Characterization of Redox Environment and Tryptophan Catabolism through Kynurenine Pathway in Military Divers’ and Swimmers’ Serum Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Military Training Program
2.3. Physical Fitness Criteria
2.4. Materials
2.4.1. Determination of Redox Status and KP Metabolites in Serum
2.4.2. Serum GSH and GSSG Determination
2.4.3. Lipid Peroxidation
2.4.4. Kynurenines Determination
2.4.5. Serum Neopterin
2.5. Statistical Analysis
3. Results
3.1. Description of the Study Population
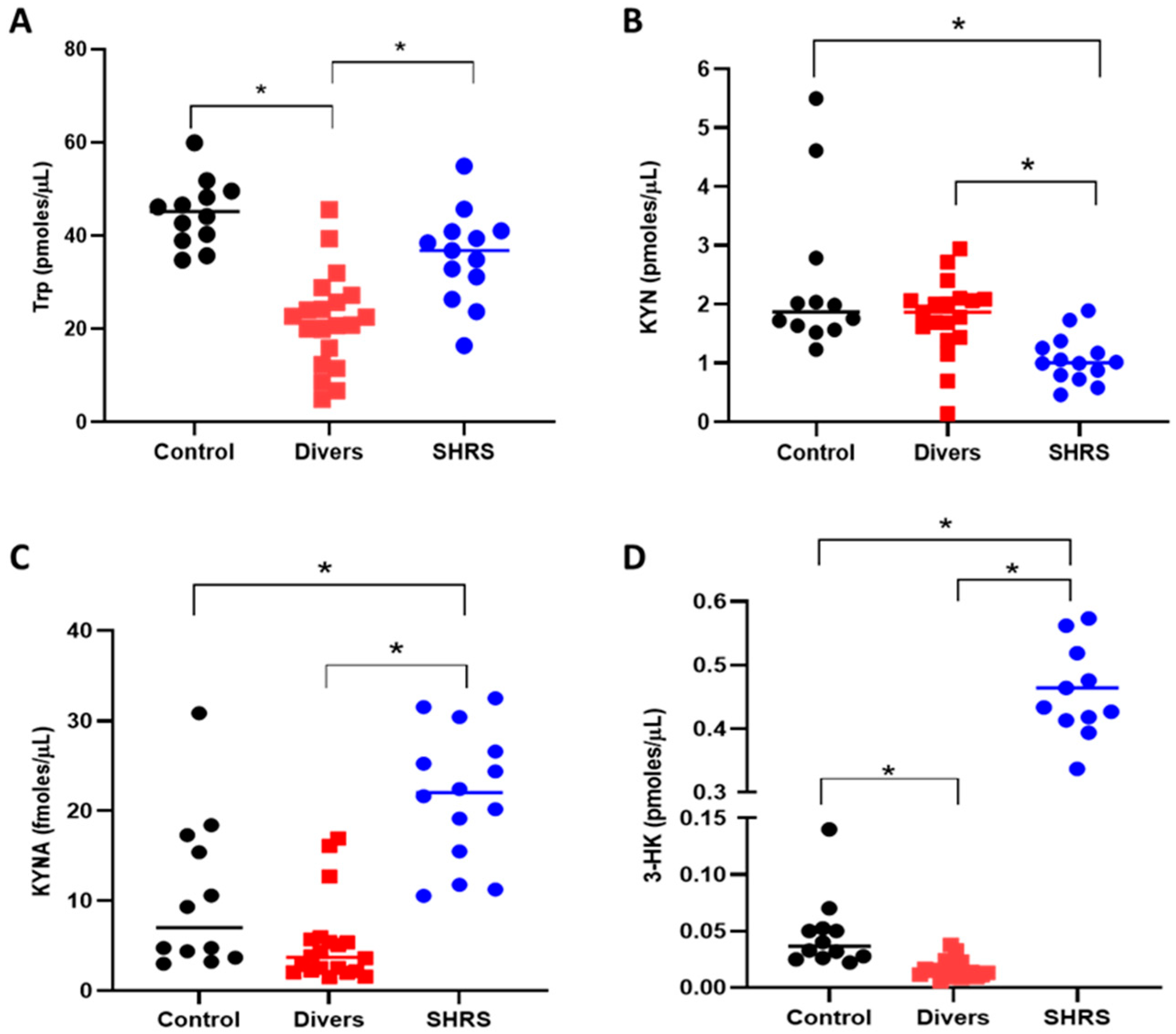
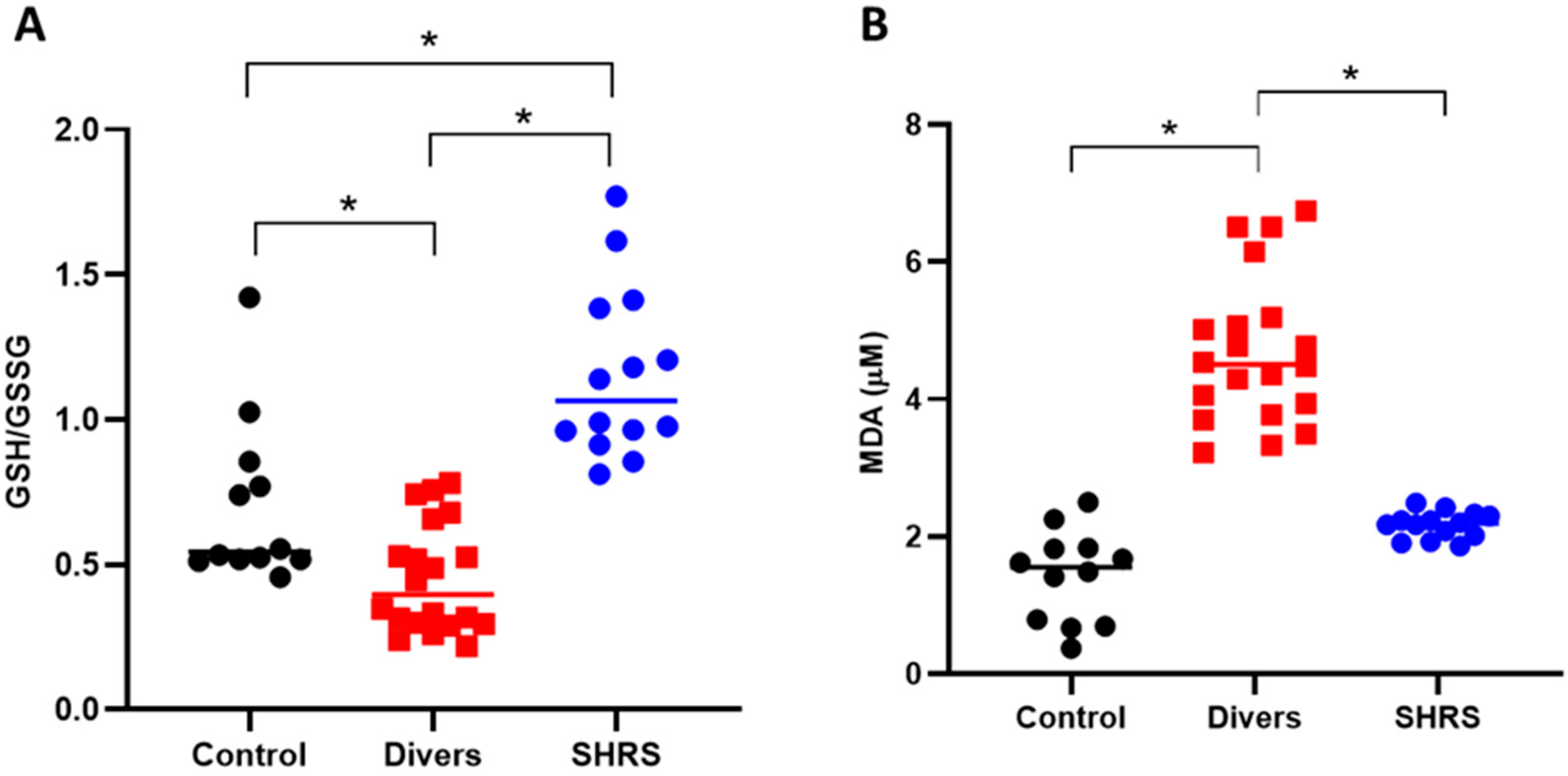
3.2. BC, Trp Catabolism and Redox Environment Parameters at the Beginning up to 6 Months after the Military Training Program in Divers’ and SHRS’ Serum
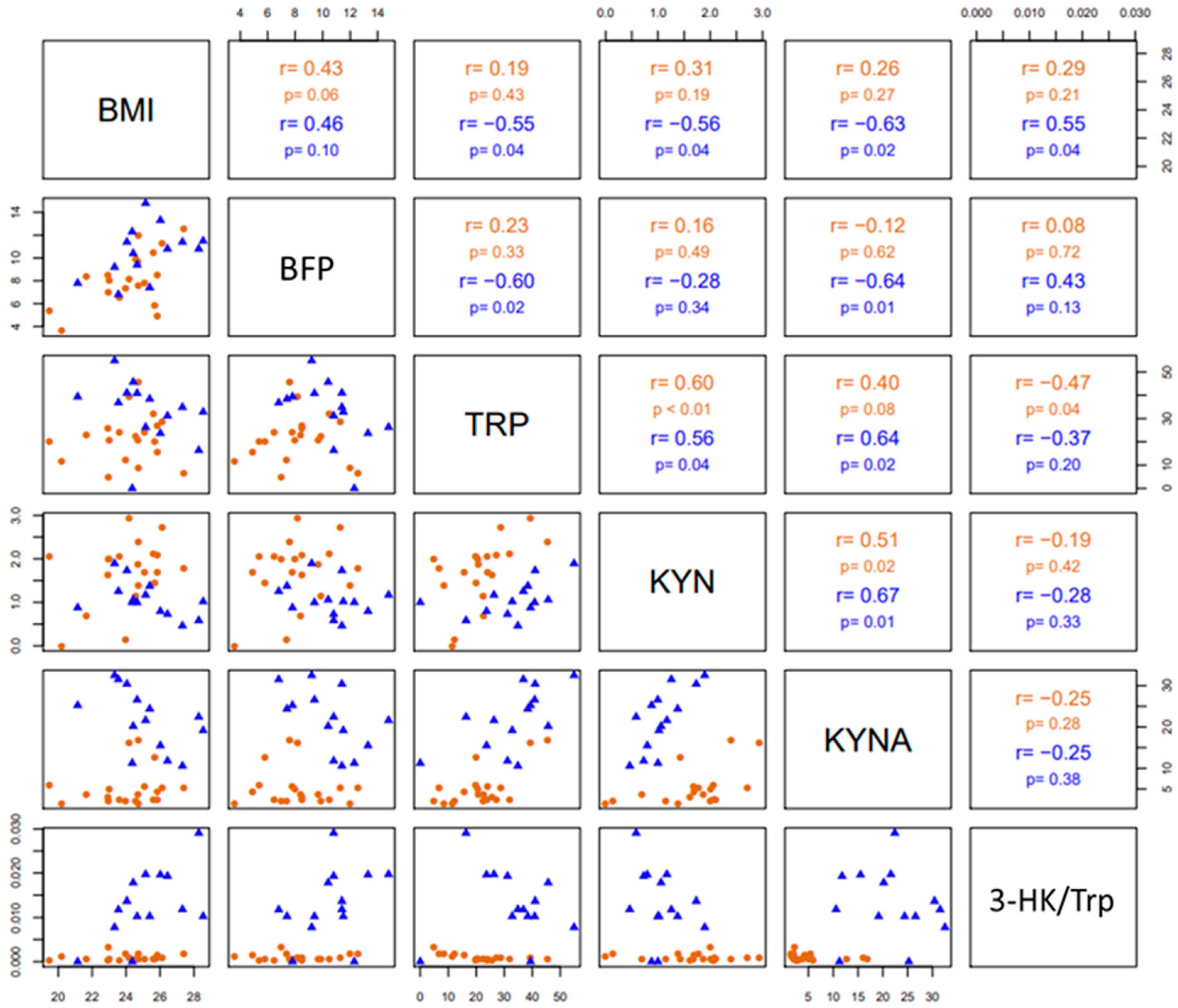
3.3. Changes in Trp Catabolism and Redox Environment Induced by the Type of Exercise
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Sanchis-Gomer, F.; Martinez-Bello, V.; Gomez-Cabrera, M.C. Exercise acts as a drug; the pharmacological benefits of exercise. Br. J. Pharmacol. 2012, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Deslandes, A.; Moraes, H.; Ferreira, C.; Veiga, H.; Silveira, H.; Mouta, R.; Pompeu, F.A.; Coutinho, E.S.; Laks, J. Exercise and mental health: Many reasons to move. Neuropsychobiology 2009, 59, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Landen, S.; Voisin, S.; Craig, J.M.; McGee, S.L.; Lamon, S.; Eynon, N. Genetic and epigenetic sex-specific adaptations to endurance exercise. Epigenetics 2019, 14, 523–535. [Google Scholar] [CrossRef]
- Braithwaite, A.W.; Royds, J.A.; Jackson, P. The p53 story: Layers of complexity. Carcinogenesis 2005, 26, 1161–1169. [Google Scholar] [CrossRef]
- Monteiro, P.A.; Chen, K.Y.; Lira, F.S.; Saraiva, B.T.; Antunes, B.M.; Campos, E.Z.; Freitas, I.F., Jr. Concurrent and aerobic exercise training promote similar benefits in body composition and metabolic profiles in obese adolescents. Lipids Health Dis. 2015, 14, 153. [Google Scholar] [CrossRef]
- Kolodziej, F.; O’Halloran, K.D. Re-Evaluating the Oxidative Phenotype: Can Endurance Exercise Save the Western World? Antioxidants 2021, 10, 609. [Google Scholar] [CrossRef]
- Ferraro, E.; Giammarioli, A.M.; Chiandotto, S.; Spoletini, I.; Rosano, G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: Redox signaling and role of autophagy. Antioxid. Redox Signal. 2014, 21, 154–176. [Google Scholar] [CrossRef]
- Ji, L.L.; Kang, C.; Zhang, Y. Exercise-induced hormesis and skeletal muscle health. Free Radic. Biol. Med. 2016, 98, 113–122. [Google Scholar] [CrossRef]
- Arany, Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr. Opin. Genet. Dev. 2008, 18, 426–434. [Google Scholar] [CrossRef]
