Abstract
Guanidine acetic acid (GAA) has been reported to improve growth performance, nutrient utilization, and meat quality in livestock. This study aimed to investigate whether coated GAA (CGAA) in comparison with uncoated GAA (UGAA) could have different effects on rumen fermentation, antioxidant capacity, and microflora composition in the rumen. Seventy-two lambs were randomly arranged in a 2 × 3 factorial experiment design with two diets of different forage type (OH: oaten hay; OHWS: oaten hay plus wheat silage) and three GAA treatments within each diet (control, diet without GAA addition; UGAA, uncoated GAA; CGAA, coated GAA). The whole feeding trial lasted for 120 days. The lambs in the OH group presented lower total volatile fatty acid (VFA), alpha diversity, Firmicutes, NK4A214_group, and Lachnospiraceae_NK3A20_group than those on the OHWS diet in the last 60 days of the feeding stage (p < 0.05). Regardless of what GAA form was added, dietary GAA supplementation increased the total VFA, microbial crude protein (MCP), adenosine triphosphate (ATP), and antioxidant capacity in rumen during lamb feedlotting (p < 0.05). However, molar propionate proportion, acetate:propionate ratio (A:P), and relative Succiniclasticum abundance decreased with GAA addition in the first 60 days of the growing stage, while the molar butyrate proportion and NK4A214_group (p < 0.05) in response to GAA addition increased in the last 60 days of feeding. These findings indicated that dietary GAA enhanced antioxidant capacity and fermentation characteristics in the rumen, but the addition of uncoated GAA in diets might cause some dysbacteriosis of the rumen microbiota.
1. Introduction
Guanidine acetic acid (GAA), with a molecular formula of C3H7N3O2 and a molecular weight of 117.11, is synthesized in the kidney, liver, and pancreas from L-arginine and glycine, and then converted to creatine to participate in the metabolism of energy and proteins [1]. As a nutritive feed additive, GAA has been used to improve growth performance, carcass characteristics, and meat quality in pigs [2], chickens [3], bulls [4], and sheep [5]. Unlike monogastric animals, host ruminants and rumen microorganisms are mutually beneficial and symbiotic [6]. Rumen microbial fermentation of feeds produces volatile fatty acids (VFA) and microbial protein to provide most of the available energy and protein required by host ruminants [7]. A previous study in Angus bulls noted that dietary GAA addition shifted ruminal fermentation towards greater propionate production [4]. The addition of GAA increased rumen total VFA production and microbial populations [8], with the ruminal degradation rate of GAA reported to be 47–49% in cattle [9]. However, it is unclear whether the coated GAA could sacrifice the aforementioned effects on rumen fermentation.
Oxidative stress in the rumen is detrimental to ruminant health [10]. Dietary additives with antioxidant features are often applied to avoid oxidative stress, especially during high-concentrate feeding [11]. Common antioxidant supplements include probiotic-based [12], selenium [13], and vitamin E [14]. As a nutritive additive, GAA donates an electron from its conjugate base and generates superoxide, a strong free radical [15], and may therefore be a direct pro-oxidant. However, GAA metabolites (e.g., creatine and arginine) might be able to quench free radicals after GAA ingestion [16,17,18]. A previous study on growing lambs reported that dietary GAA addition elevated the activities of serum catalase (CAT) and glutathione peroxidase (GSH-Px) and decreased malondialdehyde (MDA) content in skeletal muscle tissue [5]. However, it is not clear whether dietary GAA addition could present antioxidant capacity in the rumen, or what difference there could be compared with the coated GAA.
It is well known that forage type is an important factor affecting rumen fermentation as growth performance in sheep feeding practice. For instance, feeding sheep oaten hay as a forage source was found to maintain the stable state of their rumen internal environment and the growth of rumen microorganisms [19]. Wheat silage (WS) can provide an interim forage during the period when the previous year’s hay or silage has run out and the present year’s has not been harvested. A previous study in finishing beef cattle noted that feeding WS was found to decrease the acetate:propionate ratio in rumen and presented greater growth performance [20]. However, relevant research on sheep is scarce. Considering sheep are less tolerant to acid whole silage as sole forage, lamb diets with WS plus OH in comparison with sole OH as forage type were applied in the present lamb feedlotting trial.
The primary objective was to elucidate whether or not the coated GAA in comparison with uncoated GAA addition could improve rumen fermentation, antioxidant capacity, and how they affect rumen microflora in rapid-growing lambs, depending on the diet with different forage type sources.
2. Materials and Methods
2.1. Animal Ethics Statement
The experimental animals, design, and animal management in the present study followed the Guidelines of the Beijing Municipal Council on Animal Care (with protocol CAU20171014-1) and were in accordance with the recommendations of the academy’s guidelines for animal research.
2.2. Guanidinoacetic Acid Products
The uncoated GAA (available content of 984 g/kg, the average rumen degradation rate of UGAA is 50.9%) and coated GAA (available content of 600 g/kg, the packaging material was mainly fat powder, the average rumen degradation rate of CGAA is 15.8%). added in this study were in powder form and provided by Hebei Guang rui Company (Shijiazhuang, China).
2.3. Experimental Animals, Diets, and Design
Seventy-two two-month-old healthy male small-tailed Chinese Han lambs (a breed of sheep native to Shandong Province of China, with a rate of reproduction reaching 229%) initially weighing 12 ± 1.6 kg in body weight (BW) were chosen as experimental animals and fed total mixed rations (TMR). A 2 × 3 factorial feeding experimental design was applied to divide the animals into two forage types of TMRs (OH: oaten hay; OHWS: oaten hay plus wheat silage), and three GAA addition groups (GAA: 0 g/kg; UGAA: uncoated GAA, 1 g/kg; CGAA: coated GAA, 1 g/kg). GAA (UGAA and CGAA) was added to the concentrates, then mixed with the corresponding forage, and divided into two daily feeds (08:00 and 16:00). Each treatment was randomly divided into four bamboo-slotted bedding pens, and each pen was arranged with three lambs, while clean, fresh water was available at all times. All rations were formulated to satisfy the nutrient requirement of 300 g gain/day [21]. The composition and nutrient levels of the experimental diet are shown in Table A1. The study periods consisted of 7 days of adaptation and 120 days of a 2-stage data collection.
2.4. Sample Collection and Analyses
2.4.1. Rumen Fluid Sampling
Rumen fluid samples were collected at the end of every stage (d 60 and d 120). One hour after the morning meal, an oral stomach cannula (MDW15, Colebo Equipment Co., Ltd., Wuhan, China) and a 200 mL syringe were used to collect rumen fluid samples. The first 2 tubes of rumen fluid were discarded to avoid saliva contamination [8], and a 100 mL rumen fluid sample was collected from 8 lambs in each group. Ruminal pH was immediately determined by a digital pH meter (Testo205 type, Testo AG, Lenzkirch, Germany). Subsequently, the samples were strained through four layers of cheesecloth. A total of 0.25 mL of metaphosphoric acid (25 g/100 mL) was added to aliquots of 1 mL rumen fluid, which were centrifuged at 20,000× g at 4 °C for 15 min to determine the VFA, and two aliquots of 2 mL samples were taken to determine ammonia nitrogen (NH3-N) concentration and microbial protein (MCP). Three aliquots of 1 mL samples were taken to determine GAA, creatine, guanidinoacetate N-methyltransferase (GAMT), L-Arginine: glycine amidino-transferase catalyzes (AGAT), adenosine triphosphate (ATP), superoxide dismutase (SOD), catalase (CAT), gluta-thione peroxidase (GSH-Px), malondialdehyde (MDA), total antioxidant capacity (T-AOC), glutathione (GSH), and microbiota.
