Preventive Effects of β-Cryptoxanthin, a Potent Antioxidant and Provitamin A Carotenoid, on Lifestyle-Related Diseases—A Central Focus on Its Effects on Non-Alcoholic Fatty Liver Disease (NAFLD)
Abstract
:1. Introduction
2. Relationship between the Intake of β-Cryptoxanthin and Risk of Lifestyle-Related Diseases in Terms of Epidemiological Studies
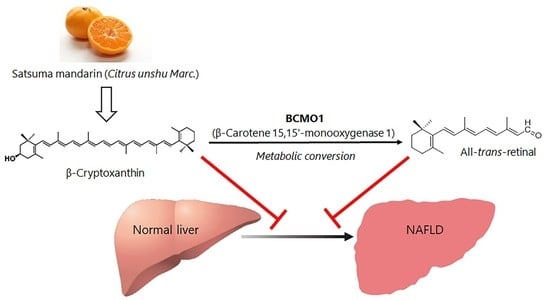
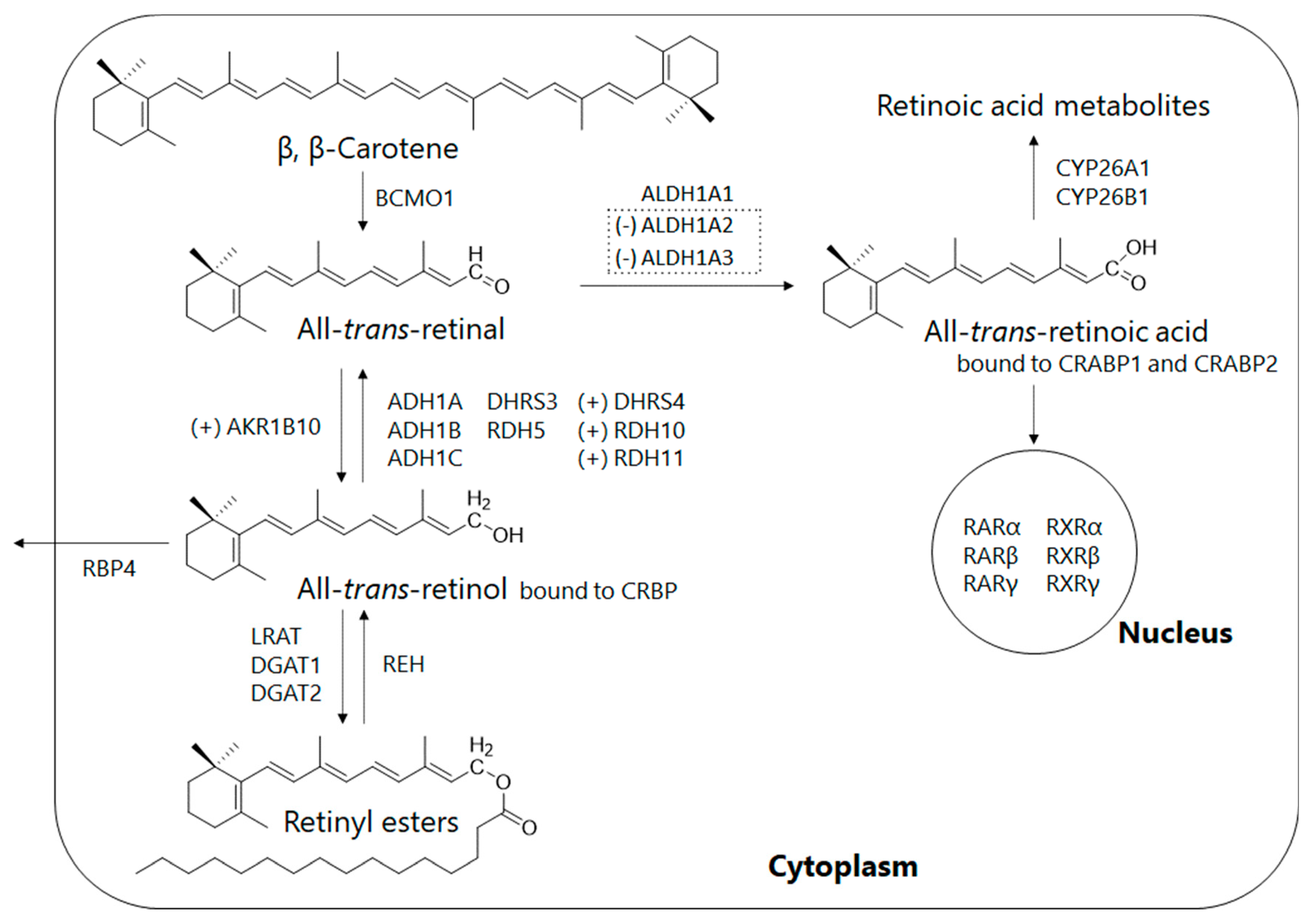
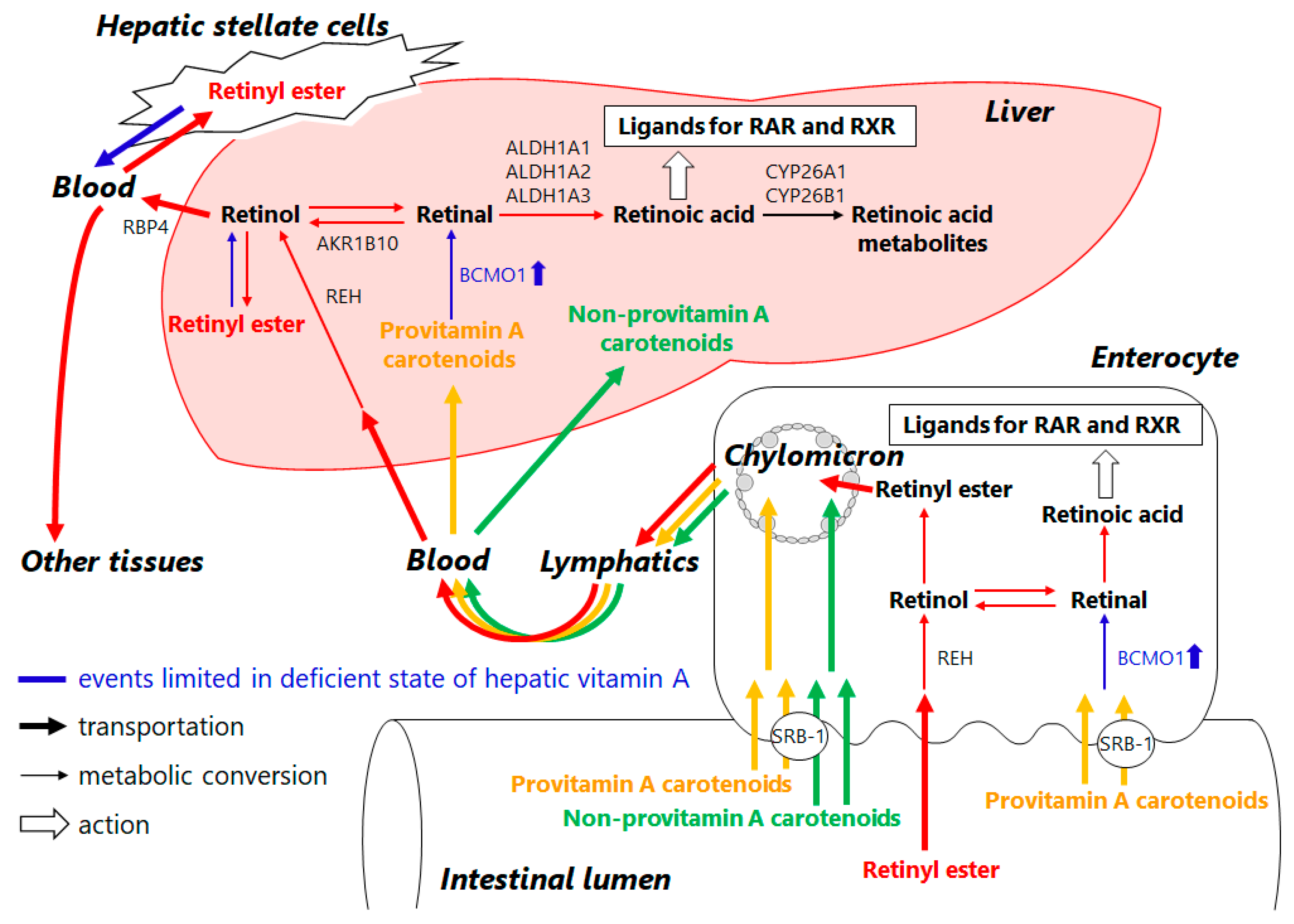
3. Retinoid Metabolic Disorders in Non-Alcoholic Fatty Liver Disease (NAFLD)
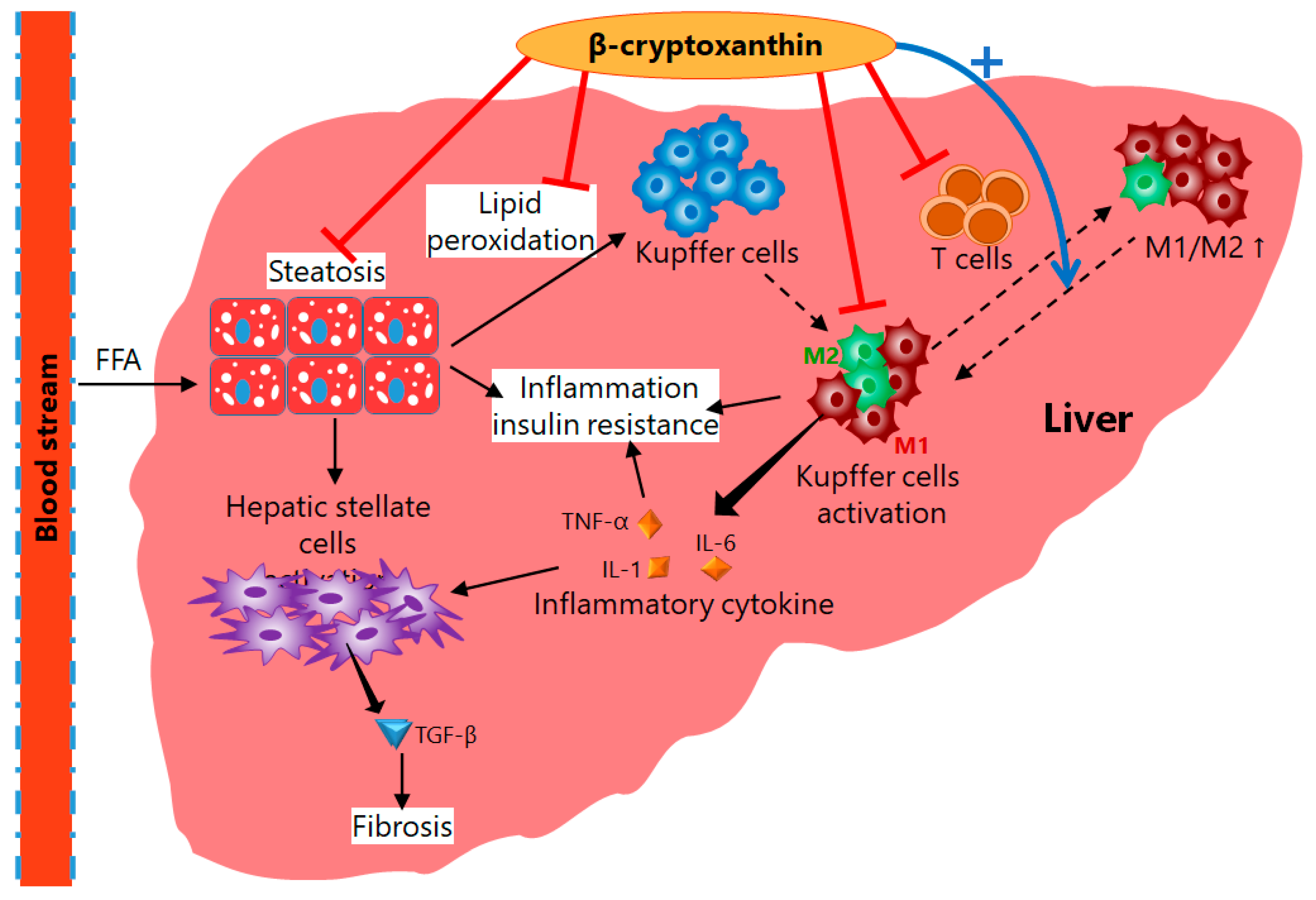
4. Mechanisms of Action of β-Cryptoxanthin for Treatment of NAFLD
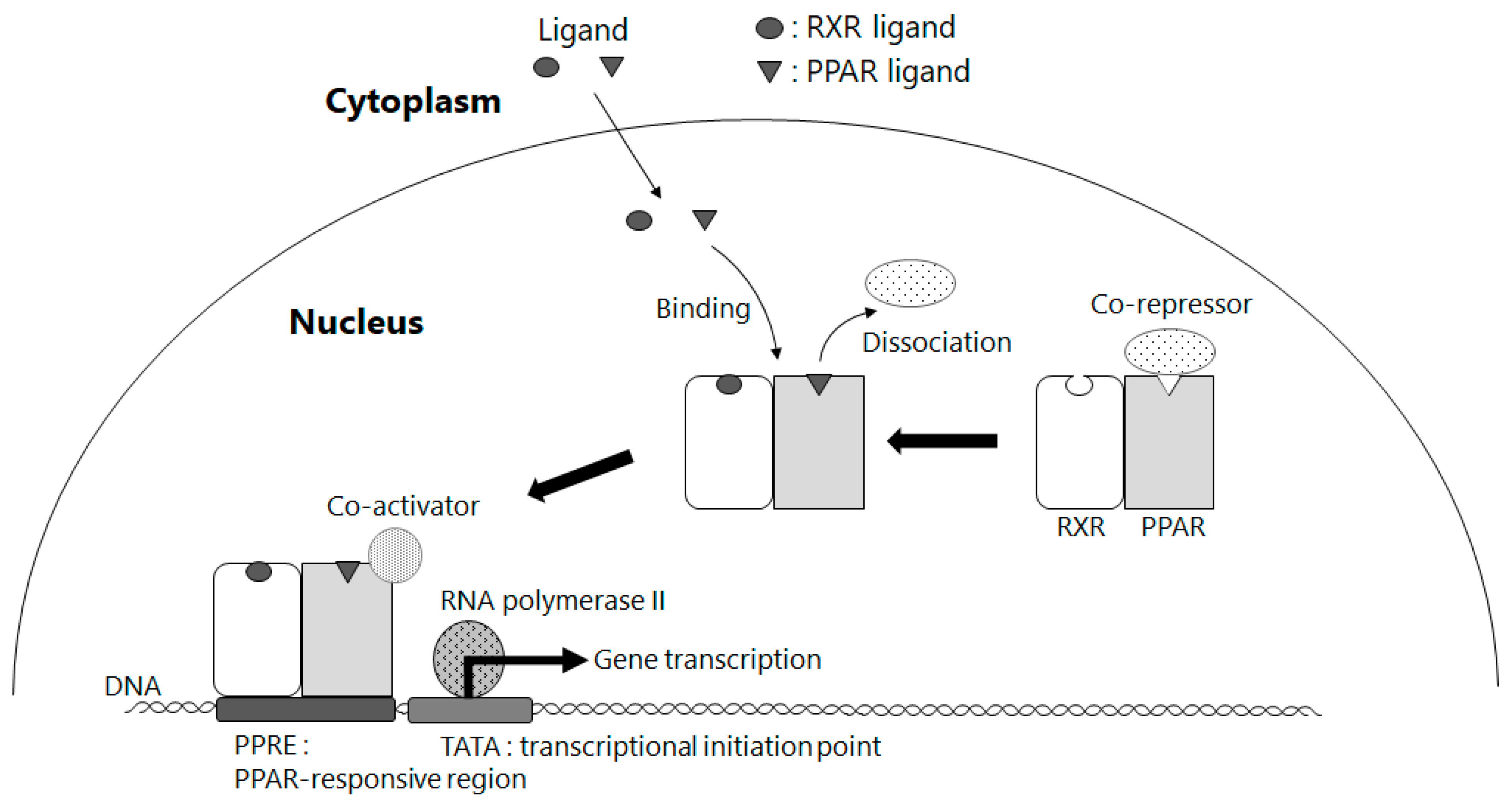
5. Amelioration of NAFLD by Way of Activating PPAR γ through BCMO1-Mediated Retinoid Conversion of β-Cryptoxanthin
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADH | alcohol dehydrogenase |
| AKR1B10 | aldo-keto reductase family 1 member B10 |
| ALDH1A | retinaldehyde dehydrogenase 1 family, member A |
| ALT | alanine aminotransferase |
| AST | aspartate amino group transferase |
| BCMO1 | β-carotene 15,15′-monooxygenase 1 |
| BMI | body mass index |
| CRABP | cellular retinoic acid-binding protein |
| CRBP | cellular retinol-binding protein |
| CYP | cytochrome P450 family members |
| DGAT | diacylglycerol acyltransferase |
| DHRS | membrane-bound short-chain dehydrogenases/reductases |
| γ-GTP | γ-glutamyl transpeptidase |
| IL-1 | interleukin 1 |
| IL-6 | interleukin 6 |
| LDL | low density lipoprotein |
| LRAT | lecithin retinol acyltransferase |
| MS | metabolic syndrome |
| NAFL | non-alcoholic fatty liver |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| PPAR | peroxisome proliferator-activated receptor |
| PPRE | peroxisome proliferator response element |
| RAR | retinoic acid receptor |
| RBP4 | retinol-binding protein 4 |
| RDH | retinal dehydrogenase |
| REH | retinyl ester hydrolase |
| RXR | retinoid X receptor |
| SOAC | singlet oxygen absorption capacity |
| SRB-1 | scavenger receptor class B type 1 |
