Effects of Nitrite Stress on the Antioxidant, Immunity, Energy Metabolism, and Microbial Community Status in the Intestine of Litopenaeus vannamei
Abstract
1. Introduction
2. Materials and Methods
2.1. Temporary Rearing of the Shrimp
2.2. Nitrite Stress Exposure and Sampling
2.3. Histomorphological Analysis
2.4. Gene Expression Analysis
2.5. Intestinal Microbial Community Analysis
2.6. Statistical Analysis
3. Results
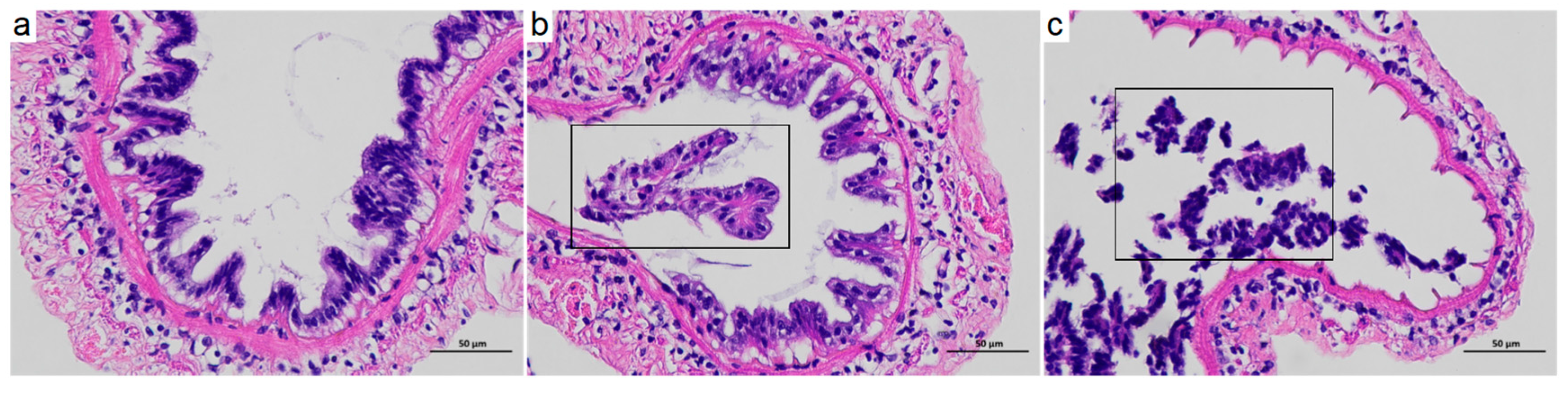
3.1. Intestinal Histomorphological Changes
3.2. Antioxidant and ER Stress Responses
3.3. Changes in the Expression of Physiological-Related Genes
3.4. Changes in the Expression of Energy Metabolism-Related Genes
3.4.1. Carbohydrate Metabolism
3.4.2. Lipid Metabolism
3.4.3. Tricarboxylic Acid (TCA) Cycle
3.4.4. Electron Transfer Chain
3.5. Changes in Intestinal Microbial Community
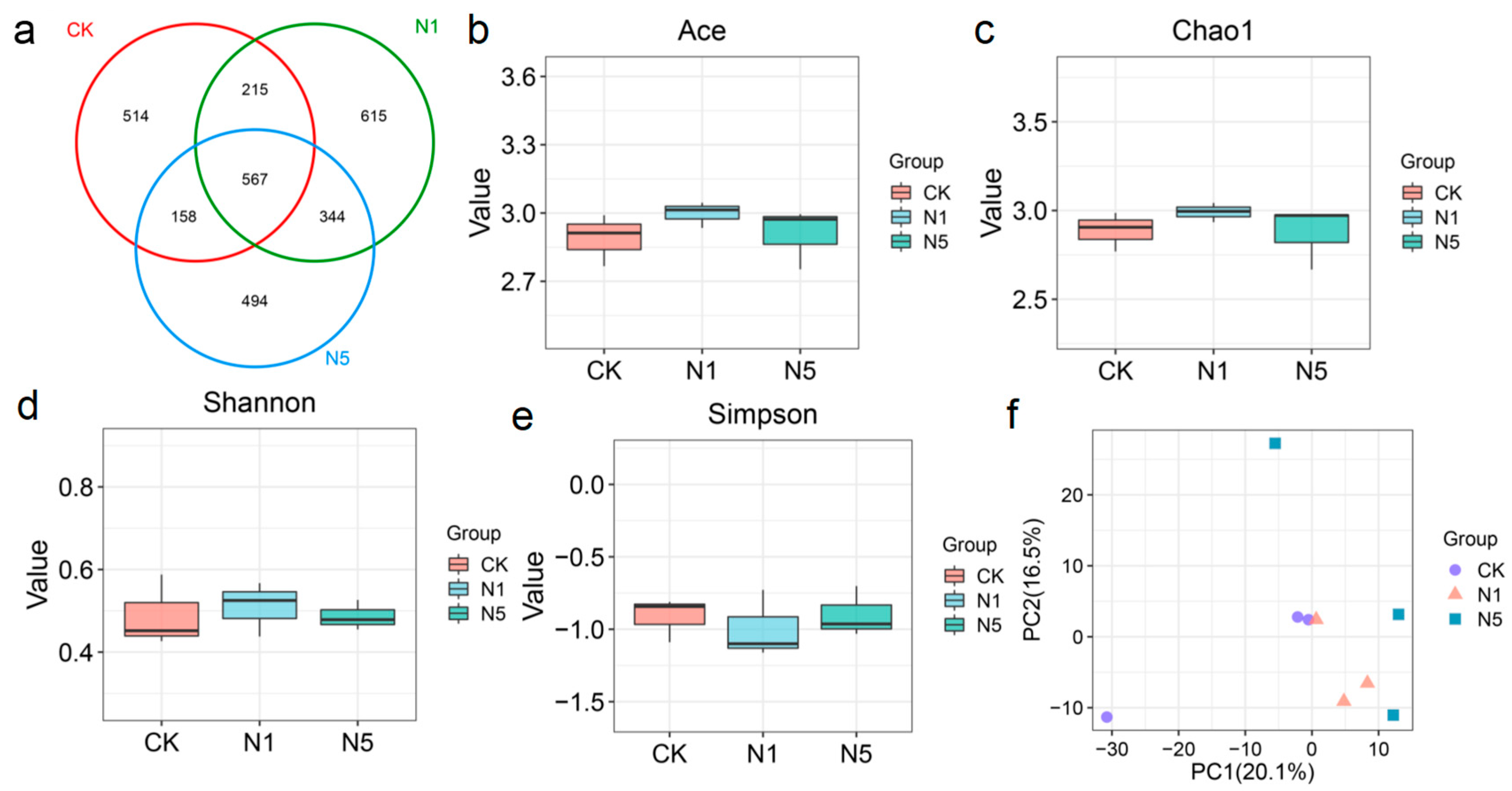
3.5.1. Intestinal Microbial Diversity Alterations
3.5.2. Changes in Intestinal Bacterial Composition
3.5.3. Changes in Intestinal Microbial Phenotypes
3.5.4. Changes in Intestinal Microbial Metabolic Function
4. Discussion
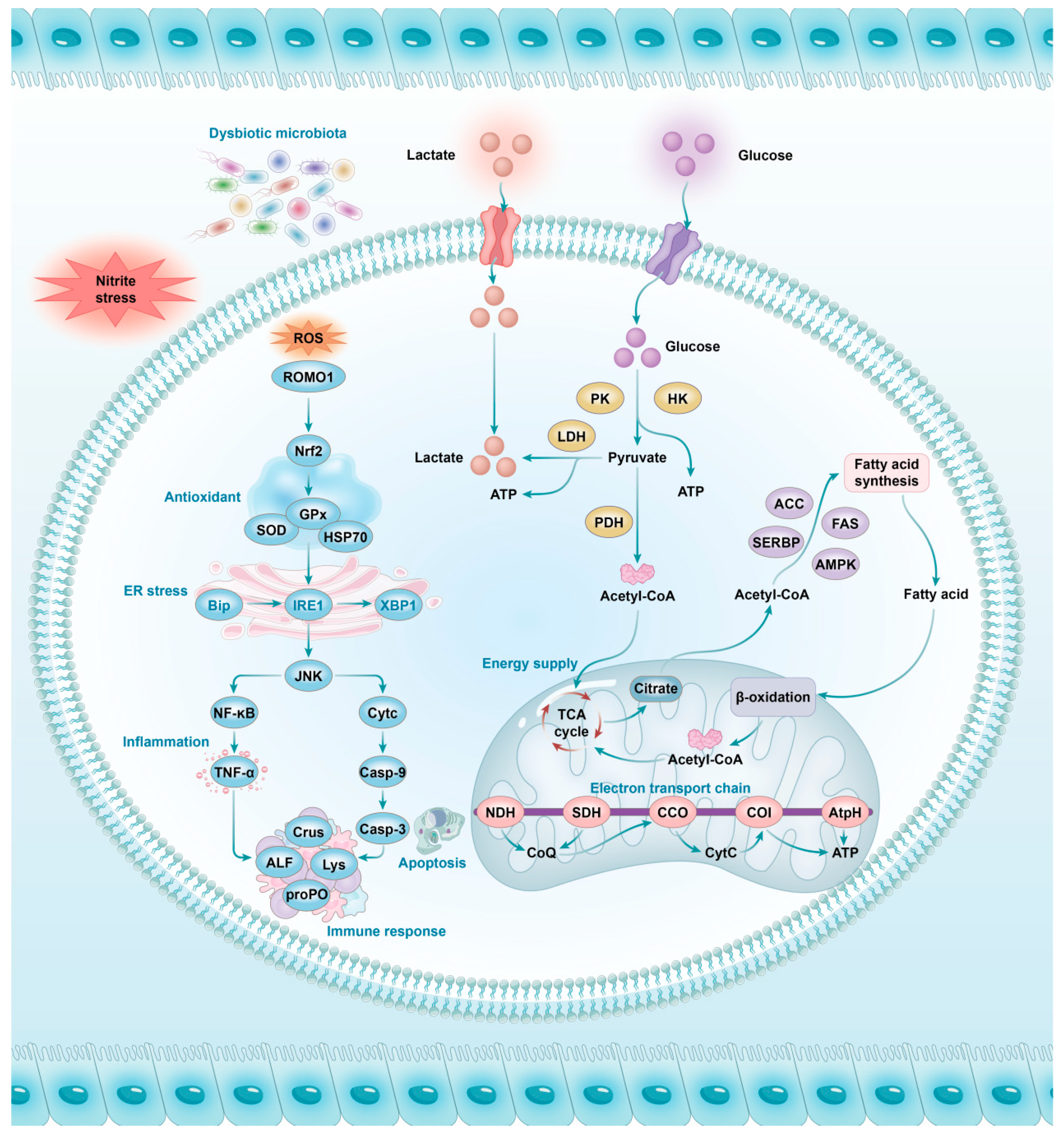
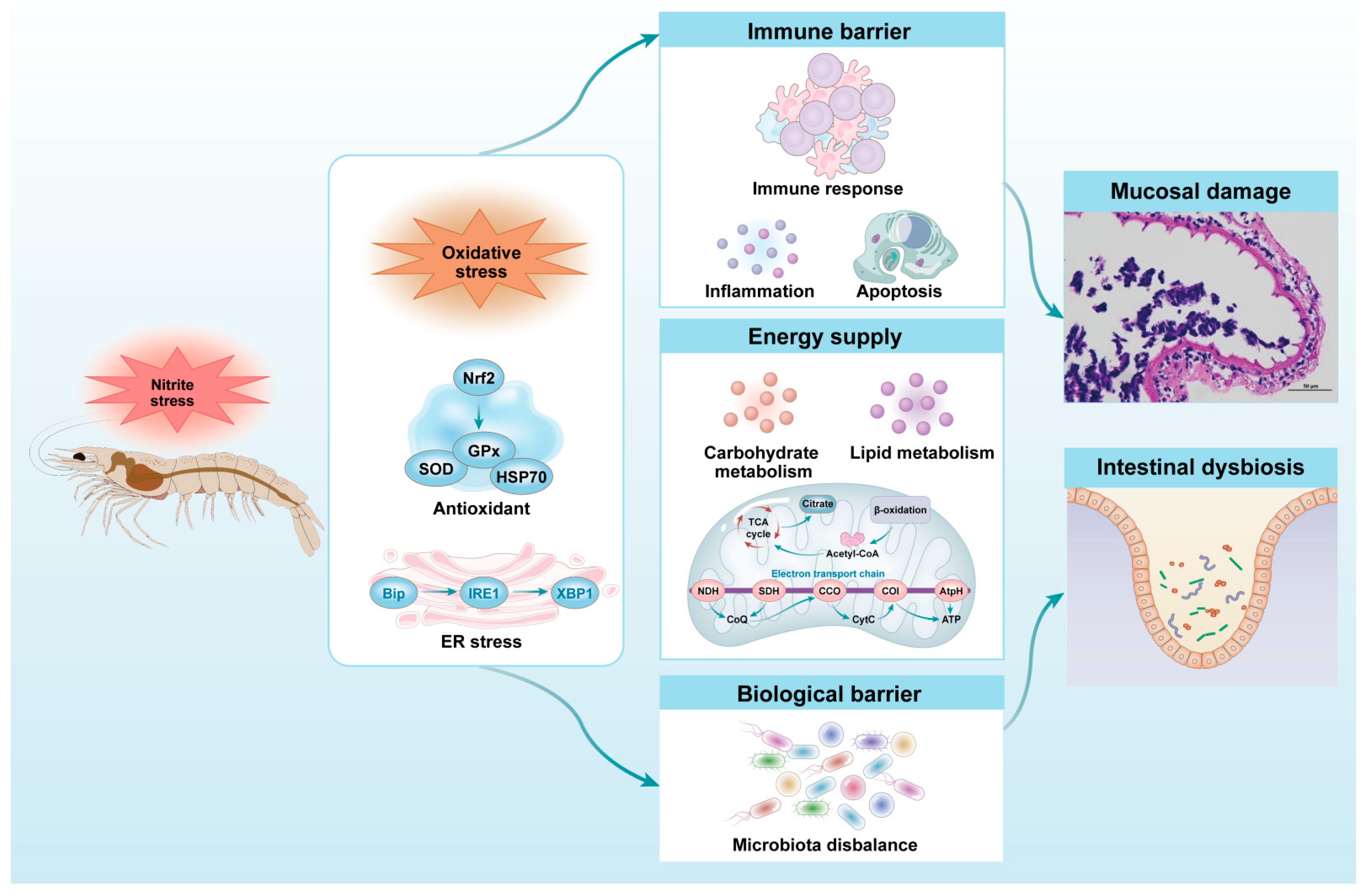
4.1. Intestinal Stress Response of Shrimps to Nitrite Stress
4.2. Intestinal Immune Response of Shrimps to Nitrite Stress
4.3. Intestinal Energy Metabolism Response of Shrimps to Nitrite Stress
4.4. Intestinal Microbiota Response of Shrimps to Nitrite Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, N.; Han, J.; Zhou, Z.; Ma, Z.; Deng, H.; Wan, L.; Xie, K. Environmental factors in shrimp culture ponds related with diseases outbreaks. Fish. Sci. 2004, 23, 5–8. [Google Scholar]
- Yu, Y.B.; Choi, J.H.; Kang, J.C.; Kim, H.J.; Kim, J.H. Shrimp bacterial and parasitic disease listed in the OIE: A review. Microb. Pathog. 2022, 166, 105545. [Google Scholar] [CrossRef] [PubMed]
