Abstract
In recent years, the evaluation of many plant-derived compounds as potential new drugs or functional foods has become an active research topic. The morphological characteristics of quinces of the genera Cydonia sp., Chaenomeles sp., and Pseuocydonia sp. are largely similar, which is why these fruits are often confused. Although they have been appreciated in Asia for centuries as a valuable component of local ethnomedicine, they are less known in Western countries, and scientific knowledge about their health benefits remains fragmentary. This literature review summarizes studies on the content of chemical compounds responsible for the health-promoting and functional properties of the quince fruit. It focuses on the content of carotenoids, vitamins, minerals, and carboxylic acids, although the main emphasis is on the content and diversity of bioactive polyphenols, which are extremely abundant in these fruits. The quince fruits are rich in antioxidants and compounds with proven anti-inflammatory, anticancer, antiallergic, and immunomodulatory effects. Their phytochemicals effectively regulate glycemia and improve the blood lipid profile, suggesting potential antidiabetic and cardioprotective benefits. Analysis of chemical characteristics showed that the Chaenomeles fruits. are underestimated as functional food ingredients. Studies on the molecular effects of their bioactive compounds and species-specific genomic analyses are sorely lacking in the scientific literature.
1. Introduction
For the consumer, the fruits of Cydonia oblonga Mill., Pseudocydonia sinensis (Thouin) Schneid., and Chaenomeles sp. Lindl. are similar: they are yellow, hard, fragrant, have a sour taste, and are used in similar ways. For growers and gardeners, these are plants with different purposes (consumption, medicinal, ornamental) and different requirements. In some languages, there are no separate names for the fruits of Cydonia, Pseudocydonia, or Chaenomeles. In English, adjectives are sometimes added, such as Turkish, Chinese, Japanese, Persian, etc. Not surprisingly, their fruits are confused even in the professional and scientific literature [1]. Distinguishing the fruits is further complicated by the number of Latin synonyms. The relative ease of obtaining hybrid forms within the genus Chaenomeles results in additional difficulties in identification.
The aim of this work was to present the chemical composition of their fruits and a large number of medical applications as well as to improve the distinguishability of the various quince taxa, especially in Western countries where these plants are still poorly recognized. Due to the vastness of the subject, the article omits detailed aspects of biology, cultivation, or culinary use and, in describing the composition, focuses mainly on the polyphenolic compounds in which the fruits of these species are rich, and which are largely responsible for their various health-promoting properties.
2. Characteristics of Quince Plants, Their Biology, Cultivation and Culinary Use
The plant Cydonia oblonga Mill. originates from Western Asia, more precisely from the Transcaucasian region [2]. The species is widespread due to its cultivated forms and is naturalized in the area of the Mediterranean Sea. It is a monotypic genus of the family Rosaceae.
Species of the genus Chaenomeles have been known in China for thousands of years, and their fruits are used in traditional Chinese medicine (TCM). Interest in the fruits has increased in recent decades due to the possibility of cultivating several species of these plants in Europe, mainly in the Baltic countries [3]. The genus Chaenomeles, a member of the Rosaceae family, consists of four species, namely Chaenomeles speciosa (Sweet) Nakai, Chaenomeles thibetica Yü, Chaenomeles cathayensis Schneid., and Chaenomeles japonica (Thunb.) Lindl. ex Spach., which are naturally distributed in eastern Asia [4]. These taxa can easily interbreed, both spontaneously and as a result of deliberate hybridization—More than 500 varieties with ornamental flowers have been described in the literature; therefore, Chaenomeles shrubs are sometimes called “flowering quince”. Another member of the Rosaceae family is Pseudocydonia sinensis Schneid., the only species of the genus Pseudocydonia. Its taxonomic status has changed over time and remains controversial [5]. This species was placed in the genus Cydonia and later in the genus Chaenomeles (C. sinensis Kochne). Recent advanced and comprehensive LM and SEM studies of pollen morphology confirmed the placement of this species in the monotypic genus Pseudocydonia [5]. This species is native to southern and eastern China but has also been introduced to Japan, Korea, and the USA.
Many taxonomists emphasize that the genera Chaenomeles and Cydonia are closely related, but in the genus Chaenomeles, many hybrids have been described, while in the genus Cydonia hybridization can only occur within C. oblonga and P. sinensis [6].
C. oblonga is a shrub or, more commonly, a small tree. Its flowers are white or pink, large and solitary, and the fruits are spherical to oblong (8–12 cm in diameter), with an average weight of 100–250 g (Figure 1). The color of the epidermis changes from brown to light green in the early stages of development and turns yellow when ripe [2]. The flesh is yellowish, consistent, slightly sweet, often astringent. Due to the high content of organic acids, the pulp of most cultivars is not eaten raw but in the form of jams, marmalades, juices, jellies, puddings, compotes, and cakes [7,8,9,10,11,12]. The naturally dried fruits of Cydonia have been used as tea in Asia Minor for centuries [13]. A unique product from the Iberian Peninsula named “quince cheese”, is a reddish, hard, sticky, and sweet paste. The use of C. oblonga fruits as an ingredient of alcohols is also widespread, including for the production of a brandy-type “rakija”, famous in the Balkans, or as an ingredient in other alcoholic beverages [14]. Attempts have also been made to improve beer with a macerate of Cydonia fruit, which enhances its sensory qualities [15].

Figure 1.
Flowers and fruits of Cydonia oblonga Mill. Source: photo by Monika Bieniasz, Univ. of Agriculture in Kraków, Poland (A,C); photo by the author (B).
Plants of the Chaenomeles genus are shrubs, usually growing to 2–3 m in height; only C. cathayensis grows as a small tree up to 6 m tall, while C. japonica is usually shorter. Chaenomeles flowers have unique decorative value; they appear before the leaves unfold and may be white, pink, orange, or red (Figure 2). The fruits are smaller, up to 100 g, spherical, and very sour (a titratable acidity of 47.5% malic eq. was measured for C. japonica) [16], which makes them unsuitable for raw consumption. However, due to their unique sensory properties, including intense aroma, they are used as additives that can enrich other products with valuable properties, e.g., teas, yogurts, cold drinks, liqueurs, ice cream, cocktails, and cottage cheese [17,18,19]. They are highly valued as dried candied fruits [20,21]. Freeze-dried fruits were added to cookies in order to improve their volatile characteristics and acceptability by consumers and to maintain quality during storage due to the strong antioxidant properties of the fruits [22]. Nawirska-Olszańska et al. [17,23] analyzed the properties of pumpkin jam mixed with C. japonica fruits and proved that such an additive enriched it with phenolic compounds and ascorbic acid as well as improved the volatile profile more significantly than using other fruits for this purpose.

Figure 2.
Flowers and fruits of Chaenomeles japonica (Thunb.) Lindl. ex Spach. Source: photo by the author (A,B); Monika Bieniasz, Univ. of Agriculture in Kraków, Poland (C).
Psudocydonia sinensis is an attractive ornamental tree growing up to 18 m high. What distinguishes it from the genus Chaenomeles is the lack of thorns, single, unclustered flowers (Figure 3), and the exfoliating bark. The flowers are decorative and appear later than in most Chaenomeles shrubs. The fruit itself resembles Cydonia; it is an ovoid pome 12–17 cm long but without tufts. It is also hard and astringent, but after frost, these properties weaken. Nevertheless, the fruit is considered unpalatable due to the high lignin content (24.5% dm) [24]. Therefore, it is processed into jams, syrups, liqueurs, wine, and jellies [25,26].

Figure 3.
Flowers and fruit of Psudocydonia sinensis Schneid. Source: photo, Dalgial, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0, accessed on 21 December 2023), via Wikimedia Commons (A) and Tusbra (public domain, Wikimedia Commons) (B).
In the culinary and pharmaceutical uses of Cydonia, Chaemnomeles, and Pseudocydonia, an important point to keep in mind is the content of cyanogenic glycosides (mainly amygdalin) in their seeds. The potential toxicity of amygdalin results from enzymatic degradation in the human digestive system leading to the production of toxic HCN [27]. However, according to the literature, C. oblonga seeds contain low levels of amygdalin, making this fruit suitable for new applications in food technology and functional food design [28,29]. Detailed studies have been carried out on C. japonica seeds. Mierina et al. [30,31] determined the HCN content to be at the level of 0.3 and 0.7 mg/g in seeds. This concentration is similar to that found in apple seeds, which can range from 0.9 to 3.9 mg/g of seed [32]. Therefore, direct consumption of Chaenomeles seeds without pretreatment may be hazardous to human health. On the other hand, tests on cold-pressed oil did not show any amygdalin content [33]. Analyses of different fractions obtained from pressed C. japonica seed residues revealed the absence of amygdalin in all oil extracts obtained with or without ethanol as a co-solvent. Amygdalin was extracted together with polyphenols at levels up to 118 μg/mL, corresponding to 3080 μg/g dm of defatted C. japonica residue. In contrast, protein isolates obtained with or without tannin removal were free of amygdalin [34].
3. Chemical Composition of Quince Fruits and Seeds
3.1. Phenolic Compounds
Many reports show the content of phenolic phytochemicals in Cydonia fruits: in their pulp [35,36,37,38,39,40], peel [35,36,39,40,41,42], and seeds [38,41,43]. A few papers focused on beneficial compounds in leaves [39,44,45,46,47,48]. Some authors used whole fruits for analysis [49,50] or their callus [51]. The data on the content of polyphenols in fresh and dried pulp, peel, and seeds are summarized in Table 1, Table 2 and Table 3.

Table 1.
Phenolic contents in C. oblonga fresh and dried pulp.

Table 2.
Phenolic contents in C. oblonga fresh and dried peel.

Table 3.
Phenolic contents in C. oblonga fresh and dried seed (extracted with methanol).
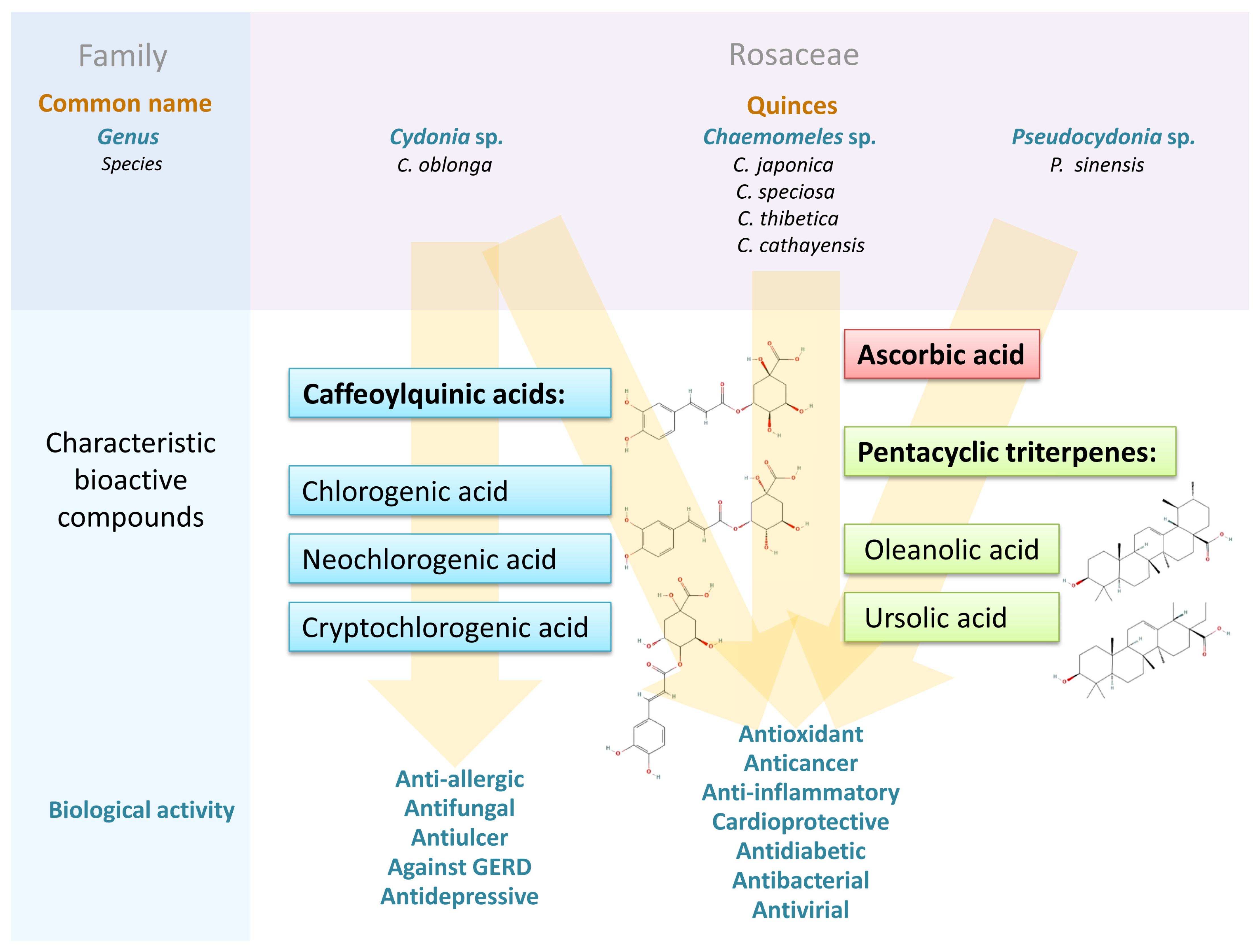
Importantly, C. oblonga fruit contained approximately twice as much total phenolics as apple fruit, both of which are used as raw materials [56]. All Cydonia organs contained significant amounts of phenolic acids; especially neochlorogenic (3-O-caffeoylquinic), cryptochlorogenic (4-O-caffeoylquinic), and chlorogenic (5-O-caffeoylquinic) acids have been widely reported. Since the numbering of chlorogenic acid atoms remains ambiguous, this review follows the nomenclature adopted by the authors of the cited papers.
Research by Andrade et al. [52] showed that the pulp contained significantly more chlorogenic acid (6770 mg/kg) than the closely related apples and pears. Sut et al. [40] showed 411 mg/kg chlorogenic acid in the peel, which was significantly less than in the pulp, while in several apple cultivars, the amounts ranged from 5 to 305 mg/kg. Typical Cydonia flavonoids were kaempferol, quercetin, and their various glycosidic derivatives; however, they appeared to be less abundant components compared to procyanidins and chlorogenic acid derivatives [40,57]. Analysis of flavonoid content showed that quince is a rich source of quercetin-O-3-galactoside, quercetin-O-3-rhamnoside, and quercetin-O-3-rutinoside (rutin) compared to their content in apple and pear pulp. In the case of rutin, about four times more was found in the pulp than in apple pulp [52].
While the content of phenolic acids and flavonoids in various quince tissues has been relatively well documented, there are few data on the content of tannins. Based on the data presented by Sharma et al. [58], we know that they are found in fruit juice at a level of 0.8%. The content of procyanidin B1 in the pulp was significantly higher (65 mg/kg) than in the pulp of apple fruits (2–19 mg/kg).
In general, a higher content of bioactive compounds was found in the peel of C. oblonga than in the pulp [37]. Both pulp and peel contained large amounts of caffeoylquinic acids, mainly types 3- and 5-, but the peel turned out to be a reservoir of flavonoid compounds. However, the differences in their amounts reported in the literature are significant. The final result of phenol content depends on the choice of cultivar, growing conditions, including soil pH, humus, and mineral content, length of the growing season, weather conditions, and the overall physiological state of plants. In addition, the fruit ripeness, duration, and storage conditions are very important. Quinces are known for being able to be stored for a long time, but the storage process affects the content of individual bioactive compounds. Each research group individually selects the extractant, which has a significant impact on the number and proportions of extracted compounds. In addition, variations in drying and extraction methods, as well as differences in measurement techniques, account for the differences observed in Table 1, Table 2, Table 3 and Table 4. According to research on the extraction of C. japonica phenolics, the most effective extractants among the nine tested were 50% ethanol, 100% methanol, and 50–70% acetone [59].
C. oblonga seeds, in turn, contained significantly fewer phenolics in total, but their more considerable diversity was observed. Among them, less common compounds could be distinguished, such as flavonoid di-C-glycosides: schaftoside, isoschaftoside, lucenin-2, stellarin-2, and vicenin-2 [36,38,41].
One research article is particularly important in that it compares the polyphenol content in the fruits (and leaves) of C. oblonga, C. japonica, and apple [47]. The authors identified 2909 mg of phenolic compounds in C. oblonga, as much as 7643 mg in C. japonica, while apples contained only 1312 mg per 100 g of dry mass (dm), which means that C. oblonga fruits were twice as rich in phenolics as apples and, which is worth emphasizing, C. japonica fruits were about three times richer in phenolic compounds than the Cydonia fruits.
The analysis of leaf phenolics revealed that they were more abundant in the leaves of C. oblonga than in the leaves of other species known for their high phenolic content, such as chokeberry, cranberry, blueberry, and blackcurrant. However, the more significant differences in the content of specific phenolics were observed in the case of mono-, di-, and oligomeric flavan-3-ols; C. japonica fruits contained 4595 mg/100 g dm, while C. oblonga fruits contained more than 50 times less. In turn, both the fruits and the leaves of C. oblonga were about three times richer in phenolic acids (273 and 3894 mg/100 g dm, respectively) than the fruits and leaves of C. japonica. The contents of flavonols in the leaves and fruits of the two compared quince species were similar [47].
