The Role of the Myokine Irisin in the Protection and Carcinogenesis of the Gastrointestinal Tract
Abstract
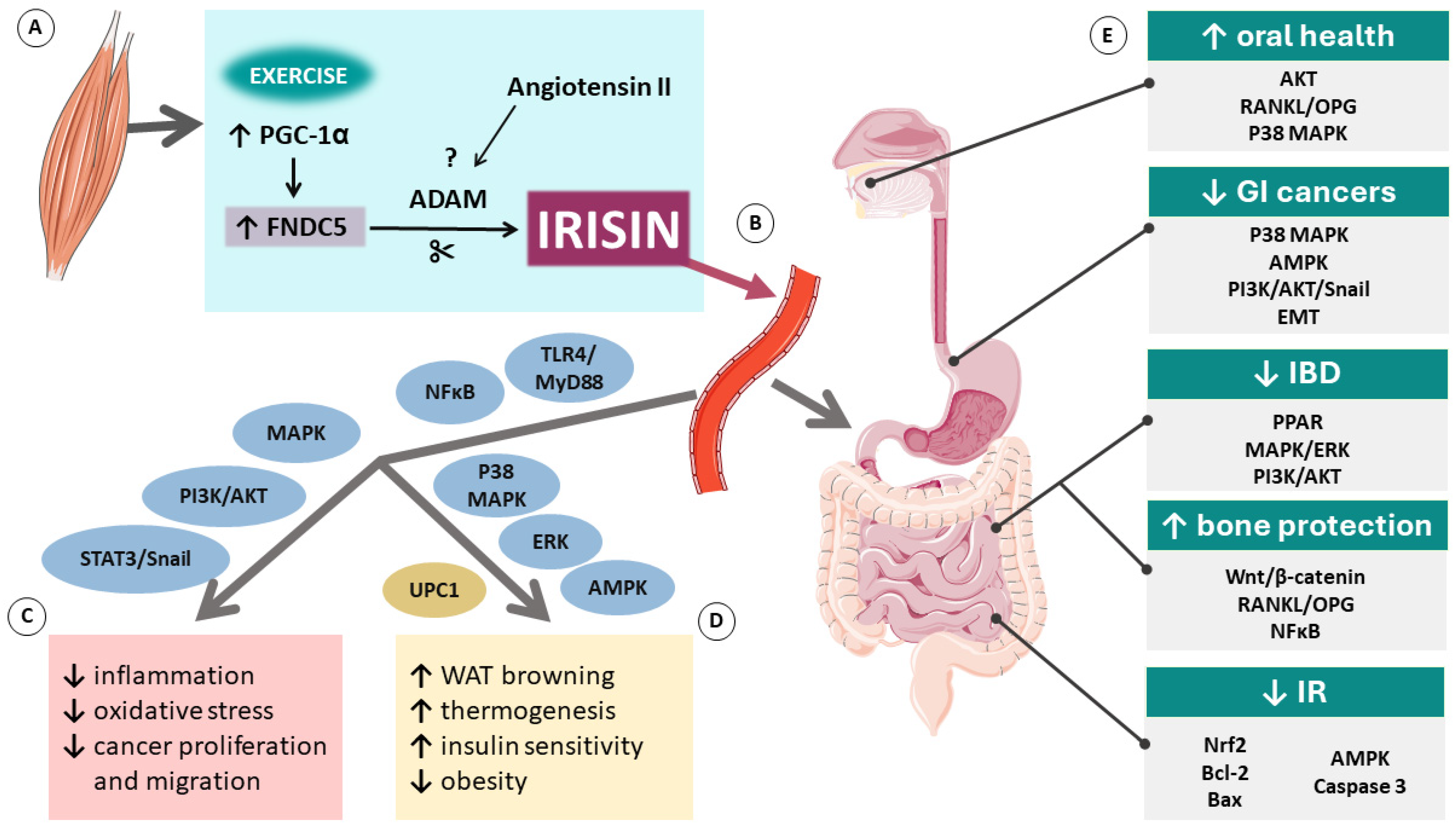
:1. Introduction
2. Protective Role of Exercise in the Gastrointestinal Tract
3. A Novel Myokine—Irisin
4. Detection of Irisin
5. Irisin’s Secretion and Mechanisms of Action
6. Irisin and Inflammatory Bowel Disease
7. Irisin and the Gut Microbiome
8. Irisin and Oral Health
9. Irisin and Intestinal Injury
10. Irisin and Gastrointestinal Malignancies
11. Current Limits and Perspectives
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Neufer, P.D.; Bamman, M.M.; Muoio, D.M.; Bouchard, C.; Cooper, D.M.; Goodpaster, B.H.; Booth, F.W.; Kohrt, W.M.; Gerszten, R.E.; Mattson, M.P.; et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015, 22, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y. The Role of Exercise-Induced Myokines in Regulating Metabolism. Arch. Pharm. Res. 2018, 41, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in Skeletal Muscle Physiology and Metabolism: Recent Advances and Future Perspectives. Acta Physiol. 2020, 228, 13367. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Magierowski, M.; Kwiecien, S.; Wojcik, D.; Ptak-Belowska, A.; Surmiak, M.; Targosz, A.; Magierowska, K.; Brzozowski, T. Exploiting Significance of Physical Exercise in Prevention of Gastrointestinal Disorders. Curr. Pharm. Des. 2018, 24, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.U.; Yassine, H.M.; Sohail, A.; Al Thani, A.A. Impact of Physical Exercise on Gut Microbiome, Inflammation, and the Pathobiology of Metabolic Disorders. Rev. Diabet. Stud. 2019, 15, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; You, Y.; Huang, J.; Guan, C.; Chen, Z.; Fang, M.; Yao, F.; Han, J. Association between Physical Activity and Digestive-System Cancer: An Updated Systematic Review and Meta-Analysis. J. Sport. Health Sci. 2021, 10, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, Q.; Liu, J.; Jia, S. Irisin, an Exercise-Induced Myokine as a Metabolic Regulator: An Updated Narrative Review. Diabetes Metab. Res. Rev. 2016, 32, 51–59. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, C.; Wu, H.; Ma, Z.; Tang, Q. Fibronectin Type III Domain-Containing 5 in Cardiovascular and Metabolic Diseases: A Promising Biomarker and Therapeutic Target. Acta Pharmacol. Sin. 2021, 42, 1390–1400. [Google Scholar] [CrossRef]
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A.; et al. Irisin—A Myth Rather than an Exercise-Inducible Myokine. Sci. Rep. 2015, 5, 8889. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Rioux, B.V.; Goulet, E.D.B.; Johanssen, N.M.; Swift, D.L.; Bouchard, D.R.; Loewen, H.; Sénéchal, M. Effect of an Acute Exercise Bout on Immediate Post-Exercise Irisin Concentration in Adults: A Meta-Analysis. Scand. J. Med. Sci. Sports 2018, 28, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Schering, L.; Buck, F.; Vlach, K.; Schober, H.C.; Drevon, C.A.; Maak, S. Irisin: Still Chasing Shadows. Mol. Metab. 2020, 34, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Sun, X.; Shan, M.; Zhao, X.; Zhang, R.; Zhao, Y.; Yang, Q. The Production, Detection, and Origin of Irisin and Its Effect on Bone Cells. Int. J. Biol. Macromol. 2021, 178, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Mantzoros, C.S. An Update on the Validity of Irisin Assays and the Link between Irisin and Hepatic Metabolism. Metabolism 2015, 64, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. Three New Players in Energy Regulation: Preptin, Adropin and Irisin. Peptides 2014, 56, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Cosio, P.L.; Crespo-Posadas, M.; Velarde-Sotres, Á.; Pelaez, M. Effect of Chronic Resistance Training on Circulating Irisin: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 2476. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, F.; Yang, M.; Sun, J.; Zhao, Y.; Tang, D. The Effect of Irisin as a Metabolic Regulator and Its Therapeutic Potential for Obesity. Int. J. Endocrinol. 2021, 2021, 6572342. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via AV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Mu, A.; Wales, T.E.; Zhou, H.; Draga-Coletă, S.-V.; Gorgulla, C.; Blackmore, K.A.; Mittenbühler, M.J.; Kim, C.R.; Bogoslavski, D.; Zhang, Q.; et al. Irisin Acts through Its Integrin Receptor in a Two-Step Process Involving Extracellular Hsp90α. Mol. Cell 2023, 83, 1903–1920.e12. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wu, Z.; Lv, Y.; et al. Irisin Reverses Intestinal Epithelial Barrier Dysfunction during Intestinal Injury via Binding to the Integrin AVβ5 Receptor. J. Cell Mol. Med. 2020, 24, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Myint, P.K.; Ito, A.; Appiah, M.G.; Darkwah, S.; Kawamoto, E.; Shimaoka, M. Integrin-Ligand Interactions in Inflammation, Cancer, and Metabolic Disease: Insights Into the Multifaceted Roles of an Emerging Ligand Irisin. Front. Cell Dev. Biol. 2020, 8, 588066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, L.; Gao, H.; Zhai, J.; Song, Y. Potential Role of Irisin in Digestive System Diseases. Biomed. Pharmacother. 2023, 166, 115347. [Google Scholar] [CrossRef] [PubMed]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Ding, D.; Ji, C.; Wu, Q.; Xia, Y.; Zhou, L.; Yang, L.; Ba, G.; Chang, Q.; Fu, Q.; et al. Relationships Between Circulating Irisin Response to Ice Swimming and Body Composition in People With Regular Exercise Experience. Front. Physiol. 2021, 11, 596896. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine Response to High-Intensity Interval vs. Resistance Exercise: An Individual Approach. Front. Physiol. 2018, 9, 416035. [Google Scholar] [CrossRef] [PubMed]
- Torabi, A.; Reisi, J.; Kargarfard, M.; Mansourian, M. Differences in the Impact of Various Types of Exercise on Irisin Levels: A Systematic Review and Meta-Analysis. Int. J. Prev. Med. 2024, 15, 11. [Google Scholar] [CrossRef]
- Kazeminasab, F.; Sadeghi, E.; Afshari-Safavi, A. Comparative Impact of Various Exercises on Circulating Irisin in Healthy Subjects: A Systematic Review and Network Meta-Analysis. Oxid. Med. Cell Longev. 2022, 2022, 8235809. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, R.A.L.; Improta-Caria, A.C.; Souza, B.S. de F. Exercise–Linked Irisin: Consequences on Mental and Cardiovascular Health in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 2199. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.R.; Lomba, G.S.B.; Gonçalves-de-Albuquerque, C.F.; Burth, P. Irisin, Exercise, and COVID-19. Front. Endocrinol. 2022, 13, 879066. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisløff, U.; Tjønna, A.E.; Raastad, T.; et al. Evidence against a Beneficial Effect of Irisin in Humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef] [PubMed]
- Witmer, N.H.; Linzer, C.R.; Boudreau, R.L. Fndc5 Is Translated from an Upstream ATG Start Codon and Cleaved to Produce Irisin Myokine Precursor Protein in Humans and Mice. Cell Metab. 2024; ahead of print. [Google Scholar] [CrossRef]
