Antioxidative Potential and Ameliorative Effects of Rice Bran Fermented with Lactobacillus against High-Fat Diet-Induced Oxidative Stress in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fermentation Medium and Fermentation Broth
2.3. Comparison of the Antioxidant Activity of the Fermentation Products and Optimum Fermentation Time for Different Strains
2.4. Composition Analysis of MF423 Fermentation Product from Rice Bran
2.5. The Fermentation Products Were Detected by HPLC
2.6. Cell Cytotoxicity and Intracellular ROS Assessments
2.7. The Protective Effect of FLRB on Oxidative Stress in C. elegans
2.7.1. C. elegans Treatment Protocol
2.7.2. Toxic Effects of Fermentation Products on C. elegans
2.7.3. Influence of FLRB on the ROS Levels and Activities of Antioxidant Enzymes in C. elegans
2.7.4. Influence of FLRB on the Antioxidative Stress Capacity of C. elegans
2.8. Animals and Experimental Design
2.9. Transcriptomic Analysis
RNA Isolation, Library Construction, and Sequencing
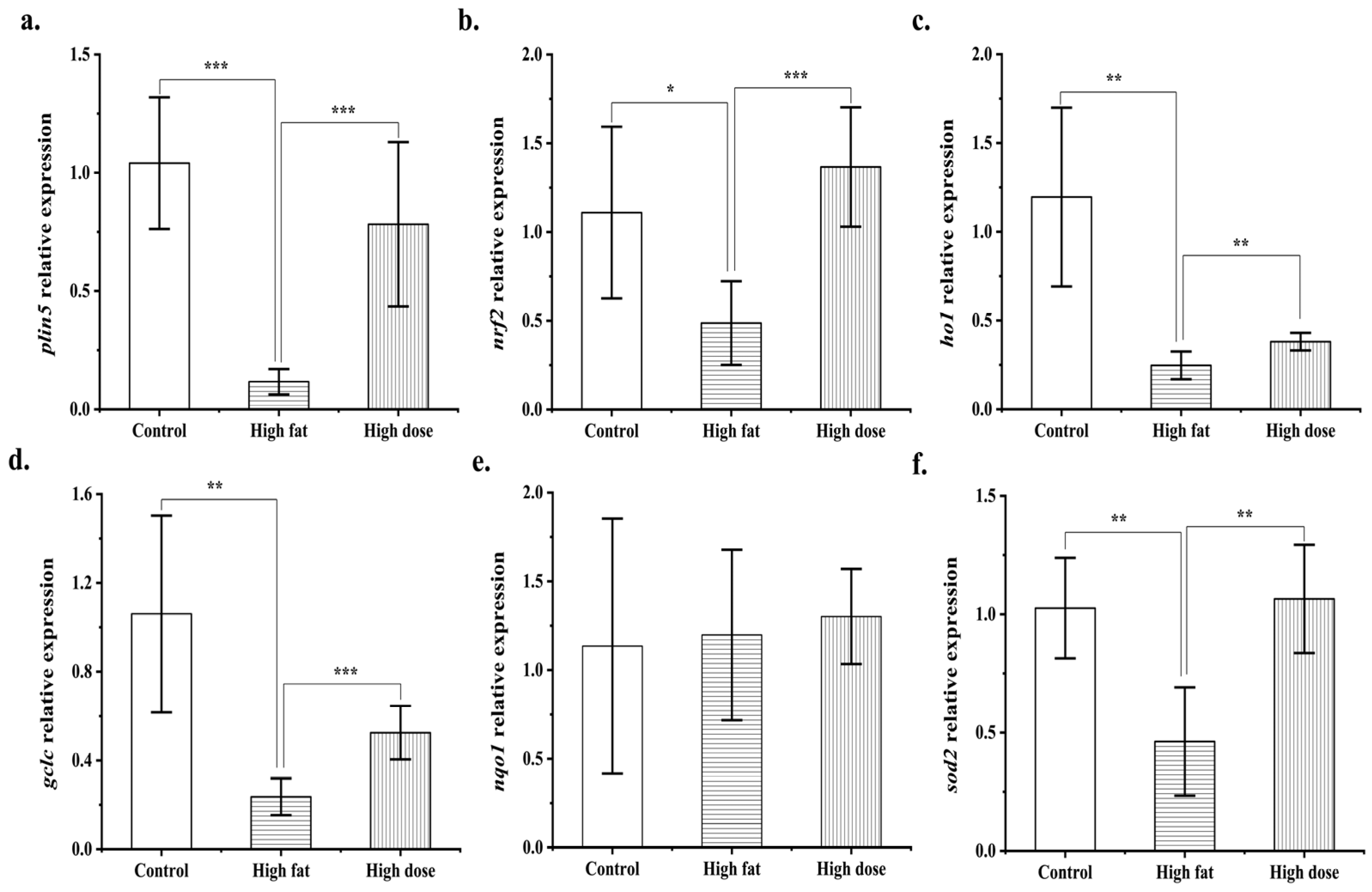
2.10. Real-Time Fluorescence Quantitative PCR
2.11. Statistical Analysis
3. Results and Discussion
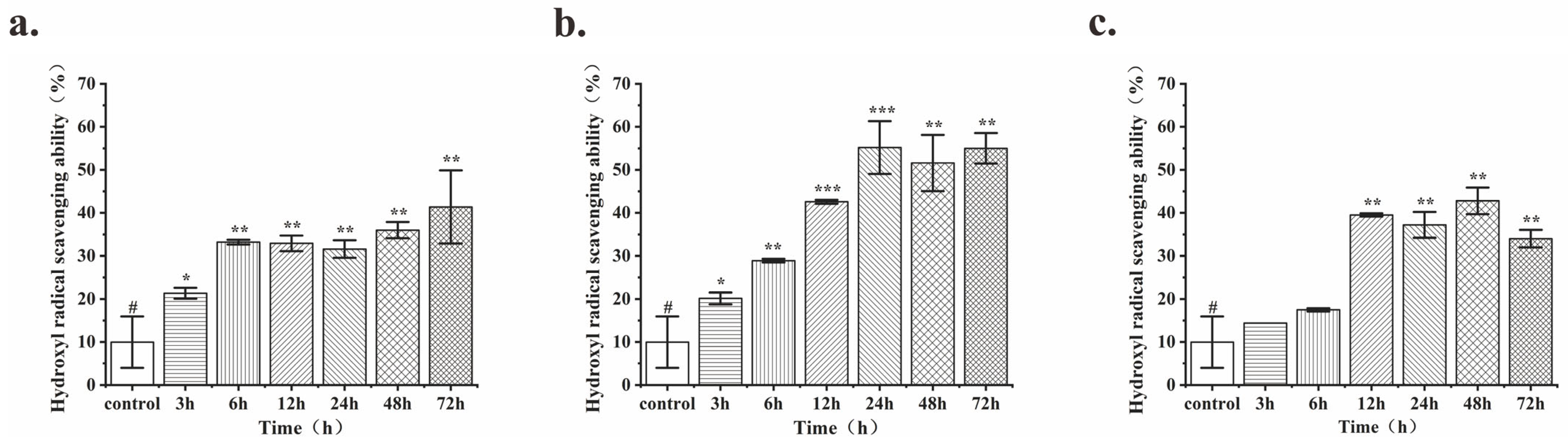
3.1. Comparison of the Antioxidant Activity of Fermentation Products and the Optimum Fermentation Time for Different Strains
