Abstract
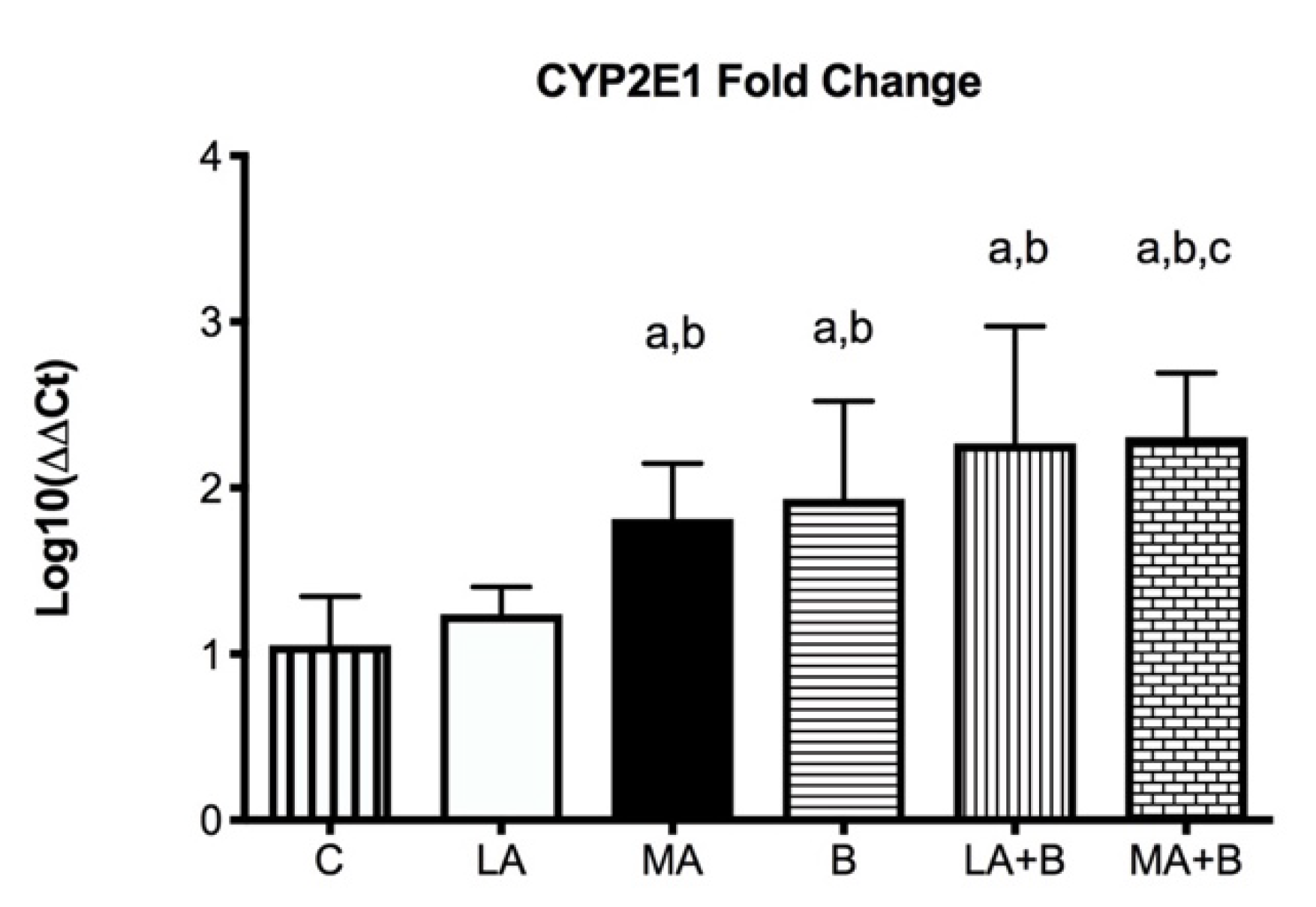
One of the key routes through which ethanol induces oxidative stress appears to be the activation of cytochrome P450 2E1 at different levels of ethanol intake. Our aim was to determine if oral β-carotene intake had an antioxidant effect on CYP2E1 gene expression in mice that had previously consumed ethanol. C57BL/6 mice were used and distributed into: control (C), low-dose alcohol (LA), moderate-dose alcohol (MA), β-carotene (B), low-dose alcohol+β-carotene (LA + B), and moderate-dose alcohol+β-carotene (MA + B). Animals were euthanized at the end of the experiment, and liver tissue was taken from each one. CYP2E1 was measured using qPCR to detect liver damage. The relative expression level of each RNA was estimated using the comparative threshold cycle (Ct) technique (2−ΔΔCT method) by averaging the Ct values from three replicates. The LA+B (2267 ± 0.707) and MA+B (2.307 ± 0.384) groups had the highest CYP2E1 fold change values. On the other hand, the C (1.053 ± 0.292) and LA (1.240 ± 0.163) groups had the lowest levels. These results suggest that ethanol feeding produced a fold increase in CYP2E1 protein in mice as compared to the control group. Increased CYP2E1 activity was found to support the hypothesis that β-carotene might be dangerous during ethanol exposure in animal models. Our findings imply that β-carotene can increase the hepatic damage caused by low and high doses of alcohol. Therefore, the quantity of alcohol ingested, the exposure period, the regulatory mechanisms of alcoholic liver damage, and the signaling pathways involved in the consumption of both alcohol and antioxidant must all be considered.
1. Introduction
Excessive alcohol drinking has been linked to several deadly illnesses, including cancer, cirrhosis of the liver, vascular disease, neuropsychiatric illness, as well as diabetes [1,2,3]. In addition, that increased oxidative stress can produce hepatic damage in people has been demonstrated [4,5].
Alcoholic fatty liver, alcoholic hepatitis, and cirrhosis are all caused by ethanol metabolism [6,7]. In the hepatic metabolization, enzymes such CYP450 2E1 (CYP2E1), alcohol dehydrogenase (ADH), and catalase (CAT) are involved in the oxidative pathway, whereas through the non-oxidative pathway the fatty acid ethyl ester (FAEE) synthase creates FAEEs [8,9].