- Bao, J.-F.; She, Q.-Y.; Hu, P.-P.; Jia, N.; Li, A. Irisin, a Fascinating Field in Our Times. Trends Endocrinol. Metab. 2022, 33, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Darkwah, S.; Park, E.J.; Myint, P.K.; Ito, A.; Appiah, M.G.; Obeng, G.; Kawamoto, E.; Shimaoka, M. Potential Roles of Muscle-Derived Extracellular Vesicles in Remodeling Cellular Microenvironment: Proposed Implications of the Exercise-Induced Myokine, Irisin. Front. Cell Dev. Biol. 2021, 9, 634853. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, F.; Lachinani, L.; Ghaedi, S.; Nasr-Esfahani, M.H.; Megraw, T.L.; Ghaedi, K. New Insights into the Cellular Activities of Fndc5/Irisin and Its Signaling Pathways. Cell Biosci. 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y.; et al. Irisin Exerts Dual Effects on Browning and Adipogenesis of Human White Adipocytes. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E530–E541. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, J.; Zhang, H.; Wang, Y.; Shi, H.; Ge, Y.; Yu, X.; Wang, H.; Dong, Y. Irisin Promotes the Browning of White Adipocytes Tissue by AMPKα1 Signaling Pathway. Res. Vet. Sci. 2022, 152, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.B.; Sahar, N.E.; Jeong, M.; Huh, J.Y. Irisin Exerts Inhibitory Effect on Adipogenesis Through Regulation of Wnt Signaling. Front. Physiol. 2019, 10, 1085. [Google Scholar] [CrossRef]
- Shaw, A.; Tóth, B.B.; Király, R.; Arianti, R.; Csomós, I.; Póliska, S.; Vámos, A.; Korponay-Szabó, I.R.; Bacso, Z.; Győry, F.; et al. Irisin Stimulates the Release of CXCL1 From Differentiating Human Subcutaneous and Deep-Neck Derived Adipocytes via Upregulation of NFκB Pathway. Front. Cell Dev. Biol. 2021, 9, 737872. [Google Scholar] [CrossRef] [PubMed]
- Slate-Romano, J.J.; Yano, N.; Zhao, T.C. Irisin Reduces Inflammatory Signaling Pathways in Inflammation-Mediated Metabolic Syndrome. Mol. Cell Endocrinol. 2022, 552, 111676. [Google Scholar] [CrossRef]
- Liu, J.; Qi, B.; Gan, L.; Shen, Y.; Zou, Y. A Bibliometric Analysis of the Literature on Irisin from 2012–2021. Int. J. Env. Res. Public Health 2022, 19, 6153. [Google Scholar] [CrossRef] [PubMed]
- Anand Narayanan, S.; Metzger, C.E.; Bloomfield, S.A.; Zawieja, D.C. Inflammation-Induced Lymphatic Architecture and Bone Turnover Changes Are Ameliorated by Irisin Treatment in Chronic Inflammatory Bowel Disease. FASEB J. 2018, 32, 4848–4861. [Google Scholar] [CrossRef]
- Metzger, C.E.; Narayanan, S.A.; Elizondo, J.P.; Carter, A.M.; Zawieja, D.C.; Hogan, H.A.; Bloomfield, S.A. DSS-Induced Colitis Produces Inflammation-Induced Bone Loss While Irisin Treatment Mitigates the Inflammatory State in Both Gut and Bone. Sci. Rep. 2019, 9, 15144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ocansey, D.K.W.; Liu, L.; Olovo, C.V.; Zhang, X.; Qian, H.; Xu, W.; Mao, F. Implications of Lymphatic Alterations in the Pathogenesis and Treatment of Inflammatory Bowel Disease. Biomed. Pharmacother. 2021, 140, 111752. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.I.; Brzozowski, B.; Magierowski, M.; Jasnos, K.; Krzysiek-Maczka, G.; Urbanczyk, K.; Ptak-Belowska, A.; Zwolinska-Wcislo, M.; Mach, T.; et al. Moderate Exercise Training Attenuates the Severity of Experimental Rodent Colitis: The Importance of Crosstalk between Adipose Tissue and Skeletal Muscles. Mediat. Inflamm. 2015, 2015, 605071. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.; Bilski, J.; Wojcik, D.; Brzozowski, B.; Surmiak, M.; Hubalewska-Mazgaj, M.; Chmura, A.; Magierowski, M.; Magierowska, K.; Mach, T.; et al. Beneficial Effect of Voluntary Exercise on Experimental Colitis in Mice Fed a High-Fat Diet: The Role of Irisin, Adiponectin and Proinflammatory Biomarkers. Nutrients 2017, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Hajj Hussein, I.; Dosh, L.; Al Qassab, M.; Jurjus, R.; El Masri, J.; Abi Nader, C.; Rappa, F.; Leone, A.; Jurjus, A. Highlights on Two Decades with Microbiota and Inflammatory Bowel Disease from Etiology to Therapy. Transpl. Immunol. 2023, 78, 101835. [Google Scholar] [CrossRef]