Unfortunately, we have much less data on the polyphenol contents of both fresh and dried Chaenomeles and P. sinensis fruits (Table 4). The analysis is complicated by the fact that sometimes extracts were prepared from whole fruits after removal of the seed core, probably due to the smaller size of the fruits, and then freeze-dried, in which case the results of all measurements were expressed on a dry weight basis, while other researchers used extracts from fresh pulp. Urbanavičiūtė et al. [59] analyzed the total phenolic content in C. japonica extracts and found that it ranged from 4523 to 6785 mg/100 g dm. Significant differences in the obtained values resulted from specific combinations of parameters (i.e., type of solvent, time, power, and temperature of ultrasonic extraction). Tarko et al. [60], reported that the total phenolic content was 924 mg catechin equivalents per 100 g dm, which is approximately50% higher than that found in the fruits of cornelian cherry and black mulberry, both of which are known for their high phenolic content. Several studies identify numerous phenolic compounds in these fruits [3,28,47,61,62,63,64], but only a few of them provide numerical values. Among those already mentioned, there are phytochemical studies that showed the presence of flavonoids [63,65], lignan glycosides [66], biphenyl derivatives [67], as well as essential oils [68], pentacyclic triterpenes [62,63] and sesquiterpenoids [62], the last three outside the polyphenol group. In turn, in the group of C. japonica flavonols, those that were highly abundant in fruits and leaves were indicated, i.e., (+)-catechin, (–)-epicatechin, and procyanidins B1, B2, B3, and C1 [47]. Among the bioactive compounds, polyphenols (mainly phenolic acids and flavonoids) and triterpenes were considered to be the major classes of phytochemicals in C. speciosa [4].
An interesting study was carried out by Hellín et al. [69], who used fruit juice from five taxa of the genus Chaenomeles (C. japonica, C. speciosa, C. cathayensis, C. japonica × C. speciosa, and C. × superba) and determined 210–592 mg of phenolic compounds in 100 mL of juice obtained from C. japonica fruits collected from different locations. Juices of other Chaenomeles species contained even more phenolics, i.e., 591 mg in 100 mL for C. cathayensis. These amounts were significantly higher than in apple juice (339 mg/100 mL) [70]. Du et al. [62] presented a comparison of the amounts of major phenolic compounds in fruits of five Chaenomeles species. They showed an abundance of chlorogenic acid in C. speciosa, C. thibetica, and C. cathayensis and a low content in P. sinensis and C. japonica. Catechin and procyanidin B1 were abundant in C. thibetica and C. cathayensis and moderate in C. speciosa. On the contrary, epicatechin and procyanidin B2 were predominant in C. speciosa, P. sinensis, and C. japonica. Research by Vila et al. [71] confirmed that Chaenomeles fruits from southern growing areas contained significantly more phenolic compounds than those from northern growing areas. During ripening, the total phenolic content showed a slight tendency to decrease from two weeks before harvest. This pattern is consistent with observations made for many Rosaceae fruits.

Table 4.
Phenolic contents in Chaenomeles sp. and Pseudocydonia sinensis fresh and dried fruits (extracted with water:acetone).
Table 4.
Phenolic contents in Chaenomeles sp. and Pseudocydonia sinensis fresh and dried fruits (extracted with water:acetone).
| Compound | Species | Content | Ref. | |
|---|---|---|---|---|
| Apigenin | C. japonica | 19.66 | mg/100 g dm | [28] |
| 3-CQA (5-O-caffeoylquinic acid) neochlorogenic acid | P. sinensis | 5.00 | mg/100 g fm | [53] |
| 4-CQA (5-O-caffeoylquinic acid) cryptochlorogenic acid | P. sinensis | 1.20 | mg/100 g fm | [53] |
| 5-CQA (5-O-caffeoylquinic acid) chlorogenic acid | C. japonica | 818.55 | mg/100 g dm | [28] |
| 10.00 | mg/100 g fm ** | [62] | ||
| 12.17 | mg/100 g dm * | [72] | ||
| C. speciosa | 182.00 | mg/100 g fm ** | [62] | |
| C. thiberica | 117.00 | mg/100 g fm ** | [62] | |
| C. cathayensis | 119.00 | mg/100 g fm ** | [62] | |
| P. sinensis | 9.00 | mg/100 g fm ** | [62] | |
| 0.50 | mg/100 g fm | [53] | ||
| (+)-Catechin | C. japonica | 15.75 | mg/100 g dm * | [72] |
| Catechin | C. japonica | 121.12 | mg/100 g dm | [28] |
| C. speciosa | 54.00 | mg/100 g fm ** | [62] | |
| C. thiberica | 156.00 | mg/100 g fm ** | [62] | |
| C. cathayensis | 113.00 | mg/100 g fm ** | [62] | |
| P. sinensis | 5.00 | mg/100 g fm ** | [62] | |
| 2.90 | mg/100 g fm | [53] | ||
| trans-Cinnamic acid | C. japonica | 18.72 | mg/100 g dm | [28] |
| p-Coumaric acid | C. japonica | 5.72 | mg/100 g dm | [28] |
| (–)-Epicatechin | C. japonica | 348.44 | mg/100 g dm * | [72] |
| Epicatechin | C. japonica | 102.00 | mg/100 g fm ** | [62] |
| C. speciosa | 235.00 | mg/100 g fm ** | [62] | |
| P. sinensis | 54.00 | mg/100 g fm ** | [62] | |
| 11.90 | mg/100 g fm | [53] | ||
| 2,5-di-Hydroxybenzoic acid | C. japonica | 2.01 | mg/100 g dm | [28] |
| 4-Hyrdoxybenzoic acid | C. japonica | 1.92 | mg/100 g dm | [28] |
| Ferulic acid | C. japonica | 2.17 | mg/100 g dm | [28] |
| Isoquercitrin | C. japonica | 3.82 | mg/100 g dm | [72] |
| Naringenin | C. japonica | 5.92 | mg/100 g dm | [28] |
| Procyanidin B1 | C. speciosa | 83.00 | mg/100 g fm ** | [62] |
| C. thiberica | 222.00 | mg/100 g fm ** | [62] | |
| C. cathayensis | 145.00 | mg/100 g fm ** | [62] | |
| P. sinensis | 13.00 | mg/100 g fm ** | [62] | |
| 9.80 | mg/100 g fm | [53] | ||
| Procyanidin B2 | C. japonica | 98.00 | mg/100 g dm ** | [62] |
| C. speciosa | 296.00 | mg/100 g fm ** | [62] | |
| P. sinensis | 16.80 | mg/100 g fm | [53] | |
| 40.00 | mg/100 g fm ** | [62] | ||
| Quercitin | C. japonica | 5.03 | mg/100 g dm | [28] |
| Q-3-R (quercetin-3-O-rutinoside) rutin | C. japonica | 107.09 | mg/100 g dm | [28] |
| 5.40 | mg/100 g dm * | [72] | ||
| Sinapic acid | C. japonica | 27.99 | mg/100 g dm * | [72] |
| Syringic acid | C. japonica | 0.03 | mg/100 g dm | [28] |
| Vanilic acid | C. japonica | 13.69 | mg/100 g dm | [28] |
fm—fresh mass, dm—dry mass. * Results for water: ethanol extract. ** Results for the pulp.
The seed sockets of C. japonica fruits are large compared to the size of the mesocarp. It is therefore not surprising that attempts have been made to use the seeds. Dried seeds contain approximately 6–16% oil [73]. Cold pressing resulted in an oil with promising health-promoting properties. It contained the highest amount of polyphenols (64 mg/kg) compared to sesame, poppy, peanut, flaxseed, pumpkin, sunflower, almond, hazelnut, and walnut oils [34]. Six phenolic compounds were found in it, viz., 4-hydroxybenzoic acid, vanillic acid, vanillin, p-coumaric acid, ferulic acid, and trans-cinnamic acid [74]. In turn, Turkiewicz et al. [75] studied the content of essential phytochemicals in Chaenomeles leaves and concluded that they could be a good material for obtaining extracts rich in phenolics, mainly procyanidins, and quercetin and its glycosides.
Our understanding of the polyphenolic components of P. sinensis fruit is limited. The phenolic total content, which was measured via the Folin–Ciocalteu assay, was found to be 1280 mg/100 g of fresh mass (fm). It was about four times higher than that of C. oblonga fresh fruit (303 mg/100 g) and 20 times higher than that of apple fresh fruit (61 mg/100 g) [53]. A more detailed study showed that P. sinensis fruit contained 24 phenolic compounds, of which 20 were flavan-3-ols such as catechin, epicatechin, and procyanidins, which accounted for 94–99% of the total polyphenols [53,76]. Research by Hamauzu and colleagues [25] showed the presence of polyphenols in the aqueous solution of P. sinensis, including procyanidin B3, (+)-catechin, procyanidin B4, procyanidin B2, (–)-epicatechin, and oligomeric and polymeric procyanidins. It is worth emphasizing that procyanidins are compounds found in the fruits of all described genera, with a great diversity of them found in the fruits of Chaenomeles sp. and P. sinensis. However, because the fruits of C. oblonga are generally the best known, procyanidins are often associated with them. As shown, the content of polymeric procyanidins decreased during heat treatment. Changing the ratio of polymeric to oligomeric and monomeric forms improved the ability to absorb protocatechuic acid in the small intestine and the susceptibility to metabolization by the microbiome [25].
3.2. Ascorbic Acid, Carotenoids, and Other Antioxidants
A characteristic feature of Cydonia and Chaenomeles fruits is the high content of vitamin C (ascorbic + dehydroascorbic acids) compared to the more common fruits of the Rosaceae family, such as apples, pears, or plums. The study by Souci et al. [77] determined 13 mg/100 g fm, which is only slightly more than in apples, while Sharma et al. [58] found a slightly higher value, i.e., 17 mg/100 g fm.
The literature shows higher vitamin C content in Chaenomeles than in C. oblonga fruits and significantly higher than in other common fruits [78]. Vila et al. [71] found 18–50 mg per 100 mL of Chaenomeles juice obtained from fruits harvested in the southern growing areas where its increased production was observed. Hellín et al. [69] obtained 45–78.5 mg of ascorbic acid in 100 mL of C. japonica juice, but significantly more in C. speciosa, C. cathayensis, and C. × superba fruit juices (102, 103, and 109 mg/100 mL, respectively). Bieniasz et al. [79] found it in a wide range of 68–207 mg/100 g fm depending on genotype and season, Hallmann et al. [80] measured it at 63 mg/100 g fm, while Zhang et al. [78] obtained values in a similar range of 69–159 mg/100 g. In turn, Baranowska-Bosiacka et al. [16] confirmed that fruits contain a substantial amount of ascorbic acid (55–92 mg/100 g fm) and this content remains relatively stable during storage and processing. Mezhenskij [81] found that fresh C. × superba fruits contain 60–150 mg of this acid per 100 g, based on data collected over eight years. The values obtained by Hallmann et al. [80] and mentioned above were only about twice lower than those of fruits considered to be unique sources of this vitamin, i.e., wild rose (Rosa rugosa Thunb.), and about 60% lower than those of rowan berries (Sorbus aucuparia L.).
The fruits of Cydonia contain carotenoids, which are antioxidants known to quench reactive oxygen species, including very harmful singlet oxygen. Souci et al. [77] determined 0.05 mg of carotene in 100 g fm and 5.5 µg of its derivative, retinol (vitamin A). The fruit material also contained thiamine (vitamin B1, 30 μg/100 g), riboflavin (vitamin B2, 30 µg/100 g), and niacin (vitamin B3, 0.2 mg/100 g), but not biotin and folic acid as found in an apple. Legua et al. [82] showed that the total concentration of carotenoids was higher in the peel (0.16–0.86 mg/100 g, depending on the clone) than in the pulp (0.04–0.42 mg/100 g) and that the color of the peel did not correlate with the color of the pulp. In recent studies by Najman et al. [83,84], the authors compared the total trans carotenoid content in fresh, dried, and processed fruits and obtained higher values. The β-carotene content was 13.6 mg/100 g fm, and the xanthophyll levels were significantly lower: 3.5 and 1.4 mg/100 g fm for lutein and zeaxanthin, respectively. Drying the fruit at 50 °C and 70 °C, freeze-drying, cooking, and frying increased the content of zeaxanthin and β-carotene by about five times. Lutein was more sensitive to conventional drying, but all types of processing also contributed to the increase in this xanthophyll.
A paper by Hallmann et al. [80] showed that among the carotenoids, C. japonica fruits contained mainly lutein (40 µg/g fm), lycopene (20.5 μg/g fm), and a small amount of β-carotene (1.7 µg/g fm). In a study by Turkiewicz et al. [85], Chaenomeles fruits of three species, i.e., C. × superba, C. japonica, and C. speciosa, and 19 cultivars contained 32–315 mg/kg dm of carotenoids (and some cultivars of C. × superba were the richest in carotenoids), 5.5–38 mg/kg dm of tocopherols, and 2–42 mg/kg dm of tocotrienols (both groups of vitamin E activity). Five carotenoids (all-trans-lutein, all-trans-β-cryptoxanthin, all-trans-α-carotene, all-trans-β-carotene, and 9- or 9′-cis-β-carotene), as well as four isomers of tocopherols and four tocotrienols, were identified in the fruits tested, regardless of cultivar. The predominant carotenoid was β-carotene and the predominant tocopherol was α-tocopherol, making these fruits a valuable source of provitamin A and vitamin E. Subsequent investigations by Turkiewicz et al. [75] have shown that Chaenomeles leaves can also be a good material for obtaining a tocopherol-rich extract whose content values ranged from 0.7 to 10.7 IU depending on the cultivar (100 g dm of leaves cover on average 24% of the daily requirement for vitamin E). On the other hand, the product obtained from C. japonica seeds, i.e., cold-pressed seed oil, contained the highest amounts of tocopherols (726 mg/kg) and β-carotene (11 mg/kg) compared to sesame, poppy, peanut, flaxseed, pumpkin, sunflower, almond, hazelnut, and walnut oils [33].
Among the compounds with proven biological effects, including antioxidant activity, pentacyclic triterpenes also play an essential role. Ursolic and oleanolic acids are characteristic chemical markers of Chaenomeles, which can be used to evaluate and classify the quality of this plant [86]. The presence of a new acylated triterpene (3-(O-(E)-3,5-dihydroxycinnamoylursolic acid) together with ursolic, oleanolic, and pomolic acids was demonstrated by Xu et al. [87].
3.3. Minerals
C. oblonga fruits are rich in mineral elements, especially Ca, K, and P, making them almost twice as rich in minerals as an apple [1,29]. Other studies, however, showed average amounts compared to the most commonly consumed fruits in Europe, i.e., K: 248 mg/100 g; P: 26 mg/100 g; Na: 8 mg/100 g; and Ca: 18 mg/100 g [57].
C. japonica fruits are also rich in minerals compared to other Rosaceae fruits, especially Fe and Mo, making them one of the richest sources of these elements. Also noteworthy are the high contents of Mg, Na, Cu, Zn, and P [3,88], although these contents were similar to those determined in C. oblonga fruits. The analysis by Baranowska-Bosiacka et al. [16] confirmed the high content of micro (Fe, Cu, Zn, Mn, and Mo) and macro (Mg, Ca, P, K, and Na) elements. The contents of Fe and Mo in these tests were 0.516 mg and 0.02 mg per 100 g dm, respectively. There are significant differences in the content of individual minerals in the fruits of related genera: Chaenemeles and Pseudocydonia are also interesting. For example, the content of K in the fruits of C. japonica was 249 mg/100 g, in C. speciosa it was much lower (up to 147 mg/100 g), and in P. sinensis K was not detected at all [88]. A study by Hellín et al. [69] found similar concentrations of K, ranging from 153 mg (C. cathayensis) to 241 mg (C. speciosa) in 100 mL of juice. C. japonica fruits were the most abundant in Mg, as confirmed by Hellín et al. [69], while P. sinensis contained the highest amounts of Fe and Mn (2.6 and up to 3.1 mg/100 g, respectively). The contents of Cu, Zn, and Ca were similar in all fruits of these species [78,88,89].
3.4. Carboxylic Acids
The fruits of all the species discussed in this review owe their distinctive flavor to their high content of organic acids. The presence of citric, malic, and fumaric acids has been confirmed in both the peel and pulp of Cydonia fruits [35,44]. Of these, citric acid was the most quantitatively determined, followed by malic and oxalic acids [44]. According to Silva et al. [54], in quince pulp and peel, the sum of malic and quinic acids represented 93%, so all other acids were present in minimal amounts, less than 0.5%, with the exception of citric and ascorbic acids. The sum of all quantified acids was about 7 g/kg in both pulp and peel, which is in agreement with the previously reported results [35].
