Abstract
Lycopene is a bioactive red pigment found in plants, especially in red fruits and vegetables, including tomato, pink guava, papaya, pink grapefruit, and watermelon. Several research reports have advocated its positive impact on human health and physiology. For humans, lycopene is an essential substance obtained from dietary sources to fulfil the body requirements. The production of reactive oxygen species (ROS) causing oxidative stress and downstream complications include one of the major health concerns worldwide. In recent years, oxidative stress and its counter strategies have attracted biomedical research in order to manage the emerging health issues. Lycopene has been reported to directly interact with ROS, which can help to prevent chronic diseases, including diabetes and neurodegenerative and cardiovascular diseases. In this context, the present review article was written to provide an accumulative account of protective and ameliorative effects of lycopene on coronary artery disease (CAD) and hypertension, which are the leading causes of death worldwide. Lycopene is a potent antioxidant that fights ROS and, subsequently, complications. It reduces blood pressure via inhibiting the angiotensin-converting enzyme and regulating nitrous oxide bioavailability. It plays an important role in lowering of LDL (low-density lipoproteins) and improving HDL (high-density lipoproteins) levels to minimize atherosclerosis, which protects the onset of coronary artery disease and hypertension. Various studies have advocated that lycopene exhibited a combating competence in the treatment of these diseases. Owing to all the antioxidant, anti-diabetic, and anti-hypertensive properties, lycopene provides a potential nutraceutical with a protective and curing ability against coronary artery disease and hypertension.
1. Introduction
Bioactive components can be found in plant-based natural products derived through food processing [1]. Many of these plant metabolites aid in the reduction of oxidative stress, making them potentially useful in the treatment of a wide range of severe illnesses. Despite the availability of numerous medications to treat oxidative stress-related chronic diseases, the high profile of drug side effects necessitates the use of alternative and complementary treatment options for diabetes and cardiovascular diseases (CVDs), such as coronary artery disease (CAD) and blood pressure control [2,3]. To avoid or treat chronic disorders, lifestyle adjustments and dietary interventions, such as increasing fruit and vegetable consumption, are frequently advocated [4,5,6]. Lycopene is a red-colored compound found in colored fruits and vegetables, including tomato, papaya, pink guava, and watermelon, and is responsible for their reddish hue. Tomatoes and tomato-based products are the most common sources of lycopene [7,8]. Tomato sauce and ketchup are better sources of sources of lycopene as compared to natural raw tomatoes [9]. Lycopene is a natural substance that may be used in high doses as a dietary supplement without causing harm to human health or physiology [10,11,12]. In accordance with these findings, lycopene has gotten a lot of interest as a possible nutraceutical for disease prevention and therapy, notably for improving vascular function and lowering blood pressure [13,14,15]. Biological and biomedical researchers are becoming increasingly interested in the expanding body of evidence indicating lycopene’s disease-preventive properties. Lycopene-rich diets have been inversely associated with heart diseases and malignancies by several in vitro, ex vivo, and in vivo studies [16,17,18]. The buildup of ROS, which is accompanied by abnormalities such as inflammation and irregular lipid metabolism, is a critical risk factor for the increasing occurrence of metabolic disorders [19,20]. ROS, also known as free radicals, are highly reactive, unstable oxygen-containing molecules that can cause cell death by damaging deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and proteins [21,22]. The fundamental biological function of lycopene is the protestation of DNA from oxidative stress by quenching ROS and inhibiting mutations that might cause chronic diseases [23]. The elongated carbon chain with conjugated double bonds have made lycopene as the most potent single oxygen and free radical scavenger among 600 naturally occurring carotenoids [24,25,26]. It is more efficient in shielding cells and tissues from ROS-induced damage [9,27].
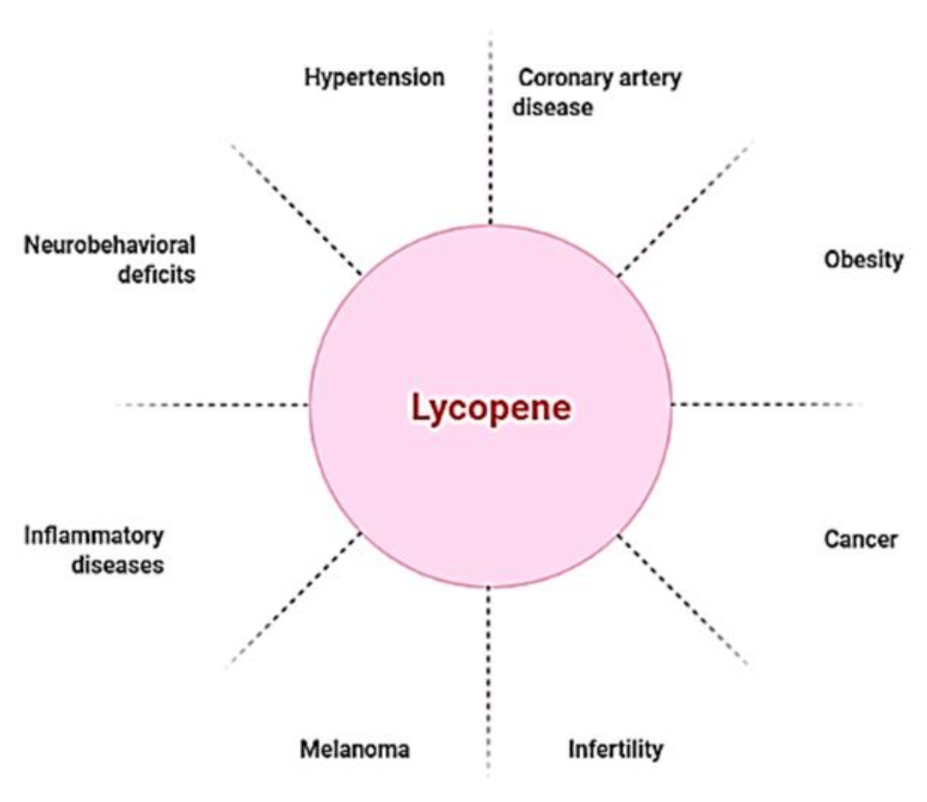
Though lycopene is beneficial for a range of ailments (Figure 1), it is especially useful in the treatment of cardiovascular diseases (CVDs), the leading cause of mortality globally. CVDs are exacerbated by high blood pressure, high cholesterol, and smoking [28,29]. Blood flow to the heart and central nervous system is often restricted, resulting in arterial remodeling and atherosclerosis, which are the principal causes of coronary artery disease [30]. Hypertension, one of the most common causes of cardiovascular morbidity and death, is caused by a restriction of blood flow caused by modified arteries. As a result, hypertension and coronary artery disease have a strong and frequent relationship [31]. A number of pathophysiologic pathways are shared by both disorders. Endothelial dysfunction, which aggravates atherosclerosis and makes atherosclerotic plaques more unstable, is caused by hypertension [32,33,34]. Several studies on lycopene supplementation have showed promising results in lowering blood pressure and coronary artery disease [35,36]. There were no harmful effects at high lycopene consumption levels, according to safety evaluation studies [37,38]. Because lycopene is a lipid-soluble antioxidant, cholesterol-lowering drugs, such as probucol and cholestyramine, diminish lycopene blood concentrations, owing to gastrointestinal absorption issues [39].
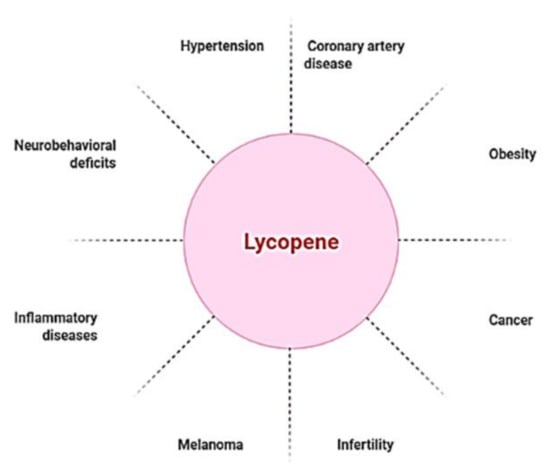
Figure 1.
Lycopene as a nutraceutical compound has applications against multiple diseased conditions.
Since natural medications are being given increasing attention due to the widespread usage of natural chemicals rather than synthetic drugs to treat ailments, and lycopene is a promising nutraceutical in treating a variety of diseases by blocking disease pathways. This review has focused on the potential effects of lycopene on coronary artery disease and hypertension.
2. Discovery, Chemical Structure, Properties, Biosynthesis and Physiological Role of Lycopene
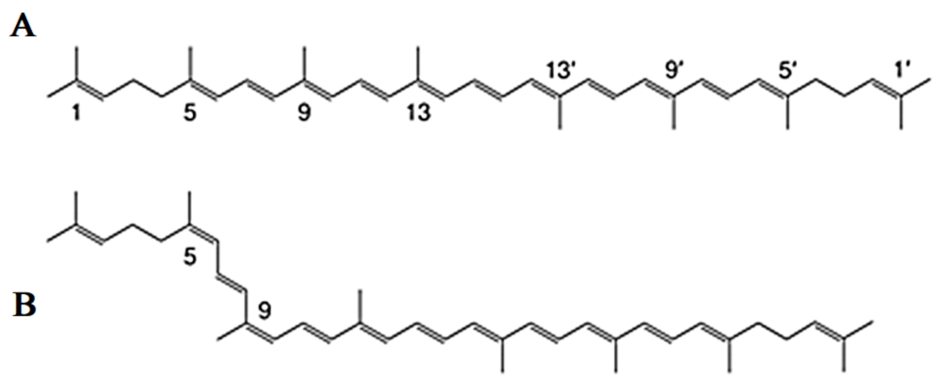
Lycopene is a carotenoid with a molecular formula (C40H56) that gives the Solanum lycopersicum L. fruits their red color [40]. Chemically, the lycopene molecule has 11 conjugated double bonds, and its structures can have over 70 Z-isomers [41,42]. According to estimations, all-E-lycopene contains 80 percent to 97 percent lycopene in tomato fruit. However, Z-isoforms account for more than half of the lycopene present in human blood and tissues [43,44] (Figure 2).
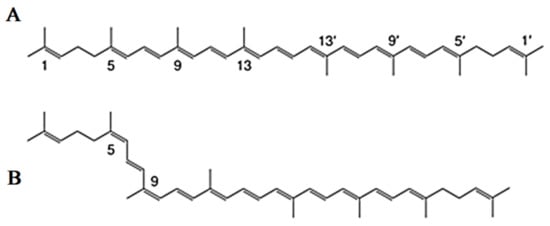
Figure 2.
Common isomers of lycopene. (A) all-E-lycopene isomer, (B) Z-lycopene isomer.