The microsomal respiratory chain and CYP2E1-dependent microsomal monooxygenase system are the main sources of ROS during alcohol intake. As to its ability to produce a diversity of hepatotoxic substrates, such as N-nitrosodimethylamine, alcohol, acetaminophen, and carbon tetrachloride, CYP2E1 is of particular interest [10]. According to this theory, alcohol-induced activation of CYP2E1 is one of the primary mechanisms by which alcohol produces oxidative stress. Furthermore, CYP2E1 oxidizes ethanol to form a very reactive particle that might contribute to alcohol’s harmfulness, acetaldehyde [11].
The primary contributor to the development of alcohol-mediated liver damage, extracellular matrix changes, and inflammation has been identified as acetaldehyde [12,13]. The generation of reactive oxygen species (ROS) and a redox potential imbalance (NAD/NADH) generate its effects. It also links to DNA, producing oncogenic chemicals like 1,N2-(3-hydroxypropane)-2′-deoxyguanosine, and creates protein aggregates in hepatocytes, restricting protein synthesis and promoting hepatomegaly. It also forms salsolinol when it reacts with dopamine, which can contribute to alcohol dependency [14,15].
Alcohol-mediated oxidative stress and toxicity have previously been examined in animal models and in vitro studies [16,17]. In consequence, these results have sparked fresh research into new pathophysiological targets that may be used to treat alcoholic liver disease (ALD). In effect, enzymatic mechanisms such as catalase, superoxide dismutase, and glutathione peroxidase and reductase, as well as non-enzymatic mechanisms, might be used to block the hepatocyte’s antioxidant defense [18,19,20]. Many antioxidants, including silymarin, N-acetylcysteine, vitamin E, and S-adenosylmethionine (SAMe), have been examined in recent clinical investigations, although the results have been inconsistent [18,19,21,22]. Therefore, this study aimed to examine the consequences of β-carotene supplementation on CYP2E1 activity in C57BL/6 mice exposed to alcohol consumption.
2. Materials and Methods
2.1. Animals
Thirty male C57BL/6 mice were used (Mus musculus), 50 days old, from the Chilean Public Health Institute. They were kept for 30 days under standardized conditions and a 12 h light/dark cycle (08:00 a.m.–08:00 p.m./08:00 p.m–8:00 a.m.), with a standard laboratory diet (AIN-93M) and water ad libitum to help them adjust to their new environment in the Animal Facility of the Center of Excellence in Morphological and Surgical Studies (CEMyQ) at the Universidad de La Frontera. The animals were handled according to the recommendations published by the Institute for Laboratory Animal Research [23]. The Scientific Ethics Committee of the Universidad de La Frontera has approved this project (Nº051/2020).
The mice were split into six groups on the first day of the experiment (day 1): 1. control (C); 2. low-dose alcohol (LA): low-dose alcohol consumption (3% v/v ad libitum) for 28 days [24]; 3. moderate-dose alcohol (MA): moderate-dose alcohol consumption (7% v/v ad libitum) for 28 days [24]; 4. β-carotene (B): administration of 0.52 mg/kg body weight/day of β-carotene for 28 days [25]; 5. low-dose alcohol + β-carotene (LA + B): low-dose alcohol consumption plus administration of 0.52 mg/kg body weight/day of β-carotene for 28 days; and 6. moderate-dose alcohol + β-carotene (MA + B): moderate-dose alcohol consumption plus administration of 0.52 mg/kg body weight/day of β-carotene for 28 days.
Chronic ethanol administration was given according to the modified liquid diet of Lieber-DeCarli [24,26]. β-carotene was orally administered at a dose of 0.52 mg/kg body weight/day [25].
2.2. Euthanasia
On day 28, at the end of the experiment, the animals were fasted for 6 h and euthanized with sodium pentobarbital.
2.3. Liver Tissue
Each animal’s liver tissue (n = 30) was obtained after euthanasia. Liver samples were extracted as soon as possible and placed in autoclaved microtubes containing lysis solution (RNeasy Mini Kit, QIAGEN) for RNA stabilization. They were then stored at room temperature for 30 min. The samples were then frozen in liquid nitrogen before being carried to the freezer room. The frozen samples were then transferred to an ultra-freezer and stored at −80 °C until they were utilized.
2.4. Extraction of RNA and cDNA Synthesis
The liver sample was crushed into a fine powder in liquid nitrogen with a prechilled mortar and pestle, then combined with the TRIzol (QIAGEN Diagnostics GmbH, Germany)/lysis buffer given with the kits and extracted according to the methodology previously described [27]. Using High-Capacity cDNA Reverse Transcription Kits, the mRNA strand was reverse transcribed into single-stranded cDNA (Applied Biosystems, Waltham, MA, USA). The cDNA was subsequently amplified using TaqManTM Universal PCR Master Mix in a quantitative PCR (qPCR) (Applied Biosystems, Waltham, MA, USA).
2.5. Quantification of RNA from Liver Tissue
The amount and integrity of the isolated total RNA were analyzed using the QubitTM 4.0 Fluorometer (Life Technologies, Thermo Fisher Scientific Inc., Waltham, MA, USA). The RNA IQ# was estimated from the fraction of large and small RNA in the sample. The RNA IQ# is a number that ranges from 1 to 10, where a high number suggests that most of the RNA in the sample is large and/or organized. On the other hand, a low IQ# indicates that the sample is largely small RNA with little tertiary structure. The manufacturer’s instructions were followed while using the standard QubitTM RNA HS Assay Kit (Life Technologies, Thermo Fisher Scientific Inc.). The QubitTM’s functioning solution was developed in accordance with the manufacturer’s standards. We added to each assay tube 180 μL of working solution, up to 20 μL of RNA, and enough water to make the final volume 200 μL. For the standard tubes, 10 μL of QubitTM RNA Standard solutions were placed into the tubes. The assay tubes were vortexed for 2–3 s, centrifuged for 5 s, and then left at room temperature for 2 min before being measured with the QubitTM Fluorometer. For the Qubit™ Assay, RNA sample concentration was calculated as: [Concentration of your sample] = QF value (the value given by the Qubit® 4.0 Fluorometer) × (200/the number of microliters of sample put into the assay tube). Three different measurements were taken on each sample.