There is a limited amount of research on the carboxylic acid content of Chaenomeles fruits. It is known that the fruits of C. × superba contain 4–5% organic acids (data collected over eight years) [81]. In a study by Hellín et al. [69], three acids (malic, quinic, and succinic) were detected in the fruit juices of five Chaenomeles taxa: C. japonica, C. speciosa, C. cathayensis, C. japonica × C. speciosa, and C. × superba. The typical organic acids such as citric, oxalic, tartaric, and galacturonic acids were not found in detectable amounts. The concentration of malic acid was similar in all tested juices, ranging from 3.06 to 5.09 g/100 mL. Slightly more significant differences were observed in the case of quinic acid, the juice from C. speciosa fruit containing significantly more of it. The same juice was particularly rich in succinic acid (174 mg/100 mL). In comparison, the other juices contained no more than 27.1 mg/100 mL (C. japonica and C. japonica × C. speciosa) and 52.5 mg/100 mL (C. cathayensis). Notably, Baranowska-Bosiacka et al. [16] showed a low oxalate content (8.21 mg/100 g fm) in the fruits of C. japonica.
3.5. Carbohydrates Including Fiber
Analyses by Lesińska et al. [90] showed that fresh C. oblonga fruits contained 7.18% of sugars, while Sharma et al. [58] determined 9% of total sugars, including 5% of reducing sugars in the juice. According to Rasheed et al. [91], 100 g of pulp contained 13.4 g of carbohydrates, of which 5.15 g was reducing sugars. HPLC analyses revealed the presence of monosaccharides: rhamnose, mannose, glucose, arabinose, and galactose [92,93]. Lesińska et al. [90] indicated that fructose was the dominant sugar (61.6%), followed by glucose, which accounted for 22.4%. The authors also showed that the total sugar content in C. oblonga was lower than in apples, pears, plums, and cherries.
Chaenomeles fruits contained about twice as much sugar as Cydonia fruits (3.8% bm) [90]. Nine carbohydrates were identified in their juice, i.e., stachyose, raffinose, sucrose, glucose, xylose, rhamnose, fructose, inositol, and sorbitol [69]. The dominant sugar was fructose, followed by glucose [69,90]. Considering the sugar content in fruit juices of different taxa, C. cathayensis is noteworthy as it contained 2–3 times more glucose and about twice more fructose than other juices tested [69].
The fruit of C. oblonga is known for its high pectin content, which makes it suitable for use in the food industry as a gelling ingredient, and for its crude fiber, which is beneficial to the digestive system, alleviating gastrointestinal disorders and cardiovascular diseases, and inhibiting the formation of some gastrointestinal cancers [1,91]. The average content of pectin in fruits of different varieties was 2 g/100 g [1] or 1.8 g/100 g [58]. For crude fiber, it ranged from 1.56 to 1.65 g/100 g [90]. Similar values of 1.6% and 1.9% were found by Sharma et al. [58] and Hegedus et al. [94].
Studies on fiber in Chaenomeles fruit have yielded more inconsistent results. Thomas et al. [95] distinguished three groups of quince genotypes: a low-fiber group (three genotypes, 28–30 g/100 g dm), a medium-fiber group (nine genotypes, 30–36 g/100 g dm), and an isolated genotype (Chaenomeles speciosa) that contained a considerable amount of fiber (38 g/100 g dry matter). Studies on cell wall polysaccharides showed that 100 g of dry fruit contained 11 g of pectins, 3 g of hemicelluloses, and 18 g of cellulose residues [96]. Later research by Thomas et al. [97] confirmed the above-mentioned pectin contents in C. japonica fruits, i.e., 11 g per 100 g dm and 1.4 g per 100 g fm. Hellín et al. [98] showed the high content of dietary fibers and pectins in C. japonica fruits, which encouraged them to use the juice to improve the quality of bread. P. sinensis fruits have been shown by Qin et al. to contain a high amount of polysaccharides, accounting for 11% of the dry pulp [99]. According to the authors, this fruit can be used as a source of commercial pectin due to its high pectin content. On the other hand, Baranowska-Bosiacka et al. [16] found only 4.7% dietary fiber in fresh C. japonica fruit. Similarly, a study by Mezhenskij [81] showed that the fruits of C. × superba contained a lower amount of pectins and significantly less than those of C. oblonga, i.e., only 0.6%. These differences were probably due to the different maturity of the fruits. The highest pectin content was found in unripe fruits.
4. Biological Activity of Quince Fruits
The fruits of C. oblonga have been used since ancient times in the Middle East and the Mediterranean region. It is an essential plant in Iranian traditional medicine (ITM) and modern phytotherapy, which is used to prevent or treat many diseases such as cancer, diabetes, hepatitis, ulcers, and respiratory and urinary tract infections [93,100]. In this review, I have focused on the properties of the fruits, but a literature analysis shows that most of the works describing the health-promoting properties of C. oblonga concern the leaves [101]. The dried fruits of Chaenomeles are one of the most important drugs in traditional Chinese medicine (TCM). They have been used for thousands of years to treat asthma, colds, sore throats, tuberculosis, mastitis, and hepatitis [102]. In TCM, C. speciosa fruit is used to treat gastric disorders, dyspepsia, dysentery, enteritis, influenza, and rheumatic inflammation [103,104]. P. sinensis fruit has been used alone or in combination with other herbs to treat diarrhea, vomiting, muscle aches, and colds [105], as well as an antitussive, antiflatulent, and diuretic. It is also known for its expectorant activity [106] and its extract is traditionally used to treat viral infections [107].
In this review, the biological activities of the analyzed fruits, described in the available literature, have been segregated and presented below, taking into account the dominant ones, and realizing that due to the complexity of the molecular mechanisms leading to the disorder development, this could be prepared and discussed in many ways. The relevant data are extracted in Table 5 and summarized in Figure 4 [108].

Table 5.
Biological activities of quince (C. oblonga, Chaenomeles sp., and P. sinensis) fruits.
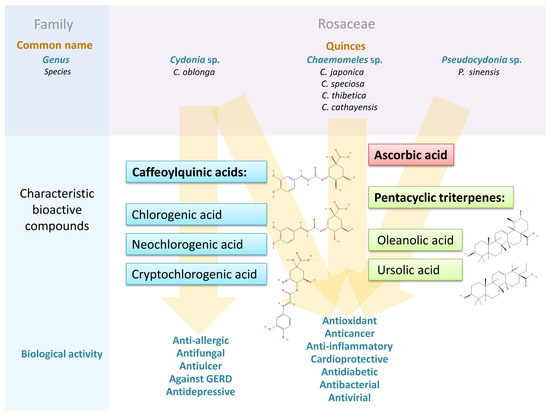
Figure 4.
The characteristic bioactive compounds and biological effects of quince (C. oblonga, Chaenomeles sp., and P. sinsnsis) fruits [108].
4.1. Antioxidant Properties
In general, antioxidant activity is attributed to radical scavenging, prevention of chain reaction initiation, binding of transition metal ion catalysts, decomposition of peroxides, and prevention of continuous hydrogen uptake [159]. In addition to cell-produced antioxidant enzymes and low-molecular-weight antioxidants such as Cys or glutathione (GSH), valuable components of the human diet are plant antioxidants derived from a large group of secondary metabolites that may be helpful in the treatment of diseases associated with the overproduction of reactive oxygen and nitrogen species (ROS/RNS). Numerous studies have shown that low-molecular-weight antioxidants may play an important role in the prevention and treatment of many human diseases, including those resulting from a highly processed diet, the presence of environmental pollutants, and inappropriate lifestyle choices [160,161]. Therefore, the various effects of food isolates, including those from quince, may play a cocktail role in therapies whose common features are oxidative stress and inflammation. The topic of using quince fruit extracts appears most often in the literature due to their significant content of antioxidants [36,37,53,61,111,162,163,164,165,166], including polyphenols and ascorbic acid. However, it is worth mentioning that these fruits also contain other potent antioxidants such as carotenoids, tocopherols, and tocotrienols, as well as minerals (including Fe and Mn) that are cofactors of antioxidant enzymes.
Numerous studies have demonstrated that C. oblonga tissues are rich in phenolic acids and flavonoids, which are potent antioxidants [36,37,38,39,41,43,164,167]. Phenolics can act as antioxidants in several ways: as reducing agents, hydrogen donors, free radical scavengers, and singlet oxygen quenchers [158]. One of the first studies using TBARS (thiobarbituric acid reactive substances) and ABTS+ (3-ethyl-benzothiazoline-6-sulfonate) assays showed that its fruits were among the 5 fruits with the highest antioxidant/antiradical capacity out of 28 tested [111]. Scavenging activities against DPPH (2,2-diphenyl-1-picrylhydrazyl) and peroxyl radicals as well as protective activities against erythrocyte damage were observed by Costa et al. [46]. They showed significantly greater reducing power than that of green tea, which is often used as a reference plant, but the antioxidant properties varied significantly depending on the extraction method and extractant. Torres and colleagues [56] analyzed the antiradical capacities of C. oblonga and apple fruits using DPPH and ORAC (oxygen radical absorbance capacity) assays. The results showed that quince had 40% higher scavenging DPPH radicals expressed in Trolox equivalents and 50% higher ORAC values, respectively, than apple.
In turn, Baroni et al. [109] analyzed acidified extracts from C. oblonga pulp and jam made from the pulp. They obtained high values of scavenging DPPH radicals (2166 µM Trolox/100 g fresh pulp) and FRAP (ferric ion reducing antioxidant power; 2433 µM Trolox/100 g fresh pulp). The processing of quince did not significantly affect the antioxidant capacity as measured by the above two tests, since it was 60% of the starting material. In their subsequent work, extracts obtained similarly to the above were evaluated in terms of the effect of processing and simulated digestion on the antioxidant properties of quince jam. Oral digestion showed that only 30% of its phenolics were bioavailable. After gastric digestion, this percentage increased to 44%. In turn, after digestion and absorption in the small intestine, only 2.7% and 24% of the original phenolics were detected in the dialyzed and non-dialyzed fractions, respectively. Quinic acids were found to be the most resistant to digestion [55].
Yildirim et al. [112] analyzed the antioxidant activity and reducing power of aqueous, ethanolic, and ethereal extracts of C. oblonga leaves and showed that the latter had the highest total antioxidant activity although it had low reducing power. On the other hand, the ethanolic extracts had the highest reducing power while the ethereal extracts had the lowest. Methanol leaf extract obtained before fruit ripening has also been successfully used in studies to alleviate hematotoxic stress induced by UV-A radiation [44].
In a study by Pacifico et al. [113], aqueous fermented C. oblonga fruit extract effectively scavenged DPPH and the anion superoxide radical with ID50 values of 69 µg/mL and 74 µg/mL, respectively. In contrast, quince lipophylic wax extract was more effective in preventing the formation of thiobarbituric acid reactive species (TBARS) with an ID50 of 49 µg/mL.
Scientific data on the antioxidant properties of Chaenomeles plants are less documented. One of the older works [115] showed that powder processed from C. speciosa showed good scavenging activity against DPPH, with a scavenging rate of 945 µg/g and 700 U/mL, and a FRAP value of 173 µmol Fe2+/g. In the work by Du et al. [59], the antioxidant activity of the extracts was investigated by ABTS+, FRAP, and DPPH assays. The highest value of Trolox equivalent antioxidant capacity (TEAC) was obtained for C. speciosa, which was 310 and 97 µmol/g fm with ABTS+ and FRAP, respectively, while C. thibetica extract was slightly less effective, exhibiting TEAC values of 254 and 84 µmol/g fm with ABTS+ and FRAP, respectively. It is worth noting that the fruits of both species showed more significant antioxidant properties than goji (Lycium ruthenicum Murray) and guava. C. japonica extracts had the lowest TEAC values (118 and 19 µmol/g with ABTS+ and FRAP, respectively). P. sinensis fruit extract was also the most effective in scavenging the DPPH radicals, followed by C. speciosa, while C. japonica extract was the least effective. Among the five extracts, the values obtained for four (excluding C. japonica) were between those of standard antioxidants such as ascorbic acid and BHT but were higher than those of Trolox [62]. Pearson correlation analysis confirmed that polyphenols, including proanthocyanins, are potent antioxidant and radical scavenging compounds in quince extracts [62,168]. The work by Baranowska-Bosiacka et al. [16] showed a significant content of antioxidants in C. japonica fruits and demonstrated that its aqueous extract had a hepatoprotective effect, observed as a decrease in the concentration of lipid peroxides. According to a recent study [72], the juice and pomace exhibited radical scavenging capacities of 15 and 70 µmol TE/100 g and 152 and 938 µmol TE/100 g, respectively, as measured by DPPH and ABTS+.
A study by Zhang et al. [123] showed that 2 of the 13 components isolated from C. speciosa fruits, i.e., 3,4-dihydroxybenzoic acid and quercetin, exhibited the highest DPPH scavenging activity, with IC50 values of 1.02 and 3.82 μg/mL, respectively. On the other hand, Deng et al. [116] found two peptides (RHAKF and NNRYE) in C. speciosa seeds after protein hydrolysis. RHAKF has been shown to scavenge DPPH radicals and superoxide anions, inhibit lipid peroxidation, and, in addition, inhibit tyrosinase [114], which may be an ingredient in cosmetics due to its involvement in melanogenesis. In terms of cosmetological applications, a partially similar potential use was found for P. sinensis. The sarcocarp extract was characterized by an effect similar to superoxide dismutase (SOD) and a pronounced collagenase inhibitory activity. The extract contained condensed tannins as the main polyphenolic components [169]. A type IV collagenase inhibitory effect was also observed for C. japonica fruit extract [131]. According to the authors, the main bioactive constituents were proanthocyanidins. There are several reports in the literature on the antioxidative properties of various products and by-products obtained from quince, e.g., polysaccharides from P. sinensis seed meal, which is a by-product of oil processing, used as fertilizer and animal feed [170]. Ma et al. [117] demonstrated that a 70% ethanolic extract of C. thiberica fruit, which is rich in phenolic compounds and has in vitro antioxidant activity, increased the levels of catalase (CAT), SOD, and GSH in rats. Additionally, the extract reduced MDA, a product of free-radical-induced oxidation of unsaturated fatty acids, which is treated as an indicator of lipid peroxidation. It also showed a protective effect on rats with chronic liver injury injected with CCl4 via the mitogen-activated protein kinase/nuclear factor (erythroid-derived 2)-like2 (MAPK/Nrf2) pathway.
4.2. Anti-Inflammatory, Anti-Allergic, and Various Immunomodulatory Effects
Recent studies on plant secondary metabolites give hope for the development of naturally derived drugs that could contribute to the treatment of diseases associated with chronic inflammation, such as rheumatoid arthritis, gastritis, inflammatory bowel disease, atherosclerosis, cancer, and many others [110].
Kawahara and Iizuka [119] evaluated the effect of a crude hot water extract of C. oblonga fruit on IgE-dependent late-phase immune responses of mast cells using an in vitro system. The extract reduced the induction of intracellular cyclooxygenase (COX)-2 expression but not COX-1 expression in mouse bone marrow-derived mast cells. It also reduced the elevation of interleukin (IL)-13 and tumor necrosis factor (TNF)-α expression levels and suppressed these cytokine expressions as well as leukotriene C4 and prostaglandin D2 production in the cells tested [3,119].
C. oblonga hot water extract was also found to have an inhibitory effect on type I allergies by suppressing immunoglobulin E (IgE) production and IgE-mediated degranulation [120]. NC/Nga mice fed with the extract showed a significant decrease in the development of atopic dermatitis-like skin lesions under conventional conditions. Serum IgE concentration was reduced in a dose-dependent manner, and the release of β-hexosaminidase from the rat basophilic leukemia cell line RBL-2H3 was inhibited. The extract fraction with masses below 3 kDa reduced the mRNA expression of the high-affinity subunit of the IgE receptor γ (Fc″RI) [120].
A combination of lemon juice and aqueous C. oblonga extract (Gencydo®) is traditionally used in anthroposophical medicine for the treatment of allergic rhinitis and asthma by down-regulating soluble mediators that are essential for the initiation of allergic reactions. It has been proven that Gencydo® reduced the degranulation and histamine release of IgE-activated basophils and mast cells and inhibited the increase in IL-8, TNF-α, and granulocyte–macrophage colony-stimulating factor (GM-CSF) production in mast cells. In addition, it partially blocked eotaxin release from human bronchial epithelial cells, but did not affect the viability and activation of GM-CSF-activated eosinophil granulocytes [121].
Although the anti-inflammatory activity of C. oblonga fruits has been confirmed in many studies, the mechanisms of action of individual compounds often remain unclear. Recent studies using network pharmacology proved to be useful in the prediction of the anti-inflammatory mechanism of the quinic acids in which the C. oblonga fruit is extraordinarily abundant, namely chlorogenic, neochlorogenic, and cryptochlorogenic acids, as well as their four metabolites. They demonstrated anti-inflammatory effects through 52 common targets. Their analysis indicated that a chlorogenic acid homolog and its metabolites could act as signal molecules binding to these targets and regulating the biological functions of related targets. Enrichment analysis showed that the top ten pathways were p75 (NTR)-mediated signaling, MAPK-related pathways, glutathione conjugation, S1P2 pathway, TNF receptor signaling pathway, p38 MAPK signaling pathway, ALK1 pathway, Phase II conjugation, biological oxidations, and dopamine degradation [171].
