Distillers’ Dried Grains with Solubles (DDGS). Carbohydr. Polym. 2009, 76, 521–527. [Google Scholar] [CrossRef]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Ray, A.; Ghosh, S. Chemometrics for Functional Group Distribution, and UV Absorption Potential of Aloe Vera L. Gel at Different Growth Periods. Mater. Today Proc. 2018, 5, 22245–22253. [Google Scholar] [CrossRef]
- Pastorova, I.; Botto, R.E.; Arisz, P.W.; Boon, J.J. Cellulose Char Structure: A Combined Analytical Py-GC-MS, FTIR, and NMR Study. Carbohydr. Res. 1994, 262, 27–47. [Google Scholar] [CrossRef]
- Borbalan, A.M.A.; Zorro, L.; Guillen, D.A.; Barroso, C.G. Study of the Polyphenol Content of Red and White Grape Varieties by Liquid Chromatography–Mass Spectrometry and Its Relationship to Antioxidant Power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar] [CrossRef]
- Cheng, G.; Zhou, S.H.; Liu, Y.; Yue, T.X.; Zhang, Z.W. Effect of Bearing Position on Phenolics Profiles in the Skins of Four Cultivars of Grapevine (Vitis Vinifera L.). J. Hortic. Sci. Biotechnol. 2015, 90, 356–363. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Zagorac, D.Č.D.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and Quantification of Phenolic Compounds in Berry Skin, Pulp, and Seeds in 13 Grapevine Varieties Grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Almanza-Oliveros, A.; Bautista-Hernández, I.; Castro-López, C.; Aguilar-Zárate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.R.; Martínez-Ávila, G.C.G. Grape Pomace—Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods 2024, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Peirano-Bolelli, P.; Heller-Fuenzalida, F.; Cuneo, I.F.; Peña-Neira, Á.; Cáceres-Mella, A. Changes in the Composition of Flavonols and Organic Acids during Ripening for Three Cv. Sauvignon Blanc Clones Grown in a Cool-Climate Valley. Agronomy 2022, 12, 1357. [Google Scholar] [CrossRef]
- Eder, R.; Soural, I.; Wendelin, S.; Hanak, K.; Philipp, C.; Balik, J. Comparison of Phenolic Composition, Ripeness Parameters and Antioxidative Capacity of 33 Table Grape Cultivars. Appl. Fruit. Sci. 2025, 67, 28. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, R.; Stefanini, M.; Velasco, R. Metabolite Profiling of Grape: Flavonols and Anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutié Rrez, I. Flavonol Profiles of Vitis Vinifera White Grape Cultivars. J. Food Compos. Anal. 2010, 23, 699–705. [Google Scholar] [CrossRef]
- Li, H.; Gao, X.; Wang, Y.; Lu, H.; Tian, M.; Duan, C.; Wang, J. Artificial Shading of Teinturier Grape Kolor Clusters Reveals Light-Responsive Biomarkers: Focus on Flavonoid Profiles and Metabolism. Front. Plant Sci. 2024, 15, 1356799. [Google Scholar] [CrossRef]
- Kropek, I.E.; Štefan, M.B.; Rajkovača, K.; Petković, T.; Cvetnić, M.; Bolanča, T.; Vladimir-Knežević, S. Comparative Phenolic Profiles of Monovarietal Wines from Different Croatian Regions. Appl. Sci. 2023, 13, 3031. [Google Scholar] [CrossRef]
- Fontana, A.; Antoniolli, A.; María, M.; D’, A.; Fernández, A.; Fernández, F.; Rub´, R.; Bottini, R. Phenolics Profiling of Pomace Extracts from Different Grape Varieties Cultivated in Argentina. RSC Adv. 2017, 7, 29446–29457. [Google Scholar] [CrossRef]
