Protein Fractions from Flaxseed: The Effect of Subsequent Extractions on Composition and Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
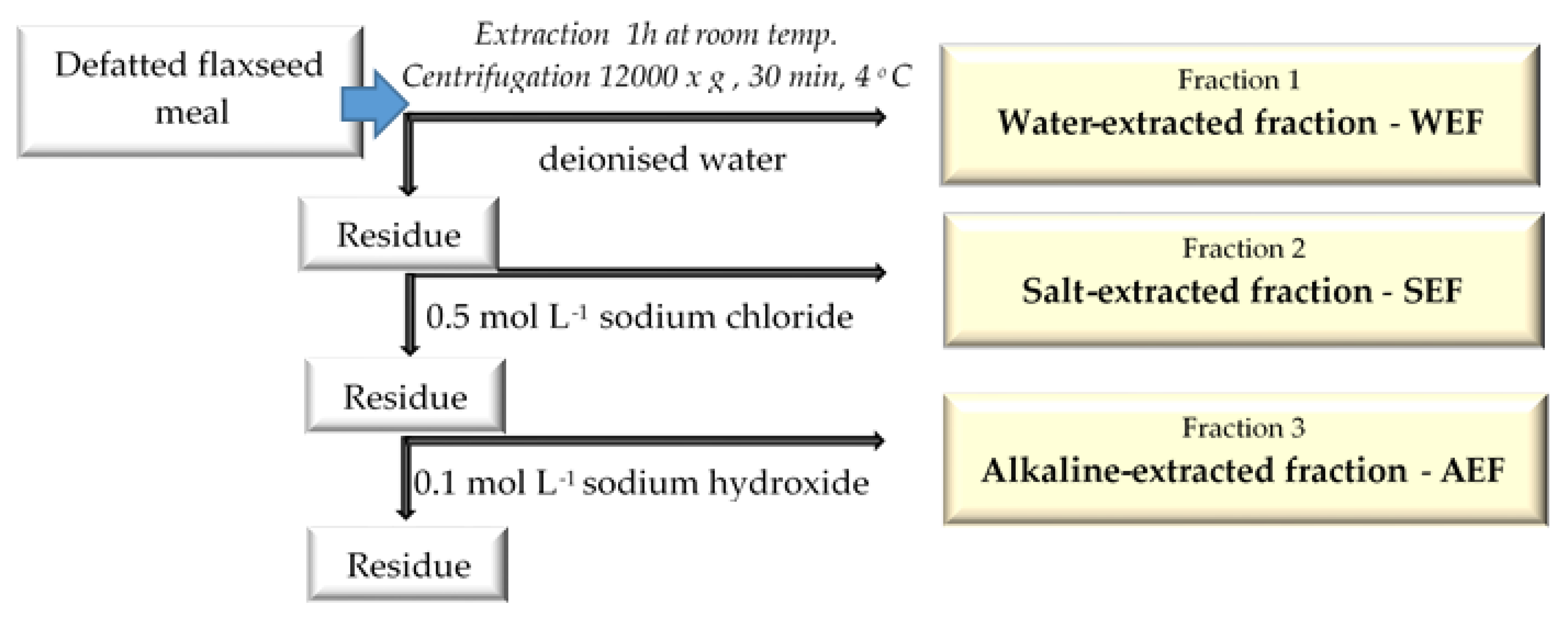
2.2. Extraction Process
2.3. Amino Acid and Electrophoretic Profile Analyses
2.4. SDG and Phenolic Acid Analysis
2.5. Spectroscopic Characterisation of the Protein Fractions
2.5.1. Fourier Transform Infrared (FT-IR) Analysis
2.5.2. Fluorometric Analysis
2.6. Functional Properties of Flaxseed Protein Fractions
2.6.1. Protein Surface Hydrophobicity (Ho)
2.6.2. Antiradical Activity (ORAC_FL Assay)
2.6.3. Total Phenol Content (TPC)
2.6.4. Ability to Inhibit Oxidation in Emulsion System
2.7. Statistical Analysis
3. Results and Discussion
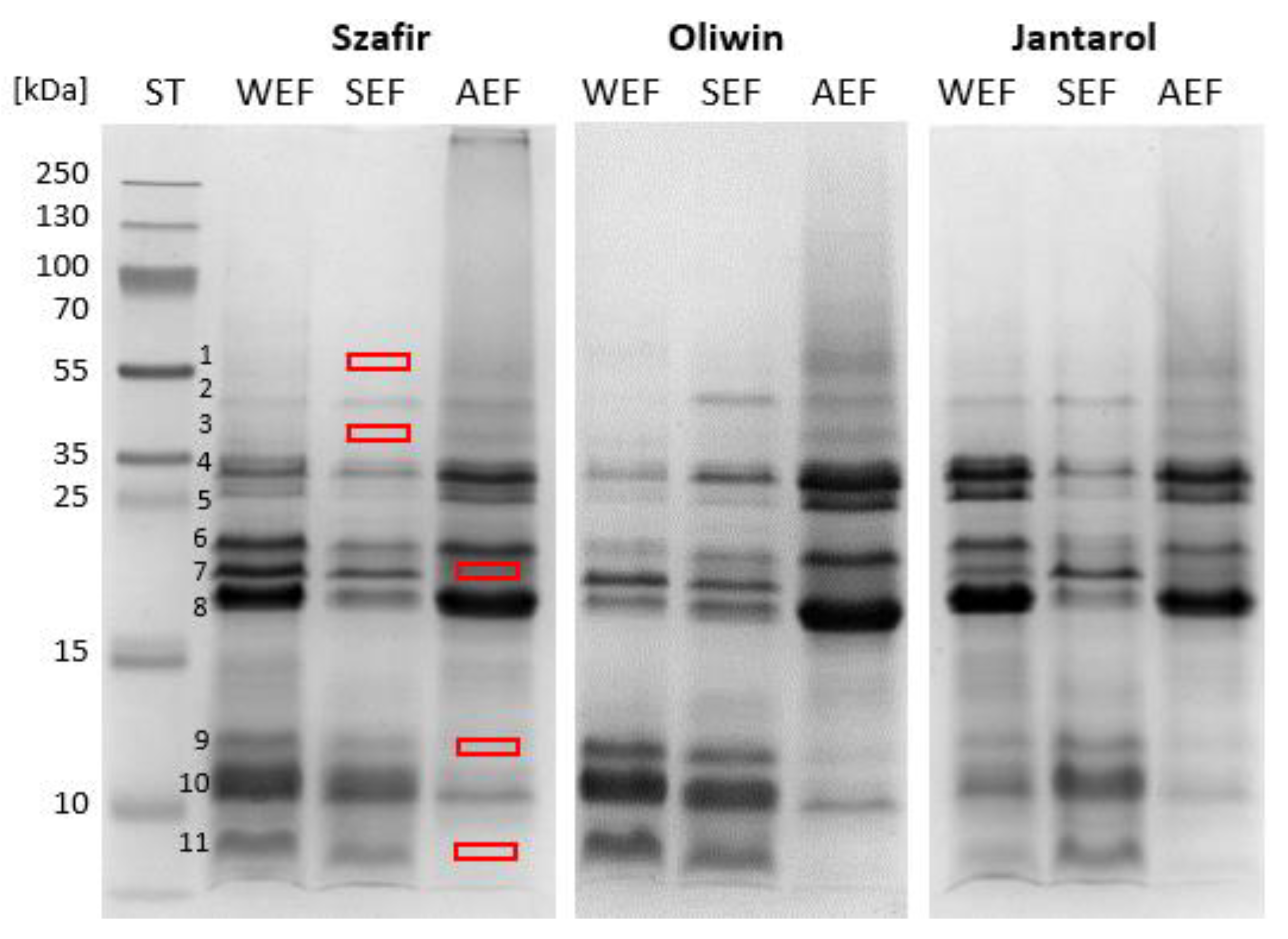
3.1. Electrophoretic and Amino Acid of Profiles of the Flaxseed Protein Fractions
3.2. SDG and Phenolic Acid Contents of the Flaxseed Protein Fractions
3.3. Spectroscopic Characterisation of the Flaxseed Protein Fractions
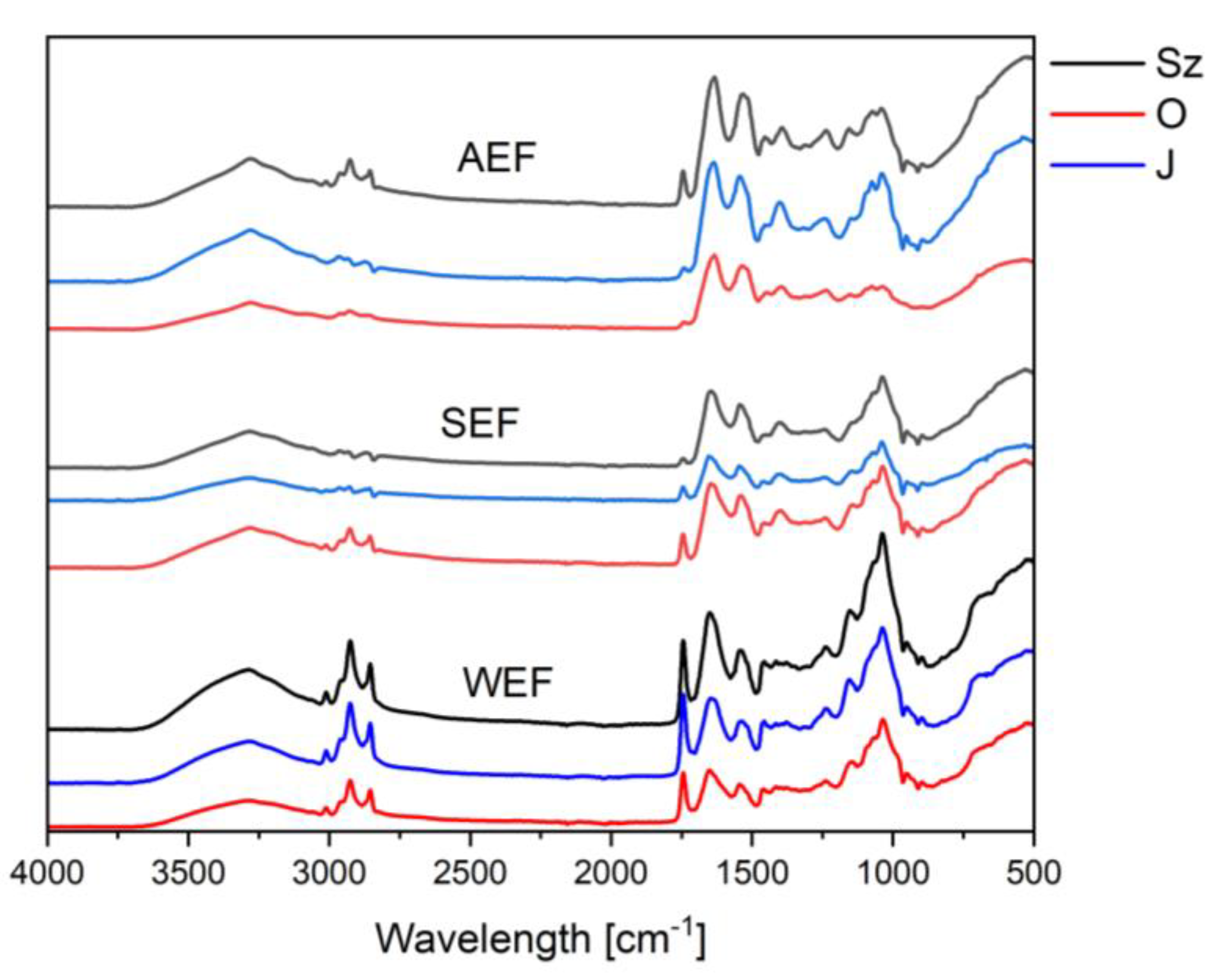
3.3.1. FT-IR Spectra Analysis
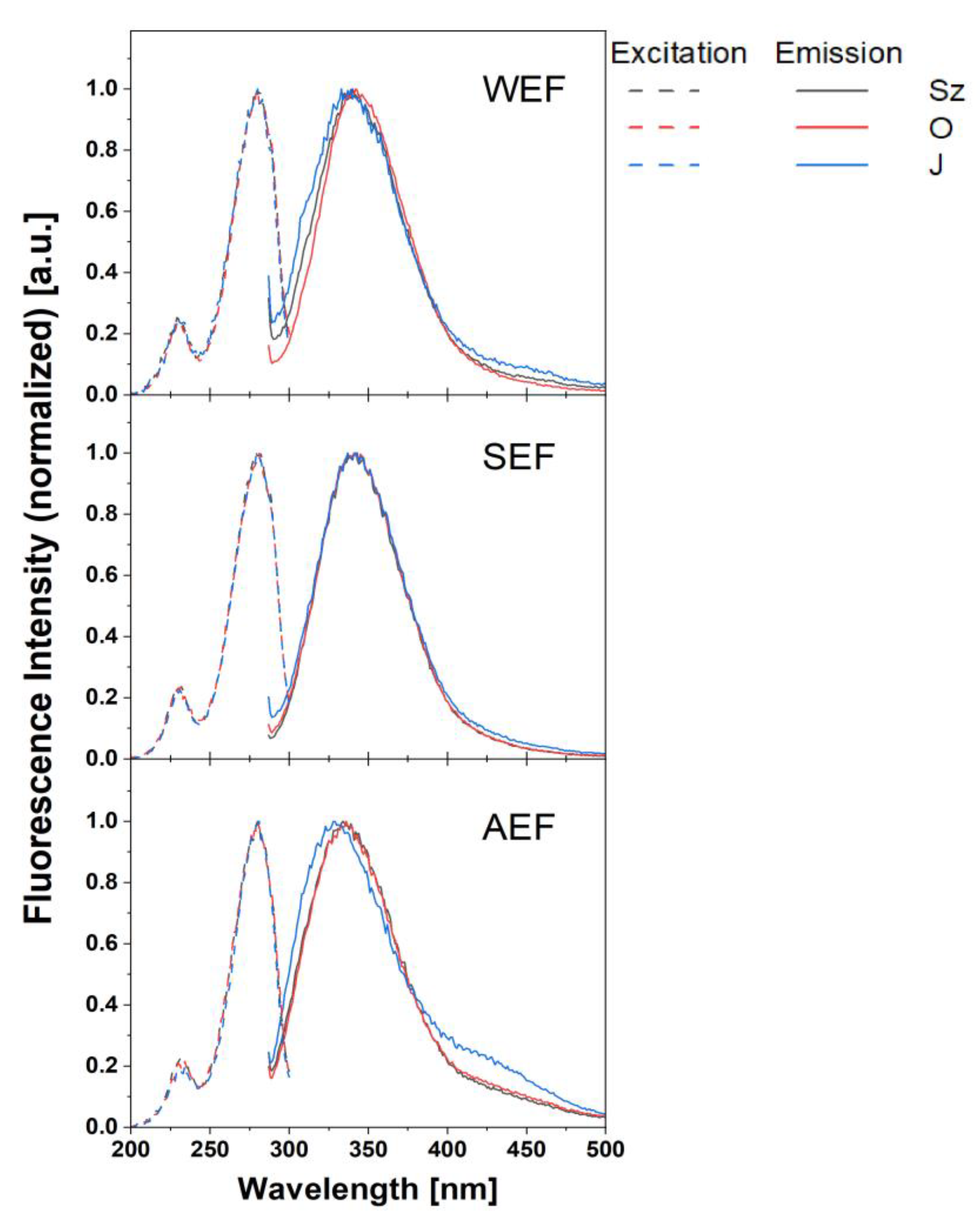
3.3.2. Fluorimetric Characterisation
3.4. Functional Properties of the Flaxseed Protein Fractions
3.5. Ability of Flaxseed Protein Fractions to Inhibit Oxidation in Emulsion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Mueed, A.; Shibli, S.; Korma, S.A.; Madjirebaye, P.; Esatbeyoglu, T.; Deng, Z. Flaxseed Bioactive Compounds: Chemical composition, functional properties, food applications and health benefits-related gut microbes. Foods 2022, 11, 3307. [Google Scholar] [CrossRef]
- Yawale, P.; Upadhyay, N.; Ganguly, S.; Singh, A.K. A Comprehensive review on recent novel food and industrial application of flaxseed: 2014 Onwards. Food Feed Res. 2022, 49, 67–95. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Van Remoortel, V.; Danthine, S.; Paquot, M.; Blecker, C. Flaxseed proteins: Food uses and health benefits. Int. J. Food Sci. Technol. 2011, 46, 221–228. [Google Scholar] [CrossRef]