2.4.2. Rumen Fermentation Index Measurement
The centrifuged sample referred to above was filtered using a 0.22 mm syringe filter. The VFA was quantified using a high-performance gas chromatograph (HPGC; GC-128; INESA Corporation) equipped with a hydrogen flame detector and a capillary column (FFAP, Zhonghuida Instruments Co., Ltd., Dalian, China; 50 m long, 0.32 mm diameter, 0.50 µm film). The VFA was identified and quantified from the chromatograph peak areas using calibration with external standards [22]. The rumen liquid NH3-N was measured according to Bremner and Keeney’s (1965) method [23], which calls for the use of a spectrophotometer (UV-6100, Mapada Instruments Co., Ltd., Shanghai, China). The microbial protein was then quantified using the purine derivative method [24].
2.4.3. Ruminal GAA, Creatine, Enzyme Activity, and Antioxidant Capacity Related to GAA Metabolism
GAA and creatine in rumen liquid were determined reference to the study by Wada et al. [25]. To this end, 1 mL of rumen liquid was aliquoted into another centrifuge tube, and 3 mL 5% aqueous solution of sodium sulfosalicylate was added and mixed into the rumen liquid to precipitate the protein. The mixture was incubated at room temperature for 10 min and then centrifuged at 12,000× g for 10 min. Subsequently, the centrifuged sample mentioned above was filtered using a 0.22 mm syringe filter for the determination. The determination conditions were as follows: C18 weak acid cation exchange column (4.6 mm × 250 mm, 5 μm) was used; the flow rate was 0.6 mL/min; the column temperature was 30 °C; the detection wavelength was 210 nm; the elution mode was one-time linear elution; and the sample size was 10 μL.
Thiobarbituric acid reactive substances (TBARS) assay measured MDA as a product of lipid peroxidation. The activity of enzymes SOD, CAT, and GSH-PX, and the contents of T-AOC, GSH, and ATP in rumen were measured using an assay kit (Jiancheng Biochemical Reagent Co., Nanjing, China) according to the manufacturer’s instructions. The GAMT and AGAT activities were measured in ruminal fluid of the lambs using a corresponding enzyme-linked immunosorbent assay kit (JinHaiKeYu Biochemical Reagent Co., Beijing, China).
2.4.4. Ruminal Microorganism DNA Extraction, PCR Amplification, and Sequencing
A Fast DNA® soil DNA Kit (MP Biomedicals, Santa Ana, CA, USA) was used to extract the total microbial DNA from 60 rumen fluid samples according to the manufacturer’s instructions. DNA purity and concentration were detected with a NanoDrop2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, DE, USA), and DNA integrity was assessed with 1% agarose gel electrophoresis. The following amplification primers for 16 rRNA (V3 + V4) were used: 341F: 5′CCTAYGGGGBGCASCAG3′; 806R: 3′GGACTACNNGGGTATCTAAT5′. The PCR amplification of the 16S rRNA gene is shown in Table A2, and the PCR system is shown in Table A3. The PCR product was extracted from 2% agarose gel and purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions and quantified using a Quantus™ Fluorometer (Promega, Madison, WI, USA). Sequencing was conducted on a MiSeq PE300 platform (Illumina, San Diego, CA, USA) according to the standard protocols of Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (PRJNA846357).
2.4.5. Processing of Sequencing Data
Raw sequencing data were merged using the sliding window method and paired using FLASH (version 1.2.11, https://ccb.jhu.edu/software/FLASH/index.shtml (accessed on 27 September 2021)) on the basis of overlapping bases. The allowed mismatches of barcode and primer mismatch were 0 and 2, respectively. UPARSE software (version 7.0.1090, http://drive5.com/uparse/ (accessed on 9 October 2021)) was used to perform operational taxonomic unit (OTU) sequence clustering, and their relative abundances were used to calculate rarefaction curves and values of the Shannon Diversity Index using UPARSE version 7.1 (http://drive5.com/uparse/ (accessed on 8 November 2021)). The ribosomal database project (RDP) classifier (http://rdp.cme.msu.edu/ (accessed on 8 November 2021)) was used to classify and annotate each sequence through comparison to Silva (Release132, http://www.arb-silva.de/ (accessed on 8 November 2021)) with a comparison threshold of 70% [26]. Alpha diversity analysis that included the Sobs, Shannon, Ace and Coverage indices at the OTU level was conducted using Mothur software (version 1.30.2, https://www.mothur.org/wiki/Download_mothur (accessed on 8 November 2021)). The beta diversity analysis and the principal coordinates analysis (PCoA) was analyzed at the OTU level with the distance algorithm of weighted normalized UniFrac. The differential bacteria were analyzed using the linear discriminant analysis effect size (LEfSe) software (http://huttenhower.sph.harvard.edu/galaxy/root?Tool_id¼lefse_Upload (accessed on 8 November 2021)). Bar plots and non-metric multidimensional scaling (NMDS) were generated using R software.
2.5. Statistical Analysis
Data for each feeding stage were analyzed using the MIXED procedure of the Statistical Analysis System Institute [27]. Ruminal antioxidant capacity, rumen fermentation, and GAA metabolism were analyzed. The model was applied as follows:
where Yijk is the dependent variable, µ is the overall mean, Gi is the fixed effect of GAA products (i = 3: control, uncoated GAA, coated GAA), Fj is the fixed effect of total mixed ration type with different forage types (OH and OHWS), and G × F is the interaction of GAA and ration type. Rk is the random effect of animals (k = 12 per treatment) or pens (k = 3 per treatment), and eijk is the residual error term. The least squares means and standard errors of the means were calculated using the LSMEANS statement of the SAS software. Significance was declared at p ≤ 0.05, unless otherwise noted.
3. Results
3.1. Rumen Fermentation
At stage 1, interaction between forage type and GAA addition was found for the total VFA, acetate, and A:P (Table 1). UGAA and CGAA addition in the OH group increased the total VFA (p < 0.05), whereas only CGAA addition increased the total VFA in OHWS group (p < 0.05). The UGAA supplementation in the OH group increased the acetate proportion and decreased the A:P (p < 0.05), whereas there was no difference in the OHWS group (p > 0.05). Similarly, no difference was observed for rumen fermentation parameters with the two forage types (p > 0.05). Both UGAA and CGAA increased the content of total VFA (p < 0.001), A:P (p = 0.011), NH3-N (p = 0.025), and MCP (p = 0.015), but decreased the propionate proportion (p = 0.009) and pH value (p = 0.023). The addition of CGAA to the OH diet increased the butyrate proportion compared to the UGAA and control (p < 0.05).

Table 1.
Effects of forage type and GAA addition on rumen fermentation in feedlotting lambs.