- Ferreira, R.; Lourenço, S.; Lopes, A.; Andrade, C.; Câmara, J.S.; Castilho, P.; Perestrelo, R. Evaluation of Fatty Acids Profile as a Useful Tool towards Valorization of By-Products of Agri-Food Industry. Foods 2021, 10, 2867. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The Effect of Palmitic Acid on Inflammatory Response in Macrophages: An Overview of Molecular Mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Ribani, R.H.; Francisco, T.M.G.; Soares, A.A.; Pontarolo, R.; Haminiuk, C.W.I. Profile of Bioactive Compounds from Grape Pomace (Vitis Vinifera and Vitis Labrusca) by Spectrophotometric, Chromatographic and Spectral Analyses. J. Chromatogr. B 2015, 1007, 72–80. [Google Scholar] [CrossRef]
- Kolláthová, R.; Hanušovský, O.; Gálik, B.; Biro, D. Fatty Acid Profile Analysis of Grape By-Products from Slovakia and Austria. Acta Fytotech. Zootech. 2020, 23, 78–84. [Google Scholar] [CrossRef]
- Dimić, I.; Teslić, N.; Putnik, P.; Kovačević, D.B.; Zeković, Z.; Šojić, B.; Mrkonjić, Ž.; Čolović, D.; Montesano, D.; Pavlić, B. Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.M.; Jacobs, J.L.; Hannah, M.C.; Moate, P.J.; Dunshea, F.R.; Leury, B.J. In Vitro Evaluation of the Methane Mitigation Potential of a Range of Grape Marc Products. Anim. Prod. Sci. 2017, 57, 1437–1444. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Cruz, R.; Pereira, J.A.; Ramalhosa, E. Seed Oils of Ten Traditional Portuguese Grape Varieties with Interesting Chemical and Antioxidant Properties. Food Res. Int. 2013, 50, 161–166. [Google Scholar] [CrossRef]
- Hassanein, M.M.M.; Abedel-Razek, A.G. Chromatographic Quantitation of Some Bioactive Minor Components in Oils of Wheat Germ and Grape Seeds Produced as By-Products. J. Oleo Sci. 2009, 58, 227–233. [Google Scholar] [CrossRef]
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S.; Winkelmann, W. Quantitation of the Main Constituents of Some Authentic Grape-Seed Oils of Different Origin. J. Agric. Food Chem. 2006, 54, 6261–6265. [Google Scholar] [CrossRef]
- Tangolar, S.G.; Özoǧul, Y.; Tangolar, S.; Torun, A. Evaluation of Fatty Acid Profiles and Mineral Content of Grape Seed Oil of Some Grape Genotypes. Int. J. Food Sci. Nutr. 2009, 60, 32–39. [Google Scholar] [CrossRef]
- Wen, X.; Zhu, M.; Hu, R.; Zhao, J.; Chen, Z.; Li, J.; Ni, Y. Characterisation of Seed Oils from Different Grape Cultivars Grown in China. J. Food Sci. Technol. 2016, 53, 3129–3136. [Google Scholar] [CrossRef]
- Demirtas, I.; Pelvan, E.; Ozdemir, I.S.; Alasalvar, C.; Ertas, E. Lipid Characteristics and Phenolics of Native Grape Seed Oils Grown in Turkey. Eur. J. Lipid Sci. Technol. 2013, 115, 641–647. [Google Scholar] [CrossRef]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (α-, β + γ- and δ-Tocopherol) Levels in Plant Oils. Flavour. Fragr. J. 2020, 35, 504–510. [Google Scholar] [CrossRef]
- Yuan, C.; Xie, Y.; Jin, R.; Ren, L.; Zhou, L.; Zhu, M.; Ju, Y. Simultaneous Analysis of Tocopherols, Phytosterols, and Squalene in Vegetable Oils by High-Performance Liquid Chromatography. Food Anal. Methods 2017, 10, 3716–3722. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Bondioli, P.; Folegatti, L.; Rovellini, P. Oils Rich in Alpha Linolenic Acid: Chemical Composition of Perilla (Perilla Frutescens) Seed Oil. Ocl-J. 2020, 27, 67. [Google Scholar] [CrossRef]
- Sabir, A.; Unver, A.; Kara, Z. The Fatty Acid and Tocopherol Constituents of the Seed Oil Extracted from 21 Grape Varieties (Vitis Spp.). J. Sci. Food Agric. 2012, 92, 1982–1987. [Google Scholar] [CrossRef]
- Gómez, M.E.; Igartuburu, J.M.; Pando, E.; Rodríguez Luis, F.; Mourente, G. Lipid Composition of Lees from Sherry Wine. J. Agric. Food Chem. 2004, 52, 4791–4794. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Chemical, Rheological and Nutritional Characteristics of Sesame and Olive Oils Blended with Linseed Oil. Adv. Pharm. Bull. 2018, 8, 107. [Google Scholar] [CrossRef]