- Marambe, H.K.; Wanasundara, J.P.D. Chapter 8—Protein from flaxseed (Linum usitatissimum L.). In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L.B.T.-S.P.S., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 133–144. ISBN 978-0-12-802778-3. [Google Scholar]
- Peng, D.; Ye, J.; Jin, W.; Yang, J.; Geng, F.; Deng, Q. A Review on the utilization of flaxseed protein as interfacial stabilizers for food applications. J. Am. Oil Chem. Soc. 2022, 99, 723–737. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Qi, W.; Guo, Q. Bioactive protein/peptides of flaxseed: A review. Trends Food Sci. Technol. 2019, 92, 184–193. [Google Scholar] [CrossRef]
- Lorenc, F.; Jarošová, M.; Bedrníček, J.; Smetana, P.; Bárta, J. Structural characterization and functional properties of flaxseed hydrocolloids and their application. Foods 2022, 11, 2304. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; McKnight, S.; Barrow, C.J.; Wang, B.; Adhikari, B. Preparation, characterization and functional properties of flax seed protein isolate. Food Chem. 2016, 197, 212–220. [Google Scholar] [CrossRef]
- Tirgar, M.; Silcock, P.; Carne, A.; Birch, E.J. Effect of extraction method on functional properties of flaxseed protein concentrates. Food Chem. 2017, 215, 417–424. [Google Scholar] [CrossRef]
- Qin, X.; Li, L.; Yu, X.; Deng, Q.; Xiang, Q.; Zhu, Y. Comparative composition structure and selected techno-functional elucidation of flaxseed protein fractions. Foods 2022, 11, 1820. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Physicochemical and emulsification properties of flaxseed (Linum usitatissimum) albumin and globulin fractions. Food Chem. 2018, 255, 216–225. [Google Scholar] [CrossRef]
- Puligundla, P.; Lim, S. A Review of extraction techniques and food applications of flaxseed mucilage. Foods 2022, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, Y.; Mai, Y.; Gao, L.; Ou, S.; Wang, Y.; Liu, L.; Peng, X. Flaxseed gum reduces body weight by regulating gut microbiota. J. Funct. Foods 2018, 47, 136–142. [Google Scholar] [CrossRef]
- Thakur, G.; Mitra, A.; Pal, K.; Rousseau, D. Effect of flaxseed gum on reduction of blood glucose and cholesterol in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2009, 60, 126–136. [Google Scholar] [CrossRef]
