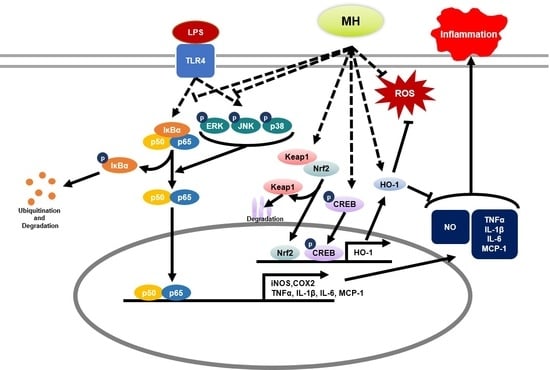
Menthae Herba Attenuates Neuroinflammation by Regulating CREB/Nrf2/HO-1 Pathway in BV2 Microglial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
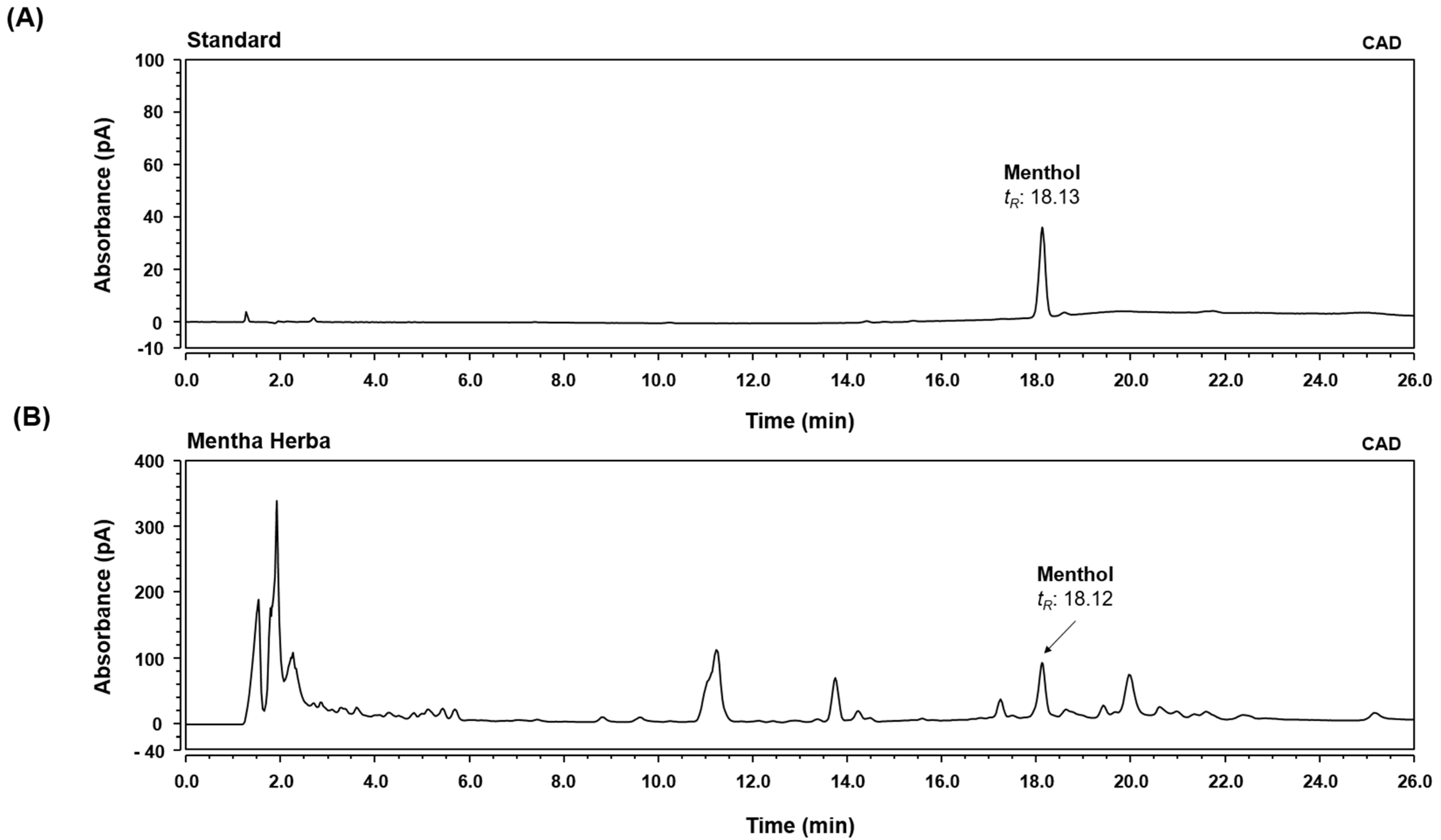
2.2. High-Performance Liquid Chromatography-Charged Aerosol Detector (HPLC-CAD) Analysis
2.3. Cell Culture and Drug Treatment
2.4. Cell Viability Test
2.5. Determination of NO Production
2.6. ELISA for the Analysis of Inflammatory Cytokine Secretion
2.7. Western Blotting for Protein Expression Measurement
2.8. RNA Isolation and RT-qPCR
2.9. Immunofluorescence
2.10. ROS Measurement
2.11. Statistical Analysis
3. Results
3.1. HPLC-CAD Analysis of MH
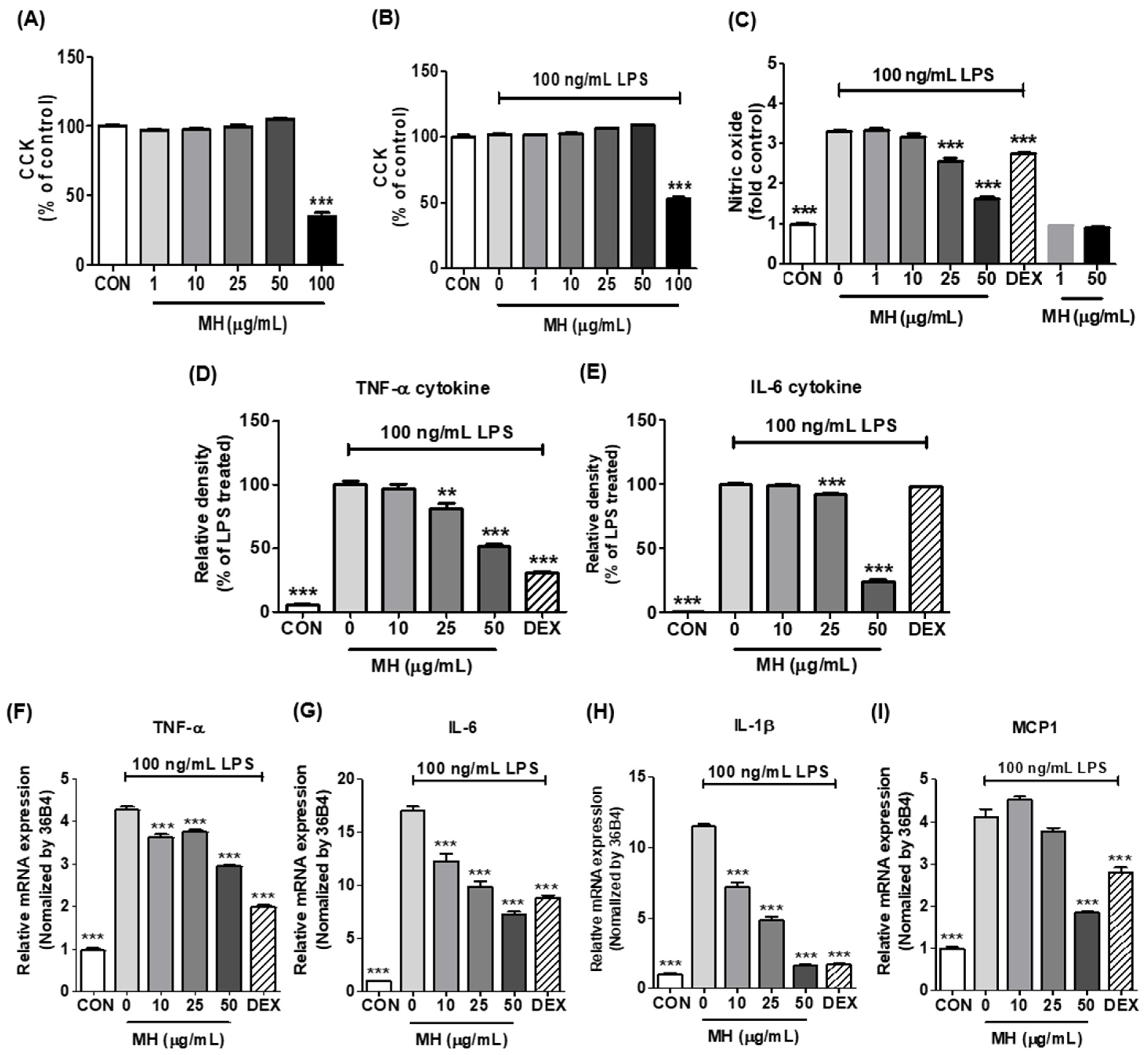
3.2. Effects of MH on Cell Viability of BV2 Cells
3.3. MH Reduces NO Production in LPS-Stimulated BV2 Cells
3.4. MH Decreases Inflammatory Cytokines and Chemokine in LPS-Stimulated BV2 Cells
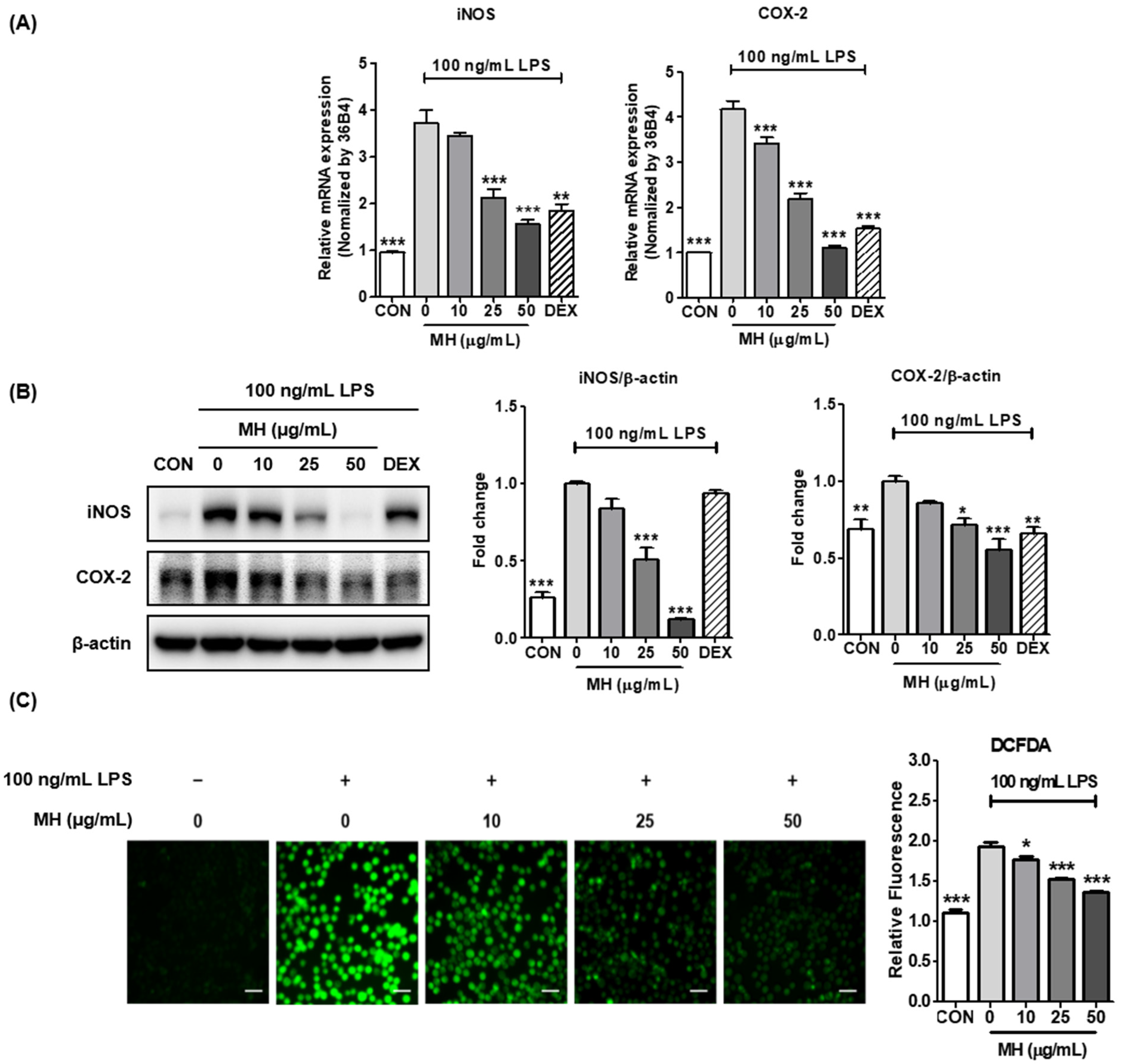
3.5. MH Represses LPS-Mediated Expression of iNOS and COX-2
3.6. MH Inhibits ROS Production in LPS-Treated BV2 Cells
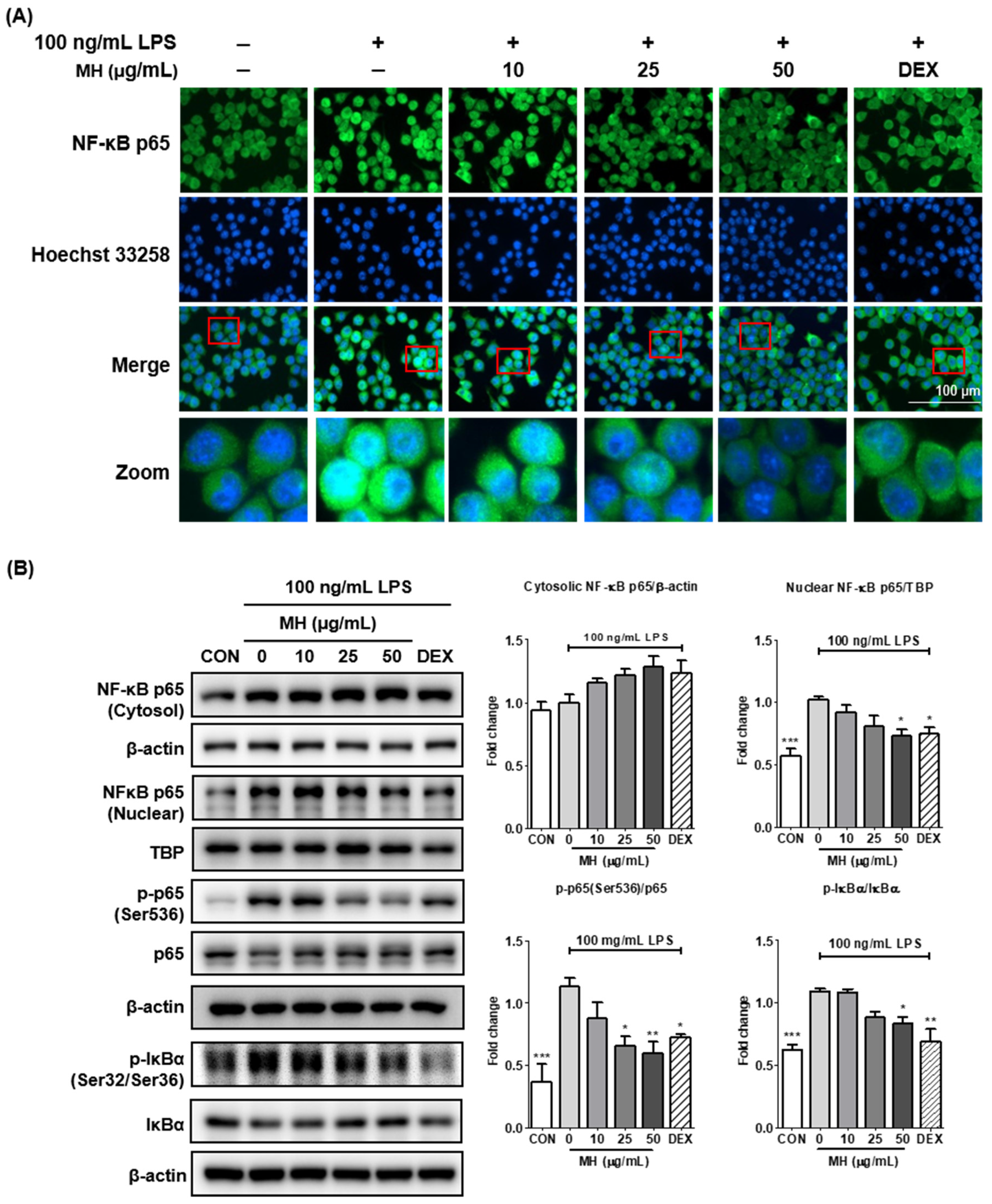
3.7. MH Suppresses the Transcriptional Activity of NF-κB
3.8. MH Inactivates MAPK Signaling
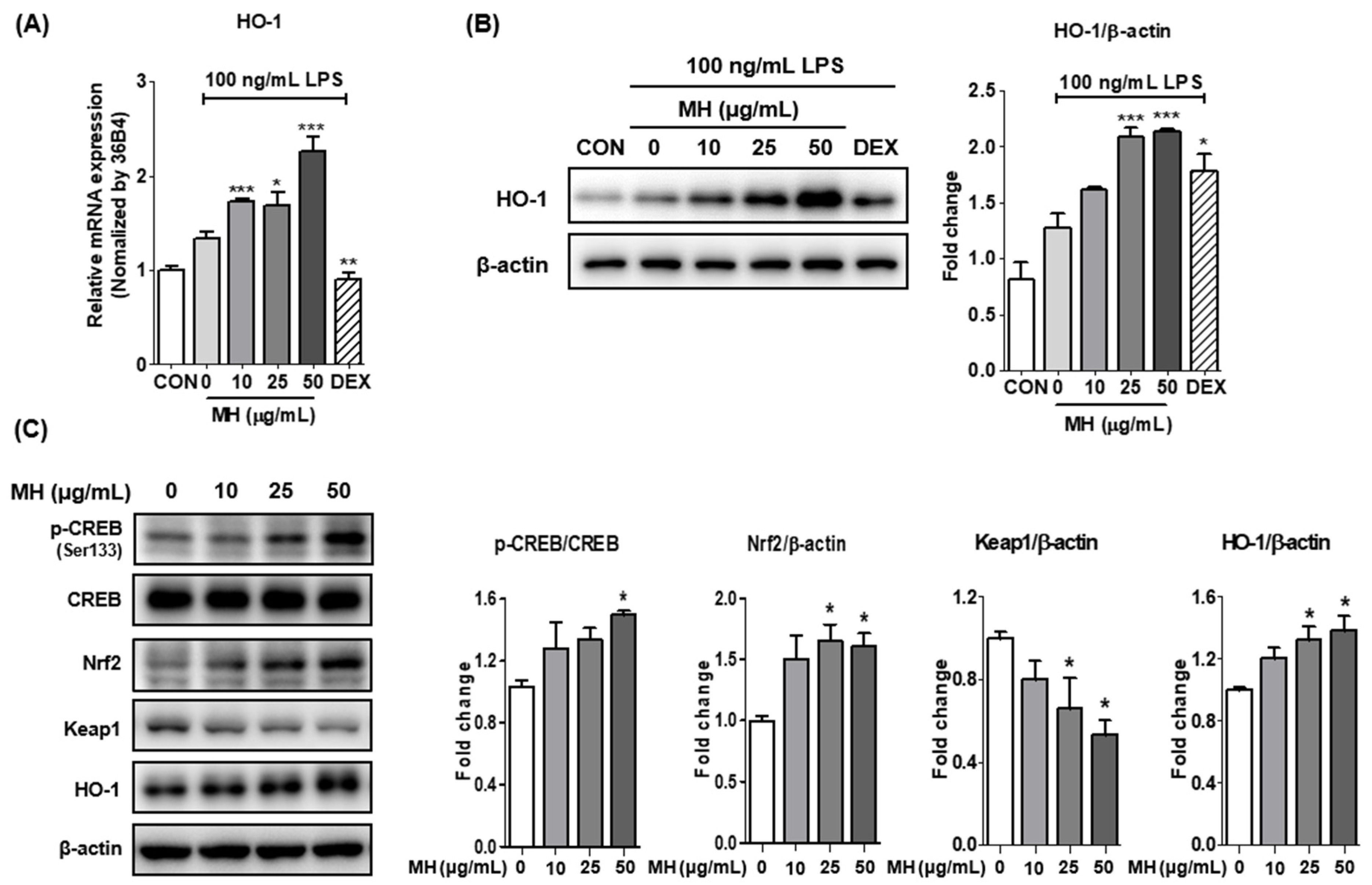