3.2. HPLC Detection of FLRB
3.3. Component Analysis of FLRB
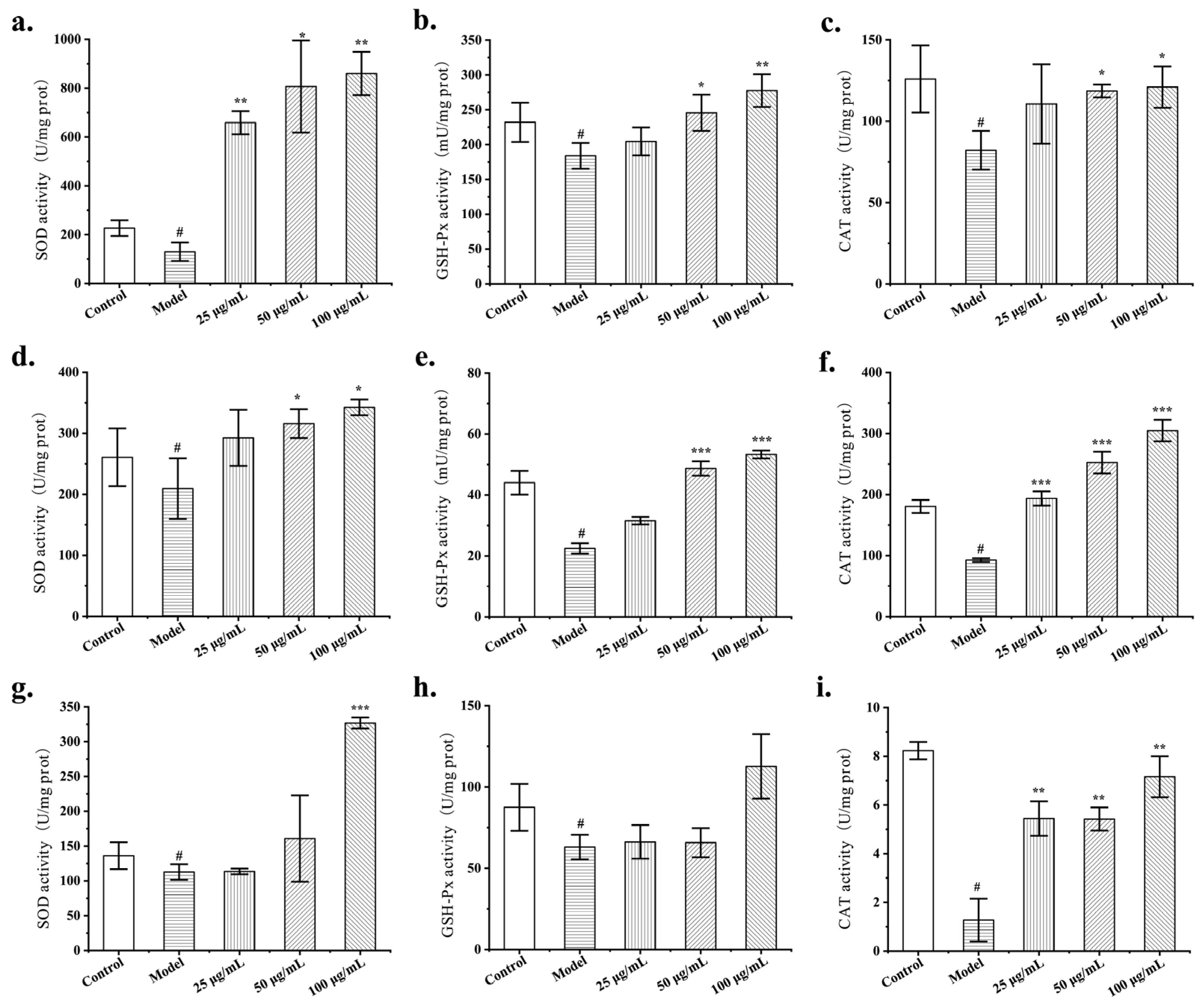
3.4. Protective Effect of FLRB against Cellular Oxidative Stress
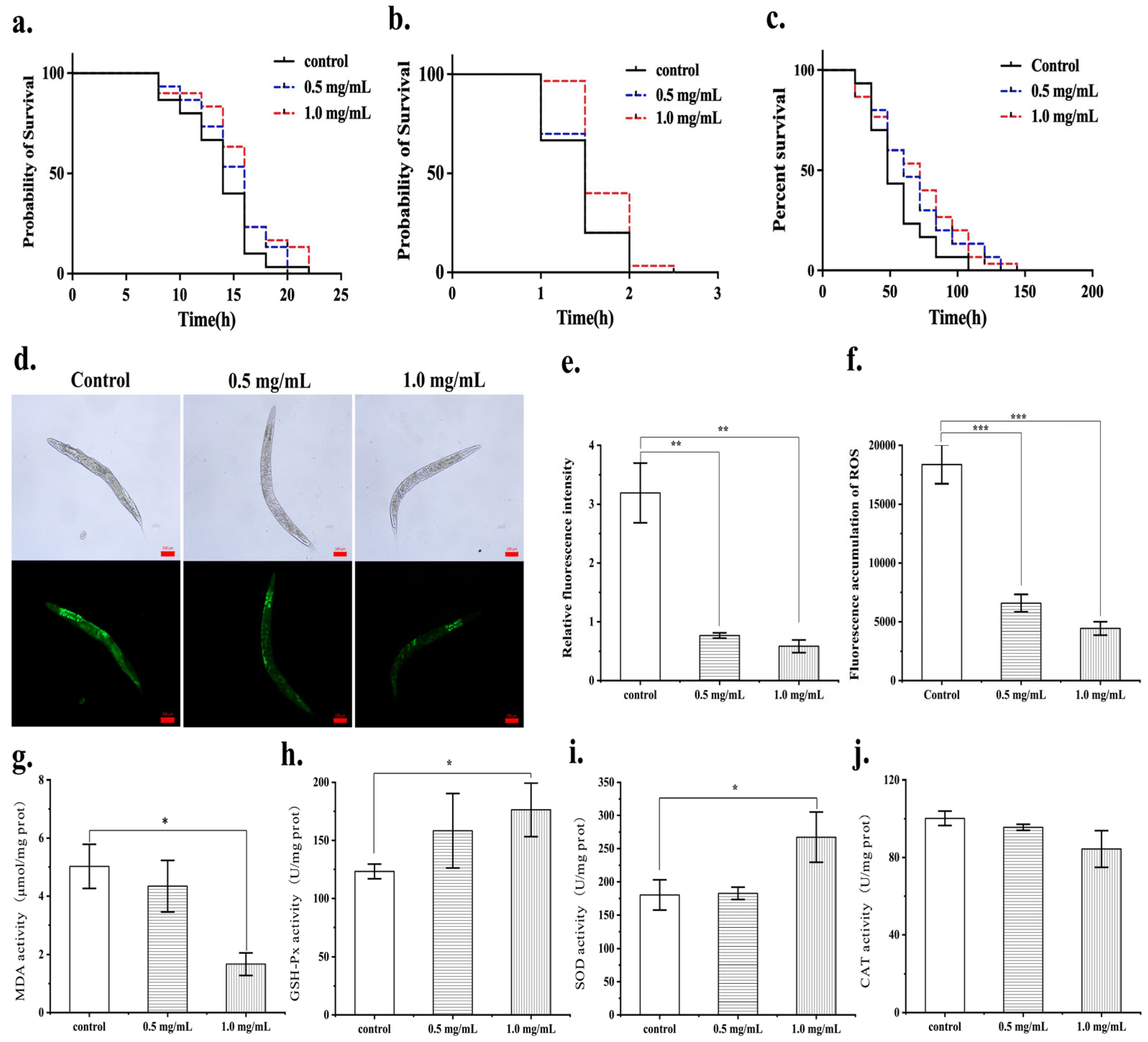
3.5. The Protective Effects of the MF423 Fermentation Product on Oxidative Stress in C. elegans
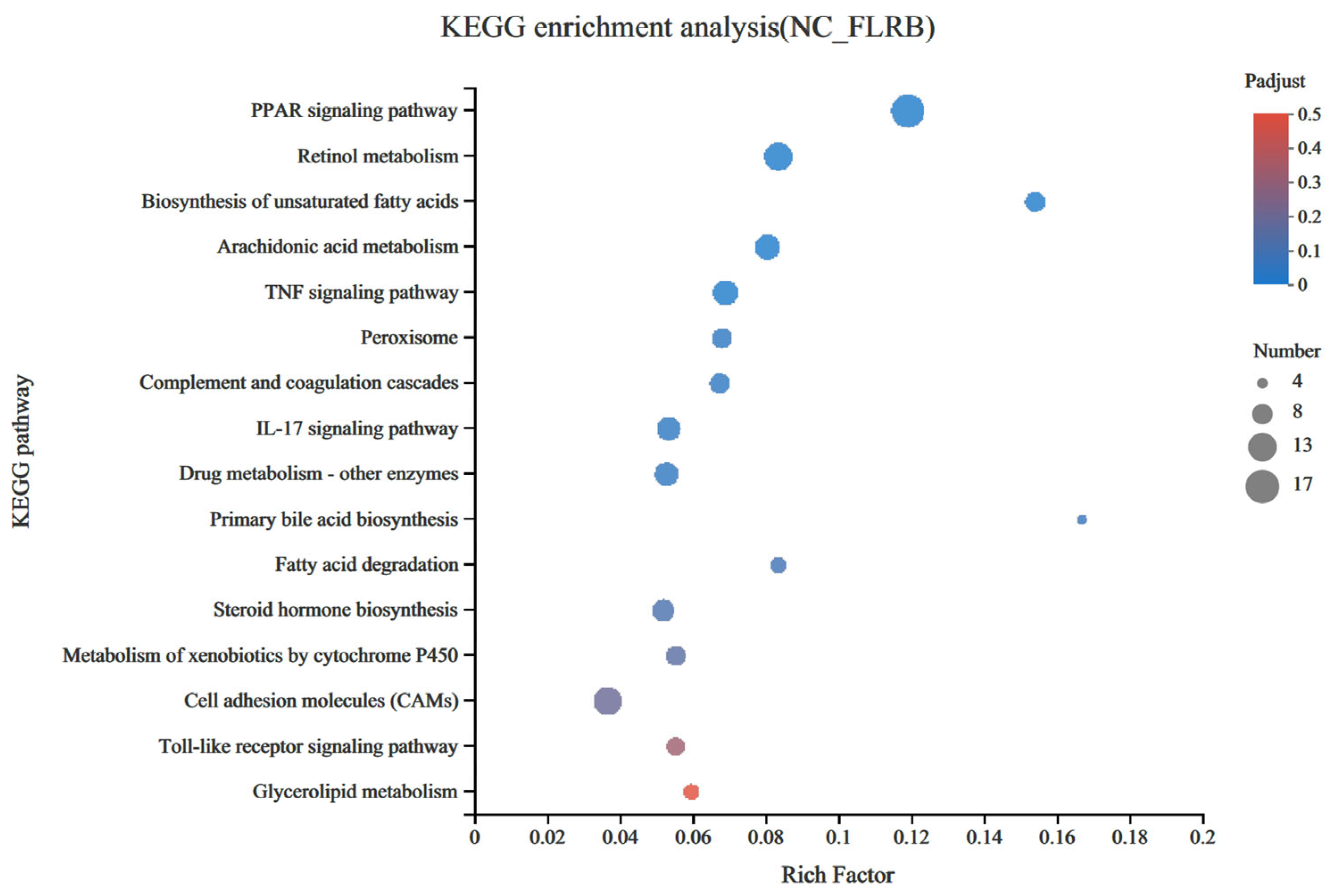
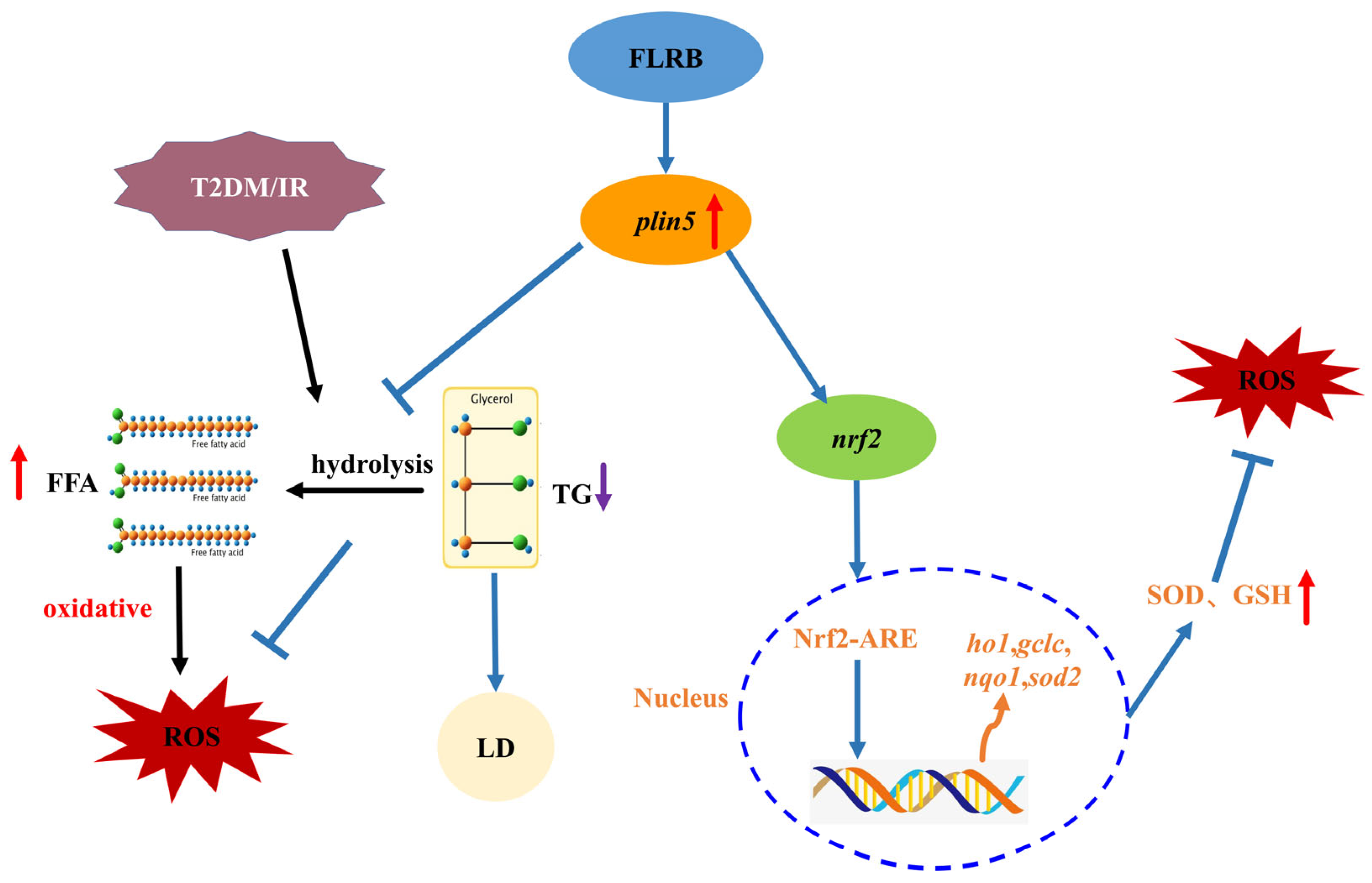
3.6. Comparison of Mouse Liver Transcriptomes between the Control Group and the FLRB-Group
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, M.; Cisneros, L.C.; Cho, E.J.; Alexander, M.; Kimelman, F.A.; Swentek, L.; Ferrey, A.; Tantisattamo, E.; Ichii, H. Nrf2 Pathway and Oxidative Stress as a Common Target for Treatment of Diabetes and Its Comorbidities. Int. J. Mol. Sci. 2024, 25, 821. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Talahalli, R.R. Aging and Hyperglycemia Intensify Dyslipidemia-Induced Oxidative Stress and Inflammation in Rats: Assessment of Restorative Potentials of Ala and Epa + Dha. Inflammation 2019, 42, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Lakshmanan, J. The Role of Antioxidants and Other Agents in Alleviating Hyperglycemia Mediated Oxidative Stress and Injury in Liver. Food Funct. 2013, 4, 1148–1184. [Google Scholar] [CrossRef] [PubMed]