- Baar, K.; Wende, A.R.; Jones, T.E.; Marison, M.; Nolte, L.A.; Chen, M.; Kelly, D.P.; Holloszy, J.O. Adaptations of skeletal muscle to exercise: Rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002, 16, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.S.; Azzolini, M.; Ruas, J.L. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Ferreira, D.M.S.; Dadvar, S.; Cervenka, I.; Ketscher, L.; Izadi, M.; Zhengye, L.; Furrer, R.; Handschin, C.; Venckunas, T.; et al. Skeletal muscle PGC-1alpha1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat. Commun. 2019, 10, 2767. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Schlittler, M.; Goiny, M.; Agudelo, L.Z.; Venckunas, T.; Brazaitis, M.; Skurvydas, A.; Kamandulis, S.; Ruas, J.L.; Erhardt, S.; Westerblad, H.; et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol. Cell Physiol. 2016, 310, C836–C840. [Google Scholar] [CrossRef]
- González Esquivel, D.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; Pérez de la Cruz, V. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology 2017, 112, 331–345. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. 2015, 20, 1116–1143. [Google Scholar]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Dang, Y.; Dale, W.E.; Brown, O.R. Comparative effects of oxygen on indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase of the kynurenine pathway. Free Radic. Biol. Med. 2000, 28, 615–624. [Google Scholar] [CrossRef]
- Amori, L.; Guidetti, P.; Pellicciari, R.; Kajii, Y.; Schwarcz, R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J. Neurochem. 2009, 109, 316–325. [Google Scholar] [CrossRef]
- Torok, N.; Tanaka, M.; Vecsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and mental health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef]
- Lewis, G.D.; Farrell, L.; Wood, M.J.; Martinovic, M.; Arany, Z.; Rowe, G.C.; Souza, A.; Cheng, S.; McCabe, E.L.; Yang, E.; et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010, 2, 33–37. [Google Scholar] [CrossRef]
- Isung, J.; Granqvist, M.; Trepci, A.; Huang, J.; Schwieler, L.; Kierkegaard, M.; Erhardt, S.; Jokinen, J.; Piehl, F. Differential effects on blood and cerebrospinal fluid immune protein markers and kynurenine pathway metabolites from aerobic physical exercise in healthy subjects. Sci. Rep. 2021, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Joisten, N.; Kummerhoff, F.; Koliamitra, C.; Schenk, A.; Walzik, D.; Hardt, L.; Knoop, A.; Thevis, M.; Kiesl, D.; Metcalfe, A.J.; et al. Exercise and the Kynurenine pathway: Current state of knowledge and results from a randomized cross-over study comparing acute effects of endurance and resistance training. Exerc. Immunol. Rev. 2020, 26, 24–42. [Google Scholar] [PubMed]
- Koliamitra, C.; Javelle, F.; Joisten, N.; Shimabukuro-Vornhagen, A.; Bloch, W.; Schenk, A.; Zimmer, P. Do Acute Exercise-Induced Activations of the Kynurenine Pathway Induce Regulatory T-Cells on the Long-Term?—A Theoretical Frame Work Supported by Pilot Data. J. Sports Sci. Med. 2019, 18, 669–673. [Google Scholar]