Millardet identified lycopene in 1876 and named it as ‘soanorubin’; later on, it was named lycopene and it was purified [45]. Tomato is a major source of natural lycopene; however, it is found in many plants at variable concentrations [46,47]. The extended conjugated double bond system of these compounds is a significant property of the carotenoids and is responsible for their attractive colors [48,49]. Lycopene produces the light-absorbing chromophore, and the extended conjugated double bond system of these compounds is a significant property of the carotenoids that is responsible for their attractive colors. In order for a molecule to have visible color, it must have at least seven conjugated double bonds. The maximum absorption wavelength increases as the number of conjugated double bonds increases [50,51]. Lycopene is not a precursor molecule for vitamin A because it lacks the terminal b-ionic ring found in vitamin A’s core structure. Lycopene is the most effective singlet oxygen quencher among the carotenoids, with the number of conjugated double bonds and, to a lesser degree, the presence of cyclic or acyclic end groups dictating its quenching ability [52]. Furthermore, its biological properties, such as oxidative sensitivity, are impacted by its chain structure, which includes a large conjugated polyene system [53,54]. Lycopene is found in nature as an all trans form with seven double bonds that can be isomerized to mono-cis or poly-cis when exposed to high temperatures, light, oxygen, acids, catalysts, and metal ions. Lycopene is a lipophilic molecule with hydrophobic properties due to its acyclic structure and 11 linear conjugated double bonds, making it more soluble in organic solvents, such as chloroform, benzene, hexane, methylene chloride, acetone, and petroleum ether [55]. Lycopene is a vivid red pigment that is water insoluble [56]. Lycopene is present in the chloroplasts of fresh fruit, a plant cell organelle that is rarely eaten [57]. [57]. Thermal food processing, particularly in the presence of cooking oils, causes lycopene to micellize and enhance its intestinal absorption rate by a factor of ten [58].
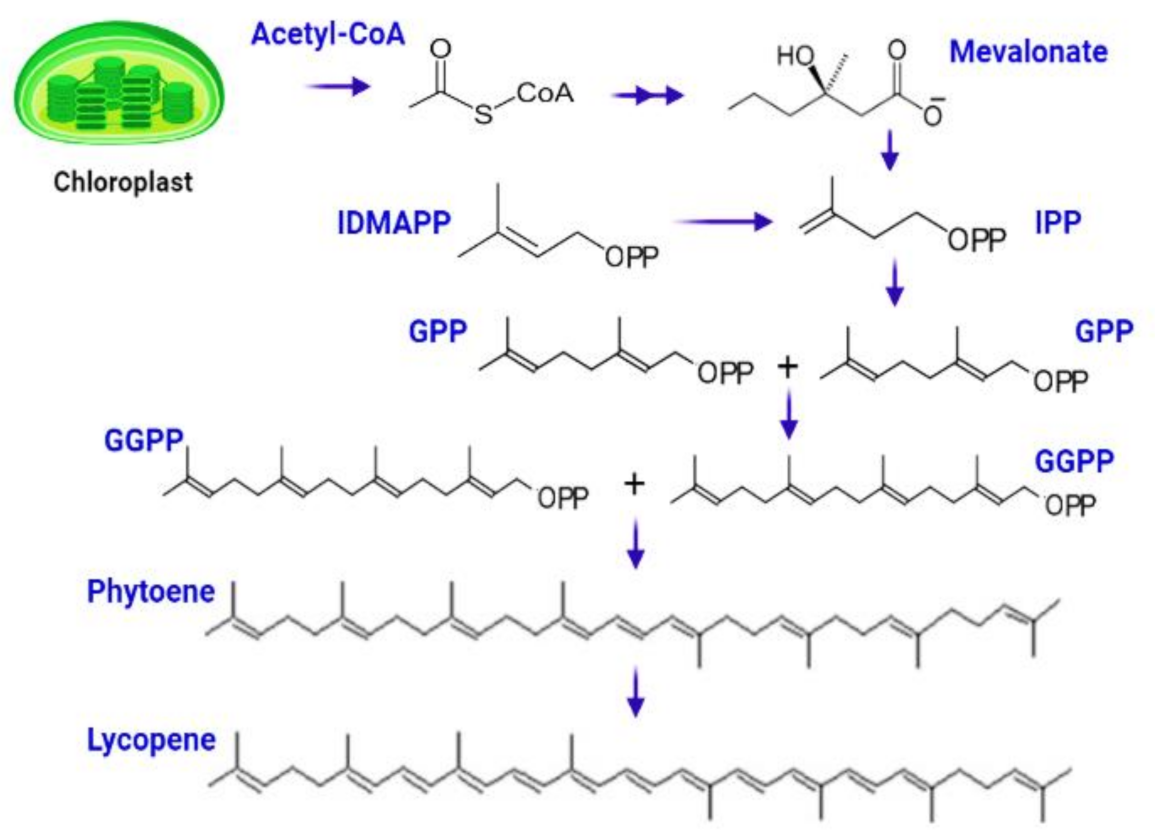
The complicated process of lycopene manufacturing begins when chlorophyll degrades to produce white-colored leucoplast, which produces particular red-colored pigmented organelles called chromoplast. The biosynthetic process begins with the conversion of acetyl-Co-A to isopentenyl diphosphate (IPP) via the mevalonate route [59]. IPP (5C) interacts with DMAPP (5C) to form geranyl diphosphate (GPP), a ten-carbon molecule [60]. The next step entails adding two IPP molecules one at a time, resulting in the synthesis of geranylgeranyl diphosphate (GGPP), a 20-carbon complex. Two molecules of GGPP are joined head-to-head in a condensation process to generate phytoene, a 40-carbon chemical that is then converted to lycopene via a mechanism mediated by phytoene desaturase [61,62] (Figure 3).
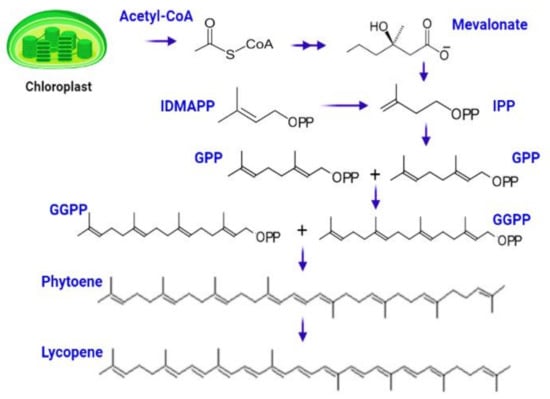
Figure 3.
Biosynthesis of lycopene starts from central metabolite Acetyle-co-A, which is subsequently converted to IPP, GPP, GGPP, phytoene, and lycopene.
The brilliant crimson hue of lycopene crystals in the shape of small globules hang throughout the fruit [63]. Lycopene is located in the thylakoid membranes as a protein lycopene complex at the cellular level, owing to its lipophilic nature. Despite the fact that lycopene is not an essential ingredient, it has been discovered to provide a variety of health advantages. Because it is a substantial carotenoid in human blood, it can protect lipids, proteins, and DNA from oxidative stress [21]. Lycopene’s antioxidant activity may be enhanced by the absence of the b ionone ring structure. Due to stereochemical variations, lycopene differs from other regularly eaten carotenoids in that it can only be found in particular subcellular locations. The human body directly absorbs a substantial amount of intact lycopene, which circulates through the body’s plasma, liver, and peripheral organs. It accumulates in the human tissues but is not evenly distributed [64]. Adipose tissues, adrenal glands, testes, and liver, for example, have larger concentrations, while the kidneys, prostate, lungs, and ovaries have lower concentrations [56]. Plasma lycopene has a half-life of 12–33 days in the human body [65]. Lycopene must be absorbed and incorporated into the plasma and tissues in order to be used as a dietary supplement. Tomato lycopene is not easily absorbed since it is integrated into the nutritional matrix. Clinical research demonstrates that heat-processed tomato products absorb lycopene more quickly than raw sources, and that adding oil increases absorption [66].
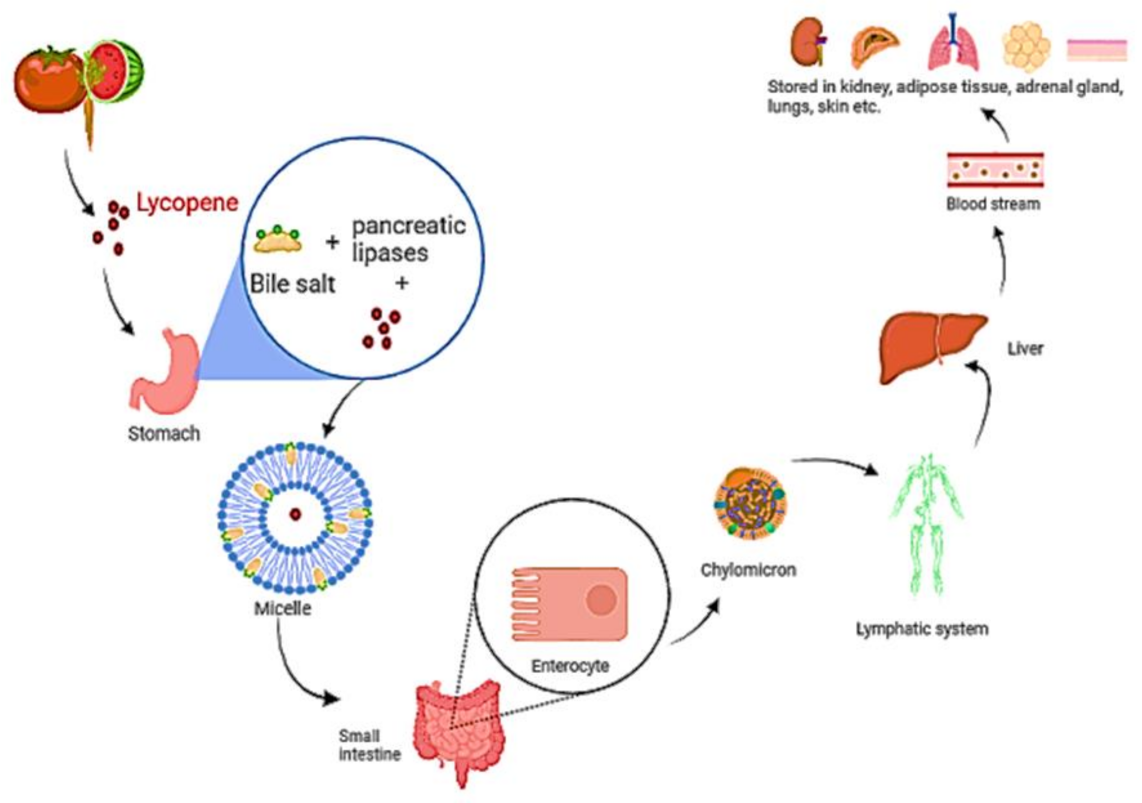
Isomerization of lycopene at low pH in the stomach has been described [67]. Before the lycopene is integrated into mixed micelles, it must be freed from the food matrix. Micelles include bile salts, cholesterol, and fatty acids from the meal and their amphiphilic shape aids in keeping the lipophilic nutrients soluble in the watery digesta [68]. The micelles approach the apical side of intestinal enterocytes’ unstirred water layer, where lycopene diffuses passively over the apical membrane [69]. Lycopene is considered to be absorbed in the same way that dietary lipids are, via passive diffusion [70,71]. According to research, the scavenger receptor class B type I (SR-BI) cholesterol membrane transporter aids in lycopene absorption. It has also been observed that various additional transporters are linked to lycopene absorption. [72,73]. Once within the enterocyte, lycopene is bound with dietary lipids to form chylomicrons [69], which are then transported via the basolateral membrane, into the lymphatic system, and finally discharged into the circulation (Figure 4). Lycopene transport and distribution are aided by plasma lipoproteins. It continues to be found in the lipophilic area of lipoproteins, which is the hydrophobic molecule’s core [18,74]. Lycopene is mostly transported by low-density lipoproteins [75,76]. Furthermore, cis isomers of lycopene have been found to have a stronger capacity to integrate into lipoprotein and other proteins than all trans isomers, owing to their shorter chain length [77].