3.9. MH Increases HO-1 Expression through Nrf2/CREB Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dansokho, C.; Heneka, M.T. Neuroinflammatory responses in Alzheimer’s disease. J. Neural Transm. 2018, 125, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, Y.; Chen, C.; Wang, H.; Ai, Q.; Lin, M.; Zeng, Q.; Zhang, Y.; Gao, Y.; Li, X.; et al. The Anti-Neuroinflammatory Effect of Fuzi and Ganjiang Extraction on LPS-Induced BV2 Microglia and Its Intervention Function on Depression-Like Behavior of Cancer-Related Fatigue Model Mice. Front. Pharmacol. 2021, 12, 670586. [Google Scholar] [CrossRef] [PubMed]
- Kanegawa, N.; Collste, K.; Forsberg, A.; Schain, M.; Arakawa, R.; Jucaite, A.; Lekander, M.; Olgart Hoglund, C.; Kosek, E.; Lampa, J.; et al. In vivo evidence of a functional association between immune cells in blood and brain in healthy human subjects. Brain Behav. Immun. 2016, 54, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [Green Version]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s Disease, Inflammation, and the Role of Antioxidants. J. Alzheimers Dis. Rep. 2020, 4, 175–183. [Google Scholar] [CrossRef]
- Nakahira, K.; Takahashi, T.; Shimizu, H.; Maeshima, K.; Uehara, K.; Fujii, H.; Nakatsuka, H.; Yokoyama, M.; Akagi, R.; Morita, K. Protective role of heme oxygenase-1 induction in carbon tetrachloride-induced hepatotoxicity. Biochem. Pharmacol. 2003, 66, 1091–1105. [Google Scholar] [CrossRef] [Green Version]