At stage 2, the significant Forage × GAA interaction was observed on total VFA, acetate, and A:P. Dietary UGAA and CGAA addition in the OH group increased the total VFA (p < 0.05) and A:P (p < 0.05), but this phenomenon did not occur in the OHWS group. However, UGAA and CGAA decreased the acetate proportion in the OHWS group, and there was no change in the OH group. Compared to the OHWS diet, the lambs fed the OH diet had lower total VFA (p = 0.018). The concentration of total VFA (p < 0.001) and percentage of butyrate (p = 0.008) and MCP (p = 0.011) were higher with the addition of UGAA or CGAA, and the pH value showed a tendency to decrease (p = 0.082). The other indicators did not alter in response to the addition of GAA.
3.2. Ruminal GAA, Creatine, ATP, and Related Metabolic Enzymes
As shown in Table 2, the forage × GAA interaction was not significant for GAA, creatine, ATP, and related metabolic enzymes at stage 1 and stage 2.

Table 2.
Effects of forage type and GAA addition on ruminal GAA, creatine, ATP, and related metabolic enzymes.
At stage 1, no difference was observed for ruminal GAA, creatine, GAMT and AGAT in the two forage types (p > 0.05). UGAA or CGAA addition increased GAA (p < 0.001), GAMT (p = 0.002), and ATP (p = 0.014) but decreased AGAT (p = 0.025) activity compared to the control.
At stage 2, the concentration of GAA, creatine, ATP, and the activity of GAMT and AGAT did not differ between the two forage types (p > 0.05). Furthermore, regardless of the form of GAA added, the lambs presented greater rumen GAA (p < 0.001) and ATP (p = 0.001) than the control. No significant differences were observed among the treatments in the rumen creatine, or in the activity of GAMT and AGAT (p > 0.05).
3.3. Ruminal Microbiota
The alpha diversity was not affected by forage type or GAA addition, except that the lambs in the OH group, in comparison with the OHWS group, presented lower sobs (p = 0.017), ace (p = 0.011), and chao (p = 0.010) in stage 2 (Supplementary Table S1).
In stage 1, the effects of the interaction between forage type and GAA addition were found on the Bacteroidota (Table 3). Dietary CGAA in the OH group presented greater Bacteroidota than dietary CGAA (p < 0.05), whereas there was no difference in the OHWS group (p > 0.05). The relative abundance of Firmicutes, Bacteroidota, Synergistota, Patescibacteria, and Proteobacteria did not alter in response to either forage type or GAA addition.

Table 3.
Microbial community analysis at the phylum level (relative abundance > 1%) of microbiomes obtained from rumen fluids of lambs at different feeding stages.
In stage 2, the effects of the interaction between forage type and GAA addition were found on the Firmicutes and Bacteroidota. Dietary UGAA and CGAA in the OH group presented greater Firmicutes compared with the control group (p < 0.05); dietary UGAA in the OH group presented lower Bacteroidota compared with the control group (p < 0.05), whereas there was no difference in the OHWS group (p > 0.05). Compared with the OHWS diet, the lambs fed the OH diet had lower (p = 0.039) Firmicutes. The relative abundance of microbiota did not alter in response to GAA addition (p > 0.05). It is worth mentioning that whatever form of GAA was added, the lambs fed the OHWS diet presented lower Patescibacteria levels (p < 0.05).
At the genus level (Table 4), forage type and GAA addition interacted (p = 0.040) to affect the abundance of the NK4A214_group at stage 1. Dietary UGAA in the OH group presented a lower NK4A214_group compared with the control group (p < 0.05), whereas there was no difference in the OHWS group (p > 0.05). The forage type did not affect the microbial composition at the genus level (p > 0.05). Regardless of the form of GAA added, the population of Succiniclasticum decreased (p = 0.049) compared to the control. Furthermore, dietary CGAA in the OH group presented higher (p < 0.05) Prevotella abundance compared with dietary CGAA and the control. The relative abundances of norank_f_norank_o_Clostridia_UCG-014 decreased (p < 0.05) with UGAA addition in the OH group compared to the control.

Table 4.
Microbial community analysis at the genus level (relative abundance > 5%) of microbiomes obtained from rumen fluids of lambs at different feeding stages.
Interaction between forage type and GAA addition affected the abundance of Prevotella (p = 0.042), Ruminococcus (p = 0.027), and Succiniclasticum (p = 0.006) at stage 2; the relative abundances of Prevotella decreased with UGAA and CGAA addition (p < 0.05); the relative abundances of Succiniclasticum decreased and NK3A20 increased with UGAA addition compared to the control in the OH group (p < 0.05), whereas there was no difference in the OHWS group (p > 0.05). However, the abundances of Ruminococcus decreased with the addition of UGAA compared to the control and NK4A214_group increased with the UGAA and CGAA addition in OHWS group (p < 0.05), whereas there was no difference in the OH group (p > 0.05). The lambs in the OH group in comparison with the OHWS group presented lower NK4A214_group (p = 0.008) and NK3A20 (p = 0.038). Both forms of GAA resulted in greater NK4A214_group abundances (p = 0.040). The other genera did not alter in response to either forage type or GAA addition (p > 0.05).