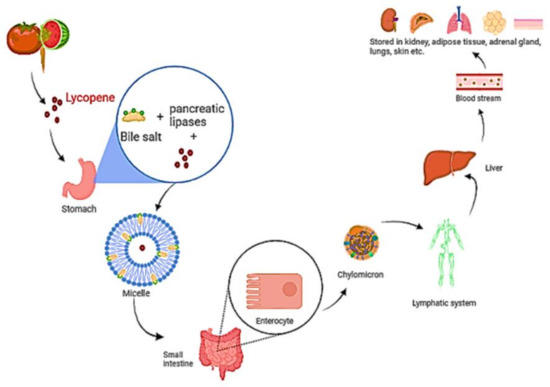
Figure 4.
A diagram depicting the digestion and absorption of lycopene. Food releases lycopene, which is then integrated into micelles containing bile salt, cholesterol, and fatty acids. The micelle approaches enterocytes, and lycopene diffuses over the apical membrane in a passive manner. Lycopene is packed with other dietary lipids inside the enterocyte to form chylomicrons, which are carried over the basolateral membrane, into the lymphatic system, and subsequently discharged into the blood.
3. Lycopene as an Antioxidant
A stressor is any agent that induces stress [78,79]. Stress is an organism’s general response to negative stimuli. Stress affects the physiological balance by causing a biological reaction to stimuli [80]. Oxidative stress, which is induced by highly reactive free radicals, is one of the primary causes of chronic illness [81,82]. Antioxidants have been identified as a varied set of chemicals that inhibit oxidation in various ways [83,84,85,86]. Only a few lipophilic natural oxidants exist, and lycopene is one of them. As a powerful singlet oxygen quencher, it can stop lipid oxidation in its early stages. Lycopene’s ability to protect against oxidative stress has been established [87,88]. Lycopene has been found to be more potent in this activity as compared to other carotenoids, such as tocopherol, ß-cryptoxanthin, carotene, lutein, and zeaxanthin [9]. B-carotene and a-tocopherol, two more lipophilic antioxidants, had double and 100-fold lower rates, respectively [89]. Lycopene’s major biological purpose is to protect DNA from oxidative stress in order to prevent mutations that might lead to chronic diseases [90,91,92]. ycopene is the most potent free radical and single oxygen scavenger among 600 naturally occurring carotenoids because of its long chain with conjugated double bonds [93,94]. It modulates phase I and II detoxifying enzymes, which affect cell proliferation, immunological response, and gene transcription [24]. It activates the antioxidant response element (ARE), which causes the cellular enzymes glutathione S-transferase (GST), superoxide dismutase (SOD), and quinone reductase to be synthesized [95,96,97,98]. HO-1, GST, NQO1, and SOD are antioxidant and detoxifying enzymes that are sometimes referred to as phase II cytoprotective enzymes [46,99]. ARE is located in the promoter regions of inducible genes that code for phase II enzymes, and it promotes overexpression of these genes when it binds to Nrf2. Lycopene inhibits Nrf2/Keapl binding in heat stressed birds, allowing Nrf2 to be transported to the nucleus and upregulate phase II enzyme synthesis [100].
The electrophile response element transcription system (EpRE) or antioxidant response element transcription system (ARE) are related to the cis-regulatory portions in the promoter region of detoxifying enzymes [26,101]. By activating the ARE transcription pathway [26], lycopene can affect xenobiotic metabolism by disrupting the cytosolic linkages between the major ARE-activating Nrf2 and its inhibitor (Keap1) [26,102]. Once liberated of Keap1, Nrf2 translocates to the nucleus, where it induces phase II enzyme expression [26,103]. According to some research, overexpression of phase II detoxification enzymes, in addition to blocking phase I metabolism and metabolic activation of aflatoxin B1, enhances lycopene’s anti-aflatoxin actions [104,105]. Lycopene operates in three methods to produce reactive oxygen species (ROS): first, radical addition (adduct formation), then electron transfer to the radical, and finally, allylic hydrogen abstraction [106]. Two processes that contribute to lycopene’s antioxidative impact are the formation of adducts and allylic hydrogen abstraction [107,108]. The type of the reacting free radical, the structural characteristics of lycopene, and the positioning and direction of lycopene inside the membrane in biological systems are all elements that impact these potential interactions [109,110,111].
The non-polarity of cell membranes or micelles is aided by the polar environment, which assists in the production of adducts and allylic hydrogen abstraction [107]. Lycopene and free radical reactions can occur in a variety of ways at the same time [109]. Lycopene can increase the cellular antioxidant defense system by regenerating non-enzymatic antioxidants, such as vitamins E and C, from their radicals [26]. Vitamin E is suggested to be protected by lycopene [87,112,113]. Lycopene serves as an antioxidant in systems that create singlet oxygen, but as a pro-oxidant in systems that generate peroxide. Lycopene serves as an antioxidant due to its redox potential [114,115,116]. Indeed, lycopene behaves as a pro-oxidant in high doses while acting as an antioxidant in low ones [117]. Many factors impact pro-oxidant potency, including tissue oxygen tension, lycopene concentration, and interactions with other antioxidants [115]. As a pro-oxidant, lycopene may have both good and negative impacts in biological systems, as well as influence the course of human illnesses. If lycopene works as a pro-oxidant in previously damaged cells, it may help prevent the creation and progression of cancerous lesions as well as tumor cytotoxicity. Carotenoids’ pro-oxidant effects can be limited by antioxidant connections, enhancing the antioxidant capabilities of these bioactive molecules [115].
4. Lycopene in Human Health and Diseases
Humans benefit from lycopene in a number of ways [26]. A lycopene-rich diet may help to prevent or lower the risk of cardiovascular disease and some malignancies [118]. According to study [119], 5 to 7 mg of lycopene per day may be sufficient to gain the benefits. Higher doses of lycopene (35–75 mg/day) may be provided in the occurrence of cancer or cardiovascular disease [120]. When combined with prostaglandins and phospholipids in cell membranes, lycopene can improve skin defense mechanisms [121]. Lycopene has been linked to the prevention and treatment of a wide range of ailments (Table 1).

Table 1.
An illustration of the nutraceutical impact of lycopene against diseases.
5. Lycopene in Cardiovascular Diseases (CVDs)
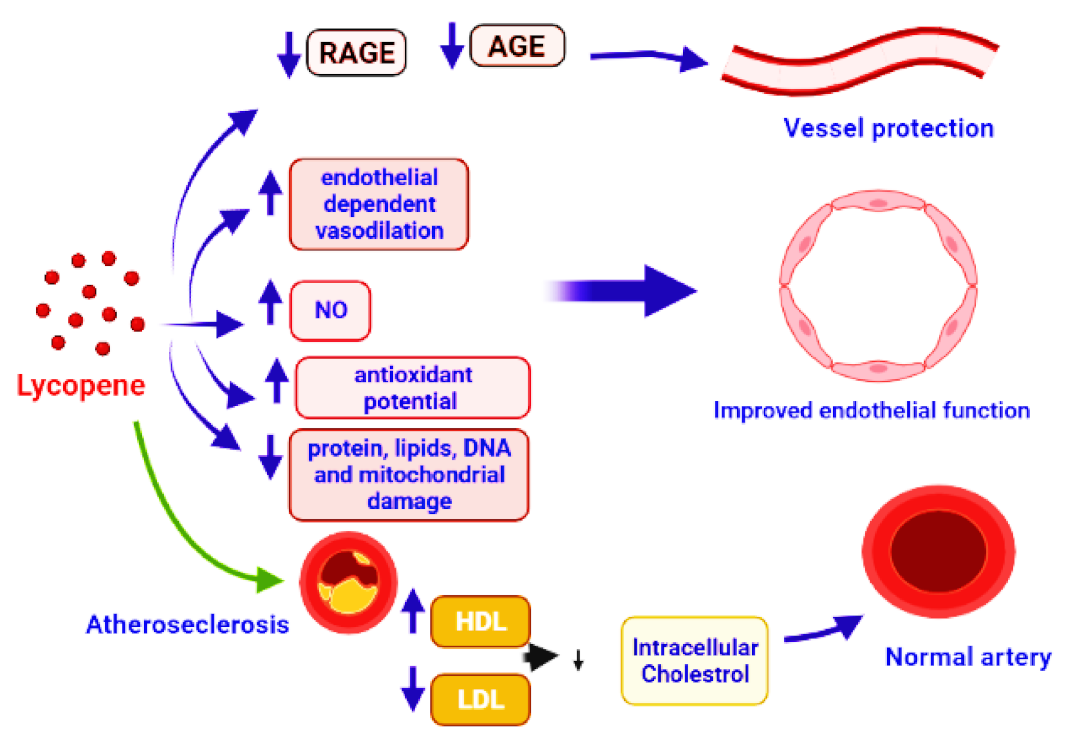
CVDs are the leading cause of illness and death around the world. High blood pressure, cholesterol, and smoking are all major risk factors for cardiovascular disease. Damage and remodeling of blood vessels impede blood flow, and atherosclerosis is the most prevalent cause of CVDs, which affect the heart and brain [156]. In comparison to Mediterranean nations, Europe and the United States have the greatest rate of CVDs [157]. The lower rates of CVD have been related to a diet heavy in vegetables, such as tomatoes, tomato derivatives, and olive oil. Small concentrations of lycopene in the blood, on the other hand, have been associated to hypertension, myocardial infarction, stroke, and atherosclerosis. Lycopene concentrations in the blood have been proven to reduce the risk of serious cardiac events. [158]. Epidemiological studies strongly advocate the preventive role of lycopene in CVDs. Low blood lycopene levels have been linked to all-cause mortality and poor cardiovascular disease outcomes. Lycopene supplementation has been shown to increase blood lycopene levels, reduce oxidative stress markers, and improve antioxidant status [76]. Reduction in the pro-inflammatory cytokines, adhesion molecules, inhibition of leukocyte migration and inflammation-related genes, problems in the interaction of monocyte with endothelium, activation of T-lymphocytes, and cyclooxygenase-2 downregulation are all anti-inflammatory mechanisms. Lycopene inhibited TNF-induced NF-kB activation and monocyte-endothelial cell interaction [159]. VCAM-1 and LDL were found inversely linked to serum lycopene [160]. Supplementation with lycopene can improve microvascular function by lowering sVCAM and sICAM concentrations, reducing DNA damage, and increasing superoxide dismutase (SOD) activity [161,162,163]. Advanced glycation end products (AGE) include a diverse group of adducts generated by the glycoxidation or glycation of DNA or protein molecules by reducing sugars [164]. Interaction of AGEs with their corresponding receptors RAGEs is the major cause of several disorders. AGE and RAGE interaction initiates oxidative stress via several pathways, such as activation of NF-kB, upregulation of gene expression for cytokines, and stimulation of NOX enzymes. Oxidative stress causes several diseases, including renal failure [165,166]. The reduction in NO production, impairment of endothelium, increased rate of mRNA degradation, neurodegenerative diseases, damage to blood vessels, and diabetes are some other subsequent effects of oxidative stress [167,168]. In this context, the natural or synthesized molecules with the potential to inhibit or reduce the production or interaction of AGEs and RAGEs have a great significance in current biomedical research. According to research reports, lycopene can reduce the production of AGE and RAGE, which aids in vessel protection [6,137,169]. The use of lycopene can promote the function of endothelial cells, as indicated by preclinical studies. Lycopene has the ability to improve the NO bioavailability, endothelium-regulated vasodilation [170], reduce the damage to proteins, DNA, and lipids, and improve mitochondrial functioning, through its antioxidant activity [171]. Lycopene supplementation boosted mitochondrial gene expression and lowered mitochondrial dysfunction [6,172]. Lycopene and tomato products were found to decrease the total cholesterol and low-density lipoprotein cholesterol (LDL-C) in clinical investigations [173,174,175]. In healthy postmenopausal women, lycopene supplementation can decrease total and LDL cholesterol [176]. In rats given lycopene supplements [177], HDL was increased significantly and LDL, triglycerides, and total cholesterol were decreased. A significant decrease in TG in lycopene-supplemented hamsters [178] and a reduction in oxidized LDL in lycopene-supplemented rats have also been reported [179]. IMT is a well-established biomarker of arterial structural change [180], and it has been associated to the presence of cardiovascular risk factors, notably in the carotid artery [181]. The thickness of the intima-media is inversely linked to serum lycopene levels [182]. The combination of lutein and lycopene (20 mg each) therapy resulted in a decrease in IMT after 12 months, with the combination showing to be more helpful than lutein alone.