- Siow, R.C.M.; Sato, H.; Mann, G.E. Heme oxygenase–carbon monoxide signalling pathway in atherosclerosis: Anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc. Res. 1999, 41, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Kim, C.-S.; Tu, T.H.; Kim, M.-S.; Goto, T.; Kawada, T.; Choi, M.-S.; Park, T.; Sung, M.-K.; Yun, J.W.; et al. Quercetin Protects Obesity-Induced Hypothalamic Inflammation by Reducing Microglia-Mediated Inflammatory Responses via HO-1 Induction. Nutrients 2017, 9, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.F.; Geng, C.A.; Huang, X.Y.; Ma, Y.B.; Zhang, X.M.; Chen, J.J. Chemical Constituents from Mentha haplocalyx Briq. (Mentha canadensis L.) and Their alpha-Glucosidase Inhibitory Activities. Nat. Prod. Bioprospect. 2019, 9, 223–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-H.; Seo, C.-S.; Shin, H.-K. Simultaneous Determination of (-)-Menthone and (-)-Menthol in Menthae Herba by Gas Chromatography and Principal Component Analysis. Nat. Prod. Sci. 2010, 16, 180–184. [Google Scholar]
- Cao, G.; Shan, Q.; Li, X.; Cong, X.; Zhang, Y.; Cai, H.; Cai, B. Analysis of fresh Mentha haplocalyx volatile components by comprehensive two-dimensional gas chromatography and high-resolution time-of-flight mass spectrometry. Analyst 2011, 136, 4653–4661. [Google Scholar] [CrossRef]
- She, G.; Xu, C.; Liu, B.; Shi, R. Two New Monoterpenes from Mentha haplocalyx Briq. Helv. Chim. Acta 2010, 93, 2495–2498. [Google Scholar] [CrossRef]
