Comparative Bioaccesibility Study of Cereal-Based Nutraceutical Ingredients Using INFOGEST Static, Semi-Dynamic and Dynamic In Vitro Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Sample Preparation
2.4. Digestion
2.4.1. Static Digestion Model
2.4.2. Semi-Dynamic Digestion Model
2.4.3. Dynamic Digestion Model
2.5. Extraction
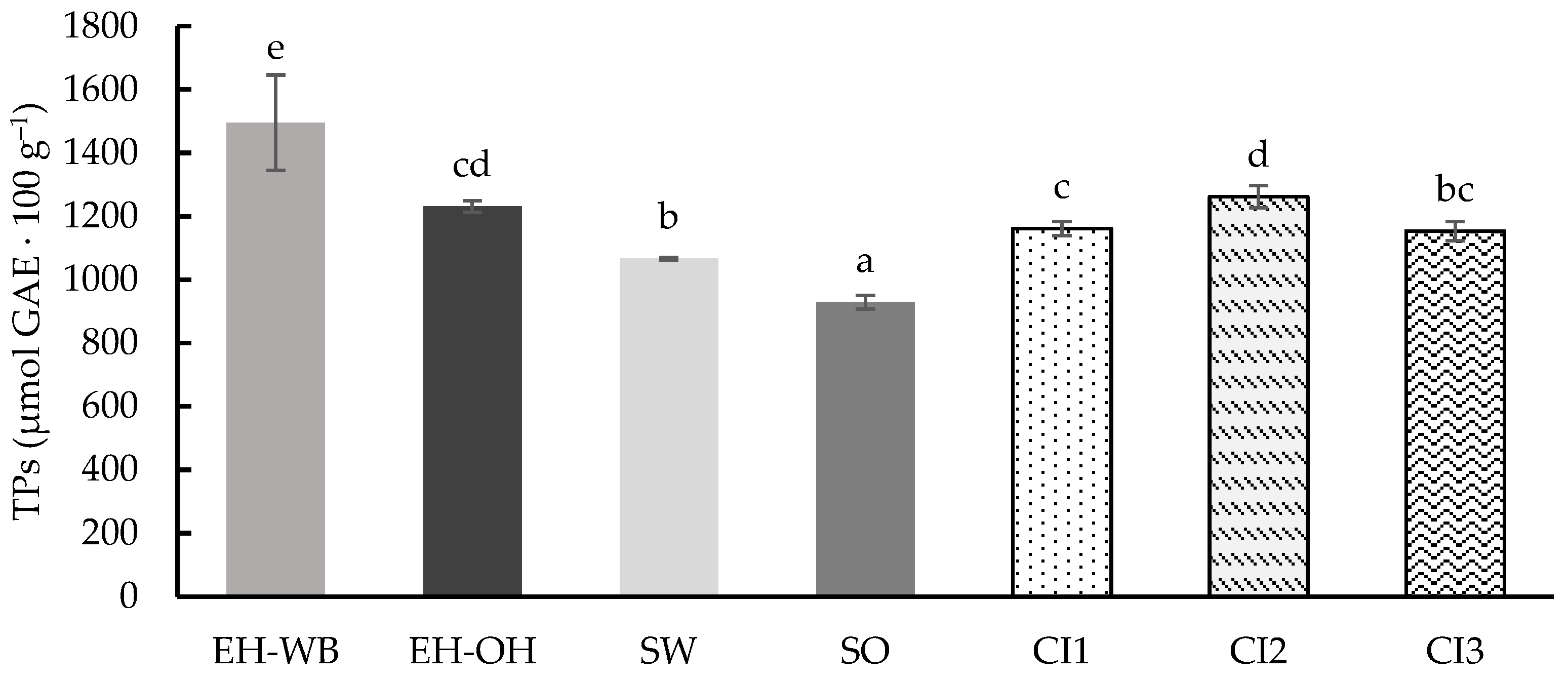
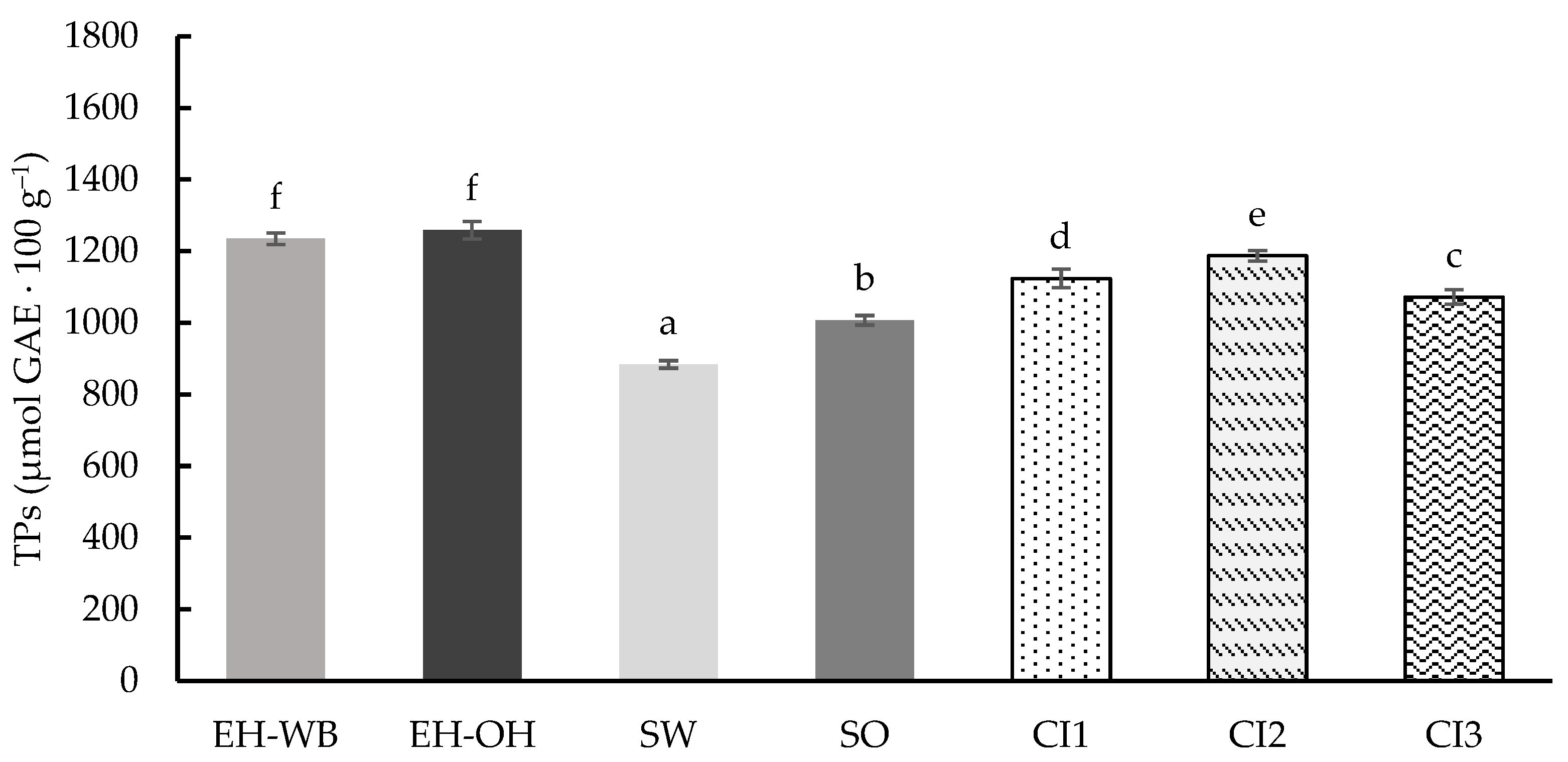
2.6. Total Phenolics Content (TPs)
2.7. Determination of Phenolic Compounds by HPLC
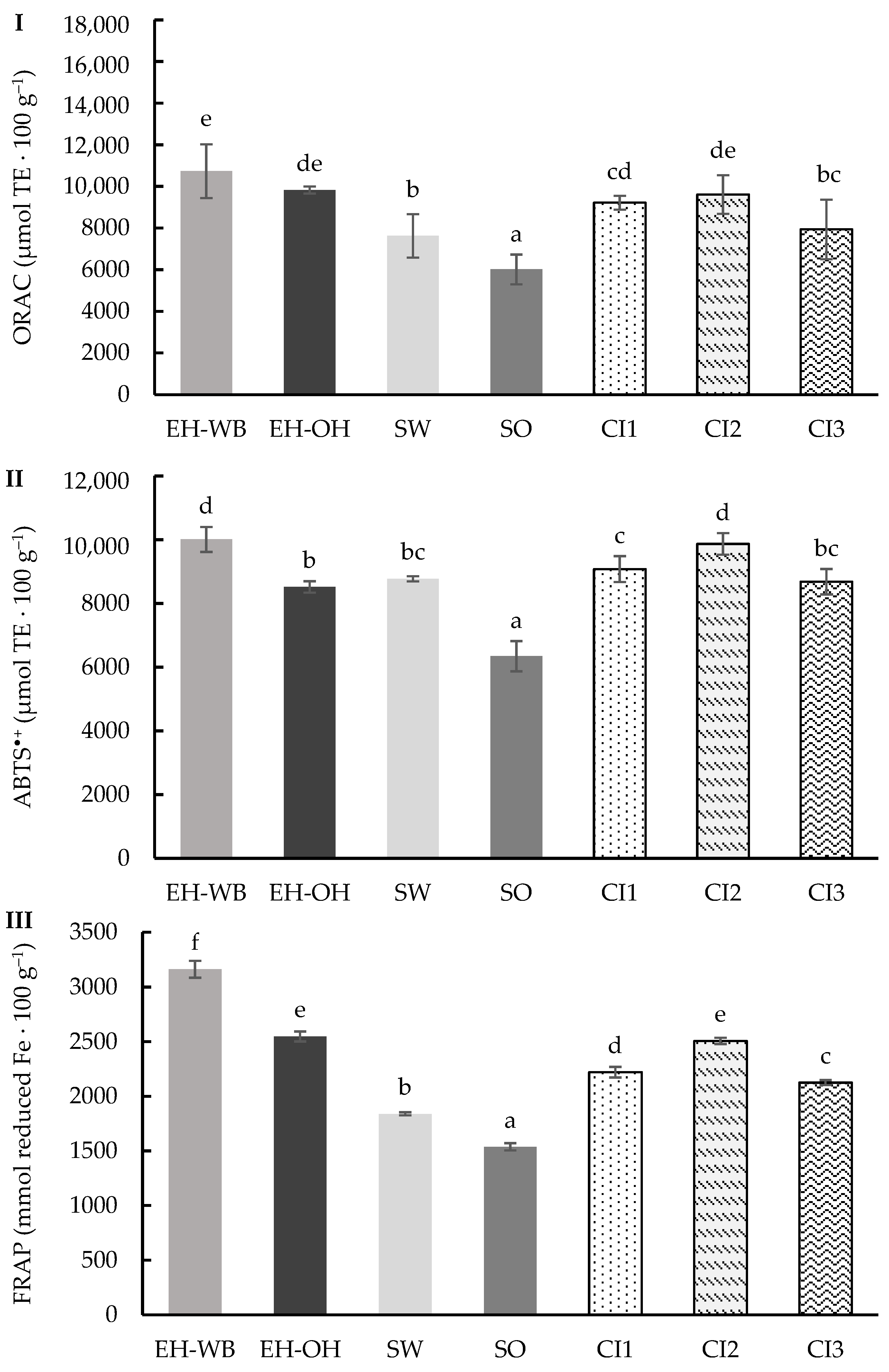
2.8. Total Antioxidant Capacity (TAC)
2.8.1. Oxygen Radical Absorbance Capacity (ORAC)
2.8.2. ABTS•+ Radical Cation Scavenging Activity
2.8.3. Ferric Reducing Antioxidant Power (FRAP)
2.9. Statistical Analysis
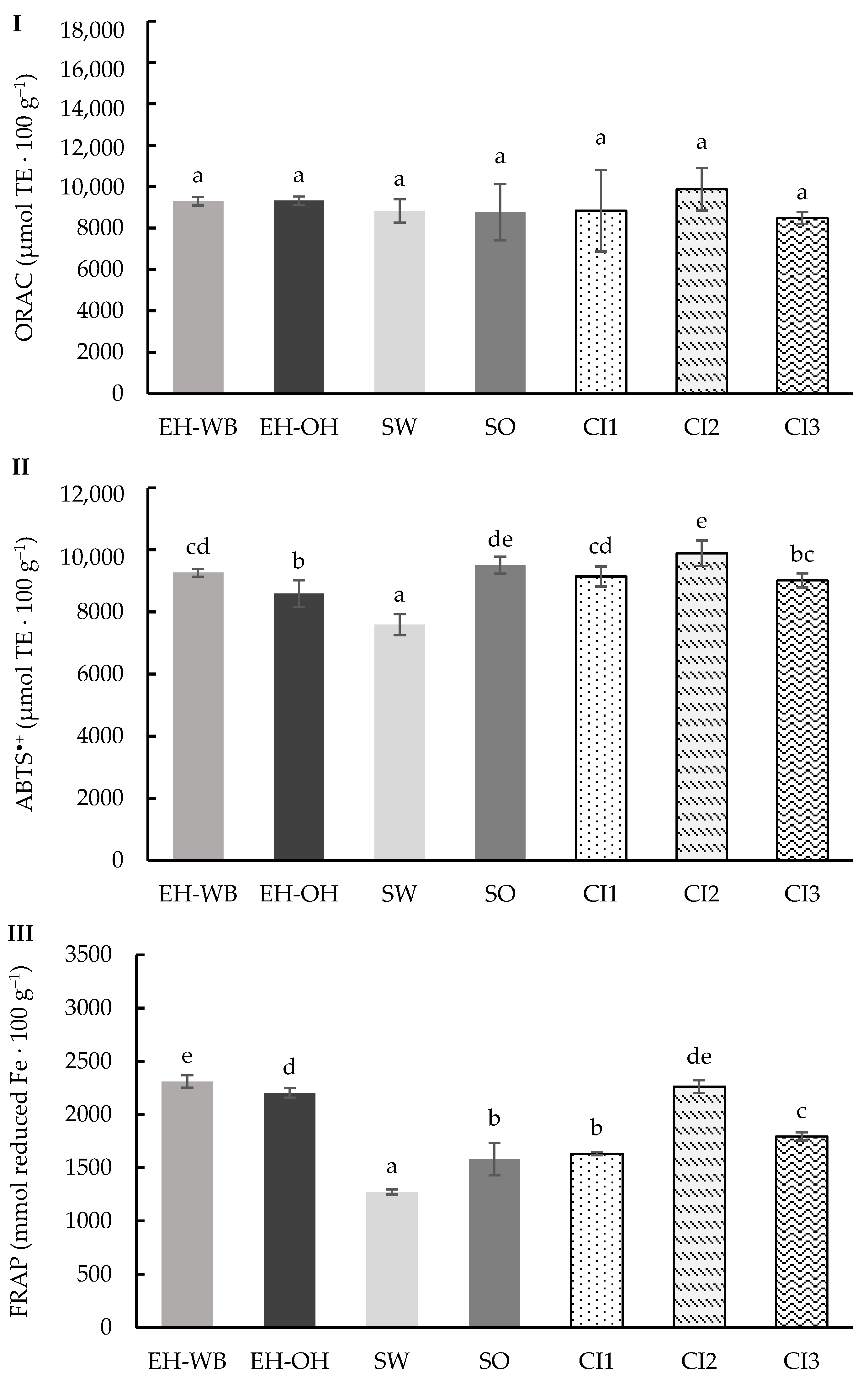
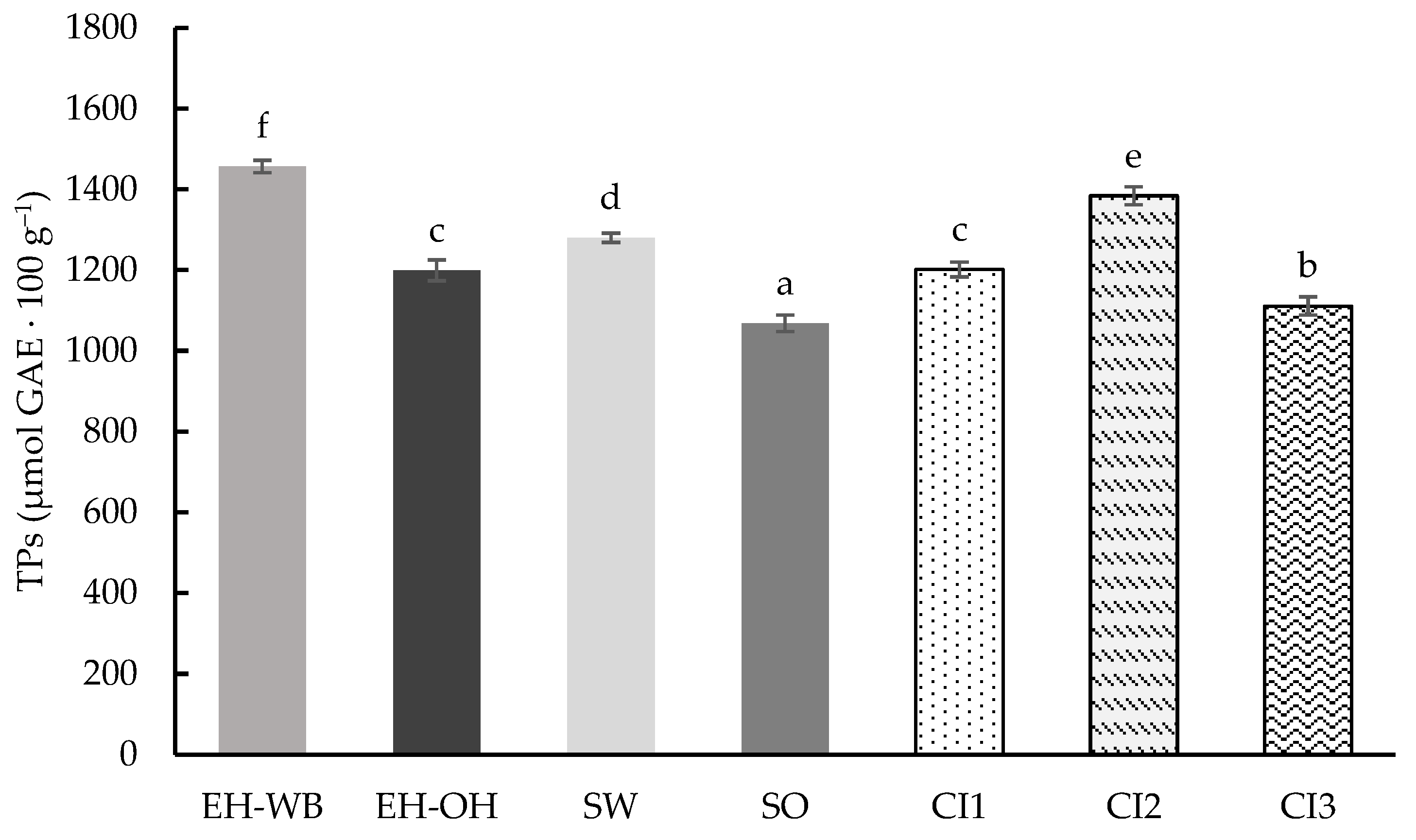
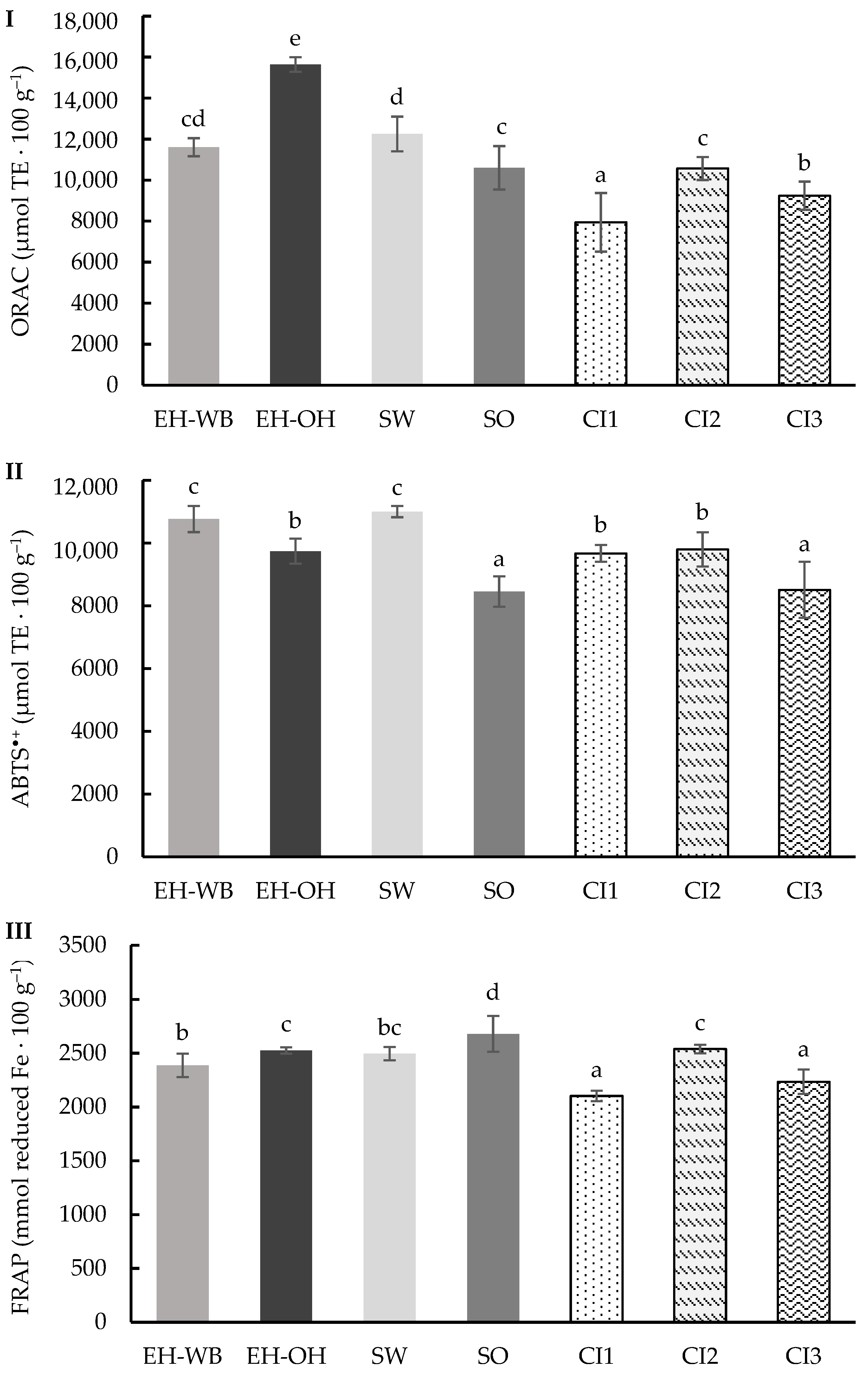
3. Results
3.1. Digestion in Static Model
3.2. Digestion in Semi-Dynamic Model
3.3. Digestion in Dynamic Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Björck, I.; Östman, E.; Kristensen, M.; Mateo Anson, N.; Price, R.K.; Haenen, G.R.M.M.; Havenaar, R.; Bach Knudsen, K.E.; Frid, A.; Mykkänen, H.; et al. Cereal Grains for Nutrition and Health Benefits: Overview of Results from in Vitro, Animal and Human Studies in the HEALTHGRAIN Project. Trends Food Sci. Technol. 2012, 25, 87–100. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole Grain Consumption and Risk of Cardiovascular Disease, Cancer, and All Cause and Cause Specific Mortality: Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. BMJ 2016, i2716. [Google Scholar] [CrossRef] [PubMed]