- Ciji, A.; Akhtar, M.S. Nitrite implications and its management strategies in aquaculture: A review. Rev. Aquac. 2020, 12, 878–908. [Google Scholar] [CrossRef]
- Chen, J.C.; Chin, T.S.; Lee, C.K. Effects of ammonia and nitrite on larval development of the shrimp (Penaeus monodon). In The First Asian Fisheries Forum; MacLean, J.L., Dizon, L.B., Hosillos, L.V., Eds.; Asian Fisheries Society: Manila, Philippines, 1986; pp. 657–662. [Google Scholar]
- Tseng, I.T.; Chen, J.C. The immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus under nitrite stress. Fish Shellfish Immunol. 2022, 17, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xian, J.A.; Li, B.; Ye, C.X.; Wang, A.L.; Miao, Y.T.; Liao, S.A. Gene expression of apoptosis-related genes, stress protein and antioxidant enzymes in hemocytes of white shrimp Litopenaeus vannamei under nitrite stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2013, 157, 366–371. [Google Scholar] [CrossRef]
- Huang, M.; Xie, J.; Yu, Q.; Xu, C.; Zhou, L.; Qin, J.G.; Chen, L.; Li, E. Toxic effect of chronic nitrite exposure on growth and health in Pacific white shrimp Litopenaeus vannamei. Aquaculture 2020, 529, 735664. [Google Scholar] [CrossRef]
- Xiao, J.; Luo, S.S.; Du, J.H.; Liu, Q.Y.; Huang, Y.; Wang, W.F.; Chen, X.L.; Chen, X.H.; Liu, H.; Zhou, X.Y.; et al. Transcriptomic analysis of gills in nitrite-tolerant and -sensitive families of Litopenaeus vannamei. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 253, 109212. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Y.; Zhuo, H.; Li, J.; Fu, S.; Zhou, X.; Wu, G.; Guo, C.; Liu, J. Integrated histological, physiological, and transcriptome analysis reveals the post-exposure recovery mechanism of nitrite in Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2024, 281, 116673. [Google Scholar] [CrossRef]