- Singh, A.; Raina, V.; Naqvi, A.; Patra, N.; Kumar, B.; Ram, P.; Khanuja, S. Essential oil composition and chemoarrays of menthol mint (Mentha arvensis L. F. piperascens Malinvaud ex. Holmes) cultivars. Flavour Fragr. J. 2005, 20, 302–305. [Google Scholar] [CrossRef]
- Shaikh, K.; Patil, S.D. Sensitive and selective method for the analysis of menthol from pharmaceutical products by RP-HPLC with refractive index detector. J. Pharm. Bioallied Sci. 2010, 2, 360. [Google Scholar] [CrossRef]
- Ganesan, P.; Kim, B.; Ramalingam, P.; Karthivashan, G.; Revuri, V.; Park, S.; Kim, J.S.; Ko, Y.T.; Choi, D.-K. Antineuroinflammatory Activities and Neurotoxicological Assessment of Curcumin Loaded Solid Lipid Nanoparticles on LPS-Stimulated BV-2 Microglia Cell Models. Molecules 2019, 24, 1170. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Cao, X.; Li, N.; Xu, Y.; Wu, Q.; Bai, J.; Yin, Z.; Luo, L.; Lan, L. Forsythin inhibits lipopolysaccharide-induced inflammation by suppressing JAK-STAT and p38 MAPK signalings and ROS production. Inflamm. Res. 2014, 63, 597–608. [Google Scholar] [CrossRef]
- Li, J.; Shui, X.; Sun, R.; Wan, L.; Zhang, B.; Xiao, B.; Luo, Z. Microglial Phenotypic Transition: Signaling Pathways and Influencing Modulators Involved in Regulation in Central Nervous System Diseases. Front. Cell. Neurosci. 2021, 15, 736710. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, S.; Xuan, Z.; Ge, D.; Chen, X.; Zhang, J.; Wang, Q.; Wu, Y.; Liu, B. The Phenolic Fraction of Mentha haplocalyx and Its Constituent Linarin Ameliorate Inflammatory Response through Inactivation of NF-κB and MAPKs in Lipopolysaccharide-Induced RAW264.7 Cells. Molecules 2017, 22, 811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-Y.; Lee, J.-A.; Seo, C.-S.; Ha, H.; Lee, N.-H.; Shin, H.-K. Protective Effects of Mentha haplocalyx Ethanol Extract (MH) in a Mouse Model of Allergic Asthma. Phytother. Res. 2011, 25, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Mitomo, K.; Watanabe, T.; Okamoto, S.; Yamamoto, K. Involvement of a NF-kappa B-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol. Cell. Biol. 1990, 10, 561–568. [Google Scholar] [PubMed] [Green Version]
- Morgan, M.J.; Liu, Z.-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huante-Mendoza, A.; Silva-García, O.; Oviedo-Boyso, J.; Hancock, R.; Baizabal Aguirre, V.M. Peptide IDR-1002 Inhibits NF-κB Nuclear Translocation by Inhibition of IκBα Degradation and Activates p38/ERK1/2–MSK1-Dependent CREB Phosphorylation in Macrophages Stimulated with Lipopolysaccharide. Front. Immunol. 2016, 7, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevtsova, E.F.; Vinogradova, D.V.; Neganova, M.E.; Avila-Rodriguez, M.; Ashraf, G.M.; Barreto, G.E.; Bachurin, S.O.; Aliev, G. Mitochondrial Permeability Transition Pore as a Suitable Targ e t for Neuroprotective Agents Against Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 677–685. [Google Scholar] [CrossRef]