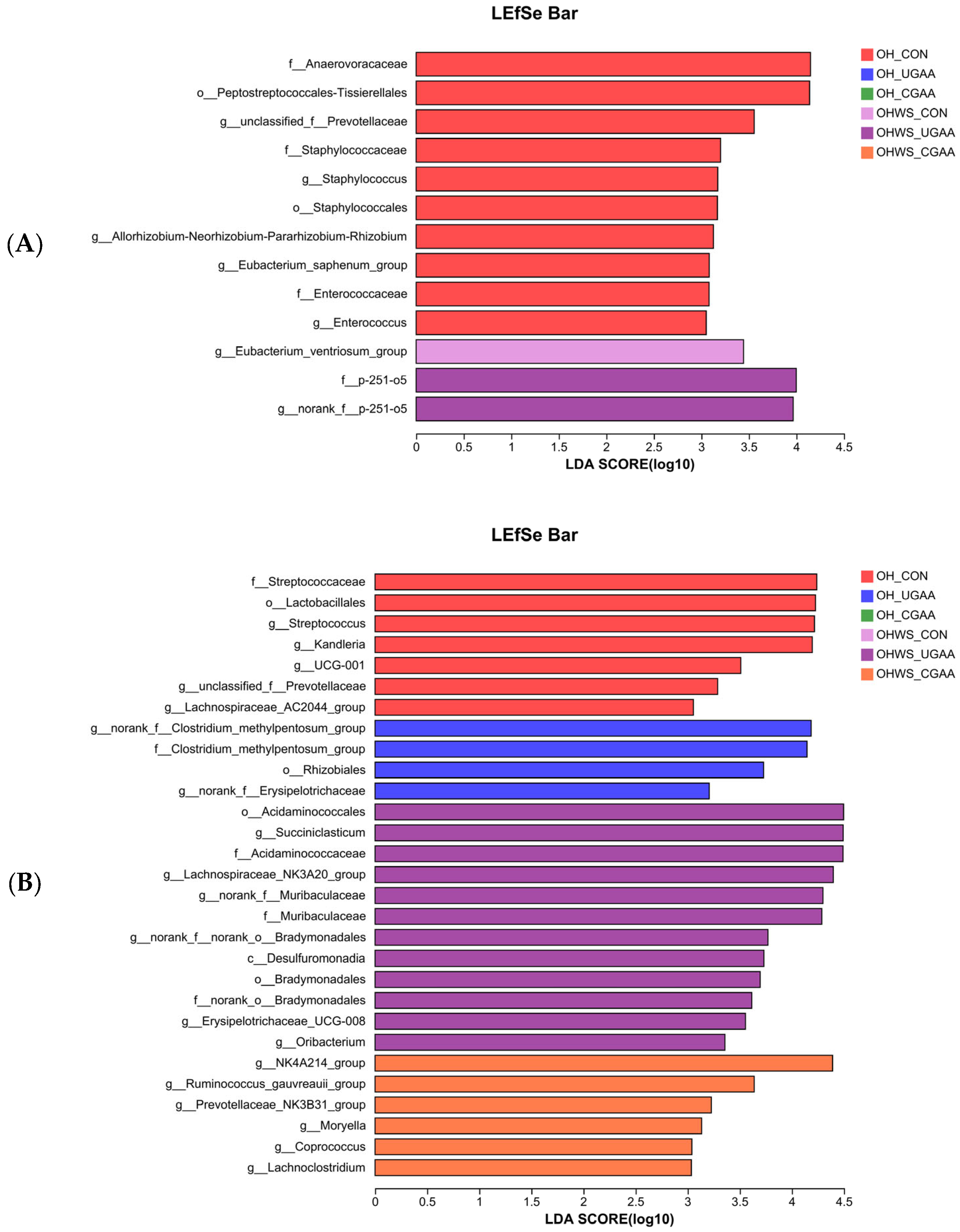
At stage 1, 13 bacterial taxa were identified by LEfSe as significantly enriched in the rumen, comprising 10 (f_Anaerovoracaceae, o_Peptostreptococcales-Tissierellales, g_unclassified_f_Prevotellaceae, f_Staphylococcaceae, g_Staphylococcus, o_Staphylococcales, g_Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium, g_Eubacterium_saphenum_group, f_Enterococcaceae, and g_Enterococcus) in the control group with the OH diet, and 1 (g_Eubacterium_ventriosum_group) and 2 (g_norank_f_p-251-o5, f_p-251-o5) in the control and UGAA addition with the OHWS diet, respectively (Figure 1A).
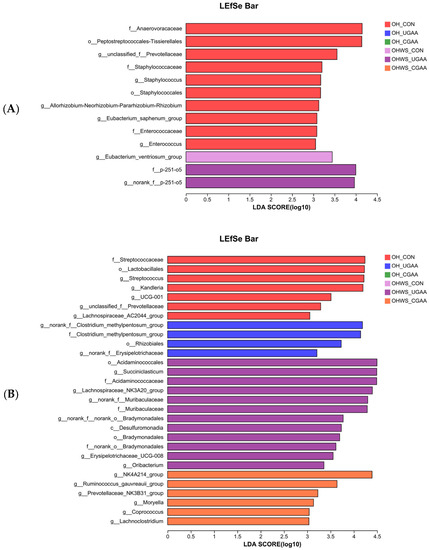
Figure 1.
LEfSe bar showing the taxonomic differences in the rumen of lambs in stage 1 (A) and stage 2 (B). All identified taxonomy was significantly different based on LDA score (B) larger than 3.0.
At stage 2, 29 bacterial taxa were identified by LEfSe as significantly enriched in the rumen, comprising 7 (f_Streptococcaceae, o_Lactobacillales, g_Streptococcus, g_Kandleria, g_UCG-001, g_unclassified_f_Prevotellaceae, and g_Lachnospiraceae_AC2044_group) and 4 (g_norank_f_Clostridium_methylpentosum_group, f_Clostridium_methylpentosum_group, o_Rhizobiales, and g_norank_f_Erysipelotrichaceae) in the control and with UGAA addition in the OH group, respectively, and 12 (o_Acidaminococcales, g_Succiniclasticum, f_Acidaminococcaceae, g_Lachnospiraceae_NK3A20_group, g_norank_f_Muribaculaceae, f_Muribaculaceae, g_norank_f_norank_o_Bradymonadales, c_Desulfuromonadia, o_Bradymonadales, f_norank_o_Bradymonadales, g_Erysipelotrichaceae_UCG008, g_Oribacterium) and 6 ((g_NK4A214, g_Ruminococcus_gauvreauii_group, g_Prevotellaceae_NK3B31_group, g_Moryella, g_Coprococcus, g_Lachnoclostridium) in the control and with CGAA addition in the OHWS group, respectively (Figure 1B).
3.4. Rumen Antioxidant Capacity
As shown in Table 5, at stage 1, the Forage × GAA interaction effect was exerted on the SOD, CAT, and GSH-Px activities but not on the levels of T-AOC, GSH, and MDA. For the OH diet, the highest T-AOC content and SOD and CAT activities were observed with the CGAA addition, whereas for the OHWS diet, UGAA addition resulted in the maximum amount of T-AOC content and SOD and CAT activities. The forage type did not affect antioxidant capacity. GAA (UGAA and CGAA) supplementation in the rumen increased the T-AOC (p < 0.001), SOD (p < 0.001), CAT (p < 0.001), GSH-Px (p < 0.001), and GSH (p < 0.001), whereas the level of MDA (p < 0.001) decreased with the addition of GAA.

Table 5.
Effects of forage type and GAA addition on ruminal antioxidant capacity.
At stage 2, an interaction effect was observed in the T-AOC, MDA levels and SOD, and GSH-Px activities. CGAA addition had the highest SOD activity, GSH-Px activity, and T-AOC content, and the lowest MDA content in the OH diet, but in the OHWS diet, UGAA addition achieved similar results. The type of forage did not affect the antioxidant capacity. Moreover, the T-AOC (p < 0.001), SOD (p < 0.001), CAT (p = 0.048), GSH-Px (p < 0.001), and GSH (p = 0.009) increased but the level of MDA (p < 0.001) decreased with GAA addition.
4. Discussion
Oxidative stress is a dysregulation between the production of reactive oxygen species and the endogenous antioxidant defense mechanisms [28]. For ruminants, high-concentrate diets have been widely associated with oxidative stress by increasing the metabolic rate [29]. In general, SOD, CAT, and GSH-Px activity, T-AOC, GSH, and MDA contents in serum are usually measured to reflect whether or not oxidative stress has occurred in the body. However, it is unknown whether oxidative stress could be reflected by measuring the aforementioned indices in rumen liquid. In a previous study, both CAT and GPx4 activities were elevated and the MDA content was decreased in serum when dietary GAA addition was applied in growing lambs [5]. In a subsequent study with Holstein dairy cows, dietary GAA addition was found to promote the growth of rumen microorganisms [30]. However, it is not clear whether the above responses to GAA could be associated with the rumen fermentation characteristics and microbial stability of the environment inside the rumen and how their relationship with antioxidant capacity is directly measured in the rumen fluids.