- Lai, K.W.; How, Y.H.; Mohd Ghazali, H.; Pui, L.P. Preliminary evaluation of potential prebiotic capacity of selected legumes and seed mucilage on the probiotic strain Lactobacillus Rhamnosus GG. Asia Pacific J. Mol. Biol. Biotechnol. 2021, 29, 60–72. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Mikołajczak, B.; Gliszczyńska-Świgło, A.; Niedźwiedzińska, K. Effect of thermal pre-treatment on the phenolic and protein profiles and oil oxidation dynamics of golden flaxseeds. Int. J. Food Sci. Technol. 2020, 55, 1272–1280. [Google Scholar] [CrossRef]
- Strandås, C.; Kamal-Eldin, A.; Andersson, R.; Åman, P. Composition and properties of flaxseed phenolic oligomers. Food Chem. 2008, 110, 106–112. [Google Scholar] [CrossRef]
- De Silva, S.F.; Alcorn, J. Flaxseed lignans as important dietary polyphenols for cancer prevention and treatment: Chemistry, pharmacokinetics, and molecular targets. Pharmaceuticals 2019, 12, 68. [Google Scholar] [CrossRef]
- Yu, X.; Huang, S.; Nie, C.; Deng, Q.; Zhai, Y.; Shen, R. Effects of atmospheric pressure plasma jet on the physicochemical, functional, and antioxidant properties of flaxseed protein. J. Food Sci. 2020, 85, 2010–2019. [Google Scholar] [CrossRef]
- Vieira, J.M.; Mantovani, R.A.; Raposo, M.F.J.; Coimbra, M.A.; Vicente, A.A.; Cunha, R.L. Effect of extraction temperature on rheological behavior and antioxidant capacity of flaxseed gum. Carbohydr. Polym. 2019, 213, 217–227. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Mikołajczak, B. The effect of roasting on the protein profile and antiradical capacity of flaxseed meal. Foods 2020, 9, 1383. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.; Park, K.H.; Rhee, K.C. Fractionation and characterization of proteins from coconut (Cocos Nucifera L.). J. Agric. Food Chem. 1996, 44, 1741–1745. [Google Scholar] [CrossRef]
- Kwanyuen, P.; Burton, J.W. A modified amino acid analysis using PITC derivatization for soybeans with accurate determination of cysteine and half-cystine. J. Am. Oil Chem. Soc. 2010, 87, 127–132. [Google Scholar] [CrossRef]