- Robert, W. Nutrient Composition and Nutritional Quality of Oats and Comparisons with Other Cereals. In Oats: Chemistry and Technology, 2nd ed.; Webster, H.F., Wood, P.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 95–108. [Google Scholar]
- Kaur, A.; Yadav, M.P.; Singh, B.; Bhinder, S.; Simon, S.; Singh, N. Isolation and Characterization of Arabinoxylans from Wheat Bran and Study of Their Contribution to Wheat Flour Dough Rheology. Carbohydr. Polym. 2019, 221, 166–173. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- de Punder, K.; Pruimboom, L. The Dietary Intake of Wheat and Other Cereal Grains and Their Role in Inflammation. Nutrients 2013, 5, 771–787. [Google Scholar] [CrossRef]
- Hui, S.; Liu, K.; Lang, H.; Liu, Y.; Wang, X.; Zhu, X.; Doucette, S.; Yi, L.; Mi, M. Comparative Effects of Different Whole Grains and Brans on Blood Lipid: A Network Meta-Analysis. Eur. J. Nutr. 2019, 58, 2779–2787. [Google Scholar] [CrossRef]
- Huang, W.; Tian, F.; Wang, H.; Wu, S.; Jin, W.; Shen, W.; Hu, Z.; Cai, Q.; Liu, G. Comparative Assessment of Extraction, Composition, and in Vitro Antioxidative Properties of Wheat Bran Polyphenols. Lebenson. Wiss. Technol. 2023, 180, 114706. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, B.; Huang, K.; Li, S.; Cao, H.; Guan, X. Bound Polyphenols of Oat Bran Released by Gut Microbiota Mitigate High Fat Diet-Induced Oxidative Stress and Strengthen the Gut Barrier via the Colonic ROS/Akt/Nrf2 Pathway. J. Agric. Food Chem. 2024, 72, 13099–13110. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary Factors and Low-Grade Inflammation in Relation to Overweight and Obesity. Br. J. Nutr. 2011, 106 (Suppl. 3), S1–S78. [Google Scholar] [CrossRef]
- Chethan Kumar, P.; Amutha, S.; Oberoi, H.S.; Kanchana, S.; Azeez, S.; Rupa, T.R. Germination Induced Changes in Bioactive Compounds and Nutritional Components of Millets. J. Food Sci. Technol. 2022, 59, 4244–4252. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pulido, I.J.; Rico, D.; Martinez-Villaluenga, C.; Pérez-Jiménez, J.; Luis, D.D.; Martín-Diana, A.B. Sprouting and Hydrolysis as Biotechnological Tools for Development of Nutraceutical Ingredients from Oat Grain and Hull. Foods 2022, 11, 2769. [Google Scholar] [CrossRef] [PubMed]
- Mencin, M.; Jamnik, P.; Mikulič Petkovšek, M.; Veberič, R.; Terpinc, P. Enzymatic Treatments of Raw, Germinated and Fermented Spelt (Triticum spelta L.) Seeds Improve the Accessibility and Antioxidant Activity of Their Phenolics. LWT Food Sci. Technol. 2022, 169, 114046. [Google Scholar] [CrossRef]
- Qie, X.; Cheng, Y.; Chen, Y.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; Li, W.; He, Z. In Vitro Phenolic Bioaccessibility of Coffee Beverages with Milk and Soy Subjected to Thermal Treatment and Protein–Phenolic Interactions. Food Chem. 2022, 375, 131644. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B. Nutracéuticos y Alimentos Funcionales Aliados Para La Salud: La Necesidad de Un Diseño “a Medida”. Nutr. Clin. Med. 2023, 17, 103–118. [Google Scholar]