An ethanolic extract of P. sinensis fruit, long used as a folk medicine for cough, revealed significant inhibitory effects on the pruritogenic compound 48/80 calcium oxalate monohydrate (COM)-induced scratching behavior in mice. Quercetin, catechin, and apigenin derivatives (apigenin-7-glucronide and apigenin-9-methoxy-7-glucronide, first found in P. sinensis fruits) showed significant inhibitory effects on COM-induced scratching behavior. The active fraction and these compounds also inhibited scratching induced by serotonin, platelet-activating factor, and prostaglandin E2, confirming that P. sinensis fruit can relieve itching in allergic patients [106].
As many reports have shown, there are potent antioxidants among the polyphenols of C. oblonga peel extract. In addition, a study by Essafi-Benkhadir et al. [110] also showed its anti-inflammatory properties, inhibiting TNF-α and IL-8 in a dose-dependent manner and increasing the levels of the anti-inflammatory IL-10 secreted by lipopolysaccharide (LPS)-treated macrophages. Analyses showed that this extract inhibited LPS-mediated activation of three major cellular pro-inflammatory effectors: nuclear factor-κB (NF-κB), p38MAPK, and protein kinase B (Akt).
The group of Li [124] found that chlorogenic acid was one of the components responsible for the anti-inflammatory effect of C. speciosa, which is confirmed by the results presented above [122]. Its 10% ethanolic fraction showed significant anti-inflammatory effects in the xylene-induced ear edema test, the acetic-acid-induced peritoneal capillary permeability test, and the cotton pellet granuloma test in mice or rats; it also showed marked analgesic activity in the acetic acid-induced abdominal contraction test and the formalin-induced paw licking test in mice and rats. Chlorogenic acid has also been found in the fruits of other Chaenomeles taxa and, as mentioned above, in large amounts in the pulp of C. oblonga.
Three compounds isolated from the ethanolic extract of C. speciosa, namely 3,4-dihydroxybenzoic acid, quercetin, and methyl-3-hydroxybutanedioic acid ester, were found to inhibit the production of TNF-α in RAW264.7 macrophage leukemia virus-transformed cells. In addition, quercetin was found to be active in the release of IL-6 with an inhibition rate of 39.8% [125]. The studies performed on the whole ethanol extract of C. speciosa showed significant inhibition of the activity of both COX-1 and COX-2, but the extract was more than twice as active against COX-2 as against COX-1 [125].
In general, C. speciosa has long been used as an herbal medicine for the treatment of various inflammatory diseases such as rheumatoid arthritis, prosopalgia, and hepatitis. Several pentacyclic triterpenoids, such as oleanolic, ursolic, betulinic, and maslinic acids, are known for their anti-inflammatory properties [126,172]. Recent work by Fallon et al. [173] demonstrated that plant pentacyclic triterpenes, including oleanolic acid, can modulate the expression of the nuclear bile acid receptor, farnesoid X receptor (FXR), which is a regulator of several intestinal functions. FXR activation reduces the production of pro-inflammatory cytokines, thereby contributing to reduced epithelial permeability. Highlighting the importance of the discovery, the authors proposed introducing a common name describing the new functional class of triterpenes as “FXR-targeted” nutraceuticals.
The glucoside fraction from C. speciosa was found to exert an anti-inflammatory effect in the collagen-induced arthritis rat model by suppressing the inflammatory response and restoring the body weight and immune organ weight of the rats. These glucosides also reduced lymphocyte proliferation and IL-1, IL-2, and TNF-α production in peritoneal macrophages and synoviocytes [126]. Additional studies have also confirmed that C. speciosa glycosides have antinociceptive effects related to their inhibitory effects on peripheral inflammatory mediators. The glycosides were shown to reduce the levels of PGE2 and TNF-α in the synovial cells of rats with adjuvant arthritis [127]. Recent research [128] showed that C. speciosa polysaccharides suppressed the secretion of pro-inflammatory cytokines (TNF-α and IL-1β) and COX-2, as well as the phosphorylation of c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK1/2) in LPS-stimulated cells. Thus, the secretion of pro-inflammatory cytokines and the downregulation of MAPK signaling promoted the analgesic and anti-arthritic effects of C. speciosa polysaccharides [128].
The anti-inflammatory activity of C. japonica in lipopolysaccharide (LPS)-activated murine macrophages was also investigated using a polyphenol-rich leaf extract. The studies confirmed its involvement in reducing the expression of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α), inflammatory mediators (COX-2 and iNOS), and both NF-κB p65 and p-NF-κB p65 in LPS-stimulated cells [122].
The inhibitory effects of P. sinensis fruit extract on the inflammatory response of human mast cells were investigated by Kim et al. [107]. The authors found that the fruit extract inhibited the migration of human mast cell line (HMC-1) cells, which play an important role in various inflammatory diseases, in response to stem cell factor (SCF). The extract also inhibited TNF-α expression by blocking the activation of ERK, p38MAPK, and JNK in HMC-1 cells. It also suppressed the expression of IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1) in human monocytic THP-1 cells as well as the secretion of IL-6 in human keratinocytic HaCaT cells.
4.3. Anticancer Activity
A study by Carvalho et al. [38] showed that C. oblonga fruit and leaf extracts exhibited significant antiproliferative activities, showing concentration-dependent growth inhibitory activity against human colon cancer cells (IC50 = 239.7 for 43.2 μg/mL), while no effect was observed in renal adenocarcinoma cells. The authors suggested that chlorogenic acid, present in all tested extracts of C. oblonga fruits and leaves, was responsible for this effect. The aqueous extract of fermented fruits, in addition to its antioxidant properties mentioned above, also exerted various proliferative and cytotoxic effects on several human cancer cell lines, such as HepG2, A549, and HeLa [38].
Riahi-Chebbi et al. [129] demonstrated that a polyphenolic extract from the peel of C. oblonga induced proliferation arrest and apoptosis of LS174 colon cancer cells and that such an effect was at least partially mediated by the inhibition of NF-κB activation. The extract also reduced the expression and secretion of VEGF-A by tumor cells, which could lead to the inhibition of tumor-induced angiogenesis. The authors attributed the observed effect to numerous polyphenols in the extract. According to Adiban et al. [130], an aqueous extract of C. oblonga fruit reduced serum biomarkers of liver damage in rats with hepatocellular carcinoma, including α-fetoprotein (AFP), γ-glutamyl transpeptidase (GGT), ALT, and AST. In addition, the extract showed antioxidant activity in vivo, increasing GSH levels and preventing lipid peroxidation in liver tissue.
The studies showed that water-soluble polysaccharides extracted from C. speciosa inhibited sarcoma 180 tumor growth in mice in a dose-dependent manner. Additionally, they increased the relative spleen index and body weight, concanavalin A- and lipopolysaccharide-induced splenocyte proliferation, and peritoneal macrophage phagocytosis. In addition, treatment with water-soluble polysaccharides could alleviate delayed-type hypersensitivity and promote the secretion of IL-2, TNF-α, and IFN-γ in the serum of tumor-bearing mice [174]. C. speciosa fruits, being rich in pentacyclic triterpenoids, have long been the subject of research using cancer cells. Several of them, including ursolic, oleanolic, and maslinic acids, typical for Chaenomeles, exhibit significant anticancer effects through the modulation of a diverse range of molecular targets and signaling pathways to induce cell cycle arrest and apoptosis as well as inhibiting cancer cell proliferation, progression, angiogenesis, tissue invasion, and metastasis [175].
Recent studies have demonstrated numerous anticancer properties of ursolic acid, particularly in breast cancer, hepatocellular carcinoma, cervical cancer, lung cancer, melanoma, gallbladder cancer, and prostate cancer [176]. On the other hand, oleanolic acid has been used in the treatment of various cancer cell lines, such as MCF-7 and MCF-7/ADR human breast cancer cells, the 1321N1 astrocytoma cell line, hepatocellular carcinoma, and HCT-116 colorectal cancer cells [177]. The third of the most widely distributed C. speciosa triterpenoids, maslinic acid, also showed pronounced inhibitory effects against various cancer cell lines, including stomach, pancreatic, and human colon cancer cells [178].
Procyanidin extract from C. japonica fruit influenced the activity of matrix metalloproteinases (MMP-2 and MMP-9) secreted into the culture medium by human peripheral blood mononuclear cells and by human leukemia HL-60, which may make these condensed tannins promising chemopreventive agents [131]. A flavanol-rich preparation from C. japonica fruit induced favorable changes in the Bax/Bcl-2 mRNA ratio, making normal and cancer cells more resistant and sensitive to apoptosis, respectively. The most favorable Bax/Bcl-2 ratio was found in DU145 human prostate cancer cells. The growth and invasiveness of MDA-MB-231 human breast cancer cells were strongly inhibited by the C. japonica preparation. This was accompanied by a reduction in MMP-9 activity and stimulation of tissue inhibitor of metalloproteinases, TIMP-1, expression (MMP-9/TIMP-1 ratio is an indicator in the assessment of invasion and metastasis) [132]. A similar flavanol preparation from the fruit of C. japonica, rich in mono- and oligomers of procyanidins, inhibited the expression of COX-2, MMP-9, and NF-κB, suggesting that it has cytotoxic, anti-inflammatory, and antiproliferative effects against SW-480 colon cancer cells [133]. In a study by Zvikas et al. [134], sixteen phenolics were detected in C. japonica leaves, with chlorogenic acid being the predominant compound. Incubation with the extracts reduced the viability of HROG36 glioblastoma cells with an efficiency similar to that of temozolomide, a drug used to treat glioblastoma. In the case of C6 glioblastoma cells, the extracts were even more effective than temozolomide.
Although studies on the anticancer properties of C. japonica have mainly been conducted on the fruit, there are also reports on the leaves. Crude phenolic leaf extract and purified phenolic-rich extracts contained 33 and 36 phenolics, respectively, of which chlorogenic acid and naringenin hexoside were found to be the major components. FRAP and ABTS+ tests showed that the purified phenolic-rich extract had two times higher antioxidant activity and exhibited higher cytotoxic activity against colon cancer cells (SW-480 and HT-29) than the crude phenolic extract. In addition, the purified phenolic-rich extracts had more potent cytotoxic effects on the colon cancer cell lines (SW-480 and HT-29) than on normal intestinal cells [135].
Gao et al. [136] investigated the anticancer activity of 22 functional constituents (including triterpenoids, flavonoids, and lignans) isolated from P. sinensis against human anaplastic large cell lymphoma (JB6) cells. This primary screening selected several compounds with promising anticancer activity.
Natural products of plant origin, including extracts rich in polyphenols, are able to alter cell signaling through epigenetic changes, including DNA methylation and histone modifications. These changes lead to altered expression of microRNA (miRNA) which are involved in the regulation of gene expression post-transcriptionally. A single miRNA is able to target more than one hundred genes. Moreover, each gene contains multiple binding sides for miRNAs. The possibility of developing a phytotherapeutic approach based on miRNAs isolated from medicinal plants may be the next step toward new-generation therapies. So far, the pharmaceutical industry has focused on plant secondary metabolites, excluding the concept of exploring potential biological functions at the expense of exogenous miRNAs [179]. However, the research confirms that they are involved in multiple signaling pathways, including the deeply studied cancer regulation processes, especially cell proliferation, and migration, metastasis, apoptosis, and cell differentiation [180]. Among the species tested, only the C. oblonga genome was analyzed for miRNAs. By using a trained SVM classifier, the identification of 600 putative pre-miRNA coding loci was carried out. Subsequent homology searches identified 33 matches, including 28 pre-miRNAs from M. domestaina, two from Glycine max, two from Vitis vinifera, and one pre-miRNA from Paeonia lactiflora [181]. As many studies have confirmed, miRNA can be regulated by many compounds obtained from medicinal plants. They are also found in C. oblonga fruits: e.g., caffeic acid, which can affect breast cancer and hepatocarcinoma cells, catechin, influencing lung and prostate cancers, hepatoma, and neuroblastoma, and quercetin, which is believed to have an effect on lung and pancreatic cancer cells [180]. Oleanolic acid, which is a valuable component of Chaenomeles and Pseudocydonia fruits, has been found to exhibit antiproliferative potential by upregulating tumor suppressor miR-122 both in in vitro and in vivo models of lung carcinoma [182]. Unfortunately, the genomes of the last-mentioned genera are poorly studied.
4.4. Cardioprotective Effects
Early prevention of hyperlipidemia is an important factor in reducing the incidence of cerebral and cardiovascular diseases. It has been shown that total flavonoid extracts from C. oblonga fruits and leaves could regulate blood lipid metabolism in rats by scavenging oxygen free radicals and improving antioxidant potential [138]. Working on hyperlipidemic rats, the authors showed that total flavonoids from the extract significantly reduced the concentration of total cholesterol, triglycerides, and low-density lipoprotein cholesterol (LDL cholesterol), and increased high-density lipoprotein cholesterol (HDL cholesterol) in serum.
C. oblonga leaf extracts are used in TCM to treat or prevent cardiovascular disease. This type of C. oblonga activity has been experimentally supported by research by Abliz et al. [137], Zhou et al. [139,140,141], and Abulizi et al. [142]. In the studies by Abliz et al. [137], ethanolic leaf extract reduced total cholesterol, triglycerides, LDL-cholesterol, and MDA, inhibited the activity of aminotransferase (ALT), aspartate aminotransferase (AST), and lipopolysaccharides, while it increased the HDL-cholesterol content and the activity of SOD, glutathione peroxidase (GSH-Px), lipoprotein lipase (LPL), and hepatic lipase (HL) in the serum of hyperlipidemic rats fed with the extract. The total flavonoid preparations of C. oblonga fruits and leaves were also effective in reducing ALT and AST, showing their involvement in hepatocyte protection. They also improved the activity of SOD and GSH-Px in liver tissues, which inhibited the formation of MDA [138]. On the other hand, Abulizi et al. [142] investigated the possibility of using aqueous extracts of C. oblonga in the treatment of atherosclerosis. They concluded that the extracts could reduce the degree of aortic injury and hemodynamic indices, regulate blood lipid levels, and improve liver function in rats with atherosclerosis. The authors also observed an increased activity of SOD and GSH-Px and a decreased content of MDA in the serum of atherosclerotic rats. Furthermore, they specified the active compounds among the 14 identified in the extract and the mechanisms underlying the anti-atherosclerotic effects of the extract using a molecular docking approach.
Hypertensive disease, its causes, and numerous consequences, including those on the functioning of the circulatory system, are widely discussed in the literature. The magnitude of this problem is still underestimated, while, according to projections, in 2025 there will be 1.5 billion people living with hypertension in the world [183]. By feeding C. oblonga ethanolic extracts to renal hypertensive rats, Zhou et al. [139] selected a dose that produced similar effects to captopril, a drug used to treat essential or renovascular hypertension. The extracts significantly reduced whole blood viscosity and improved erythrocyte deformability. Subsequent attempts to use C. oblonga extract by Zhou et al. [140] concerned its effect on renal hypertension, which is a common cause of secondary hypertension in humans, usually as a result of renal artery stenosis or hypertrophy. The study analyzed the dose–response effect of ethanolic leaf extracts on hypertension and biomarkers related to blood pressure control. It was observed that it lowered the concentration of peptides: angiotensin II (AII), one of the most effective blood pressure regulators, which causes intense contraction of the muscles of small blood vessels and significantly increases blood pressure, thus accelerating the heart rate, as well as endothelin (ET). The authors concluded that these extracts have properties similar to those of angiotensin-converting enzyme (ACE) inhibitors and captopril. Subsequently, Zhou et al. [141] demonstrated the antithrombotic activity of an aqueous extract of C. oblonga leaves in mice and rats, probably at least partly related to an antithromboxane effect. Their results were compared with those of acetylsalicylic acid and showed that the quince extracts dose-dependently prolonged the thrombosis occlusion time, reduced the weight of arterial and venous thrombosis, decreased the plasma concentrations of thromboxane B2, and increased that of 6-keto-prostaglandin F1α. As suggested by the authors, their effect on the prostacyclin/thromboxane balance was probably beneficial in their antithrombotic activity.
C. oblonga extract has been shown to be effective in alleviating cardiotoxicity caused by the use of a popular drug, doxorubicin (DOX), which is effective in the treatment of various types of cancer. It has been suggested that mitochondria play a critical role in these mechanisms of toxicity. A study by Gholami et al. [143] showed that C. oblonga fruit ameliorated the impairment of cardiac mitochondrial function in DOX-treated rats by preventing mitochondrial ROS generation, lipid peroxidation, swelling, membrane potential decrease (%ΔΨm), and cytochrome c release, and also by increasing mitochondrial GSH and complex II activity. Hanan et al. [144] performed an in vivo study to evaluate DOX-induced cardiotoxicity. Rats were orally administered quince peel extracts at doses of 160 and 320 mg/kg bm for 30 days, and ECG analysis was performed at the end of the experiment. In addition, lipid profile, blood serum parameters (creatine kinase MB (CK-MB), LDH, and AST), and tissue parameters (MDA, SOD, GSH, CAT) were analyzed. The groups of pretreated rats significantly attenuated DOX-induced changes in all parameters. In addition, improvement in histopathologic changes in cardiac tissue was also observed in the pretreated groups, indicating regression of cardiac injury.