- Su, C.H.; Chuang, H.C.; Hong, C.J. Physical exercise prevents mice from L-Kynurenine-induced depression-like behavior. Asian J. Psychiatr. 2020, 48, 101894. [Google Scholar] [CrossRef]
- Mudry, J.M.; Alm, P.S.; Erhardt, S.; Goiny, M.; Fritz, T.; Caidahl, K.; Zierath, J.R.; Krook, A.; Wallberg-Henriksson, H. Direct effects of exercise on kynurenine metabolism in people with normal glucose tolerance or type 2 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 754–761. [Google Scholar] [CrossRef]
- Leger, L.A.; Mercier, D.; Gadoury, C.; Lambert, J. The multistage 20 metre shuttle run test for aerobic fitness. J. Sports Sci. 1988, 6, 93–101. [Google Scholar] [CrossRef]
- Marfell-Jones, M.J.; Sewart, A.D.; de Ridder, J.H. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Wellington, New Zealand, 2012. [Google Scholar]
- Drinkwater, D.T.; Ross, W.D. Anthropometric Fractionation of Body Mass. In International Series of Sports Science, Kinanthropometry, 2nd ed.; Ostyn, G.B.M., Ed.; University Park Press: Will County, IL, USA, 1980. [Google Scholar]
- Ross, W.D.; Kerr, D.A. Fraccionamiento de la masa corporal: Un nuevo método para utilizar en nutrición, clínica y medicina deportiva. Apunts 1993, 28, 175–188. [Google Scholar]
- Pons, V.; Riera, J.; Galilea, P.A.; Drobnic, F.; Banquells, M.; Ruiz, O. Características antropométricas, composición corporal y somatotipo por deportes. Datos de referencia del CAR de San Cugat, 1989–2013. Apunts Med. Esport. 2015, 50, 65–72. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [PubMed]
- Ferguson, B. ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. 2014. J. Can. Chiropr. Assoc. 2014, 58, 328. [Google Scholar]
- Macias, N.; Quezada, A.D.; Flores, M.; Valencia, M.E.; Denova-Gutiérrez, E.; Quiterio-Trenado, M.; Gallegos-Carrillo, K.; Barquera, S.; Salmerón, J. Accuracy of body fat percent and adiposity indicators cut off values to detect metabolic risk factors in a sample of Mexican adults. BMC Public Health 2014, 14, 341. [Google Scholar] [CrossRef]
- Senft, A.P.; Dalton, T.P.; Shertzer, H.G. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal. Biochem. 2000, 280, 80–86. [Google Scholar] [CrossRef]
- Ramos-Chavez, L.A.; Rendón-López, C.R.; Zepeda, A.; Silva-Adaya, D.; Del Razo, L.M.; Gonsebatt, M.E. Neurological effects of inorganic arsenic exposure: Altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front. Cell Neurosci. 2015, 9, 21. [Google Scholar] [CrossRef]
- Dasgupta, A.; Kein, K. Methods for Measuring Oxidative Stress in the Laboratory, in Antioxidants in Food, Vitamins and Supplements; Chapter 2; Dasgupta, A., Klein, K., Eds.; Elsevier: San Diego, Ca, USA, 2014; pp. 19–40. [Google Scholar]
