Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity
Abstract
:1. Introduction
2. Materials and Methods
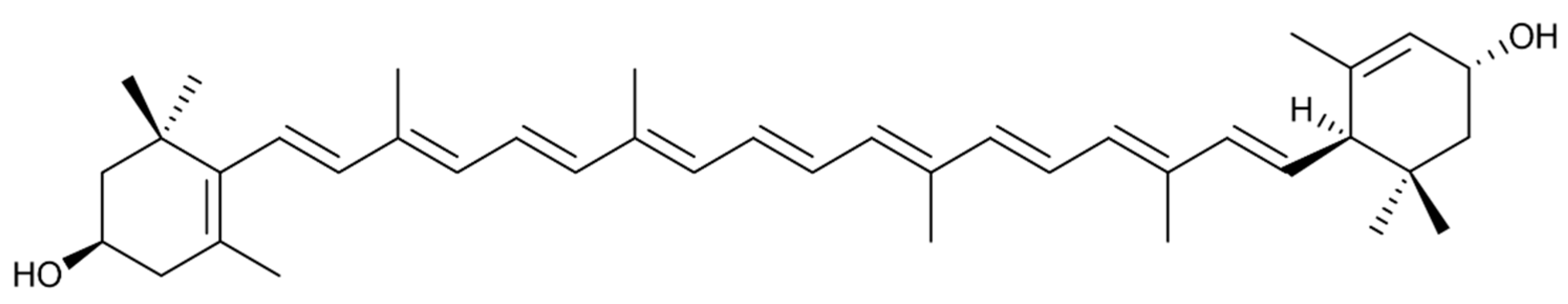
2.1. Isolation of Lutein
2.2. Cell Culture and Treatments
2.3. Cell Viability Assay
2.4. Detection of the Reactive Oxygen Species
2.5. Detection of Catalase Activity
2.6. Detection of Superoxide Dismutase Activity
2.7. Enzyme-Linked Immunosorbent Assay (ELISA) Measurements
2.8. Real-Time PCR
2.9. Western Blot
2.10. Measurements of Total Iron
2.11. Measurements of Heme Concentration
2.12. Statistical Analysis
3. Results
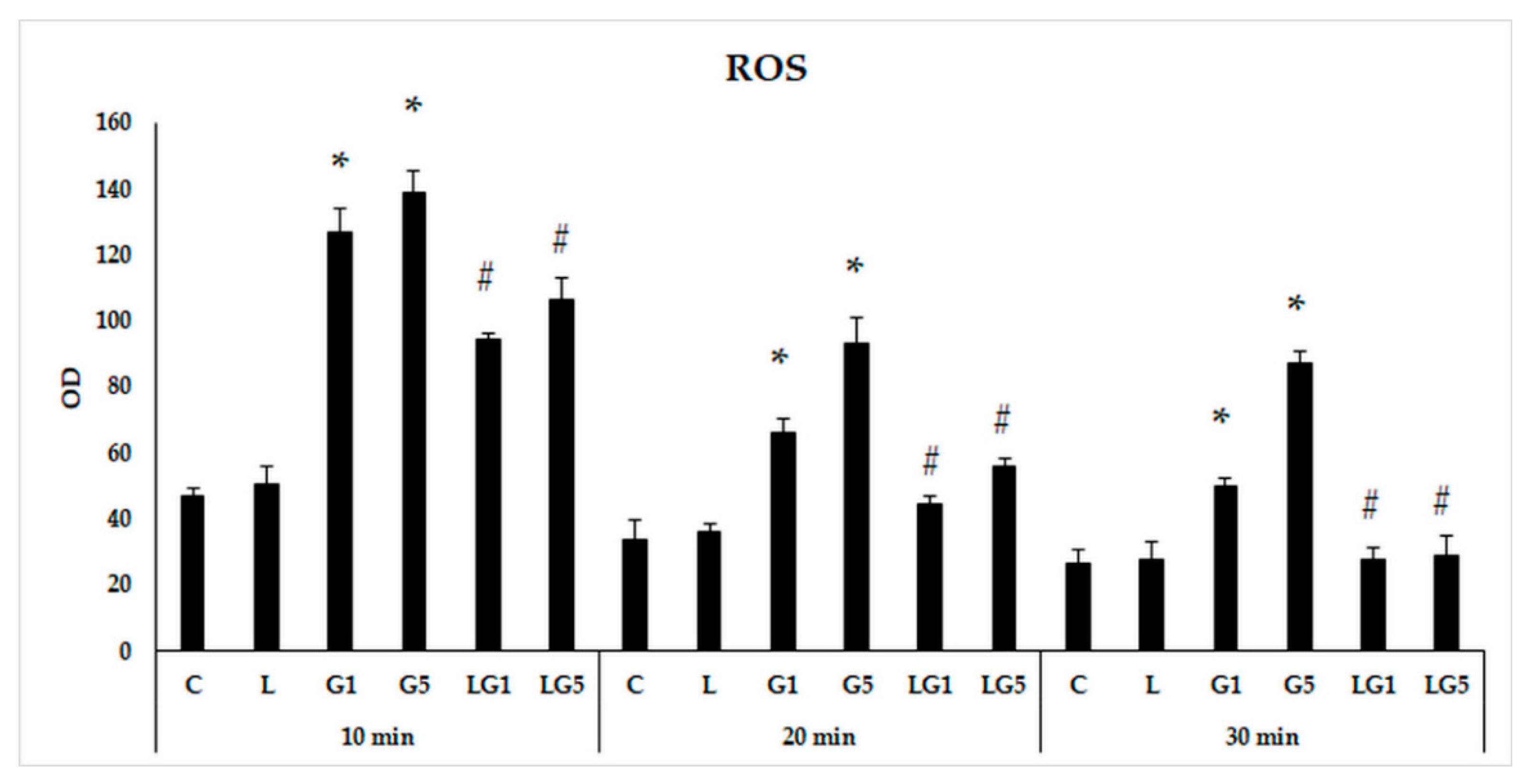
3.1. Effects of Lutein on Reactive Oxygen Species (ROS) Generated by Glutamate
3.2. Effects of Lutein on Antioxidant Enzyme Activities of Glutamate-Treated SH-SY5Y
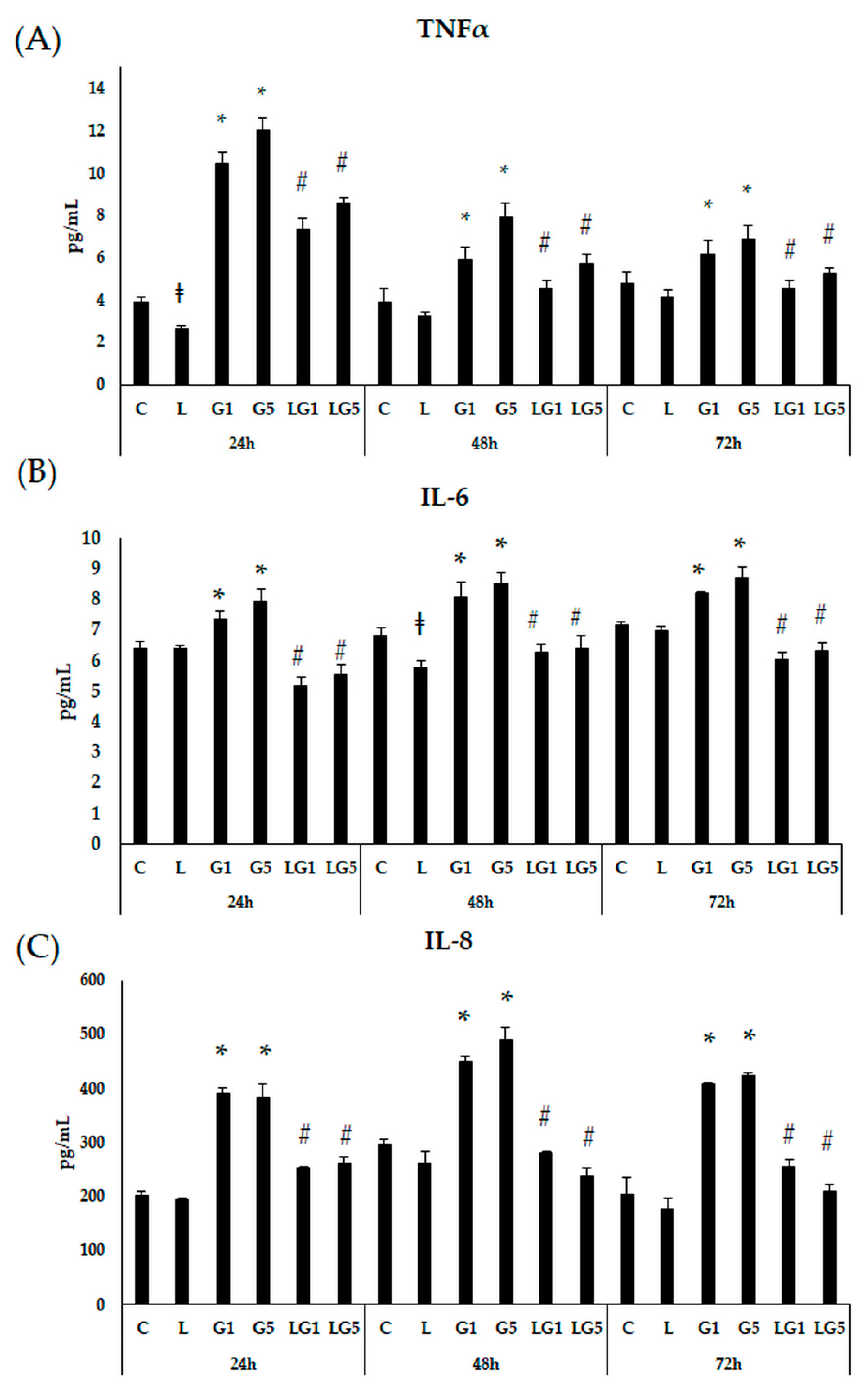
3.3. Effects of Lutein on Proinflammatory Cytokine Secretion Induced by Glutamate in SH-SY5Y Cells
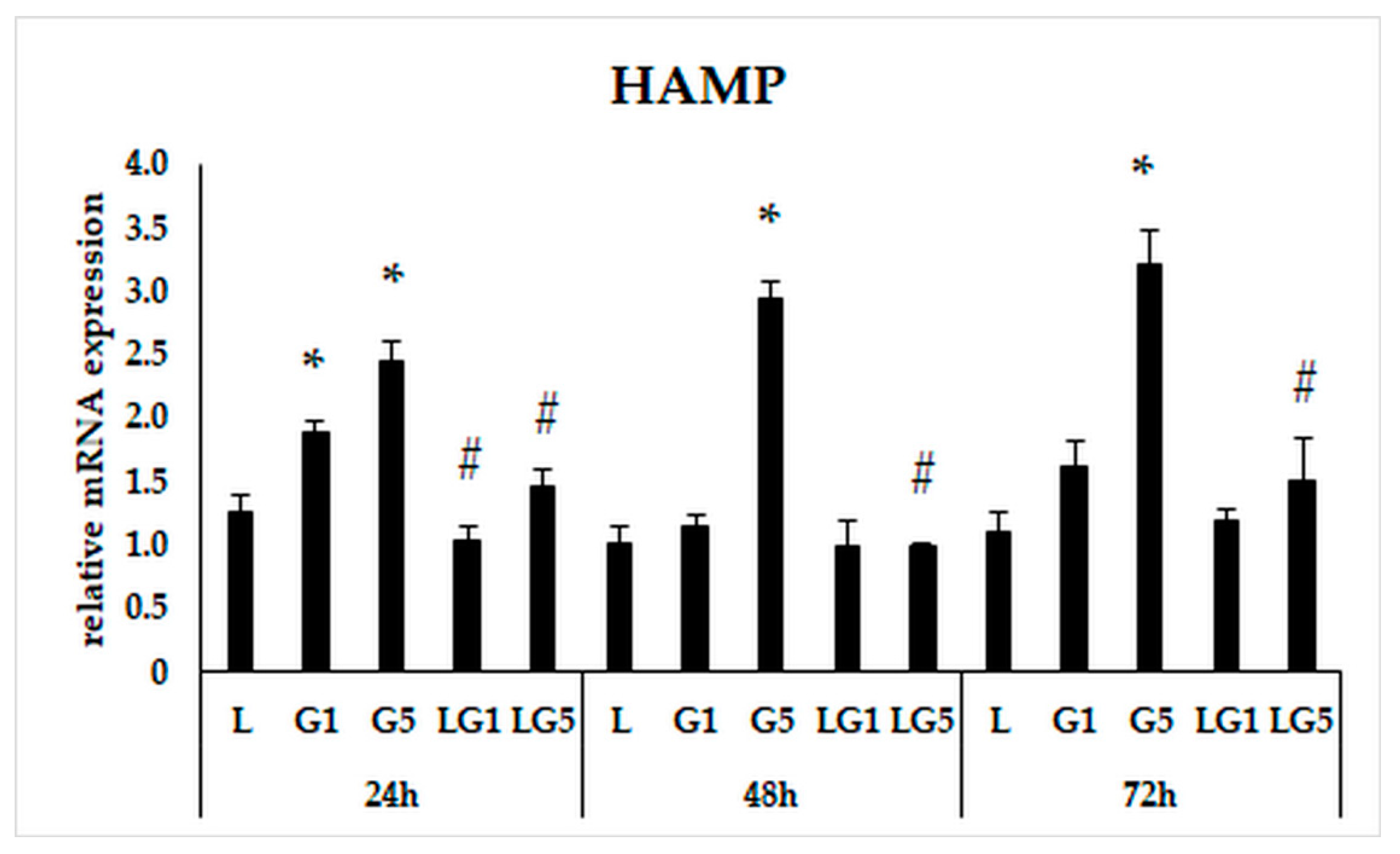
3.4. Lutein Ameliorates the Effect of Glutamate on the mRNA Expression of the Iron Regulatory Hormone Hepcidin
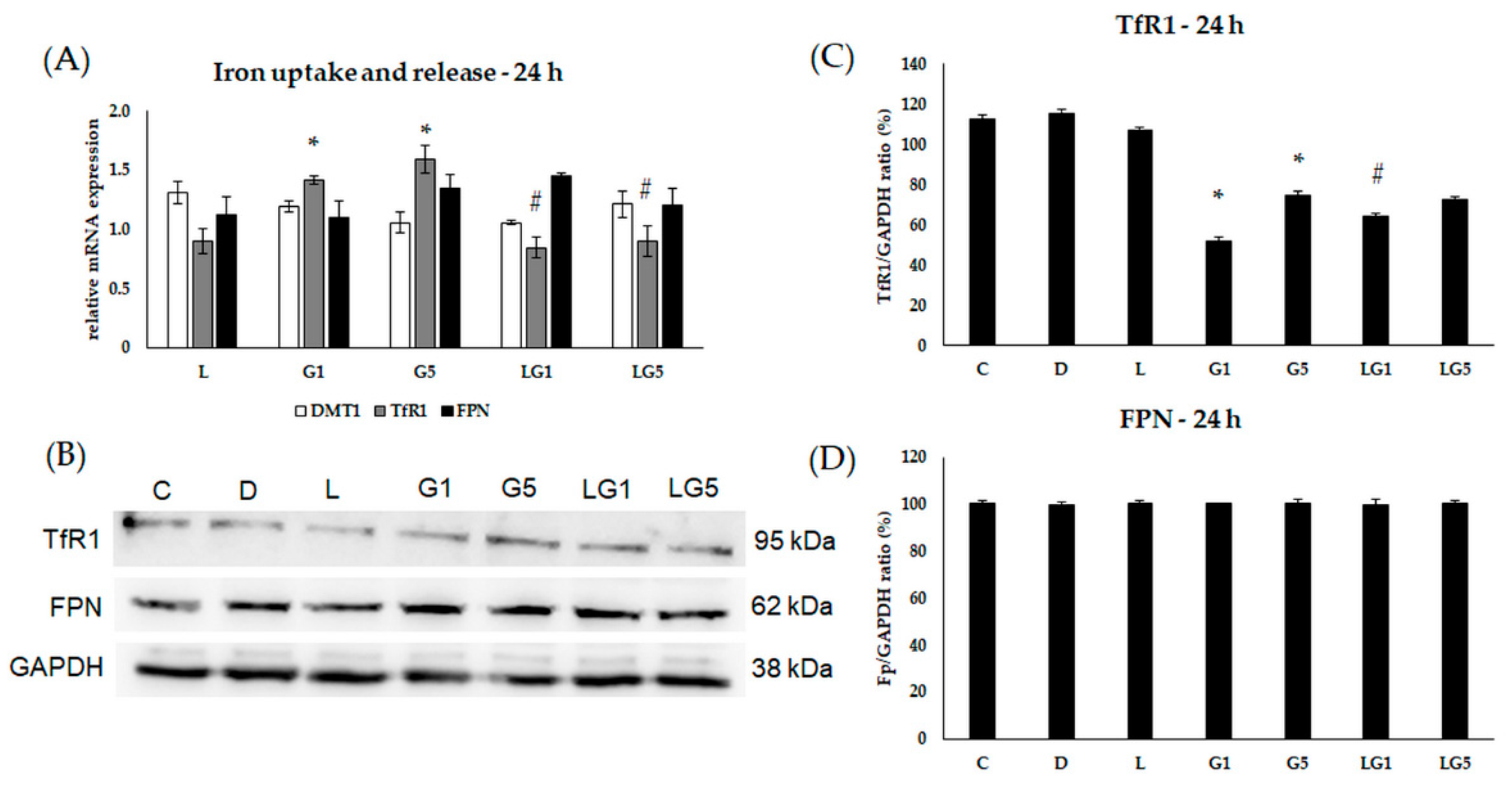
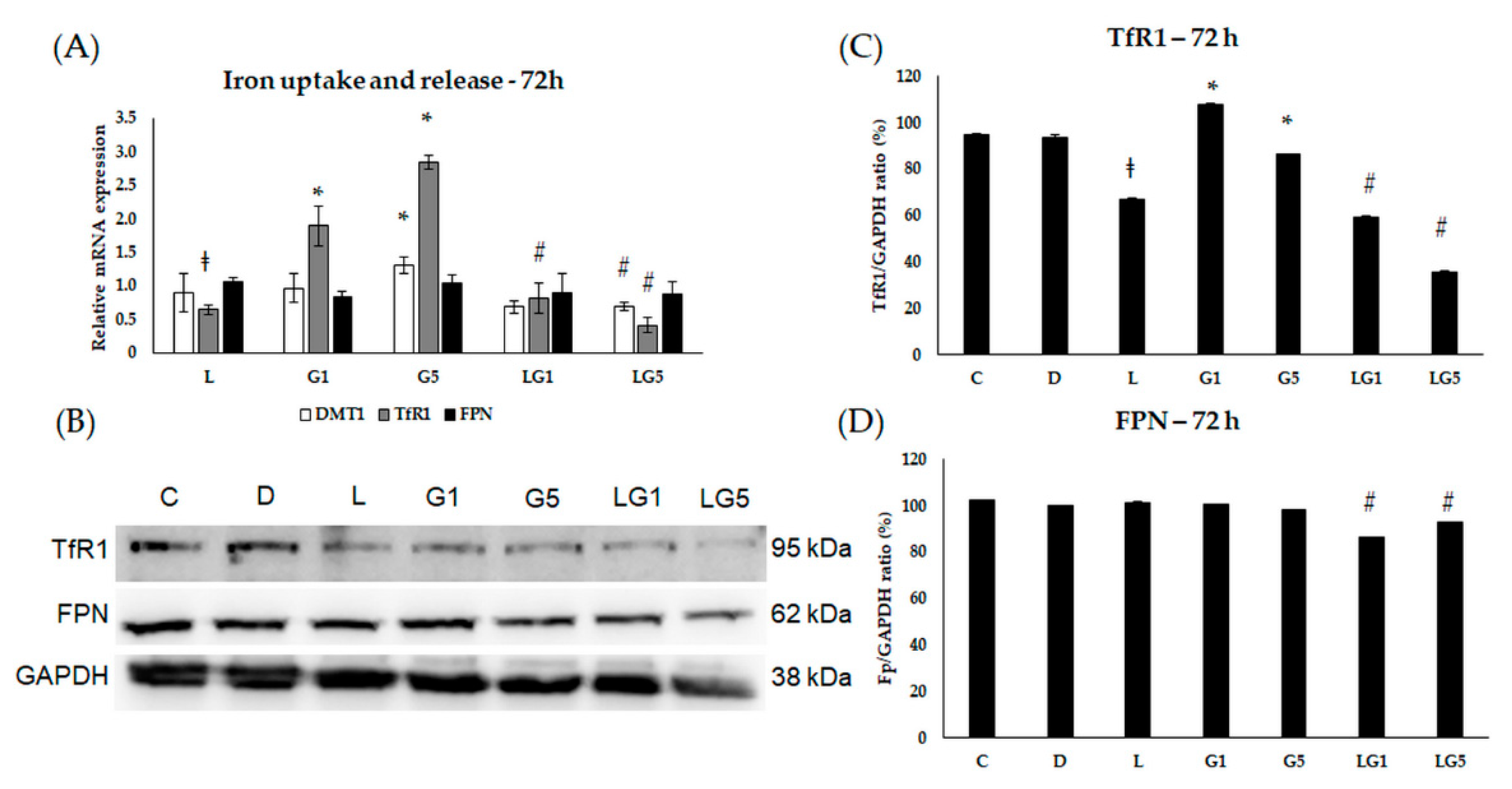
3.5. Lutein Modifies the Glutamate’s Effect on Iron Uptake and Release
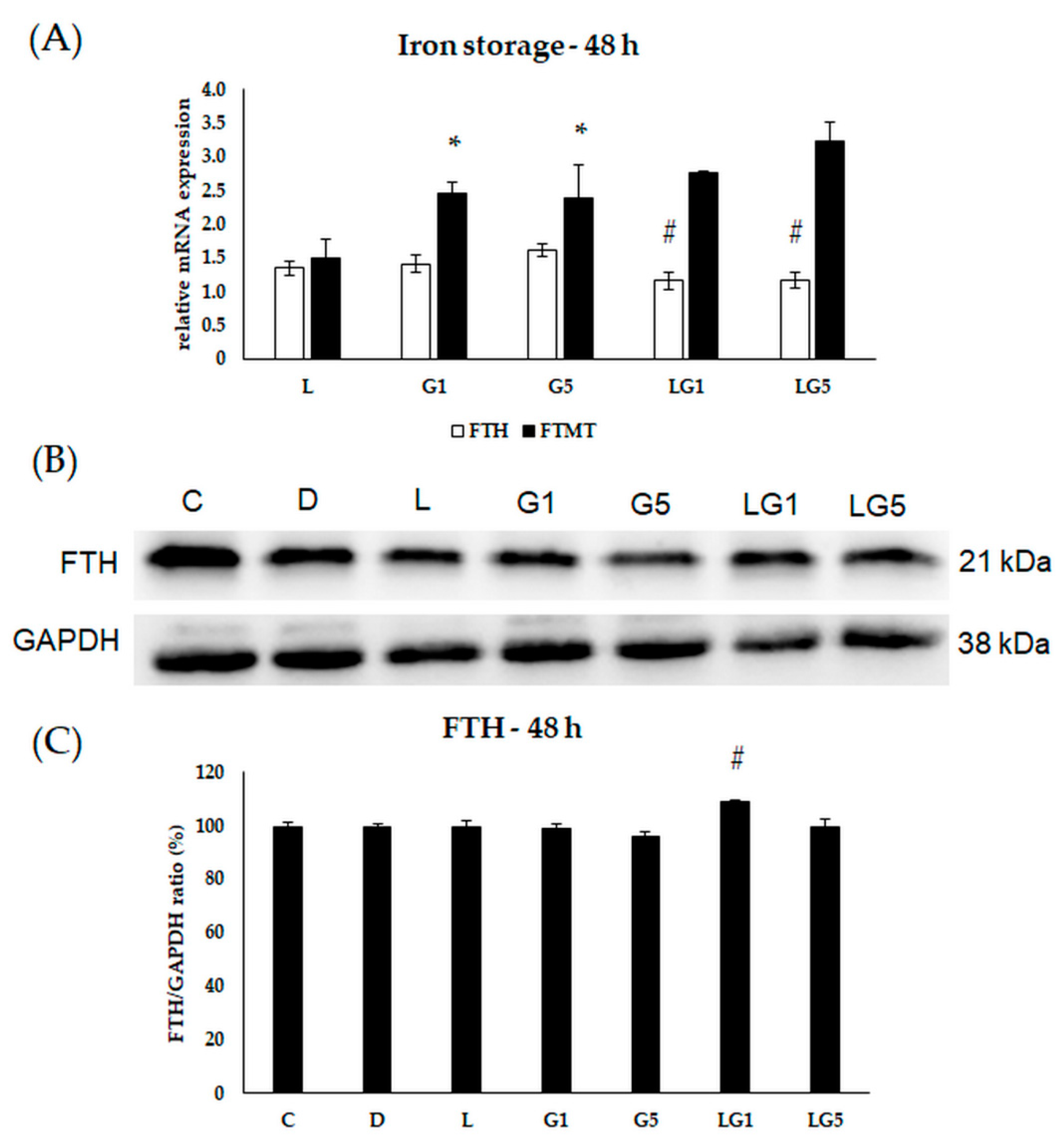