- Sherif, I.O. The Effect of Natural Antioxidants in Cyclophosphamide-Induced Hepatotoxicity: Role of Nrf2/Ho-1 Pathway. Int. Immunopharmacol. 2018, 61, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Lee, K.W.; Choi, H.D. Rice Bran Constituents: Immunomodulatory and Therapeutic Activities. Food Funct. 2017, 8, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Andriani, R.; Subroto, T.; Ishmayana, S.; Kurnia, D. Enhancement Methods of Antioxidant Capacity in Rice Bran: A Review. Foods 2022, 11, 2994. [Google Scholar] [CrossRef]
- Yi, C.; Xu, L.; Luo, C.; He, H.; Ai, X.; Zhu, H. In Vitro Digestion, Fecal Fermentation, and Gut Bacteria Regulation of Brown Rice Gel Prepared from Rice Slurry Backfilled with Rice Bran. Food Hydrocoll. 2022, 133, 107986. [Google Scholar] [CrossRef]
- Sekyere, J.O.; Maningi, N.E.; Fourie, P.B. Mycobacterium Tuberculosis, Antimicrobials, Immunity, and Lung-Gut Microbiota Crosstalk: Current Updates and Emerging Advances. Ann. N. Y. Acad. Sci. 2020, 1467, 21–47. [Google Scholar] [CrossRef]
- Ai, X.; Wu, C.; Yin, T.; Zhur, O.; Liu, C.; Yan, X.; Yi, C.; Liu, D.; Xiao, L.; Li, W.; et al. Antidiabetic Function of Lactobacillus fermentum Mf423-Fermented Rice Bran and Its Effect on Gut Microbiota Structure in Type 2 Diabetic Mice. Front. Microbiol. 2021, 12, 682290. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Grasso, S.; Purewal, S.S.; Kaur, M.; Siroha, A.K.; Kumar, K.; Kumar, V.; Kumar, M. Aspergillus oryzae Fermented Rice Bran: A Byproduct with Enhanced Bioactive Compounds and Antioxidant Potential. Foods 2020, 10, 70. [Google Scholar] [CrossRef]
- Wang, M.; Lei, M.; Samina, N.; Chen, L.; Liu, C.; Yin, T.; Yan, X.; Wu, C.; He, H.; Yi, C. Impact of Lactobacillus plantarum 423 Fermentation on the Antioxidant Activity and Flavor Properties of Rice Bran and Wheat Bran. Food Chem. 2020, 330, 127156. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and Anti-Freezing Peptides from Salmon Collagen Hydrolysate Prepared by Bacterial Extracellular Protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xin, Q.; Wei, P.; Hua, Y.; Zhang, Y.; Su, Z.; She, G.; Yuan, R. Antioxidant and Anti-Aging Activities of Longan Crude and Purified Polysaccharide (Lp-a) in Nematode Caenorhabditis Elegans. Int. J. Biol. Macromol. 2024, 267, 131634. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Xie, L.; Cao, Z.; Quan, K.; Zhu, H.; Yuan, J. Effects of Rice Bran Fermented with Lactobacillus plantarumon Palatability, Volatile Profiles, and Antioxidant Activity of Brown Rice Noodles. Int. J. Food Sci. Technol. 2022, 57, 5048–5056. [Google Scholar] [CrossRef]