- Stewart, R.J.C.; Morton, H.; Coad, J.; Pedley, K.C. In Vitro Digestion for Assessing Micronutrient Bioavailability: The Importance of Digestion Duration. Int. J. Food Sci. Nutr. 2019, 70, 71–77. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, H.; McClements, D.J. Application of Static in Vitro Digestion Models for Assessing the Bioaccessibility of Hydrophobic Bioactives: A Review. Trends Food Sci. Technol. 2022, 122, 314–327. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—an International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Egger, L.; Portmann, R.; Ménard, O.; Marze, S.; Minekus, M.; Le Feunteun, S.; Sarkar, A.; Grundy, M.M.-L.; Carrière, F.; et al. A Standardised Semi-Dynamic in Vitro Digestion Method Suitable for Food—an International Consensus. Food Funct. 2020, 11, 1702–1720. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tan, Y.; McClements, D.J. Applications of the INFOGEST in Vitro Digestion Model to Foods: A Review. Annu. Rev. Food Sci. Technol. 2023, 14, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pulido, I.J.; Martín-Diana, A.B.; Tomé-Sánchez, I.; de Luis, D.; Martínez-Villaluenga, C.; Rico, D. Boosting Synergistic Antioxidant and Anti-Inflammatory Properties Blending Cereal-Based Nutraceuticals Produced Using Sprouting and Hydrolysis Tools. Foods 2024, 13, 1868. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Siddeeg, A.; AlKehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; Al-Farga, A.M. Mode of Action and Determination of Antioxidant Activity in the Dietary Sources: An Overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644. [Google Scholar] [CrossRef]
