Effects of Antioxidant Intake on Fetal Development and Maternal/Neonatal Health during Pregnancy
Abstract
:1. Introduction
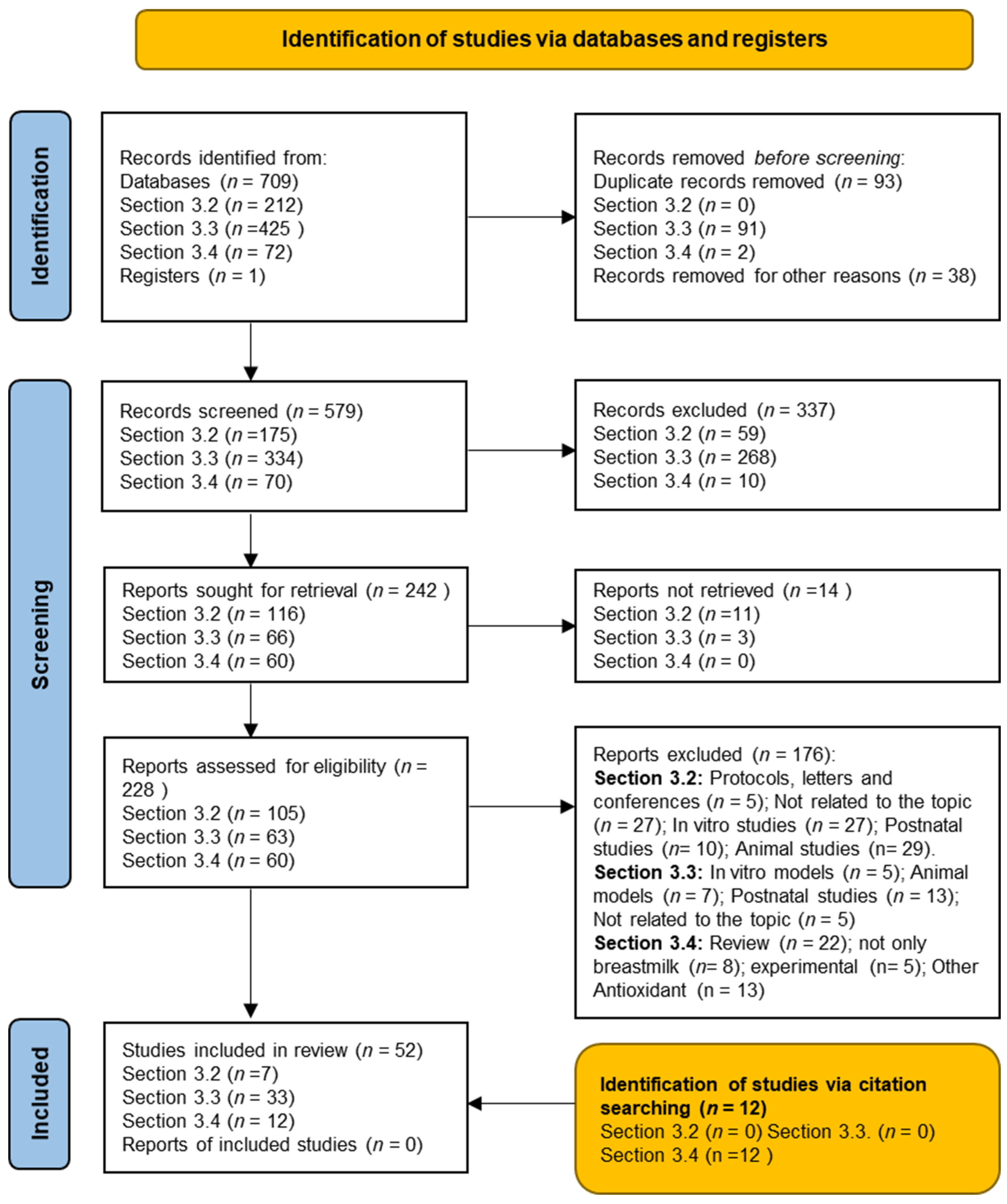
2. Materials and Methods
3. Results
3.1. Characteristics of the Studies Included
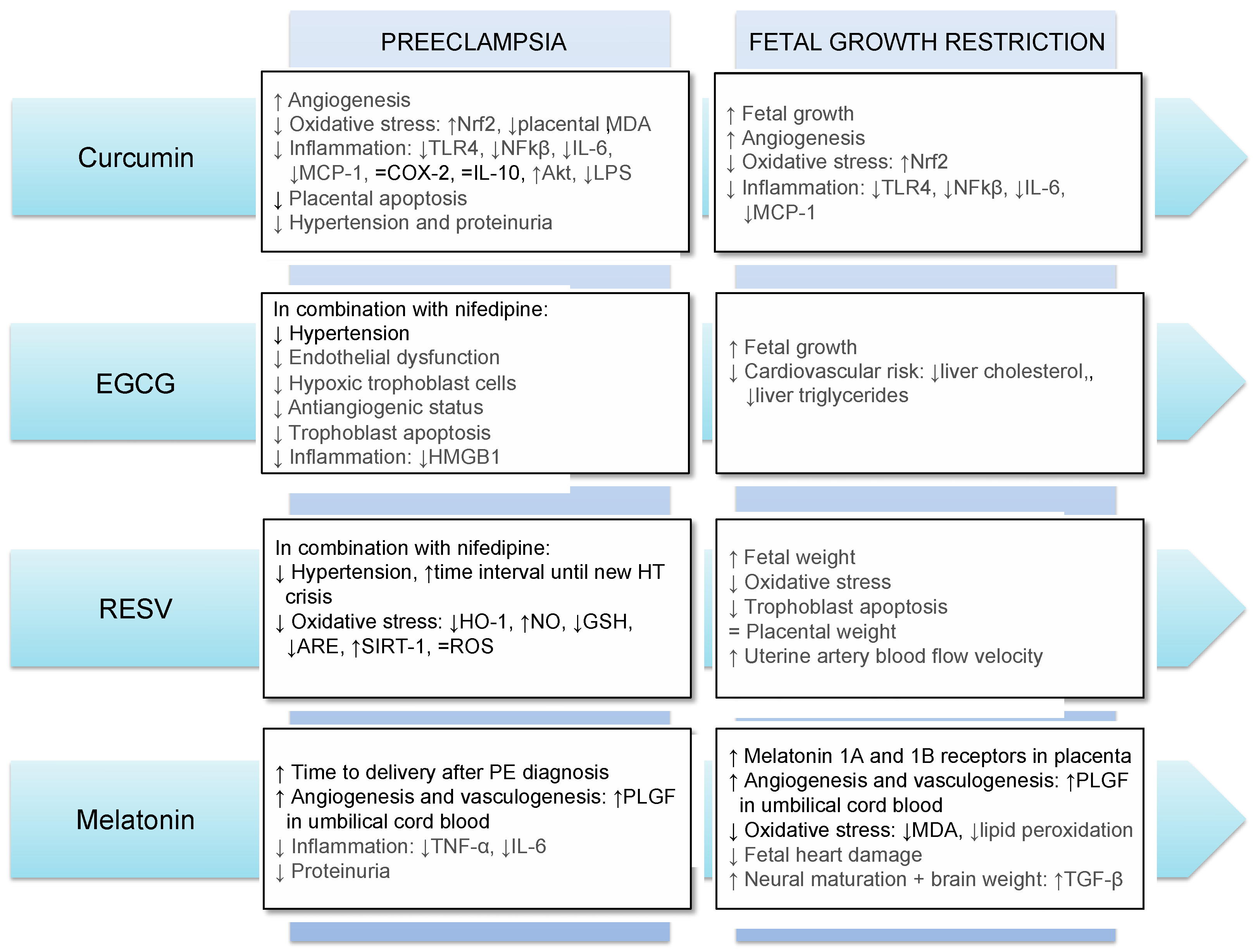
3.2. Antioxidants’ Use during Pregnancy: Effects on Preeclampsia and Fetal Growth Restriction
3.2.1. Curcumin
3.2.2. EGCG
3.2.3. Resveratrol
3.2.4. Melatonin
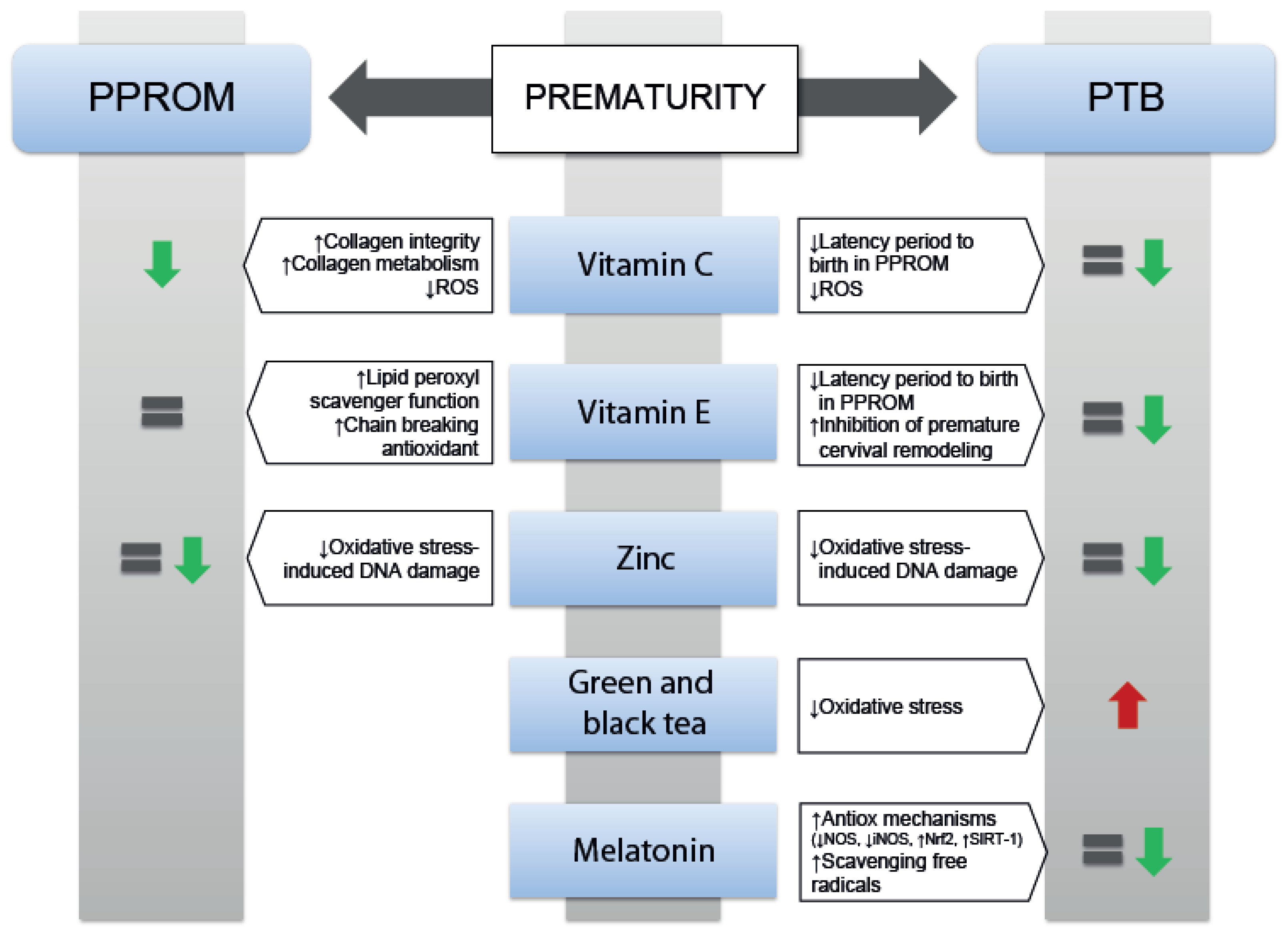
3.3. Effects of Antioxidants on Prematurity
3.3.1. Effects of Vitamin C and Vitamin E on Prematurity
3.3.2. Zinc Supplementation on Prematurity
3.3.3. Black and Green Tea
3.3.4. Use of Melatonin on Prematurity
3.4. Effects of Antioxidants in Human Milk and Neonatal Outcomes
3.4.1. Vitamin C and Vitamin E in Lactation
3.4.2. Selenium and Zinc
3.4.3. Use of Melatonin in Lactation
4. Conclusions
Supplementary Materials
Author Contributions
Funding

Conflicts of Interest
Abbreviations
| AGA | Appropriate for gestational age |
| Akt | Protein kinase B |
| ARE | Antioxidant Response Element |
| COX-2 | Cyclooxygenase-2 |
| EGCG | Epigallocatechin-3-gallate |
| FGR | Fetal growth restriction |
| GD | Gestational day |
| GRAS | Generally recognized as a safe |
| GSH | Glutathion |
| HMGB1 | High mobility group box 1 |
| HO-1 | Heme oxygenase-1 |
| HUVECs | Human umbilical vein endothelial cells |
| LP | Low-protein |
| LPS | Lipopolysaccharides |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDA | Malondialdehyde |
| NF-κB | Nuclear Factor κB |
| NO | Nitric oxide |
| NRF2 | NFE2-related factor-2 |
| PE | Preeclampsia |
| PLGF | Placental growth factor |
| PPROM | Preterm premature rupture of membranes |
| PTB | Preterm birth |
| RCT | Randomized controlled trial |
| RESV | Resveratrol |
| ROS | Reactive oxygen species |
| RUPP | Reduced uterine perfusion pressure |
| SGA | Small for gestational age |
| SIRT-1 | Sirtuin-1 |
| sPTB | Spontaneous PTB |
| TGF-β | Transforming growth factor-beta |
| TLR4 | Toll Like Receptor 4 |
| TNF-α | Tumor necrosis factor-alpha. |
| WHO | World health organization |
References
- Duley, L. The Global Impact of Pre-eclampsia and Eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Steegers, E.A.P.; Von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Ødegård, R.A.; Vatten, L.J.; Nilsen, S.T.; Salvesen, K.Å.; Austgulen, R. Preeclampsia and fetal growth. Obstet. Gynecol. 2000, 96, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Suhag, A.; Berghella, V. Intrauterine Growth Restriction (IUGR): Etiology and Diagnosis. Curr. Obstet. Gynecol. Rep. 2013, 2, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Fall, C.H.D. Fetal programming and the risk of noncommunicable disease. Indian J. Pediatr. 2013, 80 (Suppl. 1), S13–S20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garite, T.J.; Clark, R.; Thorp, J.A. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am. J. Obstet. Gynecol. 2004, 191, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef] [Green Version]
- Walani, S.R. Global burden of preterm birth. Int. J. Gynecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Cave, C.; Hanson, C.; Schumacher, M.; Lyden, E.; Furtado, J.; Obaro, S.; Delair, S.; Kocmich, N.; Rezac, A.; Izevbigie, N.I.; et al. A comparison of vitamin E status and associated pregnancy outcomes in maternal–infant dyads between a Nigerian and a United States population. Nutrients 2018, 10, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, D.M. Preterm birth. BMJ Clin. Evid. 2011, 2011, 1404. [Google Scholar]
- Hansson, S.R.; Nääv, Å.; Erlandsson, L. Oxidative stress in preeclampsia and the role of free fetal hemoglobin. Front. Physiol. 2015, 5, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guvendag Guven, E.S.; Karcaaltincaba, D.; Kandemir, O.; Kiykac, S.; Mentese, A. Cord blood oxidative stress markers correlate with umbilical artery pulsatility in fetal growth restriction. J. Matern. Neonatal Med. 2013, 26, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, Ö.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nikaido, T.; Toki, T.; Kita, N.; Kanai, M.; Ashida, T.; Ohira, S.; Konishi, I. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004, 444, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, N.; Celik, E.; Kumbak, B. Maternal plasma levels of interleukin-6, C-reactive protein, vitamins C, E and A, 8-isoprostane and oxidative status in women with preterm premature rupture of membranes. J. Matern. Neonatal Med. 2015, 28, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Zarban, A.; Taheri, F.; Chahkandi, T.; Sharifzadeh, G.; Khorashadizadeh, M. Antioxidant and radical scavenging activity of human colostrum, transitional and mature milk. J. Clin. Biochem. Nutr. 2009, 45, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Yuksel, S.; Yigit, A.A.; Cinar, M.; Atmaca, N.; Onaran, Y. Oxidant and antioxidant status of human breast milk during lactation period. Dairy Sci. Technol. 2015, 95, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; Debeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; De Vries, R.B.M.; Ritskes-Hoitinga, M.; Rovers, M.M.; Leeflang, M.M.; IntHout, J.; Wever, K.E.; Hooft, L.; de Beer, H.; Kuijpers, T.; et al. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS ONE 2018, 13, e0187271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, S.; Madan, K. The role of Safranal and saffron stigma extracts in oxidative stress, diseases and photoaging: A systematic review. Heliyon 2021, 7, e06117. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Vakili, S.; Akbari, M.; Mirhosseini, N.; Lankarani, K.B.; Rahimi, M.; Mobini, M.; Jafarnejad, S.; Vahedpoor, Z.; Asemi, Z. The effects of curcumin-containing supplements on biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Phyther. Res. 2019, 33, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Rahban, M.; Habibi-Rezaei, M.; Mazaheri, M.; Saso, L.; Moosavi-Movahedi, A.A. Anti-viral potential and modulation of nrf2 by curcumin: Pharmacological implications. Antioxidants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Basak, S.; Srinivas, V.; Mallepogu, A.; Duttaroy, A.K. Curcumin stimulates angiogenesis through VEGF and expression of HLA-G in first-trimester human placental trophoblasts. Cell Biol. Int. 2020, 44, 1237–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, L.; Jiang, J.; Zhang, J.; Zhang, L.; Wang, T. Curcumin protects human trophoblast HTR8/SVneo cells from H2O2-induced oxidative stress by activating nrf2 signaling pathway. Antioxidants 2020, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Fadinie, W.; Lelo, A.; Wijaya, D.W.; Lumbanraja, S.N. Curcumin’s effect on COX-2 and IL-10 serum in preeclampsia’s patient undergo sectio caesarea with spinal anesthesia. Open Access Maced. J. Med. Sci. 2019, 7, 3376–3379. [Google Scholar] [CrossRef]
- Sun, J.; Zhong, W.; Gu, Y.; Groome, L.J.; Wang, Y. 1,25(OH)2D3 suppresses COX-2 up-regulation and thromboxane production in placental trophoblast cells in response to hypoxic stimulation. Placenta 2014, 35, 143–145. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.; Liu, M.; Hong, G.; Li, Y.; Xue, P.; Zheng, M.; Wu, M.; Shen, L.; Yang, M.; Diao, Z.; et al. Curcumin improves LPS-induced preeclampsia-like phenotype in rat by inhibiting the TLR4 signaling pathway. Placenta 2016, 41, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Miao, H.; Li, X.; Hu, Y.; Sun, H.; Hou, Y. Curcumin inhibits placental inflammation to ameliorate LPS-induced adverse pregnancy outcomes in mice via upregulation of phosphorylated Akt. Inflamm. Res. 2017, 66, 177–185. [Google Scholar] [CrossRef]
- Qi, L.; Jiang, J.; Zhang, J.; Zhang, L.; Wang, T. Maternal curcumin supplementation ameliorates placental function and fetal growth in mice with intrauterine growth retardation. Biol. Reprod. 2020, 102, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.D.; Guo, J.J.; Zhou, L.; Wang, N. Epigallocatechin gallate enhances treatment efficacy of oral nifedipine against pregnancy-induced severe pre-eclampsia: A double-blind, randomized and placebo-controlled clinical study. J. Clin. Pharm. Ther. 2018, 43, 21–25. [Google Scholar] [CrossRef]
- Hobson, S.R.; Gurusinghe, S.; Lim, R.; Alers, N.O.; Miller, S.L.; Kingdom, J.C.; Wallace, E.M. Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early-onset preeclampsia. J. Pineal Res. 2018, 65, e12508. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Kang, Y.; Fan, Y.; Chen, Q. Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy-induced preeclampsia. Endocr. Connect. 2017, 6, 595–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldeira-Dias, M.; Montenegro, M.F.; Bettiol, H.; Barbieri, M.A.; Cardoso, V.C.; Cavalli, R.C.; Sandrim, V.C. Resveratrol improves endothelial cell markers impaired by plasma incubation from women who subsequently develop preeclampsia. Hypertens. Res. 2019, 42, 1166–1174. [Google Scholar] [CrossRef]
- Caldeira-Dias, M.; Viana-Mattioli, S.; de Souza Rangel Machado, J.; Carlström, M.; de Carvalho Cavalli, R.; Sandrim, V.C. Resveratrol and grape juice: Effects on redox status and nitric oxide production of endothelial cells in in vitro preeclampsia model. Pregnancy Hypertens. 2021, 23, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Yawno, T.; Alers, N.O.; Castillo-Melendez, M.; Supramaniam, V.G.; Vanzyl, N.; Sabaretnam, T.; Loose, J.M.; Drummond, G.R.; Walker, D.W.; et al. Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J. Pineal Res. 2014, 56, 283–294. [Google Scholar] [CrossRef]