5.1. Coronary Artery Disease (CAD) and Lycopene
Cardiovascular diseases with more that 17 million deaths every year include one of the major causes of death worldwide. CAD, which has become almost epidemic in many societies, is the most prominent CVD [183]. It is a chronic inflammatory disease caused by the remodeling of coronary arteries due to the narrowing of internal passage and hardening of vessels by plaque formation [184,185]. In addition to the above, the activation of platelets and inflammatory factors also contribute to reduce the blood flow to the heart muscles, reducing the supply of oxygen and nutrients [186], especially during vigorous exertion. Atherosclerosis is a silent and gradual process demonstrated by the accumulation of low-density lipids and inflammatory factors in the arteries [187,188,189,190]. Oxidized low-density lipoprotein cholesterol LDL-C is the main contributor to the development of atherosclerosis and subsequent CAD via the activation and differentiation of monocytes to macrophages [191]. Macrophages interact with LDL-C, and the production of interleukins, cytokines, and tumor necrosis factors is induced. All these molecules contribute to the formation of first lesion of atherosclerosis [192,193]. The smooth muscle cells are migrated to the intima from the medial layer of the artery, leading to the formation of a fibrous cap over the streak of lipid materials and subsequent formation of second lesion of atherosclerosis (plaque) [194]. The nature of the fibrous cap determines the properties of the plaque. Stable plaque is composed of an intact cap of smooth muscle cells in combination with collagen type I and III. Such a type of plaque results in stenosis, reduces the blood flow, and results in ischemia [195]. The second type of plaque, thin vulnerable plaques, is made up of type I collagen in combination with only a few smooth muscle cells. However, it contains a major proportion of proinflammatory molecules, macrophages, and prothrombotic molecules [196,197]. These plaques can erode or rapture, while the coagulatory proteins circulating in the blood can interact with them, resulting in thrombosis and acute coronary syndrome [198,199]. Chronic CAD can cause heart failure [200], altered heart rate [201], myocardial ischemia, and left ventricular dysfunction [202]. In epidemiological research, the risk pattern for coronary artery disease has been thoroughly investigated. Age, male sex, raised LDL-C levels, low HDL-C levels, diabetes mellitus, food, genetics, and cigarette smoking are prominent risk factors for coronary heart disease [203,204,205,206]. Obesity and metabolic disorders are also considered as the risk factors for CAD [36,207].
Lycopene suppresses the formation of vascular smooth muscle cells (VSMCs) and foam cells, both of which have anti-atherosclerotic characteristics [208,209,210]. Contractile VSMC change into proliferative and migratory cells during the atherosclerotic process, allowing them to move into the intima and build the plaque’s extracellular matrix [211]. Phenotypic modulation refers to phenotypic alterations that are important in vascular remodeling, which might result in atherosclerosis, hypertension, or diabetic macroangiopathy [212]. Lycopene prevents G1 phase cells from entering the S phase of the cell cycle [213], not by inhibiting matrix metalloproteinase [214]. It was also found that minimally oxidized LDL-C can cause VSMC phenotypic modification; to suppress this process, lycopene may inhibit oxidized LDL formation [215,216]. Direct binding to platelet-derived growth factor (PDGF) and reducing PDGF signaling [217] or acting as an antioxidant, since reactive oxygen species speed up the switch from contractile to synthetic phenotype [218], were observed to limit VSMC proliferation and migration. Lycopene also suppresses apoptosis in endothelial cells in vitro by interrupting the upregulation of p53 and caspase 3 mRNA, and prevents cluster of differentiation 14 (CD14) and toll-like receptor-4 expression in the endothelium membrane [219,220]. Circulating plasma lycopene is thought to protect against atherosclerosis (Figure 5), especially in smokers [221,222]. In high-fat diet rabbits, lycopene decreased the development of atherosclerotic plaques in the aorta and improved the lipid profile compared to the control group [223]. In hypertensive individuals, a short-term therapy with antioxidant-rich tomato extract (250 mg/day for eight weeks) can reduce blood pressure [224]. According to a research, lycopene consumption and carotid artery intima-media thickness, a risk factor for CVD, have an inverse association. [225].
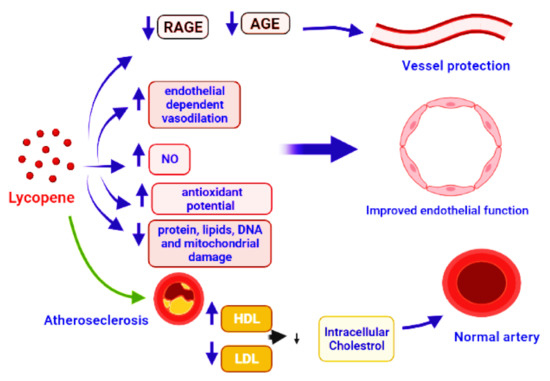
Figure 5.
Demonstration of vessel protection by the application of lycopene that caused the inhibition of AGE and RAGE production. It also leads to vasodilation, protection of proteins, DNA, lipids, and mitochondrial damage, by boosting the antioxidant activity. Improved endothelial function is reached by increasing nitric oxide (NO) bioavailability, ensuring positive changes in the lipid profile and healthy arteries, and preventing atherosclerosis.
5.2. Hypertension and Lycopene
A systolic blood pressure of more than 140 mmHg or a diastolic blood pressure of more than 90 mmHg, or both, is characterized as systemic arterial hypertension (HT), often known as high blood pressure [226]. As the silent killer, promoted by low-density lipoprotein cholesterol and smoking, it remains the third-leading cause of mortality from cardiovascular disease. Hypertension is also a major cause of stroke and renal failure. Hypertension is associated to oxidative stress and inflammatory processes [227,228]. Chronic hypertension can cause renal failure, left ventricular hypertrophy (LVH), chronic heart disease (CHD), heart failure (HF), peripheral vascular disease, stroke, and retinopathy.
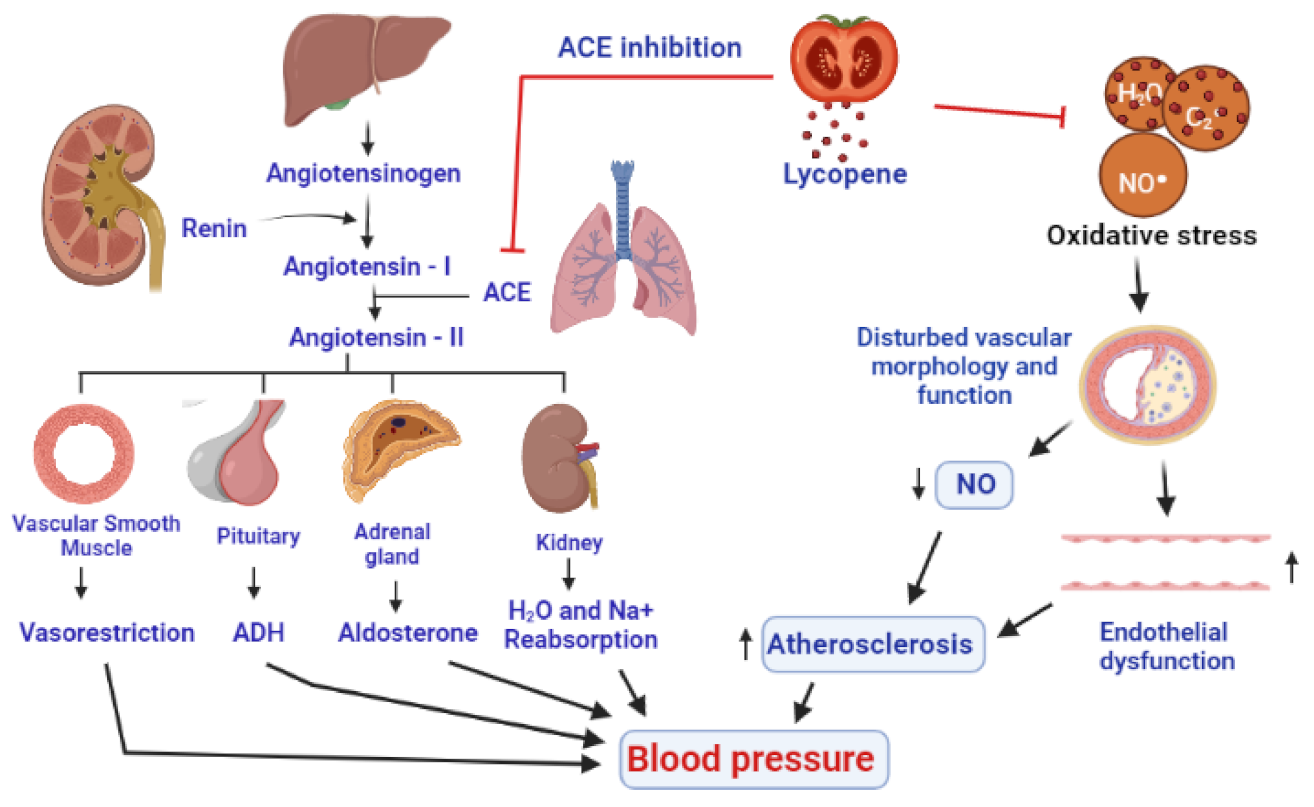
The pathophysiology of hypertension is highly complex and multifactorial. A complex interaction of environmental and pathophysiological variables that impact many systems have been reported to contribute to the development of hypertension. According to one opinion, as described by some research reports, hypertension is developed by the influence of oxidative stress. Oxidative stress makes changes in the structure and function of blood vessels by lowering the nitric oxide (NO), resulting in endothelial dysfunction and vascular cell proliferation, migration, and apoptosis, all of which promote hypertension [229]. The blood pressure-lowering effects of ROS scavengers, antioxidants, and NOX inhibitors also support the role of oxidative stress in the pathogenesis of hypertension [230]. NO is produced by endothelial cells that operate in coordination with prostacyclin to inhibit the production of adhesion molecules and aggregation of platelets. Hence, in the absence of NO, the components and mechanisms of atherosclerosis are promoted. The pathogenesis of hypertension is also well described by the renin-angiotensin system (RAS), which also provides the potential targets for the therapies against hypertension [230]. Angiotensinogen, a precursor of angiotensin, is produced in the liver, while renin is the enzyme produced by the juxtaglomerular cells of the kidneys, which catalyzes the first step in the processing of angiotensinogen into angiotensin I [231]. The angiotensin-converting enzyme (ACE), mainly produced in the lungs, is responsible for the conversion of angiotensin I into angiotensin II [232], which interacts with corresponding receptors and leads to the production of multiple biological molecules including ADH (antidiuretic hormone), aldosterone, and potent agents for vesorestriction from smooth muscle cells of vessels, adrenal, and pituitary glands. The combined effects of all these developments include the reabsorption of sodium and water by kidney, decrease in the amount of urine, narrowing of blood vessels, and subsequent onset of hypertension [233] (Figure 6).