Regrettably, there is limited research on this subject with regard to other species, as outlined in this review. C. speciosa powder dietary supplement at concentrations of 5 and 10% was administered to mice and significantly reduced serum low-density lipoprotein cholesterol and total cholesterol levels. A significant increase in GSH-Px activity and total antioxidant capacity and a decrease in the relative atherosclerotic plaque area of the aortic sinus and arch were observed compared to the control group [115]. A triterpenoid 28-O-β-D-glucopyranosyl-2α,3β-dihydroxyolean-12-ene-24,28-dioic acid, named chaenomelosid A, and its aglycone, chaenomelogenin A, isolated for the first time from the fruit of P. sinensis, showed tissue tromboplastin (TF) inhibitory activity, which may be helpful in the regulation of blood coagulation [145].
4.5. Antidiabetic Activity
The increasing incidence of diabetes mellitus is alarming and is becoming one of the most significant health problems worldwide, mainly associated with hyperglycemia and abnormal lipid and antioxidant profiles [118]. In addition to the involvement of antioxidant and anti-inflammatory compounds present in C. oblonga extract in the reduction of factors contributing to the development of ischemic heart disease, their activity in the treatment of type II diabetes has been widely described. Polysaccharides from C. oblonga fruit have also been found to inhibit tyrosine phosphatase activity (IC50 for 2.07 µg/mL), indicating its ability to treat type II diabetes and obesity [93,112]. In the in vitro study by Tang et al. [146], C. oblonga seed extract stimulated glucose metabolism by activating the PI3K/AKT insulin signaling pathway in L6 myotubes. Recent studies have shown promising results regarding the use of C. oblonga fruit extract and suggest that it can be used as an anti-obesity agent [147] by reducing body weight, body fat mass, and serum insulin, triglyceride, and leptin concentrations. However, it increased serum adiponectin and HDL cholesterol levels in high-fat diet-induced C57BL/6 mice. The extract increased AMPK activation and inhibited adipogenesis [147].
There are several papers showing the antidiabetic effect of C. oblonga leaves. They are used as a folk remedy for the treatment of this disease in Turkey. Oral administration of hydroethanolic extracts (500 mg/kg) for 5 days to diabetic rats reduced blood glucose levels by 34%. It induced a significant antioxidant effect on heart tissue as measured by TBARS concentration. The observed effects were more pronounced than those obtained with Jerusalem artichoke (Helianthus tuberosus L.) tuber and leek (Allium porrum L.) bulb extracts [148]. The same effect as at doses of 250 mg/kg and 500 mg/kg was observed during the use of an antidiabetic drug (tolbutamide) at a dose of 100 mg/kg. According to recent work by He et al. [184], the therapeutic effect of chlorogenic acid isoforms contained in Pyrrosia petiolosa (Christ) Ching, a traditional Chinese medicine used in the treatment of diabetes with good effectiveness, is realized by promoting insulin secretion and pancreatic tissue repair, which results in a strong hypoglycemic effect.
Based on recent data, Zakłos-Szyda and Pawlik [149] concluded that C. japonica polyphenols could be suitable for the prevention of pre-diabetes, type II diabetes, and metabolic syndrome. Here, C. japonica polyphenolic extract was tested on glucose metabolism in a human hepatoma HepG2 cell line cultured under non-metabolically altered and hyperglycemic conditions. Pretreatment with the preparation caused a decrease in intracellular ROS generation and affected mitochondrial membrane polarization, which appeared to lead to AMP-activated protein kinase (AMPK) activation. Other effects observed in HepG2 cells were associated with an increase in glucose uptake and glycogen content as well as alleviation of gluconeogenesis through modulation of PEPCK, PTP1B, enzymes, FOXO1 transcription factor, and glucose transporter GLUT2/4 expression.
However, a more recent paper by Loza-Rodríguez et al. [185] showed that not only the polyphenol fraction can contribute to the regulation of glycemia, but also one of the most common triterpenoids of the Chaenomeles genus, i.e., oleanic acid. In myoblasts, oleanolic acid increased peroxisome proliferator-activated receptors γ/α (PPARγ/α) expression of mRNA and their regulated genes. Protein expression of PPARγ, GLUT4, and fatty acid transport protein 1 (FATP1) was also increased, and GLUT4 translocation was observed.
The group of Sancheti [150] found that the constituents of P. sinensis fruit are an effective glycosidase inhibitor. The crude 80% methanolic extract and its fractions were tested for α- and β-glucosidase and α- and β-galactosidase inhibitory activities. The results concluded that this fruit contains α-glucosidase and β-glucosidase inhibitors and can be used as a powerful natural drug in the treatment of type II diabetes by controlling glucose absorption. Subsequent studies by this group showed that oral administration of P. sinensis extract (500 mg/kg bm) significantly inhibited the progression of streptozotocin (STZ)-induced diabetes in rats, and this effect may be related to its hypoglycemic effect, modulation of lipid metabolism, and ability to scavenge free radicals. The authors observed increased liver glycogen content, SOD, GSH, and CAT levels, and decreased fasting blood glucose, blood urea nitrogen, serum total cholesterol, triglycerides, LDL cholesterol, ALT, and AST concentrations [118]. Using the ethyl acetate fraction of P. sinensis extracts, Sancheti et al. [151] demonstrated an ameliorative effect on impaired blood glucose, lipid, acetylcholinesterase, and antioxidant levels in STZ-induced diabetic rats. According to the authors, these effects could be mediated via the inhibition of glucose transporter, α- and β-glucosidase, amylase, and lipase, and its significant antioxidant potential.
4.6. Antiviral and Antibacterial Activity
The use of neuraminidase (NA) inhibitors is one of the most common approaches in the development of anti-influenza drugs. Three compounds isolated from the ethanolic extract of C. speciosa, namely 3,4-dihydroxybenzoic acid, methyl-3-hydroxybutanedioic acid ester, and vomifoliol, exhibited significant dose-dependent inhibition of NA activity with IC50 values of 1.27, 1.90, and 2.33 μg/mL, respectively. The studies also showed that most of the 13 compounds isolated from the extract inhibited the production of NO (which can exacerbate lung injury after influenza virus pneumonia) by more than 25% at 5 μg/mL in RAW264.7 cells [123].
Several studies have shown that the anti-influenza effect of fruits and vegetables depends on the presence of certain polyphenols, and the mechanisms of inhibition vary depending on the molecular structure. The antiviral role of P. sinensis fruit polyphenols is appreciated in TCM, but so far poorly documented in the scientific literature [107,186,187]. Pretreatment with a polyphenol-rich P. sinensis extract was shown to slightly reduce cell binding, hemagglutination, and hemolytic activity in influenza A-infected Madin–Darby canine kidney epithelial cells, as well as the synthesis of viral cRNA, vRNA, and secondary mRNA [187]. High-molecular-weight polyphenols from P. sinensis fruit have also been shown to neutralize influenza virus by inhibiting heme agglutination activity and suppressing influenza NS2 protein synthesis [186].
C. oblonga peel extract was found to be the most active in inhibiting bacterial and yeast growth (Gram-positive Staphylococcus aureus and Gram-negative Pseudomonas aeruginosa, somewhat less so in the cases of Escherichia coli and yeast Candida albicans) with minimum inhibitory and bactericidal concentrations in the range of 102 × 103–105 × 103 mg polyphenol/mL [37].
A study by Alizadeh et al. [152] showed that the extract of C. oblonga can be helpful against diarrhea and in controlling Enterobacteriaceae infections; the ethanolic seed extract was the most effective against E. coli, while the aqueous fruit extract showed an antimicrobial effect only on Escherichia aerogenes. The crude extract of polyphenols from C. oblonga fruits showed antibacterial activity against E. coli. Five polyphenols were isolated and tested for their activity, namely 5-O-caffeoylquinic acid, quinic acid, a derivative of quinic acid, proanthocyanin B1, and methyl 5-O-caffeoylquinate, revealing the strong inhibitory properties of quinic acid and its derivative [188].
Interestingly, C. oblonga fruit extract has been found to be an effective agent in supporting the treatment of COVID-19. It was recommended by the Indian Ministry of AYUSH, Government of India, as an ingredient in a mixture against the SARS-CoV-2 virus and was described as an antioxidant, immunomodulatory, anti-allergic, smooth muscle relaxant, and anti-influenza agent [153].
Urbanavičiūtė et al. [72] demonstrated various antibacterial properties of C. japonica fruits. Strong inhibition of the growth of Gram-positive bacteria (E. faccalis, B. subtilis, and S. aureus) was observed, which, thanks to the analysis of three cultivars, was correlated with a high content of rutin and epicatechin. E. coli and P. aeruginosa were inhibited to a lesser extent. However, the activity of the extract against the fungus C. albicans, observed in tests with C. oblonga extracts, was not observed here at all. The antimicrobial activity of C. speciosa extract was evaluated against 18 Gram-negative or Gram-positive bacteria and a fungal strain and showed that the extract had a better inhibitory effect on S. aureus, S. typhimurium, MRSA, E. coli, P. aeruginosa, S. epidermidis, Y. enterocolitica, and C. albicans than ampicillin sodium salt, fluconazole, and berberine chloride. The better inhibitory effect of C. speciosa extract was especially noticeable for drug-resistant bacteria [72]. Another study highlighted differences in the antibacterial activity of 23 main compounds of this fruit against S. aureus and E. coli [103].
Procyanidins, which are considered one of the main fractions of C. oblonga polyphenols and found in great diversity in Chaenomeles, are believed to modulate the intestinal microbiome. They act as inhibitors of digestive enzymes and show a direct, strong antimicrobial effect. Furthermore, the health benefits of dietary fiber in plants are believed to depend on its interactions with phytochemicals, including tannins [189].
4.7. Other Health-Promoting Properties
In Persian medicine, heated extract of C. oblonga has been used to treat gastroesophageal reflux disease (GERD), the most common esophageal disorder. It is defined as the reflux of stomach contents into the esophagus with troublesome symptoms such as heartburn, vomiting, abdominal pain, recurrent pneumonia, and erosive esophagitis. In the work of Zohalinezhad et al. [156], C. oblonga syrup was helpful in the treatment of GERD in pediatrics as a gastric tonic and ulcer healing agent. Quince syrup significantly improved the patient’s condition and symptomatic status and was effective for at least two weeks after drug administration. Shakeri et al. [155] administered concentrated C. oblonga fruit extract, also known as “quince sauce”, to 137 pregnant women with GERD and found that the efficacy of quince sauce for the treatment of pregnancy-related GERD was similar to that of the popular drug ranitidine. Later, a randomized, double-blind clinical trial was conducted in 96 children with suspected GERD, and the results showed that the administration of quince syrup was also helpful in improving GERD in children, and its efficacy was similar to that of ranitidine [154].
Pretreatment of rats with P. sinensis jelly from boiling water fruit extracts protected gastric tissues against HCl/ethanol-induced gastric injury, as evidenced by a reduction in gastric lesion index and TNF-α levels. The authors suggested that the pronounced gastroprotective activity of P. sinensis jelly may be due to the synergistic effect of components such as pectin and highly polymerized procyanidins, which have a strong binding affinity to the gastric mucosa [190].
Biologically active compounds from C. oblonga have been shown to be helpful in the treatment of gynecological disorders. A randomized, triple-blind, controlled clinical trial of 146 women with menorrhagia showed that the C. oblonga pill was as effective in reducing menstrual bleeding and increasing hemoglobin levels as mefenamic acid, a nonsteroidal anti-inflammatory drug (NSAID) known for its side effects [157]. C. oblonga syrup was found to be significantly more effective against nausea and vomiting during pregnancy than vitamin B6, which is often used to relieve morning sickness. The beneficial effects of quince were even more significant because the treatment was characterized by high safety [191].
In turn, research by Din Ganaie et al. [158] demonstrated the antidepressant effects of aqueous and ethanolic extracts of C. oblonga fruit in rats using the forced swim test (FST) and tail suspension test (TST), which were compared with the effects of treatment with a standard drug (imipramine). In addition, antidepressant activity was confirmed by a gradual increase in serum enzymatic antioxidant levels. Co-administration of C. oblonga with imipramine has been introduced as a novel therapeutic approach in the treatment of depression.
5. Conclusions
The review presented here revealed an extensive literature on the bioactive compound contents and uses of fruits of Cydonia oblonga, Chaenomeles sp., and Pseudocydonia sinensis.
Cydonia oblonga fruit is relatively well-known and appreciated culinarily. Its anti-inflammatory, immunomodulatory, and anticancer properties, as well as its protective effect against disorders of lipid and carbohydrate metabolism, and thus of the cardiovascular system, are attributed to the presence of numerous polyphenols, including protocyanidins and caffeoylquinic acids. Their use in the treatment of GERD and allergies seems unique. The analysis of the literature showed that most of the papers describing the health-promoting properties of C. oblonga focused on leaves.
There are fewer studies on the medical and culinary uses of the genus Chaenomeles, but C. speciosa seems to be the most studied for its potential medicinal applications. In addition to its potent anti-inflammatory effects, the fruits have been characterized for their ability to lower glycemia and improve lipid metabolism. In turn, C. japonica is the most valuable additive that improves the taste of food products. The fruits of Chaenomeles seem to be unappreciated in terms of their use.
P. sinensis fruit is an ingredient of many medicines used in TCM. There is quite a bit of research on the biological effects of its fruits, mainly immunomodulatory and antidiabetic, but much of the work is old and inaccessible. Unfortunately, despite its beneficial health value, the plant seems to have a more local significance.
6. Future Perspectives
A comparison of the properties of the fruits of the three genera shows that the potential of Chaenomeles sp. and Pseudocydonia fruits is still poorly recognized. Overall, they contain more phenolic compounds and ascorbic acid than Cydonia fruits and are rich in carotenoids and tocopherols, organic acids, and minerals (Fe and Mo) as well as triterpenes with proven anticancer activity. The chemical characteristics of these fruits open the way to the potential use of their extracts or individual compounds in pharmacology as an alternative to conventionally used drugs. The sensory characteristics of Chaenomeles sp., largely related to the high content of organic acids, are unique and indicate that these fruits could be used on a larger scale. Due to their remarkable antioxidant properties, more significant than those of C. oblonga fruits, they could be used as natural preservatives in the food and pharmaceutical industries. In view of these advantages, it should be concluded that Chaenomeles sp. fruits are valuable functional food ingredients; however, little is known about the molecular action of their phytochemicals. Progressive pharmacology such as miRNA therapies may be the next best step towards identifying new therapeutic options using these medicinal plants. Future research efforts should delve deeper into the systematic investigation of potentially active secondary metabolites and biological effects exerted by fruit extracts and, in parallel, undertake species-specific genomic analysis.
Funding
This research received no external funding.