2.6. Quantitative Real-Time PCR
The expression levels of CYP2E1, family 2, subfamily e, polypeptide 1, β-actin (ACTB), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were analyzed using qPCR. The data were normalized according to the mRNA expression levels of housekeeping genes, such as ACTB and GAPDH. CYP2E1 was the target gene. All qPCR experiments were performed using the QuantStudio3 system (Applied Biosystems, Waltham, MA, USA). All the amplifications were done using TaqManTM Universal PCR Master Mix (Applied Biosystems, Waltham, MA, USA). The TaqMan gene expression test is a ready-to-use 5’-3’ Taq polymerase assay containing TaqMan® dye-labeled probes (FAM/MGB) and desired primers, as presented in Table 1. In addition, housekeeping genes are presented in Table 2.

Table 1.
Primers for gene targeting.

Table 2.
Housekeeping genes for quantitative PCR.
After a 10-min denaturation phase at 95 °C, 40 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s were performed. A melting curve analysis of each qPCR was carried out after each cycle. The number of times the reporter dye in the PCR reaction crossed a software-defined threshold, which was computed automatically by the QuantStudioTM Design & Analysis Software, is referred to as the ‘Ct,’ or threshold cycle (version 1.3, Applied Biosystems, Waltham, MA, USA). The relative expression level of each RNA was estimated using the comparative threshold cycle (Ct) technique (2−ΔΔCt method) by averaging the Ct values from three replicates. We utilized the threshold cycle values automatically generated by the qPCR equipment for the 2−ΔΔCt technique.
2.7. Statistical Analysis
The Kolmogorov–Smirnov test (data normality analysis) and Levene’s test were used to assess differences in quantitative data (homoscedasticity of the variances). One-way ANOVA was used to analyze the differences among the groups. Tukey’s HSD test or Dunnett’s T3 test were used to realize such a post-hoc test, as applicable. p < 0.05 was considered statistically significant (GraphPad Prism 6, GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. Statistical Analysis
RNA was extracted from liver samples and was analyzed for integrity and quality. The assay kit was prepared to be precise concerning initial RNA sample concentrations of 0.5 to 1200 ng/L, yielding a detection range of 10 to 1200 ng, depending on sample volume. A total of 18 specimens had sufficient yield (RNA IQ# >8) to proceed to amplification through a quantitative PCR (qPCR) using TaqManTM Universal PCR Master Mix (Applied Biosystems, CA, USA). It has been described that RNA is pure and satisfactory for downstream studies if RIN > 7 [28].