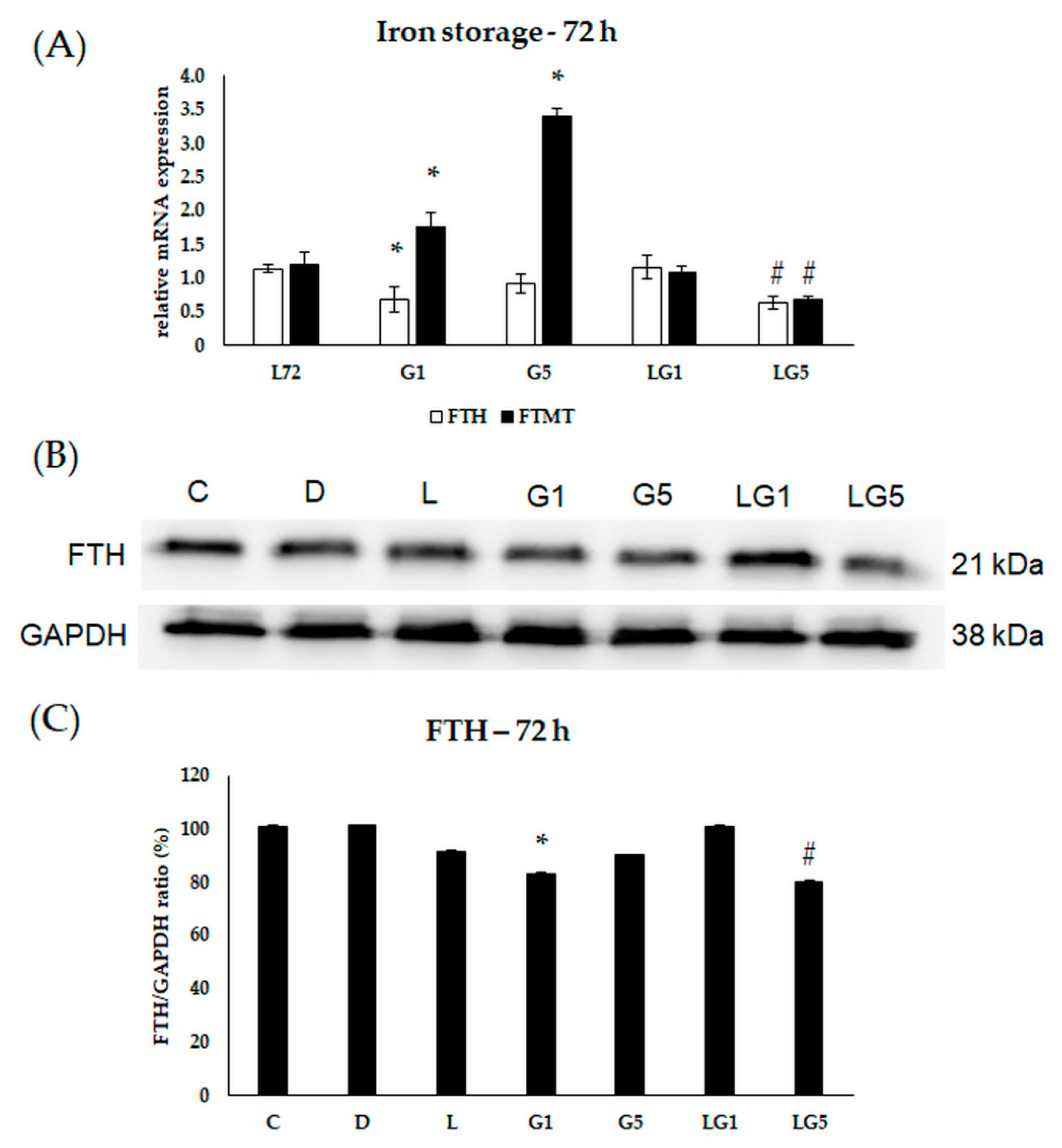
3.6. Effects of Glutamate and Lutein on the Iron Storage of the SH-SY5Y Cells
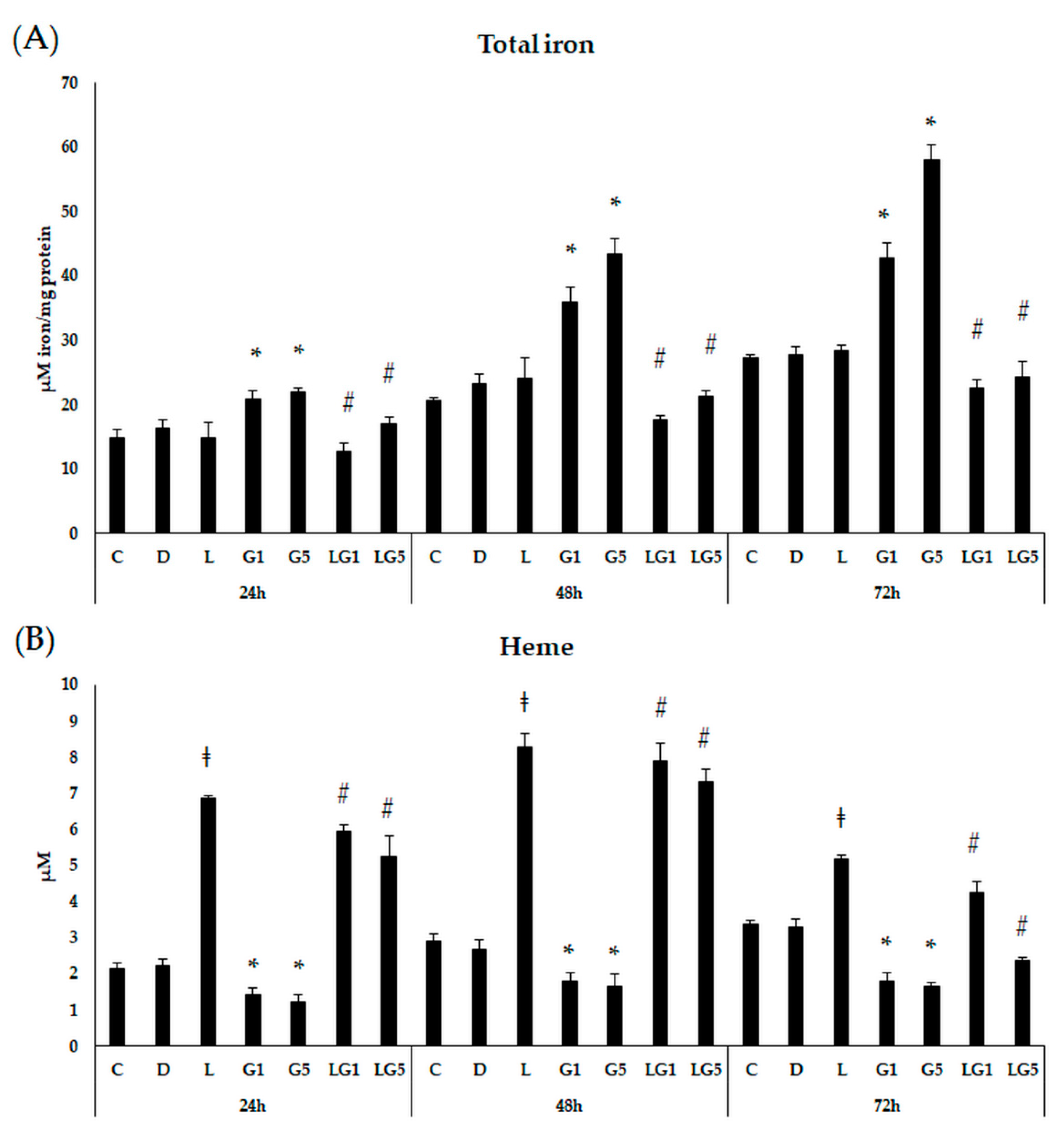
3.7. Effect of Lutein on the Iron Levels in the Glutamate-Treated SH-SY5Y Cells
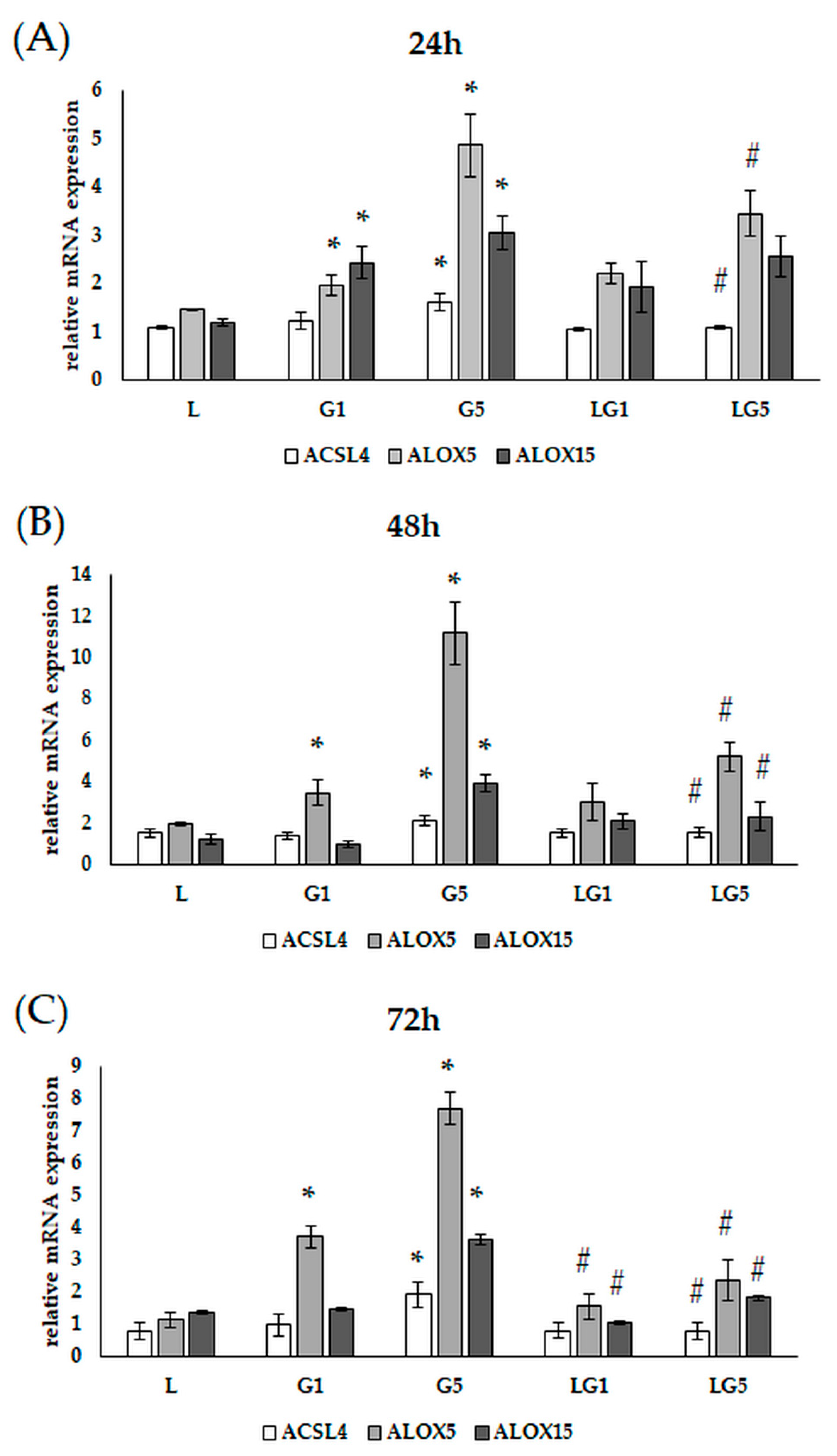
3.8. Lutein Downregulates the Glutamate-Induced Upregulation of Lipoxygenases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The effect of lutein on eye and extra-eye health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef] [Green Version]
- Koushan, K.; Rusovici, R.; Li, W.; Ferguson, L.R.; Chalam, K.V. The role of lutein in eye-related disease. Nutrients 2013, 5, 1823–1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary Carotenoids, Vitamins A, C, and E, and Advanced Age-Related Macular Degeneration. JAMA J. Am. Med. Assoc. 1994, 272, 1413–1420. [Google Scholar] [CrossRef]
- Yagi, A.; Nouchi, R.; Butler, L.; Kawashima, R. Lutein has a positive impact on brain health in healthy older adults: A systematic review of randomized controlled trials and cohort studies. Nutrients 2021, 13, 1746. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Agócs, A.; Deli, J. Lutein exerts antioxidant and anti-inflammatory effects and influences iron utilization of bv-2 microglia. Antioxidants 2021, 10, 363. [Google Scholar] [CrossRef]