- Çevikkalp, S.A.; Löker, G.B.; Yaman, M.; Amoutzopoulos, B. A simplified HPLC method for determination of tryptophan in some cereals and legumes. Food Chem. 2016, 193, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Waszkowiak, K.; Mikołajczak, B.; Kmiecik, D. Changes in oxidative stability and protein profile of flaxseeds resulting from thermal pre-treatment. J. Sci. Food Agric. 2018, 98, 5459–5469. [Google Scholar] [CrossRef] [PubMed]
- Renouard, S.; Hano, C.; Corbin, C.; Fliniaux, O.; Lopez, T.; Montguillon, J.; Barakzoy, E.; Mesnard, F.; Lamblin, F.; Lainé, E. Cellulase-assisted release of secoisolariciresinol from extracts of flax (Linum usitatissimum) hulls and whole seeds. Food Chem. 2010, 122, 679–687. [Google Scholar] [CrossRef]
- Fuentealba, C.; Figuerola, F.; Estévez, A.M.; González-Munõz, A.; Munõz, O. Optimization of secoisolariciresinol diglucoside extraction from flaxseed (Linum usitatissimum L.) and isolation by a simple HPLC-UV method. CYTA-J. Food 2015, 13, 273–281. [Google Scholar] [CrossRef]
- Li-Chan, E.; Nakai, S.; Wood, D.F. Hydrophobicity and solubility of meat proteins and their relationship to emulsifying properties. J. Food Sci. 1984, 49, 345–350. [Google Scholar] [CrossRef]
- Monahan, F.J.; German, J.B.; Kinsella, J.E. Effect of pH and temperature on protein unfolding and thiol/disulfide interchange reaction during heat-induced gelation of whey proteins. J. Agric. Food Chem. 1995, 43, 46–52. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Gliszczyńska-Świgło, A. Binary ethanol–water solvents affect phenolic profile and antioxidant capacity of flaxseed extracts. Eur. Food Res. Technol. 2016, 242, 777–786. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Barthet, V.J. Characterization of a partially purified extract from flax (Linum usitatissimum L.) Seed. J. Am. Oil Chem. Soc. 2015, 92, 1183–1194. [Google Scholar] [CrossRef]
- AOCS Official Method Th 1a-64 Spectrometric determination of conjugated dienoic acid. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaing, IL, USA, 2017.