- Alexiou, A.; Soursou, G.; Chatzichronis, S.; Gasparatos, E.; Kamal, M.A.; Yarla, N.S.; Perveen, A.; Barreto, G.E.; Ashraf, G.M. Role of GTPases in the Regulation of Mitochondrial Dynamics in Alzheimer’s Disease and CNS-Related Disorders. Mol. Neurobiol. 2019, 56, 4530–4538. [Google Scholar] [CrossRef]
- Nitti, M.; Piras, S.; Brondolo, L.; Marinari, U.M.; Pronzato, M.A.; Furfaro, A.L. Heme oxygenase 1 in the nervous system: Does it favor neuronal cell survival or induce neurodegeneration? Int. J. Mol. Sci. 2018, 19, 2260. [Google Scholar] [CrossRef] [Green Version]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Ko, H.-M.; Jeong, Y.-H.; Park, E.-M.; Kim, H.-S. β-Lapachone suppresses neuroinflammation by modulating the expression of cytokines and matrix metalloproteinases in activated microglia. J. Neuroinflamm. 2015, 12, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.-S.; Shin, J.A.; Park, E.-M.; Lee, J.-E.; Kang, Y.-S.; Min, S.-W.; Kim, D.-H.; Hyun, J.-W.; Shin, C.-Y.; Kim, H.-S. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: Critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J. Neurochem. 2010, 115, 1668–1680. [Google Scholar] [CrossRef]