- Palatianou, M.E.; Karamanolis, G.; Tsentidis, C.; Gourgiotis, D.; Papaconstantinou, I.; Vezakis, A.; Tzouvala, M. Signaling Pathways Associated with Bone Loss in Inflammatory Bowel Disease. Ann. Gastroenterol. 2023, 36, 132. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, K.; Zhao, S.; Geng, T.; Fan, X.; Sun, S.; Zheng, M.; Jin, Q. Irisin Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Activating Autophagy via the Wnt//β-Catenin Signal Pathway. Cytokine 2020, 136, 155292. [Google Scholar] [CrossRef]
- Huangfu, L.; Cai, X.; Yang, J.; Wang, H.; Li, Y.; Dai, Z.; Yang, R.; Lin, X. Irisin Attenuates Inflammation in a Mouse Model of Ulcerative Colitis by Altering the Intestinal Microbiota. Exp. Ther. Med. 2021, 22, 1433. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, Q.; Xu, T.; Yuan, Q.; Hu, Q.; Hu, N.; Sun, W.; Bai, Y.; Liu, L.; Feng, J.; et al. Fndc5/Irisin Deficiency Leads to Dysbiosis of Gut Microbiota Contributing to the Depressive-like Behaviors in Mice. Brain Res. 2023, 1819, 148537. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Du, Y.; Yang, J.; He, Q.; Wang, H.; Lin, X. Anti-Inflammatory Effect of Irisin on LPS-Stimulated Macrophages through Inhibition of MAPK Pathway. Physiol. Res. 2023, 72, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, B.; Lee, C.; Joung, H.; Kim, B.K.; Choi, I.S.; Hyun, C.K. Comprehensive Amelioration of High-Fat Diet-Induced Metabolic Dysfunctions through Activation of the PGC-1α Pathway by Probiotics Treatment in Mice. PLoS ONE 2020, 15, e0228932. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C. The Role of Oral Health in Gastrointestinal Malignancies. J. Gastrointest. Oncol. 2021, 12, S311–S315. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.L.; Kamada, N. Pathogenic Associations between Oral and Gastrointestinal Diseases. Trends Mol. Med. 2022, 28, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Hwang, B.-O.; Lim, M.; Ok, S.-H.; Lee, S.-K.; Chun, K.-S.; Park, K.-K.; Hu, Y.; Chung, W.-Y.; Song, N.-Y.; et al. Oral–Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Ferreira, R.; Correâ, M.G.; Magno, M.B.; Sousa Carvalho Almeida, A.P.C.P.; Fagundes, N.C.F.; Rosing, C.K.; Maia, L.C.; Lima, R.R. Physical Activity Reduces the Prevalence of Periodontal Disease: Systematic Review and Meta Systematic Review and Meta-Analysis. Front. Physiol. 2019, 10, 437689. [Google Scholar] [CrossRef]
- Pu, R.; Fu, M.; Yang, G.; Jiang, Z. The Association of Work Physical Activity and Recreational Physical Activity with Periodontitis in the NHANES (2009–2014). J. Periodontol. 2023, 94, 1220–1230. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, J.; Wang, X.; Cao, Z. Irisin Attenuates P. Gingivalis-Suppressed Osteogenic/Cementogenic Differentiation of Periodontal Ligament Cells via P38 Signaling Pathway. Biochem. Biophys. Res. Commun. 2022, 618, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Pullisaar, H.; Colaianni, G.; Lian, A.M.; Vandevska-Radunovic, V.; Grano, M.; Reseland, J.E. Irisin Promotes Growth, Migration and Matrix Formation in Human Periodontal Ligament Cells. Arch. Oral. Biol. 2020, 111, 104635. [Google Scholar] [CrossRef]