- De la Torre, R.; de Sola, S.; Hernandez, G.; Farré, M.; Pujol, J.; Rodriguez, J.; Espadaler, J.M.; Langohr, K.; Cuenca-Royo, A.; Principe, A.; et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 801–810. [Google Scholar] [CrossRef]
- Quezada-Fernández, P.; Trujillo-Quiros, J.; Pascoe-González, S.; Trujillo-Rangel, W.A.; Cardona-Müller, D.; Ramos-Becerra, C.G.; Barocio-Pantoja, M.; Rodríguez-de la Cerda, M.; Nérida Sánchez-Rodríguez, E.; Cardona-Muñóz, E.G.; et al. Effect of green tea extract on arterial stiffness, lipid profile and sRAGE in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 2019, 70, 977–985. [Google Scholar] [CrossRef]
- Fernandes, R.C.; Araújo, V.A.; Giglio, B.M.; Mota, J.F.; Teixeira, K.I.S.S.; Monteiro, P.A.; Lira, F.S.; Pimentel, G.D. Acute epigallocatechin 3 gallate (EGCG) supplementation delays gastric emptying in healthy women: A randomized, double-blind, placebo-controlled crossover study. Nutrients 2018, 10, 1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef]
- Fernández, V.A.; Toledano, L.A.; Lozano, N.P.; Tapia, E.N.; Roig, M.D.G.; Fornell, R.D.L.T.; Algar, Ó.G. Bioavailability of epigallocatechin gallate administered with different nutritional strategies in healthy volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Su, S.; Yu, X.; Li, Y. Dietary epigallocatechin 3-gallate supplement improves maternal and neonatal treatment outcome of gestational diabetes mellitus: A double-blind randomised controlled trial. J. Hum. Nutr. Diet. 2017, 30, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Peng, J.; Xiang, L.; Yang, X.; Wang, X.; Zhu, Y. Epigallocatechin gallate (EGCG) improves anti-angiogenic state, cell viability, and hypoxia-induced endothelial dysfunction by downregulating high mobility group Box 1 (HMGB1) in preeclampsia. Med. Sci. Monit. 2020, 26, e926924. [Google Scholar] [CrossRef]
- Sava, R.I.; March, K.L.; Pepine, C.J. Hypertension in pregnancy: Taking cues from pathophysiology for clinical practice. Clin. Cardiol. 2018, 41, 220–227. [Google Scholar] [CrossRef]
- Bellos, I.; Karageorgiou, V.; Kapnias, D.; Karamanli, K.E.; Siristatidis, C. The role of interleukins in preeclampsia: A comprehensive review. Am. J. Reprod. Immunol. 2018, 80, e13055. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Toledano, L.; Andreu-Fernández, V.; Aras-López, R.; García-Algar, Ó.; Martínez, L.; Gómez-Roig, M.D. Epigallocatechin gallate ameliorates the effects of prenatal alcohol exposure in a fetal alcohol spectrum disorder-like mouse model. Int. J. Mol. Sci. 2021, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Wu, M.; Hu, X.H.; Wang, Y.F. Effect of epigallocatechin-3-gallate on liver lipid metabolism in rats with intrauterine growth restriction and related mechanism. Chin. J. Contemp. Pediatr. 2020, 22, 65–70. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, A.; Malhotra, V.K.; Agarwal, P.S.; Thirupuram, S.; Gaind, B. Cord blood lipid levels in low birth weight newborns. Indian Pediatr. 1989, 26, 571–574. [Google Scholar]
- Molina, S.M.; Casanueva, E.V.; Cid, C.X.; Ferrada, N.M.C.; Pérez, V.R.; Dios, T.G.; Reyes, R.M.; Venegas, B.H.; Cid, S.L. Lipid profile in newborns with intrauterine growth retardation. Rev. Med. Chile 2000, 128, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, V.H.J.; Pound, L.D.; Thorn, S.R.; Gillingham, M.B.; Thornburg, K.L.; Friedman, J.E.; Frias, A.E.; Grove, K.L. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 2014, 28, 2466–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourque, S.L.; Dolinsky, V.W.; Dyck, J.R.B.; Davidge, S.T. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta 2012, 33, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.B.; Punihaole, D.; Levine, T.B. Characterization of the role of nitric oxide and its clinical applications. Cardiology 2012, 122, 55–68. [Google Scholar] [CrossRef]
- George, E.M.; Granger, J.P. Heme oxygenase in pregnancy and preeclampsia. Curr. Opin. Nephrol. Hypertens. 2013, 22, 156–162. [Google Scholar] [CrossRef]
- Poudel, R.; Stanley, J.L.; Rueda-Clausen, C.F.; Andersson, I.J.; Sibley, C.P.; Davidge, S.T.; Baker, P.N. Effects of Resveratrol in Pregnancy Using Murine Models with Reduced Blood Supply to the Uterus. PLoS ONE 2013, 8, e64401. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Zuo, Q.; Huang, S.; Yu, X.; Jiang, Z.; Zou, S.; Fan, M.; Sun, L. Resveratrol inhibits trophoblast apoptosis through oxidative stress in preeclampsia-model rats. Molecules 2014, 19, 20570–20579. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Li, S.; Wu, D.; Xu, Y.; Wang, S.; Jiang, Y.; Liu, F.; Jiang, Z.; Qu, H.; Yu, X.; et al. Resveratrol promotes trophoblast invasion in pre-eclampsia by inducing epithelial-mesenchymal transition. J. Cell. Mol. Med. 2019, 23, 2702–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darby, J.R.T.; Saini, B.S.; Soo, J.Y.; Lock, M.C.; Holman, S.L.; Bradshaw, E.L.; McInnes, S.J.P.; Voelcker, N.H.; Macgowan, C.K.; Seed, M.; et al. Subcutaneous maternal resveratrol treatment increases uterine artery blood flow in the pregnant ewe and increases fetal but not cardiac growth. J. Physiol. 2019, 597, 5063–5077. [Google Scholar] [CrossRef]
- Rodrigues Helmo, F.; Etchebehere, R.M.; Bernardes, N.; Meirelles, M.F.; Galvão Petrini, C.; Penna Rocha, L.; Gonçalves dos Reis Monteiro, M.L.; Souza de Oliveira Guimarães, C.; de Paula Antunes Teixeira, V.; dos Reis, M.A.; et al. Melatonin treatment in fetal and neonatal diseases. Pathol. Res. Pract. 2018, 214, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Okamoto, K.; Hayashi, K.; Wakatsuki, A.; Tamura, S.; Sagara, Y. Maternal-fetal transfer of melatonin in pregnant women near term. J. Pineal Res. 1998, 25, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Lin, B.; Cheng, H.; Wang, C.; Zhao, M.; Zhang, J.; Wu, J. The reduction of melatonin levels is associated with the development of preeclampsia: A meta-analysis. Hypertens. Pregnancy 2019, 38, 65–72. [Google Scholar] [CrossRef]
- Berbets, A.M.; Davydenko, I.S.; Barbe, A.M.; Konkov, D.H.; Albota, O.M.; Yuzko, O.M. Melatonin 1A and 1B Receptors’ Expression Decreases in the Placenta of Women with Fetal Growth Restriction. Reprod. Sci. 2021, 28, 197–206. [Google Scholar] [CrossRef]
- Berbets, A.M.; Barbe, A.M.; Andriiets, O.A.; Andriiets, A.V.; Yuzko, O.M. Melatonin Levels Decrease in the Umbilical Cord in Case of Intrauterine Growth Restriction. J. Med. Life 2020, 13, 548–553. [Google Scholar] [CrossRef]
- Berbets, A.; Koval, H.; Barbe, A.; Albota, O.; Yuzko, O. Melatonin decreases and cytokines increase in women with placental insufficiency. J. Matern. Neonatal Med. 2021, 34, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Hobson, S.R.; Lim, R.; Gardiner, E.E.; Alers, N.O.; Wallace, E.M. Phase I pilot clinical trial of antenatal maternally administered melatonin to decrease the level of oxidative stress in human pregnancies affected by pre-eclampsia (PAMPR): Study protocol. BMJ Open 2013, 3, e003788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Malkey, N.F.; Aref, M.; Emam, H.; Khalil, S.S. Impact of Melatonin on Full-Term Fetal Brain Development and Transforming Growth Factor-β Level in a Rat Model of Preeclampsia. Reprod. Sci. 2021, 28, 2278–2291. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Jiang, Z. Melatonin attenuates hypertension and oxidative stress in a rat model of L-NAME-induced gestational hypertension. Vasc. Med. 2020, 25, 295–301. [Google Scholar] [CrossRef]
- Uzun, M.; Gencer, M.; Turkon, H.; Oztopuz, R.O.; Demir, U.; Ovali, M.A. Effects of Melatonin on Blood Pressure, Oxidative Stress and Placental Expressions of TNFα, IL-6, VEGF and sFlt-1 in RUPP Rat Model of Preeclampsia. Arch. Med. Res. 2017, 48, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Doğanlar, O.; Doğanlar, Z.B.; Ovali, M.A.; Güçlü, O.; Demir, U.; Doğan, A.; Uzun, M. Melatonin regulates oxidative stress and apoptosis in fetal hearts of pinealectomised RUPP rats. Hypertens. Pregnancy 2020, 39, 429–443. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N.; Lee, C. Te Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxid. Med. Cell. Longev. 2014, 2014, 283180. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Leu, S.; Wu, K.L.H.; Lee, W.C.; Chan, J.Y.H. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014, 57, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Shi, X.T.; Xu, X.F.; Zhou, G.X.; Xiong, Y.W.; Yi, S.J.; Liu, W.B.; Dai, L.M.; Cao, X.L.; Xu, D.X.; et al. Melatonin protects against environmental stress-induced fetal growth restriction via suppressing ROS-mediated GCN2/ATF4/BNIP3-dependent mitophagy in placental trophoblasts. Redox Biol. 2021, 40, 101854. [Google Scholar] [CrossRef]
- Tare, M.; Parkington, H.C.; Wallace, E.M.; Sutherland, A.E.; Lim, R.; Yawno, T.; Coleman, H.A.; Jenkin, G.; Miller, S.L. Maternal melatonin administration mitigates coronary stiffness and endothelial dysfunction, and improves heart resilience to insult in growth restricted lambs. J. Physiol. 2014, 592, 2695–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sales, F.; Peralta, O.A.; Narbona, E.; Mccoard, S.; González-Bulnes, A.; Parraguez, V.H. Rapid Communication: Maternal melatonin implants improve fetal oxygen supply and body weight at term in sheep pregnancies. J. Anim. Sci. 2019, 97, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Lemley, C.O.; Meyer, A.M.; Camacho, L.E.; Neville, T.L.; Newman, D.J.; Caton, J.S.; Vonnahme, K.A. Melatonin supplementation alters uteroplacental hemodynamics and fetal development in an ovine model of intrauterine growth restriction. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2012, 302, R454–R467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemley, C.O.; Camacho, L.E.; Meyer, A.M.; Kapphahn, M.; Caton, J.S.; Vonnahme, K.A. Dietary melatonin supplementation alters uteroplacental amino acid flux during intrauterine growth restriction in ewes. Animal 2013, 7, 1500–1507. [Google Scholar] [CrossRef] [Green Version]
