Baicalein Ameliorates Insulin Resistance of HFD/STZ Mice Through Activating PI3K/AKT Signal Pathway of Liver and Skeletal Muscle in a GLP-1R-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Cell Culture and Treatment
2.3. Glucose Uptake Assay
2.4. Glucose Consumption Assay
2.5. Ca2+ Mobility Determination
2.6. Small Interfering RNA (siRNA) and Transfection
2.7. In Vivo Pharmacological Experiment
2.8. Intraperitoneal Glucose Tolerance Test (IPGTT) and Insulin Tolerance Test (ITT)
2.9. RNA Isolation, cDNA Synthesis and Real Time PCR
2.10. Quantifications of Plasm Insulin and Homeostasis Model Assessment Insulin Resistance (HOMA-IR) in Mice
2.11. Glycogen Content Determination Assay
2.12. Western Blotting Analysis
2.13. Immunofluorescence
2.14. Network Pharmacology
2.14.1. Gene Collection of Insulin Resistance
2.14.2. Prediction and Identification of Potential Targets of Baicalein
2.14.3. Gene Ontology (GO) Functional Enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
2.14.4. Key Target Discovery Based on Protein–Protein Interaction (PPI) Network Analysis
2.15. Statistical Analyses
3. Results
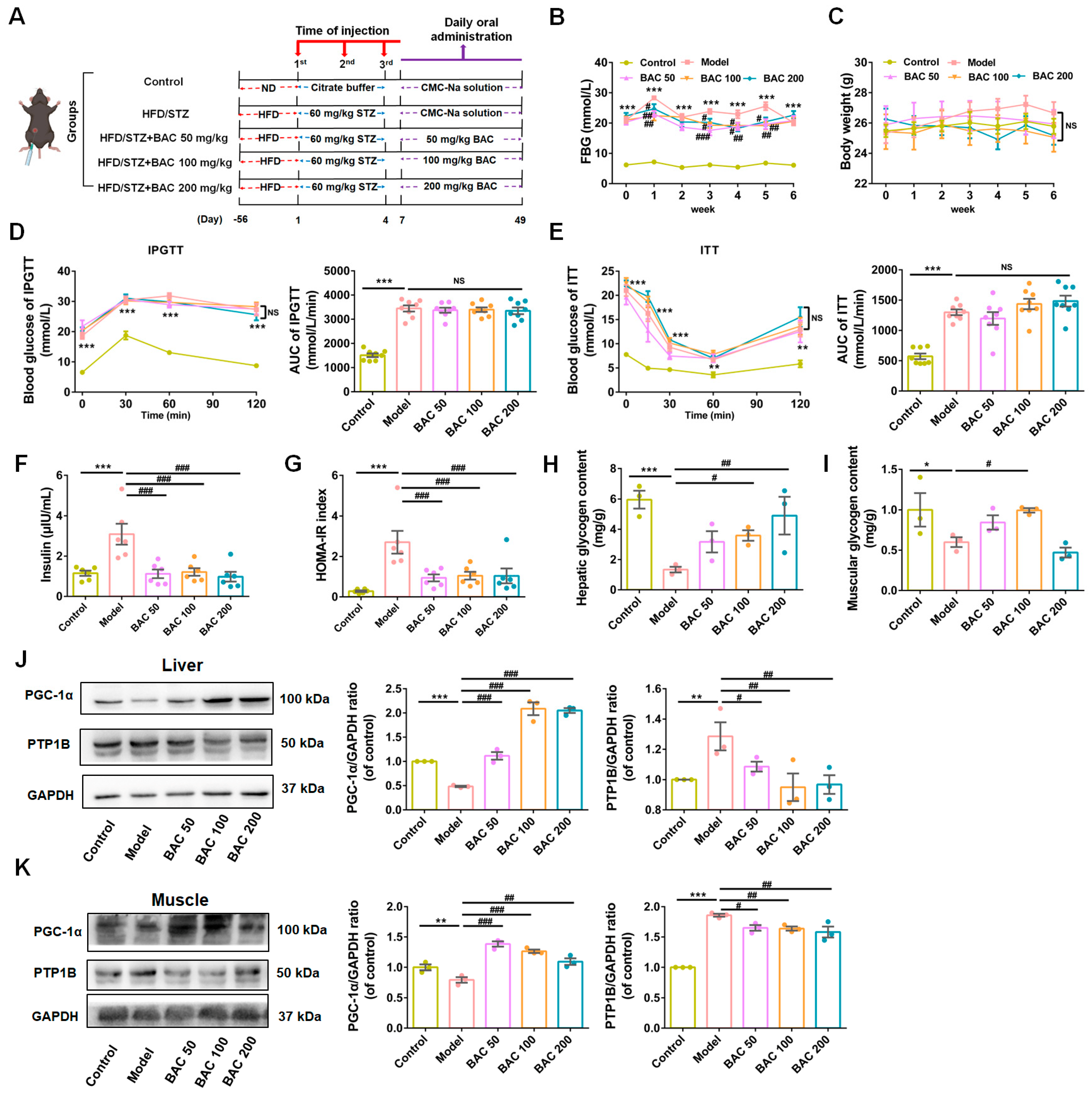
3.1. Baicalein Ameliorated Hyperglycemia of T2DM Mice by Alleviating IR
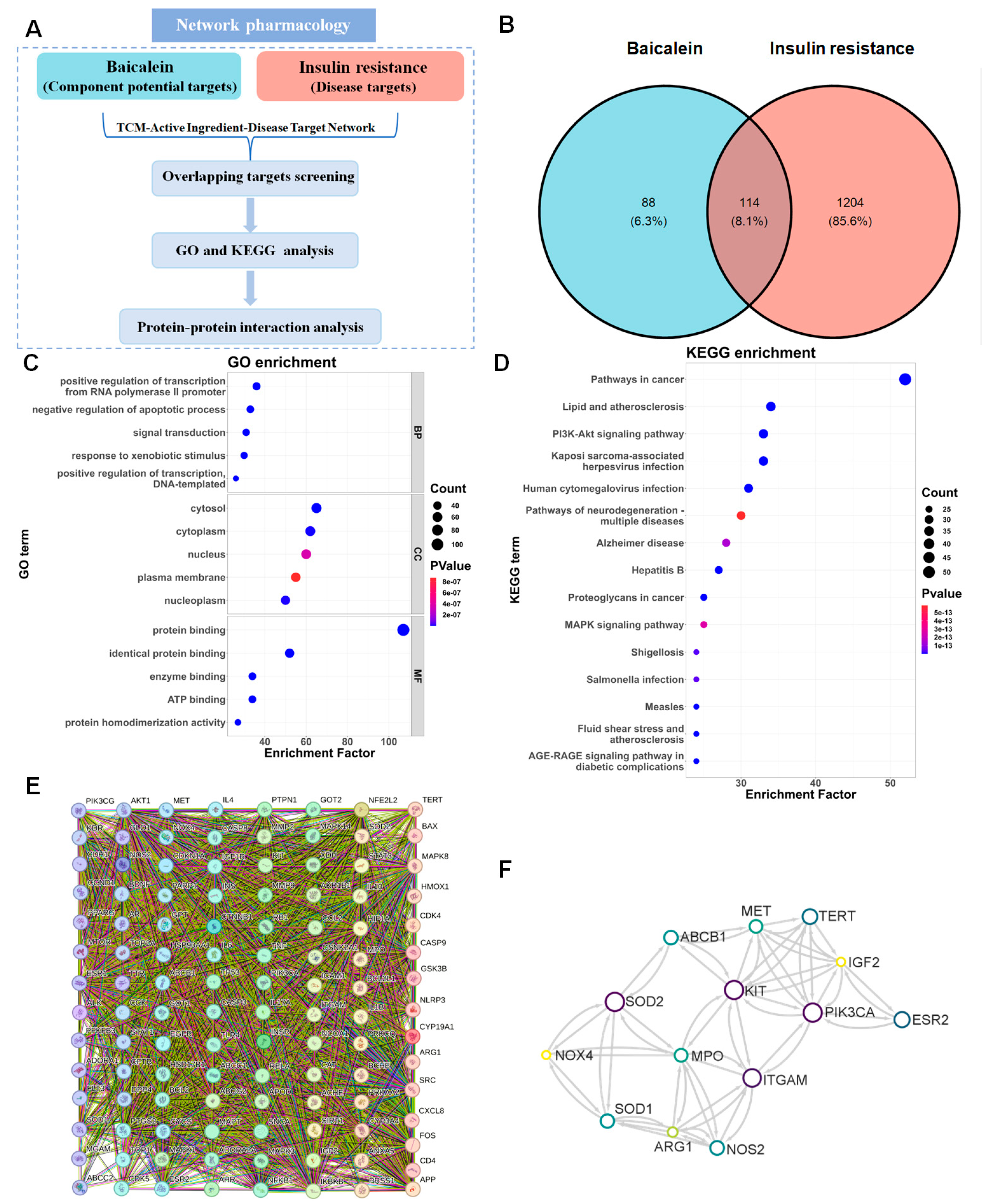
3.2. Network Pharmacology Analysis Applied to Clarify the Potential Signal Pathway Involved in the IR Improvement Effect of Baicalein
3.2.1. Prediction of the Target Proteins
3.2.2. GO and KEGG Enrichment Analyses
3.2.3. PPI Network
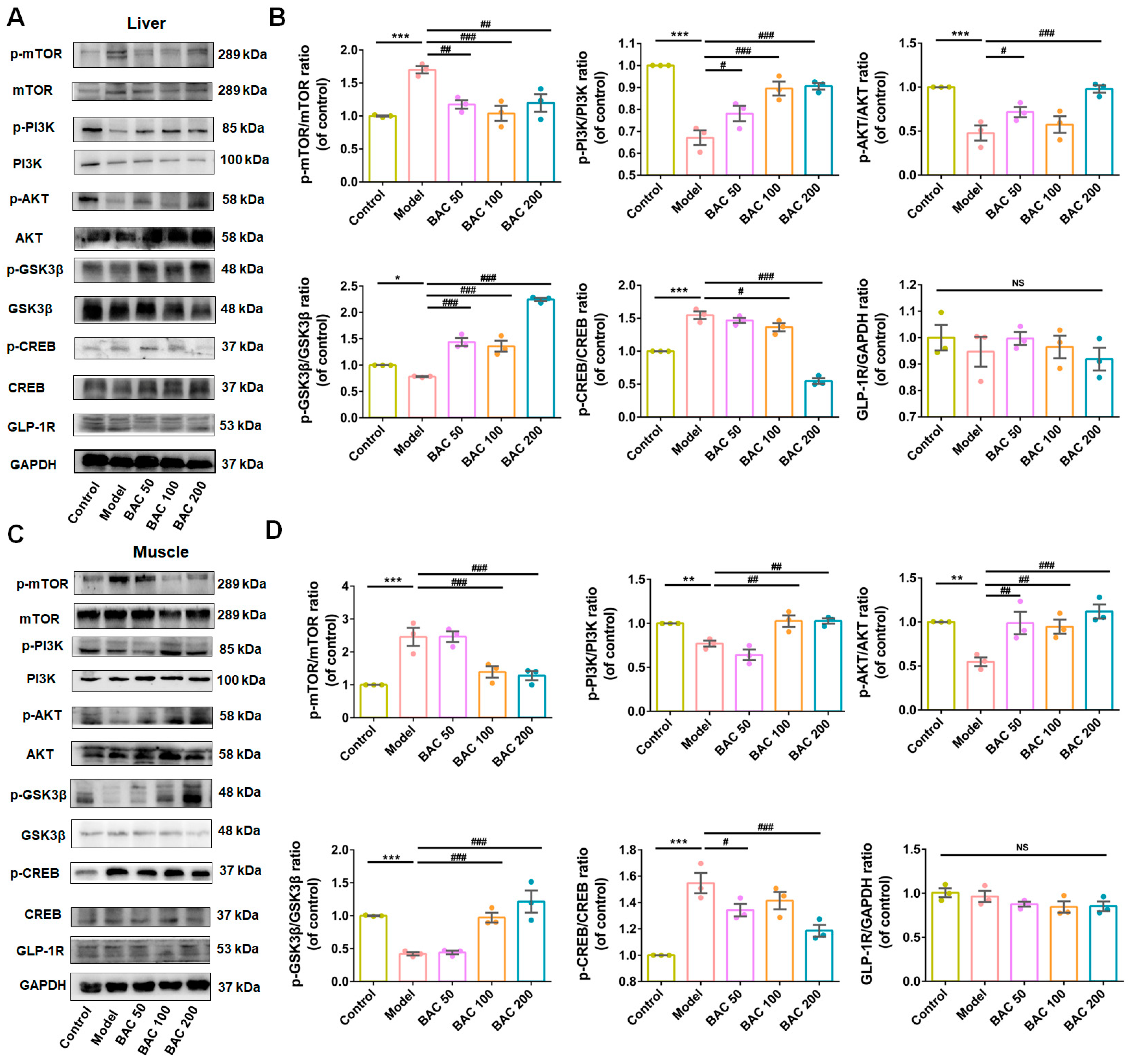
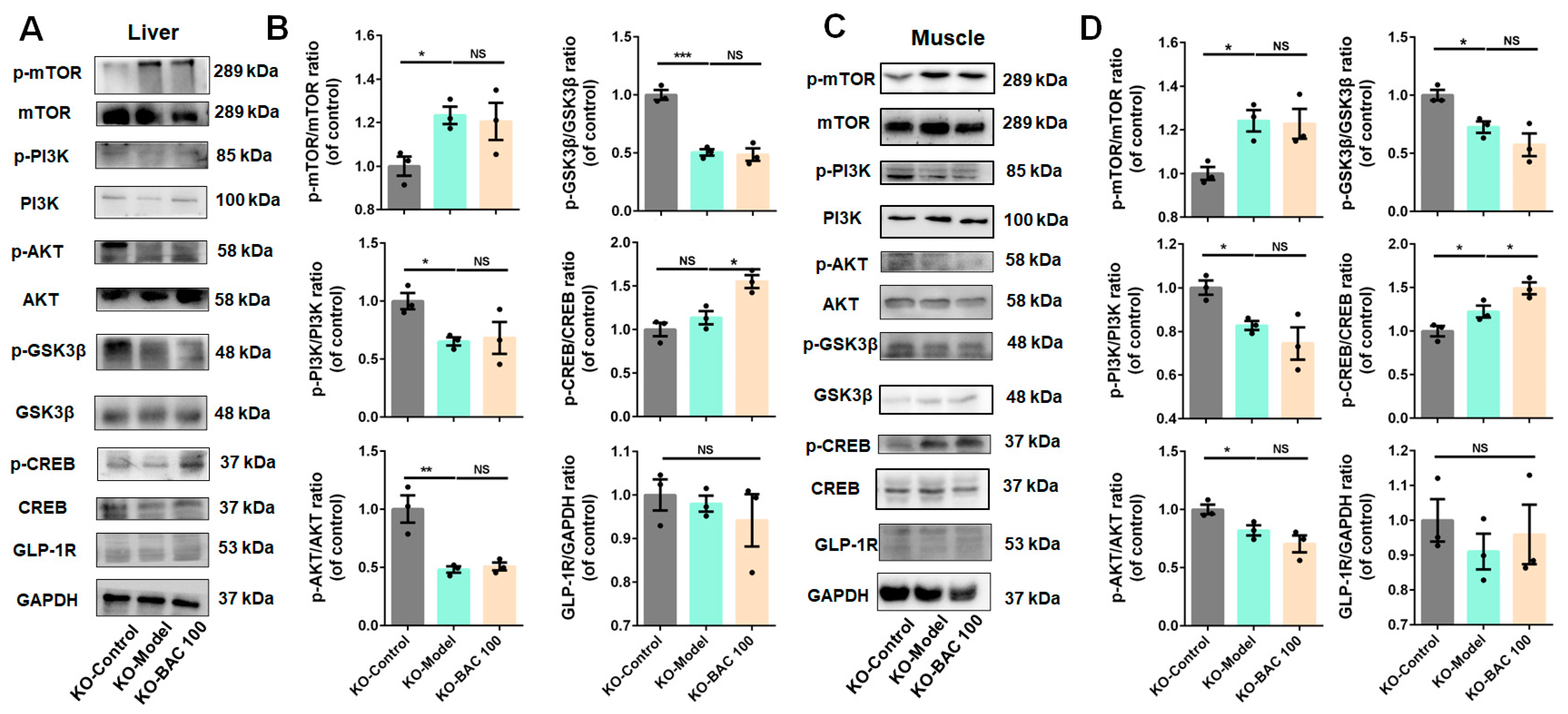
3.3. Baicalein Improved Glucose Disposal of Liver and Skeletal Muscle Tissues Through PI3K/AKT Insulin Signaling Pathway
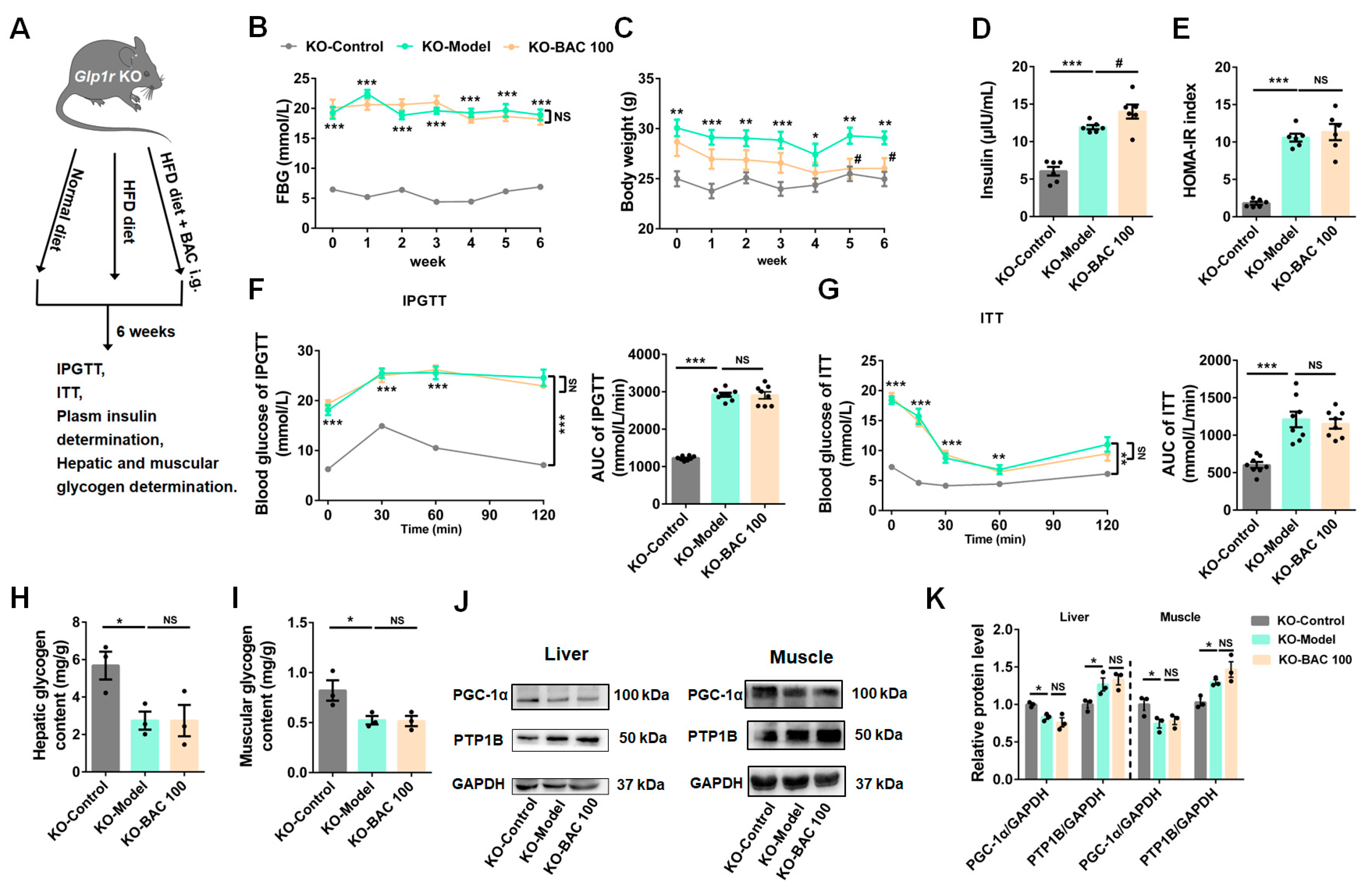
3.4. Glp1r KO Disrupted the Beneficial Effects of Baicalein on Glucose Metabolism
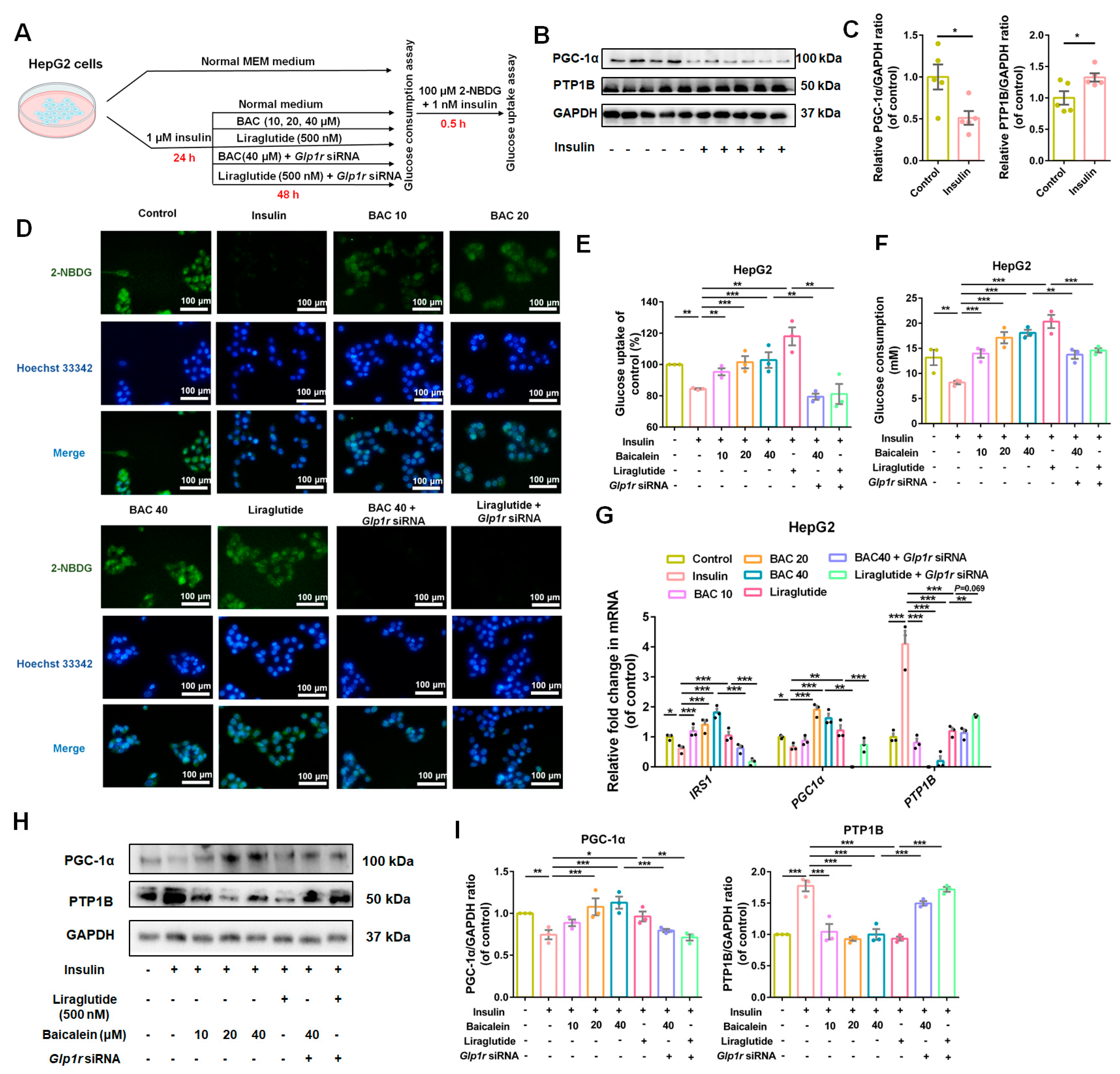
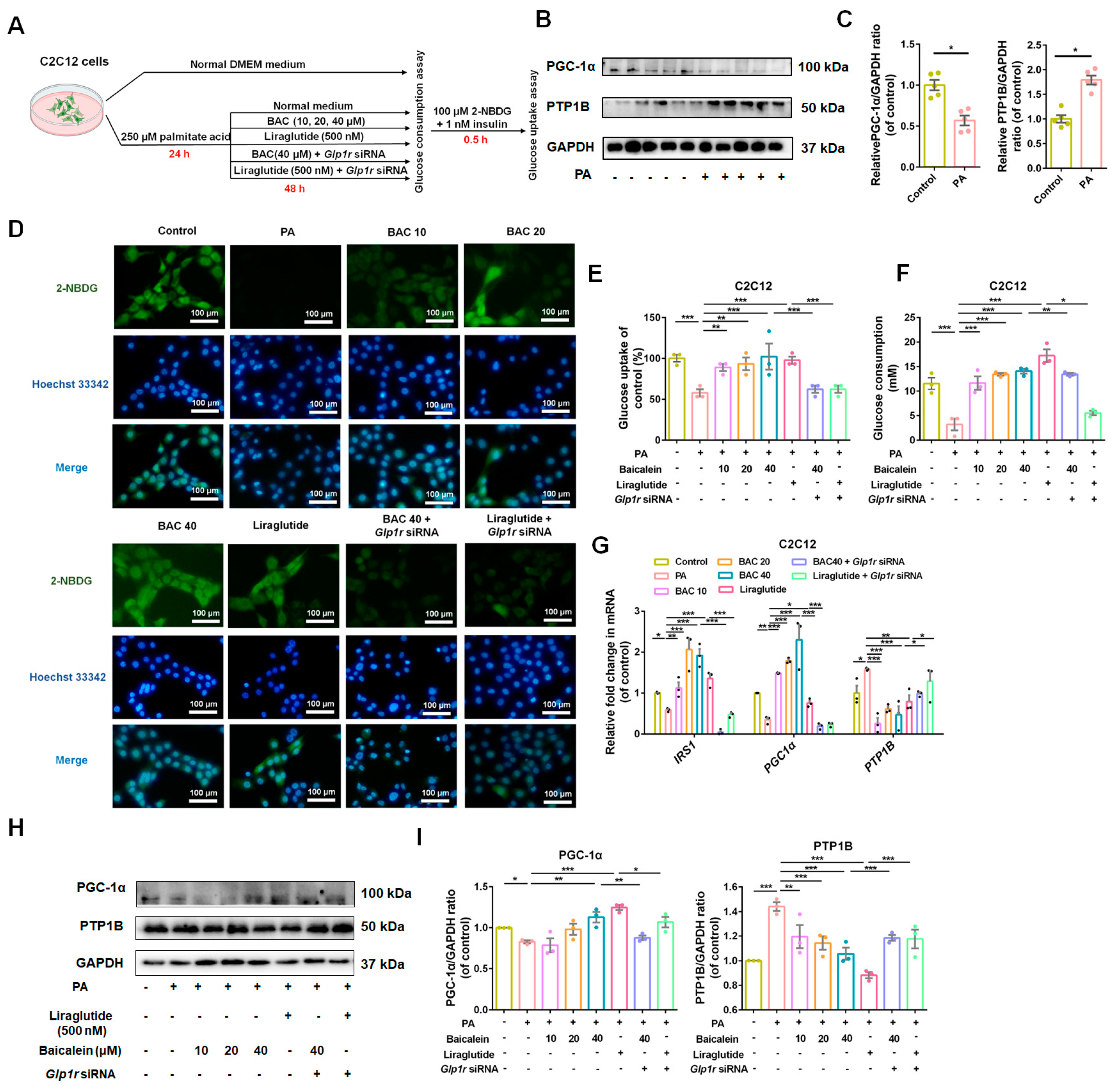
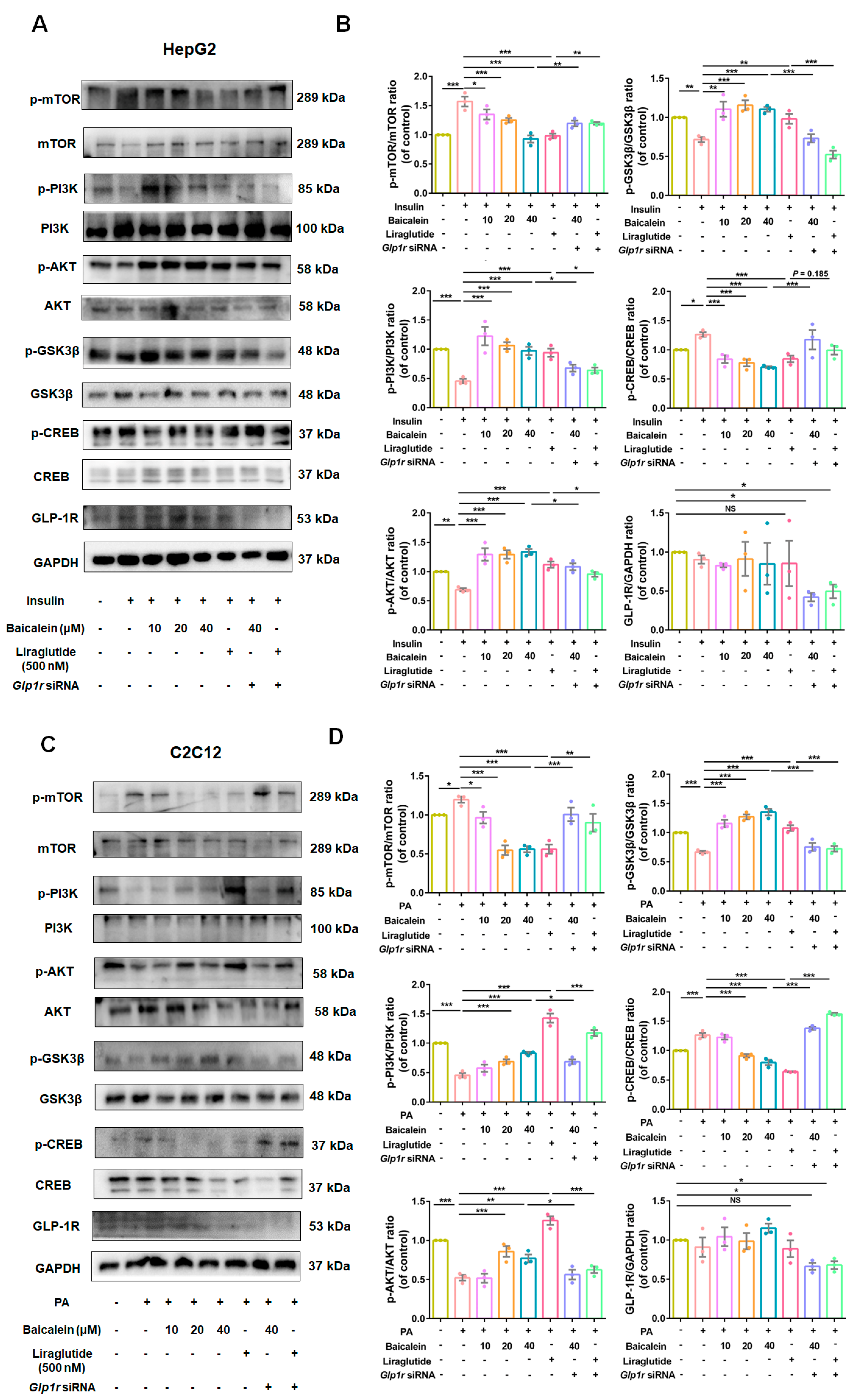
3.5. GLP-1R Was Indispensable for Baicalein to Improve Glucose Utilization in IR-HepG2 and IR-C2C12 Cells
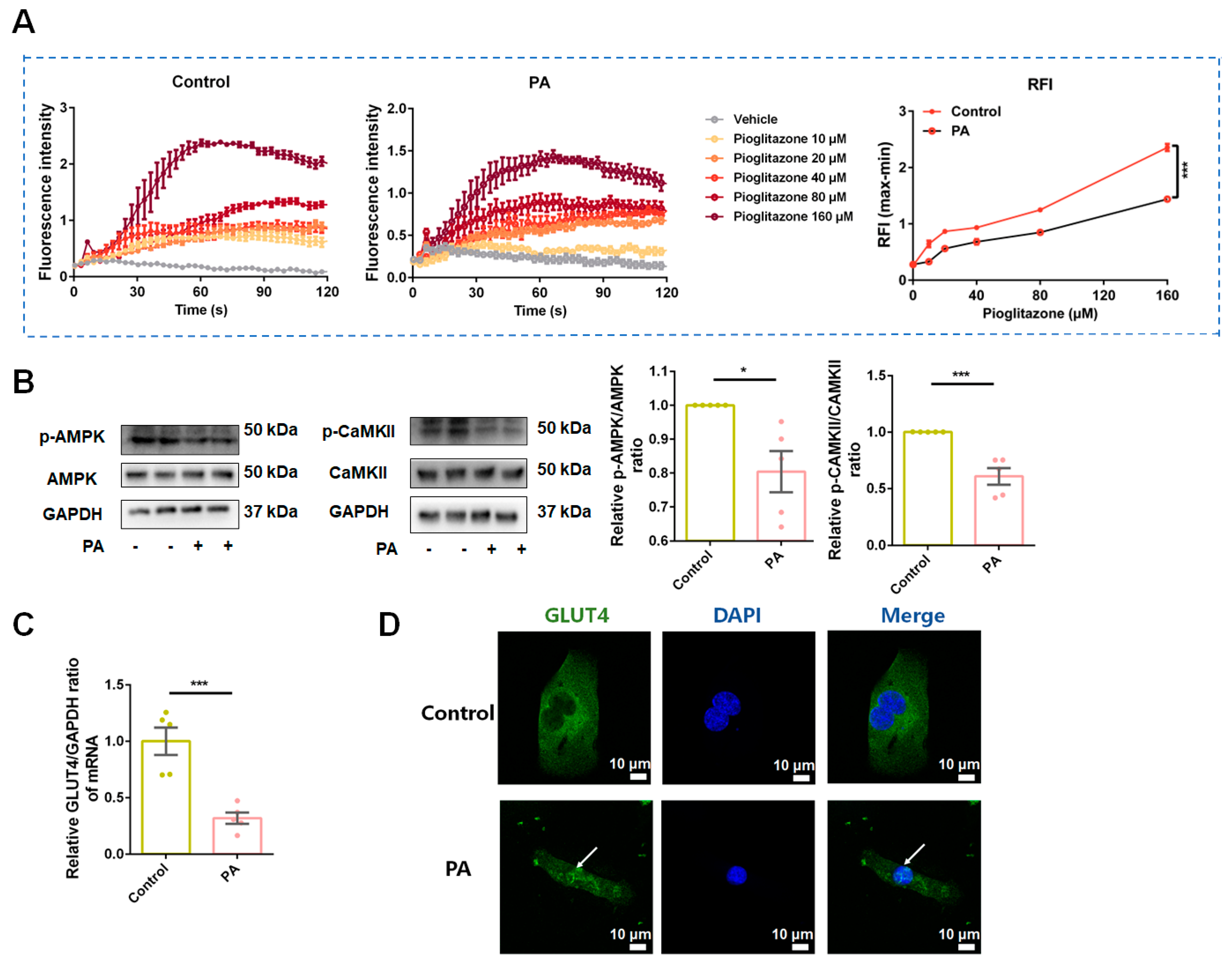
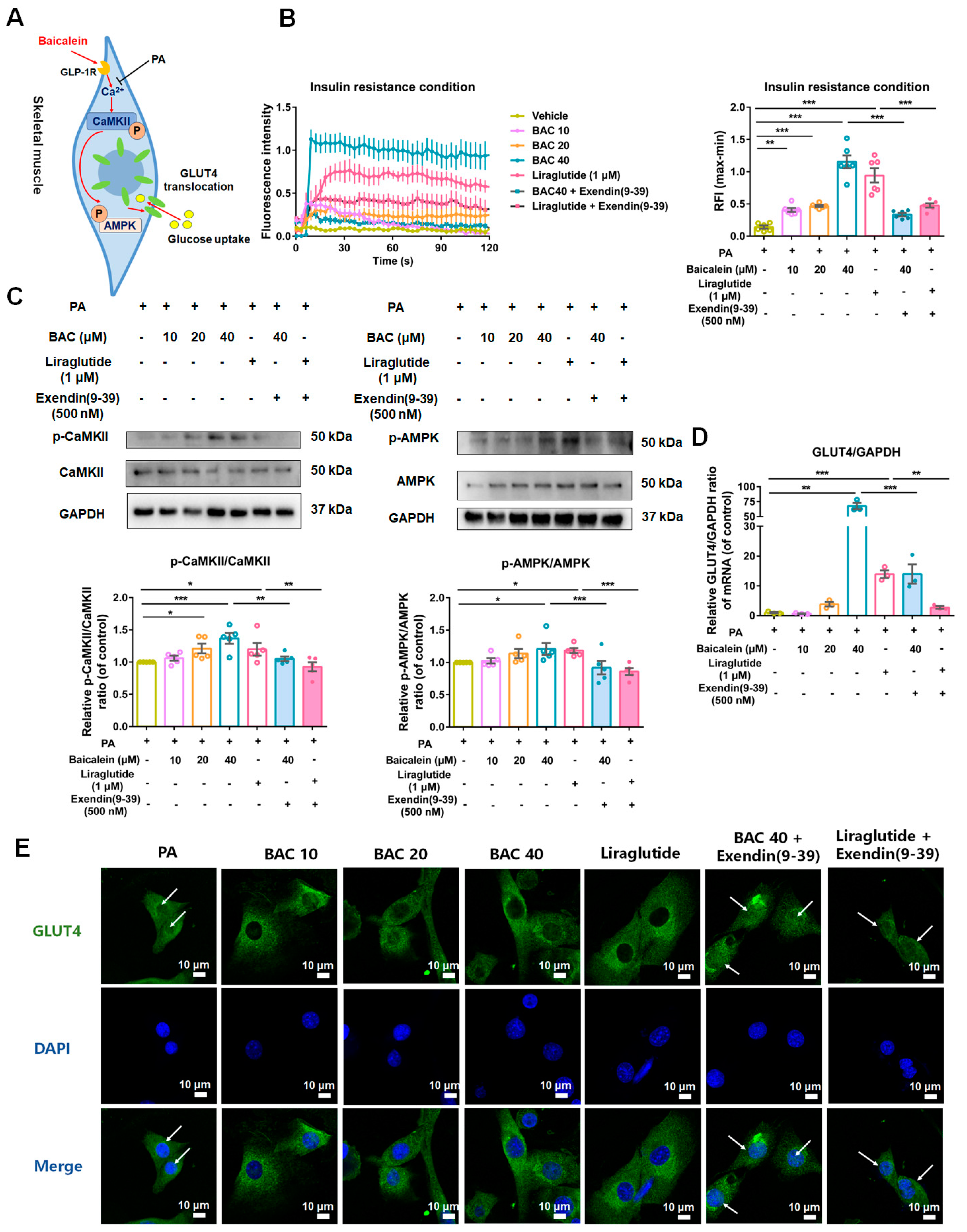
3.6. Baicalein Increased Intracellular Calcium (Ca2+) Levels, Activated CaMKII and AMPK, and Led Glucose Transporter 4 (GLUT4) Translocation from Nucleus to Cytomembrane in Skeletal Muscle Cells
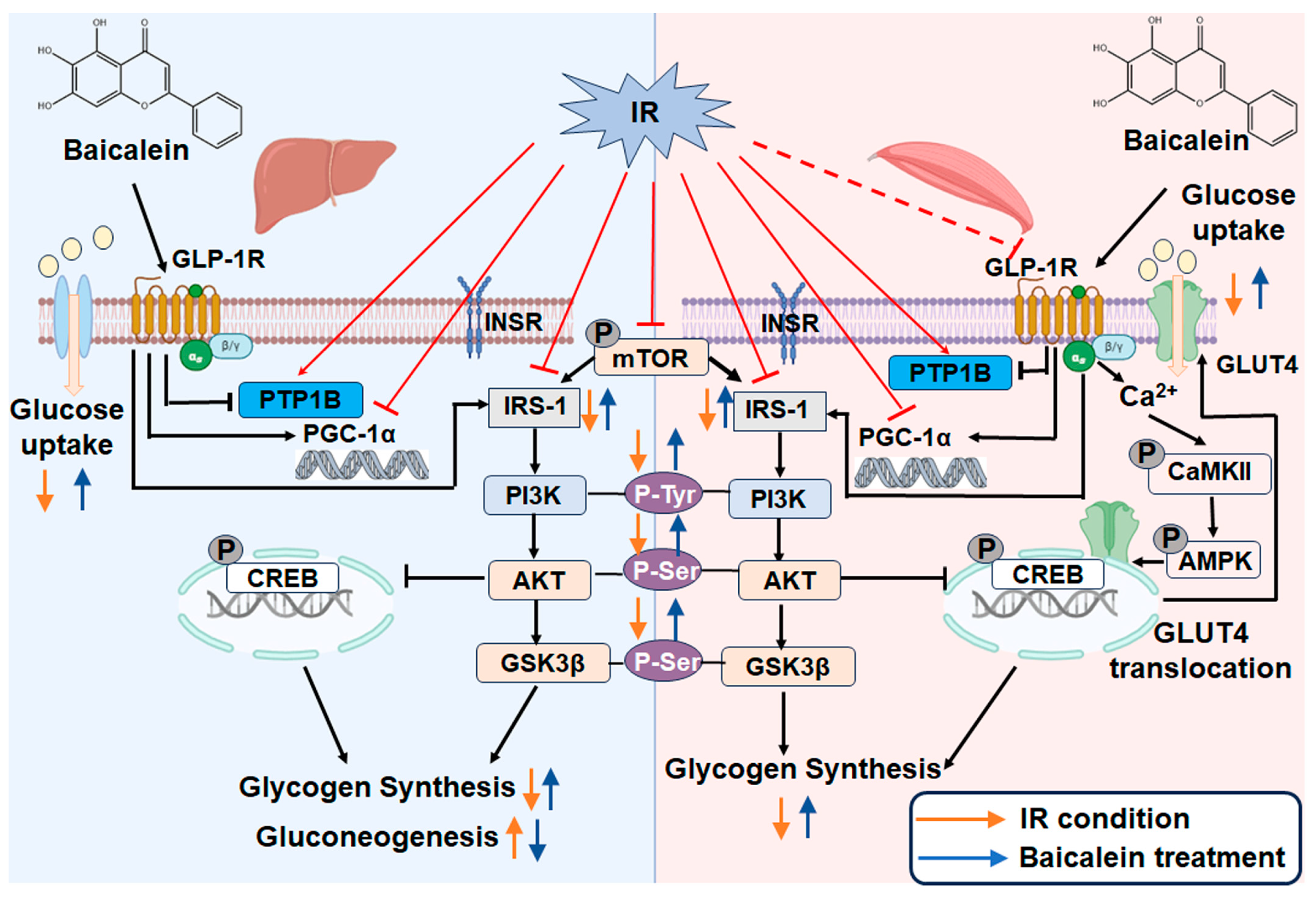