- Posa, F.; Colaianni, G.; Di Cosola, M.; Dicarlo, M.; Gaccione, F.; Colucci, S.; Grano, M.; Mori, G. The Myokine Irisin Promotes Osteogenic Differentiation of Dental Bud-Derived MSCs. Biology 2021, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Son, J.W.; Choi, S.H.; Jang, J.H.; Koh, J.T.; Oh, W.M.; Hwang, Y.C.; Lee, B.N. Irisin Promotes Odontogenic Differentiation and Angiogenic Potential in Human Dental Pulp Cells. Int. Endod. J. 2021, 54, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, J.; Liu, L.; Pang, Y.; Li, Z.; Mu, H. Recombinant Irisin Protects Against Alveolar Bone Destruction During Orthodontic Tooth Movement. Inflammation 2023, 46, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Reseland, J.E.; Pullisaar, H. Expression and Regulation of FNDC5/Irisin in Periodontium and Dental Pulp. Arch. Dent. 2022, 4, 8–10. [Google Scholar] [CrossRef]
- Yang, Y.; Pullisaar, H.; Landin, M.A.; Heyward, C.A.; Schröder, M.; Geng, T.; Grano, M.; Reseland, J.E. FNDC5/Irisin Is Expressed and Regulated Differently in Human Periodontal Ligament Cells, Dental Pulp Stem Cells and Osteoblasts. Arch. Oral. Biol. 2021, 124, 105061. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, T.; Samara, A.; Olstad, O.K.; He, J.; Agger, A.E.; Skallerud, B.H.; Landin, M.A.; Heyward, C.A.; Pullisaar, H.; et al. Recombinant Irisin Enhances the Extracellular Matrix Formation, Remodeling Potential, and Differentiation of Human Periodontal Ligament Cells Cultured in 3D. J. Periodontal Res. 2023, 58, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pullisaar, H.; Stunes, A.K.; Nogueira, L.P.; Syversen, U.; Reseland, J.E. Irisin Reduces Orthodontic Tooth Movement in Rats by Promoting the Osteogenic Potential in the Periodontal Ligament. Eur. J. Orthod. 2023, 45, 842. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Y.; Cao, Z.; Zhu, J.; He, H. Effect of Irisin on the Expression of Osteoclast-Related Genes in Cementoblasts. Eur. J. Orthod. 2022, 44, 420–426. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Cao, Z.; Du, M.; Hao, Y.; Pan, J.; He, H. Irisin Promotes Cementoblast Differentiation via P38 MAPK Pathway. Oral. Dis. 2020, 26, 974–982. [Google Scholar] [CrossRef]
- Li, G.; Qin, H.; Zhou, M.; Zhang, T.; Zhang, Y.; Ding, H.; Xu, L.; Song, J. Knockdown of SIRT3 Perturbs Protective Effects of Irisin against Bone Loss in Diabetes and Periodontitis. Free Radic. Biol. Med. 2023, 200, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Ghafoor, S.; Khaliq, S.; Syed, A.R. Salivary Irisin and Periodontal Clinical Parameters in Patients of Chronic Periodontitis and Healthy Individuals: A Novel Salivary Myokine for Periodontal Disease. J. Pak. Med. Assoc. 2021, 72, 27–32. [Google Scholar] [CrossRef]
- Turkmen, E.; Uzun, E.V.; Bozaba, F.; Balci, N.; Toygar, H. Salivary Irisin Level Is Higher and Related with Interleukin-6 in Generalized Periodontitis. Clin. Oral. Investig. 2023, 27, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Fan, X.; Yang, B.; Chen, Y.; Liu, K.X.; Zhou, J. Irisin Pretreatment Ameliorates Intestinal Ischemia/Reperfusion Injury in Mice through Activation of the Nrf2 Pathway. Int. Immunopharmacol. 2019, 73, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.-F.; Wang, M.-Z.; Bi, J.-B.; Zhang, J.; Zhang, L.; Liu, W.-M.; Wei, S.-S.; Lv, Y.; Wu, Z.; Wu, R.-Q. Irisin Attenuates Intestinal Injury, Oxidative and Endoplasmic Reticulum Stress in Mice with L-Arginine-Induced Acute Pancreatitis. World J. Gastroenterol. 2019, 25, 6653–6667. [Google Scholar] [CrossRef]