| TATA | transcriptional initiation point |
| TNF-α | tumor necrosis factor-α |
| UV | ultraviolet light |
References
- Fukuzawa, K.; Inokami, Y.; Tokumura, A.; Terao, J.; Suzuki, A. Rate constants for quenching singlet oxygen and activities for inhibiting lipid peroxidation of carotenoids and α-tocopherol in liposomes. Lipids 1998, 33, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G.; Rancan, F.; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W. Vitamins E and C, β-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995, 62, 1315–1321. [Google Scholar] [CrossRef]
- Aizawa, K.; Iwasaki, Y.; Ouchi, A.; Inakuma, T.; Nagaoka, S.; Terao, J.; Mukai, K. Development of singlet oxygen absorption capacity (SOAC) assay method. 2. measurements of the SOAC values for carotenoids and food extracts. J. Agric. Food Chem. 2011, 59, 3717–3729. [Google Scholar] [CrossRef]
- Wawrzyniak, A.; Hamułka, J.; Friberg, E.; Wolk, A. Dietary, anthropometric, and lifestyle correlates of serum carotenoids in postmenopausal women. Eur. J. Nutr. 2013, 52, 1919–1926. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Hatade, K.; Sookwong, P.; Arai, H.; Miyazawa, T. Antioxidant effect of lutein towards phospholipid hydroperoxidation in human erythrocytes. Br. J. Nutr. 2009, 102, 1280–1284. [Google Scholar] [CrossRef] [Green Version]
- Tang, G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am. J. Clin. Nutr. 2010, 91, 1468–1473. [Google Scholar] [CrossRef] [Green Version]
- Min, K.B.; Min, J.Y. Serum carotenoid levels and risk of lung cancer death in US adults. Cancer Sci. 2014, 105, 736–743. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, B.; Pan, M.X.; Mo, X.F.; Chen, Y.M.; Zhang, C.X. Specific carotenoid intake is inversely associated with the risk of breast cancer among Chinese women. Br. J. Nutr. 2014, 111, 1686–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silaste, M.L.; Alfthan, G.; Aro, A.; Kesäniemi, Y.A.; Hörkkö, S. Tomato juice decreases LDL cholesterol levels and increases LDL resistance to oxidation. Br. J. Nutr. 2007, 98, 1251–1258. [Google Scholar] [CrossRef] [Green Version]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Suzuki, T.; Arai, H.; Miyazawa, T. Significance of Lutein in red blood cells of Alzheimer’s disease patients. J. Alzheimer’s Dis. 2012, 28, 593–600. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [Green Version]
- Yeum, K.J.; Shang, F.; Schalch, W.; Russell, R.M.; Taylor, A. Fat-soluble nutrient concentrations in different layers of human cataractous lens. Curr. Eye Res. 1999, 19, 502–505. [Google Scholar] [CrossRef]
- Takagi, T.; Hayashi, R.; Nakai, Y.; Okada, S.; Miyashita, R.; Yamada, M.; Mihara, Y.; Mizushima, K.; Morita, M.; Uchiyama, K.; et al. Dietary intake of carotenoid-rich vegetables reduces visceral adiposity in obese Japanese men—A randomized, double-blind trial. Nutrients 2020, 12, 2342. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.S.; Sharp, S.J.; Imamura, F.; Chowdhury, R.; Gundersen, T.E.; Steur, M.; Sluijs, I.; Van Der Schouw, Y.T.; Agudo, A.; Aune, D.; et al. Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-interact case-cohort study in eight European countries. BMJ 2020, 370, m2194. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, M. Recent findings from nutritional epidemiologic studies about the relationship of β-cryptoxanthin and risk of lifestyle-related disease. Oleoscience 2012, 12, 515–523. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Sugiura, M.; Kato, M.; Matsumoto, H.; Nagao, A.; Yano, M. Serum concentration of β-cryptoxanthin in Japan reflects the frequency of satsuma mandarin (Citrus unshiu Marc.) consumption. J. Health Sci. 2002, 48, 350–353. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High serum carotenoids associated with lower risk for the metabolic syndrome and its components among Japanese subjects: Mikkabi cohort study. Br. J. Nutr. 2015, 114, 1674–1682. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High-serum carotenoids associated with lower risk for developing type 2 diabetes among Japanese subjects: Mikkabi cohort study. BMJ Open Diabetes Res. Care 2015, 3, e000147. [Google Scholar] [CrossRef] [Green Version]