- Andreu-Coll, L.; Cano-Lamadrid, M.; Sendra, E.; Carbonell-Barrachina, Á.; Legua, P.; Hernández, F. Fatty Acid Profile of Fruits (Pulp and Peel) and Cladodes (Young and Old) of Prickly Pear [Opuntia Ficus-Indica (L.) Mill.] from Six Spanish Cultivars. J. Food Compos. Anal. 2019, 84, 103294. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Islam, M.M.; Bostami, A.B.M.R.; Mun, H.S.; Kim, Y.J.; Yang, C.J. Meat Composition, Fatty Acid Profile and Oxidative Stability of Meat from Broilers Supplemented with Pomegranate (Punica Granatum L.) by-Products. Food Chem. 2015, 188, 481–488. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Shi, F.; Chowdhury, R.; Sofianopoulou, E.; Koulman, A.; Sun, L.; Steur, M.; Aleksandrova, K.; Dahm, C.C.; Schulze, M.B.; van der Schouw, Y.T.; et al. Association of Circulating Fatty Acids with Cardiovascular Disease Risk: Analysis of Individual-Level Data in Three Large Prospective Cohorts and Updated Meta-Analysis. Eur. J. Prev. Cardiol. 2025, 32, 233–246. [Google Scholar] [CrossRef]
- Ouahhoud, S.; Khoulati, A.; Kadda, S.; Bencheikh, N.; Mamri, S.; Ziani, A.; Baddaoui, S.; Eddabbeh, F.E.; Lahmass, I.; Benabbes, R.; et al. Antioxidant Activity, Metal Chelating Ability and DNA Protective Effect of the Hydroethanolic Extracts of Crocus Sativus Stigmas, Tepals and Leaves. Antioxidants 2022, 11, 932. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Omer, H.A.A.; Caprioli, G.; Abouelenein, D.; Mustafa, A.M.; Uba, A.I.; Ak, G.; Ozturk, R.B.; Zengin, G.; Yagi, S. Phenolic Profile, Antioxidant and Enzyme Inhibitory Activities of Leaves from Two Cassia and Two Senna Species. Molecules 2022, 27, 5590. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in Vivo and in Vitro Methods Evaluation of Antioxidant Activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoǧlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, Ş.; Gülçin, I. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Solvent Effects on the Antioxidant Capacity of Lipophilic and Hydrophilic Antioxidants Measured by CUPRAC, ABTS/Persulphate and FRAP Methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef]
- Becker, E.M.; Nissen, L.R.; Skibsted, L.H. Antioxidant Evaluation Protocols: Food Quality or Health Effects. Eur. Food Res. Technol. 2004, 219, 561–571. [Google Scholar] [CrossRef]
- Rock, P.A. The Standard Oxidation Potential of the Ferrocyanide-Ferricyanide Electrode at 25° and the Entropy of Ferrocyanide Ion. J. Phys. Chem. 1966, 70, 576–580. [Google Scholar] [CrossRef]
- Burgess, J. Redox Potentials. In Ions in Solution; Elsevier: Amsterdam, The Netherlands, 1999; pp. 93–105. [Google Scholar]
- Kabtamu, D.M.; Lin, G.Y.; Chang, Y.C.; Chen, H.Y.; Huang, H.C.; Hsu, N.Y.; Chou, Y.S.; Wei, H.J.; Wang, C.H. The Effect of Adding Bi3+ on the Performance of a Newly Developed Iron–Copper Redox Flow Battery. RSC Adv. 2018, 8, 8537–8543. [Google Scholar] [CrossRef]
- Cheah, M.H.; Chernev, P. Electrochemical Oxidation of Ferricyanide. Sci. Rep. 2021, 11, 23058. [Google Scholar] [CrossRef]