- Aynaoglu Yildiz, G.; Yildiz, D.; Yapca, O.E.; Suleyman, B.; Arslan, Y.K.; Kurt, N.; Suleyman, H. Effect of diazepam, sertraline and melatonin on the stress-induced reproductive disorders and intrauterine growth restriction in female rats. J. Matern. Neonatal Med. 2021, 34, 4103–4109. [Google Scholar] [CrossRef]
- Castillo-Melendez, M.; Yawno, T.; Sutherland, A.; Jenkin, G.; Wallace, E.M.; Miller, S.L. Effects of Antenatal Melatonin Treatment on the Cerebral Vasculature in an Ovine Model of Fetal Growth Restriction. Dev. Neurosci. 2017, 39, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Polglase, G.R.; Barbuto, J.; Allison, B.J.; Yawno, T.; Sutherland, A.E.; Malhotra, A.; Schulze, K.E.; Wallace, E.M.; Jenkin, G.; Ricardo, S.D.; et al. Effects of antenatal melatonin therapy on lung structure in growth-restricted newborn lambs. J. Appl. Physiol. 2017, 123, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- González-Candia, A.; Veliz, M.; Araya, C.; Quezada, S.; Ebensperger, G.; Serón-Ferré, M.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Potential adverse effects of antenatal melatonin as a treatment for intrauterine growth restriction: Findings in pregnant sheep. Am. J. Obstet. Gynecol. 2016, 215, 245.e1–245.e7. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.R.; Mockler, J.C.; Davies-Tuck, M.L.; Miller, S.L.; Goergen, S.K.; Fahey, M.C.; Anderson, P.J.; Groom, K.M.; Wallace, E.M. Protect-me: A parallel-group, triple blinded, placebo-controlled randomised clinical trial protocol assessing antenatal maternal melatonin supplementation for fetal neuroprotection in early-onset fetal growth restriction. BMJ Open 2019, 9, e028243. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.S.; Goldenberg, R.L. Global burden of prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79. [Google Scholar] [CrossRef]
- Martin, C.L.; Sotres-Alvarez, D.; Siega-Riz, A.M. Maternal dietary patterns during the second trimester are associated with preterm birth. J. Nutr. 2015, 145, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Ghomian, N.; Hafizi, L.T.Z. The role of vitamin C in prevention of preterm premature rupture of membranes. Iran. Red Crescent Med. J. 2013, 15, 113–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Gaikwad, H.S.; Nath, B.; Batra, A. Can vitamin C and interleukin 6 levels predict preterm premature rupture of membranes: Evaluating possibilities in North Indian population. Obstet. Gynecol. Sci. 2020, 63, 432–439. [Google Scholar] [CrossRef]
- Sharma, R.; Mehta, S. Ascorbic Acid Concentration and Preterm Premature Rupture of Membranes. J. Obstet. Gynecol. India 2014, 64, 417–420. [Google Scholar] [CrossRef] [Green Version]
- Harville, E.W.; Lewis, C.E.; Catov, J.M.; Jacobs, D.R.; Gross, M.D.; Gunderson, E.P. A longitudinal study of pre-pregnancy antioxidant levels and subsequent perinatal outcomes in black and white women: The CARDIA study. PLoS ONE 2020, 15, e0229002. [Google Scholar] [CrossRef] [Green Version]
- Bártfai, L.; Bártfai, Z.; Nedeczky, I.; Puho, E.H.; Bánhidy, F.; Czeizel, A.E. Rate of preterm birth in pregnant women with vitamin e treatment: A population-based study. J. Matern. Neonatal Med. 2012, 25, 575–580. [Google Scholar] [CrossRef]
- Carmichael, S.; Yang, W.; Shaw, G. Maternal dietary nutrient intake and risk of preterm delivery. Am. J. Perinatol. 2013, 30, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Gungorduk, K.; Asicioglu, O.; Gungorduk, O.C.; Yildirim, G.; Besimoǧlu, B.; Ark, C. Does vitamin C and vitamin E supplementation prolong the latency period before delivery following the preterm premature rupture of membranes? A randomized controlled study. Am. J. Perinatol. 2014, 31, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Paknahad, Z.; Khoigani, M. The relationship between macro- and micro-nutrients intake and risk of preterm premature rupture of membranes in pregnant women of Isfahan. Adv. Biomed. Res. 2016, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Hauth, J.C.; Clifton, R.G.; Roberts, J.M.; Spong, C.Y.; Myatt, L.; Leveno, K.J.; Pearson, G.D.; Varner, M.W.; Thorp, J.M.; Mercer, B.M.; et al. Vitamin C and E supplementation to prevent spontaneous preterm birth: A randomized controlled trial. Obstet. Gynecol. 2010, 116, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Ilhan, N.; Aygun, B.K.; Gungor, H. The relationship between the latency period, infection markers, and oxidant and antioxidant states in women with preterm premature rupture of membranes. Ir. J. Med. Sci. 2017, 186, 965–970. [Google Scholar] [CrossRef]
- Koenig, M.D.; McFarlin, B.L.; Steffen, A.D.; Tussing-Humphreys, L.; Giurgescu, C.; Engeland, C.G.; Kominiarek, M.A.; Ciezczak-Karpiel, C.; O’Brien, W.D.; White-Traut, R. Decreased Nutrient Intake Is Associated With Premature Cervical Remodeling. JOGNN-J. Obstet. Gynecol. Neonatal Nurs. 2017, 46, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhou, H.; Perkins, A.; Wang, Y.; Sun, J. Maternal dietary nutrient intake and its association with preterm birth: A case-control study in Beijing, China. Nutrients 2017, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Charkamyani, F.; Khedmat, L.; Hosseinkhani, A. Decreasing the main maternal and fetal complications in women undergoing in vitro fertilization (IVF) trained by nutrition and healthy eating practices during pregnancy. J. Matern. Neonatal Med. 2021, 34, 1855–1867. [Google Scholar] [CrossRef]
- Nga, H.T.; Quyen, P.N.; Chaffee, B.W.; Diep Anh, N.T.; Ngu, T.; King, J.C. Effect of a nutrient-rich, food-based supplement given to rural Vietnamese mothers prior to and/or during pregnancy on birth outcomes: A randomized controlled trial. PLoS ONE 2020, 15, e0232197. [Google Scholar] [CrossRef] [PubMed]
- Nossier, S.A.; Naeim, N.E.; El-Sayed, N.A.; Abu Zeid, A.A. The effect of zinc supplementation on pregnancy outcomes: A double-blind, randomised controlled trial, Egypt. Br. J. Nutr. 2015, 114, 274–285. [Google Scholar] [CrossRef] [Green Version]
- Zahiri Sorouri, Z.; Sadeghi, H.; Pourmarzi, D. The effect of zinc supplementation on pregnancy outcome: A randomized controlled trial. J. Matern. Neonatal Med. 2016, 29, 2194–2198. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Belo, S.; Souteiro, P.; Neves, J.S.; Magalhães, D.; Silva, R.B.; Oliveira, S.C.; Freitas, P.; Varela, A.; Queirós, J.; et al. Pregnancy after bariatric surgery: Maternal and fetal outcomes of 39 pregnancies and a literature review. J. Obstet. Gynaecol. Res. 2018, 44, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Kucukaydin, Z.; Kurdoglu, M.; Kurdoglu, Z.; Demir, H.; Yoruk, I.H. Selected maternal, fetal and placental trace element and heavy metal and maternal vitamin levels in preterm deliveries with or without preterm premature rupture of membranes. J. Obstet. Gynaecol. Res. 2018, 44, 880–889. [Google Scholar] [CrossRef]
- Shen, P.J.; Gong, B.; Xu, F.Y.; Luo, Y. Four trace elements in pregnant women and their relationships with adverse pregnancy outcomes. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4690–4697. [Google Scholar] [PubMed]
- Chen, L.W.; Fitzgerald, R.; Murrin, C.M.; Mehegan, J.; Kelleher, C.C.; Phillips, C.M. Associations of maternal caffeine intake with birth outcomes: Results from the Lifeways Cross Generation Cohort Study. Am. J. Clin. Nutr. 2018, 108, 1301–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Lerro, C.; Yang, T.; Li, J.; Qiu, J.; Qiu, W.; He, X.; Cui, H.; Lv, L.; Xu, R.; et al. Maternal tea consumption and the risk of preterm delivery in urban China: A birth cohort study. BMC Public Health 2016, 16, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.H.; He, J.R.; Shen, S.Y.; Wei, X.L.; Chen, N.N.; Yuan, M.Y.; Qiu, L.; Li, W.D.; Chen, Q.Z.; Hu, C.Y.; et al. Does tea consumption during early pregnancy have an adverse effect on birth outcomes? Birth 2017, 44, 281–289. [Google Scholar] [CrossRef]
- Okubo, H.; Miyake, Y.; Tanaka, K.; Sasaki, S.; Hirota, Y. Maternal total caffeine intake, mainly from Japanese and Chinese tea, during pregnancy was associated with risk of preterm birth: The Osaka Maternal and Child Health Study. Nutr. Res. 2015, 35, 309–316. [Google Scholar] [CrossRef]
- Moussally, K.; Bérard, A. Exposure to herbal products during pregnancy and the risk of preterm birth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 150, 107–108. [Google Scholar] [CrossRef]
- Sengpiel, V.; Elind, E.; Bacelis, J.; Nilsson, S.; Grove, J.; Myhre, R.; Haugen, M.; Meltzer, H.M.; Alexander, J.; Jacobsson, B.; et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: Results from a large prospective observational cohort study. BMC Med. 2013, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Sindiani, A.M.; Khader, Y.; Amarin, Z. The association between coffee and tea consumption during pregnancy and preterm delivery: Case–control study. J. Multidiscip. Healthc. 2020, 13, 2011–2019. [Google Scholar] [CrossRef]
- Biran, V.; Decobert, F.; Bednarek, N.; Boizeau, P.; Benoist, J.F.; Claustrat, B.; Barré, J.; Colella, M.; Frérot, A.; Garnotel, R.; et al. Melatonin levels in preterm and term infants and their mothers. Int. J. Mol. Sci. 2019, 20, 2077. [Google Scholar] [CrossRef] [Green Version]