- Montonen, J.; Knekt, P.; Järvinen, R.; Reunanen, A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care 2004, 27, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High serum carotenoids are associated with lower risk for developing elevated serum alanine aminotransferase among Japanese subjects: The Mikkabi cohort study. Br. J. Nutr. 2016, 115, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.L.; Chen, G.D.; Zeng, F.F.; Qiu, R.; Shi, W.Q.; Lin, J.S.; Cao, Y.; Li, H.B.; Ling, W.H.; Chen, Y.M. Higher serum carotenoids associated with improvement of non-alcoholic fatty liver disease in adults: A prospective study. Eur. J. Nutr. 2019, 58, 721–730. [Google Scholar] [CrossRef]
- Matsuura, B.; Miyake, T.; Yamamoto, S.; Furukawa, S.; Hiasa, Y. Usefulness of beta-cryptoxanthin for non-alcoholic fatty liver diseases. J. Food Nutr. Disord. 2016, 5, 1000196. [Google Scholar]
- Shirakami, Y.; Lee, S.A.; Clugston, R.D.; Blaner, W.S. Hepatic metabolism of retinoids and disease associations. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of non-alcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Day, C.P.; James, O.F.W. Steatohepatitis: A tale of two “Hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Palczewski, K.; Kumasaka, T.; Hori, T.; Behnke, C.A.; Motoshima, H.; Fox, B.A.; Le Trong, I.; Teller, D.C.; Okada, T.; Stenkamp, R.E.; et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 2000, 289, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Sommer, A.; Vyas, K.S. A global clinical view on vitamin A and carotenoids. Am. J. Clin. Nutr. 2012, 96, 1204–1206. [Google Scholar] [CrossRef] [Green Version]
- Nagao, A. Absorption and metabolism of dietary carotenoids. BioFactors 2011, 37, 83–87. [Google Scholar] [CrossRef]