- Furdak, P.; Kut, K.; Bartosz, G.; Sadowska-Bartosz, I. Comparison of Various Assays of Antioxidant Activity/Capacity: Limited Significance of Redox Potentials of Oxidants/Indicators. Int. J. Mol. Sci. 2025, 26, 7069. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, I.D.; Sarteshnizi, R.A.; Udenigwe, C.C.; Aluko, R.E. A Concise Review of Current In Vitro Chemical and Cell-Based Antioxidant Assay Methods. Molecules 2021, 26, 4865. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Xu, C.; Huang, K.; Lu, J.; Zhang, Y. Evaluation of Phenolic Compounds, Antioxidant and Antiproliferative Activities of 31 Grape Cultivars with Different Genotypes. J. Food Biochem. 2019, 43, e12626. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; Troncoso, D.; Mackenna, M.J.; Urzúa, C.; Pérez, D.; Dicenta, S.; de la Cerda, P.M.; Amigo, L.; Carreño, J.C.; Echeverría, G.; et al. The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial. Nutrients 2018, 10, 1388. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; de Diego, E.H.; Riomoros-Arranz, M.; Reynoso-Camacho, R.; Saura-Calixto, F.; Pérez-Jiménez, J. Effects of Acute Intake of Grape/Pomegranate Pomace Dietary Supplement on Glucose Metabolism and Oxidative Stress in Adults with Abdominal Obesity. Int. J. Food Sci. Nutr. 2020, 71, 94–105. [Google Scholar] [CrossRef]
- Urquiaga, I.; D’Acuña, S.; Pérez, D.; Dicenta, S.; Echeverría, G.; Rigotti, A.; Leighton, F. Wine Grape Pomace Flour Improves Blood Pressure, Fasting Glucose and Protein Damage in Humans: A Randomized Controlled Trial. Biol. Res. 2015, 48, 49. [Google Scholar] [CrossRef]
- Barona, J.; Aristizabal, J.C.; Blesso, C.N.; Volek, J.S.; Fernandez, M.L. Grape Polyphenols Reduce Blood Pressure and Increase Flow-Mediated Vasodilation in Men with Metabolic Syndrome. J. Nutr. 2012, 142, 1626–1632. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Léniz, A.; Martínez-Maqueda, D.; Amézqueta, S.; Fernández-Quintela, A.; Hereu, M.; Torres, J.L.; Portillo, M.P.; Pérez-Jiménez, J. Inter-Individual Variability in Insulin Response after Grape Pomace Supplementation in Subjects at High Cardiometabolic Risk: Role of Microbiota and MiRNA. Mol. Nutr. Food Res. 2021, 65, 2000113. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of Grape Pomace Polyphenolic Extract (Taurisolo® ) in Reducing Tmao Serum Levels in Humans: Preliminary Results from a Randomized, Placebo-Controlled, Cross-over Study. Nutrients 2019, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Taladrid, D.; De Celis, M.; Belda, I.; Bartolomé, B.; Moreno-Arribas, M.V. Hypertension- and Glycaemia-Lowering Effects of a Grape-Pomace-Derived Seasoning in High-Cardiovascular Risk and Healthy Subjects. Interplay with the Gut Microbiome. Food Funct. 2022, 13, 2068–2082. [Google Scholar] [CrossRef] [PubMed]
| Cultivar | Skin Color | Usage | Genetic Origin | |
|---|---|---|---|---|
| Blasius (BL), created and homologated at SCDVV Blaj, 1994 | White | Grapevine cultivar for white wine | Vitis vinifera ssp. sativa L. (Traminer roz x Iordană) (Raisin de Saint Piere x Perlă de Csaba) |  |
| Riesling de Rhin (RR) | White | Grapevine cultivar for white wine | Vitis vinifera ssp. sativa H. ? x Heunisch Weiss |  |
| Roze Blaj (RB), created and homologated at SCDVV Blaj, 2020 | Rosé | Grapevine cultivar for white wine | Sexuate intercrossing of two elites 8-33-44 (Iordană x Traminer roz) x 51-19 (Raisin de Saint Pierre x Perla de Csaba). |  |
| Astra (AS), created and homologated at SCDVV Blaj, 1995 | White | Grapevine cultivar for white wine | Vitis vinifera ssp. sativa L.; Fetească regală x Pinot gris |  |
| Traminer roz (TR) | Rosé | Grapevine cultivar for white wine | Vitis vinifera ssp. sativa H. Sauvignon Blanc mutation |  |
| Johanniter (JO) | White | Grapevine cultivar for white wine | Vitis vinifera ssp. sativa H. Riesling weiss x Freiburg 589-54 |  |
| Neuburger (NE) | White | Grapevine cultivar for white wine | Vitis vinifera ssp. sativa H. Veltliner Rot x Sylvaner |  |