- Yang, S.; Luo, J.; Huang, Y.; Yuan, Y.; Cai, S. Effect of sub-lethal ammonia and nitrite stress on autophagy and apoptosis in hepatopancreas of Pacific whiteleg shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2022, 130, 72–78. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Shieh, L.W.; Chen, J.C. Changes in hemolymph oxyhemocyanin, acid–base balance, and electrolytes in Marsupenaeus japonicus under combined ammonia and nitrite stress. Aquat. Toxicol. 2013, 130–131, 132–138. [Google Scholar] [CrossRef]
- Li, Z.; Ma, S.; Shan, H.; Wang, T.; Xiao, W. Responses of hemocyanin and energy metabolism to acute nitrite stress in juveniles of the shrimp Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2019, 186, 109753. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Castañeda, G.; Frías-Espericueta, M.G.; Vanegas-Pérez, R.C.; Chávez-Sánchez, M.C.; Páez-Osuna, F. Physiological changes in the hemolymph of juvenile shrimp Litopenaeus vannamei to sublethal nitrite and nitrate stress in low-salinity waters. Environ. Toxicol. Pharmacol. 2020, 80, 103472. [Google Scholar] [CrossRef] [PubMed]
- Biju, N.; Sathiyaraj, G.; Raj, M.; Shanmugam, V.; Baskaran, B.; Govindan, U.; Kumaresan, G.; Kasthuriraju, K.; Chellamma, T. High prevalence of Enterocytozoon hepatopenaei in shrimps Penaeus monodon and Litopenaeus vannamei sampled from slow growth ponds in India. Dis. Aquat. Org. 2016, 120, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.C.; Bass, D.; Stentiford, G.D.; van der Giezen, M. Understanding the role of the shrimp gut microbiome in health and disease. J. Invertebr. Pathol. 2021, 186, 107387. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, S.; Xiong, J.; Hou, D.; Zhou, R.; Xing, C.; Wei, D.; Deng, X.; Yu, L.; Wang, H.; et al. Microecological Koch’s postulates reveal that intestinal microbiota dysbiosis contributes to shrimp white feces syndrome. Microbiome 2020, 8, 1–13. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Swelum, A.A.; Ghanima, M.M.A.; Shukry, M.; Omar, A.A.; Taha, A.E.; Salem, H.M.; El-Tahan, A.M.; El-Tarabily, K.A.; El-Hack, M.E.A. Shrimp production, the most important diseases that threaten it, and the role of probiotics in confronting these diseases: A review. Res. Veter-Sci. 2022, 144, 126–140. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Q.; Wang, Y.; Zhang, J.; Xiong, D. Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish Shellfish Immunol. 2018, 78, 279–288. [Google Scholar] [CrossRef]
- Xiong, J.; Dai, W.; Qiu, Q.; Zhu, J.; Yang, W.; Li, C. Response of host-bacterial colonization in shrimp to developmental stage, environment and disease. Mol. Ecol. 2018, 27, 3686–3699. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, J.; Wang, Y.; Liu, Q.; Xiong, D. Nitrite stress disrupts the structural integrity and induces oxidative stress response in the intestines of Pacific white shrimp Litopenaeus vannamei. J. Exp. Zoöl. Part A Ecol. Integr. Physiol. 2018, 329, 43–50. [Google Scholar] [CrossRef]
- Hou, D.; Li, H.; Wang, S.; Weng, S.; He, J. Nitrite nitrogen stress disrupts the intestine bacterial community by altering host-community interactions in shrimp. Sci. Total Environ. 2024, 925, 171536. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- He, H.H.; Chi, Y.M.; Yuan, K.; Li, X.Y.; Weng, S.P.; He, J.G.; Chen, Y.H. Functional characterization of a reactive oxygen species modulator 1 gene in Litopenaeus vannamei. Fish Shellfish Immunol. 2017, 70, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Lee, J.H.; Jegal, K.H.; Cho, I.J.; Kim, Y.W.; Kim, S.C. Oxyresveratrol abrogates oxidative stress by activating ERK–Nrf2 pathway in the liver. Chem. Interact. 2016, 245, 110–121. [Google Scholar] [CrossRef]