- Jiménez-Pulido, I.J.; Rico, D.; De Luis, D.; Martín-Diana, A.B. Combined Strategy Using High Hydrostatic Pressure, Temperature and Enzymatic Hydrolysis for Development of Fibre-Rich Ingredients from Oat and Wheat by-Products. Foods 2024, 13, 378. [Google Scholar] [CrossRef]
- Engert, N.; John, A.; Henning, W.; Honermeier, B. Effect of Sprouting on the Concentration of Phenolic Acids and Antioxidative Capacity in Wheat Cultivars (Triticum aestivum ssp. Aestivum L.) in Dependency of Nitrogen Fertilization. J. Appl. Bot. Food Qual. 2011, 84, 111–118. [Google Scholar]
- Nguyen, T.H.D.; Nguyen, L.L.P.; Baranyai, L. Influence of Sprouting on Phenolic Acids, Carotenoids, and Antioxidant Activity of Millet Varieties. J. Agric. Food Res. 2023, 14, 100810. [Google Scholar] [CrossRef]
- Aborus, N.E.; Šaponjac, V.T.; Čanadanović-Brunet, J.; Ćetković, G.; Hidalgo, A.; Vulić, J.; Šeregelj, V. Sprouted and Freeze-dried Wheat and Oat Seeds—Phytochemical Profile and in Vitro Biological Activities. Chem. Biodivers. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, R.F.; de Lima Pena, F.; Fabiano, G.A.; de Oliveira Lopes, J.; Ponte, L.G.S.; da Cunha, D.T.; Bezerra, R.M.N.; Antunes, A.E.C. Changes in Phenolic Compound and Antioxidant Activity of Germinated Broccoli, Wheat, and Lentils during Simulated Gastrointestinal Digestion. Plant Foods Hum. Nutr. 2022, 77, 233–240. [Google Scholar] [CrossRef]