4.1. Rumen Fermentation
Total VFA concentration, pH value, NH3-N, and MCP levels are the main internal environmental indicators of rumen fermentation [8]. Among them, total VFA production was believed to account for over 70% of the energy requirement [31]. In one of our previous studies with growing lambs, increasing the foxtail millet silage replacement of peanut vine hay in rations exhibited a lower pH value and greater total VFA production [32]. In the present study, regardless of what form of GAA was applied, the lambs in the OHWS group in comparison with those in the OH group presented greater total VFA production during the last 60 days of the feeding stage, suggesting that WS in comparison with OH might exhibit rumen digestibility.
In one of our previous studies, the application of GAA was confirmed to improve growth performance and apparent total tract nutrient digestibility in lamb feeding practice [33]. In the present study, GAA feeding resulted in higher total VFA assessed at two stages. These results could be partly explained by the stimulation of nutrient degradation with dietary GAA supplementation in the rumen. Similarly, the addition of GAA has also been shown to increase rumen total VFA production in Holstein dairy cows [30]. The decrease in ruminal pH was commonly believed to be associated with an increase in the total VFA concentration. In the present study, the relatively low ruminal pH of 6.74 was observed for UGAA added to the OHWS diet group, and this pH was not low enough to have a negative impact on ruminal microorganisms [34]. Previous studies have reported that addition of GAA (0.6 or 0.9 g/kg basal diet) increased molar propionate proportion and A:P in Holstein dairy cows [30]. This phenomenon was not observed in the present study, and was probably a result of the differences in lamb species, ages, and basal diets. Researchers have also indicated that GAA can be used by microbes as an N source to synthesize their proteins [4], as observed in the present study in two stages.
4.2. Ruminal GAA, Creatine, ATP, and Related Metabolic Enzymes
In the present study, rumen GAA, creatine, and ATP levels, as well as GAMT and AGAT activities, did not vary with forages. This was probably due to the same ratio of concentrate to forage in both TMRs, as replacing 36% OH with WS had little effect on indicators related to energy metabolism in the rumen.
A previous study noted that GAA in the diet was absorbed by the gut through the portal blood into the liver for creatine synthesis [35], and GAMT and AGAT were key enzymes in this process [36]. Decreased rumen AGAT activity was observed with the addition of GAA at stage 1 in the present study. This was consistent with the finding that AGAT can be feedback-inhibited by GAA [37]. Most metabolic studies on GAA have focused on the liver, blood, urea, and small intestinal segment mucosa [8,36,38]. The addition of GAA increased the rumen GAA content at both stages 1 and 2, confirming that GAA was degraded in the rumen and significantly correlated with the degradation rate (UGAA, 50.9%; CGAA, 15.8%). Furthermore, ATP level improved with GAA addition compared to the control. It also supports the theory that GAA can provide energy to microbes [4]. Interestingly, we did not observe a difference in ATP content between the two forms of GAA, suggesting that not all of the GAA degradation was used to provide energy, and would even be a waste, thus necessitating coating.
4.3. Ruminal Microbiota
The alpha diversity analysis of lamb rumen flora in our experiment showed that the coverage of each group was higher than 99% in two stages, indicating that the sequencing results truly reflected the species and structural diversity of the lamb rumen bacterial community. Forages did not affect alpha diversity in stage 1, which is consistent with previous research [39]. However, compared with the OHWS group, the sobs, ace, and chao index decreased with the OH diet in stage 2. These results indicate that rumen microbial richness varies with diet and host [40]. In the present study, no difference emerged in the alpha diversity with the addition of GAA, indicating that GAA did not affect the richness or evenness of lamb rumen microorganisms.
In this study, at the phylum level, Firmicutes and Bacteroidota were the dominant phyla, which are known to be involved in carbohydrate and protein degradation [33]. The interaction between forage type and GAA addition was found to affect the abundance of Bacteroidota. We suspected that suitable GAA (CGAA) could promote Bacteroidota enrichment in the rumen, and a high concentration (UGAA) would affect the abundance of Bacteroidota. At stage 2, compared with the OHWS diet, the lambs fed the OH diet had a lower relative abundance of Firmicutes, which represented the predominant bacterium within the rumen, mainly comprising diverse fibrolytic and cellulolytic bacterial genera [41]. Similar to stage 1, dietary UGAA in the OH group presented a lower relative abundance of Bacteroidota, compared with the control group, which contributed to the release of energy from dietary fiber and starch [42].
At the genus level, Prevotella participates in the hydrolysis of proteins and the absorption of peptides in the rumen [43] and propionate production [44]. Prevotella enrichment helps to increase the antioxidant capacity in rumen fluid [45]. In the present study, the CGAA addition exhibited a higher relative abundance of Prevotella than the UGAA addition and the control with the OH diet. This corresponds to the highest propionate proportion. This also confirms that the addition of GAA can improve antioxidant capacity in rumen fluid. Furthermore, the addition of UGAA decreased the abundance of NK4A214_group, Succiniclasticum, and Clostridia_UCG-014 in the OH diet at stage 1. This suggests that the degradation of GAA in the rumen may lead to partial microbiota disorders. The same phenomenon was manifested in Ruminococcus and Succiniclasticum at stage 2. Higher relative abundances of NK4A214_group and NK3A20 were observed with the OHWS diet at stage 2. This may be because whole wheat silage contains higher starch than oaten hay. Zhang et al. [46] also found that NK4A214_group and NK3A20 enhanced starch and sucrose metabolism. Furthermore, NK4A214_group and NK3A20 are butyric acid-producing bacterium [47,48], thus explaining the corresponding increase in butyric acid with the addition of GAA.
Apart from norank_f_p-251-o5 and f_p-251-o5 in the UGAA with the OHWS diet, no differential bacteria were identified in stage 1 by LEfSe analysis. However, at stage 2, the OH diet with the UGAA group was enriched with norank_f_Clostridium_methylpentosum_group, f_Clostridium_methylpentosum_group, o_Rhizobiales, and norank_f_Erysipelotrichaceae. Other researchers have reported that Clostridium_methylpentosum is a ring-shaped intestinal bacterium that ferments only methylpentoses and pentoses [49]. The norank_f_Erysipelotrichaceae was reported to relate to metabolic disorder and inflammation-related gastrointestinal diseases [50]. This indicates that the addition of CGAA may lead to disorder of the internal environment and even inflammatory reaction in rumen. In the present study, the NK4A214_group, Ruminococcus_gauvreauii_group, Prevotellaceae_NK3B31_group, Moryella, Coprococcus, and Lachnoclostridium were enriched in the group fed with the OHWS and CGAA diet. Previous research has shown that Moryella, Coprococcus, and Lachnoclostridium are butyric acid-producing bacteria [51,52]. Previous studies have shown that butyrate treatment of the colon increases glutathione content [53]. The increase in butyric acid-producing bacteria may also provide indirect evidence that GAA improves antioxidant capacity in this study.
