Dexmedetomidine as a Protective Agent Against X-Ray Ionizing Radiation-Induced Small Intestinal Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochemical Analysis
2.1.1. Tissue Sampling and Homogenization
2.1.2. Malondialdehyde Analysis
2.1.3. Glutathione Analysis
2.2. Histopathological Analysis
2.3. Immunohistochemical (IHC) Staining Procedure
2.4. Semi-Quantitative Evaluation
2.5. Statistical Analysis
3. Results
3.1. Biochemical Analysis
3.2. Histopathological Analysis
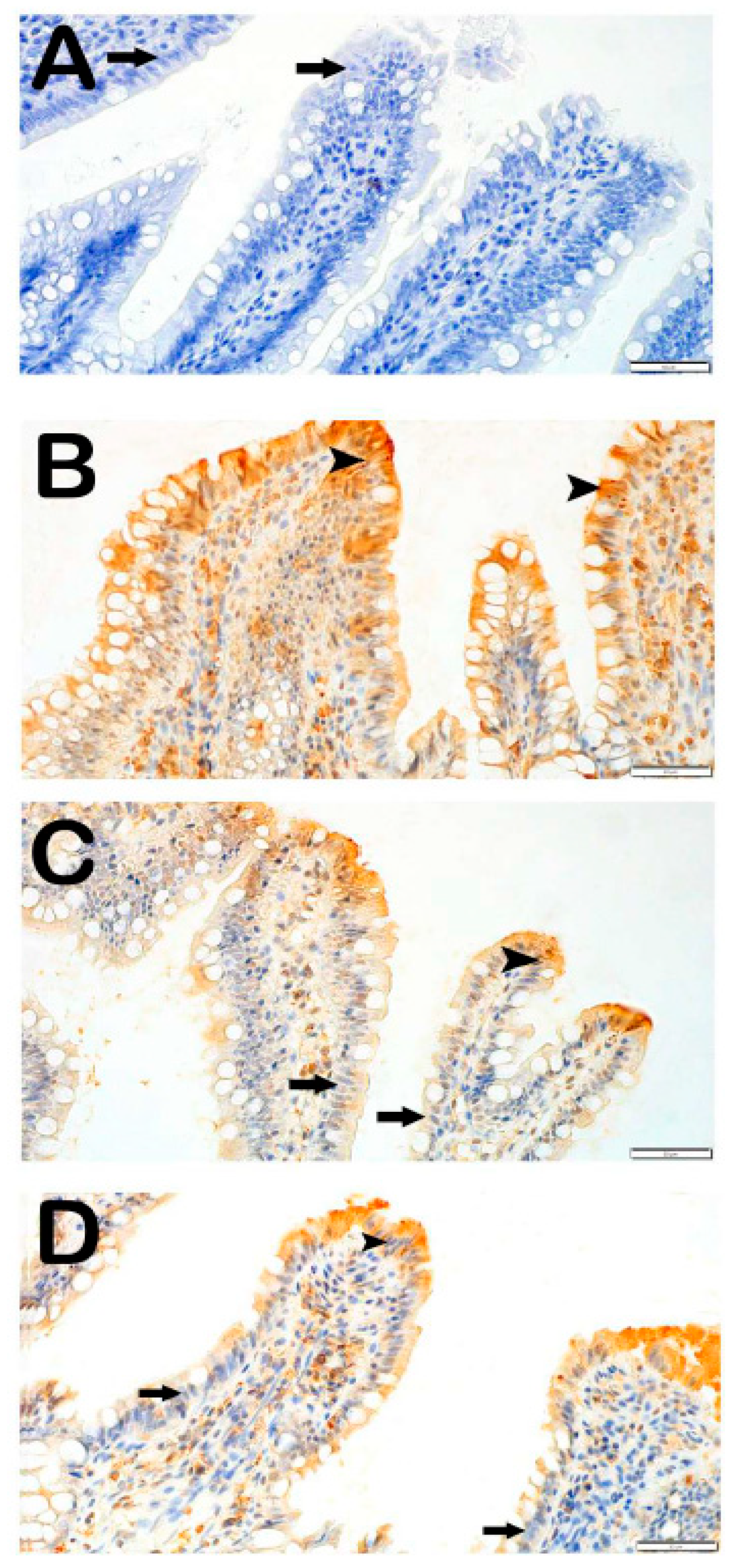
3.3. IHC Analysis
3.3.1. Caspase-3 Positivity
3.3.2. 8-OHdG Positivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yilmaz, H.; Karakoc, Y.; Tumkaya, L.; Mercantepe, T.; Sevinc, H.; Yilmaz, A.; Yılmaz Rakıcı, S. The Protective Effects of Red Ginseng and Amifostine against Renal Damage Caused by Ionizing Radiation. Hum. Exp. Toxicol. 2022, 41, 1–12. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted Agents and Immunotherapies: Optimizing Outcomes in Melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for Combining Targeted and Conventional Cancer Therapy with Immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Han, Y.; Zhang, H.; Tu, W.; Zhang, S. Radiotherapy-Induced Digestive Injury: Diagnosis, Treatment and Mechanisms. Front. Oncol. 2021, 11, 757973. [Google Scholar] [CrossRef] [PubMed]
- Thariat, J.; Hannoun-Levi, J.M.; Sun Myint, A.; Vuong, T.; Gérard, J.P. Past, Present, and Future of Radiotherapy for the Benefit of Patients. Nat. Rev. Clin. Oncol. 2013, 10, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Emami, B.; Lyman, J.; Brown, A.; Coia, L.; Goitein, M.; Munzenrider, J.; Shank, B.; Solin, L.; Wesson, M. Tolerance of Normal Tissue to Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 21, 109–122. [Google Scholar] [CrossRef]
- Filiz, S.; Gürsel, Ş.B.; Coştur Biyiksiz, P.; Yörüker, S.; Gonca, S.; Gelenli, E.; Dalçik, H. Immunohistochemical Evaluation of the Protective Effect of Ginkgo Biloba, Probiotic Saccharomyces Boulardii and N-Acetylcysteine on Radiation-Induced Small Intestine Injury. Turkiye Klin. J. Med. Sci. 2010, 30, 1433–1440. [Google Scholar] [CrossRef][Green Version]
- Zhao, W.; Robbins, M. Inflammation and Chronic Oxidative Stress in Radiation-Induced Late Normal Tissue Injury: Therapeutic Implications. Curr. Med. Chem. 2008, 16, 130–143. [Google Scholar] [CrossRef]