- Cheng, Y.; Jiang, N.; Diao, J.; Zheng, L. Achieving Cinnamic Acid Amides in Water by a Variant of Acyltransferase from Mycobacterium Smegmatis and Its Immobilized Form Using Ni-Nta Modified Aspen Powder as a Carrier. Int. J. Biol. Macromol. 2024, 261 Pt 2, 129849. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, F.; Mohammadi, S.; Kosari-Nasab, M.; Asgharian, P. The Impact of Microcrystalline and Nanocrystalline Cellulose on the Antioxidant Phenolic Compounds Level of the Cultured Artemisia absinthium. Sci. Rep. 2024, 14, 2692. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Li, J.; Wang, A.; Li, G.; Ke, S.; Wang, X.; Ning, M.; Sheng, Z.; Wang, B.; Zhou, Z. Structurally Manipulated Antioxidant Peptides Derived from Wheat Bran: Preparation and Identification. Food Chem. 2024, 442, 138465. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Cutruzzolá, F.; Antonov, A.; Agostini, M.; Melino, G. Serine and Glycine Metabolism in Cancer. Trends Biochem. Sci. 2014, 39, 191–198. [Google Scholar] [CrossRef]
- Kim, C.H.; Jeong, S.S.; Yoon, J.Y.; Yoon, J.U.; Yu, S.B.; Kim, E.J. Remifentanil Reduced the Effects of Hydrogen Peroxide-Induced Oxidative Stress in Human Keratinocytes via Autophagy. Connect. Tissue Res. 2017, 58, 597–605. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Mo, Q.; You, S.; Fu, H.; Wang, D.; Zhang, J.; Wang, C.; Li, M. Purification and Identification of Antioxidant Peptides from Rice Fermentation of Lactobacillus plantarum and Their Protective Effects on Uva-Induced Oxidative Stress in Skin. Antioxidants 2022, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, B.; Wu, C.; Li, D.; Wu, Y.; Ye, L.; Ye, L.; Chen, X.; Li, P.; Yuan, Y.; et al. Acidic Fibroblast Growth Factor Attenuates Type 2 Diabetes-Induced Demyelination via Suppressing Oxidative Stress Damage. Cell Death Dis. 2021, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ji, Y.; Guo, Y.; Wang, H.; Wu, Z.; Li, H.; Wang, H. Dietary Supplementation of Apple Phlorizin Attenuates the Redox State Related to Gut Microbiota Homeostasis in C57bl/6j Mice Fed with a High-Fat Diet. J. Agric. Food Chem. 2021, 69, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Jin, Y.; Wang, Q.; Huang, J.; Wu, X.; Ren, Z. Perilipin 5 Protects against Cellular Oxidative Stress by Enhancing Mitochondrial Function in Hepg2 Cells. Cells 2019, 8, 1241. [Google Scholar] [CrossRef]
- Sanchez, P.B.M.; Krizanac, M.; Weiskirchen, R.; Asimakopoulos, A. Understanding the Role of Perilipin 5 in Non-Alcoholic Fatty Liver Disease and Its Role in Hepatocellular Carcinoma: A Review of Novel Insights. Int. J. Mol. Sci. 2021, 22, 5284. [Google Scholar] [CrossRef]