- Pegg, R.B. Lipid oxidation/stability. Measurement of primary lipid oxidation products. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2001; pp. D2.1.1–D2.1.15. [Google Scholar]
- Lingnert, H.; Vallentin, K.; Eriksson, C.E. Measurement of antioxidative effect in model system. J. Food Process. Preserv. 1979, 3, 87–103. [Google Scholar] [CrossRef]
- Madhusudhan, K.T.; Singh, N. Isolation and characterization of a small molecular weight protein of linseed meal. Phytochemistry 1985, 24, 2507–2509. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, B.; Liu, Y.; Xiong, Y.L. Interfacial structural role of pH-shifting processed pea protein in the oxidative stability of oil/water emulsions. J. Agric. Food Chem. 2014, 62, 1683–1691. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Q.; Xiong, Y.L. A PH Shift Approach to the improvement of interfacial properties of plant seed proteins. Curr. Opin. Food Sci. 2018, 19, 50–56. [Google Scholar] [CrossRef]
- Gerstenmeyer, E.; Reimer, S.; Berghofer, E.; Schwartz, H.; Sontag, G. Effect of thermal heating on some lignans in flax seeds, sesame seeds and rye. Food Chem. 2013, 138, 1847–1855. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Ereifej, K.; Brewer, S.; Alli, I. Phenolic-protein interactions in oilseed protein isolates. Food Res. Int. 2013, 52, 178–184. [Google Scholar] [CrossRef]
- Struijs, K.; Vincken, J.P.; Verhoef, R.; Voragen, A.G.J.; Gruppen, H. Hydroxycinnamic acids are ester-linked directly to glucosyl moieties within the lignan macromolecule from flaxseed hulls. Phytochemistry 2008, 69, 1250–1260. [Google Scholar] [CrossRef]
- Kasote, D.M. Flaxseed phenolics as natural antioxidants. Int. Food Res. J. 2013, 20, 27–34. [Google Scholar]
- Mishra, O.P.; Simmons, N.; Tyagi, S.; Pietrofesa, R.; Shuvaev, V.V.; Valiulin, R.A.; Heretsch, P.; Nicolaou, K.C.; Christofidou-Solomidou, M. Synthesis and antioxidant evaluation of (S,S)- and (R,R)-secoisolariciresinol diglucosides (SDGs). Bioorg. Med. Chem. Lett. 2013, 23, 5325–5328. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, F.S.; Muir, A.D.; Westcott, N.D.; Krol, E.S. Antioxidant capacity of flaxseed lignans in two model systems. J. Am. Oil Chem. Soc. 2006, 83, 835–840. [Google Scholar] [CrossRef]
- Andronie, L.; Pop, I.D.; Sobolu, R.; Diaconeasa, Z.; Truţă, A.; Hegeduş, C.; Rotaru, A. Characterization of flax and hemp using spectrometric methods. Appl. Sci. 2021, 11, 8341. [Google Scholar] [CrossRef]
- Safdar, B.; Pang, Z.; Liu, X.; Jatoi, M.A.; Mehmood, A.; Rashid, M.T.; Ali, N.; Naveed, M. Flaxseed gum: Extraction, bioactive composition, structural characterization, and its potential antioxidant activity. J. Food Biochem. 2019, 43, e13014. [Google Scholar] [CrossRef]
- Kanicky, J.R.; Shah, D.O. Effect of degree, type, and position of unsaturation on the PKa of long-chain fatty acids. J. Colloid Interface Sci. 2002, 256, 201–207. [Google Scholar] [CrossRef]
- Yang, J.; Zamani, S.; Liang, L.; Chen, L. Extraction methods significantly impact pea protein composition, structure and gelling properties. Food Hydrocoll. 2021, 117, 106678. [Google Scholar] [CrossRef]