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Quarta, C.; Stemmer, K.; Novikoff, A.; Yang, B.; Klingelhuber, F.; Harger, A.; Bakhti, M.; Bastidas-Ponce, A.; Baugé, E.; Campbell, J.E.; et al. GLP-1-mediated delivery of tesaglitazar improves obesity and glucose metabolism in male mice. Nat. Metab. 2022, 4, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Samms, R.J.; Zhang, G.; He, W.; Ilkayeva, O.; Droz, B.A.; Bauer, S.M.; Stutsman, C.; Pirro, V.; Collins, K.A.; Furber, E.C.; et al. Tirzepatide induces a thermogenic-like amino acid signature in brown adipose tissue. Mol. Metab. 2022, 64, 101550. [Google Scholar] [CrossRef]
- Parlevliet, E.T.; de Leeuw van Weenen, J.E.; Romijn, J.A.; Pijl, H. GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E318–E324. [Google Scholar] [CrossRef]
- Tsai, S.F.; Chen, C.H. Management of Diabetes Mellitus in Normal Renal Function, Renal Dysfunction and Renal Transplant Recipients, Focusing on Glucagon-Like Peptide-1 Agonist: A Review Based upon Current Evidence. Int. J. Mol. Sci. 2019, 20, 3152. [Google Scholar] [CrossRef]
- Hoffman, S.; Alvares, D.; Adeli, K. GLP-1 attenuates intestinal fat absorption and chylomicron production via vagal afferent nerves originating in the portal vein. Mol. Metab. 2022, 65, 101590. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, M.; Nian, H.; Mayfield, D.; Devin, J.K.; Gamboa, J.L.; Yu, C.; Silver, H.J.; Niswender, K.; Luther, J.M.; Brown, N.J. Weight Loss-Independent Effect of Liraglutide on Insulin Sensitivity in Individuals With Obesity and Prediabetes. Diabetes 2024, 73, 38–50. [Google Scholar] [CrossRef]
- Yu, W.; Fan, L.; Wang, M.; Cao, B.; Hu, X. Pterostilbene Improves Insulin Resistance Caused by Advanced Glycation End Products (AGEs) in Hepatocytes and Mice. Mol. Nutr. Food Res. 2021, 65, e2100321. [Google Scholar] [CrossRef]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44. [Google Scholar] [CrossRef]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Winden, K.D.; Ebrahimi-Fakhari, D.; Sahin, M. Abnormal mTOR Activation in Autism. Annu. Rev. Neurosci. 2018, 41, 1–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, N.Q.; Yan, F.; Jin, H.; Zhou, S.Y.; Shi, J.S.; Jin, F. Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef]
- Montminy, M.R.; Bilezikjian, L.M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature 1987, 328, 175–178. [Google Scholar] [CrossRef]