3.2. Quantitative Real-Time PCR
In gene quantification analysis, data normalization in qPCR is a critical step [29,30]. Indeed, depending on the experimental settings and pathophysiology of the examined tissue, mRNA levels of the required housekeeping genes, ACTB and GAPDH, are likely to change to the point where normalization becomes erroneous and/or deceptive.
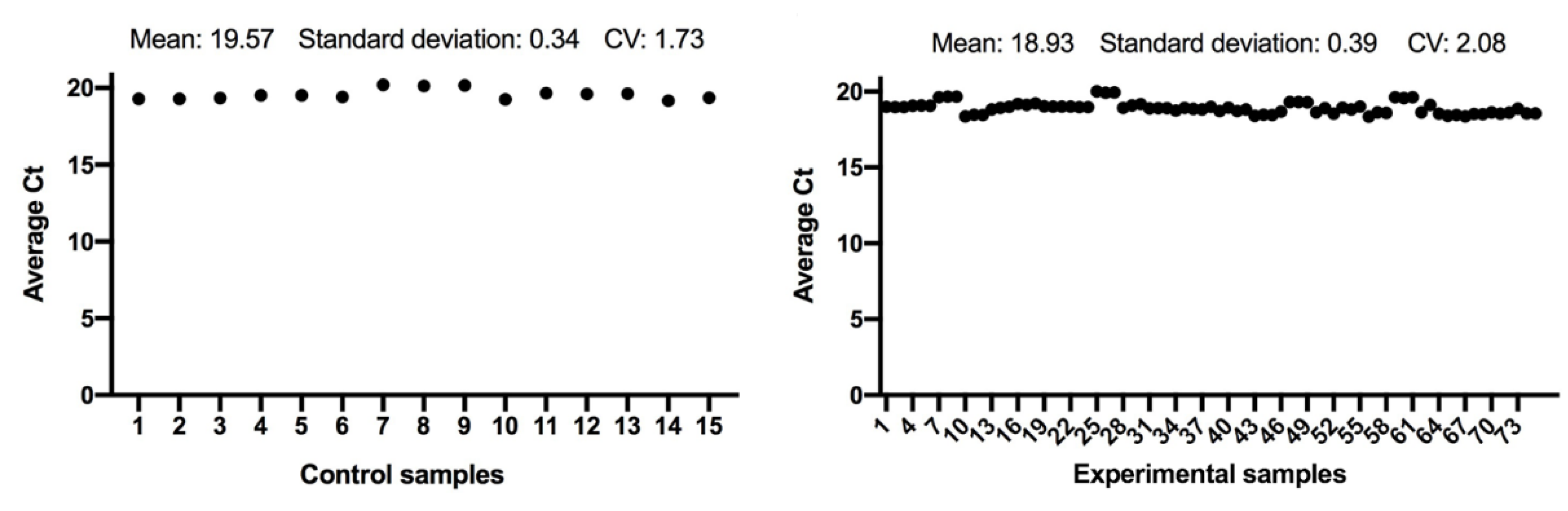
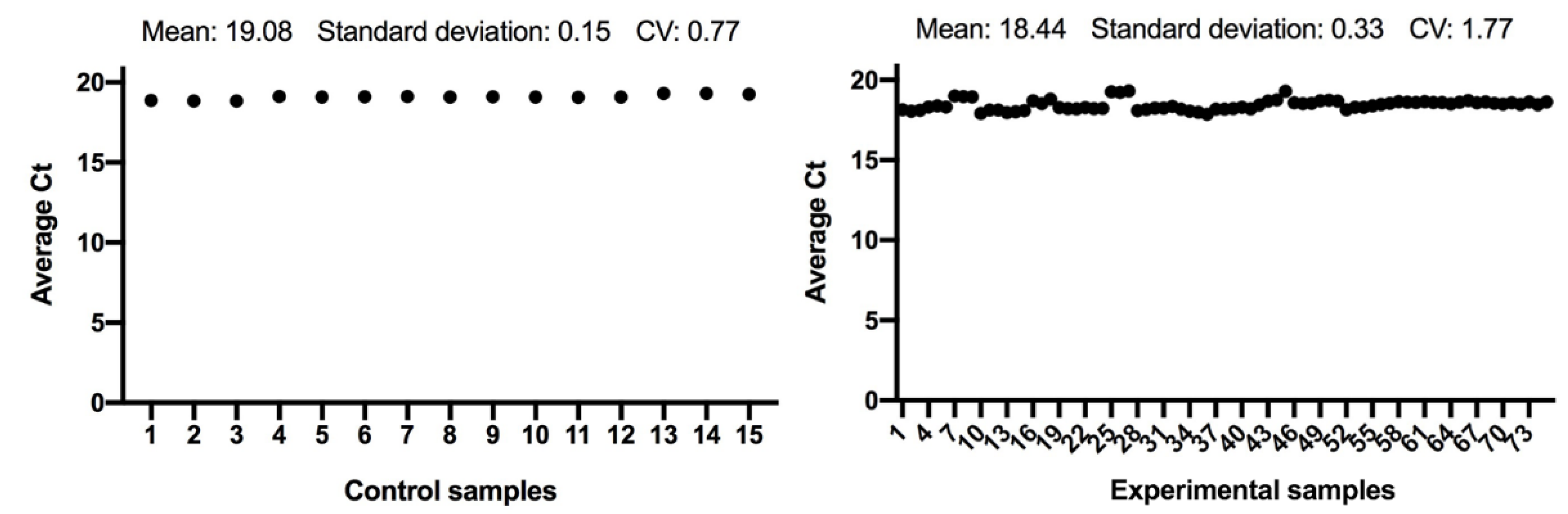
The expression of the ACTB and GAPDH genes changes very little between the control and the experimental samples, as seen in Figure 1 and Figure 2; and has a low variability of expression. As a result, the internal control genes ACTB and GAPDH may be used to provide accurate and consistent findings. ACTB and GAPDH resolve differences in templates starting with the amount and operational loading errors [31].
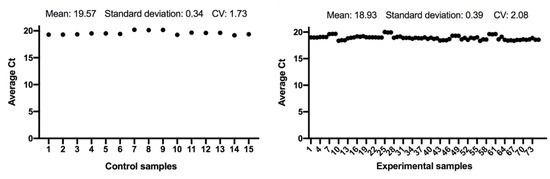
Figure 1.
Comparison of ACTB gene expression from control and experimental groups, respectively.
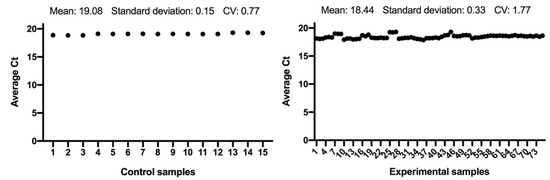
Figure 2.
Comparison of GAPDH gene expression from control and experimental groups, respectively.
The reference RNA utilized in the standard curve approach is highly efficient and stable. Moreover, this RNA has been useful to determine absolute comparative quantification of target genes by qPCR.
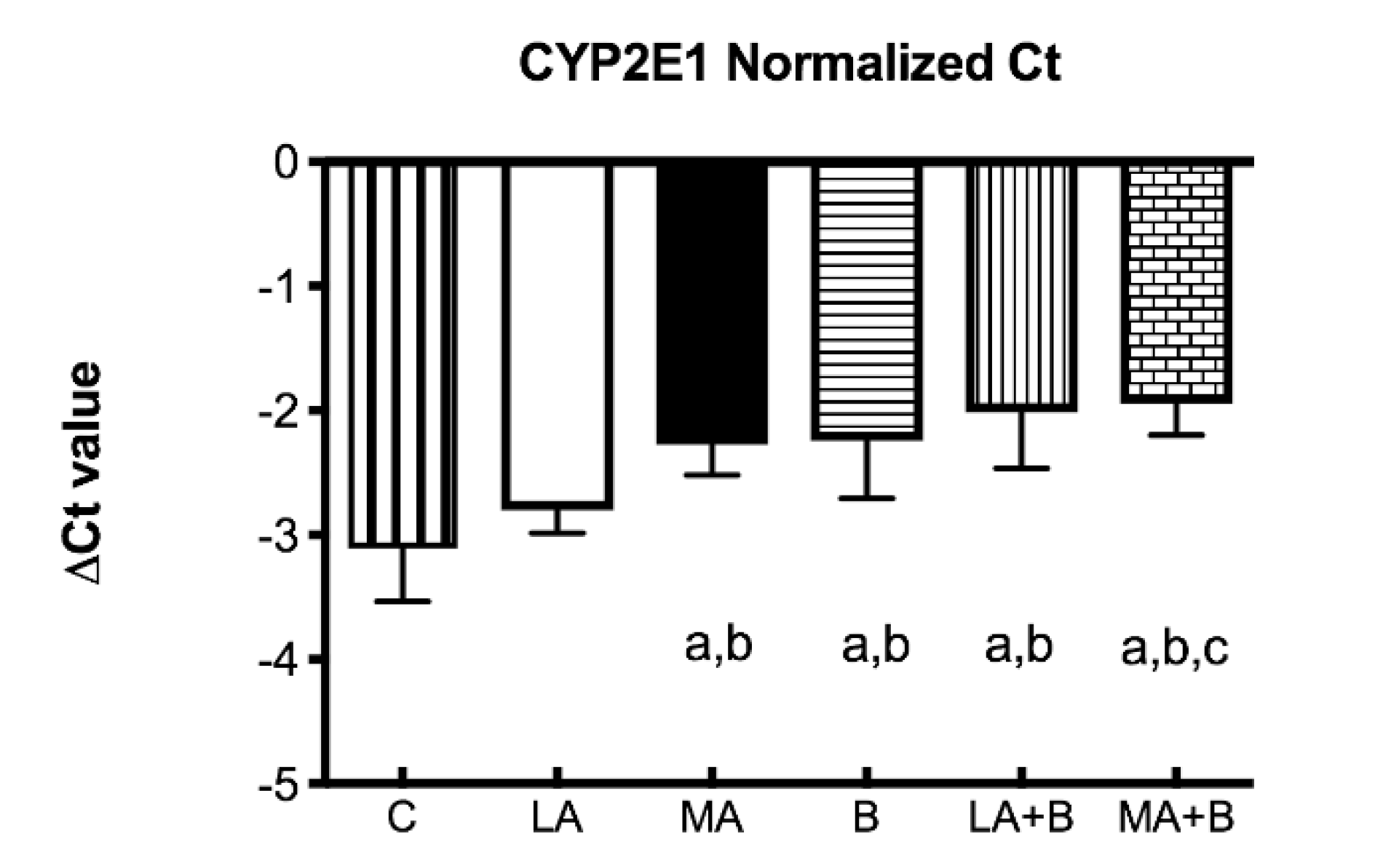
Since chronic ethanol feeding elevates CYP450 2E1, the ΔCT values were determined for CYP2E1 mRNA expression profiles of control and experimental groups after 28 days of ethanol and/or ß-carotene administration (Figure 3). The ΔCT value is the gap among the target gene and housekeeping genes, described as:
∆CT = average CT (a target gene) − average CT (housekeeping genes)
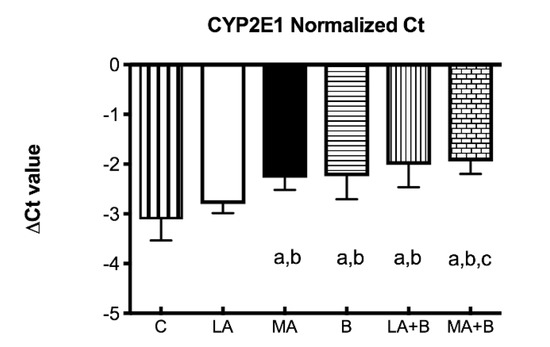
Figure 3.
Delta Ct values of CYP2E1 mRNA of control and experimental groups. Bars represent mean ± SD values of ∆Ct per group; a: significant differences (p < 0.05) with the C group; b: significant differences (p < 0.05) with the LA group; c: significant differences (p < 0.05) with the MA group.
The 2−ΔΔCT comparative approach was used to estimate relative gene expression. All data were regulated to ACTB and GAPDH mRNA content. Comparative RNA expression study in experimental versus control groups (calibrator) was performed as follows:
Experimental groups: ΔCt = Ct (target) − Ct (housekeeping genes)
Control group: ΔCt = Ct (target) − Ct (housekeeping genes)
ΔΔCt = ΔCt (experimental groups) − ΔCt (control group)
Ratio = 2−ΔΔCt
Control group: ΔCt = Ct (target) − Ct (housekeeping genes)
ΔΔCt = ΔCt (experimental groups) − ΔCt (control group)
Ratio = 2−ΔΔCt
The average ΔCt value of housekeeping gene RNA was subtracted from the average Ct value of the control and experimental groups, yielding the Ct value. The ΔΔCt value was obtained by subtracting the control group’s ΔCt value from the experimental groups’ ΔCt value [32]. The ratio 2−ΔΔCt was used to determine the fold of enrichment values.
Figure 4 displays that following 28 days of alcohol intake, levels of CYP2E1 were bigger 2267 ± 0.707-fold in LA+B and 2.307 ± 0.384-fold in MA+B groups after ethanol and β-carotene exposure. No significant differences were found between C and LA groups.
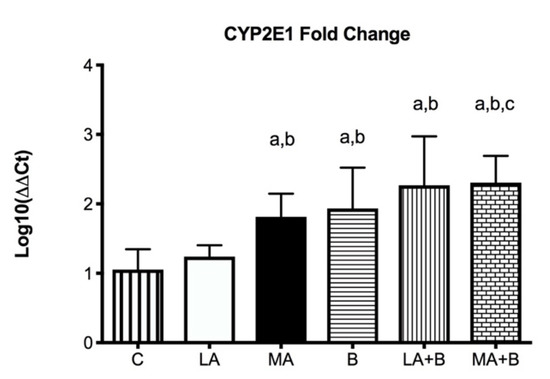
Figure 4.
CYP2E1 mRNA fold change is expressed as fold change using the ΔΔCt method in experimental groups with respect to the control group (calibrator). Bars represent mean ± SD values of fold change per group; a: significant differences (p < 0.05) with the C group; b: significant differences (p < 0.05) with the LA group; c: significant differences (p < 0.05) with the MA group.
The housekeeping genes allow the target gene’s gene expression pattern to be normalized against the quantity of input RNA or cDNA. They were adjusted for probable RNA degradation, sample management variations, reverse-transcription efficacy differences, the existence of inhibitors in the RNA sample and RNA content, and sample handling differences. The comparative approach (ratio 2−ΔΔCT) was applied, with ACTB and GAPDH serving as housekeeping genes and the control group serving as a calibrator [33].
4. Discussion
4.1. Summary of Key Findings and Interpretation
For reference samples, the relative gene expression is commonly adjusted to 1 since CT equals 0 and hence 20 equals 1. The 2−ΔΔCT method approach assumes that all samples have a consistent PCR amplification efficiency of 100 percent [33,34]. The number 2 is 1 plus the PCR amplification efficiency (100 %). This assumption simplifies the method and ensures that it is valid in ideal circumstances. However, since there are variables such as the existence of PCR inhibitors or enhancers, extraction of RNA, and various primers, probes, and enzymes, PCR efficiency cannot be guaranteed.