- Domínguez Rubio, A.P.; Sordelli, M.S.; Salazar, A.I.; Aisemberg, J.; Bariani, M.V.; Cella, M.; Rosenstein, R.E.; Franchi, A.M. Melatonin prevents experimental preterm labor and increases offspring survival. J. Pineal Res. 2014, 56, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Song, H.; Dash, O.; Park, M.; Shin, N.E.; McLane, M.W.; Lei, J.; Hwang, J.Y.; Burd, I. Administration of melatonin for prevention of preterm birth and fetal brain injury associated with premature birth in a mouse model. Am. J. Reprod. Immunol. 2019, 82, e13151. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Cortijo, D.; de la Calle, M.; Rodríguez-Rodríguez, P.; López de Pablo, Á.L.; López-Giménez, M.R.; Aguilera, Y.; Martín-Cabrejas, M.A.; González, M.D.C.; Arribas, S.M. Maternal antioxidant status in early pregnancy and development of fetal complications in twin pregnancies: A pilot study. Antioxidants 2020, 9, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Specht, I.O.; Hammer, P.E.C.; Flachs, E.M.; Begtrup, L.M.; Larsen, A.D.; Hougaard, K.S.; Hansen, J.; Hansen, Å.M.; Kolstad, H.A.; Rugulies, R.; et al. Night work during pregnancy and preterm birth—A large register-based cohort study. PLoS ONE 2019, 14, e0215748. [Google Scholar] [CrossRef] [PubMed]
- Robins, J.C.; Marsit, C.J.; Padbury, J.F.; Sharma, S.S. Endocrine disruptors, environmental oxygen, epigenetics and pregnancy. Front. Biosci.-Elit. Ed. 2011, 3, 690–700. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Cindrova-Davies, T.; Yung, H.W.; Jauniaux, E. Hypoxia and reproductive health: Oxygen and development of the human placenta. Reproduction 2021, 161, F53–F65. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, S.; Plösch, T.; Teller, I.C. Antioxidative molecules in human milk and environmental contaminants. Antioxidants 2021, 10, 550. [Google Scholar] [CrossRef]
- Hoppu, U.; Rinne, M.; Salo-Väänänen, P.; Lampi, A.M.; Piironen, V.; Isolauri, E. Vitamin C in breast milk may reduce the risk of atopy in the infant. Eur. J. Clin. Nutr. 2005, 59, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zarban, A.; Toroghi, M.M.; Asli, M.; Jafari, M.; Vejdan, M.; Sharifzadeh, G. Effect of vitamin C and e supplementation on total antioxidant content of human breastmilk and infant Urine. Breastfeed. Med. 2015, 10, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Daneel-Otterbech, S.; Davidsson, L.; Hurrell, R. Ascorbic acid supplementation and regular consumption of fresh orange juice increase the ascorbic acid content of human milk: Studies in European and African lactating women. Am. J. Clin. Nutr. 2005, 81, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Friel, J.K.; Diehl-Jones, W.L.; Suh, M.; Tsopmo, A.; Shirwadkar, V.P. Impact of iron and vitamin C-containing supplements on preterm human milk: In vitro. Free Radic. Biol. Med. 2007, 42, 1591–1598. [Google Scholar] [CrossRef]
- Da Silva, A.L.C.; Da Silva Ribeiro, K.D.; De Melo, L.R.M.; Bezerra, D.F.; De Queiroz, J.L.C.; Lima, M.S.R.; Pires, J.F.; Bezerra, D.S.; Osório, M.M.; Dimenstein, R. Vitamin E in human milk and its relation to the nutritional requirement of the term newborn. Rev. Paul. Pediatr. 2017, 35, 158–164. [Google Scholar] [CrossRef] [Green Version]
- De Melo, L.R.M.; Clemente, H.A.; Bezerra, D.F.; Dantas, R.C.S.; Ramalho, H.M.M.; Dimenstein, R. Effect of maternal supplementation with vitamin E on the concentration of α-tocopherol in colostrum. J. Pediatr. 2017, 93, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires Medeiros, J.F.; Ribeiro, K.D.D.S.; Lima, M.S.R.; Das Neves, R.A.M.; Lima, A.C.P.; Dantas, R.C.S.; Da Silva, A.B.; Dimenstein, R. α-Tocopherol in breast milk of women with preterm delivery after a single postpartum oral dose of vitamin e. Br. J. Nutr. 2016, 115, 1424–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keikha, M.; Shayan-Moghadam, R.; Bahreynian, M.; Kelishadi, R. Nutritional supplements and mother’s milk composition: A systematic review of interventional studies. Int. Breastfeed. J. 2021, 16, 1. [Google Scholar] [CrossRef]
- Darlow, B.A.; Austin, N. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst. Rev. 2003, 2003, CD003312. [Google Scholar] [CrossRef] [PubMed]
- Strambi, M.; Longini, M.; Vezzosi, P.; Berni, S.; Buoni, S. Selenium status, birth weight, and breast-feeding: Pattern in the first month. Biol. Trace Elem. Res. 2004, 99, 71–81. [Google Scholar] [CrossRef]
- Shaaban, S.Y.; El-Hodhod, M.A.A.; Nassar, M.F.; Hegazy, A.E.T.; El-Arab, S.E.; Shaheen, F.M. Zinc status of lactating Egyptian mothers and their infants: Effect of maternal zinc supplementation. Nutr. Res. 2005, 25, 45–53. [Google Scholar] [CrossRef]
- Loui, A.; Raab, A.; Wagner, M.; Weigel, H.; Grüters-Kieslich, A.; Brätter, P.; Obladen, M. Nutrition of very low birth weight infants fed human milk with or without supplemental trace elements: A randomized controlled trial. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 346–353. [Google Scholar] [CrossRef]
- Sánchez-Barceló, E.J.; Mediavilla, M.D.; Reiter, R.J. Clinical Uses of Melatonin in Pediatrics. Int. J. Pediatr. 2011, 2011, 892624. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Shi, W.; Zhuang, J.; Liu, Y.; Tang, L.; Bu, J.; Sun, J.; Bei, F. Variations in melatonin levels in preterm and term human breast milk during the first month after delivery. Sci. Rep. 2019, 9, 17984. [Google Scholar] [CrossRef]
- Honorio-França, A.C.; Hara, C.C.P.; Ormonde, J.V.S.; Nunes, G.T.; França, E.L. Human colostrum melatonin exhibits a day-night variation and modulates the activity of colostral phagocytes. J. Appl. Biomed. 2013, 11, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Clemente, H.A.; Ramalho, H.M.M.; Lima, M.S.R.; Grilo, E.C.; Dimenstein, R. Maternal supplementation with natural or synthetic vitamin E and its levels in human colostrum. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Simon-szabo, Z.; Fogarasi, E.; Nemes-Nagy, E.; Denes, L.; Croitoru, M.; Szabo, B. Oxidative stress and peripartum outcomes (Review). Exp. Ther. Med. 2021, 22, 771. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-κB is suppressed by curcumin (diferulolylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef] [Green Version]
- Walsh, S.W. Preeclampsia: An imbalance in placental prostacyclin and thromboxane Production. Am. J. Obstet. Gynecol. 1985, 152, 335–340. [Google Scholar] [CrossRef]
- Cas, M.D.; Ghidoni, R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [Green Version]
- Guillier, C.; Carrière, D.; Pansiot, J.; Maroni, A.; Billion, E.; Ringot, M.; Benoist, J.F.; Jacques, S.; Matrot, B.; Jarreau, P.H.; et al. Nebulized curcumin protects neonatal lungs from antenatal insult in rats. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 321, L545–L552. [Google Scholar] [CrossRef]
- Der Hsuuw, Y.; Chang, C.K.; Chan, W.H.; Yu, J.S. Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts. J. Cell. Physiol. 2005, 205, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ganiger, S.; Malleshappa, H.N.; Krishnappa, H.; Rajashekhar, G.; Ramakrishna Rao, V.; Sullivan, F. A two generation reproductive toxicity study with curcumin, turmeric yellow, in Wistar rats. Food Chem. Toxicol. 2007, 45, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Malvasi, A.; Kosmas, I.; Mynbaev, O.A.; Sparic, R.; Gustapane, S.; Guido, M.; Tinelli, A. Can trans resveratrol plus d-chiro-inositol and myo-inositol improve maternal metabolic profile in overweight pregnant patients? Clin. Ter. 2017, 168, e240–e247. [Google Scholar] [CrossRef] [PubMed]
- Viana-Mattioli, S.; Cinegaglia, N.; Bertozzi-Matheus, M.; Bueno-Pereira, T.O.; Caldeira-Dias, M.; Cavalli, R.C.; Sandrim, V.C. SIRT1-dependent effects of resveratrol and grape juice in an in vitro model of preeclampsia. Biomed. Pharmacother. 2020, 131, 110659. [Google Scholar] [CrossRef]
- Dai, B.; Liu, T.; Zhang, B.; Zhang, X.; Wang, Z. The polymorphism for endothelial nitric oxide synthase gene, the level of nitric oxide and the risk for pre-eclampsia: A meta-analysis. Gene 2013, 519, 187–193. [Google Scholar] [CrossRef]
- Cui, L.; Xu, F.; Wang, S.; Jiang, Z.; Liu, L.; Ding, Y.; Sun, X.; Du, M. Melatonin-MT1 signal is essential for endometrial decidualization. Reproduction 2021, 162, 161–170. [Google Scholar] [CrossRef]
- Von Dadelszen, P.; Ornstein, M.P.; Bull, S.B.; Logan, A.G.; Koren, G.; Magee, L.A. Fail in mean arterial pressure and fetal growth restriction in pregnancy hypertension: A meta-analysis. Lancet 2000, 355, 87–92. [Google Scholar] [CrossRef]
- Ottosen, L.D.M.; Hindkjær, J.; Husth, M.; Petersen, D.E.; Kirk, J.; Ingerslev, H.J. Observations on intrauterine oxygen tension measured by fibre-optic microsensors. Reprod. Biomed. Online 2006, 13, 380–385. [Google Scholar] [CrossRef]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [Green Version]
- Shkayeva, M.; Gregory, P.; Pickering, M.; Hein, D.; Hu, J.; Rodriguez, A. Green Tea Product Epigallocatechin Gallate (EGCG) Content and Label Information: A Descriptive Analysis. J. Nutr. Ther. 2015, 4, 81–84. [Google Scholar] [CrossRef]
- Rossi, D.; Guerrini, A.; Bruni, R.; Brognara, E.; Borgatti, M.; Gambari, R.; Maietti, S.; Sacchetti, G. Trans-resveratrol in nutraceuticals: Issues in retail quality and effectiveness. Molecules 2012, 17, 12393–12405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraloglu, O.; Engin-Ustun, Y.; Tonguç, E.; Var, T.; Tapisiz, Ö.L.; Ergün, H.; Guvenc, T.; Gacar, A. The effect of resveratrol on blood pressure in a rat model of preeclampsia. J. Matern. Neonatal Med. 2012, 25, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Renshall, L.J.; Morgan, H.L.; Moens, H.; Cansfield, D.; Finn-Sell, S.L.; Tropea, T.; Cottrell, E.C.; Greenwood, S.; Sibley, C.P.; Wareing, M.; et al. Melatonin increases fetal weight in wild-type mice but not in mouse models of fetal growth restriction. Front. Physiol. 2018, 9, 1141. [Google Scholar] [CrossRef] [PubMed]