- Bilski, J.; Pinkas, M.; Wojcik-Grzybek, D.; Magierowski, M.; Korbut, E.; Mazur-Bialy, A.; Krzysiek-Maczka, G.; Kwiecien, S.; Magierowska, K.; Brzozowski, T. Role of Obesity, Physical Exercise, Adipose Tissue-Skeletal Muscle Crosstalk and Molecular Advances in Barrett’s Esophagus and Esophageal Adenocarcinoma. Int. J. Mol. Sci. 2022, 23, 3942. [Google Scholar] [CrossRef] [PubMed]
- Askari, H.; Rajani, S.F.; Poorebrahim, M.; Haghi-Aminjan, H.; Raeis-Abdollahi, E.; Abdollahi, M. A Glance at the Therapeutic Potential of Irisin against Diseases Involving Inflammation, Oxidative Stress, and Apoptosis: An Introductory Review. Pharmacol. Res. 2018, 129, 44–55. [Google Scholar] [CrossRef]
- Sumsuzzman, D.M.; Jin, Y.; Choi, J.; Yu, J.-H.; Lee, T.H.; Hong, Y. Pathophysiological Role of Endogenous Irisin against Tumorigenesis and Metastasis: Is It a Potential Biomarker and Therapeutic? Tumor Biol. 2019, 41, 101042831989279. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Kozlowska, K.; Pochec, E.; Bilski, J.; Brzozowski, T. Myokine Irisin-Induced Protection against Oxidative Stress in Vitro. Involvement of Heme Oxygenase-1 and Antioxidizing Enzymes Superoxide Dismutase-2 and Glutathione Peroxidase. J. Physiol. Pharmacol. 2018, 69, 117–125. [Google Scholar] [CrossRef]
- Waseem, R.; Shamsi, A.; Mohammad, T.; Hassan, M.I.; Kazim, S.N.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, F.; Islam, A. FNDC5/Irisin: Physiology and Pathophysiology. Molecules 2022, 27, 1118. [Google Scholar] [CrossRef] [PubMed]
- Pazgan-Simon, M.; Zuwala-Jagiello, J.; Menzyk, T.; Bator, M.; Derra, A.; Lekstan, A.; Grzebyk, E.; Simon, K.; Kukla, M. Serum Betatrophin and Irisin Levels in Hepatocellular Carcinoma. J. Physiol. Pharmacol. 2020, 71, 113–123. [Google Scholar] [CrossRef]
- Moon, H.S.; Mantzoros, C.S. Regulation of Cell Proliferation and Malignant Potential by Irisin in Endometrial, Colon, Thyroid and Esophageal Cancer Cell Lines. Metabolism 2014, 63, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Kuloglu, T.; Ozercan, M.; Albayrak, S.; Aydin, S.; Bakal, U.; Yilmaz, M.; Kalayci, M.; Yardim, M.; Sarac, M.; et al. Irisin Immunohistochemistry in Gastrointestinal System Cancers. Biotech. Histochem. 2016, 91, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Okechukwu, C.; Okechukwu, C.; Agag, A.; Naushad, N.; Abbas, S.; Deb, A. Hypothesized Biological Mechanisms by Which Exercise-Induced Irisin Mitigates Tumor Proliferation and Improves Cancer Treatment Outcomes. MGM J. Med. Sci. 2021, 8, 452. [Google Scholar] [CrossRef]
- Wozniak, S.; Nowinska, K.; Chabowski, M.; Dziegiel, P. Significance of Irisin (FNDC5) Expression in Colorectal Cancer. Vivo 2022, 36, 180. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, M.; Zhang, N.; Pan, H.; Lin, G.; Li, N.; Wang, L.; Yang, H.; Yan, K.; Gong, F. Serum and Adipose Tissue MRNA Levels of ATF3 and FNDC5/Irisin in Colorectal Cancer Patients with or without Obesity. Front. Physiol. 2018, 9, 1125. [Google Scholar] [CrossRef] [PubMed]
- Celik, Z.; Baygutalp, N.K.; Kilic, A.F.; Tekin, S.B.; Bakan, E.; Gul, M.A.; Yuce, N. Serum Irisin Levels in Colorectal Cancer Patients. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Tumer, A.; Rashid, F. The Relationship between Circulating Irisin and Oxidative Stress in Gastric and Colorectal Cancer Patients. Asian Pac. J. Cancer Prev. 2022, 23, 2649–2654. [Google Scholar] [CrossRef]
- Uzun, M.; Ilhan, Y.S.; Bozdag, A.; Yilmaz, M.; Artas, G.; Kuloglu, T. Asprosin, Irisin, and Meteorin-like Protein Immunoreactivity in Different Stages of Colorectal Adenocarcinoma. Pathol. Res. Pract. 2023, 245, 154432. [Google Scholar] [CrossRef]