- Zhang, L.; García-Pérez, P.; Martinelli, E.; Giuberti, G.; Trevisan, M.; Lucini, L. Different Fractions from Wheat Flour Provide Distinctive Phenolic Profiles and Different Bioaccessibility of Polyphenols Following in Vitro Digestion. Food Chem. 2023, 404, 134540. [Google Scholar] [CrossRef]
- Zhang, Q.; Xing, B.; Sun, M.; Zhou, B.; Ren, G.; Qin, P. Changes in Bio-Accessibility, Polyphenol Profile and Antioxidants of Quinoa and Djulis Sprouts during in Vitro Simulated Gastrointestinal Digestion. Food Sci. Nutr. 2020, 8, 4232–4241. [Google Scholar] [CrossRef]
- Ed Nignpense, B.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Effect of Gastrointestinal Digestion on the Stability, Antioxidant Activity, and Caco-2 Cellular Transport of Pigmented Grain Polyphenols. J. Food Sci. 2024, 89, 2701–2715. [Google Scholar] [CrossRef]
- Martínez-Medina, G.A.; Carranza-Méndez, R.; Amaya-Chantaca, D.P.; Ilyna, A.; Gaviria-Acosta, E.; Hoyos-Concha, J.L.; Chávez-González, M.L.; Govea-Salas, M.; Prado-Barragán, L.A.; Aguilar-González, C.N. Bioactive Peptides from Food Industrial Wastes. In Bioactive Peptides: Production, Bioavailability, Health Potential and Regulatory Issues, 1st ed.; Onuh, J.O., Selvamuthukumaran, M., Pathak, Y.V., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 169–203. ISBN 9781003052777. [Google Scholar]