- Erdman, J.W.; Smith, J.W.; Kuchan, M.J.; Mohn, E.S.; Johnson, E.J.; Rubakhin, S.S.; Wang, L.; Sweedler, J.V.; Neuringer, M. Lutein and brain function. Foods 2015, 4, 547–564. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [Green Version]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Cui, Y.; An, Z.; Yang, Q.; Zou, X.; Yu, N. Attenuated glutamate induced ROS production by antioxidative compounds in neural cell lines. RSC Adv. 2019, 9, 34735–34743. [Google Scholar] [CrossRef] [Green Version]
- Atlante, A.; Calissano, P.; Bobba, A.; Giannattasio, S.; Marra, E.; Passarella, S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 2001, 497, 1–5. [Google Scholar] [CrossRef]
- Al-Nasser, M.N.; Mellor, I.R.; Carter, W.G. Is L-Glutamate Toxic to Neurons and Thereby Contributes to Neuronal Loss and Neurodegeneration? A Systematic Review. Brain Sci. 2022, 12, 577. [Google Scholar] [CrossRef] [PubMed]
- Kritis, A.A.; Stamoula, E.G.; Paniskaki, K.A.; Vavilis, T.D. Researching glutamate—Induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front. Cell. Neurosci. 2015, 9, e00091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohidpour, A.; Morgun, A.V.; Boitsova, E.B.; Malinovskaya, N.A.; Martynova, G.P.; Khilazheva, E.D.; Kopylevich, N.V.; Gertsog, G.E.; Salmina, A.B. Neuroinflammation and infection: Molecular mechanisms associated with dysfunction of neurovascular unit. Front. Cell. Infect. Microbiol. 2017, 7, e00276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liba, Z.; Nohejlova, H.; Capek, V.; Krsek, P.; Sediva, A.; Kayserova, J. Utility of chemokines CCL2, CXCL8, 10 and 13 and interleukin 6 in the pediatric cohort for the recognition of neuroinflammation and in the context of traditional cerebrospinal fluid neuroinflammatory biomarkers. PLoS ONE 2019, 14, e0219987. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef]
- Ying, J.-F.; Lu, Z.-B.; Fu, L.-Q.; Tong, Y.; Wang, Z.; Li, W.-F.; Mou, X.-Z. The role of iron homeostasis and iron-mediated ROS in cancer. Am. J. Cancer Res. 2021, 11, 1895–1912. [Google Scholar]
- Batista-Nascimento, L.; Pimentel, C.; Andrade Menezes, R.; Rodrigues-Pousada, C. Iron and neurodegeneration: From cellular homeostasis to disease. Oxid. Med. Cell. Longev. 2012, 2012, 128647. [Google Scholar] [CrossRef] [Green Version]
- Angelova, P.R.; Choi, M.L.; Berezhnov, A.V.; Horrocks, M.H.; Hughes, C.D.; De, S.; Rodrigues, M.; Yapom, R.; Little, D.; Dolt, K.S.; et al. Alpha synuclein aggregation drives ferroptosis: An interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 2020, 27, 2781–2796. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Choi, E.H.; Chaudhary, S.; Kim, M.H. Molecular dynamic simulations of oxidized skin lipid bilayer and permeability of reactive oxygen species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Haldar, S.; Tripathi, A.K.; Horback, K.; Wong, J.; Sharma, D.; Beserra, A.; Suda, S.; Anbalagan, C.; Dev, S.; et al. Brain Iron Homeostasis: From molecular mechanisms to clinical significance and therapeutic opportunities. Antioxid. Redox Signal. 2014, 20, 1324–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codazzi, F.; Pelizzoni, I.; Zacchetti, D.; Grohovaz, F. Iron entry in neurons and astrocytes: A link with synaptic activity. Front. Mol. Neurosci. 2015, 8, e00018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinert, A.; Morawski, M.; Seeger, J.; Arendt, T.; Reinert, T. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 2019, 20, 25. [Google Scholar] [CrossRef]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, C.; Wen, X.; Li, C.; Xiong, S.; Yue, T.; Long, P.; Shi, J.; Zhang, Z. ACSL4 as a Potential Target and Biomarker for Anticancer: From Molecular Mechanisms to Clinical Therapeutics. Front. Pharmacol. 2022, 13, e949863. [Google Scholar] [CrossRef]
- Kowal-Bielecka, O.; Distler, O.; Neidhart, M.; Künzler, P.; Rethage, J.; Nawrath, M.; Carossino, A.; Pap, T.; Müller-Ladner, U.; Michel, B.A.; et al. Evidence of 5-lipoxygenase overexpression in the skin of patients with systemic sclerosis: A newly identified pathway to skin inflammation in systemic sclerosis. Arthritis Rheum. 2001, 44, 1865–1875. [Google Scholar] [CrossRef]
- Kelavkar, U.P.; Nixon, J.B.; Cohen, C.; Dillehay, D.; Eling, T.E.; Badr, K.F. Overexpression of 15-lipoxygenase-1 in PC-3 human prostate cancer cells increases tumorigenesis. Carcinogenesis 2001, 22, 1765–1773. [Google Scholar] [CrossRef] [Green Version]
- AlFadly, E.D.; Elzahhar, P.A.; Tramarin, A.; Elkazaz, S.; Shaltout, H.; Abu-Serie, M.M.; Janockova, J.; Soukup, O.; Ghareeb, D.A.; El-Yazbi, A.F.; et al. Tackling neuroinflammation and cholinergic deficit in Alzheimer’s disease: Multi-target inhibitors of cholinesterases, cyclooxygenase-2 and 15-lipoxygenase. Eur. J. Med. Chem. 2019, 167, 161–186. [Google Scholar] [CrossRef]
- Siddiqui, A.; Akhtar, S.; Shah, Z.; Othman, I.; Kumari, Y. Inflammation Drives Alzheimer’s Disease: Emphasis on 5-lipoxygenase Pathways. Curr. Neuropharmacol. 2020, 19, 885–895. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 10 October 2022).