- Shi, J.; Fu, M.; Zhao, C.; Zhou, F.; Yang, Q.; Qiu, L. Characterization and function analysis of Hsp60 and Hsp10 under different acute stresses in black tiger shrimp, Penaeus monodon. Cell Stress Chaperon 2016, 21, 295–312. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Zheng, J.; Mao, Y.; Su, Y.; Wang, J. Effects of nitrite stress on mRNA expression of antioxidant enzymes, immune-related genes and apoptosis-related proteins in Marsupenaeus japonicus. Fish Shellfish Immunol. 2016, 58, 239–252. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Ganz, T. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 1999, 11, 23–27. [Google Scholar] [CrossRef]
- Sangsuriya, P.; Charoensapsri, W.; Chomwong, S.; Senapin, S.; Tassanakajon, A.; Amparyup, P. A shrimp pacifastin light chain-like inhibitor: Molecular identification and role in the control of the prophenoloxidase system. Dev. Comp. Immunol. 2016, 54, 32–45. [Google Scholar] [CrossRef]
- Wan, F.; Lenardo, M.J. The nuclear signaling of NF-κB: Current knowledge, new insights, and future perspectives. Cell Res. 2010, 20, 24–33. [Google Scholar] [CrossRef]
- Ammendrup, A.; Maillard, A.; Nielsen, K.; Andersen, N.A.; Serup, P.; Madsen, O.D.; Mandrup-Poulsen, T.; Bonny, C. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes 2000, 49, 1468–1476. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Su, Y.L.; Ma, H.L.; Deng, Y.Q.; Feng, J.; Chen, X.L.; Jie, Y.K.; Guo, Z.X. Effect of nitrite exposure on oxidative stress, DNA damage and apoptosis in mud crab (Scylla paramamosain). Chemosphere 2020, 239, 124668. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, C.Y.T.; Kong, A.N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharmacal Res. 2005, 28, 249–268. [Google Scholar] [CrossRef]
- Nan, Y.; Xiao, M.; Duan, Y.; Yang, Y. Toxicity of ammonia stress on the physiological homeostasis in the gills of Litopenaeus vannamei under seawater and low-salinity conditions. Biology 2024, 13, 281. [Google Scholar] [CrossRef]
- Shan, H.; Geng, Z.; Ma, S.; Wang, T. Comparative study of the key enzymes and biochemical substances involved in the energy metabolism of Pacific white shrimp, Litopenaeus vannamei, with different ammonia-N tolerances. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 221, 73–81. [Google Scholar] [CrossRef]