- Heyes, M.P.; Quearry, B.J. Quantification of 3-hydroxykynurenine in brain by high-performance liquid chromatography and electrochemical detection. J. Chromatogr. 1988, 428, 340–344. [Google Scholar] [CrossRef]
- Perez-de la Cruz, V.; Amori, L.; Sathyasaikumar, K.V.; Wang, X.D.; Notarangelo, F.M.; Wu, H.Q.; Schwarcz, R. Enzymatic transamination of D-kynurenine generates kynurenic acid in rat and human brain. J. Neurochem. 2012, 120, 1026–1035. [Google Scholar] [CrossRef]
- Blanco Ayala, T.; Lugo Huitrón, R.; Carmona Aparicio, L.; Ramírez Ortega, D.; González Esquivel, D.; Pedraza Chaverrí, J.; Pérez de la Cruz, G.; Ríos, C.; Schwarcz, R.; Pérez de la Cruz, V. Alternative kynurenic acid synthesis routes studied in the rat cerebellum. Front. Cell Neurosci. 2015, 9, 178. [Google Scholar] [CrossRef]
- Widner, B.; Sepp, N.; Kowald, E.; Ortner, U.; Wirleitner, B.; Fritsch, P.; Baier-Bitterlich, G.; Fuchs, D. Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology 2000, 201, 621–630. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.J.; Koliamitra, C.; Javelle, F.; Bloch, W.; Zimmer, P. Acute and chronic effects of exercise on the kynurenine pathway in humans—A brief review and future perspectives. Physiol. Behav. 2018, 194, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Wingelaar, T.T.; van Ooij, P.A.M.; van Hulst, R.A. Oxygen Toxicity and Special Operations Forces Diving: Hidden and Dangerous. Front. Psychol. 2017, 8, 1263. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz-García, M.J.; Albaladejo, M.D.; Acevedo, C.; Olea, A.; Zamora, S.; Martínez, P.; Parra, S. Effects of hyperoxia on biomarkers of oxidative stress in closed-circuit oxygen military divers. J. Physiol. Biochem. 2008, 64, 135–141. [Google Scholar] [CrossRef]
- Doubt, T.J. Cardiovascular and thermal responses to SCUBA diving. Med. Sci. Sports Exerc. 1996, 28, 581–586. [Google Scholar]
- Ferrer, M.D.; Sureda, A.; Batle, J.M.; Tauler, P.; Tur, J.A.; Pons, A. Scuba diving enhances endogenous antioxidant defenses in lymphocytes and neutrophils. Free Radic. Res. 2007, 41, 274–281. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress: Relationship with exercise and training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- Sureda, A.; Ferrer, M.D.; Batle, J.M.; Tauler, P.; Tur, J.A.; Pons, A. Scuba diving increases erythrocyte and plasma antioxidant defenses and spares NO without oxidative damage. Med. Sci. Sports Exerc. 2009, 41, 1271–1276. [Google Scholar] [CrossRef]
- Sureda, A.; Batle, J.M.; Ferrer, M.D.; Mestre-Alfaro, A.; Tur, J.A.; Pons, A. Scuba diving activates vascular antioxidant system. Int. J. Sports Med. 2012, 33, 531–536. [Google Scholar] [CrossRef]