- Sekar, R.; Motzler, K.; Kwon, Y.; Novikoff, A.; Jülg, J.; Najafi, B.; Wang, S.; Warnke, A.L.; Seitz, S.; Hass, D.; et al. Vps37a regulates hepatic glucose production by controlling glucagon receptor localization to endosomes. Cell Metab. 2022, 34, 1824–1842.e9. [Google Scholar] [CrossRef]
- Benchoula, K.; Parhar, I.S.; Madhavan, P.; Hwa, W.E. CREB nuclear transcription activity as a targeting factor in the treatment of diabetes and diabetes complications. Biochem. Pharmacol. 2021, 188, 114531. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Pacini, G. Glucose effectiveness: Lessons from studies on insulin-independent glucose clearance in mice. J. Diabetes Investig. 2021, 12, 675–685. [Google Scholar] [CrossRef]
- Magnone, M.; Emionite, L.; Guida, L.; Vigliarolo, T.; Sturla, L.; Spinelli, S.; Buschiazzo, A.; Marini, C.; Sambuceti, G.; De Flora, A.; et al. Insulin-independent stimulation of skeletal muscle glucose uptake by low-dose abscisic acid via AMPK activation. Sci. Rep. 2020, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Meng, Z.; Yu, S.; Li, J.; Wang, Y.; Yang, W.; Wu, H. Baicalein ameliorates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via regulating GPX4/ACSL4/ACSL3 axis. Chem. Biol. Interact. 2022, 366, 110137. [Google Scholar] [CrossRef]
- Tuli, H.S.; Aggarwal, V.; Kaur, J.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Tuorkey, M.; Kaur, G.; Savla, R.; Sak, K.; et al. Baicalein: A metabolite with promising antineoplastic activity. Life Sci. 2020, 259, 118183. [Google Scholar] [CrossRef]
- Chagas, M.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxidative Med. Cell Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Zhang, B.W.; Li, X.; Sun, W.L.; Xing, Y.; Xiu, Z.L.; Zhuang, C.L.; Dong, Y.S. Dietary Flavonoids and Acarbose Synergistically Inhibit α-Glucosidase and Lower Postprandial Blood Glucose. J. Agric. Food Chem. 2017, 65, 8319–8330. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Deravi, N.; Forouhar, K.; Rahimi, R.; Kheirandish, A.; Bahramsoltani, R. Endoplasmic reticulum as a therapeutic target in type 2 diabetes: Role of phytochemicals. Int. Immunopharmacol. 2023, 114, 109508. [Google Scholar] [CrossRef]
- Riaz, S.; Siddiqui, S.; Abul Qais, F.; Mateen, S.; Moin, S. Inhibitory effect of baicalein against glycation in HSA: An in vitro approach. J. Biomol. Struct. Dyn. 2023, 42, 935–947. [Google Scholar] [CrossRef]