| Number | Retention Time (min) | Molecular Weight (mass) | Component |
|---|---|---|---|
| 1 | 4.395 | 148.0519 | 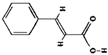 Cinnamic acid (C9H8O2) |
| 2 | 11.028 | 396.1564 | 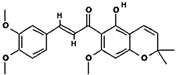 Glychalcone (C23H24O6) |
| 3 | 11.563 | 340.1302 |  Chalcone (C20H20O5) |
| 4 | 6.255 | 424.17 | Pro His Gly Asp |
| 5 | 6.605 | 507.2448 | Ala Asp Phe Arg |
| 6 | 6.629 | 561.2473 | Cys Phe His Arg |
| 7 | 7.516 | 434.2191 | Ala Thr Ile Met |
| 8 | 7.522 | 353.1408 | Met Phe Gly |
| 9 | 7.571 | 442.2062 | Thr Pro Pro Glu |
| 10 | 7.578 | 524.2575 | Tyr Thr Asn Lys |
| 11 | 7.592 | 567.2904 | Phe His His Lys |
| 12 | 8.202 | 396.2007 | Ser Tyr Lys |
| 13 | 8.245 | 439.2427 | Ile Asn Pro Pro |
| GO ID | Term Type | Description | Number of Genes |
|---|---|---|---|
| GO:0009987 | Biological process | cellular process | 333 |
| GO:0065007 | biological regulation | 302 | |
| GO:0050896 | response to stimulus | 209 | |
| GO:0008152 | metabolic process | 202 | |
| GO:0044464 | Cellular component | cell part | 386 |
| GO:0043226 | organelle | 273 | |
| GO:0016020 | membrane | 236 | |
| GO:0044422 | organelle part | 207 | |
| GO:0044425 | membrane part | 206 | |
| GO:0032991 | protein-containing complex | 115 | |
| GO:0005488 | Molecular function | binding | 344 |