- Shmarakov, I.; Fleshman, M.K.; D’Ambrosio, D.N.; Piantedosi, R.; Riedl, K.M.; Schwartz, S.J.; Curley, R.W.; von Lintig, J.; Rubin, L.P.; Harrison, E.H.; et al. Hepatic stellate cells are an important cellular site for β-carotene conversion to retinoid. Arch. Biochem. Biophys. 2010, 504, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, R.; Taschler, U.; Preiss-Landl, K.; Wongsiriroj, N.; Zimmermann, R.; Lass, A. Retinyl ester hydrolases and their roles in vitamin A homeostasis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senoo, H.; Yoshikawa, K.; Morii, M.; Miura, M.; Imai, K.; Mezaki, Y. Hepatic stellate cell (vitamin A-storing cell) and its relative—Past, present and future. Cell Biol. Int. 2010, 34, 1247–1272. [Google Scholar] [CrossRef]
- Pettinelli, P.; Arendt, B.M.; Teterina, A.; McGilvray, I.; Comelli, E.M.; Fung, S.K.; Fischer, S.E.; Allard, J.P. Altered hepatic genes related to retinol metabolism and plasma retinol in patients with non-alcoholic fatty liver disease. PLoS ONE 2018, 13, e0205747. [Google Scholar] [CrossRef]
- Chaves, G.V.; Pereira, S.E.; Saboya, C.J.; Spitz, D.; Rodrigues, C.S.; Ramalho, A. Association between liver vitamin A reserves and severity of non-alcoholic fatty liver disease in the class III obese following bariatric surgery. Obes. Surg. 2014, 24, 219–224. [Google Scholar] [CrossRef]
- Endo, S.; Xia, S.; Suyama, M.; Morikawa, Y.; Oguri, H.; Hu, D.; Ao, Y.; Takahara, S.; Horino, Y.; Hayakawa, Y.; et al. Synthesis of potent and selective inhibitors of aldo-keto reductase 1B10 and their efficacy against proliferation, metastasis, and cisplatin resistance of lung cancer cells. J. Med. Chem. 2017, 60, 8441–8455. [Google Scholar] [CrossRef]
- Tsuchiya, H. Retinoids as promising treatment for non-alcoholic fatty liver disease. Yakugaku Zasshi 2012, 132, 903–909. [Google Scholar] [CrossRef] [Green Version]
- Penniston, K.L.; Tanumihardjo, S.A. The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [CrossRef]
- Ramkumar, S.; Moon, J.; Golczak, M.; von Lintig, J. LRAT coordinates the negative-feedback regulation of intestinal retinoid biosynthesis from β-carotene. J. Lipid Res. 2021, 62, 100055. [Google Scholar] [CrossRef]