Acknowledgments
I thank my university colleagues, Monika Bieniasz, from the Department of Horticulture, and Przemysław Petryszak, from the Department of Plant Biology and Biotechnology, for their numerous valuable comments during the preparation of this manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- Rop, O.; Balik, J.; Řezniček, V.; Jurikova, T.; Škardova, P.; Salaš, P.; Sochor, J.; Mlček, J.; Kramařova, D. Chemical characteristics of fruits of some selected quince (Cydonia oblonga Mill.) cultivars. Czech J. Food Sci. 2011, 29, 65–73. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The medical importance of Cydonia oblonga—A review. IOSR J. Pharm. 2016, 6, 87–99. [Google Scholar]
- Nahorska, A.; Dzwoniarska, M.; Thiem, B. Fruits of Japanese quince (Chaenomeles japonica Thunb. Lindl. ex Spach) as a sourcen of bioactive compounds. Post. Fitoter. 2014, 4, 239–246. (In Polish) [Google Scholar]
- Gao, R.; Xiong, S.; Zhang, T.; Deng, X.; Li, J.; Liao, M. Two new quinic acid derivatives from the fruits of Chaenomeles speciose. Biochem. Syst. Ecol. 2020, 93, e104167. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Ma, W.; Liu, J.; Tian, L.; Zhou, Y.; Shang, F.; Guo, P. Comparative Pollen Morphology of the Genus Chaenomeles Lindl. (Rosaceae): Diagnostic Features and Implications for Taxonomy. Diversity 2023, 15, 960. [Google Scholar] [CrossRef]
- Mabberley, D.J. The Plant Book, a Portable Dictionary of the Higher Plants, 4th ed.; Cambridge University Press: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Martins, R.C.; Seabra, R.M.; Ferreira, M.A. Principal component analysis as tool of characterization of quince (Cydonia oblonga Miller) jam. Food Chem. 2006, 94, 504–512. [Google Scholar] [CrossRef]
- Trigueros, L.; Pérez-Alvarez, J.A.; Viuda-Martos, M.; Sendra, E. Production of low-fat yogurt with quince (Cydonia oblonga Mill.) scalding water. LWT—Food Sci. Technol. 2011, 44, 1388–1395. [Google Scholar] [CrossRef]
- Wojdyło, A.; Teleszko, M.; Oszmiański, J. Antioxidant property and storage stability of quince juice phenolic compounds. Food Chem. 2014, 152, 261–270. [Google Scholar] [CrossRef]
- Mir, S.A.; Wani, S.M.; Wani, T.A.; Ahmad, M.; Gani, A.; Masoodi, F.A.; Nazir, A. Comparative Evaluation of the Proximate Composition and Antioxidant Properties of Processed Products of Quince (Cydonia oblonga). Int. J. Food Res. 2016, 23, 816–821. [Google Scholar] [CrossRef]
- De Almeida Lopes, M.M.; Guimarães Sanches, A.; de Souza, K.O.; de Oliveira Silva, E. Quince—Cydonia oblonga. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 363–368. [Google Scholar] [CrossRef]
- Cascales, E.V.; García, J.M.R. Characteristics of the raw fruit, industrial pulp, and commercial jam elaborated with Spanish quince (Cydonia oblonga Miller). Emir. J. Food Agric. 2020, 32, 623–633. [Google Scholar] [CrossRef]
- Gheisari, H.R.; Abhari, K.H. Drying method effects on the antioxidant activity of quince (Cydonia oblonga Miller) tea. Acta Sci. Pol. Technol. Aliment. 2014, 13, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Barrachina, A.A.; Szychowski, P.J.; Vásquez, M.V.; Hernández, F.; Wojdyło, A. Technological aspects as the main impact on quality of quince liquors. Food Chem. 2015, 167, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.J.; Martínez-Esplá, A.; Gironés-Vilaplana, A.; Santos-Lax, D.; Noguera-Artiaga, L.; Carbonell-Barrachina, A.A. Phenolic, volatile, and sensory profiles of beer enriched by macerating quince fruits. LWT—Food Sci. Technol. 2019, 103, 139–146. [Google Scholar] [CrossRef]
- Baranowska-Bosiacka, I.; Bosiacka, B.; Rast, J.; Gutowska, I.; Wolska, J.; Rębacz-Maron, E.; Dębia, K.; Janda, K.; Korbecki, J.; Chlubek, D. Macro- and Microelement Content and Other Properties of Chaenomeles japonica L. Fruit and Protective Effects of Its Aqueous Extract on Hepatocyte Metabolism. Biol. Trace Elem. Res. 2017, 178, 327–337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nawirska-Olszańska, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Biesiada, A. Quality Assasment of pumpkin jams enriched with Japanese quince, Cornellian cherry and strawberries. Acta Sci. Pol. Technol. Aliment. 2010, 1, 40–48. (In Polish) [Google Scholar]
- Wojdyło, A.; Oszmiański, J.; Teleszko, M.; Sokół-Łętowska, A. Composition and quantification of major polyphenolic compounds, antioxidant activity and colour properties of quince and mixed quince jams. Int. J. Food Sci. Nutr. 2013, 64, 749–756. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Teleszko, M.; Samoticha, J. Sensory attributes and changes of physicochemical properties during storage of smoothies prepared from selected fruit. LWT—Food Sci Technol. 2016, 71, 102–109. [Google Scholar] [CrossRef]
- Seglina, D.; Krasnova, I.; Heidemane, G.; Ruisa, S. Influence of drying technology on the quality of dried candied Chaenomeles japonica during storage. Lat. J. Agron. 2009, 12, 113–118. [Google Scholar]
- Krasnova, I.; Seglina, D.; Pole, V. The effect of pre-treatment methods on the quality of dehydrated candied Japanese quince fruits during storage. J. Food Sci. Technol. 2018, 55, 4468–4476. [Google Scholar] [CrossRef]
- Antoniewska, A.; Rutkowska, J.; Martinez Pineda, M. Antioxidative, sensory and volatile profiles of cookies enriched with freeze-dried Japanese quince (Chaenomeles japonica) fruits. Food Chem. 2019, 286, 376–387. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Content of biactive compounds and antioxidant capacity of pumpkin puree enriched with Japanese quince Cornellian cherry, strawberry and apples. Acta Sci. Pol. Technol. Aliment. 2011, 10, 51–60. [Google Scholar] [PubMed]
- Hamauzu, Y.; Mizuno, Y. Non-extractable Procyanidins and Lignin are Important Factors in the Bile Acid Binding and Radical Scavenging Properties of Cell Wall Material in some Fruits. Plant Foods Hum. Nutr. 2011, 66, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Hamauzu, Y.; Takedachi, N.; Miyasaka, R.; Makabe, H. Heat treatment of Chinese quince polyphenols increases rat plasma levels of protocatechuic and vanillic acids. Food Chem. 2010, 118, 757–763. [Google Scholar] [CrossRef]
- Cheng, X.-C.; Guo, X.-R.; Qin, Z.; Wang, X.-D.; Liu, H.-M.; Liu, Y.-L. Structural features and antioxidant activities of Chinese quince (Chaenomeles sinensis) fruits lignin during auto-catalyzed ethanol organosolv pretreatment. Int. J. Biol. Macromol. 2020, 164, 4348–4358. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P. Comprehensive characterization of Chaenomeles seeds as a potential source of nutritional and biologically active compounds. J. Food Comp. Anal. 2021, 102, e104065. [Google Scholar] [CrossRef]
- Byczkiewicz, S.; Szwajgier, D.; Kobus-Cisowska, J.; Szczepaniak, O.; Szulc, P. Comparative examination of bioactive phytochemicals in quince (Chaenomeles) fruits and their in vitro antioxidant activity. EJFA 2021, 3, 293–302. [Google Scholar] [CrossRef]
- Al-Zughbi, I.; Krayem, M. Quince fruit Cydonia oblonga Mill nutritional composition, antioxidative properties, health benefits and consumers preferences towards some industrial quince products: A review. Food Chem. 2022, 393, e133362. [Google Scholar] [CrossRef]
- Mierina, I.; Seržane, R.; Strele, M.; Moskaluka, J.; Seglina, D.; Jure, M. Extracts of Japanese quince seeds—Potential source of antioxidants. In Proceedings of 6th Baltic Conference on Food Science and Technology: Innovations for Food Science and Production; Latvia University of Agriculture: Jelgava, Latvia, 2011; p. 75. [Google Scholar]
- Mierina, I.; Seržane, R.; Strele, M.; Moskaļuka, J.; Ivdre, E.; Jure, M. Investigation of the oil and Meal of Japanese Quince (Chaenomeles japonica) Seeds. Proc. Latv. Acad. Sci. Sect. B 2013, 67, 405–410. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem. 2015, 170, 437–442. [Google Scholar] [CrossRef]
- Górnaś, P.; Siger, A.; Juhņeviča, K.; Lācis, G.; Šnē, E.; Segliņa, D. Cold-pressed Japanese quince (Chaenomeles japonica (Thunb.) Lindl. ex Spach) seed oil as a rich source of α-tocopherol, carotenoids and phenolics: A comparison of the composition and antioxidant activity with nine other plant oils. Eur. J. Lipid Sci. Technol. 2014, 116, 563–570. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Bleive, U.; Kaldmäe, H.; Aluvee, A.; Rätsep, R.; Karp, K.; Maciel, L.S.; Herodes, K.; Rinken, T. Phytochemical characterization of oil and protein fractions isolated from Japanese quince (Chaenomeles japonica) wine by-product, LWT—Food Sci. Technol. 2023, 178, e114632. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Ferreres, F.; Domingues, A.L.; Seabra, R.M.; Ferreira, M.A. Phenolic Profile of Quince Fruit (Cydonia oblonga Miller) (Pulp and Peel). J. Agric. Food Chem. 2002, 50, 4615–4618. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Valentão, P.; Ferreres, F.; Seabra, R.M.; Ferreira, M.A. Quince (Cydonia oblonga Miller) Fruit (Pulp, Peel, and Seed) and Jam: Antioxidant Activity. J. Agric. Food Chem. 2004, 52, 4705–4712. [Google Scholar] [CrossRef] [PubMed]
- Fattouch, S.; Caboni, P.; Coroneo, V.; Tuberoso, C.I.G.; Angioni, A.; Dessi, S.; Marzouki, N.; Cabras, P. Antimicrobial activity of tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J. Agric. Food Chem. 2007, 55, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Silva, B.M.; Silva, R.; Valentão, P.; Andrade, P.B.; Bastos, M.L. First report on Cydonia oblonga Miller anticancer potential: Differential antiproliferative effect against human kidney and colon cancer cells. J. Agric. Food Chem. 2010, 58, 3366–3370. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, B.T.; Mitić, S.S.; Stojanović, G.S.; Mitić, M.N.; Kostić, D.A.; Paunović, D.D.; Arsić, B.B.; Pavlović, A.N. Phenolic profiles and metal ions analyses of pulp and peel of fruits and seeds of quince (Cydonia oblonga Mill.). Food Chem. 2017, 232, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Dall’Acqua, S.; Poloniato, G.; Maggi, F.; Malagoli, M. Preliminary evaluation of quince (Cydonia oblonga Mill.) fruit as extraction source of antioxidant phytoconstituents for nutraceutical and functional food applications. J. Sci. Food Agric. 2019, 99, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.S.; Silva, B.M.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M. Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food Chem. Toxicol. 2009, 47, 1372–1377. [Google Scholar] [CrossRef]
- Alesiani, D.; Canini, A.; D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Mastellone, C.; Pacifico, S. Antioxidant and antiproliferative activities of phytochemicals from Quince (Cydonia vulgaris) peels. Food Chem. 2010, 118, 199–207. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Ferreres, F.; Seabra, R.M.; Beatriz, M.; Oliveira, P.P.; Ferreira, M.A. Composition of Quince (Cydonia oblonga Miller) seeds: Phenolics, organic acids and free amino acids. Nat. Prod. Res. 2005, 19, 275–281. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Seabra, R.M.; Silva, B.M. Organic acids composition of Cydonia oblonga Miller leaf. Food Chem. 2008, 111, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.; Costa, R.M.; Magalhães, A.S.; Pereira, J.A.; Carvalho, M.; Valentão, P.; Andrade, P.B.; Silva, B.M. Targeted metabolites and biological activities of Cydonia oblonga Miller leaves. Food Res. Int. 2012, 46, 496–504. [Google Scholar] [CrossRef]
- Costa, R.M.; Magalhães, A.S.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M.; Silva, B.M. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: A comparative study with green tea (Camellia sinensis). Food Chem. Toxicol. 2009, 47, 860–865. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves, J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Ashrafi, H.; Ghabili, K.; Alihemmati, A.; Jouyban, A.; Shoja, M.M.; Aslanabadi, S.; Adl, F.H.; Ghavimi, H.; Hajhosseini, L. The effect of quince leaf (Cydonia oblonga Miller) decoction on testes in hypercholesterolemic rabbits: A pilot study. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2013, 10, 277–282. [Google Scholar] [CrossRef]
- Szychowski, P.J.; Lech, K.; Sendra-Nadal, E.; Hernández, F.; Figiel, A.; Wojdyło, A.; Carbonell-Barrachina, A.A. Kinetics, biocompounds, antioxidant activity, and sensory attributes of quinces as affected by drying metod. Food Chem. 2018, 255, 157–164. [Google Scholar] [CrossRef]
- Blanda, G.; Rodriguez-Roque, M.J.; Comandini, P.; Flores-Cordova, M.A.; Salas-Salazar, N.A.; Cruz-Alvarez, O.; Soto-Caballero, M.C. Phenolic profile and physicochemical characterization of quince (Cydonia oblonga Mill) fruits at different maturity index. Not. Bot. Horti. Agrobot. Cluj Napoca 2020, 48, 2306–2315. [Google Scholar] [CrossRef]
- De Bellis, R.; Chiarantini, L.; Potenza, L.; Gorassini, A.; Verardo, G.; De Marco, R.; Benayada, L.; Stocchi, V.; Albertini, M.C.; Fraternale, D. High Production of Secondary Metabolites and Biological Activities of C. oblonga Mill. Pulp Fruit Callus. J. Funct. Foods 2022, 94, 105133. [Google Scholar] [CrossRef]
- Andrade, P.B.; Carvalho, A.R.F.; Seabra, R.M.; Ferreira, M.A. A previous study of phenolic profiles of quince, pear, and apple purees by HPLC diode array detection for the evaluation of quince puree genuineness. J. Agric. Food Chem. 1998, 46, 968–972. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Yasui, H.; Inno, T.; Kume, C.; Omanyuda, M. Phenolic profile, antioxidant property, and anti-influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga Mill.), and apple (Malus domestica Mill.) fruits. J. Agric. Food Chem. 2005, 53, 928–934. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Gonçalves, A.C.; Seabra, R.M.; Oliveira, M.B.; Ferreira, M.A. Influence of jam processing upon the contents of phenolics, organic acids and free amino acids in quince fruit (Cydonia oblonga Miller). Eur. Food Res. Technol. 2004, 218, 385–389. [Google Scholar] [CrossRef]
- Baroni, M.V.; Fabani, M.P.; Adan, F.; Podio, N.S.; Wunderlin, D.A. Effect of geographical location, processing and simulated digestion on antioxidant characteristics of quince (Cydonia oblonga). Heliyon 2022, 8, e11435. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.A.; Romero, L.A.; Diaz, R.I. Quality and sensory attributes of apple and quince leathers made without preservatives and with enhanced antioxidant activity. LWT—Food Sci. Technol. 2015, 62, 996–1003. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Polyphenolic Composition, Antioxidant Activity, and Polyphenol Oxidase (PPO) Activity of Quince (Cydonia oblonga Miller) Varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Joshi, V.K.; Rana, J.C. Nutritional Composition and Processed Products of Quince (Cydonia oblonga). J. Asian Nat. Prod. Res. 2011, 2, 354–357. [Google Scholar]
- Urbanavičiūtė, I.; Viškelis, P. Biochemical Composition of Japanese Quince (Chaenomeles japonica) and Its Promising Value for Food, Cosmetic, and Pharmaceutical Industries. In Fruit Industry; Kahramanoğlu, İ., Wan, C., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Satora, P.; Sroka, P.; Pogoń, P.; Machalica, J. Chaenomeles japonica, Cornus mas, Morus nigra fruits characteristics and their processing potential. J. Food Sci. Technol. 2014, 51, 3934–3941. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Inno, T.; Kume, C.; Irie, M.; Hiramatsu, K. Antioxidant and Antiulcerative Properties of Phenolics from Chinese Quince, Quince, and Apple Fruits. J. Agric. Food Chem. 2006, 54, 765–772. [Google Scholar] [CrossRef]
- Du, H.; Wu, J.; Li, H.; Zhong, P.-X.; Xu, Y.-J.; Li, C.-H.; Ji, K.-X.; Wang, L.-S. Polyphenols and triterpenes from Chaenomeles fruits: Chemical analysis and antioxidant activities assessment. Food Chem. 2013, 141, 4260–4268. [Google Scholar] [CrossRef]