- Faulkner, J.; Pye, C.; Al-Shabrawey, M.; Elmarakby, A.A. Inhibition of 12/15-Lipoxygenase Reduces Renal Inflammation and Injury in Streptozotocin-Induced Diabetic Mice. J. Diabetes Metab. 2015, 6, 555. [Google Scholar]
- Liu, N.; Cui, X.; Yan, W.; Guo, T.; Wang, Z.; Wei, X.; Sun, Y.; Liu, J.; Xian, C.; Ma, W.; et al. Baicalein: A potential GLP-1R agonist improves cognitive disorder of diabetes through mitophagy enhancement. J. Pharm. Anal. 2024, 14, 100968. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Kang, Y.J.; Sung, B.; Jang, J.Y.; Hwang, N.L.; Oh, H.J.; Ahn, Y.R.; Kim, H.J.; Shin, J.H.; Yoo, M.A.; et al. Folic acid is necessary for proliferation and differentiation of C2C12 myoblasts. J. Cell. Physiol. 2018, 233, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Lee, S.H.; Kim, H.C.; Bang, J.S.; Abd El-Aty, A.M.; Hacımüftüoğlu, A.; Shin, Y.K.; Jeong, J.H. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Liao, Q.; Zhang, T.; Pan, R.; Lin, L. Myricanol modulates skeletal muscle-adipose tissue crosstalk to alleviate high-fat diet-induced obesity and insulin resistance. Br. J. Pharmacol. 2019, 176, 3983–4001. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Einwallner, E. Study of In Vivo Glucose Metabolism in High-fat Diet-fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J. Vis. Exp. 2018, 131, e56672. [Google Scholar]
- Agrawal, N.; Dhakrey, P.; Pathak, S. A comprehensive review on the research progress of PTP1B inhibitors as antidiabetics. Chem. Biol. Drug Des. 2023, 102, 921–938. [Google Scholar] [CrossRef]
- Gu, L.; Ding, X.; Wang, Y.; Gu, M.; Zhang, J.; Yan, S.; Li, N.; Song, Z.; Yin, J.; Lu, L.; et al. Spexin alleviates insulin resistance and inhibits hepatic gluconeogenesis via the FoxO1/PGC-1α pathway in high-fat-diet-induced rats and insulin resistant cells. Int. J. Biol. Sci. 2019, 15, 2815–2829. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020, 8, e14607. [Google Scholar] [CrossRef]
- Wang, M.; Chang, S.Q.; Tian, Y.S.; Zhang, G.Q.; Qi, J. Zengye Decoction Ameliorates Insulin Resistance by Promoting Glucose Uptake. Rejuvenation Res. 2020, 23, 367–376. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, H.R.; Yan, Z. AMPK and the Adaptation to Exercise. Annu. Rev. Physiol. 2022, 84, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Ojuka, E.O. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc. Nutr. Soc. 2004, 63, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, S.K.; Taylor, C.G.; Siemens, L.; Zahradka, P. Acute exposure of L6 myotubes to cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid isomers stimulates glucose uptake by modulating Ca2+/calmodulin-dependent protein kinase II. Int. J. Biochem. Cell Biol. 2012, 44, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Douros, J.D.; Mokrosinski, J.; Finan, B. The GLP-1R as a model for understanding and exploiting biased agonism in next-generation medicines. J. Endocrinol. 2024, 261, e230226. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, Q.; Wu, X.; Zhang, L.; Liu, Q.; Wang, L. The Utility of Exosomes in Diagnosis and Therapy of Diabetes Mellitus and Associated Complications. Front. Endocrinol. 2021, 12, 756581. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, Q.W.; Yang, X.Y.; Yang, W.; Li, D.R.; Jin, J.Y.; Zhang, H.C.; Zhang, X.F. GLP-1 receptor agonists for the treatment of obesity: Role as a promising approach. Front. Endocrinol. 2023, 14, 1085799. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Ruze, R.; Xu, Q.; Liu, G.; Li, Y.; Chen, W.; Cheng, Z.; Xiong, Y.; Liu, S.; Zhang, G.; Hu, S.; et al. Central GLP-1 contributes to improved cognitive function and brain glucose uptake after duodenum-jejunum bypass on obese and diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E392–E409. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Sun, B.; Yoshino, H.; Feng, D.; Suzuki, Y.; Fukazawa, M.; Nagao, S.; Wainscott, D.B.; Showalter, A.D.; Droz, B.A.; et al. Structural basis for GLP-1 receptor activation by LY3502970, an orally active nonpeptide agonist. Proc. Natl. Acad. Sci. USA 2020, 117, 29959–29967. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Pieber, T.R.; Hartoft-Nielsen, M.L.; Hansen, O.K.H.; Jabbour, S.; Rosenstock, J. Effect of Oral Semaglutide Compared with Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients with Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2017, 318, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Shakour, N.; Hoseinpoor, S.; Rajabian, F.; Azimi, S.G.; Iranshahi, M.; Sadeghi-Aliabadi, H.; Hadizadeh, F. Discovery of non-peptide GLP-1r natural agonists for enhancing coronary safety in type 2 diabetes patients. J. Biomol. Struct. Dyn. 