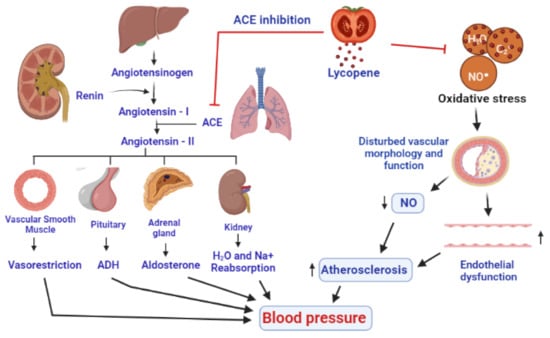
Figure 6.
A presentation of antihypertensive effects of lycopene by inhibition of ACE (angiotensin-converting enzyme) and through an antioxidant activity to inhibit the action of ROS on the linings of the blood vessels, resulting in the improvement of nitric oxide levels and the functioning of endothelium. Eventually, a decrease in vasoconstriction, inhibition of antidiuretic hormone and aldosterone, and decrease in the reabsorption of water and Na+ by the kidneys decrease the blood pressure.
Lycopene reduces oxidative stress indirectly increases nitric oxide (NO) generation in the endothelium, acting as an antioxidant and decreases blood pressure. After 6 weeks of tomato extract supplementation, a significant reduction in both systolic and diastolic blood pressure was found in 54 patients suffering from moderate hypertension, already using ACE inhibitors or calcium channel blockers, indicating a contributory role of lycopene in the management of hypertension [229]. In a meta-analysis, lycopene supplementation (above 12 mg/day) was found to lower systolic blood pressure in prehypertensive and hypertension patients, while it has shown no effect on diastolic blood pressure [234,235]. Lycopene can impede the angiotensin II by inhibiting the ACE [2]. Due to the antioxidant and anti-inflammatory properties, lycopene supplementation prevented changes in hemodynamic parameters, biochemical and inflammatory markers, apoptotic alterations, and reduced the extent of myocardial infarction. A study on 299 Korean men found that they had a significant reduction in their blood pressure [230], after 8 weeks of 15 mg/day lycopene supplementation [236] (Figure 6).
6. Conclusions
Lycopene has been shown to have a variety of biological effects in epidemiologic research and animal and cell culture investigations. These findings have prompted more research into the role of lycopene and its derivatives in the development of chronic illnesses. More research is certainly needed to identify and describe additional lycopene metabolites as well as their biological activities, which could provide vital insight into the processes behind lycopene’s positive benefits in humans, especially in terms of chronic disease prevention. Furthermore, higher lycopene consumption is linked to a lower risk of death from cardiovascular disease-, stroke-, and hypertension-related injuries. It will be determined in the future whether lycopene with increased bioavailability can maintain its antioxidant effect on lipoprotein oxidation and cardiovascular markers over time. Research with a longer duration of lycopene supplementation and a placebo control group are needed, as well as the correct amount, as there are a variety of studies demonstrating varying benefits with different doses, but no clear criteria to identify an accurate dose for hypertension and CAD patients. It is also crucial to see how this lycopene treatment affects other clinical manifestations of hypertension and coronary artery disease. Additionally, it is important to look into the effects of different lycopene isomers on oxidative and cardiovascular markers. Nonetheless, our findings imply that lycopene supplementation has a lot of potential in the treatment of cardiovascular disease and could be used to improve the inflammatory state and cardiovascular parameters in hypertension and coronary artery disease patients.
Author Contributions
Conceptualization, M.S.N., M.N.B.-J. and I.K.; Writing—original draft preparation, M.S.N. and I.U.; Writing review and editing, critical revision, M.N.B.-J., F.A.A.-A., S.J.G., B.M., M.M.G., S.I.A. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ketnawa, S.; Reginio, F.C., Jr.; Thuengtung, S.; Ogawa, Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Masson, L.F.; Rudd, A.; Vaughan, N.; Tsang, C.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Horgan, G.W.; Duthie, G. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1013–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kario, K.; Kagitani, H.; Hayashi, S.; Hanamura, S.; Ozawa, K.; Kanegae, H.A. Japan nationwide web-based survey of patient preference for renal denervation for hypertension treatment. Hypertens. Res. 2021, 17, 1–9. [Google Scholar] [CrossRef]
- Abdalla, A.A. Knowledge, attitude and practice towards therapeutic lifestyle changes in the management of hypertension in Khartoum State. Cardiovasc. J. Afr. 2021, 2, 1–6. [Google Scholar] [CrossRef]
- Couch, S.C.; Saelens, B.E.; Khoury, P.R.; Dart, K.B.; Hinn, K.; Mitsnefes, M.M.; Daniels, S.R.; Urbina, E.M. Dietary approaches to stop hypertension dietary intervention improves blood pressure and vascular health in youth with elevated blood pressure. Hypertension 2021, 77, 241–251. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Ma, H.; Yin, H.; Wang, P.; Bai, B.; Guo, L.; Geng, Q. Associations between depression, nutrition, and outcomes among individuals with coronary artery disease. Nutrition 2021, 86, 111157. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.L.; Schwartz, S.J. Lycopene: Chemical and biological properties. Food Technol. 1999, 53, 38–45. [Google Scholar]
- Mehta, D.N. Lycopene: Structure, pharmacokinetics and role in oral cancer precancerous lesions. J. Res. Adv. Dent. 2012, 1, 44–49. [Google Scholar]
- Heber, D.; Lu, Q.Y. Overview of mechanisms of action of lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food sources, biological activities, and human health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—A critical review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Hedayati, N.; Oskouei, Z.; Tabeshpour, J.; Naeini, M.B. Berberine and lycopene as alternative or add-on therapy to metformin and statins, a review. Eur. J. Pharmacol. 2021, 913, 174590. [Google Scholar] [CrossRef]
- John, J.H.; Ziebland, S.; Yudkin, P.; Roe, L.S.; Neil, H.A.; Oxford, F.; Vegetable Study, G. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: A randomised controlled trial. Lancet 2002, 359, 1969–1974. [Google Scholar] [CrossRef]
- Song, B.; Liu, K.; Gao, Y.; Zhao, L.; Fang, H.; Li, Y.; Pei, L.; Xu, Y. Lycopene and risk of cardiovascular diseases: A meta-analysis of observational studies. Mol. Nutr. Food Res. 2017, 61, 1601009. [Google Scholar] [CrossRef] [PubMed]
- Puah, B.P.; Jalil, J.; Attiq, A.; Kamisah, Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef]
- Kong, K.W.; Khoo, H.E.; Prasad, K.N.; Ismail, A.; Tan, C.P.; Rajab, N.F. Revealing the power of the natural red pigment lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapio, S.; Little, M.P.; Kaiser, J.C.; Impens, N.; Hamada, N.; Georgakilas, A.G.; Simar, D.; Salomaa, S. Ionizing radiation-induced circulatory and metabolic diseases. Environ. Int. 2021, 146, 106235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Tambunan, R.Z.; Rusmarilin, H.; Kaban, J. Antioxidant activity of tomato juice rich in lycopene antioxidant as degenerative chemopreventive agents using citrus aurantifolia juice as a preservative. IOP Conf. Ser. Earth Environ. Sci. 2018, 205, 012035. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, J.; Dong, B.; Cai, X.; Jiang, J.; Xue, R.; Liu, C. Lycopene protects against pressure overload-induced cardiac hypertrophy by attenuating oxidative stress. J. Nutr. Biochem. 2019, 66, 70–78. [Google Scholar] [CrossRef]
- Goralczyk, R.; Siler, U. The role of lycopene in health and disease. Phytochem. Health Dis. 2004, 285–309. [Google Scholar]
- Palozza, P.; Catalano, A.; Simone, R.; Cittadini, A. Lycopene as a guardian of redox signalling. Acta Biochim. Pol. 2012, 59, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Breemen, R.B.; Pajkovic, N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008, 269, 339–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelkel, M.; Schumacher, M.; Dicato, M.; Diederich, M. Antioxidant and anti-proliferative properties of lycopene. Free. Radic. Res. 2011, 45, 925–940. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kováčik, A.; Tušimová, E.; Paál, D.; Mackovich, A.; Alimov, J.; Lukáč, N. Antioxidant efficiency of lycopene on oxidative stress-induced damage in bovine spermatozoa. J. Anim. Sci. Biotechnol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lima, T.R.; Martins, P.C.; Guerra, P.H.; Santos Silva, D.A. Muscular strength and cardiovascular risk factors in adults: A systematic review. Physician Sportsmed. 2021, 49, 18–30. [Google Scholar] [CrossRef]
- Lind, L.; Ingelsson, M.; Sundstrom, J.; Ärnlöv, J. Impact of risk factors for major cardiovascular diseases: A comparison of life-time observational and Mendelian randomisation findings. Open Heart 2021, 8, e001735. [Google Scholar] [CrossRef]
- Blaum, C.; Brunner, F.J.; Kröger, F.; Braetz, J.; Lorenz, T.; Goßling, A.; Ojeda, F.; Koester, L.; Karakas, M.; Zeller, T.; et al. Modifiable lifestyle risk factors and C-reactive protein in patients with coronary artery disease: Implications for an anti-inflammatory treatment target population. Eur. J. Prev. Cardiol. 2021, 28, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Niaz, S.; Latif, J.; Hussain, S. Serum resistin: A possible link between inflammation, hypertension and coronary artery disease. Pak. J. Med Sci. 2019, 35, 641–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konukoglu, D.; Uzun, H. Endothelial dysfunction and hypertension. Hypertension: From basic research to clinical practice. Adv. Exp. Med. Biol. 2016, 956, 511–540. [Google Scholar]
- Escobar, E. Hypertension and coronary heart disease. J. Hum. Hypertens. 2002, 16, S61–S63. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Waqar, A.B.; Yan, H.; Wang, Y.; Liang, J.; Fan, J. Renovascular hypertension aggravates atherosclerosis in cholesterol-fed rabbits. J. Vasc. Res. 2019, 56, 28–38. [Google Scholar] [CrossRef]