- Huang, G.-H.; Xi, Z.-X.; Li, J.-L.; Chen, C.; Yang, G.-J.; Sun, L.; Chen, W.-S.; Zhu, H.-Y. Isolation of two new phenolic compounds from the fruit of Chaenomeles speciosa (Sweet) Nakai. Phytochem. Lett. 2013, 6, 526–530. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Lech, K.; Tkacz, K.; Nowicka, P. Influence of different drying methods on the quality of Japanese quince fruit. LWT—Food Sci. Technol. 2019, 114, e108416. [Google Scholar] [CrossRef]
- Lee, M.H.; Son, Y.K.; Han, Y.N. Tissue factor inhibitory flavonoids from the fruits of Chaenomeles sinensis. Arch. Pharm. Res. 2002, 25, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Lignan glycosides from the twigs of Chaenomeles sinensis and their biological activities. J. Nat. Prod. 2015, 78, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.S.; Park, K.J.; Kim, D.H.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Lee, K.R. A biphenyl derivative from the twigs of Chaenomeles speciosa. Bioorg. Chem. 2017, 72, 156–160. [Google Scholar] [CrossRef]
- Xie, X.; Cai, X.; Zhu, S.; Zou, G. Chemical composition and antimicrobial activity of essential oils of Chaenomeles speciosa from China. Food Chem. 2007, 100, 1312–1315. [Google Scholar] [CrossRef]
- Hellín, P.; Vila, R.; Jordán, M.J.; Laencina, J.; Rumpunen, K.; Ros, J.M. Characteristics and Composition of Chaenomeles Fruit Juice. In Japanese Quince—Potential Fruit Crop for Northern Europe. Department of Crop Science; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2003; pp. 127–139. [Google Scholar]
- Gardner, P.T.; White, T.A.C.; McPhail, D.B.; Duthie, G.G. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000, 68, 471–474. [Google Scholar] [CrossRef]
- Vila, R.; Granados, M.V.; Hellín, P.; Kauppinen, S.; Laencina, J.; Rumpunen, K.; Ros, J.M. Biochemical changes in chaenomeles fruits and fruit juice during ripening and storage. In Japanese Quince—Potential Fruit Crop for Northern Europe; Department of Crop Science, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2003; pp. 159–168. [Google Scholar]
- Urbanavičiūtė, I.; Liaudanskas, M.; Bobinas, Č.; Šarkinas, A.; Rezgienė, A.; Viskelis, P. Japanese Quince (Chaenomeles japonica) as a Potential Source of Phenols: Optimization of the Extraction Parameters and Assessment of Antiradical and Antimicrobial Activities. Foods 2020, 9, 1132. [Google Scholar] [CrossRef]
- Osman, A. Chapter 49—Cold pressed Japanese quince (Chaenomeles japonica (Thunb.) Lindl. ex Spach) seed oil. In Cold Pressed Oils; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 541–546. [Google Scholar]
- Górnaś, P.; Siger, A.; Segliņa, D. Physicochemical characteristics of the cold-pressed Japanese quince seed oil: New promising unconventional bio-oil from by-products for the pharmaceutical and cosmetic industry. Ind. Crops Prod. 2013, 48, 178–182. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P. UPLC/ESI-Q-TOF-MS analysis of (poly)phenols, tocols and amino acids in Chaenomeles leaves versus in vitro anti-enzyme activities. Ind. Crop. Prod. 2022, 181, e114829. [Google Scholar] [CrossRef]
- Marat, N.; Danowska-Oziewicz, M.; Narwojsz, A. Chaenomeles Species-Characteristics of Plant, Fruit and Processed Products: A Review. Plants 2022, 11, 3036. [Google Scholar] [CrossRef]
- Souci, S.W.; Fachmann, W.; Kraut, H. Food Composition and Nutrition Tables, 7th ed.; MedPharm: Stuttgart, Germany, 2008; pp. 743–1238. [Google Scholar]
- Zhang, H.; Geng, Y.L.; Wang, D.J.; Liu, J.H.; Wang, X.; Du, J.H.; Li, S.B. Research on nutrient components of different species of Chaenomeles speciosa Nakai. Shandong Sc. 2011, 24, 24–27. [Google Scholar]
- Bieniasz, M.; Dziedzic, E.; Kaczmarczyk, E. The effect of storage and processing on vitamin C content in Japanese quince fruit. Folia Hortic. 2017, 29, 83–93. [Google Scholar] [CrossRef]
- Hallmann, E.; Orpel, E.; Rembiałkowska, E. The content of biologically active compounds in some fruits from natural state. Veg. Crop Res. Bull. 2011, 75, 81–90. [Google Scholar] [CrossRef]
- Mezhenskij, V.L. Research, Cultivation and Processing of Japanese Quince, Chaenomeles spp. in Ukraine. Verksamhetsberättelse 1992–1994: Report; Balsgård-Deparment of Horticultural Plant Breeding, Swedish University of Agricultural Sciences: Uppsala, Sweden, 1996; pp. 193–195. [Google Scholar]
- Legua, P.; Serrano, M.; Melgarejo, P.; Valero, D.; Martínez, J.J.; Martínez, R.; Hernández, F. Quality parameters, biocompounds and antioxidant activity in fruits of nine quince (Cydonia oblonga Miller) accessions. Sci. Hortic. 2013, 154, 61–65. [Google Scholar] [CrossRef]
- Najman, K.; Adrian, S.; Hallmann, E.; Sadowska, A.; Buczak, K.; Waszkiewicz-Robak, B.; Szterk, A. Effect of Various Drying Methods on Physicochemical and Bioactive Properties of Quince Fruit (Cydonia oblonga Mill.). Agriculture 2023, 13, 446. [Google Scholar] [CrossRef]
- Najman, K.; Adrian, S.; Sadowska, A.; Świąder, K.; Hallmann, E.; Buczak, K.; Waszkiewicz-Robak, B.; Szterk, A. Changes in Physicochemical and Bioactive Properties of Quince (Cydonia oblonga Mill.) and Its Products. Molecules 2023, 28, 3066. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P. Carotenoids, chlorophylls, vitamin E and amino acid profile in fruits of nineteen Chaenomeles cultivars. J. Food Comp. Anal. 2020, 93, e103608. [Google Scholar] [CrossRef]
- Zhang, R.; Li, S.; Zhu, Z.; He, J. Recent advances in valorization of Chaenomeles fruit: A review of botanical profile, phytochemistry, advanced extraction technologies and bioactivities. Trend. Food Sci. Technol. 2019, 91, 467–482. [Google Scholar] [CrossRef]
- Xu, N.Y.; Kim, J.S.; Kang, S.S.; Son, K.H.; Kim, H.P.; Chang, H.W.; Bae, K. A new acylated triterpene from the roots of Chaenomeles japonica. Chem. Pharm. Bull. 2002, 50, 1124–1125. [Google Scholar] [CrossRef]
- Lesińska, E. Zawartość składników mineralnych w owocach pigwowca. Zesz. Nauk. AR W Krakowie Rol. 1985, 25, 175–183. (In Polish) [Google Scholar]
- Watychowicz, K.; Janda, K.; Jakubczyk, K.; Wolska, J. Chaenomeles—Health Promoting Benefits. Rocz. Panstw. Zakl. Hig. 2017, 68, 217–227. [Google Scholar]
- Lesińska, E. Characteristics of sugars and acids in the fruits of East Asian quince. Die Nahrung 1987, 31, 763–765. [Google Scholar] [CrossRef]
- Rasheed, M.; Hussain, I.; Rafiq, S.; Hayat, I.; Qayyum, A.; Ishaq, S.; Awan, M.S. Chemical composition and antioxidant activity of quince fruit pulp collected from different locations. Int. J. Food Propert. 2018, 21, 2320–2327. [Google Scholar] [CrossRef]
- Hopur, H.; Asrorov, A.M.; Qingling, M.; Yili, A.; Ayupbek, A.; Nannan, P.; Aisa, H.A. HPLC Analysis of Polysaccharides in Quince (Cydonia Oblonga Mill. var. maliformis) Fruit and PTP1B Inhibitory Activity. Nat. Prod. J. 2011, 1, 146–150. [Google Scholar] [CrossRef]
- Ashraf, M.U.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A. Cydonia oblonga M., A Medicinal Plant Rich in Phytonutrients for Pharmaceuticals. Front. Pharmacol. 2016, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, A.; Nora, P.; Eva, S.B. A Review of Nutritional Value and Putative Health—Effects of Quince (Cydonia oblonga) Fruit. Int. J. Hortic. Sci. 2013, 3, 29–32. [Google Scholar] [CrossRef]
- Thomas, M.; Crépeau, M.J.C.; Rumpunen, K.; Thibault, J.-F. Dietary fibre and cell-wall polysaccharides in the fruits of Japanese quince (Chaenomeles japonica). LWT—Food Sci. Technol. 2000, 33, 124–131. [Google Scholar] [CrossRef]
- Thomas, M.; Thibault, J.-F. Cell-wall polysaccharides in the fruits of Japanese quince (Chaenomeles japonica): Extraction and preliminary characterisation. Carbohydr. Polym. 2002, 49, 345–355. [Google Scholar] [CrossRef]
- Thomas, M.; Guillemin, F.; Guillon, F.; Thibault, J.F. Pectins in the fruits of Japanese Quince (Chaenomeles japonica). Carbohyd. Polym. 2003, 53, 361–372. [Google Scholar] [CrossRef]
- Hellín, P.; Jordán, M.J.; Vila, R.; Gustafsson, M.; Göransson, E.; Ĺkesson, B.; Gröön, I.; Laencina, J.; Ros, J.M. Processing and products of Japanese quince (Chaenomeles japonica) fruits. In Japanese Quince—Potential Fruit Crop for Northern Europe; Department of Crop Science, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2003; pp. 169–175. [Google Scholar]
- Qin, Z.; Liu, H.-M.; Lv, T.-T.; Wang, X.-D. Structure, rheological, thermal and antioxidant properties of cell wall polysaccharides from Chinese quince fruits. Int. J. Biol. Macromol. 2019, 147, 1146–1155. [Google Scholar] [CrossRef]
- Aliasl, F.; Toliyat, T.; Mohammadi, A.; Minaee, B.; Samadi, N.; Aliasl, J.; Sadeghpour, O. Medicinal Properties of Cydonia Oblonga Mill Fruit (Pulp and Peel) in Iranian Traditional Medicine and Modern Phytothrapy. Trad Integr. Med. 2016, 1, 122–128. [Google Scholar]
- Khoubnasabjafari, M.; Jouyban, A. A review of phytochemistry and bioactivity of quince (Cydonia oblonga Mill.). J. Med. Plant. Res. 2011, 5, 3577–3594. [Google Scholar] [CrossRef]
- Yang, G.; Fen, W.; Lei, C.; Xiao, W.; Sun, H. Study on determination of pentacyclic triterpenoids in Chaenomeles by HPLC-ELSD. J. Chromatogr. Sci. 2009, 47, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-J.; Jin, D.-N.; Zhou, Y.; Sang, X.-Y.; Zhu, Y.-Y.; He, Y.-J.; Xie, T.-Z.; Dai, Z.; Zhao, Y.-L.; Luo, X.-D. Bioactivity Ingredients of Chaenomeles speciosa against Microbes: Characterization by LC-MS and Activity Evaluation. J. Agric. Food Chem. 2021, 69, 4686–4696. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zheng, H.; Fu, G.; Yin, M.; Jiang, L.; Zhao, Y.; Zha, L.; Chu, S.; Peng, H.; Huang, L. Integrated untargeted metabolome, full-length sequencing, and transcriptome analyses reveal insights into the fruit quality at different harvest times of Chaenomeles speciosa. Food Res. Int. 2023, 164, 112314. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.M.; Kim, D.H.; Subedi, L.; Khan, Z.; Choi, S.U.; Kim, S.Y.; Kim, C.S. Chemical constituents of Chaenomeles sinensis twigs and their biological activity. Beilstein J. Org. Chem. 2020, 16, 3078–3085. [Google Scholar] [CrossRef] [PubMed]
- Oku, H.; Ueda, Y.; Ishiguro, K. Antipruritic effects of the fruits of Chaenomeles sinensis. Biol. Pharm. Bull. 2003, 26, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, J.-S.; Yun, C.-Y.; Kim, D.-H.; Kim, I.S. Chinese quince (Chaenomeles sinensis) extract inhibits cell migration and cytokine release in HMC-1 cells. Food Sci. Biotechnol. 2013, 22, 501–506. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1794427, Chlorogenic Acid; CID 5280633, Neochlorogenic Acid; CID 9798666, Cryptochlorogenic Acid; CID 64945, Ursolic Acid, and CID 10494, Oleanolic Acid. 2023. Available online: http://pubchem.ncbi.nlm.nih.gov (accessed on 21 December 2023).
- Baroni, M.V.; Gastaminza, J.; Podio, N.S.; Lingua, M.S.; Wunderlin, D.A.; Rovasio, J.L.; Dotti, R.; Rosso, J.C.; Ghione, S.; Ribotta, P.D. Changes in the antioxidant properties of quince fruit (Cydonia oblonga Miller) during jam production at industrial scale. J. Food Qual. 2018, 2018, e1460758. [Google Scholar] [CrossRef]
- Essafi-Benkhadir, K.; Refai, A.; Riahi, I.; Fattouch, S.; Karoui, H.; Essafi, M. Quince (Cydonia oblonga Miller) peel polyphenols modulate LPS-induced inflammation in human THP-1-derived macrophages through NF-κB, p38MAPK and Akt inhibition. Biochem. Biophys. Res. Commun. 2012, 418, 180–185. [Google Scholar] [CrossRef]
- García-Alonso, M.; de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J. Evaluation of the antioxidant properties of fruits. Food Chem. 2004, 84, 13–18. [Google Scholar] [CrossRef]
- Yildirim, A.; Oktay, M.; Bilaloglu, V. The antioxidant activity of the leaves of Cydonia vulgaris. Turk. J. Med. Sci. 2001, 31, 23–27. [Google Scholar]
- Pacifico, S.; Gallicchio, M.; Fiorentino, A.; Fischer, A.; Meyer, U.; Stintzing, F.C. Antioxidant properties and cytotoxic effects on human cancer cell lines of aqueous fermented and lipophilic quince (Cydonia oblonga Mill.) preparations. Food Chemical Toxicol. 2012, 50, 4130–4135. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Kim, B.J.; Kim, J.H.; Heo, M.Y.; Kim, H.P. Biological screening of 100 plant extracts for cosmetic use (I): Inhibitory activities of tyrosinase and DOPA auto-oxidation. Int. J. Cosmet. Sci. 1997, 19, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yu, X.; Mi, M.; Zhao, J.; Wang, J.; Zhang, T. Antioxidative property and antiatherosclerotic effects of the powder processed from Chaenomeles speciosa in APOE-/- mice. J. Food Biochem. 2010, 34, 535–548. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Liu, L. Skin-care functions of peptides prepared from Chinese quince seed protein: Sequences analysis, tyrosinase inhibition and molecular docking study. Ind. Crops Prod. 2020, 148, e112331. [Google Scholar] [CrossRef]
- Ma, B.; Wang, J.; Tong, J.; Zhou, G.; Chen, Y.; He, J.; Wang, Y. Protective effects of Chaenomeles thibetica extract against carbon tetrachloride-induced damage via the MAPK/Nrf2 pathway. Food Funct. 2016, 7, 1492–1500. [Google Scholar] [CrossRef]
- Sancheti, S.; Sancheti, S.; Bafna, M.; Seo, S.Y. Antihyperglycemic, antihyperlipidemic, and antioxidant effects of Chaenomeles sinensis fruit extract in streptozotocin-induced diabetic rats. Eur. Food Res. Technol. 2010, 231, 415–421. [Google Scholar] [CrossRef]
- Kawahara, T.; Iizuka, T. Inhibitory effect of hotwater extract of quince (Cydonia oblonga) on immunoglobulin E-dependent late-phase immune reactions of mast cells. Cytotechnology 2011, 63, 143–152. [Google Scholar] [CrossRef]
- Shinomiya, F.; Hamauzu, Y.; Kawahara, T. Anti-allergic effect of a hot-water extract of quince (Cydonia oblonga). Biosci. Biotechnol. Biochem. 2009, 73, 1773–1778. [Google Scholar] [CrossRef]
- Gründemann, C.; Papagiannopoulos, M.; Lamy, E.; Mersch-Sundermann, V.; Huber, R. Immunomodulatory properties of a lemon-quince preparation (Gencydo®) as an indicator of anti-allergic potency. Phytomedicine 2011, 18, 760–768. [Google Scholar] [CrossRef]
- Chojnacka, K.; Owczarek, K.; Caban, M.; Caban, M.; Sosnowska, D.; Polka, D.; Koziołkiewicz, M.; Fichna, J.; Lewandowska, U. Japanese quince (Chaenomeles japonica) leaf phenol extract as modulator of the inflammatory response in lipopolysaccharide-triggered murine macrophage raw 264.7 cells. J. Physiol. Pharmacol. 2020, 71, 833–843. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Y.X.; Liu, A.L.; Wang, H.D.; Wang, Y.L.; Du, G.H. Antioxidant, anti-inflammatory and anti-influenza properties of components from Chaenomeles speciosa. Molecules 2010, 15, 8507–8517. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.-B.; Yang, Q.; Sun, L.-N.; Chen, W.-S. Anti-Inflammatory and Analgesic Activities of Chaenomeles speciosa Fractions in Laboratory Animals. J. Med. Food. 2009, 12, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Cyboran-Mikołajczyk, S.; Dudra, A.; Mizgier, P.; Kucharska, A.Z.; Olejniczak, T.; Gabrielska, J. Biological activity of Japanese quince extract and its interactions with lipids, erythrocyte membrane, and human albumin. J. Membr. Biol. 2016, 249, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wei, W. Effects and mechanisms of glucosides of chaenomeles speciosa on collagen-induced arthritis in rats. Int. Immunopharmacol. 2003, 3, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.P.; Dai, M.; Wang, H.; Zhang, L.L.; Wei, W. Antinociceptive effect of glucosides of Chaenomeles speciosa. Chin. J. Pharmacol. Toxicol. 2005, 19, 169–174. [Google Scholar]
- Huang, D.; Jiang, S.; Du, Z.; Chen, Y.; Xue, D.; Wang, X.; Li, M.; Zhang, F.; Chen, W.; Sun, L. Analgesic and AntiArthritic Activities of Polysaccharides in Chaenomeles speciosa. Front. Pharmacol. 2022, 13, e744915. [Google Scholar] [CrossRef]
- Riahi-Chebbi, I.; Haoues, M.; Essafi, M.; Zakraoui, O.; Fattouch, S.; Karoui, H.; Essafi-Benkhadir, K. Quince peel polyphenolic extract blocks human colon adenocarcinoma LS174 cell growth and potentiates 5-fluorouracil efficacy. Cancer Cell Int. 2015, 16, e1. [Google Scholar] [CrossRef]