- Mercantepe, F.; Topcu, A.; Rakici, S.; Tumkaya, L.; Yilmaz, A. The Effects of N-Acetylcysteine on Radiotherapy-Induced Small Intestinal Damage in Rats. Exp. Biol. Med. 2019, 244, 372–379. [Google Scholar] [CrossRef]
- Yahyapour, R.; Motevaseli, E.; Rezaeyan, A.; Abdollahi, H.; Farhood, B.; Cheki, M.; Rezapoor, S.; Shabeeb, D.; Musa, A.E.; Najafi, M.; et al. Reduction–Oxidation (Redox) System in Radiation-Induced Normal Tissue Injury: Molecular Mechanisms and Implications in Radiation Therapeutics. Clin. Transl. Oncol. 2018, 20, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Dexmedetomidine: Present and Future Directions. Korean J. Anesthesiol. 2019, 72, 323–330. [Google Scholar] [CrossRef]
- Liu, M.W.; Duan, S.X.; Zhao, X.Y.; Wang, Q.F.; Yang, S.L.; Ma, N.; Li, X. Research Status and Advances in Dexmedetomidine for Sepsis-induced Multiple Organ Dysfunction Syndrome (Review). Int. J. Mol. Med. 2025, 55, 94. [Google Scholar] [CrossRef] [PubMed]
- Mercantepe, F.; Tumkaya, L.; Mercantepe, T.; Rakici, S.Y.; Ciftel, S.; Ciftel, S. Radioprotective Effects of A2-Adrenergic Receptor Agonist Dexmedetomidine on X-Ray Irradiation-Induced Pancreatic Islet Cell Damage. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023, 396, 1827–1836. [Google Scholar] [CrossRef]
- Bao, N.; Tang, B. Organ-Protective Effects and the Underlying Mechanism of Dexmedetomidine. Mediat. Inflamm. 2020, 2020, 6136105. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.; Bakke, J.; Riccio, E.; Javitz, H.S.; Nishita, D.; Kapur, S.; Bunin, D.I.; Chang, P.Y. The Progression of Radiation Injury in a Wistar Rat Model of Partial Body Irradiation with ~5% Bone Marrow Shielding. Int. J. Radiat. Biol. 2023, 99, 1080–1095. [Google Scholar] [CrossRef]
- Kutanis, D.; Erturk, E.; Besir, A.; Demirci, Y.; Kayir, S.; Akdogan, A.; Vanizor Kural, B.; Bahat, Z.; Canyilmaz, E.; Kara, H. Dexmedetomidine Acts as an Oxidative Damage Prophylactic in Rats Exposed to Ionizing Radiation. J. Clin. Anesth. 2016, 34, 577–585. [Google Scholar] [CrossRef]