- Han, G.M.; Liu, P. Higher serum lycopene is associated with reduced prevalence of hypertension in overweight or obese adults. Eur. J. Integr. Med. 2017, 13, 34–40. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and vascular health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Aggarwal, S. Lycopene in oral diseases. Guident 2012, 5, 73–74. [Google Scholar]
- Trumbo, P.R. Are there adverse effects of lycopene exposure? J. Nutr. 2005, 135, 2060S–2061S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elinder, L.S.; Hadell, K.; Johansson, J.; Mølgaard, J.; Holme, I.; Olsson, A.G.; Walldius, G. Probucol treatment decreases serum concentrations of diet derived antioxidants. Arter. Thromb. Vasc. Biol. 1995, 15, 1057–1063. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Chasse, G.A.; Mak, M.L.; Deretey, E.; Farkas, I.; Torday, L.L.; Papp, J.G.; Sarma, D.S.; Agarwal, A.; Chakravarthi, S.; Agarwal, S.; et al. An ab initio computational study on selected lycopene isomers. J. Mol. Struct. Theochem. 2001, 571, 27–37. [Google Scholar] [CrossRef]
- Guo, W.H.; Tu, C.Y.; Hu, C.H. Cis-trans isomerizations of β-carotene and lycopene: A theoretical study. J. Phys. Chem. B. 2008, 112, 12158–12167. [Google Scholar] [CrossRef]
- Honda, M.; Murakami, K.; Ichihashi, K.; Takada, W.; Goto, M. Enriched (Z)-lycopene in Tomato Extract via Co-Extraction of Tomatoes and Foodstuffs Containing Z-isomerization-accelerating Compounds. Catalysts 2021, 11, 462. [Google Scholar] [CrossRef]
- Schierle, J.; Bretzel, W.; Bühler, I.; Faccin, N.; Hess, D.; Steiner, K.; Schüep, W. Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem. 1997, 59, 459–465. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M.; Karabelas, A.J. Natural origin lycopene and its “green” downstream processing. Crit. Rev. Food Sci. Nutr. 2016, 56, 686–709. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.A.; Mei, Z.; Hassan, F.U.; Saeed, M.; Alagawany, M.; Shar, A.H.; Rajput, I.R. Lycopene: A natural antioxidant for prevention of heat-induced oxidative stress in poultry. World’s Poult. Sci. J. 2018, 74, 89–100. [Google Scholar] [CrossRef]
- Suwanaruang, T. Analyzing lycopene content in fruits. Agric. Agric. Sci. Procedia 2016, 11, 46–48. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M. Carotenoids in Foods. In Harvestplus Handbook for Carotenoid Analysis; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2004; p. 2. [Google Scholar]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; pp. 1–45. [Google Scholar]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [Green Version]
- Islamian, J.P.; Mehrali, H. Lycopene as a carotenoid provides radioprotectant and antioxidant effects by quenching radiation-induced free radical singlet oxygen: An overview. Cell J. 2015, 16, 386. [Google Scholar]
- Shi, J.; Le Maguer, M.; Bryan, M. Lycopene from tomatoes. Funct. Foods Biochem. Process. Asp. 2002, 2, 135–167. [Google Scholar]
- Fernandes, R.F.; Maia, L.F.; Couri, M.R.; Costa, L.A.S.; de Oliveira, L.F.C. Raman spectroscopy as a tool in differentiating conjugated polyenes from synthetic and natural sources. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 434–441. [Google Scholar] [CrossRef]
- Roldán-Gutiérrez, J.M.; Dolores Luque de Castro, M. Lycopene: The need for better methods for characterization and determination. Trends Anal. Chem. 2007, 26, 163–170. [Google Scholar] [CrossRef]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An Update on the Health Effects of Tomato Lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [Green Version]
- Petyaev, I.M. Lycopene deficiency in ageing and cardiovascular disease. Oxidative Med. Cell. Longev. 2016, 3218605. [Google Scholar] [CrossRef] [Green Version]
- Dhuique-Mayer, C.; Servent, A.; Descalzo, A.; Mouquet-Rivier, C.; Amiot, M.J.; Achir, N. Culinary practices mimicking a polysaccharide-rich recipe enhance the bioaccessibility of fat-soluble micronutrients. Food Chem. 2016, 210, 182–188. [Google Scholar] [CrossRef]
- Marshall, J.H. Production of Secondary Metabolites from Acetyl Co-A Precursors in Bacterial and Fungal Hosts. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2004. [Google Scholar]
- Thulasiram, H.V.; Poulter, C.D. Farnesyl diphosphate synthase: The art of compromise between substrate selectivity and stereoselectivity. J. Am. Chem. Soc. 2006, 128, 15819–15823. [Google Scholar] [CrossRef] [Green Version]
- Shahbani, Z.H.; Akbari, N.K.; Samoudi, M.; Omid, Y.N.; Abou Alhasanirad, S.; Safari, A.; Hosseini, F.; Hajhosseini, R. Effect of concomitant lycopene biosynthesis on CoQ10 accumulation in transformed Escherichia coli strains. Iran. J. Biotechnol. 2009, 7, 224–232. [Google Scholar]
- Hong, J.; Park, S.H.; Kim, S.; Kim, S.W.; Hahn, J.S. Efficient production of lycopene in Saccharomyces cerevisiae by enzyme engineering and increasing membrane flexibility and NAPDH production. Appl. Microbiol. Biotechnol. 2019, 103, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Siddiqui, M.W.; Tlili, I.; Montefusco, A.; Piro, G.; Hdider, C.; Lenucci, M.S. When color really matters: Horticultural performance and functional quality of high-lycopene tomatoes. Crit. Rev. Plant Sci. 2018, 37, 15–53. [Google Scholar] [CrossRef]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.Y.; Katz, N.B. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp. Biol. Med. 2002, 227, 845–851. [Google Scholar] [CrossRef]
- Liu, C.; Lian, F.; Smith, D.E.; Russell, R.M.; Wang, X.D. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003, 63, 3138–3144. [Google Scholar] [PubMed]
- Stahl, W.; Sies, H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J. Nutr. 1992, 122, 2161–2166. [Google Scholar] [CrossRef]
- Re, R.; Fraser, P.D.; Long, M.; Bramley, P.M.; Rice-Evans, C. Isomerization of lycopene in the gastric milieu. Biochem. Biophys. Res. Commun. 2001, 281, 576–581. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Harrison, E.H. Intestinal absorption and metabolism of carotenoids: Insights from cell culture. Arch. Biochem. Biophys. 2004, 430, 77–88. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of carotenoid intestinal absorption: Where do we stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapelli-Brahm, P.; Margier, M.; Desmarchelier, C.; Halimi, C.; Nowicki, M.; Borel, P.; Meléndez-Martínez, A.J.; Reboul, E. Comparison of the bioavailability and intestinal absorption sites of phytoene, phytofluene, lycopene and β-carotene. Food Chem. 2019, 300, 125232. [Google Scholar] [CrossRef]
- During, A.; Dawson, H.D.; Harrison, E.H. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in caco-2 cells treated with ezetimibe. J. Nutr. 2005, 135, 2305–2312. [Google Scholar] [CrossRef]
- Moussa, M.; Landrier, J.; Reboul, E.; Ghiringhelli, O.; Comera, C.; Collet, X.; Borel, P. Lycopene absorption in human intestinal cells and in mice involves scavenger receptor class B type I but not Niemann-Pick C1-like 1. J. Nutr. 2008, 138, 1432–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arballo, J.; Amengual, J.; Erdman, J.W. Lycopene: A critical review of digestion, absorption, metabolism, and excretion. Antioxidants 2021, 10, 342. [Google Scholar] [CrossRef]
- Clinton, S.K. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998, 56, 35–51. [Google Scholar] [CrossRef]
- Przybylska, S. Lycopene—A bioactive carotenoid offering multiple health benefits: A review. Int. J. Food Sci. Technol. 2020, 55, 11–32. [Google Scholar] [CrossRef]
- Boileau, P.; Krishnan, S.G.; Tinsi, L.; Walch, G.; Coste, J.S.; Molé, D. Tuberosity malposition and migration: Reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J. Shoulder Elb. Surg. 2002, 11, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Gouin, J.P.; Glaser, R.; Malarkey, W.B.; Beversdorf, D.; Kiecolt-Glaser, J. Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol. 2012, 31, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suresh, P.; Matthews, A.; Coyne, I. Stress and stressors in the clinical environment: A comparative study of fourth-year student nurses and newly qualified general nurses in Ireland. J. Clin. Nurs. 2013, 22, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Gu, C.; Chen, D.; Yu, B.; He, J. Oxidative stress-induced diseases and tea polyphenols. Oncotarget 2017, 8, 81649–81661. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [Green Version]
- Karadas, F.; Erdoğan, S.; Kor, D.; Oto, G.; Uluman, M. The effects of different types of antioxidants (Se, vitamin E and carotenoids) in broiler diets on the growth performance, skin pigmentation and liver and plasma antioxidant concentrations. Braz. J. Poult. Sci. 2016, 18, 101–116. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Antioxidants as modulators of arsenic-induced oxidative stress tolerance in plants: An overview. J. Hazard. Mater. 2021, 127891. [Google Scholar] [CrossRef]
- Amarowicz, R. Natural antioxidants as a subject of research. Eur. J. Lipid Sci. Technol. 2009, 111, 1053–1056. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.E.; Catalano, A.; Mele, M.C. Tomato lycopene and lung cancer prevention: From experimental to human studies. Cancers 2011, 3, 2333–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuorro, A.; Lavecchia, R.; Medici, F.; Piga, L. Enzyme-assisted production of tomato seed oil enriched with lycopene from tomato pomace. Food Bioprocess Technol. 2013, 6, 3499–3509. [Google Scholar] [CrossRef]
- Pennathur, S.; Maitra, D.; Byun, J.; Sliskovic, I.; Abdulhamid, I.; Saed, G.M.; Abu-Soud, H.M. Potent antioxidative activity of lycopene: A potential role in scavenging hypochlorous acid. Free Radic. Biol. Med. 2010, 49, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative stress and DNA damage: Implications in inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef]