- Moallem, S.A.; Afshar, M.; Etemad, L.; Razavi, B.M.; Hosseinzadeh, H. Evaluation of teratogenic effects of crocin and safranal, active ingredients of saffron, in mice. Toxicol. Ind. Health 2016, 32, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Strakovsky, R.S.; Pan, Y.X. In utero oxidative stress epigenetically programs antioxidant defense capacity and adulthood diseases. Antioxid. Redox Signal. 2012, 17, 237–253. [Google Scholar] [CrossRef] [PubMed]
| Antioxidant | Author (Year)/Country | Objectives | Study Design | Population | Gestational Age | Dose/Intervention Period | Variables Studied | Key Results | Conclusion | Quality of Evidence |
|---|---|---|---|---|---|---|---|---|---|---|
| Preeclampsia | ||||||||||
| Curcumin | Fadinie et al. (2019) [28]/Indonesia | To determine the effect of perioperative curcumin administration on COX-2 and IL-10. | DBRCT | PEP undergoing CS. Age: 19–40 y PG (n = 23) TG (n = 24) | Full term. | 100 mg/day 12 h before C-section. | COX-2 and IL-10 levels in serum at 90 min and 12 h. | NS differences. | Curcumin does not change COX-2 or IL-10 levels after preoperational administration. | Low ++/++++ |
| EGCG | Shi et al. (2018) [33]/China | To determine the effect of coadministration of EGCG and nifedipine on PE. | DBRCT | Severe PEP Age: 25–35 y PG (n = 156) TG (n = 148) | ~37 ± 1.5 w | 100 mg/capsule up to 5 dosages (≥98% purity)/every 15 min until normalization of BP. | Time needed to control BP. Time to new hypertensive crisis. Number of treatment doses used. | Significantly less time to control blood pressure in TG vs. PG (31.2 ± 16.7 min vs. 45.3 ± 21.9 min) Time between hypertensive crises significantly longer in the TG group (7.2 ± 2.9 h vs. 4.1 ± 3.7 h). Use of EGCG decreased the number of doses required to control BP. | EGCG potentiates the efficacy of nifedipine against severe PE | High ++++/++++ |
| Melatonin | Hobson et al. (2018) [34]/Australia | To evaluate the safety and efficacy of melatonin on PE clinical outcomes. | Open label, phase I single arm clinical trial. | 20 women with preterm PE. 48 HCC | ~32 ± 1 w | Melatonin tablets (10 mg) + vitamin B6 (10 mg)/3 times daily; from recruitment until delivery. | Maternal and perinatal safety. Prolongation of pregnancy. PE biomarkers. | Significant increase of the interval from diagnosis to delivery (6 ± 2.3 days) vs. HCC. NS differences in average mean arterial BP between groups. Melatonin significantly decreased antihypertensive medication. No disturbance in sleep patterns. | Melatonin is safe for newborns and their mothers and could provide effective adjuvant therapy to extend pregnancy duration. | Low ++/++++ |
| Resveratrol | Ding et al. (2017) [35]/China | To determine the effect of coadministration of RESV and nifedipine on PE. | DBRCT | Severe PEP Age: 21–32 y PG (n = 175) TG (n = 174) | ~34 ± 3.5 w | 50 mg/capsule up to 5 dosages/ every 15 min until normalization of BP. | Time needed to control BP.Time to new hypertensive crisis. Number of treatment doses used. | Significantly less time to control blood pressure in TG vs. PG (35.6 ± 18.7 min vs. 51.1 ± 22.4 min). Time between hypertensive crises was significantly longer in the TG group (8 ± 2.1 h vs. 5.5 ± 1.8 h). Use of RESV significantly decreased the number of doses required to control BP. | RESV potentiates the efficacy of nifedipine against severe PE. | High ++++/++++ |
| Caldeira-Dias et al. (2019) [36]/Brasil | To study the effects of serum from PE women on antioxidant defenses and vasodilator factor in HUVECs cells. | Observational | Severe PEP (n = 6). Healthy women (n = 6). | ~28 ± 4 w | No intervention, RESV was added to serum from PE and healthy patients. | ARE activation HO-1, GSH and NO levels Intracellular ROS levels. | NS differences in ARE activation in PE group. RESV decreased HO-1 levels by 15% in the PE group. RESV increased GSH and nitrite levels in the PE group. NS differences in ROS levels. | RESV could prevent alterations in HO-1 and NO markers in endothelial cells. | Very Low +/++++ | |
| Grape juice | Caldeira-Dias et al. (2021) [37]/Brasil | To compare theeffects of serum incubation in endothelial cells from PE women before and after 1 h of red grapefruit juice ingestion. | Pilot Phase I single-arm open-label clinical trial. | PEP (n = 4) | ~25 ± 3 w | 200 mL of organic whole grape juice. | Redox status and NOProduction. | Significant decrease of HO-1 and GSH levels (~17% and ~50%) in HUVEC cells compared to serum prior to juice ingestion. Significant decrease of ARE activity (~69%). Significant increase in NO levels. NS differences in ROS levels. | The biologically active molecules in grape juice restore the physiological NO balance of the endothelium. | Very Low +/++++ |
| Fetal growth restriction | ||||||||||
| Melatonin | Miller et al. (2014) [38]/Australia | To evaluate MLT as neuroprotectant. | Pilot Phase I single-arm open-label. | 12 women with severe early onset IFGR. CG (n = 6) TG (n = 6) | ~26 ± 1 w | MLT tablets (4 mg), twice daily, from recruitment until delivery. | Placental concentration of MDA. | Significantly higher levels of umbilical arterial MLT in TG vs. CG (6501 vs. 21 pg/mL). Significantly lower levels of MDA in the placenta in TG (2.4 vs. 4.6 nmol/mg tissue). | Antenatal MLT treatment reduces fetoplacental oxidative stress. | Low ++/++++ |
| Antioxidant | Author (Year)/Country | Objectives | Study Design | Population | Dose/Intervention Period | Variables Studied | Key Results | Conclusion | Quality of Evidence |
|---|---|---|---|---|---|---|---|---|---|
| Vitamin C | Martin et al. (2015), [85]/USA | To analyze the association between vitamin C intake and risk of PTB. | Prospective cohort study. | 3143 pregnant women at 26–29 GWs. | FFQ for dietary information collection. | PTB before 37 GWs. | OR of PTB 1.55, 95% CI: 1.07–2.24 in pregnant women with high consumption of vitamin C-rich drinks. | Type of diet during pregnancy, including high consumption of vitamin C-rich products is associated with PTB. | Low ++/++++ |
| Ghomian et al. (2013) [86]/Iran | To study the association of vitamin C supplementation with the risk of PPROM in women with previous PPROM. | Clinical trial | 170 singletons pregnancies at 14 GWs in women with previous PPROM. (85 controls/85 cases) | Cases: 100 mg vitamin C daily from 14 GWs. Controls: chewing a placebo tablet from 14 GWs. | Incidence of PPROM in pregnant women with history of previous PPROM. | PPROM in the control group 38 (44.7%) and 27 (31.8%) in the vitamin C supplementation group (p < 0.001). | Low vitamin C intake is associated with an increased risk of PPROM in women with history of previous PPROM. | Moderate +++/++++ | |
| Gupta et al. (2020) [87]/India | To assess the association between vitamin C deficiency and PPROM. | Prospective case control study. | 100 women aged 18–35 with singleton pregnancy between 28–36.6 GWs. (n = 50 PPROM/n = 50 control) | Blood test for ascorbic acid and IL-6 analysis. | Measurement of plasmatic ascorbic acid and Il-6 levels. PPROM before 37 GWs. | Plasmatic ascorbic acid levels in PPROM group 0.60 ± 0.35 and 1.18 ± 0.43 mg/dL in control group (p < 0.001). 33 patients (66%) with low plasmatic ascorbic acid levels in PPROM vs. 6 (12%) in control group (p < 0.001). | PPROM susceptibility is increased in pregnant women with vitamin C deficiency. | Low ++/++++ | |
| Sharma et al. (2014) [88]/India | To evaluate the association between vitamin C and PPROM. | Prospective case control study. | 40 pregnant women with singleton pregnancy between 28–37 GWs. (n = 20 controls/n = 20 PPROM cases) | Blood test for ascorbic assessment. | Maternal plasmatic ascorbic acid level analysis. | Plasmatic ascorbic acid in PPROM group 0.41 ± 0.08 vs. 0.84 ± 0.19 mg/dl in control group (p < 0.001) and inverse relationship were observed between PPROM relationship and ascorbic acid levels. A decrease in ascorbic acid levels was observed throughout pregnancy. | Antenatal vitamin C supplementation would prevent PPROM. | Low ++/++++ | |
| Vitamin E | Harville et al. (2020) [89]/USA | To check the relationship between preconceptionally antioxidant levels and obstetric adverse outcomes. | Prospective observational study. | 2787 women aged 18–30 (1638 pregnant women during the follow-up period). | Blood test for antioxidant status assessment. Follow-up at 0, 2, 5, 7, 10, 15 and 20 years. | Interviewer-administered quantitative FFQ for evaluation of antioxidant intake. Plasmatic levels of the studied antioxidants. Women self-report pregnancy outcomes. | No statistically significant differences in vitamin E levels according to PTB. | Vitamin E supplementation was not associated with decrease in PTB. | Low ++/++++ |
| Bartfai et al. (2012) [90]/Hungary | To determine the effect of vitamin E in the prevention of PTB. | Observational study. | 37,971 pregnant women (n = 35,864 no vitamin E/n = 2287 vitamin E). | Prospective record of maternal clinical history and obstetric outcomes. Retrospective maternal self-report about pregnancy supplements (estimated daily dose of 450 mg vitamin E). | PTB before 37 GWs. | Rate of PTB was 9.3% in women without vitamin E supplementation vs. 6.6% in vitamin E supplementation group (adjusted OR 0.71, 95% CI; 0.63–0.84). | Vitamin E was associated with a nearly 30% reduction in PTB. | Moderate +++/++++ | |