Ethanol dependence is a disease that progresses nearly five years after the primary alcohol use starts and it takes nearly 15–20 years for the alcohol addict to request medical care [35,36]. While much of the effort on alcohol metabolism has been on ADH, chronic alcohol consumption might raise levels of other enzymes such as CYP2E1 [15,30,37,38,39]. In effect, elevated levels of CYP2E1 in the liver of patients with alcoholic and nonalcoholic liver diseases have been shown [40]. As expected, ethanol has shown that feeding mice using the Lieber-DeCarli liquid diet results in a fold increase in CYP2E1 levels compared to control (Figure 3 and Figure 4). Under these conditions, previous studies using the same protocol have shown alcohol feeding produces fatty liver and raises LDL-C levels [39].
Following alcohol intake, the microsomal monooxygenase system, and the microsomal respiratory chain, both of which rely on CYP2E1, are the major generators of ROS in hepatocytes. Because of its propensity to process and stimulate diverse hepatotoxic substrates in the liver, cytochrome P450 2E1 is particularly essential in the prevention of carbon tetrachloride, alcohol, N-nitroso dimethylamine, and acetaminophen turning into more toxic compounds [10]. The activation of CYP2E1 by ethanol seems to be one of the keyways by which alcohol produces oxidative stress. In effect, CYP2E1 also converts ethanol to acetaldehyde, a highly reactive molecule that contributes to ethanol toxicity [11]. Furthermore, heavy alcohol use appears to be associated with CYP2E1 blood expression [41]. Due to enhanced NADPH oxidase activity and strong production of O2 and H2O2 radicals even in the absence of substrate, CYP2E1 is a potent ROS generator [42].
Previous research has established the alcohol and β-carotene dosages, as well as the treatment times [24,25]. In this regard, low doses of oral β-carotene supplementation have been shown to protect against liver damage caused by the antioxidant pathway [20,43]. Our findings have reported greater levels of CYP2E1 fold-change in the MA, LA+B, and MA+B groups in comparison to the C group and even the LA group (Figure 4, p < 0.05). The C and LA groups had the lowest levels (1.053 ± 0.292 and 1.240 ± 0.163, respectively). Hence, our findings show that ß-carotene increases the activity of CYP2E1 during ethanol consumption in low and moderate doses. Conversely, previous studies using the same protocol found it could prevent alcoholic liver disease and improve health when biochemical markers and histopathological and transmission electron microscopy are used in the evaluation [20,39]. In effect, reduced oxidative stress and reduced CYP2E1 activity have been found to support the protection provided by induced-β-carotene in a moderate alcohol intake. These discrepancies demonstrate the need to identify the action pathway of β-carotene during ethanol metabolism, which could be directly related to acetaldehyde and even acetate. Despite these findings, it is necessary to understand that these discrepancies with other studies are mainly due to the amount of alcohol drunk, the exposure period, the regulatory mechanisms of alcoholic liver damage, and the signaling pathways involved in the consumption of both alcohol and antioxidants. In fact, previous reviews have described these discrepancies using vitamins and supplements against alcoholic liver disease [44].
Although increases in CYP2E1 mRNA can occur at very high blood ethanol levels [45], ethanol induction of CYP2E1 is mostly posttranscriptional, suggesting the stability of CYP2E1 against proteosome-mediated destruction. Ethanol is both a ligand and a substrate for CYP2E1, which explains its ability to stabilize and prolong the half-life of the enzyme [46,47]. This study also shown that combining ethanol with p-carotene causes a more severe hepatic damage in C57BL/6 mice than either drug alone, raising concerns about the use of B-carotene as a source of retinol and as an anticancer agent when substantial alcohol use or abuse is present. It has been frequently suggested that a lack of carotenoids in a diet is related to an increased risk of cancer [48,49,50], while several studies have failed to show such a link [51,52].
4.2. Scope and Limitations
The aim of this research was to evaluate the consequences of β-carotene on CYP2E1 activity of C57BL/6 mice exposed to alcohol exposure. In addition, our data update the existing linkage between CYP2E1 and β-carotene therapy. However, the absence of information linking alcohol dehydrogenase and aldehyde dehydrogenase expressions with antioxidant treatments remains a limitation of this research and must be attended to in future studies. Unfortunately, previous research results provide little support for this notion. Considering these data, seems that β-carotene exposure did not improve the hepatotoxic damage induced by alcohol exposure in C57BL/6 mice.
5. Conclusions
Because CYP2E1 activity increases after moderate alcohol consumption and β-carotene, our findings imply that antioxidant therapies might be dangerous during ethanol exposure in animal models. Despite all the progress made in understanding the effects of antioxidant supplementation, future studies should use specific cell lines and clinical trials to better understand the relationship between alcohol consumption, antioxidant therapies, the signaling pathways involved, and enzymatic and non-enzymatic mechanisms.
Author Contributions
C.S., L.M., J.F., K.G. and K.A. carried out the conception and design of the research. C.S. and K.G. have participated in the experimental phase, and they did the molecular analysis using qPCR. L.M. and J.F. did the quantification of RNA from liver tissue. C.S. and K.A. participated in obtaining funding. All authors have read and agreed to the published version of the manuscript.
Funding
Universidad de La Frontera, DIUFRO Project DI22–0007 and Programa de Formación de Investigadores Postdoctorales 2022.
Institutional Review Board Statement
The animal study protocol was approved by the Scientific Ethics Committee of the Universidad de La Frontera (Nº051/2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are openly available in “figshare” at https://doi.org/10.6084/m9.figshare.19766218.v1, accessed on 4 April 2022.
Conflicts of Interest
The authors have declared no conflict of interest.
References
- Haber, P.S.; Apte, M.V.; Moran, C.; Applegate, T.L.; Pirola, R.C.; Korsten, M.A.; McCaughan, G.W.; Wilson, J.S. Non-oxidative Metabolism of Ethanol by Rat Pancreatic Acini. Pancreatology 2004, 4, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Brust, J.C.M. Ethanol and Cognition: Indirect Effects, Neurotoxicity, and Neuroprotection: A Review. Int. J. Environ. Res. Public Health 2010, 7, 1540–1557. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Alcohol. Descriptive Note Nº 349; World Health Organization: New York, NY, USA, 2011.