- Wagner, S.; Manickam, R.; Brotto, M.; Tipparaju, S.M. NAD(+) centric mechanisms and molecular determinants of skeletal muscle disease and aging. Mol. Cell Biochem. 2022, 477, 1829–1848. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Yeo, D. Maintenance of NAD+ Homeostasis in Skeletal Muscle during Aging and Exercise. Cells 2022, 11, 710. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Saito, K.; Maruta, K.; Nakagami, Y.; Koike, T.; Oguri, Y.; Nagamura, Y. Kynurenine concentration of serum was increased by exercise. Adv. Exp. Med. Biol. 1999, 467, 717–722. [Google Scholar] [PubMed]
- Trepci, A.; Imbeault, S.; Wyckelsma, V.L.; Westerblad, H.; Hermansson, S.; Andersson, D.C.; Piehl, F.; Venckunas, T.; Brazaitis, M.; Kamandulis, S.; et al. Quantification of Plasma Kynurenine Metabolites Following One Bout of Sprint Interval Exercise. Int. J. Tryptophan. Res. 2020, 13, 1178646920978241. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.J.; Nederveen, J.P.; Snijders, T.; Bell, K.E.; Kumbhare, D.; Phillips, S.M.; Parise, G.; Heisz, J.J. Exercise training impacts skeletal muscle gene expression related to the kynurenine pathway. Am. J. Physiol. Cell Physiol. 2019, 316, C444–C448. [Google Scholar] [CrossRef] [PubMed]
- Finck, B.N.; Kelly, D.P. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J. Clin. Investig. 2006, 116, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.A.; Kleinman, M.T.; Hamilton, M.; Barstow, T.J. The effect of exercise intensity on lipid peroxidation. Med. Sci. Sports Exerc. 1997, 29, 1036–1039. [Google Scholar] [CrossRef]
- Miyazaki, H.; Oh-ishi, S.; Ookawara, T.; Kizaki, T.; Toshinai, K.; Ha, S.; Haga, S.; Ji, L.L.; Ohno, H. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur. J. Appl. Physiol. 2001, 84, 1–6. [Google Scholar] [CrossRef]
- Schippinger, G.; Wonisch, W.; Abuja, P.M.; Fankhauser, F.; Winklhofer-Roob, B.M.; Halwachs, G. Lipid peroxidation and antioxidant status in professional American football players during competition. Eur. J. Clin. Investig. 2002, 32, 686–692. [Google Scholar] [CrossRef]
- Onur, E.; Kabaroğlu, C.; Günay, O.; Var, A.; Yilmaz, O.; Dündar, P.; Tikiz, C.; Güvenç, Y.; Yüksel, H. The beneficial effects of physical exercise on antioxidant status in asthmatic children. Allergol. Immunopathol. 2011, 39, 90–95. [Google Scholar] [CrossRef]
- Leelarungrayub, D.; Sawattikanon, N.; Klaphajone, J.; Pothongsunan, P.; Bloomer, R.J. Coenzyme Q10 Supplementation Decreases Oxidative Stress and Improves Physical Performance in Young Swimmers: A Pilot Study. Open Sports Med. J. 2010, 4, 1–8. [Google Scholar] [CrossRef][Green Version]
- Lugo-Huitron, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ocampo, J.; Ramírez-Ortega, D.; Cervantes, G.I.; Pineda, B.; Balderas, P.M.; González-Esquivel, D.; Sánchez-Chapul, L.; Lugo-Huitrón, R.; Silva-Adaya, D.; Ríos, C.; et al. Mitochondrial dysfunction related to cell damage induced by 3-hydroxykynurenine and 3-hydroxyanthranilic acid: Non-dependent-effect of early reactive oxygen species production. Neurotoxicology 2015, 50, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Reyes Ocampo, J.; Lugo Huitrón, R.; González-Esquivel, D.; Ugalde-Muñiz, P.; Jiménez-Anguiano, A.; Pineda, B.; Pedraza-Chaverri, J.; Ríos, C.; Pérez de la Cruz, V. Kynurenines with neuroactive and redox properties: Relevance to aging and brain diseases. Oxid. Med. Cell Longev. 2014, 2014, 646909. [Google Scholar] [CrossRef] [PubMed]