| Carmichael et al. (2013) [91]/USA | To assess maternal dietary intake and PTB. | Transversal study. | 5738 singleton pregnancies. | Shortened version FFQ.MDS and DQI for diet quality evaluation. | PTB before 37 GWs. | OR PTB < 32 GWs 1.9 (1.0–3.6) for the lowest quartile of vitamin E intake. | Vitamin E nutritional intake is not clearly associated with PTB. | Low ++/++++ | |
| Vitamin C + E | Gungorduk et al. (2014) [92]/Turkey | To study the effect of vitamin C + E supplementation on PPROM to increase the latency period before birth. | Prospective open randomized trial. | 229 pregnant women with PPROM 24–34 GWs. (n = 126 1 g vitamin C + 400 IU vitamin E/n = 123 placebo) | Diagnosis of PPROM according clinical examination, nitrazine test or Amnisure® test. | Latency period until birth. | Longer latency period before birth in vitamin C + E group (11.2 ± 6.3 days) compared with control group (6.2 ± 4.0 days), p < 0.001; and higher gestational age (31.9 ± 2.6 weeks vs. 31.0 ± 2.6 weeks), p < 0.01. | Vitamin C + E supplementation is associated with a longer latency period before birth and higher gestational age at birth. | Moderate +++/+++++ |
| Hassanzadeh et al. (2016) [93]/Iran | To evaluate the relationship between macro and micronutrients maternal intake in 3rd trimester and PPROM. | Prospective cohort study. | 620 pregnant women aged 15–49 years. | 48-h dietary recalls at 11th–15th, 26th, 34th–37th GWs. Records of physical activity and reproductive and demographic maternal characteristics. | PPROM diagnosis. | Vitamin C levels in 1st and 2nd trimester were higher in PPROM (206.2 ± 156.5 and 208.7 ± 193.1) compared with controls (147.9 ± 99.8 and 152.7 ± 105.8), p = 0.020 and p = 0.037. No statistically significant differences in vitamin E levels. | Higher vitamin C intake in 1st and 2nd trimester of pregnancy was associated with an increased risk of PPROM. | Low ++/++++ | |
| Hauth et al. (2010) [94]/USA | To assess the protective effect of vitamin C and E supplementation in PTB prevention. | Randomized, double-masked, placebo-controlled trial. | 10,154 nulliparous women with low-risk pregnancies (n = 4992 1000 mg vitamin C + 400 IU vitamin E/n = 4976 placebo). | Administration of 1000 mg ascorbic acid + 400 IU α-tocopherol acetate or placebo (mineral oil) since 9.0–16.6 GWs to birth. | Spontaneous PTB and PROM + PTB. | PROM + PTB before 32 GWs in the supplemented pregnant women (0.3%) compared to the placebo group (0.6%), adjusted OR 0.3–0.9. PPROM < 32 GWs 0.36% in supplement group vs. 0.64% in the placebo group, p < 0.046. | Maternal supplementation with vitamin C + E in low-risk pregnancies does not reduce total spontaneous PTB, but prevent PROM + PTB before 32 GWs. | High ++++/++++ | |
| Ilhan et al. (2015) [15]/Turkey | To analyze maternal oxidative status in PPROM. | Prospective cross-sectional study. | 72 pregnant women. (n = 38 PPROM/n = 34 controls) | Blood samples collection. ELISA for biomarker analysis. | Plasmatic IL-6, vitamin C, vitamin E, CRP and 8-isoprostane levels, TOS and TAS. | High TOS and low vitamin C and 8-isoprostane in PPROM group (p < 0.001). | Plasmatic vitamin C levels were associated with PPROM. | Low ++/++++ | |
| Ilhan et al. (2017) [95]/Turkey | To investigate maternal oxidative status in PPROM and the latency period to birth. | Prospective case control study. | 116 pregnant women. (n = 75 PPROM cases between 24–34 GWs/n = 41 controls) | Maternal blood for biochemical analysis. ELISA test. HPLC analysis. | Plasmatic vitamin C, vitamin E, MDA, leukocyte count and CRP levels. TOS and TAS evaluations.Latency period between PPROM and birth. | Vitamin C levels were lower in PPROM group (7.39 ± 2.37) compared to controls (13.83 ± 3.16), p < 0.001, but not statistically significant differences were found in vitamin E levels. | Vitamin C is associated with a lower risk of PPROM. | Low ++/++++ | |
| Koenig et al. (2017) [96]/USA | To assess the relationship between nutrient intake and cervix characteristics. | Longitudinal descriptive design. | 47 pregnant women. | FFQ at 19–24, 27–29 GWs. Transvaginal ultrasound examination at 19–21, 23–25, 27–29, 31–33 and 35–37 GWs. | Cervical remodeling. | Women in the less-risk group of PTB assessed by cervical length remodeling had higher vitamin E intake (p = 0.04). No differences according to vitamin C intake. | Certain nutrients, such as vitamin E, prevented PTB through the inhibition of premature cervical remodeling. | Very low +/++++ | |
| Zhang et al. (2017) [97]/China | To evaluate the association between dietary nutrients and PTB. | Prospective case-control design. | 511 pregnant women. (n = 130 PTB/n = 381 term birth) | FFQ for mother diet assessment. | PTB before 37 GWs. | Women with PTB had lower vitamin E intake (29.60 ± 9.51) than women with term birth (33.57 ± 11.30), p < 0.01. Women with PTB and BMI <18.5 had lower vitamin E intake (p < 0.05). No statistically significant differences in vitamin C intake and PTB. | Low levels of vitamin E intake were associated with PTB. | Low ++/++++ | |
| Zinc | Charkamyani et al. (2019) [98]/Iran | To study the effect of a diet modification program in IVF pregnant women to reduce PTB. | Quasi-experimental clinical trial. | 170 IVF pregnant women aged 19–45 from 2017 to 2018. | Dietary intervention promoting increased intake of lactose, fiber, magnesium, zinc, vitamin B3 and B5. | Self-developed questionnaire for demographic characteristics collection, dietary habits and lifestyle behaviors. | Zn increased intake (p = 0.017) after dietary intervention. No statistically significant differences in the rate of PTB according to Zn intake. | Zn intake is not associated with PTB in IVF pregnant women. | Low ++/++++ |
| Nga et al. (2020) [99]/Vietnam | To determine if nutrient-rich diet during pregnancy improves obstetric outcomes in low-income countries. | Randomized controlled trial. | 460 primiparous women aged 18–30 from 2011 to 2015. (n = 150 PC-T, n = 153 MG-T, n = 157 RPC) | 3 study groups: PC-T; MG-T and RPC. Nutrient-rich food-based supplement containing Zn, folate, vitamin B12, A and iron. | Zn intakes increase in PC-T and MG-T groups (p < 0.001) compared to RPC. | No statistical differences in PTB according to the intervention group. | A nutrient-rich supplement containing Zn in pregnant women from low-income countries did not improve the rate of PTB. | Moderate +++/++++ | |
| Nossier et al. (2015) [100]/Egypt | To assess the effects of Zn supplementation on obstetric outcomes. | Double-blind randomized controlled trial. | 1460 women with low serum Zn levels from 2007 to 2009 (n = 223 placebo, n = 225 Zn, n = 227 Zn + MM) | 3 study groups: (1) placebo; (2) Zn (daily 30 mg ZnSO4); (3) Zn + MM (daily 30 mg ZnSO4 + multivitamin). | FFQ for dietary intake assessment. Blood test for serum Zn quantification. | Higher Zn serum levels in the Zn group compared to placebo and Zn + MM (p < 0.001). PTB was lower in the Zn group (1%) and Zn + MM group (2%), compared to placebo (10%), p < 0.001. RR = 0.012, 95% CI 0.036–0.77) in the Zn group and RR = 0.268, 95% CI 0.119–0.603 in Zn + MM group compared to the placebo group. | Zn supplementation is effective in reducing PTB. | High ++++/++++ | |
| Zahiri et al. (2015) [101]/Iran | To investigate the effect of Zn supplementation on obstetric outcomes. | Randomized controlled trial. | 540 women from 2010–2012 (n = 270 Zn/n = 270 control). | 2 study groups (supplementation from 16 GWs until delivery): (1) daily supplementation 400 µg folic acid + 30 mg ferrous sulfate; (2) daily supplementation 400 µg folic acid + 30 mg ferrous sulfate + 15 mg Zn sulfate. | Demographic and anthropometric data, blood pressure and obstetric outcomes. | No statistically significant differences in PTB (p = 0.999) or PPROM (p = 0.630). | Daily 15 mg Zn supplementation does not reduce PTB or PPROM. | High ++++/++++ | |
| Costa et al. (2018) [102]/Portugal | To examine obstetric outcomes in women who had undergone bariatric surgery. | Retrospective descriptive observational study. | 39 pregnant women. | Pregnant women who had previously undergone bariatric surgery. Study period 2010–2014. | Maternal characteristics, type of bariatric surgery (restrictive or mixed technique), obstetric outcomes. Evaluation of Zn, iron, vitamin B12 and D prior and during pregnancy. | Zn deficiency in 12 cases (66.8%). PPROM in 2 cases. PTB in 5 cases. | No differences in obstetric outcomes were observed. Nutritional deficits are less common in restrictive bariatric surgery. | Very low +/++++ | |
| Kucukaydin et al. (2018) [103]/Turkey | To analyze trace element, heavy metals, and maternal vitamin in PTB and PPROM. | Prospective cohort study. | 68 women with PTB (n = 35 PPROM, n = 33 without PPROM). | Singleton pregnancies. Study period: 2008–2009. | Zn levels in maternal, umbilical plasma and placental tissue. | Zn lower levels in maternal and umbilical cord serum in PPROM (p < 0.01). No statistically significant differences in placental tissue Zn levels. | PPROM is associated with low maternal and fetal Zn levels. | Low ++/++++ | |
| Shen et al. (2015) [104]/China | To evaluate changes in trace elements during pregnancy and related-obstetric outcomes. | Prospective cohort study. | 1568 pregnant women. | Recruitment of women aged 18–39 in antenatal care.Study period 2013–2014. Blood tests in fasting conditions. | Measurement of plasmatic Zn levels before pregnancy, at 7–12 GWs, 24–28 GWs and 35–40 GWs. | No statistically significant differences in Zn levels during pregnancy. Zn levels were significantly lower in PPROM and PTB (p < 0.05) compared to controls. | Zn deficiencies in pregnancy may be associated with increased risk of PPROM and PTB. | Low ++/++++ | |