- Cederbaum, A.I. Cytochrome P450 2E1-dependent Oxidant Stress and Upregulation of Anti-oxidant Defense in Liver Cells. J. Gastroenterol. Hepatol. 2006, 21, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I.; Roychowdhury, S.; DiBello, P.M.; Jacobsen, D.W.; Nagy, L.E. Exogenous Thioredoxin Prevents Ethanol-induced Oxidative Damage and Apoptosis in Mouse Liver. Hepatology 2009, 49, 1709–1717. [Google Scholar] [CrossRef] [Green Version]
- García Gutiérrez, E.; Lima Mompó, G.; Aldana Vilas, L.; Casanova Carrillo, P.; Feliciano Álvarez, V. Alcoholismo y sociedad, tendencias actuales. Rev. Cub. Med. Mil. 2004, 33, 3. [Google Scholar]
- Arias, R. Reacciones fisiológicas y neuroquímicas del alcoholismo. Diversitas 2005, 1, 138–147. [Google Scholar] [CrossRef]
- Lakshman, R.; Cederbaum, A.I.; Hoek, J.B.; Konishi, M.; Koop, D.; Donohue, T.M. Use of CYP2E1-transfected Human Liver Cell Lines in Elucidating the Actions of Ethanol. Alcohol Clin. Exp. Res. 2006, 29, 1726–1734. [Google Scholar] [CrossRef]
- Wu, H.; Cai, P.; Clemens, D.L.; Jerrells, T.R.; Shakeel Ansari, G.A.; Kaphalia, B.S. Metabolic Basis of Ethanol-induced Cytotoxicity in Recombinant HepG2 Cells: Role of Nonoxidative Metabolism. Toxicol. Appl. Pharmacol. 2006, 216, 238–247. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and Oxidative Liver Injury by Alcohol. Free Rad. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Klionsky, D.J. Eaten Alive: A History of Macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.M.; Cunningham, C.C. Contribution of Mitochondria to Oxidative Stress Associated with Alcoholic Liver Disease. Free Rad. Biol. Med. 2002, 32, 11–16. [Google Scholar] [CrossRef]
- Hoek, J.B.; Cahill, A.; Pastorino, J.G. Alcohol and Mitochondria: A Dysfunctional Relationship. Gastroenterology 2002, 122, 2049–2063. [Google Scholar] [CrossRef] [Green Version]
- Tuma, D.J.; Casey, C.A. Dangerous Byproducts of Alcohol Breakdown: Focus on Adducts. Alcohol Res. Health 2003, 27, 285–290. [Google Scholar]
- Sandoval, C.; Vaásquez, B.; Mandarim-de-Lacerda, C.; del Sol, M. Ethanol Intake and Toxicity: In Search of New Treatments. Int. J. Morphol. 2017, 35, 942–949. [Google Scholar] [CrossRef] [Green Version]
- Schattenberg, J.M.; Czaja, M.J. Regulation of the Effects of CYP2E1-induced Oxidative Stress by JNK Signaling. Redox Biol. 2014, 3, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Diesinger, T.; Buko, V.; Lautwein, A.; Dvorsky, R.; Belonovskaya, E.; Lukivskaya, O.; Naruta, E.; Kirko, S.; Andreev, V.; Buckert, D.; et al. Drug Targeting CYP2E1 for the Treatment of Early-stage Alcoholic Steatohepatitis. PLoS ONE 2020, 15, e0235990. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide Dismutase Multigene Family: A Comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) Gene Structures, Evolution, and Expression. Free Rad. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Chang, P.; Cheng, E.; Brooke, S.; Sapolsky, R. Marked Differences in the Efficacy of Post-insult Gene Therapy with Catalase versus Glutathione Peroxidase. Brain Res. 2005, 1063, 27–31. [Google Scholar] [CrossRef]
- Sandoval, C.; Vásquez, B.; Souza-Mello, V.; Adeli, K.; Mandarim-de-Lacerda, C.; del Sol, M. Morphoquantitative Effects of Oral β-carotene Supplementation on Liver of C57BL/6 Mice Exposed to Ethanol Consumption. Int. J. Clin. Exp. Pathol. 2019, 12, 1713–1722. [Google Scholar]
- Schott, M.B.; Rasineni, K.; Weller, S.G.; Schulze, R.J.; Sletten, A.C.; Casey, C.A.; McNiven, M.A. β-Adrenergic Induction of Lipolysis in Hepatocytes Is Inhibited by Ethanol Exposure. J. Biol. Chem. 2017, 292, 11815–11828. [Google Scholar] [CrossRef] [Green Version]
- Werling, K. A Májbetegségek Kialakulásának Új Szempontjai—Különös Tekintettel Az Autophagiára Ès A mikro-RNS Szerepére. Orvosi Hetilap 2020, 161, 1449–1455. [Google Scholar] [CrossRef]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; Institute for Laboratory Animal Research; Division on Earth and Life Studies. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Furuya, D.T.; Binsack, R.; Machado, U.F. Low ethanol consumption increases insulin sensitivity in Wistar rats. Braz. J. Med. Biol. Res. 2003, 36, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.C.; Chen, Y.L.; Yang, S.Y.; Ho, P.Y.; Yang, S.S.; Hu, J.T.; Yang, S.C. The antiapoptotic effects of different doses of β-carotene in chronic ethanol-fed rats. Hepatobiliary Surg. Nutr. 2013, 2, 132–141. [Google Scholar] [CrossRef]
- Diao, Y.; Nie, J.; Tan, P.; Zhao, Y.; Zhao, T.; Tu, J.; Ji, H.; Cao, Y.; Wu, Z.; Liang, H.; et al. Long-term low-dose ethanol intake improves healthspan and resists high-fat diet-induced obesity in mice. Aging 2020, 12, 13128–13146. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA Using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef]
- Sheng, Q.; Vickers, K.; Zhao, S.; Wang, J.; Samuels, D.C.; Koues, O.; Shyr, Y.; Guo, Y. Multi-perspective quality control of Illumina RNA sequencing data analysis. Brief. Funct. Genom. 2017, 16, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Bustin, S.A. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002, 29, 23–29. [Google Scholar] [CrossRef]