4.4. Rumen Antioxidant Capacity
A high concentrate diet in ruminants results in a massive release of bacterial endotoxins, which cause rumen epithelial cells to generate a certain amount of reactive oxygen species and lead to oxidative stress [54]. This phenomenon causes oxidative damage and decreases the activities of GSH-Px, CAT, and SOD [55]. SOD, CAT, and GSH-Px are intracellular antioxidant enzymes involved in the enzymatic antioxidant system against oxidative stress [56]. The SOD involved in the superoxide anion free radical (O2−) scavenging process in cells [57]. MDA is one of the meta-stable end products of lipid peroxidation [58]. Consistent with our hypothesis, the forage type did not affect antioxidant capacity due to the consistent forage-to-concentrate ratio. GAA has been reported to have a direct [59] or indirect antioxidant effect [60,61]. In our study, GAA addition increased SOD, CAT, and GSH-Px activities, as well as levels of T-AOC and GSH, but decreased MDA level at both stages 1 and 2, suggesting that GAA enhanced the antioxidant capacity in rumen. There are limited studies on the antioxidant properties of GAA in rumen fluid. We speculate that there are three possible reasons for this phenomenon. The content of creatine in rumen fluid increases numerically with the addition of GAA, as creatine has been proven to have the ability to remove O2- [60]. In addition, dietary GAA supplementation can save arginine, and L-Arginine alleviates oxidative stress by modulation of intestinal microbiota in intrauterine growth-retarded suckling lambs [61]. Another possible reason is that GAA increases the proportion of butyric acid in the rumen fluid, and butyrate improves the level of oxidative stress in intestinal mucosal cells [62]. It is interesting to find that OH diets had higher antioxidant capacity with CGAA addition, while OHWS diets had more significant antioxidant capacity with the addition of UGAA. This suggests that, in practice, we can choose the appropriate form of GAA according to the different types of forage.
Examining these findings together, the authors of the present study speculated that GAA supplementation could improve antioxidant capacity and rumen fermentation via providing energy for rumen microorganisms since partial GAA was metabolized. However, GAA degradation would also cause rumen dysbacteriosis as noted by the ruminal microbiota analysis. The results obtained in the present study suggested that the antioxidant capacity of GAA could be reflected by measuring the relevant indicators in rumen fluids samples; the increase in antioxidant capacity may be related to the enrichment of butyric acid-producing bacteria.
5. Conclusions
Our findings demonstrated that dietary GAA exhibited higher antioxidant capacity, total VFA, and microbial protein production in lambs fed with different forage types. The application of GAA as a feed additive has a bright application prospect of reducing oxidative stress and providing energy to support rumen fermentation in rapid-growing lambs. However, considering the rumen microbial stability, coated GAA is necessary to avoid rumen dysbacteriosis in feeding practice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12030772/s1, Table S1: Effects of forage type and GAA addition on alpha diversity of ruminal microbiota.
Author Contributions
Conceptualization, W.L. and H.Y.; methodology, W.W.; software, W.L.; validation, Z.C., Y.J. and A.A.; formal analysis, Q.W. and F.Z.; investigation, W.W.; resources, Y.B.; data curation, F.Z.; writing—original draft preparation, W.L.; writing review and editing, W.L. and H.Y.; project administration, H.Y.; funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Ministry of Agriculture and Rural Affairs of the People’s Republic of China (202105510410447) and the National Natural Science Foundation of China Program (grant No. 31572432).
Institutional Review Board Statement
The Animal Ethics Committee of China Agricultural University approved all procedures with animals. The sampling procedures followed the Guidelines on Ethical Treatment of Experimental Animals (2006) No. 398 set by the Ministry of Science and Technology, China.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Data are available on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Ingredient and chemical composition of trial diets.
Table A1.
Ingredient and chemical composition of trial diets.
| Ingredients | Stage 1 | Stage 2 | ||
|---|---|---|---|---|
| OH | OHWS | OH | OHWS | |
| Wheat silage 1 | 0 | 90 | 0 | 70 |
| Oat hay 2 | 250 | 160 | 200 | 130 |
| Concentrate 3 | 750 | 750 | 800 | 800 |
| Nutrient level of TMR (g/kg, as Dry Matter) | ||||
| Organic matter | 893.3 | 901.8 | 905.6 | 910.5 |
| Crude protein | 191.9 | 193.7 | 184.6 | 174.5 |
| Ether extract | 36.6 | 35.1 | 38.0 | 37.5 |
| Neutral detergent fiber | 307.6 | 293.8 | 274.5 | 271.2 |
| Acid detergent fiber | 118.4 | 117.1 | 108.0 | 99.7 |
| Net energy for gain (MJ/kg) | 4.60 | 4.64 | 4.79 | 4.81 |
OH, the rations with the forage type of oaten hay; OHWS, the rations with the forage type of oaten hay plus wheat silage. Wheat silage 1: DM 329.3 g/kg; CP 120.8 g/kg; EE 32.0 g/kg; NDF 620.0 g/kg; ADF 370.5 g/kg; OM 919.0 g/kg; NEg 2.51 MJ/kg. Oat hay 2: DM 879.5 g/kg; CP 121.0 g/kg; EE 24.1 g/kg; NDF: 611.6 g/kg; ADF: 351.4 g/kg; OM 917.0 g/kg; NEg 1.67 MJ/kg. Concentrate 3: Contained per kg in stage 1: 380 g Corn meal, 150 g Soybean meal, 180 g DDGS, 40 g Premix, Contained per kg in stage 2: 500 g Corn meal, 150 g Soybean meal, 110 g DDGS, 40 g Premix. Contained per kg premix: 200–500 mg Cu, 750–17,500 mg, 750–5000 mg Mn, 1250–4250 mg Zn, 3.75–350 mg I, 2.5–17.87 mg Se, 75,000–350,000 IU vitamin A, 12,500–142,500 IU vitamin D3, 500 mg vitamin E.

Table A2.
PCR amplification of 16S rRNA gene.
Table A2.
PCR amplification of 16S rRNA gene.
| Stages | Temperature | Time | Cycles |
|---|---|---|---|
| Initial denaturation | 95 °C | 3 min | 1 |
| Denaturation | 95 °C | 30 s | 27 |
| Annealing | 55 °C | 30 s | 1 |
| Extension | 72 °C | 45 s | 1 |
| Single extension | 72 °C | 10 min | 1 |

Table A3.
PCR system.
Table A3.
PCR system.