| Tea | Chen et al. (2018) [105]/Ireland | To evaluate the association between caffeine intake (tea) and birth outcomes. | Prospective cohort study. | 941 mother-child dyads | FFQ to measure maternal tea intake during the first 12–16 weeks of pregnancy. | Average tea consumption frequency (grams per day) divided in 6 levels. PTB (before 37 weeks of gestation). | PTB (OR = 2.56 (1.14–5.75)) in highest tea intake categories compared to the lowest (p < 0.05). | Maternal tea drinking is associated with an increased risk of PTB. | Low ++/++++ |
| Huang et al. (2016) [106]/China | To study the relation between tea consumption and risk of PTB. | Prospective cohort study | 10,179 women with uncomplicated pregnancies. | Standardized and structured questionnaires within 3 days after labor to obtain information regarding tea consumption. | Amount of tea consumption. PTB: moderate PTB (32–36 weeks), very PTB (28–31 weeks) and extremely PTB (<28 weeks). | PTB (OR = 1.36, 95% CI: 1.09–1.69) in tea consumers. Moderate PTB (OR = 1.41, 95% CI: 1.12–1.79) and spontaneous PTB (OR = 1.41, 95% CI: 1.09–1.83) in tea consumers. | Tea intake (green and scented tea) during pregnancy is associated with PTB. | Low ++/++++ | |
| Lu et al. (2017) [107]/China | To assess the association between tea consumption in early pregnancy and risk of PTB. | Prospective cohort study. | 8775 pregnant women. | Self-completed questionnaire about sociodemographic variables and tea drinking at 16 weeks. | Amount of tea consumption and type of tea.PTB (before 37 weeks of gestation). | No statistically significant differences in PTB according to the amount and type of tea. | Tea drinking in early pregnancy is not associated to increased risk of PTB. | Low ++/++++ | |
| Okubo et al. (2015) [108]/Japan | To examine the association between caffeine intake with the risk of PTB. | Prospective cohort study. | 858 mother-child dyads. | Validated self-administered dietary history questionnaire (8 categories) collected through gestation. | Maternal total caffeine intake. PTB (before 37 weeks gestational age). | Maternal median caffeine intake = 258 mg/day. Tea drinking (1 cup/day) is associated with an increased risk of PTB (OR 1.16; 95% CI 1.01–1.32, p = 0.035).No differences in risk of PTB according to the trimester of caffeine intake. | Tea consumption is associated with an increased risk of PTB. | Low ++/++++ | |
| Moussally et al. (2010) [109]/Canada | To analyze the association between HP consumption (mainly green tea) and PTB. | Prospective cohort study. | 8505 pregnant women aged 15–45 years. | Self-administered questionnaire in the second or third trimester of pregnancy. | Consumption of HP (green tea) during pregnancy. PTB (before 37 weeks gestational age) | No association between green tea intake and risk of PTB (OR 0.94 (0.55–1.61)). | Green tea drinking in the second and third trimester of pregnancy is not associated with an increased risk of PTB. | Low ++/++++ | |
| Sengpiel et al. (2013) [110]/Norway | To investigate the association between maternal caffeine consumption and birth results. | Prospective cohort study. | 59,123 mother-child dyads. | Self-administered FFQ at 17, 22 and 30 weeks of pregnancy. | Caffeine calculation using FoodCalc and Norwegian Food Composition table. Spontaneous PTB (22–36 weeks of gestation). | Black tea was associated with elevated risk of early PTB (OR 1.61, 95% CI 1.10–2.35, p = 0.01), but not an association in all PTB. The other sources of caffeine were not associated with an increased risk of PTB. | Black tea is associated with the risk of PTB. Caffeine intake from other sources (coffee, caffeinated soft drinks, tea and chocolate) is not associated with PTB. | Low ++/++++ | |
| Sindiani et al. (2020) [111]/Jordan | To study the association between tea consumption and PTB. | Unmatched case-control study. | 1110 healthy pregnant women. (314 cases/796 controls) | Interviewer administered structured questionnaires in women admitted for delivery. | Average number of teacups (150 mL) consumption. PTB (before 37 weeks gestational age). | Tea drinking was not associated with elevated risk of PTB. | There is not an association between tea consumption and the risk of PTB. | Very low +/++++ | |
| Melatonin | Biran et al. (2019) [112]/France | To compare melatonin plasmatic levels in women who delivered preterm infants before 34 GW. | Prospective longitudinal multicenter study. | 169 mothers. | Recruitment of women admitted for birth and blood tests for analysis of plasma melatonin levels. | Radioimmunoassay for plasma melatonin concentration measurements. | Statistically significant lower median IQR = 7 (7–20) in mothers who delivered before 34 GW compared to median IQR 11 (7–50) in mothers who gave birth after 34 weeks (p = 0.02). | Median plasma melatonin concentration was significant lower in mothers who delivered before 34 gestational weeks. | Low ++/++++ |
| Dominguez et al. (2014) [113]/Argentina | To evaluate the effect of melatonin treatment in a mice model of inflammation-associated PTB. | BALB mice model. | 4 experimental groups (n = 10 each group): (1) control; (2) melatonin pellet; (3) LPS injection; (4) melatonin + LPS. | Sc administration of 25 mg melatonin pellet on GD 14. Ip injection of bacterial LPS twice/day on GD 15. | Gestational age at the moment of the birth. NOS activity assessment. PG radioimmunoassay. ELISA for TNFα measurement. Western blot for iNOS and COX-2 activity analysis. | Melatonin prevented 50% of LPS-induced PTB (p > 0.05). PGE2, COX-2, PGF2α levels, NOS and iNOS activity were decreased in LPS + melatonin compared to the LPS group (p < 0.05). | Melatonin has effect on inflammation-induced alterations, making it a promising agent for PTB prevention. | Very low +/++++ | |
| Lee et al. (2019) [114]/Korea | To study the immunomodulatory effect of melatonin on PTB in a murine model. | Mouse model and in vitro model. | 3 experimental groups (n = 10 for each group): (1) control; (2) LPS; (3) LPS + melatonin. | 2 mg/Kg LPS ip injection on 16.5 GD. 10 mg/Kg melatonin ip injection on 16.5 GD (30 min after LPS injection). | Gestational age at birth. Western blot analysis for SIRT1/Nrf2 analysis. RT-PCR for IL-1β, IL-6, TNF-α, COX-2 quantification. | Melatonin decreases a 30% the rate of PTB (p < 0.001). Melatonin significantly decreased TNF-α, COX-2, IL-6 and 1L-1β levels (p < 0.05) and increased SIRT1 and Nrf2 levels (p < 0.05). | The effect of melatonin in the reduction of PTB is related to its immunomodulatory effects. | Very low +/++++ | |
| Ramiro-Cortijo et al. (2020) [115]/Spain | To investigate the effect of melatonin on PTB in twin pregnancies. | Single-center prospective observational study. | 104 twin-pregnant women. | Blood test between 9–11th GW. | Spectrophotometry for antioxidant (catalase, SOSA, GSH, thiol groups, phenolic compounds) and oxidative damage biomarkers (MDA, carboxyl groups) analysis and assessment of global antioxidant status (Antiox-S, Prooxy-S). Immunoassay for melatonin quantification. | Melatonin was significant lower in women with PTB (p = 0.024) compared to full-term. No differences in Antiox-S and Prooxy-S according to PTB. | Lower melatonin levels in the first trimester were associated with PTB in twin pregnancies. | Very low +/++++ | |
| Specht et al. (2019) [116]/Denmark | To evaluate the relationship between night work during first and second trimesters of pregnancy and risk of PTB. | Prospective cohort study. | 16,501 pregnant women. | Pregnant women with nightshift (23:00–06:00) in their first (1–12 GW) or second trimester (13–22 GW) from 2007 to 2013. | Odds of PTB (23–37 GW) analysis. | Prevalence of PTB was 5.2% in night workers and 5.1% in day workers. | There was no association between the night working shift and the risk of PTB. | Low ++/++++ |
| Antioxidant | Author (Year)/Country | Objectives | Study Design | Population | Dose/Intervention Period | Variables Studied | Key Results | Conclusion | Quality of Evidence |
|---|---|---|---|---|---|---|---|---|---|
| Vitamin C | Hoppu et al. (2005) [120]/Finland | To evaluate the impact of vitamin C in breast milk on the development of atopic disease. | Cross-sectional. | 65 mothers with atopic background at the end of gestation and their infants. | Mother’s diet rich in natural supplies of vitamin C (abundant intake of fresh fruits, berries and vegetables during breastfeeding). | Concentration of antioxidants in breast milk Infants: Clinical atopy and SPT at 12 months. | Decreased risk of atopy in the infant (OR = 0.30; 95% CI 0.09–0.94; p = 0.038). | A maternal diet enriched in natural supply of vitamin C during breastfeeding may decrease the risk of atopy in high-risk infants. | Low ++/++++ |
| Vitamin C and E | Zarban et al. (2015) [121]/Iran | To examine the effects of vitamin C and E supplements in the diet of breastfeeding mothers to ameliorate antioxidant activity. | RCCT | Breastfeeding mothers. CG: 30 EG: 30 | CG: free diet. EG: free diet supplemented with effervescent tablets of vitamin C (500 mg) and chewable tablets of vitamin E (100 IU). | Antioxidant content and activity in breast milk and infants’ urine, respectively. Measurements: the ferric reducing/antioxidant properties. | EG: higher levels of antioxidants in the breast milk (610–295.5 to 716–237.5 μmol/L) and infant urine (43.2–21.8 to 75.0–49.2 μmol/mg creatinine) (p < 0.05). Free radical scavenging in infant urine after 30 days of supplementation (p < 0.05). | Supplements of vitamin C and E increase anti-oxidant content of breast milk and antioxidant activity in infant urine. | High ++++/++++ |