- Altay, D.U.; Keha, E.E.; Yaman, S.O.; Ince, I.; Alver, A.; Erdogan, B.; Canpolat, S.; Cobanoglu, U.; Mentese, A. Investigation of the Expression of Irisin and Some Cachectic Factors in Mice with Experimentally Induced Gastric Cancer. QJM 2016, 109, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Hejazi, J.; Moghimi, M.; Borji, S.; Zabihian, S.; Fathi, M. Circulating Irisin Levels and Redox Status Markers in Patients with Gastric Cancer: A Case-Control Study. Asian Pac. J. Cancer Prev. 2020, 21, 2847–2851. [Google Scholar] [CrossRef] [PubMed]
- de Castro, G.S.; Correia-Lima, J.; Simoes, E.; Orsso, C.E.; Xiao, J.; Gama, L.R.; Gomes, S.P.; Gonçalves, D.C.; Costa, R.G.F.; Radloff, K.; et al. Myokines in Treatment-Naïve Patients with Cancer-Associated Cachexia. Clin. Nutr. 2021, 40, 2443–2455. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Belli, R.; Imbimbo, G.; Carletti, R.; Amabile, M.I.; Tambaro, F.; di Gioia, C.R.T.; Belloni, E.; Ferraro, E.; Nigri, G.; et al. Evaluation of Browning Markers in Subcutaneous Adipose Tissue of Newly Diagnosed Gastrointestinal Cancer Patients with and without Cachexia. Cancers 2022, 14, 1948. [Google Scholar] [CrossRef] [PubMed]

| Disease | Organism/Model/Cell Line | Methods/Intervention | Irisin Detection Method | Findings/Effects of Irisin | Signaling Pathways | Ref. |
|---|---|---|---|---|---|---|
| IBD | Rats with TNBS-induced colitis | HFD, LFD, ND, forced exercise | Plasma irisin, ELISA | ↓ irisin in the HFD colitis group ↑ irisin in exercising groups | N/A | [48] |
| IBD | Mice with TNBS-induced colitis | HFD, ND, voluntary exercise | Plasma irisin, detection method not specified | ↓ irisin in the HFD sedentary group ↓ FNDC5 gene expression in WAT of colitis mice | N/A | [49] |
| IBD | Rats with TNBS-induced colitis | Intraperitoneal injection of irisin (18 ng/mL) | N/A | ↓ inflammatory markers ↑ colonic lymphatic structure ↑ bone formation | RANKL/OPG | [45] |
| IBD | Mice with DSS-induced colitis | Intraperitoneal injection of irisin (0.0075 µg/g) | N/A | ↓ macroscopic and histopathological scores ↓ colonic CD64+ cells ↓ plasma IL-12 and IL-23; ↑ gut microbiota | N/A | [53] |
| UC, LPS-induced inflammation | LPS-induced macrophage (RAW 264.7) cell line | Incubation with irisin (400 ng/mL) and/or LPS in vitro | N/A | ↓ inflammation ↓ cytotoxicity and apoptosis ↓ IL-12 and IL-23 | MAPK, ERK, PI3K/AKT, PPAR | [55] |
| Gut dysbiosis | FNDC5-knocked out mice | Fecal microbiota assessment | N/A | ↑ dysbiosis of the gut microbiota | N/A | [54] |
| Periodontitis | Mouse cementoblast (OCCM-30) cell line | Incubation with irisin (0–200 ng/mL) | N/A | ↑ differentiation ↑ proliferation ↑ mineralization | p38 MAPK | [72] |
| Periodontitis | Human osteoblast cell lines | Incubation with irisin (10 and 100 ng/mL) | N/A | ↑ proliferation ↑ extracellular matrix formation ↑ migration | N/A | [63] |
| Dental | Human dental pulp cells | Incubation with irisin (5, 10, 20, 40 µM) | N/A | ↑ differentiation ↑ mineralization ↑ angiogenesis | MAPK, AKT | [65] |
| Periodontitis | Human patients | Periodontitis assessment | Salivary irisin, ELISA | ↑ salivary irisin in periodontitis | N/A | [74] |
| Periodontitis | Human patients | Periodontitis assessment | Salivary irisin, ELISA | ↑ salivary irisin in periodontitis | N/A | [75] |
| IR | IR-induced mice, HR simulated rat intestinal epithelial (IEC-6) cell line | Intravenous injection of irisin (10 and 100 ng/g) in vivo; irisin pretreatment (1, 10, 100 ng/mL) in vitro. | N/A | ↓ oxidative stress ↓ apoptosis ↓ inflammatory markers | Nrf2, Bcl-2/Bax, Caspase-3 | [76] |