- Gong, E.S.; Gao, N.; Li, T.; Chen, H.; Wang, Y.; Si, X.; Tian, J.; Shu, C.; Luo, S.; Zhang, J.; et al. Effect of in Vitro Digestion on Phytochemical Profiles and Cellular Antioxidant Activity of Whole Grains. J. Agric. Food Chem. 2019, 67, 7016–7024. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H.; Brennan, M.; Guan, W.; Liu, J.; Wang, M.; Wen, X.; He, J.; Brennan, C. In Vitro Gastric Digestion Antioxidant and Cellular Radical Scavenging Activities of Wheat-Shiitake Noodles. Food Chem. 2020, 330, 127214. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, L.; Qiu, J.; Li, Z.; Wang, L. Milling of Wheat Bran: Influence on Digestibility, Hydrolysis and Nutritional Properties of Bran Protein during in Vitro Digestion. Food Chem. 2023, 404, 134559. [Google Scholar] [CrossRef]
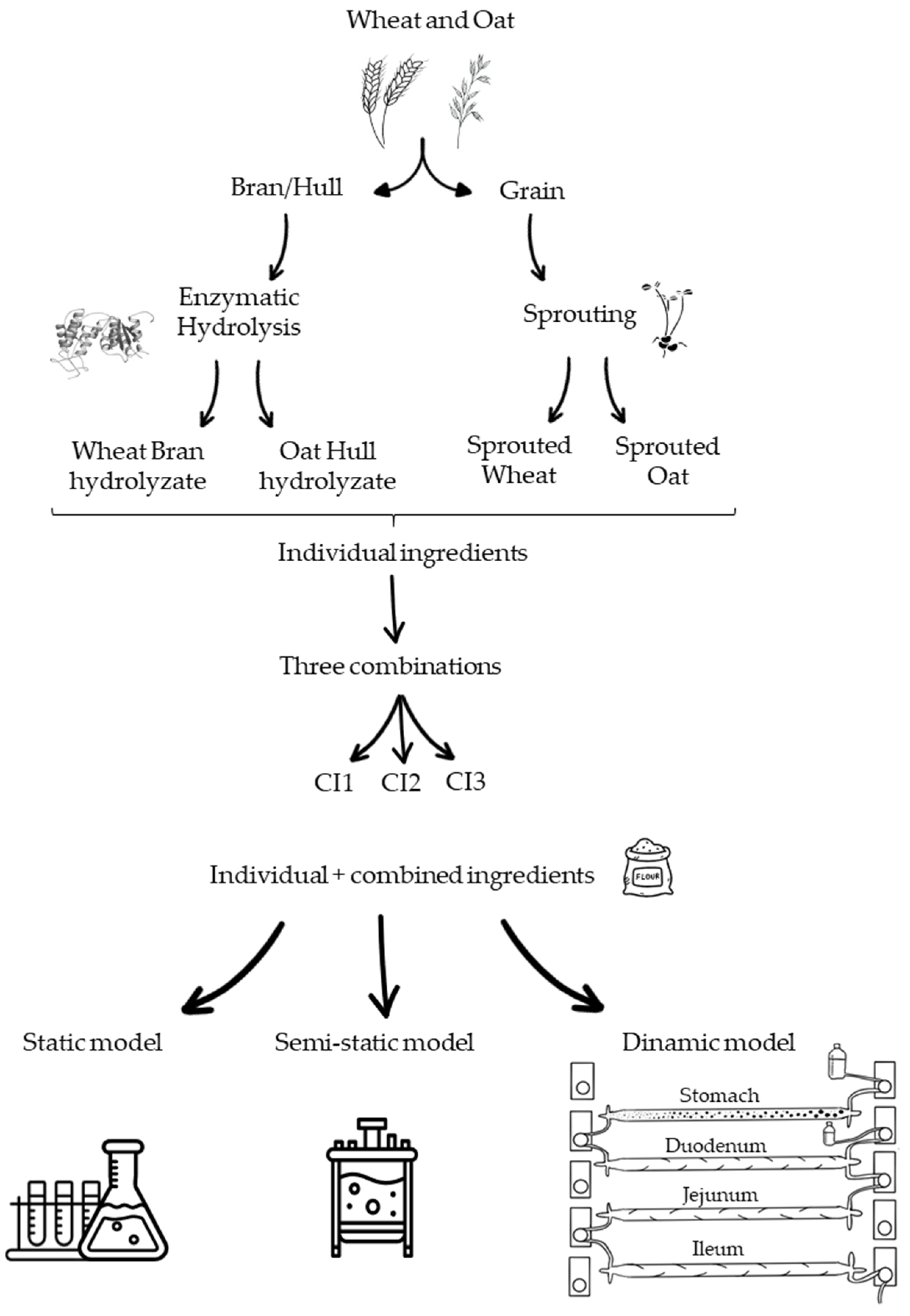
| Combined Ingredient 1 (CI1) | Combined Ingredient 2 (CI2) | Combined Ingredient 3 (CI3) | |
|---|---|---|---|
| EH-WB | 1 | 2 | 1 |
| EH-OH | 1 | 2 | 1 |
| SW | 1 | 1 | 2 |
| SO | 1 | 1 | 2 |
| Cumaric Acid | Ferulic Acid | |
|---|---|---|
| EH-WB | 0.33 ± 0.07 b | 0.16 ± 0.06 a |
| EH-OH | 0.15 ± 0.00 a | <LOD |
| SW | 1.08 ± 0.01 e | 3.26 ± 0.05 e |
| SO | <LOD | <LOD |
| CI1 | 0.63 ± 0.07 c | 1.62 ± 0.05 c |
| CI2 | 0.86 ± 0.09 d | 1.93 ± 0.17 d |
| CI3 | 0.58 ± 0.01 c | 0.60 ± 0.05 b |
| Cumaric Acid | Ferulic Acid | |
|---|---|---|
| EH-WB | 0.02 ± 0.01 a | 0.07 ± 0.03 a |
| EH-OH | <LOD | <LOD |
| SW | 1.24 ± 0.16 d | 0.87 ± 0.02 d |
| SO | 0.11 ± 0.06 b | <LOD |
| CI1 | 0.73 ± 0.05 c | 0.20 ± 0.04 b |
| CI2 | <LOD | 0.27 ± 0.02 c |
| CI3 | <LOD | <LOD |
| Cumaric Acid | Ferulic Acid | |
|---|---|---|
| EH-WB | 3.36 ± 0.02 e | 21.84 ± 0.00 e |