| Vitamin C | Friel et al. (2007) [123]/Canada | To determine if iron or iron + vitamin C or iron + TVS supplementation (vitamins A, C and D) improve lipid oxidation in human milk in vitro. | Experimental. | 81 mothers. Preterm babies: 29–37 weeks. HM samples. | Iron = 2 mg/kg/day. Vitamin C = 20 mg/kg/day. | Lipid peroxidation in HM (FOX-2 and TBARS assays). Fatty acid composition (gas chromatography). Intracellular oxidative stress or DNA damage (cell culture bioassays: Caco-2BBe and FHS-74 Int cells). | Iron; iron + vitamin C; iron + TVS: ↑ lipid oxidation products of HM ↓ mono and polyunsaturated fatty acids in HM. Iron; iron + TVS: ↑ intracellular oxidative stress in FHS-74 Int cells. All treatments increased DNA damage in Caco-2BBE cells. | Iron + vitamin C increased DNA damage if compared to iron alone.Iron supplements may provoke oxidative stress in preterm infants and should be divided from vitamin C supplementation. | Very Low +/++++ |
| Daneel-Otterbech et al. (2005) [122]/Canada | To compare human milk AA content in European and African women and to evaluate the influence of increased AA intake on human milk AA output. | RCCT | 171 African lactating women. 142 European women. | Effervescent tablets (1000 mg AA/day). | AA concentration in human milk. | After 10 d: ↑ AA concentration from 19 to 60 mg/kg (p ≤ 0.001) and from 60 to 70 mg/kg (p ≤ 0.03) in 18 African and 10 European women. In 11 African women, AA levels increased from 17 to 36 mg/kg (p ≤ 0.001) after intake of 100 mg AA/day during the same period. | AA in human milk can be increased in women with low human milk AA content at baseline | Moderate +++/++++ | |
| Vitamin E | Melo et al. (2017) [125]/Brasil | To evaluate if supplementation with vitamin E increases the concentration of α-TOH in colostrum and its supply to the newborn. | RCCT | n = 99 healthy adult pregnant women. (CG: 39; EG: 60) | The supplemented group received 400 IU of supplementary vitamin E. | Vitamin E concentrations in human milk and blood sample, before and after treatment. | Basal vitamin E levels: CG: 1509.3 ± 793.7 g/dL EG: and 1452.9 ± 808.6 g/dL After 24 h: CG: 1650.6 ± 968.7 g/dL (p > 0.05) EG: 2346.9 ± 1203.2 g/dL (p < 0.001) ↑ of vitamin E in the newborn to 9.3 mg/day. | Maternal vitamin E supplementation provides more than twice the Recommended Daily Intake of this vitamin. | High ++++/++++ |
| Medeiros et al. (2016) [126]/Brasil | To evaluate the effect of maternal vitamin E supplements on its levels in the colostrum, transitional milk and mature milk of mothers of preterm babies. | RCCT | n = 89 women (CG:51;EG:38) | 400 IU of RRR-α-tocopheryl acetate. Breast milk samples were collected 1, 7 and 30 d after delivery. | Vitamin E concentrations in HM and BS by HPLC. | No significant differences in α-TOH levels in BS at baseline in both groups.Breast milk α-TOH levels increased by 60% at 24 h in EG. Transitional milk’s levels were 35% higher in EG. Similar α-TOH in the mature milk in both groups | Maternal supplements with 400 IU of RRR-α-tocopherol increased the vitamin E levels in the colostrum and transitional milk, but not of the mature milk. The effects of megadoses are not prolonged. | High ++++/++++ | |
| Clemente et al. (2015) [135]/Brasil | To assess if supplements with a natural or synthetic form of α-TOH to lactating women increase its concentration in colostrum. | RCCT | n = 109 lactating women: CG: 36 NF:40 SF:33 | Blood and colostrum samples were collected before and after supplementation to check the nutritional status of these women. | Vitamin E concentrations in HM and BS by HPLC. | Higher levels of α-TOH in colostrum from women who received supplementation (increase of 57% and 39% in NF and SF, respectively) | Supplements of α-TOH increase vitamin E concentrations in colostrum. However, the natural form is more efficient in increasing levels. | High ++++/++++ | |
| Selenium and zinc | Strambi et al. (2004) [129]/Italy | To compare the nutritional Se status in the AGA and SGA newborns in the first month of life in relation to feeding type. | Longitudinal | n = 210 (AGA: 129; SGA: 81) Feeding type: breast milk, formula and mixed. | Breast, bottle, or mixed feeding during the study period/4 weeks. | Se status in plasma and erythrocyte concentrations. | Se plasmatic levels were lower in SGA than in AGA newborns. SGA: higher plasma concentrations in breast-fed (p = 0.013) and mixed-fed (p = 0.006). The difference was not significant in AGA neonates. | Breast-fed SGA newborns showed higher plasma Se concentrations than formula-fed newborns. Even if supplemented from birth, Se intake was not adequate in bottle-fed SGA infants. | Moderate +++/++++ |
| Loui et al. (2004) [131]/Germany | To assess mineral, trace element, thyroid status and growth of infants fed with HM fortified with calcium, phosphorus and protein, with (BMF) or without (FM 85) trace elements (zinc, copper, manganese and iodine). | RCCT | n = 62 (FM85:34; BMF:28) Age: <33 w Weight: 1000–1499 g | Fortified HM with trace elements (5% BMF) or without (3% FM85)/6 weeks. | Serum: red blood cells. HM: minerals and trace elements. Serum: alkaline phosphatase activity, TSH, T4. FT4 on the fifth day and at 3 and 6 weeks of life. Clinical evolution and anthropometric measurements. | Levels of zinc, copper, manganese, calcium, phosphorus and magnesium were higher in the BMF group (p < 0.001). Serum zinc concentrations did not differ between groups. Median alkaline phosphatase activity: 436/379 IU/L in the FM 85/BMF group at 6 weeks (p < 0.01). Significant higher weight gain in the FM 85 group (due to higher caloric and protein intake) at 3 weeks. | zinc statusdid not differ between groups after treatment. | High ++++/++++ | |
| Shaaban et al. (2005) [130]/Egypt | To assess the impact of maternal Zinc supplements on maternal and infant Zn levels and on the infants’ physical growth. | RCCT | 60 primiparous lactating mothers.(CG:30; EG:30) | 10 mg/day of Zinc sulfate capsules/2 months. | Zn levels in hair, nails and breast milk. | Zn supplements increased maternal Zn store in hair, nail, and breast milk. No differences in infant growth. | Zn supplements in lactating women increased breast milk Zn levels and maternal body stores, but it does not impact the infants’ physical growth. | High ++++/++++ | |
| Melatonin | Qin et al. (2019) [133]/China | To assess the changes in breast milk melatonin during lactation and to explore changes in melatonin levels and rhythms in preterm and term breast milk. | Longitudinal. | 392 breast milk samples from 98 healthy nursing mothers at 0 to 30 days postpartum. 32% preterm. 67% full-term. | Breast milk was collected sequentially the same day, at 03:00, 09:00, 15:00. | Melatonin concentration. | Preterm and term breast milk: melatonin showed a circadian rhythm with peak at around 03:00. Highest melatonin concentration in the colostrum. Higher concentrations of melatonin in preterm than in term breast milk in the colostrum (28.67 pg/mL vs. 25.31 pg/mL, p < 0.022), transitional breast milk (24.70 pg/mL vs. 22.55 pg/mL), and mature breast milk (22.37 pg/mL vs. 20.12 pg /mL). | Melatonin showed a clear circadian rhythm in both preterm and term breast milk during lactation stages. The peak level was highest in colostrum, decreasing during the first month after birth. | Moderate +++/++++ |
| Honorio-Franca et al. (2013) [134]/Brasil | To assess the effects of HM samples (diurnal/ nocturnal) on colostral melatonin levels and the property of this hormone to modify colostral phagocyte activity. | Experimental. | 60 Colostrum samples from 30 mothers during the day and night. | Not applicable. | Melatonin levels in colostrum and superoxide release and bacterial killing by colostral phagocytes. | Nocturnal colostrum: higher melatonin levels and increased spontaneous superoxide release; higher phagocytosis rate. Bactericidal activity of mononuclear phagocytes increased in response to melatonin, regardless of the sample type. | Melatonin levels in human colostrum follow a day-night cycle and increase phagocytic activity of colostral cells against bacteria. | Very low +/++++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastiani, G.; Navarro-Tapia, E.; Almeida-Toledano, L.; Serra-Delgado, M.; Paltrinieri, A.L.; García-Algar, Ó.; Andreu-Fernández, V. Effects of Antioxidant Intake on Fetal Development and Maternal/Neonatal Health during Pregnancy. Antioxidants 2022, 11, 648. https://doi.org/10.3390/antiox11040648
Sebastiani G, Navarro-Tapia E, Almeida-Toledano L, Serra-Delgado M, Paltrinieri AL, García-Algar Ó, Andreu-Fernández V. Effects of Antioxidant Intake on Fetal Development and Maternal/Neonatal Health during Pregnancy. Antioxidants. 2022; 11(4):648. https://doi.org/10.3390/antiox11040648
Chicago/Turabian StyleSebastiani, Giorgia, Elisabet Navarro-Tapia, Laura Almeida-Toledano, Mariona Serra-Delgado, Anna Lucia Paltrinieri, Óscar García-Algar, and Vicente Andreu-Fernández. 2022. "Effects of Antioxidant Intake on Fetal Development and Maternal/Neonatal Health during Pregnancy" Antioxidants 11, no. 4: 648. https://doi.org/10.3390/antiox11040648
APA StyleSebastiani, G., Navarro-Tapia, E., Almeida-Toledano, L., Serra-Delgado, M., Paltrinieri, A. L., García-Algar, Ó., & Andreu-Fernández, V. (2022). Effects of Antioxidant Intake on Fetal Development and Maternal/Neonatal Health during Pregnancy. Antioxidants, 11(4), 648. https://doi.org/10.3390/antiox11040648