- Zhuravlev, A.V.; Zakharov, G.A.; Shchegolev, B.F.; Savvateeva-Popova, E.V. Antioxidant Properties of Kynurenines: Density Functional Theory Calculations. PLoS Comput. Biol. 2016, 12, e1005213. [Google Scholar] [CrossRef] [PubMed]
- Javelle, F.; Bloch, W.; Knoop, A.; Guillemin, G.J.; Zimmer, P. Toward a neuroprotective shift: Eight weeks of high intensity interval training reduces the neurotoxic kynurenine activity concurrently to impulsivity in emotionally impulsive humans—A randomized controlled trial. Brain. Behav. Immun. 2021, 96, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Millischer, V.; Erhardt, S.; Ekblom, Ö.; Forsell, Y.; Lavebratt, C. Twelve-week physical exercise does not have a long-lasting effect on kynurenines in plasma of depressed patients. Neuropsychiatr. Dis. Treat. 2017, 13, 967–972. [Google Scholar] [CrossRef]
- Morris, J.K.; Vidoni, E.D.; Johnson, D.K.; Van Sciver, A.; Mahnken, J.D.; Honea, R.A.; Wilkins, H.M.; Brooks, W.M.; Billinger, S.A.; Swerdlow, R.H.; et al. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS ONE 2017, 12, e0170547. [Google Scholar] [CrossRef]
- Lattari, E.; Pascouto, A.J.C.; Oliveira, B.R.R.; Silva, L.S.; Oliveira, A.J.; Machado, S.; Neto, G.A.M. Association between Estimated Cardiorespiratory Fitness and Depression among Middle-income Country Adults: Evidence from National Health Survey. Clin. Pract. Epidemiol. Ment. Health 2021, 17, 198–204. [Google Scholar] [CrossRef]


| Controls | Divers | SHRS | |
|---|---|---|---|
| (n = 12) | (n = 20) | (n = 14) | |
| Age | 23.1 ± 0.66 | 27.2 ± 0.86 | 28.6 ± 1.14 |
| Heigh (m) | 1.75 ± 0.02 | 1.74 ± 0.01 | 1.69 ± 0.02 |
| WC (cm) | ≤90 | ≤90 | ≤90 |
| Min | Percentile 25 | Median | Percentile 75 | Max | Mean | p Values Wilcoxon Signed-Rank Test | ||
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | Beginning | 60.10 | 66.98 | 74.10 | 76.33 | 83.80 | 72.76 | 0.9345 |
| 6 months | 62.60 | 69.78 | 72.35 | 77.65 | 82.80 | 72.88 | ||
| BMI (kg/m2) | Beginning | 19.62 | 23.02 | 24.49 | 25.82 | 27.68 | 24.13 | 0.8771 |
| 6 months | 19.48 | 22.98 | 24.65 | 25.67 | 27.41 | 24.12 | ||
| MMP (%) | Beginning | 36.20 | 44.60 | 47.25 | 49.25 | 50.60 | 46.27 | 0.0001 * |
| 6 months | 40.20 | 48.10 | 49.25 | 51.63 | 54.60 | 49.07 | ||
| BFP (%) | Beginning | 4.30 | 8.925 | 10.40 | 12.55 | 20.70 | 11.02 | 0.0005 * |
| 6 months | 3.60 | 6.625 | 8.10 | 9.85 | 12.60 | 8.18 | ||
| METs | Beginning | 9.07 | 10.30 | 11.14 | 12.48 | 14.16 | 11.30 | 0.7680 |