2024, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Huang, W.; Wang, X.; Zhao, L.; Jiang, Y.; Liu, F.; Guo, W.; Sun, X.; Zhong, W.; Yuan, D.; et al. Structural insights into the activation of GLP-1R by a small molecule agonist. Cell Res. 2020, 30, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Cong, Z.; Chen, L.N.; Ma, H.; Zhou, Q.; Zou, X.; Ye, C.; Dai, A.; Liu, Q.; Huang, W.; Sun, X.; et al. Molecular insights into ago-allosteric modulation of the human glucagon-like peptide-1 receptor. Nat. Commun. 2021, 12, 3763. [Google Scholar] [CrossRef]
- Graaf, C.; Donnelly, D.; Wootten, D.; Lau, J.; Sexton, P.M.; Miller, L.J.; Ahn, J.M.; Liao, J.; Fletcher, M.M.; Yang, D.; et al. Glucagon-like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol. Rev. 2016, 68, 954–1013. [Google Scholar] [CrossRef]
- Wang, J.; Yang, D.; Cheng, X.; Yang, L.; Wang, Z.; Dai, A.; Cai, X.; Zhang, C.; Yuliantie, E.; Liu, Q.; et al. Allosteric Modulators Enhancing GLP-1 Binding to GLP-1R via a Transmembrane Site. ACS Chem. Biol. 2021, 16, 2444–2452. [Google Scholar] [CrossRef]
- Morris, L.C.; Nance, K.D.; Gentry, P.R.; Days, E.L.; Weaver, C.D.; Niswender, C.M.; Thompson, A.D.; Jones, C.K.; Locuson, C.W.; Morrison, R.D.; et al. Discovery of (S)-2-cyclopentyl-N-((1-isopropylpyrrolidin2-yl)-9-methyl-1-oxo-2,9-dihydro-1H-pyrrido [3,4-b]indole-4-carboxamide (VU0453379): A novel, CNS penetrant glucagon-like peptide 1 receptor (GLP-1R) positive allosteric modulator (PAM). J. Med. Chem. 2014, 57, 10192–10197. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Xie, Y.; Du, A.; Chen, M.; Lai, S.; Wei, X.; Ji, L.; Wang, C. Panax notoginseng saponins alleviate diabetic retinopathy by inhibiting retinal inflammation: Association with the NF-κB signaling pathway. J. Ethnopharmacol. 2024, 319 Pt 1, 117135. [Google Scholar] [CrossRef]
- Siamashvili, M.; Davis, S.N. Update on the effects of GLP-1 receptor agonists for the treatment of polycystic ovary syndrome. Expert Rev. Clin. Pharmacol. 2021, 14, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, S.; Mehan, S.; Khan, A.; Rehman, M.U. Protective role of IGF-1 and GLP-1 signaling activation in neurological dysfunctions. Neurosci. Biobehav. Rev. 2022, 142, 104896. [Google Scholar] [CrossRef] [PubMed]
- Brunner, K.T.; Henneberg, C.J.; Wilechansky, R.M.; Long, M.T. Nonalcoholic Fatty Liver Disease and Obesity Treatment. Curr. Obes. Rep. 2019, 8, 220–228. [Google Scholar] [CrossRef]