- Uyan, M.; Yilmaz, H.; Tümkaya, L.; Suzan, Z.T.; Mercantepe, T. Radioprotective Effects of Coenzyme Q10 on X-Ray Radiation-Induced Intestinal Damage via Oxidative Stress and Apoptosis. Arch. Med. Res. 2025, 56, 103181. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Elliott, T.B.; Deutz, N.E.; Gulani, J.; Koch, A.; Olsen, C.H.; Christensen, C.; Chappell, M.; Whitnall, M.H.; Moroni, M. Gastrointestinal Acute Radiation Syndrome in Göttingen Minipigs (Sus Scrofa Domestica). Comp. Med. 2014, 64, 456–463. [Google Scholar]
- Dickson, R.P. The Microbiome and Critical Illness. Lancet Respir. Med. 2016, 4, 59–72. [Google Scholar] [CrossRef]
- El-Ghazaly, M.A.; El-Hazek, R.M.; Khayyal, M.T. Protective Effect of the Herbal Preparation, STW 5, against Intestinal Damage Induced by Gamma Radiation in Rats. Int. J. Radiat. Biol. 2015, 91, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Pérez Piñero, C.; Bruzzone, A.; Sarappa, M.G.; Castillo, L.F.; Lüthy, I.A. Involvement of A2- and Β2-Adrenoceptors on Breast Cancer Cell Proliferation and Tumour Growth Regulation. Br. J. Pharmacol. 2012, 166, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Batcik, S.; Tumkaya, L.; Dil, E.; Kazancioglu, L.; Gaygusuz, E.; Yazici, Z.A.; Ozden, Z.; Kilinc, K.; Mercantepe, T. Protective Effects of Dexmedetomidine and Amifostine Against Radiotherapy-Induced Kidney Injury. Life 2025, 15, 897. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Chen, M.H.; Gong, Y.X.; Lin, F.; Sun, C. Effects of Dexmedetomidine on Oxidative Stress, Programmed Cell Death, Liver Function, and Expression of Peripheral Immune Cells in Patients with Primary Liver Cancer Undergoing Hepatectomy. Front. Physiol. 2023, 14, 1159746. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, P.; Sauvat, A.; Carnet Le Provost, K.; Liu, J.; Checcoli, A.; Pol, J.; Kepp, O.; Kroemer, G.; Bezu, L. Dexmedetomidine Induces Immunogenic Cancer Cell Death and Sensitizes Tumors to PD-1 Blockade. J. Immunother. Cancer 2025, 13, e010714. [Google Scholar] [CrossRef]
- Weerink, M.A.S.; Struys, M.M.R.F.; Hannivoort, L.N.; Barends, C.R.M.; Absalom, A.R.; Colin, P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef]
- Shukry, M.; Miller, J.A. Update on Dexmedetomidine: Use in Nonintubated Patients Requiring Sedation for Surgical Procedures. Ther. Clin. Risk Manag. 2010, 6, 111–121. [Google Scholar] [CrossRef]
- Reel, B.; Maani, C.V. Dexmedetomidine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513303 (accessed on 21 August 2025).