- Gonfloni, S.; Maiani, E.; Di Bartolomeo, C.; Diederich, M.; Cesareni, G. Oxidative stress, DNA damage, and c-Abl signaling: At the crossroad in neurodegenerative diseases? Int. J. Cell Biol. 2012, 2012, 683097. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Agarwal, A.; Ong, C.; Prashast, P. Lycopene and male infertility. Asian J. Androl. 2014, 16, 420–425. [Google Scholar]
- Müller, L.; Goupy, P.; Fröhlich, K.; Dangles, O.; Caris-Veyrat, C.; Böhm, V. Comparative study on antioxidant activity of lycopene (Z)-isomers in different assays. J. Agric. Food Chem. 2011, 59, 4504–4511. [Google Scholar] [CrossRef]
- Luo, C.; Wu, X.G. Lycopene enhances antioxidant enzyme activities and immunity function in N-Methyl-N′-nitro-N-nitrosoguanidine–induced gastric cancer rats. Int. J. Mol. Sci. 2011, 12, 3340–3351. [Google Scholar] [CrossRef] [Green Version]
- Campos, K.K.; de Oliveira Ramos, C.; Martins, T.L.; de Paula Costa, G.; Talvani, A.; Garcia, C.C.; Oliveira, L.A.; Cangussu, S.D.; Costa, D.C.; Bezerra, F.S. Lycopene mitigates pulmonary emphysema induced by cigarette smoke in a murine model. J. Nutr. Biochem. 2019, 65, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef]
- Xu, F.; Yu, K.; Yu, H.; Wang, P.; Song, M.; Xiu, C.; Li, Y. Lycopene relieves AFB1-induced liver injury through enhancing hepatic antioxidation and detoxification potential with Nrf2 activation. J. Funct. Foods 2017, 39, 215–224. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Ali, S.; Sahin, N. Tomato powder supplementation activates nrf-2 via erk/akt signalling pathway and attenuates heat stress-related responses in quails. Anim. Feed. Sci. Technol. 2011, 165, 230–237. [Google Scholar] [CrossRef]
- Otsuki, A.; Yamamoto, M. Cis-element architecture of Nrf2–sMaf heterodimer binding sites and its relation to diseases. Arch. Pharmacal Res. 2020, 43, 275–285. [Google Scholar] [CrossRef]
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory activity of β-carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo 2018, 32, 255–264. [Google Scholar] [PubMed] [Green Version]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. J. Funct. Foods 2020, 64, 103696. [Google Scholar] [CrossRef]
- Hedayati, N.; Naeini, M.B.; Nezami, A.; Hosseinzadeh, H.; Hayes, A.W.; Hosseini, S.; Imenshahidi, M.; Karimi, G. Protective effect of lycopene against chemical and natural toxins: A review. BioFactors 2019, 45, 5–23. [Google Scholar] [CrossRef] [Green Version]
- Karaca, A.; Yilmaz, S.; Kaya, E.; Altun, S. The effect of lycopene on hepatotoxicity of aflatoxin B1 in rats. Arch. Physiol. Biochem. 2019, 127, 429–436. [Google Scholar] [CrossRef]
- Park, H.-A.; Stumpf, A.; Broman, K.; Jansen, J.; Dunn, T.; Scott, M.; Crowe-White, K.M. Role of lycopene in mitochondrial protection during differential levels of oxidative stress in primary cortical neurons. Brain Disord. 2021, 3, 100016. [Google Scholar] [CrossRef]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar] [CrossRef]
- Ural, M.Ş. Chlorpyrifos-induced changes in oxidant/antioxidant status and haematological parameters of Cyprinus carpio carpio: Ameliorative effect of lycopene. Chemosphere 2013, 90, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Francisco-Marquez, M. Reactions of OOH radical with β-carotene, lycopene, and torulene: Hydrogen atom transfer and adduct formation mechanisms. J. Phys. Chem. B 2009, 113, 11338–11345. [Google Scholar] [CrossRef]
- Sgherri, C.; Pérez-López, U.; Pinzino, C. Antioxidant Properties of Food Products Containing Lycopene are Increased by the Presence of Chlorophyll. In Lycopene: Food Sources, Potential Role in Human Health and Antioxidant Effects; Bailey, J.R., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; pp. 39–90. [Google Scholar]
- Santos Sánchez, N.; Salas-Coronado, R.; Villanueva, C.; Hernandez-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Intechopen: London, UK, 2019. [Google Scholar]
- Rao, A.V.; Agarwal, S. Role of Antioxidant Lycopene in Cancer and Heart Disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Ahmed, T.A.I.; Ibrahim, A.T.A. Protective role of lycopene and vitamin E against diazinon-induced biochemical changes in Oreochromis niloticus. Afr. J. Environ. Sci. Technol. 2015, 9, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Palozza, P. Prooxidant actions of carotenoids in biologic systems. Nutr. Rev. 1998, 56, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ascenso, A.; Pedrosa, T.; Pinho, S.; Pinho, F.; De Oliveira, J.M.; Marques, H.C.; Santos, C. The effect of lycopene preexposure on UV-B-irradiated human keratinocytes. Oxidative Med. Cell. Longev. 2016, 2016, 8214631. [Google Scholar] [CrossRef]
- Zhang, F.F.; Morioka, N.; Kitamura, T.; Fujii, S.; Miyauchi, K.; Nakamura, Y.; Hisaoka-Nakashima, K.; Nakata, Y. Lycopene ameliorates neuropathic pain by upregulating spinal astrocytic connexin 43 expression. Life Sci. 2016, 155, 116–122. [Google Scholar] [CrossRef]
- Sahin, I.; Bilir, B.; Ali, S.; Sahin, K.; Kucuk, O. Soy isoflavones in integrative oncology: Increased efficacy and decreased toxicity of cancer therapy. Integr. Cancer Ther. 2019, 18, 1534735419835310. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; Lavecchia, R. Spent coffee grounds as a valuable source of phenolic compounds and bioenergy. J. Clean. Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Campos, K.K.D.; Araújo, G.R.; Martins, T.L.; Bandeira, A.C.B.; Costa, G.d.P.; Talvani, A.; Bezerra, F.S. The antioxidant and anti-inflammatory properties of lycopene in mice lungs exposed to cigarette smoke. J. Nutr. Biochem. 2017, 48, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional importance of carotenoids and their effect on liver health: A review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascenso, A.; Pedrosa, T.; Pinho, S.; Pinho, F.; Oliveira, J.; Cabral, H.; Marques, H.O.; Simões, S.; Santos, C. The Effect of UV-B Irradiation on Human Keratinocytes after Lycopene Exposure. Carr. Mediat. Dermal Deliv. Prev. Treat. Ski. Disord. 2012, 1001, 221. [Google Scholar]
- Aust, O.; Ale-Agha, N.; Zhang, L.; Wollersen, H.; Sies, H.; Stahl, W. Lycopene oxidation product enhances gap junctional communication. Food Chem. Toxicol. 2003, 41, 1399. [Google Scholar] [CrossRef]
- Mein, J.R.; Lian, F.; Wang, X.-D. Biological activity of lycopene metabolites: Implications for cancer prevention. Nutr. Rev. 2008, 66, 667–683. [Google Scholar] [CrossRef]
- Li, W.; Jiang, B.; Cao, X.; Xie, Y.; Huang, T. Protective effect of lycopene on fluoride-induced ameloblasts apoptosis and dental fluorosis through oxidative stress-mediated Caspase pathways. Chem.-Biol. Interact. 2017, 261, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.S.; Wu, W.B.; Fang, J.Y.; Chen, D.F.; Chen, B.H.; Huang, C.C.; Chen, Y.T.; Hung, C.F. Lycopene inhibits PDGF-BB-induced signaling and migration in human dermal fibroblasts through interaction with PDGF-BB. Life Sci. 2007, 81, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yang, F. The effects of lycopene supplementation on serum insulin-like growth factor 1 (IGF-1) levels and cardiovascular disease: A dose-response meta-analysis of clinical trials. Complementary Ther. Med. 2021, 56, 102632. [Google Scholar] [CrossRef]
- Chen, J.; O’Donoghue, A.; Deng, Y.F.; Zhang, B.; Kent, F.; O’Hare, T. The effect of lycopene on the PI3K/Akt signalling pathway in prostate cancer. Anti-Cancer Agents Med. Chem. 2014, 14, 800–805. [Google Scholar] [CrossRef]
- Sahin, K.; Yenice, E.; Tuzcu, M.; Orhan, C.; Mizrak, C.; Ozercan, I.H.; Sahin, N.; Yilmaz, B.; Bilir, B.; Ozpolat, B.; et al. Lycopene Protects Against Spontaneous Ovarian Cancer Formation in Laying Hens. J. Cancer Prev. 2018, 23, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Aydemir, G.; Kasiri, Y.; Bartók, E.-M.; Birta, E.; Fröhlich, K.; Böhm, V.; Mihaly, J.; Rühl, R. Lycopene supplementation restores vitamin A deficiency in mice and possesses thereby partial pro-vitamin A activity transmitted via RAR signaling. Mol. Nutr. Food Res. 2016, 60, 2413–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboubakr, M.; Elshafae, S.M.; Abdelhiee, E.Y.; Fadl, S.E.; Soliman, A.; Abdelkader, A.; Abdel-Daim, M.M.; Bayoumi, K.A.; Baty, R.S.; Elgendy, E.; et al. Antioxidant and Anti-Inflammatory Potential of Thymoquinone and Lycopene Mitigate the Chlorpyrifos-Induced Toxic Neuropathy. Pharmaceuticals 2021, 14, 940. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Y.; Zhou, X.; Pan, W.; Shi, Y.; Wang, M.; Zhang, X.; Qi, D.; Li, L.; Ma, K.; et al. Depression-like behaviors and heme oxygenase-1 are regulated by Lycopene in lipopolysaccharide-induced neuroinflammation. J. Neuroimmunol. 2016, 298, 1–8. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- Lucas, R.; Mihály, J.; Lowe, G.M.; Graham, D.L.; Szklenar, M.; Szegedi, A.; Rühl, R. Reduced carotenoid and retinoid concentrations and altered lycopene isomer ratio in plasma of atopic dermatitis patients. Nutrients 2018, 10, 1390. [Google Scholar] [CrossRef] [Green Version]
- Shah, H.; Mahajan, S.R. Screening of topical gel containing lycopene and 1417 dexamethasone against UV radiation induced photoaging in mice. Biomed. Aging Pathol. 2014, 4, 303–308. [Google Scholar] [CrossRef]
- Palozza, P.; Colangelo, M.; Simone, R.; Catalano, A.; Boninsegna, A.; Lanza, P.; Monego, G.; Ranelletti, F.O. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis 2010, 31, 1813–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Li, Q.; Lin, T. Lycopene attenuates Staphylococcus aureus-induced inflammation via inhibiting α-hemolysin expression. Microbes Infect. 2021, 23, 104853. [Google Scholar] [CrossRef]