- Ramasubbu, K.; Devi Rajeswari, V. Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases: A perspective review. Mol. Cell. Biochem. 2023, 478, 1307–1324. [Google Scholar] [CrossRef]
- Tamrakar, A.K.; Maurya, C.K.; Rai, A.K. PTP1B inhibitors for type 2 diabetes treatment: A patent review (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1101–1115. [Google Scholar] [CrossRef]
- Teimouri, M.; Hosseini, H.; ArabSadeghabadi, Z.; Babaei-Khorzoughi, R.; Gorgani-Firuzjaee, S.; Meshkani, R. The role of protein tyrosine phosphatase 1B (PTP1B) in the pathogenesis of type 2 diabetes mellitus and its complications. J. Physiol. Biochem. 2022, 78, 307–322. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxidative Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef] [PubMed]
- Ancuceanu, R.; Dinu, M.; Dinu-Pirvu, C.; Anuţa, V.; Negulescu, V. Pharmacokinetics of B-Ring Unsubstituted Flavones. Pharmaceutics 2019, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zeng, M.; Du, T.; Li, L.; Yang, G.; Hu, M.; Gao, S. Simultaneous determinations of 17 marker compounds in Xiao-Chai-Hu-Tang by LC-MS/MS: Application to its pharmacokinetic studies in mice. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1003, 12–21. [Google Scholar] [CrossRef]
- Zhu, M.L.; Liang, X.L.; Zhao, L.J.; Liao, Z.G.; Zhao, G.W.; Cao, Y.C.; Zhang, J.; Luo, Y. Elucidation of the transport mechanism of baicalin and the influence of a Radix Angelicae Dahuricae extract on the absorption of baicalin in a Caco-2 cell monolayer model. J. Ethnopharmacol. 2013, 150, 553–559. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Zhang, L.; Moro, A.; Chen, M.C.; Harris, D.M.; Eibl, G.; Go, V.L. Detection of baicalin metabolites baicalein and oroxylin-a in mouse pancreas and pancreatic xenografts. Pancreas 2012, 41, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Herath, W.; Mikell, J.R.; Hale, A.L.; Ferreira, D.; Khan, I.A. Microbial metabolism. Part 6. Metabolites of 3- and 7-hydroxyflavones. Chem. Pharm. Bull. 2006, 54, 320–324. [Google Scholar]
- Li, M.; Shi, A.; Pang, H.; Xue, W.; Li, Y.; Cao, G.; Yan, B.; Dong, F.; Li, K.; Xiao, W.; et al. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J. Ethnopharmacol. 2014, 156, 210–215. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Inzucchi, S.; Abdul-Ghani, M.; Nissen, S.E. Pioglitazone: The forgotten, cost-effective cardioprotective drug for type 2 diabetes. Diabetes Vasc. Dis. Res. 2019, 16, 133–143. [Google Scholar] [CrossRef]