- Shi, B.; Lu, J.; Hu, X.; Betancor, M.B.; Zhao, M.; Tocher, D.R.; Zhou, Q.; Jiao, L.; Xu, F.; Jin, M. Dietary copper improves growth and regulates energy generation by mediating lipolysis and autophagy in hepatopancreas of Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2021, 537, 736505. [Google Scholar] [CrossRef]
- Hardie, D.G.; Carling, D.; Carlson, M. The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 1998, 67, 821–855. [Google Scholar] [CrossRef] [PubMed]
- Eswarappa, S.M.; Fox, P.L. Citric acid cycle and the origin of MARS. Trends Biochem. Sci. 2013, 38, 222–228. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, S.; Kumar, J.; Barik, S.; Mazumder, S. Mitochondrial electron transport chain in macrophage reprogramming: Potential role in antibacterial immune response. Curr. Res. Immunol. 2024, 5, 100077. [Google Scholar] [CrossRef]
- Li, X.; Ringø, E.; Hoseinifar, S.H.; Lauzon, H.L.; Birkbeck, H.; Yang, D. The adherence and colonization of microorganisms in fish gastrointestinal tract. Rev. Aquac. 2019, 11, 603–618. [Google Scholar] [CrossRef]
- Chiquette, J.; Allison, M.; Rasmussen, M. Use of Prevotella bryantii 25A and a commercial probiotic during subacute acidosis challenge in midlactation dairy cows. J. Dairy Sci. 2012, 95, 5985–5995. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wang, Y.; Dong, H.; Ding, X.; Liu, Q.; Li, H.; Zhang, J.; Xiong, D. Changes in the intestine microbial, digestive, and immune-related genes of Litopenaeus vannamei in response to dietary probiotic Clostridium butyricum supplementation. Front. Microbiol. 2018, 9, 2191. [Google Scholar] [CrossRef] [PubMed]