| Rubin (RU), created and homologated at SCDVV Blaj, 2007 | Rosé | Grapevine cultivar for white wine | Sexuate interspecific hybridization between the Traminer roz cultivar and a hybrid descendant (Seyve Villard 12375 x Regina viilor) |  |
| Sauvignon Blanc (SB) | White | Grapevine cultivar for white wine | Vitis vinifera ssp. sativa H. Savagnin blanc x Traminer x ? |  |
| Fetească regală (FR) | White | Grapevine cultivar for white wine | Vitis vinifera ssp. sativa H. Fetească albă x Frâncușe |  |
| Radames (RA), created and homologated at SCDVV Blaj, 1993 | Rosé | Grapevine cultivar for white wine | Interspecific hybrid Traminer roz x Seyve Villard 12.375 |  |
| Brumăriu (BR), created and homologated at SCDVV Blaj, 1983 | White | Grapevine cultivar for white wine | Interspecific hybrid Saint Emilion x Rayon d’Or |  |
| Selena (SE), created and homologated at SCDVV Blaj, 1995 | Rosé | Grapevine cultivar for white wine | Vitis vinifera ssp. sativa L. Sexuate hybridization between Iordană cultivars x Traminer roz |  |
| Muscat Ottonel (MO) | White | Grapevine cultivar for white wine | Vitis vinifera ssp. sativa H. Ingram’s Muscat x Chasselas blanc |  |
| Regent (RE) | Dark red | Grapevine cultivar for red wine | Vitis vinifera ssp. sativa H. Diana x Chambourcin |  |
| Syrah (SH) | Dark red | Grapevine cultivar for red wine | Vitis vinifera ssp. sativa H. Mondeuse blanche x Dureza |  |
| Amurg (AM), created and homologated at SCDVV Blaj, 1989 | Dark red | Grapevine cultivar for red wine | Vitis vinifera ssp. sativa L. Muscat de Hamburg x Cabernet Sauvignon |  |
| GP Sample | TPC 1 (mgGAE/g GP) |
|---|---|
| BL | 46.38 ± 0.40 b |
| RR | 52.07 ± 0.83 c |
| RB | 53.82 ± 1.03 c,d |
| AS | 42.97 ± 0.20 a,b |
| TR | 79.24 ± 2.37 h |
| BR | 51.46 ± 0.43 c |
| JO | 77.59 ± 1.54 h |
| SE | 56.43 ± 0.18 d,e |
| NE | 76.72 ± 0.79 h |
| MO | 54.11 ± 0.22 c,d |
| RU | 42.38 ± 0.08 a |
| RA | 64.95 ± 0.43 f |
| SB | 56.85 ± 0.62 d,e |
| FR | 72.56 ± 1.23 g |
| AM | 51.14 ± 0.35 c |
| SH | 59.71 ± 3.19 e |
| RE | 72.05 ± 0.91 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chedea, V.S.; Tomoiagă, L.L.; Ropota, M.; Marc, G.; Ranga, F.; Muntean, M.D.; Sîrbu, A.D.; Giurca, I.S.; Comșa, M.; Bocsan, I.C.; et al. Transylvanian Grape Pomaces as Sustainable Sources of Antioxidant Phenolics and Fatty Acids—A Study of White and Red Cultivars. Antioxidants 2025, 14, 1152. https://doi.org/10.3390/antiox14101152
Chedea VS, Tomoiagă LL, Ropota M, Marc G, Ranga F, Muntean MD, Sîrbu AD, Giurca IS, Comșa M, Bocsan IC, et al. Transylvanian Grape Pomaces as Sustainable Sources of Antioxidant Phenolics and Fatty Acids—A Study of White and Red Cultivars. Antioxidants. 2025; 14(10):1152. https://doi.org/10.3390/antiox14101152
Chicago/Turabian StyleChedea, Veronica Sanda, Liliana Lucia Tomoiagă, Mariana Ropota, Gabriel Marc, Floricuta Ranga, Maria Doinița Muntean, Alexandra Doina Sîrbu, Ioana Sorina Giurca, Maria Comșa, Ioana Corina Bocsan, and et al. 2025. "Transylvanian Grape Pomaces as Sustainable Sources of Antioxidant Phenolics and Fatty Acids—A Study of White and Red Cultivars" Antioxidants 14, no. 10: 1152. https://doi.org/10.3390/antiox14101152
APA StyleChedea, V. S., Tomoiagă, L. L., Ropota, M., Marc, G., Ranga, F., Muntean, M. D., Sîrbu, A. D., Giurca, I. S., Comșa, M., Bocsan, I. C., Buzoianu, A. D., Kisher, H., & Pop, R. M. (2025). Transylvanian Grape Pomaces as Sustainable Sources of Antioxidant Phenolics and Fatty Acids—A Study of White and Red Cultivars. Antioxidants, 14(10), 1152. https://doi.org/10.3390/antiox14101152