| 6 months | 8.10 | 9.935 | 11.67 | 12.53 | 14.16 | 11.26 | ||
| Trp (pmoles/µL) | Beginning | 3.06 | 15.63 | 19.86 | 26.50 | 33.40 | 20.66 | 0.7285 |
| 6 months | 4.87 | 13.19 | 21.65 | 26.83 | 45.58 | 21.66 | ||
| KYN (pmoles/µL) | Beginning | 1.26 | 1.58 | 1.88 | 2.56 | 3.20 | 2.03 | 0.5791 |
| 6 months | 0.14 | 1.65 | 2.00 | 2.09 | 2.94 | 1.84 | ||
| KYNA (fmoles/µL) | Beginning | 2.82 | 3.50 | 5.29 | 5.88 | 8.84 | 5.02 | 0.3488 |
| 6 months | 1.51 | 2.21 | 3.72 | 5.63 | 16.93 | 5.23 | ||
| 3-HK (pmoles/µL) | Beginning | 0.003 | 0.006 | 0.008 | 0.011 | 0.02 | 0.01 | 0.0001 * |
| 6 months | 0.006 | 0.011 | 0.014 | 0.023 | 0.038 | 0.02 | ||
| KYN/Trp | Beginning | 0.054 | 0.081 | 0.087 | 0.153 | 0.629 | 0.143 | 0.3778 |
| 6 months | 0.012 | 0.064 | 0.077 | 0.100 | 0.412 | 0.104 | ||
| KYNA/Trp | Beginning | 0.095 | 0.171 | 0.203 | 0.325 | 0.672 | 0.262 | 0.8596 |
| 6 months | 0.080 | 0.113 | 0.187 | 0.371 | 0.800 | 0.265 | ||
| 3-HK/Trp | Beginning | 0.0001 | 0.0003 | 0.0004 | 0.0006 | 0.0024 | 0.0006 | 0.0689 |
| 6 months | 0.0003 | 0.0005 | 0.0007 | 0.0015 | 0.0034 | 0.0010 | ||
| MDA (µM) | Beginning | 3.22 | 3.79 | 4.48 | 4.94 | 5.61 | 4.41 | 0.3162 |
| 6 months | 3.22 | 3.82 | 4.51 | 5.16 | 6.74 | 4.69 | ||
| GSH/GSSG | Beginning | 0.03 | 0.36 | 0.42 | 0.70 | 1.21 | 0.51 | 0.4749 |
| 6 months | 0.22 | 0.30 | 0.40 | 0.63 | 0.78 | 0.45 | ||
| Neopterin (ng/mL) | Beginning | 2.09 | 3.023 | 4.11 | 4.79 | 6.25 | 4.04 | 0.0002 * |
| 6 months | 1.203 | 2.374 | 2.95 | 3.43 | 4.21 | 2.99 | ||
| Min | Percentile 25 | Median | Percentile 75 | Max | Mean | p Values Wilcoxon Signed-Rank Test | ||
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | Beginning | 60.80 | 68.40 | 72.25 | 77.38 | 92.50 | 73.89 | 0.0132 * |
| 6 months | 60.40 | 66.03 | 69.95 | 77.15 | 90.60 | 72.18 | ||
| BMI(kg/m2) | Beginning | 21.29 | 24.69 | 26.00 | 27.25 | 28.87 | 25.90 | 0.0031 * |
| 6 months | 21.15 | 23.93 | 24.90 | 26.66 | 28.54 | 25.19 | ||
| MMP (%) | Beginning | 39.70 | 45.98 | 48.10 | 52.33 | 55.80 | 48.89 | 0.8672 |
| 6 months | 43.30 | 45.53 | 49.40 | 51.43 | 55.00 | 48.89 | ||
| BFP (%) | Beginning | 8.00 | 11.10 | 13.65 | 15.00 | 18.50 | 13.19 | 0.0023 * |
| 6 months | 6.80 | 8.85 | 10.80 | 11.70 | 14.80 | 10.52 | ||
| METs | Beginning | 8.86 | 10.24 | 11.53 | 12.52 | 13.23 | 11.32 | 0.0067 * |
| 6 months | 8.01 | 11.77 | 12.63 | 13.43 | 14.53 | 12.42 | ||
| Trp (pmoles/µL) | Beginning | 19.95 | 36.50 | 43.84 | 48.76 | 64.69 | 43.52 | 0.0479 * |
| 6 months | 16.36 | 28.74 | 36.82 | 40.93 | 54.90 | 35.90 | ||
| KYN (pmoles/µL) | Beginning | 0.41 | 0.89 | 1.06 | 1.37 | 2.05 | 1.13 | 0.8077 |
| 6 months | 0.46 | 0.78 | 1.01 | 1.29 | 1.89 | 1.07 | ||
| KYNA (fmoles/µL) | Beginning | 6.61 | 13.00 | 17.10 | 21.29 | 57.26 | 19.64 | 0.1726 |
| 6 months | 10.57 | 14.57 | 22.01 | 27.54 | 32.52 | 21.65 | ||
| 3-HK (pmoles/µL) | Beginning | 0.206 | 0.345 | 0.435 | 0.693 | 0.787 | 0.500 | 0.9990 |