- Vikram, B.; Brizel, D.M. Phase III Randomized Trial of Amifostine as a Radioprotector in Head and Neck Cancer. J. Clin. Oncol. 2001, 19, 1233–1234. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, K.; Lian, Y.; Hong, S.; Zhu, Y. Dexmedetomidine Ameliorates X-Ray-Induced Myocardial Injury via Alleviating Cardiomyocyte Apoptosis and Autophagy. BMC Cardiovasc. Disord. 2024, 24, 323. [Google Scholar] [CrossRef]
- Polat, H.B.; Yılmaz, H.; Kilinc, K.; Gülhan, B.; Rakıcı, S.Y. A Dose-Dependent Study Examining Dexmedetomidine’s Possible Effects Against Oxidative, Fibrotic, and Apoptotic Damage Induced by Radiation Exposure in Spleen Tissue. Life 2025, 15, 1430. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.M.; Hu, Q.; Liu, Z.R.; Liu, Z.Y.; Zhang, H.G.; Huang, Y.L.; Chen, Q.H.; Wang, W.X.; Zhang, X.K. Dexmedetomidine Inhibits Mitochondria Damage and Apoptosis of Enteric Glial Cells in Experimental Intestinal Ischemia/Reperfusion Injury via SIRT3-Dependent PINK1/HDAC3/P53 Pathway. J. Transl. Med. 2021, 19, 463. [Google Scholar] [CrossRef]
- Zhang, X.K.; Zhou, X.P.; Qin, Z.; Feng, Z. The Preventive Effects of Dexmedetomidine against Intestinal Ischemia-Reperfusion Injury in Wistar Rats. Iran. J. Basic Med. Sci. 2015, 18, 604–609. [Google Scholar]
- He, G.Z.; Bu, N.; Li, Y.J.; Gao, Y.; Wang, G.; Kong, Z.D.; Zhao, M.; Zhang, S.S.; Gao, W. Extra Loading Dose of Dexmedetomidine Enhances Intestinal Function Recovery After Colorectal Resection: A Retrospective Cohort Study. Front. Pharmacol. 2022, 13, 806950. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Zhang, X.; Lu, X.; Wu, J.; Li, S. Reduced Mitochondrial Response Sensitivity Is Involved in the Anti-Apoptotic Effect of Dexmedetomidine Pretreatment in Cardiomyocytes. Int. J. Mol. Med. 2018, 41, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mu, X.; Zhou, X. Dexmedetomidine Alleviates Inflammatory Response and Oxidative Stress Injury of Vascular Smooth Muscle Cell via A2AR/GSK-3β/MKP-1/NRF2 Axis in Intracranial Aneurysm. BMC Pharmacol. Toxicol. 2022, 23, 81. [Google Scholar] [CrossRef]
- Lv, P.; Chen, T.; Liu, P.; Zheng, L.; Tian, J.; Tan, F.; Chen, J.; Deng, Y.; Li, J.; Cai, J.; et al. Dexmedetomidine Attenuates Orthotopic Liver Transplantation-Induced Acute Gut Injury via α 2-Adrenergic Receptor-Dependent Suppression of Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 9426368. [Google Scholar] [CrossRef] [PubMed]
- Iirola, T.; Vilo, S.; Aantaa, R.; Wendelin-Saarenhovi, M.; Neuvonen, P.J.; Scheinin, M.; Olkkola, K.T. Dexmedetomidine Inhibits Gastric Emptying and Oro-Caecal Transit in Healthy Volunteers. Br. J. Anaesth. 2011, 106, 522–527. [Google Scholar] [CrossRef]