- Pham, L.B.; Wang, B.; Zisu, B.; Adhikari, B. Complexation between flaxseed protein isolate and phenolic compounds: Effects on interfacial, emulsifying and antioxidant properties of emulsions. Food Hydrocoll. 2019, 94, 20–29. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.P.; Xu, M.F.; Tang, Z.X.; Chen, H.J.; Wu, D.T.; Wang, Z.Y.; Songzhen, Y.X.; Hao, J.; Wu, L.M.; Shi, L.E. Flaxseed protein: Extraction, functionalities and applications. Food Sci. Technol. 2022, 42, 1–13. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Siger, A.; Rudzińska, M.; Bamber, W. Effect of roasting on flaxseed oil quality and stability. J. Am. Oil Chem. Soc. 2020, 97, 637–649. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Tse, T.J.; Wang, Y.; Reaney, M.J.T. Flaxseed gum a versatile natural hydrocolloid for food and non-food applications. Trends Food Sci. Technol. 2018, 75, 146–157. [Google Scholar] [CrossRef]
- Dev, D.K.; Quensel, E. Preparation and functional properties of linseed protein products containing differing levels of mucilage. J. Food Sci. 1988, 53, 1834–1837. [Google Scholar] [CrossRef]
- Dev, D.K.; Quensel, E. Functional Properties of linseed protein products containing different levels of mucilage in selected food systems. J. Food Sci. 1989, 54, 183–186. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Poth, A.G.; Reaney, M.J.T. Conlinin in flaxseed (Linum usitatissimum L.) gum and its contribution to emulsification properties. Food Hydrocoll. 2016, 52, 963–971. [Google Scholar] [CrossRef]
| Amino Acids | Szafir Cultivar | Oliwin Cultivar | Jantarol Cultivar | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WEF | % | SEF | % | AEF | % | WEF | % | SEF | % | AEF | % | WEF | % | SEF | % | AEF | % | |
| Asx | 46.65 ± 0.57 b | 14 | 72.78 ± 1.16 c | 14.7 | 112.86 ± 0.49 e | 17.8 | 28.41 ± 0.23 a | 9.1 | 69.33 ± 0.69 c | 13.7 | 122.66 ± 4.52 f | 17.3 | 47.65 ± 0.4 b | 15.3 | 52.73 ± 0.41 b | 14.3 | 85.79 ± 0.24 d | 17.1 |
| Glx | 76.24 ± 0.96 b | 22.9 | 127.33 ± 1.34 e | 25.6 | 124.72 ± 0.58 e | 19.7 | 89.28 ± 0.88 c | 28.6 | 132.07 ± 0.54 f | 26.1 | 135.27 ± 2.41 f | 19.1 | 62.73 ± 0.20 a | 20.2 | 94.10 ± 0.23 d | 25.5 | 97.28 ± 0.60 d | 19.4 |
| Ser | 16.11 ± 0.14 b | 4.8 | 22.88 ± 0.07 c | 4.6 | 29.88 ± 0.22 e | 4.7 | 14.64 ± 0.22 a | 4.7 | 24.21 ± 0.11 d | 4.8 | 35.09 ± 0.76 f | 5 | 16.66 ± 0.20 b | 5.4 | 17.05 ± 0.21 b | 4.6 | 24.86 ± 0.29 d | 5 |
| Gly | 21.39 ± 0.06 b | 6.4 | 31.48 ± 0.18 f | 6.3 | 28.88 ± 0.08 e | 4.6 | 25.93 ± 0.03 d | 8.3 | 34.12 ± 0.07 h | 6.7 | 32.64 ± 0.47 g | 4.6 | 17.38 ± 0.06 a | 5.6 | 26.08 ± 0.15 d | 7.1 | 24.37 ± 0.29 c | 4.9 |
| His | 6.29 ± 0.35 b | 1.9 | 9.04 ± 0.07 d | 1.8 | 12.54 ± 0.03 f | 2 | 4.89 ± 0.16 a | 1.6 | 8.04 ± 0.10 c | 1.6 | 14.54 ± 0.12 g | 2.1 | 7.57 ± 0.06 c | 2.4 | 6.51 ± 0.04 b | 1.8 | 10.98 ± 0.45 e | 2.2 |
| Arg | 27.86 ± 0.11 b | 8.4 | 42.73 ± 0.61 d | 8.6 | 54.15 ± 0.10 e | 8.6 | 26.74 ± 0.11 b | 8.6 | 42.39 ± 0.04 d | 8.4 | 62.40 ± 0.63 f | 8.8 | 25.09 ± 0.56 a | 8.1 | 29.77 ± 0.23 c | 8.1 | 43.12 ± 0.59 d | 8.6 |
| Thr | 12.86 ± 0.51 b | 3.9 | 16.00 ± 0.01 c | 3.2 | 23.03 ± 0.22 e | 3.6 | 10.20 ± 0.02 a | 3.3 | 16.90 ± 0.20 c | 3.3 | 26.30 ± 0.60 f | 3.7 | 12.68 ± 0.10 a | 4.1 | 13.44 ± 0.07 b | 3.6 | 19.30 ± 0.02 d | 3.9 |
| Ala | 13.48 ± 0.18 c | 4.1 | 17.44 ± 0.18 d | 3.5 | 23.34 ± 0.05 f | 3.7 | 11.57 ± 0.20 a | 3.7 | 17.11 ± 0.04 d | 3.4 | 28.65 ± 0.40 g | 4 | 12.47 ± 0.05 b | 4 | 12.99 ± 0.18 bc | 3.5 | 19.35 ± 0.07 e | 3.9 |
| Pro | 10.98 ± 0.03 b | 3.3 | 13.78 ± 0.32 c | 2.8 | 21.65 ± 0.07 e | 3.4 | 8.32 ± 0.04 a | 2.7 | 14.66 ± 0.02 c | 2.9 | 27.36 ± 0.74 f | 3.9 | 11.44 ± 0.21 b | 3.7 | 10.65 ± 0.05 b | 2.9 | 18.50 ± 0.13 d | 3.7 |
| Tyr | 8.82 ± 0.06 b | 2.7 | 11.44 ± 0.16 c | 2.3 | 19.11 ± 0.17 e | 3 | 7.39 ± 0.12 a | 2.4 | 11.32 ± 0.19 c | 2.2 | 19.76 ± 0.85 e | 2.8 | 8.21 ± 0.03 b | 2.6 | 8.78 ± 0.07 b | 2.4 | 14.06 ± 0.48 d | 2.8 |