- Riemer, J.; Hoepken, H.H.; Czerwinska, H.; Robinson, S.R.; Dringen, R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal. Biochem. 2004, 331, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Wessling-Resnick, M. Iron homeostasis and the inflammatory response. Annu. Rev. Nutr. 2010, 30, 105–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochette, L.; Gudjoncik, A.; Guenancia, C.; Zeller, M.; Cottin, Y.; Vergely, C. The iron-regulatory hormone hepcidin: A possible therapeutic target? Pharmacol. Ther. 2015, 146, 35–52. [Google Scholar] [CrossRef]
- McClung, J.A.; Levy, L.; Garcia, V.; Stec, D.E.; Peterson, S.J.; Abraham, N.G. Heme-oxygenase and lipid mediators in obesity and associated cardiometabolic diseases: Therapeutic implications. Pharmacol. Ther. 2022, 231, 107975. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Carrì, M.T.; Ferri, A.; Cozzolino, M.; Calabrese, L.; Rotilio, G. Neurodegeneration in amyotrophic lateral sclerosis: The role of oxidative stress and altered homeostasis of metals. Brain Res. Bull. 2003, 61, 365–374. [Google Scholar] [CrossRef]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. Eur. J. Physiol. 2010, 460, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Maher, P. Chronic glutamate toxicity in neurodegenerative diseases-What is the evidence? Front. Neurosci. 2015, 9, e00469. [Google Scholar] [CrossRef] [Green Version]
- Balázs, R.; Bridges, R.J.; Cotman, C.W. Glutamate and Glutamate Receptors in Neurological Diseases. In Excitatory Amino Acid Transmission in Health and Disease; Oxford University Press: Oxford, UK, 2010; pp. 1–46. ISBN 9780199865079. [Google Scholar]
- Sun, Z.W.; Zhang, L.; Zhu, S.J.; Chen, W.C.; Mei, B. Excitotoxicity effects of glutamate on human neuroblastoma SH-SY5Y cells via oxidative damage. Neurosci. Bull. 2010, 26, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Hu, Y.; Li, J.; Chen, X.; Guo, D.H.; Li, Q.S.; Rahman, K. Protection of SH-SY5Y neuronal cells from glutamate-induced apoptosis by 3,6 ′-disinapoyl sucrose, a bioactive compound isolated from radix polygala. J. Biomed. Biotechnol. 2012, 2012, 728342. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.W. Excitotoxic cell death. J. Neurobiol. 1992, 23, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, H.; Basuroy, S.; Bhattacharya, S.; Tcheranova, D.; Qu, Y.; Regan, R.F.; Leffler, C.W. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: Contributions of HO-1 and HO-2 to cytoprotection. Am. J. Physiol.-Cell Physiol. 2006, 290, C1399–C1410. [Google Scholar] [CrossRef] [PubMed]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Kim, G.S.; Jun, M.; Song, K.S. Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells. Food Funct. 2014, 5, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Dubey, K.; Sharma, G.; Prakash, M.M.; Parihar, M.S.; Dawane, V. Effects of high glutamate concentrations on mitochondria of Human neuroblastoma SH-SY5Y cells. Ann. Pharm. Françaises, 2022; in press. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- And Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [Green Version]
- Vergun, O.; Sobolevsky, A.I.; Yelshansky, M.V.; Keelan, J.; Khodorov, B.I.; Duchen, M.R. Exploration of the role of reactive oxygen species in glutamate neurotoxicity in rat hippocampal neurones in culture. J. Physiol. 2001, 531, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Reaume, A.G.; Elliott, J.L.; Hoffman, E.K.; Kowall, N.W.; Ferrante, R.J.; Siwek, D.F.; Wilcox, H.M.; Flood, D.G.; Beal, M.F.; Brown, R.H.; et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996, 13, 43–47. [Google Scholar] [CrossRef]
- Gurney, M.E.; Liu, R.; Althaus, J.S.; Hall, E.D.; Becker, D.A. Mutant CuZn superoxide dismutase in motor neuron disease. J. Inherit. Metab. Dis. 1998, 21, 587–597. [Google Scholar] [CrossRef]
- Juliani, J.; Vassileff, N.; Spiers, J.G. Inflammatory-Mediated Neuron-Glia Communication Modulates ALS Pathophysiology. J. Neurosci. 2021, 41, 1142–1144. [Google Scholar] [CrossRef]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep. 2016, 6, 30226. [Google Scholar] [CrossRef] [PubMed]
- He, R.R.; Tsoi, B.; Lan, F.; Yao, N.; Yao, X.S.; Kurihara, H. Antioxidant properties of lutein contribute to the protection against lipopolysaccharide-induced uveitis in mice. Chin. Med. 2011, 6, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, C.C.; Hu, S. Tumor necrosis factor-alpha potentiates glutamate neurotoxicity in human fetal brain cell cultures. Dev. Neurosci. 1994, 16, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.E.; Netzeband, J.G.; Gruol, D.L. Chronic interleukin-6 exposure alters metabotropic glutamate receptor-activated calcium signalling in cerebellar Purkinje neurons. Eur. J. Neurosci. 2004, 20, 2387–2400. [Google Scholar] [CrossRef]
- Wullschleger, A.; Kapina, V.; Molnarfi, N.; Courvoisier, D.S.; Seebach, J.D.; Santiago-Raber, M.L.; Hochstrasser, D.F.; Lalive, P.H. Cerebrospinal Fluid Interleukin-6 in Central Nervous System Inflammatory Diseases. PLoS ONE 2013, 8, e72399. [Google Scholar] [CrossRef] [Green Version]
- Kummer, K.K.; Zeidler, M.; Kalpachidou, T.; Kress, M. Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine 2021, 144, 155582. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Wu, Y.; Zhang, Y.; Wang, Z.; Liu, X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-κB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol. Nutr. Food Res. 2015, 59, 1663–1673. [Google Scholar] [CrossRef]