| EH-OH | 0.80 ± 0.47 c | 3.74 ± 0.46 b |
| SW | 0.01 ± 0.00 a | 28.57 ± 0.04 f |
| SO | 0.24 ± 0.00 b | 1.44 ± 0.00 a |
| CI1 | 0.77 ± 0.04 c | 18.39 ± 0.17 d |
| CI2 | 1.39 ± 0.02 d | 22.10 ± 0.35 e |
| CI3 | 0.26 ± 0.07 b | 11.97 ± 0.20 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Pulido, I.J.; Martín-Diana, A.B.; Luis, D.d.; Rico, D. Comparative Bioaccesibility Study of Cereal-Based Nutraceutical Ingredients Using INFOGEST Static, Semi-Dynamic and Dynamic In Vitro Gastrointestinal Digestion. Antioxidants 2024, 13, 1244. https://doi.org/10.3390/antiox13101244
Jiménez-Pulido IJ, Martín-Diana AB, Luis Dd, Rico D. Comparative Bioaccesibility Study of Cereal-Based Nutraceutical Ingredients Using INFOGEST Static, Semi-Dynamic and Dynamic In Vitro Gastrointestinal Digestion. Antioxidants. 2024; 13(10):1244. https://doi.org/10.3390/antiox13101244
Chicago/Turabian StyleJiménez-Pulido, Iván Jesús, Ana Belén Martín-Diana, Daniel de Luis, and Daniel Rico. 2024. "Comparative Bioaccesibility Study of Cereal-Based Nutraceutical Ingredients Using INFOGEST Static, Semi-Dynamic and Dynamic In Vitro Gastrointestinal Digestion" Antioxidants 13, no. 10: 1244. https://doi.org/10.3390/antiox13101244
APA StyleJiménez-Pulido, I. J., Martín-Diana, A. B., Luis, D. d., & Rico, D. (2024). Comparative Bioaccesibility Study of Cereal-Based Nutraceutical Ingredients Using INFOGEST Static, Semi-Dynamic and Dynamic In Vitro Gastrointestinal Digestion. Antioxidants, 13(10), 1244. https://doi.org/10.3390/antiox13101244