| Val | 15.14 ± 0.40 b | 4.6 | 19.03 ± 0.07 c | 3.8 | 30.78 ± 0.06 e | 4.9 | 11.84 ± 0.05 a | 3.8 | 19.70 ± 0.04 c | 3.9 | 37.35 ± 1.04 f | 5.3 | 15.23 ± 0.15 b | 4.9 | 14.89 ± 0.26 b | 4 | 26.54 ± 0.48 d | 5.3 |
| Met | 5.62 ± 0.04 a | 1.7 | 7.18 ± 0.07 b | 1.4 | 10.65 ± 0.39 e | 1.7 | 5.44 ± 0.01 a | 1.7 | 8.10 ± 0.29 c | 1.6 | 13.05 ± 0.29 f | 1.8 | 5.52 ± 0.09 a | 1.8 | 6.03 ± 0.01 a | 1.6 | 9.10 ± 0.00 d | 1.8 |
| Cys | 11.12 ± 0.04 d | 3.3 | 16.63 ± 0.01 g | 3.3 | 9.94 ± 0.04 c | 1.6 | 13.62 ± 0.13 f | 4.4 | 19.34 ± 0.52 h | 3.8 | 12.36 ± 0.08 e | 1.7 | 6.73 ± 0.01 a | 2.2 | 11.32 ± 0.04 d | 3.1 | 8.47 ± 0.01 b | 1.7 |
| Ile | 12.39 ± 0.06 b | 3.7 | 16.78 ± 0.02 c | 3.4 | 36.66 ± 0.25 f | 5.8 | 9.99 ± 0.18 a | 3.2 | 17.40 ± 0.02 c | 3.4 | 31.86 ± 0.72 e | 4.5 | 13.04 ± 0.24 b | 4.2 | 12.57 ± 0.07 b | 3.4 | 22.27 ± 0.22 d | 4.5 |
| Leu | 17.01 ± 0.17 b | 5.1 | 23.83 ± 0.03 d | 4.8 | 35.06 ± 0.06 g | 5.5 | 15.18 ± 0.02 a | 4.9 | 25.38 ± 0.05 e | 5 | 40.39 ± 0.55 h | 5.7 | 18.89 ± 0.01 c | 6.1 | 18.47 ± 0.05 c | 5 | 29.62 ± 0.18 f | 5.9 |
| Phe | 12.83 ± 0.08 b | 3.9 | 19.17 ± 0.66 c | 3.9 | 28.83 ± 0.46 e | 4.6 | 9.59 ± 0.03 a | 3.1 | 18.28 ± 0.02 c | 3.6 | 34.63 ± 0.77 f | 4.9 | 14.09 ± 0.01 b | 4.5 | 12.85 ± 0.24 b | 3.5 | 23.76 ± 0.02 d | 4.7 |
| Lys | 13.56 ± 0.08 b | 4.1 | 20.41 ± 0.25 e | 4.1 | 19.47 ± 0.15 d | 3.1 | 14.32 ± 0.18 b | 4.6 | 20.74 ± 0.03 e | 4.1 | 22.84 ± 0.41 f | 3.2 | 12.06 ± 0.10 a | 3.9 | 16.84 ± 0.23 c | 4.6 | 16.44 ± 0.23 c | 3.3 |
| Trp | 4.20 ± 0.04 b | 1.3 | 8.80 ± 0.09 e | 1.8 | 11.48 ± 0.13 g | 1.8 | 4.51 ± 0.01 b | 1.4 | 6.75 ± 0.21 d | 1.3 | 10.97 ± 0.04 f | 1.5 | 3.81 ± 0.01 a | 1.2 | 4.66 ± 0.05 c | 1.3 | 6.63 ± 0.04 b | 1.3 |
| Total | 332.55 ± 2.5 a | 100 | 496.72 ± 5.18 c | 100 | 633.02 ± 2.34 d | 100 | 311.87 ± 1.6 a | 100 | 505.82 ± 2.14 c | 100 | 708.14 ± 15.1 e | 100 | 311.25 ± 1.5 a | 100 | 369.73 ± 1.88 b | 100 | 500.43 ± 4.0 c | 100 |
| SCAA | 5 | 4.8 | 3.3 | 6.1 | 5.4 | 3.6 | 3.9 | 4.7 | 3.5 | |||||||||
| BCAA | 13.4 | 12 | 16.2 | 11.9 | 12.3 | 15.5 | 15.2 | 12.4 | 15.7 | |||||||||
| HAA | 34 | 31.7 | 35.9 | 32.8 | 31.9 | 36.3 | 35.9 | 32.2 | 36 | |||||||||
| NCAA | 37 | 40.3 | 37.5 | 37.7 | 39.8 | 36.4 | 35.5 | 39.7 | 36.6 | |||||||||
| PCAA | 14.3 | 14.5 | 13.6 | 14.7 | 14.1 | 14.1 | 14.4 | 14.4 | 14.1 | |||||||||
| Fraction | Compounds | ||
|---|---|---|---|
| SDG | p-Coumaric Acid | Ferulic Acid | |
| Szafir cultivar | |||
| WEF | 2.80 ± 0.16 b | 0.10 ± 0.004 b | 0.51 ± 0.01 b |
| SEF | 6.01 ± 0.40 c | 0.20 ± 0.02 d | 0.79 ± 0.02 c |
| AEF | 14.26 ± 0.03 f | 0.69 ± 0.002 g | 2.52 ± 0.01 f |
| Oliwin cultivar | |||
| WEF | 1.72 ± 0.03 a | 0.05 ± 0.001 a | 0.39 ± 0.002 a |
| SEF | 3.43 ± 0.27 b | 0.10 ± 0.01 b | 0.52 ± 0.07 b |
| AEF | 9.53 ± 0.46 e | 0.45 ± 0.04 f | 2.32 ± 0.11 e |
| Jantarol cultivar | |||
| WEF | 5.84 ± 0.29 c | 0.10 ± 0.01 b | 0.32 ± 0.03 a |
| SEF | 7.73 ± 0.67 d | 0.14 ± 0.02 c | 0.62 ± 0.02 b |
| AEF | 19.06 ± 0.18 g | 0.38 ± 0.01 e | 1.54 ± 0.004 d |
| p | <0.001 | <0.001 | <0.001 |
| Property | Protein Fractions | p | ||
|---|---|---|---|---|
| /Cultivar | WEF | SEF | AEF | |
| Surface Hydrophobicity (Ho) 1 | ||||
| Szafir | 7.30 ± 0.51 a | 16.13 ± 0.43 c | 67.33 ± 0.32 f | |
| Oliwin | 6.52 ± 0.22 a | 14.49 ± 0.46 c | 50.80 ± 0.63 e | |
| Jantarol | 10.30 ± 0.39 b | 21.34 ± 0.88 d | 80.93 ± 0.94 g | |
| <0.001 | ||||
| Antiradical activity (ORAC_FL) 2 | ||||