- Heinken, A.; Khan, M.T.; Paglia, G.; Rodionov, D.A.; Harmsen, H.J.M.; Thiele, I. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J. Bacteriol. 2014, 196, 3289–3302. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gao, M.; Song, B.; Yan, S.; Zhao, Y.; Gong, L.; Liu, Y.; Lv, Z.; Guo, Y. Soya saponin fails to improve the antioxidation and immune function of laying hens with antibiotics treated. Poult. Sci. 2022, 101, 101921. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, W.; Yu, N.; Sun, J.; Yu, X.; Li, X.; Xing, Y.; Yan, D.; Ding, Q.; Xiu, Z.; et al. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J. Funct. Foods 2018, 46, 256–267. [Google Scholar] [CrossRef]
- Gu, X.; Sim, J.X.; Lee, W.L.; Cui, L.; Chan, Y.F.; Chang, E.D.; Teh, Y.E.; Zhang, A.N.; Armas, F.; Chandra, F.; et al. Gut Ruminococcaceae levels at baseline correlate with risk of antibiotic-associated diarrhea. iScience 2022, 25, 103644. [Google Scholar] [CrossRef]
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef]
- Mayo, B.; BelénFlórez, A.B. Lactic acid bacteria: Lactobacillus spp.: Lactobacillus plantarum. In Encyclopedia of Dairy Sciences, 3rd ed.; 2022; pp. 206–217. [Google Scholar]
- Wang, H.; Wang, C.; Tang, Y.; Sun, B.; Huang, J.; Song, X. Pseudoalteromonas probiotics as potential biocontrol agents improve the survival of Penaeus vannamei challenged with acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Aquaculture 2018, 494, 30–36. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, M.; Liu, Y.; Su, Y.; Xu, T.; Yu, M.; Zhang, X.H. Comparison of cultivable bacterial communities associated with Pacific white shrimp (Litopenaeus vannamei) larvae at different health statuses and growth stages. Aquaculture 2016, 451, 163–169. [Google Scholar] [CrossRef]