- Mylroie, H.; Dumont, O.; Bauer, A.; Thornton, C.C.; Mackey, J.; Calay, D.; Hamdulay, S.S.; Choo, J.R.; Boyle, J.J.; Samarel, A.M.; et al. PKCε-CREB-Nrf2 signalling induces HO-1 in the vascular endothelium and enhances resistance to inflammation and apoptosis. Cardiovasc. Res. 2015, 106, 509–519. [Google Scholar] [CrossRef]
- Santo, S.G.E.; Romualdo, G.R.; Santos, L.A.d.; Grassi, T.F.; Barbisan, L.F. Modifying effects of menthol against benzo (a) pyrene-induced forestomach carcinogenesis in female Swiss mice. Environ. Toxicol. 2021, 36, 2245–2255. [Google Scholar] [CrossRef]
- Liu, Y.; Li, A.; Feng, X.; Jiang, X.; Sun, X.; Huang, W.; Zhu, X.; Zhao, Z. l-Menthol alleviates cigarette smoke extract induced lung injury in rats by inhibiting oxidative stress and inflammation via nuclear factor kappa B, p38 MAPK and Nrf2 signalling pathways. RSC Adv. 2018, 8, 9353–9363. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Liu, D.; Zhang, X.; Zhou, A.; Su, Y.; He, D.; Fu, S.; Gao, F. Menthol protects dopaminergic neurons against inflamma-tion-mediated damage in lipopolysaccharide (LPS)-Evoked model of Parkinson’s disease. Int. Immunopharmacol. 2020, 85, 106679. [Google Scholar] [CrossRef]

| Target Gene | Primer Sequence (5′-3′) | Gene Bank Accession Number |
|---|---|---|
| COX-2 | F: TGAGTACCGCAAACGCTTCTC R: TGGACGAGGTTTTTCCACCAG | NM_011198.4 |
| HO-1 | F: TGAAGGAGGCCACCAAGGAGG R: AGAGGTCACCCAGGTAGCGGG | NM_010442.2 |
| IL-1β | F: ATGGCAACTGTTCCTGAACTCAACT R: CAGGACAGGTATAGATTCTTTCCTTT | NM_008361.4 |