| Items | Volume |
|---|---|
| 5 × FastPfu Buffer | 4 μL |
| 2.5 mmol/L dNTPs | 2 μL |
| Forward primer (5 mmol/L) | 0.8 μL |
| Reverse primer (5 mmol/L) | 0.8 μL |
| FastPfu Polymerase | 0.4 μL |
| Template DNA | 10 ng |
| ddH2O | 12 μL |
| Total | 20 μL |
References
- Ostojic, S.M. Tackling guanidinoacetic acid for advanced cellular bioenergetics. Nutrition 2017, 34, 55–57. [Google Scholar] [CrossRef]
- Zhu, Z.; Gu, C.; Hu, S.; Li, B.; Zeng, X.; Yin, J. Dietary guanidinoacetic acid supplementation improved carcass characteristics, meat quality and muscle fibre traits in growing-finishing gilts. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1454–1461. [Google Scholar] [CrossRef]
- Majdeddin, M.; Braun, U.; Lemme, A.; Golian, A.; Kermanshahi, H.; Smet, D.S.; Michiels, J. Guanidinoacetic acid supplementation improves feed conversion in broilers subjected to heat stress associated with muscle creatine loading and arginine sparing. Poult. Sci. 2020, 99, 4442–4453. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, C.; Wu, Z.; Liu, Q.; Guo, G.; Huo, W.; Zhang, J.; Chen, L.; Zhang, Y.; Pei, C.; et al. Effects of guanidinoacetic acid supplementation on growth performance, nutrient digestion, rumen fermentation and blood metabolites in Angus bulls. Animal 2020, 14, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Song, P.; Zhao, J.; Zhang, J.; Zhao, J. Skeletal muscle mass, meat quality and antioxidant status in growing lambs supplemented with guanidinoacetic acid. Meat Sci. 2022, 192, 108906. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, s78–s86. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Webb, M.; Ghimire, S.; Blair, A.; Olson, K.; Fenske, G.J.; Fonder, A.T.; Christopher-Hennings, J.; Brake, D.; Scaria, J. Metagenomic characterization of the effect of feed additives on the gut microbiome and antibiotic resistome of feedlot cattle. Sci. Rep. 2017, 7, 12257. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Liu, S.; Chai, S.; Meng, Q.; Zhou, Z. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef]
- Speer, H.F.; Pearl, K.A.; Titgemeyer, E.C. Relative bioavailability of guanidinoacetic acid delivered ruminally or abomasally to cattle. J. Anim. Sci. 2020, 98, skaa282. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Juniper, D.T. Revisiting Oxidative Stress and the Use of Organic Selenium in Dairy Cow Nutrition. Animals 2019, 9, 462. [Google Scholar] [CrossRef]
- Bezerra, H.V.A.; Buarque, V.L.M.; Silva, L.S.B.; Leme, P.R.P.; Vidal, A.M.C.; Vaz, A.C.N.; Gallo, S.B.; Silva, S.L.; Leme, P.R. Effect of Castor and Cashew Nut Shell Oils, Selenium and Vitamin E as Antioxidants on the Health and Meat Stability of Lambs Fed a High-Concentrate Diet. Antioxidants 2020, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Izuddin, W.I.; Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A. Dietary Postbiotic Lactobacillus plantarum Improves Serum and Ruminal Antioxidant Activity and Upregulates Hepatic Antioxidant Enzymes and Ruminal Barrier Function in Post-Weaning Lambs. Antioxidants 2020, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Mion, B.; Ogilvie, L.; Van Winters, B.; Spricigo, J.F.W.; Anan, S.; Duplessis, M.; McBride, B.W.; LeBlanc, S.J.; Steele, M.A.; Ribeiro, E.S. Effects of replacing inorganic salts of trace minerals with organic trace minerals in the pre- and postpartum diets on mineral status, antioxidant biomarkers, and health of dairy cows. J. Anim. Sci. 2023, 3, skad041. [Google Scholar] [CrossRef] [PubMed]
- Gázquez, A.; Sánchez-Campillo, M.; Arnao, M.B.; Barranco, A.; Rueda, R.; Jensen, S.K.; Chan, J.P.; Kuchan, M.J.; Larqué, E. Natural vitamin E supplementation during pregnancy in rats increases RRR-α-tocopherol stereoisomer proportion and enhances fetal antioxidant capacity, compared to synthetic vitamin E administration. Ann. Nutr. Metab. 2023. [Google Scholar] [CrossRef]
- Hiramatsu, M. A role for guanidino compounds in the brain. Mol. Cell. Biochem. 2003, 244, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Eghbali, E.; Suzuki, K. Creatine Supplementation, Physical Exercise and Oxidative Stress Markers: A Review of the Mechanisms and Effectiveness. Nutrients 2021, 13, 869. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Martinelli, C.; Colombo, E.; Barbieri, E.; Potenza, L.; Sartini, S.; Fimognari, C. Creatine as an antioxidant. Amino Acids 2011, 40, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Berikol, G.B.; Berikol, G.; Ayrik, C.; Kose, A.; Babus, S.B.; Gumus, L.T.; Arpaci, R.B.; Dag, A.; Ayan, E.; Gorur, A. Antioxidant and Neuroprotective Effects of L-arginine Administration After Traumatic Brain Injury and Hemorrhagic Shock in Rats. Turk. Neurosurg. 2022. [Google Scholar] [CrossRef]
- An, X.; Zhang, L.; Luo, J.; Zhao, S.; Jiao, T. Effects of Oat Hay Content in Diets on Nutrient Metabolism and the Rumen Microflora in Sheep. Animals 2020, 10, 2341. [Google Scholar] [CrossRef]
- Mc Geough, E.J.; O’Kiely, P.; Hart, K.J.; Moloney, A.P.; Boland, T.M.; Kenny, D.A. Methane emissions, feed intake, performance, digestibility, and rumen fermentation of finishing beef cattle offered whole-crop wheat silages differing in grain content. J. Anim. Sci. 2010, 88, 2703–2716. [Google Scholar] [CrossRef]
- National Research Council. Toxicity Testing in the 21st Century: A Vision and a Strategy; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Zhang, D.; Yang, H. Combination effects of nitrocompounds, pyromellitic diimide, and 2-bromoethanesulfonate on in vitro ruminal methane production and fermentation of a grain-rich feed. J. Agric. Food. Chem. 2012, 60, 364–371. [Google Scholar] [CrossRef]
- Bremner, M.; Keeney, D.R. Distillation Methods for Determination of Ammonium Nitrate and Nitrite. Anal. Chim. Acta 1965, 32, 485–495. [Google Scholar] [CrossRef]
- Perez, J.F.; Balcells, J.; Cebrian, J.A.; Martin, S.M. Excretion of endogenous and exogenous purine derivatives in sheep: Effect of increased concentrate intake. Br. J. Nutr. 1998, 79, 237–240. [Google Scholar] [CrossRef]
- Wada, T.; Shimbo, H.; Osaka, H. A simple screening method using ion chromatography for the diagnosis of cerebral creatine deficiency syndromes. Amino Acids 2012, 43, 993–997. [Google Scholar] [CrossRef]
- Han, H.; Zhang, L.; Shang, Y.; Wang, M.; Phillips, C.J.C.; Wang, Y.; Su, C.; Lian, H.; Fu, T.; Gao, T. Replacement of Maize Silage and Soyabean Meal with Mulberry Silage in the Diet of Hu Lambs on Growth, Gastrointestinal Tissue Morphology, Rumen Fermentation Parameters and Microbial Diversity. Animals 2022, 12, 1406. [Google Scholar] [CrossRef] [PubMed]
- Institute SAS Inc. SAS User’s Guide: Statistics; Version 9.2; SAS Institute Inc.: Cary, NC, USA, 2003. [Google Scholar]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Celi, P. The role of oxidative stress in small ruminants’ health and production. Rev. Bras. Zootec. 2010, 39, 348–363. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Wang, C.; Guo, G.; Huo, W.; Xia, C.; Chen, L.; Zhang, Y.; Pei, C.; Liu, Q. Effects of guanidinoacetic acid supplementation on lactation performance, nutrient digestion and rumen fermentation in Holstein dairy cows. J. Sci. Food Agric. 2023, 103, 1522–1529. [Google Scholar] [CrossRef]