- Mantz, J.; Josserand, J.; Hamada, S. Dexmedetomidine: New Insights. Eur. J. Anaesthesiol. 2011, 28, 3–6. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Sun, W.Z.; Ko, W.J.; Chan, W.S.; Fan, S.Z.; Tsai, J.C.; Lin, T.Y. Dexmedetomidine Prevents Alterations of Intestinal Microcirculation That Are Induced by Surgical Stress and Pain in a Novel Rat Model. Anesth. Analg. 2012, 115, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Wu, C.Y.; Cheng, Y.J.; Liu, C.M.; Hsiao, J.K.; Chan, W.S.; Wu, Z.G.; Yu, L.C.H.; Sun, W.Z. Effects of Dexmedetomidine on Intestinal Microcirculation and Intestinal Epithelial Barrier in Endotoxemic Rats. Anesthesiology 2016, 125, 355–367. [Google Scholar] [CrossRef] [PubMed]


| Score | Findings |
|---|---|
| Villous Fusion | |
| 0 | ≤5% |
| 1 | ≤25% |
| 2 | ≤50% |
| 3 | ≤75% |
| Villous Shortening | |
| 0 | ≤5% |
| 1 | ≤25% |
| 2 | ≤50% |
| 3 | ≤75% |
| Inflammation | |
| 0 | ≤5% |
| 1 | ≤25% |
| 2 | ≤50% |
| 3 | ≤75% |
| Hemorrhage | |
| 0 | ≤5% |
| 1 | ≤25% |
| 2 | ≤50% |
| 3 | ≤75% |
| Score | |
|---|---|
| 0 | None (less than 5%) |
| 1 | Mild (less than 25%) |
| 2 | Moderate (less than 50%) |
| 3 | Severe (less than 75%) |
| Group | MDA (nmol/g Tissue) | GSH (nmol/g Tissue) |
|---|---|---|
| Control | 13.46 ± 0.99 | 11.54 ± 2.45 |
| IR | 17.04 ± 2.57 c | 6.45 ± 1.04 a |
| IR+DEX 100 | 8.96 ± 2.30 d,f | 8.31 ± 2.02 |
| IR+DEX 200 | 7.66 ± 1.51 e,g | 9.86 ± 1.70 b |
| Groups | Villus Fusion | Shortening of the Villi | Inflammation | Hemorrhage | IHDS |
|---|---|---|---|---|---|
| Control | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–1) |
| IR | 2.5 (2–3) a | 2.5 (2–3) | 1 (1–1) a | 1 (1–1) a | 7.5 (6–8) a |
| IR+DEX 100 | 1 (1–1) b | 1 (0–1) b | 0 (0–0) b | 0 (0–0) b | 2 (2–3) a,b |
| IR+DEX 200 | 1 (1–1) b | 1 (1–1) b | 0 (0–0) b | 0 (0–0) b | 2 (2–3) a,b |
| Group | Caspase-3 Positivity Score | 8-OHdG Positivity Score |
|---|---|---|
| Control | 0 (0–1) | 0 (0–0) |
| IR | 3 (2–3) a | 2 (2–3) a |
| IR+DEX 100 | 1 (1–1) b | 0 (0–1) c |
| IR+DEX 200 | 1 (0–1) c | 0 (0–0) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalcan, S.; Tumkaya, L.; Mercantepe, T.; Yilmaz, H.; Karakas, S.M.; Pergel, A.; Demiral, G.; Ozdemir, A.; Rakici, S. Dexmedetomidine as a Protective Agent Against X-Ray Ionizing Radiation-Induced Small Intestinal Injury. Antioxidants 2025, 14, 1153. https://doi.org/10.3390/antiox14101153
Kalcan S, Tumkaya L, Mercantepe T, Yilmaz H, Karakas SM, Pergel A, Demiral G, Ozdemir A, Rakici S. Dexmedetomidine as a Protective Agent Against X-Ray Ionizing Radiation-Induced Small Intestinal Injury. Antioxidants. 2025; 14(10):1153. https://doi.org/10.3390/antiox14101153
Chicago/Turabian StyleKalcan, Süleyman, Levent Tumkaya, Tolga Mercantepe, Hamit Yilmaz, Sibel Mataraci Karakas, Ahmet Pergel, Gokhan Demiral, Ali Ozdemir, and Sema Rakici. 2025. "Dexmedetomidine as a Protective Agent Against X-Ray Ionizing Radiation-Induced Small Intestinal Injury" Antioxidants 14, no. 10: 1153. https://doi.org/10.3390/antiox14101153
APA StyleKalcan, S., Tumkaya, L., Mercantepe, T., Yilmaz, H., Karakas, S. M., Pergel, A., Demiral, G., Ozdemir, A., & Rakici, S. (2025). Dexmedetomidine as a Protective Agent Against X-Ray Ionizing Radiation-Induced Small Intestinal Injury. Antioxidants, 14(10), 1153. https://doi.org/10.3390/antiox14101153