- Matsumoto, A.; Mizukami, H.; Mizuno, S.; Umegaki, K.; Nishikawa, J.; Shudo, K.; Kagechika, H.; Inoue, M. β-Cryptoxanthin, a novel natural RAR ligand, induces ATP-binding cassette transporters in macrophages. Biochem. Pharmacol. 2007, 74, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Ni, Y.; Takahashi, Y.; Watanabe, N.; Sugiura, M.; Ogawa, K.; Nagashimada, M.; Kaneko, S.; Naito, S.; Ota, T. β-Cryptoxanthin alleviates diet-induced non-alcoholic steatohepatitis by suppressing inflammatory gene expression in mice. PLoS ONE 2014, 9, e98294. [Google Scholar] [CrossRef]
- Ni, Y.; Nagashimada, M.; Zhan, L.; Nagata, N.; Kobori, M.; Sugiura, M.; Ogawa, K.; Kaneko, S.; Ota, T. Prevention and reversal of lipotoxicity-induced hepatic insulin resistance and steatohepatitis in mice by an antioxidant carotenoid, β-cryptoxanthin. Endocrinology 2015, 156, 987–999. [Google Scholar] [CrossRef]
- Chylikova, J.; Dvorackova, J.; Tauber, Z.; Kamarad, V. M1/M2 macrophage polarization in human obese adipose tissue. Biomed. Pap. 2018, 162, 79–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeckmans, J.; Natale, A.; Rombaut, M.; Buyl, K.; Rogiers, V.; De Kock, J.; Vanhaecke, T.; Rodrigues, R.M. Anti-NASH drug development hitches a lift on PPAR agonism. Cells 2020, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, C.; Krey, G.; Keller, H.; Givel, F.; Helftenbein, G.; Wahli, W. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell 1992, 68, 879–887. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARγ. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Promrat, K.; Lutchman, G.; Uwaifo, G.I.; Freedman, R.J.; Soza, A.; Heller, T.; Doo, E.; Ghany, M.; Premkumar, A.; Park, Y.; et al. A pilot study of pioglitazone treatment for non-alcoholic steatohepatitis. Hepatology 2004, 39, 188–196. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for non-alcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazra, S.; Xiong, S.; Wang, J.; Rippe, R.A.; Chatterjee, V.K.K.; Tsukamoto, H. Peroxisome proliferator-activated receptor γ induces a phenotypic switch from activated to quiescent hepatic stellate cells. J. Biol. Chem. 2004, 279, 11392–11401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, K.; Kawachi, E.; Inoue, N.; Fukasawa, H.; Hashimoto, Y.; Itai, A.; Kagechika, H. Retinoidal pyrimidinecarboxylic acids. Unexpected diaza-substituent effects in retinobenzoic acids. Chem. Pharm. Bull. 2000, 48, 1504–1513. [Google Scholar] [CrossRef] [Green Version]
- Hessel, S.; Eichinger, A.; Isken, A.; Amengual, J.; Hunzelmann, S.; Hoeller, U.; Elste, V.; Hunziker, W.; Goralczyk, R.; Oberhauser, V.; et al. BCMO1 deficiency abolishes vitamin a production from β-carotene and alters lipid metabolism in mice. J. Biol. Chem. 2007, 282, 33553–33561. [Google Scholar] [CrossRef] [Green Version]
- Nishino, A.; Ichihara, T.; Sugimoto, K.; Kuriki, T.; Yasui, H.; Maoka, T. Predicting organ carotenoids levels from analysis of plasma could lead to errors: A study in cynomolgus monkeys. Nutr. Res. 2019, 61, 95–101. [Google Scholar] [CrossRef]
- Boulanger, A.; Mclemore, P.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Yu, S.S.; Gentleman, S.; Redmond, T.M. Identification of beta-carotene 15,15′-monooxygenase as a peroxisome proliferator-activated receptor target gene. FASEB J. 2003, 17, 1304–1306. [Google Scholar] [CrossRef]
- Leung, W.C.; Hessel, S.; Méplan, C.; Flint, J.; Oberhauser, V.; Tourniaire, F.; Hesketh, J.E.; Lintig, J.; Lietz, G. Two common single nucleotide polymorphisms in the gene encoding β-carotene 15,15′-monoxygenase alter β-carotene Metabolism in Female Volunteers. FASEB J. 2009, 23, 1041–1053. [Google Scholar] [CrossRef]
- Lietz, G.; Oxley, A.; Leung, W.; Hesketh, J. Single nucleotide polymorphisms upstream from the β-Carotene 15, 15’-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J. Nutr. 2012, 142, 161–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Disorder of Lipid Metabolism | Type 2 Diabetes | Non-Alcoholic Fatty Liver Disease | ||||
|---|---|---|---|---|---|---|