- Pfaffl, M.W. Quantification Strategies in Real-Time PCR. In A-Z of Quantitative PCR; International University Line: La Jolla, CA, USA, 2004. [Google Scholar]
- Finis, K.; Sültmann, H.; Ruschhaupt, M.; Buness, A.; Helmchen, B.; Ruprecht, K.; Gross, M.L.; Fink, B.; Schirmacher, P.; Poustka, A. Analysis of pigmented villonodular synovitis with genome-wide complementary DNA microarray and tissue array technology reveals insight into potential novel therapeutic approaches. Arthritis Rheum. 2006, 54, 1009–1019. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantification PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Arocho, A.; Chen, B.; Ladanyi, M.; Pan, Q. Validation of the 2-DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn. Mol. Pathol. 2006, 15, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, L.; Sonzogni, L.; De Silvestri, A. CYP Enzyme Polymorphısms and Susceptıbılıty to HCV-Related Chronıc Liver Disease and Liver Cancer. Int. J. Cancer 2003, 104, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. The Discovery of the Microsomal Ethanol Oxidizing System and Its Physiologic and Pathologic Role. Drug Metab. Rev. 2004, 36, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, C.; Vásquez, B.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; del Sol, M. Rol del consumo de alcohol y antioxidantes sobre la metilación global del ADN y cáncer. Int. J. Morphol. 2018, 36, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, C.; Carrasco, C.; Souza-Mello, V.; Sandoval, C. Effectiveness of antioxidant treatments on cytochrome P450 2E1 (CYP2E1) activity after alcohol exposure in humans and in vitro models: A systematic review. Int. J. Food Prop. 2021, 24, 1300–1317. [Google Scholar] [CrossRef]
- Sandoval, C.; Vásquez, B.; Vasconcellos, A.; Souza-Mello, V.; Adeli, K.; Mandarim-de-Lacerda, C.; del Sol, M. Oral supplementation of b-carotene benefits the hepatic structure and metabolism in mice exposed to chronic ethanol consumption. Sains Malays. 2022, 51, 285–296. [Google Scholar] [CrossRef]
- Niemelä, O.; Parkkila, S.; Pasanen, M.; Viitala, K.; Villanueva, J.A.; Halsted, C.H. Induction of cytochrome P450 enzymes and generation of protein-aldehyde adducts are associated with sex-dependent sensitivity to alcohol-induced liver disease in micropigs. Hepatology 1999, 30, 1011–1017. [Google Scholar] [CrossRef]
- Liangpunsakul, S.; Kolwankar, D.; Pinto, A.; Gotski, J.C.; Hall, S.D.; Chalasani, N. Activity of CYP2E1 and CYP3A enzymes in adults with moderate alcohol consumption: A comparison with nonalcoholics. Hepatology 2005, 41, 1144–1150. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. Cisplatin-Induced Hepatotoxicity Is Enhanced by Elevated Expression of Cytochrome P450 2E1. Toxıcol. Sci. 2006, 89, 515–523. [Google Scholar] [CrossRef]
- Lin, W.T.; Huang, C.C.; Lin, T.J.; Chen, J.R.; Shieh, M.J.; Peng, H.C.; Yang, S.C.; Huang, C.Y. Effects of beta-carotene on antioxidant status in rats with chronic alcohol consumption. Cell Biochem. Funct. 2009, 27, 344–350. [Google Scholar] [CrossRef]
- Sandoval, C.; Farías, J.; Zamorano, M.; Herrera, C. Vitamin Supplements as a Nutritional Strategy against Chronic Alcohol Consumption? An Updated Review. Antioxidants 2022, 11, 564. [Google Scholar] [CrossRef]
- Ronis, M.J.; Huang, J.; Crouch, J.; Mercado, C.; Irby, D.; Valentine, C.R.; Lumpkin, C.K.; Ingelman-Sundberg, M.; Badger, T.M. Cytochrome P450 CYP 2E1 induction during chronic alcohol exposure occurs by a two-step mechanism associated with blood alcohol concentrations in rats. J. Pharmacol. Exp. Ther. 1993, 264, 944–950. [Google Scholar]
- Eliasson, E.; Johansson, I.; Ingelman-Sundberg, M. Ligand-dependent maintenance of ethanol-inducible cytochrome P-450 in primary rat hepatocyte cell cultures. Biochem. Biophys. Res. Commun. 1988, 150, 436–443. [Google Scholar] [CrossRef]
- Lieber, C.S. Microsomal ethanol-oxidizing system (MEOS): The first 30 years (1968–1998)—A review. Alcohol. Clin. Exp. Res. 1999, 23, 991–1007. [Google Scholar] [CrossRef]
- Ziegler, R.G. A review of epidemiologic evidence that carotenoids reduce the risk of cancer. J. Nutr. 1989, 119, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, R.G. Vegetables, fruits, and carotenoids and the risk of cancer. Am. J. Clin. Nutr. 1991, 53 (Suppl. S1), 251S–259S. [Google Scholar] [CrossRef]
- Stahelin, H.B.; Gey, K.F.; Eichholzer, M.; Ludin, E. B-carotene and cancer prevention: The Base1 Study. Am. J. Clin. Nutr. 1991, 53 (Suppl. S1), 265S–269S. [Google Scholar] [CrossRef]
- Paganini-Hill, A.; Chao, A.; Ross, R.K.; Henderson, B.E. Vitamin A, @-carotene, and the risk of cancer: A prospective study. J. Natl. Cancer Inst. 1987, 79, 443–448. [Google Scholar]
- Leo, M.A.; Kim, C.; Lowe, N.; Lieber, C.S. Interaction of ethanol with beta-carotene: Delayed blood clearance and enhanced hepatotoxicity. Hepatology 1992, 15, 883–891. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).