- Adiban, H.H.; Shirazi, F.; Gholami, S.; Kamalinejad, M.; Hosseini, S.H.; Noubarani, M.; Eskandari, M.R. Chemopreventive effect of quince (Cydonia oblonga Mill.) fruit extract on hepatocellular carcinoma induced by diethylnitrosamine in rats. Int. Pharm. Acta 2019, 2, e2. [Google Scholar] [CrossRef]
- Strek, M.; Gorlach, S.; Podsędek, A.; Sosnowska, D.; Koziołkiewicz, M.; Hrabec, Z.; Hrabec, E. Procyanidin oligomers from Japanese quince (Chaenomeles japonica) fruit inhibit activity of MMP-2 and MMP-9 metalloproteinases. J. Agric. Food Chem. 2007, 55, 6447–6452. [Google Scholar] [CrossRef]
- Lewandowska, U.; Szewczyk, K.; Owczarek, K.; Hrabec, Z.; Podsędek, A.; Koziołkiewicz, M.; Hrabec, E. Flavanols from Japanese quince (Chaenomeles japonica) fruit inhibit human prostate and breast cancer cell line invasiveness and cause favorable changes in Bax/Bcl-2 mRNA Ratio. Nutr. Cancer 2013, 65, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, K.; Hrabec, E.; Fichna, J.; Sosnowska, D.; Koziołkiewicz, M.; Szymański, J.; Lewandowska, U. Flavanols from Japanese quince (Chaenomeles japonica) fruit suppress expression of cyclooxygenase-2, metalloproteinase-9, and nuclear factor-kappaB in human colon cancer cells. Acta Biochim. Pol. 2017, 64, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Zvikas, V.; Urbanaviciute, I.; Bernotiene, R.; Kulakauskiene, D.; Morkunaite, U.; Balion, Z.; Majiene, D.; Liaudanskas, M.; Viskelis, P.; Jekabsone, A.; et al. Investigation of Phenolic Composition and Anticancer Properties of Ethanolic Extracts of Japanese Quince Leaves. Foods 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Sosnowska, D.; Polka, D.; Owczarek, K.; Gorlach-Lira, K.; Oliveira de Verasa, B.; Lewandowska, U. Comparison of phenolic compounds, antioxidant and cytotoxic activity of extracts prepared from Japanese quince (Chaenomeles japonica L.) leaves. J. Physiol. Pharmacol. 2020, 71, 213–222. [Google Scholar] [CrossRef]
- Gao, H.Y.; Wu, L.J.; Kuroyanagi, M.; Harada, K.; Kawahara, N.; Nakane, T.; Umehara, K.; Hirasawa, A.; Nakamura, Y. Antitumor-promoting constituents from Chaenomeles sinensis Koehne and their activities in JB6 mouse epidermal cells. Chem. Pharm. Bull. 2003, 51, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Abliz, A.; Aji, Q.; Abdusalam, E.; Sun, X.; Abdurahman, A.; Zhou, W.; Moore, N.; Umar, A. Effect of Cydonia oblonga Mill. Leaf extract on serum lipids and liver function in a rat model of hyperlipidaemia. J. Ethnopharmacol. 2014, 151, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Iskandar, G.; Aikemu, A.; Yiming, W.; Zhou, W.; Berké, B.; Begaud, B.; Moore, N. Effects of Cydonia oblonga Miller leaf and fruit flavonoids on blood lipids and anti-oxydant potential in hyperlipidemia rats. J. Ethnopharmacol. 2015, 169, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Abdusalam, E.; Abliz, P.; Reyim, N.; Tian, S.; Aji, Q.; Issak, M.; Iskandar, G.; Moore, N.; Umar, A. Effect of Cydonia oblonga Mill. fruit and leaf extracts on blood pressure and blood rheology in renal hypertensive rats. J. Ethnopharmacol. 2014, 152, 464–469. [Google Scholar] [CrossRef]
- Zhou, W.; Abdurahman, A.; Abdusalam, E.; Yiming, W.; Abliz, P.; Aji, Q.; Issak, M.; Iskandar, G.; Moore, N.; Umar, A. Effect of Cydonia oblonga Mill. Leaf extracts or captopril on blood pressure and related biomarkers in renal hypertensive rats. J. Ethnopharmacol. 2014, 153, 635–640. [Google Scholar] [CrossRef]
- Zhou, W.; Abdurahman, A.; Umar, A.; Iskander, G.; Abdusalam, E.; Berké, B.; Bégaud, B.; Moore, N. Effects of Cydonia oblonga Miller extracts on blood hemostasis, coagulation and fibrinolysis in mice, and experimental thrombosis in rats. J. Ethnopharmacol. 2014, 154, 163–169. [Google Scholar] [CrossRef]
- Abulizi, A.; Simayi, J.; Nuermaimaiti, M.; Han, M.; Hailati, S.; Talihati, Z.; Maihemuti, N.; Nuer, M.; Khan, N.; Abudurousuli, K.; et al. Quince extract resists atherosclerosis in rats by down-regulating the EGFR/PI3K/Akt/GSK-3β pathway. Biomed. Pharmacother. 2023, 160, 114330. [Google Scholar] [CrossRef]
- Gholami, S.; Hosseini, M.J.; Jafari, L.; Omidvar, F.; Kamalinejad, M.; Mashayekhi, V.; Hosseini, S.H.; Kardan, A.; Pourahmad, J.; Eskandari, M.R. Mitochondria as a Target for the Cardioprotective Effects of Cydonia oblonga Mill. and Ficus carica L. in Doxorubicin-Induced Cardiotoxicity. Drug Res. 2017, 67, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Hanan, E.; Hasan, N.; Zahiruddin, S.; Ahmad, S.; Sharma, V.; Ahmad, F.J. Metabolite profiling and Ameliorative effect of quince (Cydonia oblonga) leaves against doxorubicin induced cardiotoxicity in Wistar rats. Food Biosci. 2023, 53, 102691. [Google Scholar] [CrossRef]
- Lee, M.H.; Han, Y.N. A new in vitro tissue factor inhibitory triterpene from the fruits of Chaenomeles sinensis. Planta Med. 2003, 69, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Xie, L.; Xin, X.; Aisa, H. Anti-diabetic action of Cydonia oblonga seed extract: Improvement of glucose metabolism via activation of PI3K/AKT signaling pathway. J. Pharmacogn. Phytochem. 2016, 4, 7–13. [Google Scholar]
- Lee, H.S.; Jeon, Y.E.; Jung, J.I.; Kim, S.M.; Hong, S.H.; Lee, J.; Hwang, J.S.; Hwang, M.O.; Kwon, K.; Kim, E.J. Anti-obesity effect of Cydonia oblonga Miller extract in high-fat diet-induced obese C57BL/6 mice. J. Funct. Foods 2022, 89, e104945. [Google Scholar] [CrossRef]
- Aslan, M.; Orhan, N.; Orhan, D.D.; Ergun, F. Hypoglycemic activity and antioxidant potential of some medicinal plants traditionally used in Turkey for diabetes. J. Ethnopharmacol. 2010, 128, 384–389. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pawlik, N. Japanese quince (Chaenomeles japonica L.) fruit polyphenolic extract modulates carbohydrate metabolism in HepG2 cells via AMP-activated protein kinase. Acta Biochim. Pol. 2018, 65, 67–78. [Google Scholar] [CrossRef]
- Sancheti, S.S.; Sancheti, S.; Seo, S. Chaemnomeles sinensis: A potent α- and β-glucosidase inhibitor. Am. J. Pharmacol. Toxicol. 2009, 4, 8–11. [Google Scholar] [CrossRef]
- Sancheti, S.; Sancheti, S.; Seo, S.Y. Antidiabetic and antiacetylcholinesterase effects of ethyl acetate fraction of Chaenomeles sinensis (Thouin) Koehne fruits in streptozotocin-induced diabetic rats. Exp. Toxicol. Pathol. 2013, 65, 55–60. [Google Scholar] [CrossRef]
- Alizadeh, H.; Rahnema, M.; Semnani, S.N.; Hajizadeh, N. Detection of compounds and antibacterial effect of quince (Cydonia oblonga Miller) extracts in vitro and in vivo. J. Biol. Active Prod. Nature 2013, 3, 303–309. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, S.B.; Singh, S. COVID-19: Environment concern and impact of Indian medicinal system. J. Environ. Chem. Eng. 2020, 8, 104–144. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, M.; Kianifar, H.; Memariani, Z.; Kamalinejad, M.; Bijani, A.; Saghebi, R.; Gorji, N. Comparison of the efficacy of ranitidine and quince syrup on gastroesophageal reflux disease in children. BMC Complement. Med. Ther. 2019, 45, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Hashempur, M.H.; Mojibian, M.; Aliasl, F.; Bioos, S.; Nejatbakhsh, F. A comparative study of ranitidine and quince (Cydonia oblonga mill) sauce on gastroesophageal reflux disease (GERD) in pregnancy: A randomised, open-label, active-controlled clinical trial. J. Obstet. Gynaecol. 2018, 38, 899–905. [Google Scholar] [CrossRef]
- Zohalinezhad, M.E.; Imanieh, M.H.; Samani, S.M.; Mohagheghzadeh, A.; Dehghani, S.M.; Haghighat, M.; Akbarzadeh, A.R. Effects of Quince syrup on clinical symptoms of children with symptomatic gastroesophageal reflux disease: A double-blind randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2015, 21, 268–276. [Google Scholar] [CrossRef]
- Rahi, P.; Mirghafourvand, M.; Mohammad-Alizadeh-Charandabi, S.; Javadzadeh, Y.; Seidi, S. Comparison of the effect of mefenamic acid and quince on the level of menstrual bleeding and hemoglobin: A randomized controlled clinical trial. Eur. J. Integr. Med. 2016, 8, 67–72. [Google Scholar] [CrossRef]
- Din Ganaie, M.U.; Behl, T.; Nijhawan, P.; Sachdeva, M.; Khan, N. Investigation of anti-depressant effect of aqueous and ethanolic extract of Cydonia oblonga in rats. Obes. Med. 2020, 18, e100202. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Mandal, A.; Hossain, U.; Sil, P.C. Chapter 5.2—Antioxidants and cardiovascular diseases. In Antioxidants Effects in Health; Nabavi, S.M., Sanches Silva, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 613–640. [Google Scholar]
- Zekrumah, M.; Begua, P.B.; Razak, A.S.; Wahab, J.; Moffo, N.; Ivane, A.; Oman, M.H.; Elrashied, H.T.; Zou, X.; Zhang, D. Dietary polyphenols—Role in non-communicable chronic disease prevention, and interactions in food systems. An overview. Nutrition 2023, 112, e112034. [Google Scholar] [CrossRef]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Mastellone, C.; Piscopo, V.; Monaco, P. Spectroscopic identification and antioxidant activity of glucosylated carotenoid metabolites from Cydonia vulgaris fruits. J. Agric. Food Chem. 2006, 54, 9592–9597. [Google Scholar] [CrossRef]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Mastellone, C.; Piccolella, S.; Monaco, P. Isolation, structure elucidation, and evaluation of cydonioside A, an unusual terpenoid from the fruits of Cydonia vulgaris. Chem. Biodivers. 2007, 4, 973–979. [Google Scholar] [CrossRef]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Mastellone, C.; Piscopo, V.; Caputo, R.; Monaco, P. Isolation and structure elucidation of antioxidant polyphenols from quince (Cydonia vulgaris) peels. J. Agric. Food Chem. 2008, 56, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.M.; Valentao, P.; Seabra, R.M.; Andrade, P.P. Quince (Cydonia oblonga Miller): An interesting source of bioactive compounds. In Food Chemistry Research Development; Papadopoulos, K.N., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2008; pp. 243–266. [Google Scholar]
- Devkota, H.P.; Dirar, A.I.; Hassan, M.M.; Logesh, R. Chapter 10—Cydonia oblonga Mill. In Himalayan Fruits and Berries, Bioactive Compounds, Uses and Nutraceutical Potential; Belwal, T., Bhatt, I., Devkota, H.P., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 91–99. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Seabra, R.M.; Silva, B.M. Phenolic profile of Cydonia oblonga Miller leaves. J. Agric. Food Chem. 2007, 55, 7926–7930. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.E.; Frederiksen, H.; Struntze Krogholm, K.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Yamaguchi, M.; Shigeyama, K.; Sakaguchi, I. The Anti-Aging Potential of Extracts from Chaenomeles sinensis. Cosmetics 2019, 6, 21. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.-M.; Qin, G.-Y. Structure characterization and antioxidant activity of polysaccharides from Chinese quince seed meal. Food Chem. 2017, 234, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.P.; Wang, Y.Q.; Shi, L.; Zhang, Z.K.; Dong, F.; Li, H.R.; Zhang, J.Y.; Man, Y.Q. Comparative metabolism study on chlorogenic acid, cryptochlorogenic acid and neochlorogenic acid using UHPLC-Q-TOF MS coupled with network pharmacology. Chin. J. Nat. Med. 2021, 19, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Han, L.Y.; Zhang, H.; Xin, H.L. Chaenomeles speciosa: A review of chemistry and pharmacology. Biomed. Rep. 2014, 2, 12–18. [Google Scholar] [CrossRef]
- Fallon, C.M.; Quach, A.; Lajczak-McGinley, N.; O’Toole, A.; Barrett, K.E.; Sheridan, H.; Keely, S.J. Pentacyclic triterpenes modulate farnesoid X receptor expression in colonic epithelial cells: Implications for colonic secretory function. J. Biol. Chem. 2022, 298, 102569. [Google Scholar] [CrossRef]
- Xie, X.; Zou, G.; Li, C. Antitumor and immunomodulatory activities of a water-soluble polysaccharide from Chaenomeles speciosa. Carbohydr. Polym. 2015, 132, 323–329. [Google Scholar] [CrossRef]
- Haque, A.; Brazeau, D.; Amin, A.R. Perspectives on natural compounds in chemoprevention and treatment of cancer: An update with new promising compounds. Eur. J. Cancer. 2021, 149, 165–183. [Google Scholar] [CrossRef]
- Shao, J.; Fang, Y.; Zhao, R.; Chen, F.; Yang, M.; Jiang, J.; Chen, Z.; Yuan, X.; Jia, L. Evolution from small molecule to nano-drug delivery systems—An emerging approach for cancer therapy of ursolic acid. Asian J. Pharm. Sci. 2020, 15, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Y.; Li, Y.; Tang, Y.T.; Ma, X.D.; Tang, Z.Y. Anticancer activity of oleanolic acid and its derivatives: Recent advances in evidence, target profiling and mechanisms of action. Biomed. Pharmacother. 2022, 145, e112397. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.X.; Poo, C.L.; Subramaniam, M.; Cordell, G.A.; Lim, Y.M. Maslinic acid exerts anticancer effects by targeting cancer hallmarks. Phytomedicine 2023, 110, e154631. [Google Scholar] [CrossRef] [PubMed]
- Villani, V.; Di Marco, G.; Iacovelli, F.; Pietrucci, D.; Canini, A.; Gismondi, A. Profile and potential bioactivity of the miRNome and metabolome expressed in Malva sylvestris L. leaf and flower. BMC Plant Biol. 2023, 23, 439. [Google Scholar] [CrossRef]
- Gezici, S.; Sekeroglu, N. Regulation of MicroRNAs by Natural Products and Bioactive Compounds Obtained from Common Medicinal Plants: Novel Strategy in Cancer Therapy. Indian J. Pharm. Educ. Res. 2017, 51, s483–s488. [Google Scholar] [CrossRef]
- Soyturk, A.; Sen, F.; Uncu, A.T.; Celik, I.; Uncu, A.O. De novo assembly and characterization of the first draft genome of quince (Cydonia oblonga Mill.). Sci. Rep. 2021, 11, 3818. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Li, D. Oleanolic acid suppresses the proliferation of lung carcinoma cells by miR-122/Cyclin G1/MEF2D axis. Mol. Cell. Biochem. 2015, 400, 1–7. [Google Scholar] [CrossRef]
- The Lancet (editorial) Hypertension: Uncontrolled and conquering the world. Lancet 2007, 370, 539. [CrossRef]
- He, H.; Wei, Q.; Chang, J.; Yi, X.; Yu, X.; Luo, G.; Li, X.; Yang, W.; Long, Y. Exploring the hypoglycemic mechanism of chlorogenic acids from Pyrrosia petiolosa (Christ) Ching on type 2 diabetes mellitus based on network pharmacology and transcriptomics strategy. J. Ethnopharmacol. 2023, 322, 117580. [Google Scholar] [CrossRef]
- Loza-Rodríguez, H.; Estrada-Soto, S.; Alarcón-Aguilar, F.J.; Huang, F.; Aquino-Jarquín, G.; Fortis-Barrera, Á.; Giacoman-Martínez, A.; Almanza-Pérez, J.C. Oleanolic acid induces a dual agonist action on PPARγ/α and GLUT4 translocation: A pentacyclic triterpene for dyslipidemia and type 2 diabetes. Eur. J. Pharmacol. 2020, 883, 173252. [Google Scholar] [CrossRef]
- Sawai, R.; Kuroda, K.; Shibata, T.; Gomyou, R.; Osawa, K.; Shimizu, K. Anti-influenza virus activity of Chaenomeles sinensis. J. Ethnopharmacol. 2008, 118, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Sawai-Kuroda, R.; Kikuchi, S.; Shimizu, Y.K.; Sasaki, Y.; Kuroda, K.; Tanaka, T.; Yamamoto, T.; Sakurai, K.; Shimizu, K. A polyphenol-rich extract from Chaenomeles sinensis (Chinese quince) inhibits influenza A virus infection by preventing primary transcription in vitro. J. Ethnopharmacol. 2013, 146, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Karar, M.G.E.; Pletzer, D.; Jaiswal, R.; Weingart, H.; Kuhnert, N. Identification, characterization, isolation and activity against Escherichia coli of quince (Cydonia oblonga) fruit polyphenols. Food Res. Int. 2014, 65, 121–129. [Google Scholar] [CrossRef]
- Armet, A.M.; Deehan, E.C.; O’Sullivan, A.F.; Mota, J.F.; Field, C.J.; Prado, C.M.; Lucey, A.J.; Walter, J. Rethinking healthy eating in light of the gut microbiome. Cell Host Microbe 2022, 30, 764–785. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Kishida, H.; Yamazaki, N. Gastroprotective property of Pseudocydonia sinensis fruit jelly on the ethanol-induced gastric lesions in rats. J. Funct. Foods 2018, 48, 275–282. [Google Scholar] [CrossRef]
- Schloss, J.; Steel, A. Quince fruit compared to Vitamin B6 for treatment of nausea and vomiting in Pregnancy. Adv. Integr. Med. 2017, 4, 80–81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).