- Duan, Y.F.; Lu, Z.J.; Zeng, S.M.; Dan, X.M.; Mo, Z.M.; Zhang, J.S.; Li, Y.W. Integration of intestinal microbioata and transcriptomic and metabolomic responses reveals the toxic responses of Litopannaeus vannamei to microcystin-LR. Ecotox. Environ. Safe. 2021, 228, 113030. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Geng, Y.; Zheng, L.; Rehman, T.; Zhao, R.; Wang, K.; OuYang, P.; Chen, D.; Huang, X.; et al. Molecular serotyping and antimicrobial susceptibility of Streptococcus agalactiae isolated from fish in China. Aquaculture 2019, 510, 84–89. [Google Scholar] [CrossRef]
- Liu, F.; Liu, G.; Li, F. Characterization of two pathogenic Photobacterium strains isolated from Exopalaemon carinicauda causing mortality of shrimp. Aquaculture 2016, 464, 129–135. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Betancourt-Lozano, M.; Morales-Covarrubias, M.S. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in Northwestern Mexico. Appl. Environ. Microbiol. 2015, 81, 1689–1699. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Zhong, G.; Nan, Y.; Yang, Y.; Xiao, M.; Li, H. Effects of Nitrite Stress on the Antioxidant, Immunity, Energy Metabolism, and Microbial Community Status in the Intestine of Litopenaeus vannamei. Antioxidants 2024, 13, 1318. https://doi.org/10.3390/antiox13111318
Duan Y, Zhong G, Nan Y, Yang Y, Xiao M, Li H. Effects of Nitrite Stress on the Antioxidant, Immunity, Energy Metabolism, and Microbial Community Status in the Intestine of Litopenaeus vannamei. Antioxidants. 2024; 13(11):1318. https://doi.org/10.3390/antiox13111318
Chicago/Turabian StyleDuan, Yafei, Guowei Zhong, Yuxiu Nan, Yukai Yang, Meng Xiao, and Hua Li. 2024. "Effects of Nitrite Stress on the Antioxidant, Immunity, Energy Metabolism, and Microbial Community Status in the Intestine of Litopenaeus vannamei" Antioxidants 13, no. 11: 1318. https://doi.org/10.3390/antiox13111318
APA StyleDuan, Y., Zhong, G., Nan, Y., Yang, Y., Xiao, M., & Li, H. (2024). Effects of Nitrite Stress on the Antioxidant, Immunity, Energy Metabolism, and Microbial Community Status in the Intestine of Litopenaeus vannamei. Antioxidants, 13(11), 1318. https://doi.org/10.3390/antiox13111318