- Wang, H.; Qin, L.; Wang, D.; Jiang, Y.; Wu, X.; Xu, T.; Liu, T.; Li, L. Mixture of five herbal extracts ameliorates pioglitazone-induced aggravation of hepatic steatosis via activating the adiponectin receptor 2/AMP-activated protein kinase signal pathway in diabetic KKAy mice. J. Tradit. Chin. Med. 2017, 37, 588–598. [Google Scholar]
| Accession Number | Forward | Reverse | Product Length | |
|---|---|---|---|---|
| IRS-1 | NM_005544.3 | ACTGGACATCACAGCAGAATGA | AGAACGTGCAGTTCAGTCAA | 199 |
| PTP1B | NM_011201.3 | TGACGGTGCTCATGACTCTT | TGCTGGCTTCTCTGGGTAAA | 141 |
| GLUT4 | NM_001359114.2 | CAACTGGACCTGTAACTTCATTGT | ACGGCAAATAGAAGGAAGACGTA | 87 |
| PGC-1α | NM_001402988.1 | CACCAAACCCACAGAAAACAG | GGGTCAGAGGAAGAGATAAAGTTG | 125 |
| GAPDH | NM_002046.7 | GAGTCAACGGATTTGGTCGT | GACAAGCTTCCCGTTCTCAG | 185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Cui, X.; Guo, T.; Wei, X.; Sun, Y.; Liu, J.; Zhang, Y.; Ma, W.; Yan, W.; Chen, L. Baicalein Ameliorates Insulin Resistance of HFD/STZ Mice Through Activating PI3K/AKT Signal Pathway of Liver and Skeletal Muscle in a GLP-1R-Dependent Manner. Antioxidants 2024, 13, 1246. https://doi.org/10.3390/antiox13101246
Liu N, Cui X, Guo T, Wei X, Sun Y, Liu J, Zhang Y, Ma W, Yan W, Chen L. Baicalein Ameliorates Insulin Resistance of HFD/STZ Mice Through Activating PI3K/AKT Signal Pathway of Liver and Skeletal Muscle in a GLP-1R-Dependent Manner. Antioxidants. 2024; 13(10):1246. https://doi.org/10.3390/antiox13101246
Chicago/Turabian StyleLiu, Na, Xin Cui, Tingli Guo, Xiaotong Wei, Yuzhuo Sun, Jieyun Liu, Yangyang Zhang, Weina Ma, Wenhui Yan, and Lina Chen. 2024. "Baicalein Ameliorates Insulin Resistance of HFD/STZ Mice Through Activating PI3K/AKT Signal Pathway of Liver and Skeletal Muscle in a GLP-1R-Dependent Manner" Antioxidants 13, no. 10: 1246. https://doi.org/10.3390/antiox13101246
APA StyleLiu, N., Cui, X., Guo, T., Wei, X., Sun, Y., Liu, J., Zhang, Y., Ma, W., Yan, W., & Chen, L. (2024). Baicalein Ameliorates Insulin Resistance of HFD/STZ Mice Through Activating PI3K/AKT Signal Pathway of Liver and Skeletal Muscle in a GLP-1R-Dependent Manner. Antioxidants, 13(10), 1246. https://doi.org/10.3390/antiox13101246