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Iron, Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 7267. [Google Scholar] [CrossRef]
- Vela, D. The dual role of hepcidin in brain iron load and inflammation. Front. Neurosci. 2018, 12, e00740. [Google Scholar] [CrossRef] [Green Version]
- Pietrangelo, A.; Dierssen, U.; Valli, L.; Garuti, C.; Rump, A.; Corradini, E.; Ernst, M.; Klein, C.; Trautwein, C. STAT3 Is Required for IL-6-gp130-Dependent Activation of Hepcidin In Vivo. Gastroenterology 2007, 132, 294–300. [Google Scholar] [CrossRef]
- Qian, Z.M.; He, X.; Liang, T.; Wu, K.C.; Yan, Y.C.; Lu, L.N.; Yang, G.; Luo, Q.Q.; Yung, W.H.; Ke, Y. Lipopolysaccharides Upregulate Hepcidin in Neuron via Microglia and the IL-6/STAT3 Signaling Pathway. Mol. Neurobiol. 2014, 50, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.; Arab, N.T.T.; Greenwood, M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 399–430. [Google Scholar] [CrossRef] [PubMed]
- Yua, J.; Guo, Y.; Sun, M.; Li, B.; Zhang, Y.; Li, C. Iron is a potential key mediator of glutamate excitotoxicity in spinal cord motor neurons. Brain Res. 2009, 1257, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020, 30, 3411–3423.e7. [Google Scholar] [CrossRef] [Green Version]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta-Bioenerg. 1996, 1275, 161–203. [Google Scholar] [CrossRef] [Green Version]
- Finazzi, D.; Arosio, P. Biology of ferritin in mammals: An update on iron storage, oxidative damage and neurodegeneration. Arch. Toxicol. 2014, 88, 1787–1802. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.D.; Pang, P.; Zhou, X.T.; Hu, F.; Xiong, W.; Chen, K.; Wang, J.; Wang, F.; Xie, D.; Hu, Y.Z.; et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021, 28, 1548–1562. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.Y.; Zhou, H.H.; Mao, X.Y. Emerging Roles of 5-Lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxid. Med. Cell. Longev. 2019, 2019, 2749173. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhou, B.; Mao, W.W.; Yin, M. Overexpression of 5-lipoxygenase increases the neuronal vulnerability of PC12 cells to aβ42. Yakugaku Zasshi 2011, 131, 1843–1853. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.L.; Hu, B.X.; Li, Z.L.; Du, T.; Shan, J.L.; Ye, Z.P.; Peng, X.D.; Li, X.; Huang, Y.; Zhu, X.Y.; et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat. Cell Biol. 2022, 24, 88–98. [Google Scholar] [CrossRef] [PubMed]



| Controls | Treatments |
|---|---|
| C-absolute control | Untreated |
| D2.5-DMSO equivalent to lutein | 2.5 ng/µL of lutein |
| D5-DMSO equivalent to lutein | 5 ng/µL of lutein |
| D7.5-DMSO equivalent to lutein | 7.5 ng/µL of lutein |
| D10-DMSO equivalent to lutein | L-10 ng/µL of lutein |
| C1-DMSO equivalent to glutamate | G1-1 mM glutamate |
| C2-DMSO equivalent to glutamate | G2-2 mM glutamate |
| C3-DMSO equivalent to glutamate | G3-3 mM glutamate |
| C4-DMSO equivalent to glutamate | G4-4 mM glutamate |
| C5-DMSO equivalent to glutamate | G5-5 mM glutamate |
| C6-DMSO equivalent to glutamate | G6-6 mM glutamate |
| C7-DMSO equivalent to glutamate | G7-7 mM glutamate |
| C8-DMSO equivalent to glutamate | G8-8 mM glutamate |
| C9-DMSO equivalent to glutamate | G9-9 mM glutamate |
| C10-DMSO equivalent to glutamate | G10-10 mM glutamate |
| C15-DMSO equivalent to glutamate | G15-15 mM glutamate |
| C20-DMSO equivalent to glutamate | G20-20 mM glutamate |
| Target Gene | Gene Accession Number | Sequence 5′ → 3 |
|---|---|---|
| β-actin forward | NM_007393.5 | AGAAAATCTGGCACCACACC |
| β-actin reverse | GGGGTGTTGAAGGTGTCAAA | |
| ACSL4 forward | NM_004458.3 | TCTTGCTTTACCTATGGCTG |
| ACSL4 reverse | CAGTACAGTCTCCTTTGCTT | |
| ALOX5 forward | NM_001256154.3 | CGCGGTGGATTCATACG |
| ALOX5 reverse | GTCTTCAGCGTGATGTACT | |
| ALOX15 forward | NM_001140.5 | GAGGAGGAGTATTTTTCGGG |
| ALOX15 reverse | AATTTCCTTATCCAGGGCAG | |
| DMT1 forward | NM_001174125.2 | GTGGTTACTGGGCTGCATCT |
| DMT1 reverse | CCCACAGAGGAATTCTTCCT | |
| FPN forward | NM_014585.6 | AAAGGAGGCTGTTTCCATAG |
| FPN reverse | TTCCTTCTCTACCTTGGTCA | |
| FTH forward | NM_002032.3 | GAGGTGGCCGAATCTTCCTTC |
| FTH reverse | TCAGTGGCCAGTTTGTGCAG | |
| FTMT forward | NM_177478.2 | AAGGTGACCCCCATTTGTGC |
| FTMT reverse | GGGGCCCCCATCTTCACTAA | |
| HAMP forward | NM_021175.4 | CAGCTGGATGCCCATGTT |
| HAMP reverse | TGCAGCACATCCCACACT | |
| TfR1 forward | NM_003234.4 | CATGTGGAGATGAAACTTGC |
| TfR1 reverse | TCCCATAGCAGATACTTCCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Nagy, T.; Agócs, A.; Deli, J. Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity. Antioxidants 2022, 11, 2269. https://doi.org/10.3390/antiox11112269
Pap R, Pandur E, Jánosa G, Sipos K, Nagy T, Agócs A, Deli J. Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity. Antioxidants. 2022; 11(11):2269. https://doi.org/10.3390/antiox11112269
Chicago/Turabian StylePap, Ramóna, Edina Pandur, Gergely Jánosa, Katalin Sipos, Tamás Nagy, Attila Agócs, and József Deli. 2022. "Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity" Antioxidants 11, no. 11: 2269. https://doi.org/10.3390/antiox11112269
APA StylePap, R., Pandur, E., Jánosa, G., Sipos, K., Nagy, T., Agócs, A., & Deli, J. (2022). Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity. Antioxidants, 11(11), 2269. https://doi.org/10.3390/antiox11112269