| Test areas | Japan [20] (Mikkabi) | Japan [21] (Mikkabi) | Finland [22] (Several areas) | Japan [23] (Mikkabi) | China [24] (Guangzhou) | Japan [25] (Ehime Univ. Hospital) |
| Subjects | General inhabitants | General inhabitants | General inhabitants | General inhabitants | General inhabitants | Patients |
| Provitamin A | ||||||
| α-carotene | Excellent | Excellent | None | Fair | Excellent | None |
| β-carotene | Excellent | None | None | Excellent | Excellent | None |
| β-cryptoxanthin | Excellent | Excellent | Excellent | Excellent | Excellent | Excellent |
| Non-provitamin A | ||||||
| Lycopene | None | None | None | None | Excellent | None |
| Lutein | None | None | None | None | Excellent | None |
| Zeaxanthin | None | None | None | None | Excellent | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishino, A.; Maoka, T.; Yasui, H. Preventive Effects of β-Cryptoxanthin, a Potent Antioxidant and Provitamin A Carotenoid, on Lifestyle-Related Diseases—A Central Focus on Its Effects on Non-Alcoholic Fatty Liver Disease (NAFLD). Antioxidants 2022, 11, 43. https://doi.org/10.3390/antiox11010043
Nishino A, Maoka T, Yasui H. Preventive Effects of β-Cryptoxanthin, a Potent Antioxidant and Provitamin A Carotenoid, on Lifestyle-Related Diseases—A Central Focus on Its Effects on Non-Alcoholic Fatty Liver Disease (NAFLD). Antioxidants. 2022; 11(1):43. https://doi.org/10.3390/antiox11010043
Chicago/Turabian StyleNishino, Azusa, Takashi Maoka, and Hiroyuki Yasui. 2022. "Preventive Effects of β-Cryptoxanthin, a Potent Antioxidant and Provitamin A Carotenoid, on Lifestyle-Related Diseases—A Central Focus on Its Effects on Non-Alcoholic Fatty Liver Disease (NAFLD)" Antioxidants 11, no. 1: 43. https://doi.org/10.3390/antiox11010043
APA StyleNishino, A., Maoka, T., & Yasui, H. (2022). Preventive Effects of β-Cryptoxanthin, a Potent Antioxidant and Provitamin A Carotenoid, on Lifestyle-Related Diseases—A Central Focus on Its Effects on Non-Alcoholic Fatty Liver Disease (NAFLD). Antioxidants, 11(1), 43. https://doi.org/10.3390/antiox11010043