| Szafir | 141 ± 1 b | 352 ± 16 d | 894 ± 35 f | |
| Oliwin | 131 ± 6 ab | 252 ± 11 c | 627 ± 15 e | |
| Jantarol | 95 ± 11 a | 317 ± 5 d | 1356 ± 22 g | |
| <0.001 | ||||
| Total phenolic content (TPC)/compounds reducing FCR 3 | ||||
| Szafir | 8.95 ± 0.54 b | 16.91 ± 0.34 e | 32.03 ± 0.95 h | |
| Oliwin | 10.67 ± 0.43 c | 15.27 ± 0.84 d | 28.44 ± 0.49 g | |
| Jantarol | 6.98 ± 0.25 a | 20.76 ± 0.41 f | 42.21 ± 1.17 i | |
| <0.001 | ||||
| Cultivar | Antioxidant/Protein Fraction | ||||||
|---|---|---|---|---|---|---|---|
| Control | BHT | WEF | SEF | AEF | p | ||
| 0.1 g L−1 | 10 g L−1 | 10 g L−1 | 10 g L−1 | ||||
| CD (g kg−1) | |||||||
| initial | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.26 ± 0.02 | 0.23 ± 0.01 | 0.25 ± 0.01 | 0.174 | |
| Szafir | after 24 h | 1.19 ± 0.12 c | 0.54 ± 0.03 b | 0.45 ± 0.01 b | 0.29 ± 0.02 a | 0.44 ± 0.05 b | <0.001 |
| PI_24 h | 2.2 | 2.6 | 4.1 | 2.7 | |||
| initial | 0.33 ± 0.02 | 0.32 ± 0.01 | 0.32 ± 0.01 | 0.31 ± 0.01 | 0.32 ± 0.01 | 0.055 | |
| Oliwin | after 24 h | 0.90 ± 0.01 c | 0.51 ± 0.04 b | 0.47 ± 0.04 b | 0.32 ± 0.03 a | 0.53 ± 0.02 b | <0.001 |
| PI_24 h | 1.8 | 1.9 | 2.8 | 1.7 | |||
| initial | 0.26 ± 0.02 | 0.26 ± 0.02 | 0.27 ± 0.01 | 0.24 ± 0.02 | 0.27 ± 0.01 | 0.24 | |
| Jantarol | after 24 h | 1.08 ± 0.13 c | 0.53 ± 0.03 b | 0.46 ± 0.01 b | 0.31 ± 0.02 a | 0.52 ± 0.04 b | <0.001 |
| PI_24 h | 2 | 2.3 | 3.5 | 2.1 | |||
| CT | |||||||
| initial | 0.19 ± 0.01 a | 0.20 ± 0.01 ab | 0.21 ± 0.02 b | 0.21 ± 0.01 ab | 0.22 ± 0.01 b | 0.002 | |
| Szafir | after 24 h | 0.46 ± 0.05 c | 0.28 ± 0.01 b | 0.26 ± 0.01 ab | 0.22 ± 0.01 a | 0.26 ± 0.01 ab | <0.001 |
| PI_24 h | 1.7 | 1.8 | 2.1 | 1.8 | |||
| initial | 0.24 ± 0.01 a | 0.26 ± 0.01 ab | 0.26 ± 0.02 ab | 0.25 ± 0.01 a | 0.28 ± 0.01 b | 0.006 | |
| Oliwin | after 24 h | 0.36 ± 0.01 c | 0.28 ± 0.01 ab | 0.28 ± 0.01 ab | 0.26 ± 0.03 a | 0.31 ± 0.01 b | <0.001 |
| PI_24 h | 1.3 | 1.3 | 1.4 | 1.2 | |||
| initial | 0.20 ± 0.01 a | 0.21 ± 0.01 a | 0.21 ± 0.01 a | 0.24 ± 0.01 b | 0.25 ± 0.01 b | 0.006 | |
| Jantarol | after 24 h | 0.42 ± 0.05 c | 0.29 ± 0.01 b | 0.26 ± 0.01 ab | 0.24 ± 0.01 a | 0.30 ± 0.01 b | <0.001 |
| PI_24 h | 1.4 | 1.6 | 1.8 | 1.4 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waszkowiak, K.; Mikołajczak, B.; Polanowska, K.; Wieruszewski, M.; Siejak, P.; Smułek, W.; Jarzębski, M. Protein Fractions from Flaxseed: The Effect of Subsequent Extractions on Composition and Antioxidant Capacity. Antioxidants 2023, 12, 675. https://doi.org/10.3390/antiox12030675
Waszkowiak K, Mikołajczak B, Polanowska K, Wieruszewski M, Siejak P, Smułek W, Jarzębski M. Protein Fractions from Flaxseed: The Effect of Subsequent Extractions on Composition and Antioxidant Capacity. Antioxidants. 2023; 12(3):675. https://doi.org/10.3390/antiox12030675
Chicago/Turabian StyleWaszkowiak, Katarzyna, Beata Mikołajczak, Katarzyna Polanowska, Marek Wieruszewski, Przemysław Siejak, Wojciech Smułek, and Maciej Jarzębski. 2023. "Protein Fractions from Flaxseed: The Effect of Subsequent Extractions on Composition and Antioxidant Capacity" Antioxidants 12, no. 3: 675. https://doi.org/10.3390/antiox12030675
APA StyleWaszkowiak, K., Mikołajczak, B., Polanowska, K., Wieruszewski, M., Siejak, P., Smułek, W., & Jarzębski, M. (2023). Protein Fractions from Flaxseed: The Effect of Subsequent Extractions on Composition and Antioxidant Capacity. Antioxidants, 12(3), 675. https://doi.org/10.3390/antiox12030675