- Castillo-Gonzalez, A.R.; Burrola-Barraza, M.E.; Dominguez-Viveros, J.; Chavez-Martinez, A. Rumen microorganisms and fermentation. Arch. De Med. Vet. 2014, 46, 349–361. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Wang, W.; Wang, Y.; Zhang, F.; Lv, L.; Yang, H. Foxtail millet (Setaria italica L.) silage compared peanut vine hay (Arachis hypogaea L.) exhibits greater feed efficiency via enhancing nutrient digestion and promoting rumen fermentation more efficiently in feedlotting lambs. Small Rumin. Res. 2022, 215, 106704. [Google Scholar] [CrossRef]
- Li, W.; Wu, Q.; Cui, Z.; Jiang, Y.; Aisikaer, A.; Zhang, F.; Chen, H.; Wang, W.; Wang, Y.; Lv, L.; et al. Guanidine acetic acid exhibited greater growth performance in younger (13–30 kg) than in older (30–50 kg) lambs under high-concentrate feedlotting pattern. Front. Vet. Sci. 2022, 9, 954675. [Google Scholar] [CrossRef]
- Mourino, F.; Akkarawongsa, R.A.; Weimer, P.J. Initial pH as a determinant of cellulose digestion rate by mixed ruminal microorganisms in vitro. J. Dairy Sci. 2001, 84, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zang, C.; Pan, J.; Ma, C.; Wang, C.; Li, X.; Cai, W.; Yang, K. Effects of dietary guanidinoacetic acid on growth performance, guanidinoacetic acid absorption and creatine metabolism of lambs. PLoS ONE 2022, 17, e0264864. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Sun, Q. Laboratory Diagnosis of Cerebral Creatine Deficiency Syndromes by Determining Creatine and Guanidinoacetate in Plasma and Urine. Methods Mol. Biol. 2022, 2546, 129–140. [Google Scholar] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xing, T.; Li, J.; Zhang, L.; Jiang, Y.; Gao, F. Guanidinoacetic acid supplementation improves intestinal morphology, mucosal barrier function of broilers subjected to chronic heat stress. J. Anim. Sci. 2022, 25, skac355. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Global Rumen Census Collaborators; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, W.; Zhu, W.; Liu, J.; Mao, S. Effect of dietary forage sources on rumen microbiota, rumen fermentation and biogenic amines in dairy cows. J. Sci. Food Agric. 2014, 94, 1886–1895. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Li, F.; Li, C.; Li, G.; Zhang, D.; Song, Q.; Li, X.; Zhao, Y.; Wang, W. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 2021, 15, 100161. [Google Scholar] [CrossRef]
- Flint, H.J. Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- McCann, J.C.; Wiley, L.M.; Forbes, T.D.; Rouquette, F.M., Jr.; Tedeschi, L.O. Relationship between the rumen microbiome and residual feed intake-efficiency of Brahman bulls stocked on bermudagrass pastures. PLoS ONE 2014, 9, e91864. [Google Scholar] [CrossRef]
- Alzahal, O.; Li, F.; Le, L.G.; Walker, N.D.; Mcbride, B.W. Factors influencing ruminal bacterial community diversity and composition and microbial fibrolytic enzyme abundance in lactating dairy cows with a focus on the role of active dry yeast. J. Dairy Sci. 2017, 100, 4377–4393. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, H.; Zhang, F.; Wang, W.; Xiong, F.; Liu, Y.; Lv, L.; Li, W.; Bo, Y.; Yang, H. Cysteamine Supplementation In Vitro Remarkably Promoted Rumen Fermentation Efficiency towards Propionate Production via Prevotella Enrichment and Enhancing Antioxidant Capacity. Antioxidants 2022, 11, 2233. [Google Scholar] [CrossRef]
- Zhang, K.; Qian, Q.; Mao, Y.; Xu, Y.; Yang, Y.; Chen, Y.; Wang, X. Characterization of growth phenotypes and gastrointestinal tract microbiota in sheep fed with caragana. J. Appl. Microbiol. 2021, 131, 2763–2779. [Google Scholar] [CrossRef]
- Ma, T.; Villot, C.; Renaud, D.; Skidmore, A.; Chevaux, E.; Steele, M.; Guan, L.L. Linking perturbations to temporal changes in diversity, stability, and compositions of neonatal calf gut microbiota: Prediction of diarrhea. ISME J. 2020, 14, 2223–2235. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, Y.; Zhang, S.; Zou, J.; Gao, X.; Song, Y.; Zhang, Y.; Hu, Y.; Huang, Y.; Jiang, Q. The Effect of Dietary Supplementation with Resveratrol on Growth Performance, Carcass and Meat Quality, Blood Lipid Levels and Ruminal Microbiota in Fattening Goats. Foods 2022, 11, 598. [Google Scholar] [CrossRef]
- Himelbloom, B.H.; Canale-Parola, E. Clostridium methylpentosum sp. nov.: A ring-shaped intestinal bacterium that ferments only methylpentoses and pentoses. Arch. Microbiol. 1989, 151, 287–293. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Wu, Q.; Chen, J.; Deng, S.; Chen, L.; Wei, D.; Liang, F. Improvement of intestinal flora: Accompany with the antihypertensive effect of electroacupuncture on stage 1 hypertension. Chin. Med. 2021, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.; Keller, D.; Verbruggen, S.; Abboud, K.Y.; Venema, K. A blend of 3 mushrooms dose-dependently increases butyrate production by the gut microbiota. Benef. Microbes 2021, 12, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.M.A.E.; Bast, A.; Vanhoutvin, S.A.L.W.; Fischer, M.A.J.G.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.J.M. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009, 28, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.M.; Hosseini, A.; Zhou, Z.; Alharthi, A.; Trevisi, E.; Osorio, J.S.; Loor, J.J. Reticulo-rumen mass, epithelium gene expression, and systemic biomarkers of metabolism and inflammation in Holstein dairy cows fed a high-energy diet. J. Dairy Sci. 2017, 100, 9352–9360. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Zhang, T.; Zhang, R.; Liu, R.; Chen, Y. Molecular mechanism on cadmium-induced activity changes of catalase and superoxide dismutase. Int. J. Biol. Macromol. 2015, 77, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Shukla, R.; Madhu, S.V.; Gambhir, J.K.; Prabhu, K.M. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin. Biochem. 2003, 36, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim. Biophys. Acta 2014, 1840, 757–767. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Stojanovic, M.D.; Olcina, G. Oxidant-Antioxidant Capacity of Dietary Guanidinoacetic Acid. Ann. Nutr. Metab. 2015, 67, 243–246. [Google Scholar] [CrossRef]
- Lawler, J.M.; Barnes, W.S.; Wu, G.; Song, W.; Demaree, S. Direct antioxidant properties of creatine. Biochem. Biophys. Res. Commun. 2002, 290, 47–52. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.; Zha, X.; Ma, Y.; Liu, X.; Elsabagh, M.; Wang, H.; Wang, M. Dietary L-Arginine or N-Carbamylglutamate Alleviates Colonic Barrier Injury, Oxidative Stress, and Inflammation by Modulation of Intestinal Microbiota in Intrauterine Growth-Retarded Suckling Lambs. Antioxidants 2022, 11, 2251. [Google Scholar] [CrossRef]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90 (Suppl. 4), 266–268. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).