| 6 months | 0.337 | 0.416 | 0.454 | 0.568 | 0.814 | 0.495 | ||
| KYN/Trp | Beginning | 0.011 | 0.186 | 0.023 | 0.038 | 0.058 | 0.028 | 0.4973 |
| 6 months | 0.013 | 0.023 | 0.034 | 0.036 | 0.045 | 0.031 | ||
| KYNA/Trp | Beginning | 0.153 | 0.291 | 0.419 | 0.727 | 0.885 | 0.478 | 0.1099 |
| 6 months | 0.303 | 0.513 | 0.641 | 0.782 | 1.370 | 0.667 | ||
| 3-HK/Trp | Beginning | 0.0042 | 0.0066 | 0.0099 | 0.0173 | 0.0379 | 0.0133 | 0.0342 * |
| 6 months | 0.0078 | 0.0102 | 0.0128 | 0.0195 | 0.0291 | 0.0151 | ||
| MDA (µM) | Beginning | 1.81 | 2.19 | 2.34 | 2.42 | 3.47 | 2.36 | 0.0652 |
| 6 months | 1.86 | 1.99 | 2.19 | 2.31 | 2.49 | 2.17 | ||
| GSH/GSSG | Beginning | 0.99 | 1.23 | 1.36 | 1.61 | 2.32 | 1.49 | 0.0494 * |
| 6 months | 0.81 | 0.95 | 1.06 | 1.39 | 1.77 | 1.16 | ||
| Neopterin (ng/mL) | Beginning | 2.41 | 2.70 | 3.91 | 4.52 | 5.51 | 3.79 | 0.0353 * |
| 6 months | 2.22 | 2.55 | 2.83 | 3.36 | 4.37 | 3.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Chapul, L.; Pérez de la Cruz, G.; Ramos Chávez, L.A.; Valencia León, J.F.; Torres Beltrán, J.; Estrada Camarena, E.; Carillo Mora, P.; Ramírez Ortega, D.; Baños Vázquez, J.U.; Martínez Nava, G.; et al. Characterization of Redox Environment and Tryptophan Catabolism through Kynurenine Pathway in Military Divers’ and Swimmers’ Serum Samples. Antioxidants 2022, 11, 1223. https://doi.org/10.3390/antiox11071223
Sánchez Chapul L, Pérez de la Cruz G, Ramos Chávez LA, Valencia León JF, Torres Beltrán J, Estrada Camarena E, Carillo Mora P, Ramírez Ortega D, Baños Vázquez JU, Martínez Nava G, et al. Characterization of Redox Environment and Tryptophan Catabolism through Kynurenine Pathway in Military Divers’ and Swimmers’ Serum Samples. Antioxidants. 2022; 11(7):1223. https://doi.org/10.3390/antiox11071223
Chicago/Turabian StyleSánchez Chapul, Laura, Gonzalo Pérez de la Cruz, Lucio Antonio Ramos Chávez, Jesús F. Valencia León, Joel Torres Beltrán, Erika Estrada Camarena, Paul Carillo Mora, Daniela Ramírez Ortega, José U. Baños Vázquez, Gabriela Martínez Nava, and et al. 2022. "Characterization of Redox Environment and Tryptophan Catabolism through Kynurenine Pathway in Military Divers’ and Swimmers’ Serum Samples" Antioxidants 11, no. 7: 1223. https://doi.org/10.3390/antiox11071223
APA StyleSánchez Chapul, L., Pérez de la Cruz, G., Ramos Chávez, L. A., Valencia León, J. F., Torres Beltrán, J., Estrada Camarena, E., Carillo Mora, P., Ramírez Ortega, D., Baños Vázquez, J. U., Martínez Nava, G., Luna Angulo, A., Martínez Canseco, C., Wences Chirino, T. Y., Ríos Martínez, J., & Pérez de la Cruz, V. (2022). Characterization of Redox Environment and Tryptophan Catabolism through Kynurenine Pathway in Military Divers’ and Swimmers’ Serum Samples. Antioxidants, 11(7), 1223. https://doi.org/10.3390/antiox11071223