| IL-6 | F: TCCAGTTGCCTTCTTGGGAC R: GTGTAATTAAGCCTCCGACTTG | NM_001314054.1 |
| iNOS | F: GGCAGCCTGTGAGACCTTTG R: GCATTGGAAGTGAAGCGTTTC | NM_001313922.1 |
| MCP1 | F: ACCTGGATCGGAACCAAATG R: CCTTAGGGCAGATGCAGTTT | NM_011333.3 |
| TNF-α | F: TTCTGTCTACTGAACTTCGGGGTGATCGGTCC R: GTATGAGATAGCAAATCGGCTGACGGTGTGGG | NM_001278601.1 |
| 36B4 | F: ACCTCCTTCTTCCAGGCTTT R: CTCCAGTCTTTATCAGCTGC | NM_007475.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.J.; Yang, H.J.; Li, W.; Oh, Y.-C.; Go, Y. Menthae Herba Attenuates Neuroinflammation by Regulating CREB/Nrf2/HO-1 Pathway in BV2 Microglial Cells. Antioxidants 2022, 11, 649. https://doi.org/10.3390/antiox11040649
Park YJ, Yang HJ, Li W, Oh Y-C, Go Y. Menthae Herba Attenuates Neuroinflammation by Regulating CREB/Nrf2/HO-1 Pathway in BV2 Microglial Cells. Antioxidants. 2022; 11(4):649. https://doi.org/10.3390/antiox11040649
Chicago/Turabian StylePark, Yeo Jin, Hye Jin Yang, Wei Li, You-Chang Oh, and Younghoon Go. 2022. "Menthae Herba Attenuates Neuroinflammation by Regulating CREB/Nrf2/HO-1 Pathway in BV2 Microglial Cells" Antioxidants 11, no. 4: 649. https://doi.org/10.3390/antiox11040649
APA StylePark, Y. J., Yang, H. J., Li, W., Oh, Y.-C., & Go, Y. (2022). Menthae Herba Attenuates Neuroinflammation by Regulating CREB/Nrf2/HO-1 Pathway in BV2 Microglial Cells. Antioxidants, 11(4), 649. https://doi.org/10.3390/antiox11040649