| IR | IR-induced mice, HR simulated human colon carcinoma (Caco-2) cell line | Intravenous injection of irisin (250 μg/kg) in vivo; irisin treatment after HR (10 nmol/L) in vitro | Western blot | ↑ gut barrier function ↓ oxidative stress | αVβ5-AMPK-UCP2 | [23] |
| IR, AP | AP-induced mice | Intraperitoneal injection of irisin (50 and 250 μg/kg) | N/A | ↓ intestinal damage ↓ apoptosis ↓ oxidative stress | N/A | [77] |
| Disease | Organism/Model/Cell Line | Methods/Intervention | Irisin Detection Method | Findings/Effects of Irisin | Ref. |
|---|---|---|---|---|---|
| Colon cancer, esophageal cancer | Human and mouse colon (HT29, MCA38) and esophageal (OE13, OE33) cell lines | Incubation with irisin (5, 10, 50, and 100 nM) | N/A | No effects on cell proliferation or malignant potential | [84] |
| Various GI cancers | Human patients | Collection and histopathological evaluation of cancer tissues | Immunohistochemical staining | ↓ irisin in GI cancer tissues | [85] |
| Gastric cancer | Mice | Carcinogen administration | Serum irisin, ELISA, Real-time PCR | No irisin/FNDC5 gene expression in gastric tissue, ↑ irisin/FNDC5 gene expression in fat tissues in the cancer group | [92] |
| Gastric cancer | Human patients | Blood sample collection and analysis | Serum irisin, ELISA | ↑ serum irisin in cancer patients | [93] |
| Gastric and colorectal cancer | Human patients | Blood and tissue sample collection and analysis | Multiplex technology with the Magpix instrument | ↑ irisin in tumor tissue of patients with cancer cachexia | [94] |
| Gastric and colorectal cancer | Human patients | Blood sample collection and analysis | Serum irisin, ELISA | No correlation between irisin and oxidative stress, ↓ serum irisin in cancer patients | [90] |
| Colorectal cancer | Human patients | Blood and tissue sample collection and analysis | Serum irisin, ELISA | ↓ serum irisin in cancer patients | [88] |
| Colorectal Cancer | Human patients, human intestinal (CCD-18Co) and intestinal cancer (CaCo-2, LoVo, HT-29) cell lines | Histopathological evaluation of colorectal samples, in vitro cell culture | Immunohistochemical staining, immunofluorescence, western blot | ↑ irisin in cancer cells ↑ irisin in initial colon cancer ↓ irisin in more advanced stages | [87] |
| Colorectal cancer | Human patients | Tissue sample collection and analysis | Immunohistochemical staining | ↑ irisin in initial cancer, ↓ irisin in more advanced stages | [91] |
| Colorectal cancer | Human patients | Blood sample collection and analysis | Serum irisin, ELISA | ↓ serum irisin in cancer patients | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinkas, M.; Brzozowski, T. The Role of the Myokine Irisin in the Protection and Carcinogenesis of the Gastrointestinal Tract. Antioxidants 2024, 13, 413. https://doi.org/10.3390/antiox13040413
Pinkas M, Brzozowski T. The Role of the Myokine Irisin in the Protection and Carcinogenesis of the Gastrointestinal Tract. Antioxidants. 2024; 13(4):413. https://doi.org/10.3390/antiox13040413
Chicago/Turabian StylePinkas, Monika, and Tomasz Brzozowski. 2024. "The Role of the Myokine Irisin in the Protection and Carcinogenesis of the Gastrointestinal Tract" Antioxidants 13, no. 4: 413. https://doi.org/10.3390/antiox13040413
APA StylePinkas, M., & Brzozowski, T. (2024). The Role of the Myokine Irisin in the Protection and Carcinogenesis of the Gastrointestinal Tract. Antioxidants, 13(4), 413. https://doi.org/10.3390/antiox13040413