| GO:0003824 | catalytic activity | 156 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, T.; Chen, Y.; Li, W.; Tang, T.; Li, T.; Xie, B.; Xiao, D.; He, H. Antioxidative Potential and Ameliorative Effects of Rice Bran Fermented with Lactobacillus against High-Fat Diet-Induced Oxidative Stress in Mice. Antioxidants 2024, 13, 639. https://doi.org/10.3390/antiox13060639
Yin T, Chen Y, Li W, Tang T, Li T, Xie B, Xiao D, He H. Antioxidative Potential and Ameliorative Effects of Rice Bran Fermented with Lactobacillus against High-Fat Diet-Induced Oxidative Stress in Mice. Antioxidants. 2024; 13(6):639. https://doi.org/10.3390/antiox13060639
Chicago/Turabian StyleYin, Tingting, Yidan Chen, Wenzhao Li, Tingting Tang, Tong Li, Binbin Xie, Dong Xiao, and Hailun He. 2024. "Antioxidative Potential and Ameliorative Effects of Rice Bran Fermented with Lactobacillus against High-Fat Diet-Induced Oxidative Stress in Mice" Antioxidants 13, no. 6: 639. https://doi.org/10.3390/antiox13060639
APA StyleYin, T., Chen, Y., Li, W., Tang, T., Li, T., Xie, B., Xiao, D., & He, H. (2024). Antioxidative Potential and Ameliorative Effects of Rice Bran Fermented with Lactobacillus against High-Fat Diet-Induced Oxidative Stress in Mice. Antioxidants, 13(6), 639. https://doi.org/10.3390/antiox13060639