- Martínez, A.; Melendez-Martínez, A.J. Lycopene, oxidative cleavage derivatives and antiradical activity. Comput. Theor. Chem. 2016, 1077, 92–98. [Google Scholar] [CrossRef]
- Soleymaninejad, M.; Joursaraei, S.G.; Feizi, F.; Jafari Anarkooli, I. The effects of lycopene and insulin on histological changes and the expression level of Bcl-2 family genes in the hippocampus of streptozotocin-induced diabetic rats. J. Diabetes Res. 2017, 2017, 4650939. [Google Scholar] [CrossRef] [Green Version]
- Tabrez, S.; Al-Shali, K.Z.; Ahmad, S. Lycopene powers the inhibition of glycation-induced diabetic nephropathy: A novel approach to halt the AGE-RAGE axis menace. Biofactors 2015, 41, 372–381. [Google Scholar] [CrossRef]
- Greish, S.M.; Kader, G.S.A.; Abdelaziz, E.Z.; Eltamany, D.A.; Sallam, H.S.; Abogresha, N.M. Lycopene is superior to moringa in improving fertility markers in diet-induced obesity male rats. Saudi J. Biol. Sci. 2021, 28, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Kumar, A. Lycopene protects against memory impairment and mito-oxidative damage induced by colchicine in rats: An evidence of nitric oxide signaling. Eur. J. Pharmacol. 2013, 721, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Mehrotra, A.; Kamboj, S.S. Lycopene prevents 3-nitropropionic acid-induced mitochondrial oxidative stress and dysfunctions in nervous system. Neurochem. Int. 2010, 57, 579–587. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Wang, J.; Li, Y.; Liu, W.; Xia, J. Lycopene supplementation protects vascular dementia gerbils against the impairment of learning and memory. Folia Neuropathol. 2021, 59, 161–173. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, C.; Wang, R.; Gao, X.; Hao, C.; Liu, C. A combination of lycopene and human amniotic epithelial cells can ameliorate cognitive deficits and suppress neuroinflammatory signaling by choroid plexus in Alzheimer’s disease rat. J. Nutr. Biochem. 2021, 88, 108558. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Chopra, K. Lycopene abrogates Aβ (1–42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer’s disease. J. Nutr. Biochem. 2015, 26, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Ratto, F.; Franchini, F.; Musicco, M.; Caruso, G.; Di Santo, S.G. A narrative review on the potential of tomato and lycopene for the prevention of Alzheimer’s disease and other dementias. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- Ning, W.J.; Lv, R.J.; Xu, N.; Hou, X.Y.; Shen, C.; Guo, Y.L.; Fan, Z.Y.; Cao, N.; Liu, X.P. Lycopene-Loaded Microemulsion Regulates Neurogenesis in Rats with Aβ-Induced Alzheimer’s Disease Rats Based on the Wnt/β-catenin Pathway. Neural Plast. 2021, 2021. [Google Scholar] [CrossRef]
- Kaur, H.; Chauhan, S.; Sandhir, R. Protective Effect of Lycopene on Oxidative Stress and Cognitive Decline in Rotenone Induced Model of Parkinson’s Disease. Neurochem. Res. 2011, 36, 1435–1443. [Google Scholar] [CrossRef]
- Prema, A.; Janakiraman, U.; Manivasagam, T.; Thenmozhi, A.J. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson’s disease in mice. Neurosci. Lett. 2015, 599, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Man, H.B.; Bi, W.P. Protective effect of lycopene in a mouse model of Parkinson’s disease via reducing oxidative stress and apoptosis. Anal. Quant. Cytopathol. Histopathol. 2018, 40, 253–258. [Google Scholar]
- Ardawi, M.-S.M.; Badawoud, M.H.; Hassan, S.M.; Rouzi, A.A.; Ardawi, J.M.; AlNosani, N.M.; Qari, M.H.; Mousa, S.A. Lycopene treatment against loss of bone mass, microarchitecture and strength in relation to regulatory mechanisms in a postmenopausal osteoporosis model. Bone 2016, 83, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.S.; Shao, M.L.; Sun, Z.; Chen, S.M.; Hu, Y.J.; Wang, H.T.; Wei, T.K.; Li, X.S.; Zheng, H.X. Lycopene ameliorates diabetic osteoporosis via anti-inflammatory, anti-oxidation, and increasing Osteoprotegerin/RANKL expression ratio. J. Funct. Foods 2021, 83, 104539. [Google Scholar] [CrossRef]
- Whelton, S.P.; McEvoy, J.W.; Shaw, L.; Psaty, B.M.; Lima, J.A.C.; Budoff, M.; Nasir, K.; Szklo, M.; Blumenthal, R.S.; Blaha, M.J. Association of Normal Systolic Blood Pressure Level with Cardiovascular Disease in the Absence of Risk Factors. JAMA Cardiol. 2020, 5, 1011–1018. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Ohukainen, P.; Virtanen, J.K.; Ala-Korpela, M. Vexed causal inferences in nutritional epidemiology—Call for genetic help. Int. J. Epidemiology 2021, in press. [Google Scholar] [CrossRef]
- Shixian, Q.; Dai, Y.; Kakuda, Y.; Shi, J.; Mittal, G.; Yeung, D.; Jiang, Y. Synergistic antioxidative effects of lycopene with other bioactive compounds. Food Rev. Int. 2005, 21, 295–311. [Google Scholar] [CrossRef]
- Castro, I.A.; Moraes Barros, S.B.; Lanfer Marquez, U.M.; Motizuki, M.; Higashi Sawada, T.C. Optimization of the antioxidant capacity of a mixture of carotenoids and α-tocopherol in the development of a nutritional supplement. Food Res. Int. 2005, 38, 861–866. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Shanely, R.A.; Zwetsloot, J.J.; Jurrissen, T.J.; Hannan, L.C.; Zwetsloot, K.A.; Needle, A.R.; Bishop, A.E.; Wu, G.; Perkins-Veazie, P. Daily watermelon consumption decreases plasma sVCAM-1 levels in overweight and obese postmenopausal women. Nutr. Res. 2020, 76, 9–19. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and cardiovascular health. Int. J. Mol. sciences 2018, 19, 3988. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Xu, J.; Liu, Y.; Zhao, X.; Deng, X.; Guo, L.; Gu, J. A novel bud mutation that confers abnormal patterns of lycopene accumulation in sweet orange fruit (Citrus sinensis L. Osbeck). J. Exp. Bot. 2007, 58, 4161–4171. [Google Scholar] [CrossRef]
- Dhar, I.; Caspar-Bell, G.; Prasad, K. Role of Advanced Glycation End Products and Its Receptors in the Pathogenesis of Cigarette Smoke-Induced Cardiovascular Disease. Int. J. Angiol. 2015, 24, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Sanajou, D.; Haghjo, A.G.; Argani, H.; Aslani, S. AGE-RAGE axis blockade in diabetic nephropathy: Current status and future directions. Eur. J. Pharmacol. 2018, 833, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Wautier, M.-P.; Guillausseau, P.-J.; Wautier, J.-L. Activation of the receptor for advanced glycation end products and consequences on health. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 305–309. [Google Scholar] [CrossRef]
- Kellow, N.; Coughlan, M.T. Effect of diet-derived advanced glycation end products on inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef]
- Van Puyvelde, K.; Mets, T.; Njemini, R.; Beyer, I.; Bautmans, I. Effect of advanced glycation end product intake on inflammation and aging: A systematic review. Nutr. Rev. 2014, 72, 638–650. [Google Scholar] [CrossRef]
- Treggiari, D.; Dalbeni, A.; Meneguzzi, A.; Delva, P.; Fava, C.; Molesini, B.; Pandolfini, T.; Minuz, P. Lycopene inhibits endothelial cells migration induced by vascular endothelial growth factor A increasing nitric oxide bioavailability. J. Funct. Foods 2018, 42, 312–318. [Google Scholar] [CrossRef]
- Watters, J.L.; A Satia, J.; Kupper, L.L.; A Swenberg, J.; Schroeder, J.C.; Switzer, B.R.; Florin, T.A.; Fryer, G.E.; Miyoshi, T.; Weitzman, M.; et al. Associations of Antioxidant Nutrients and Oxidative DNA Damage in Healthy African-American and White Adults. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1428–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scolastici, C.; de Lima, R.A.; Barbisan, L.F.; Ferreira, A.; Ribeiro, D.; Salvadori, D. Antigenotoxicity and antimutagenicity of lycopene in HepG2 cell line evaluated by the comet assay and micronucleus test. Toxicol. Vitr. 2008, 22, 510–514. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Ma, Y.; Qu, Y.; Lu, X.; Luo, H. Effects of dietary lycopene supplementation on plasma lipid profile, lipid peroxidation and antioxidant defense system in feedlot Bamei lamb. Asian-Australas. J. Anim. Sci. 2015, 28, 958. [Google Scholar] [CrossRef]
- Fachinello, M.R.; Gasparino, E.; Partyka, A.V.; de Souza Khatlab, A.; Castilha, L.D.; Huepa, L.M.; Ferreira, L.F.; Pozza, P.C. Dietary lycopene alters the expression of antioxidant enzymes and modulates the blood lipid profile of pigs. Anim. Prod. Sci. 2020, 60, 806–814. [Google Scholar] [CrossRef]
- Tinkler, J.H.; Böhm, F.; Schalch, W.; Truscott, T. Dietary carotenoids protect human cells from damage. J. Photochem. Photobiol. B Biol. 1994, 26, 283–285. [Google Scholar] [CrossRef]
- Riso, P.; Visioli, F.; Erba, D.; Testolin, G.; Porrini, M. Lycopene and vitamin C concentrations increase in plasma and lymphocytes after tomato intake. Effects on cellular antioxidant protection. Eur. J. Clin. Nutr. 2004, 58, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Visioli, F.; Grande, S.; Guarnieri, S.; Gardana, C.; Simonetti, P.; Porrini, M. Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. J. Agric. Food Chem. 2006, 54, 2563–2566. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Qu, Q.; Kakuda, Y.; Xue, S.J.; Jiang, Y.; Koide, S.; Shim, Y.-Y. Investigation of the antioxidant and synergistic activity of lycopene and other natural antioxidants using LAME and AMVN model systems. J. Food Compos. Anal. 2007, 20, 603–608. [Google Scholar] [CrossRef]
- Stahl, W.; Junghans, A.; de Boer, B.; Driomina, E.S.; Briviba, K.; Sies, H. Carotenoid mixtures protect multilamellar liposomes against oxidative damage: Synergistic effects of lycopene and lutein. FEBS Lett. 1998, 427, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Gaur, S. Lycopene: Chemistry, Biosynthesis, Health Benefits and Nutraceutical Applications. In Plant-Derived Bioactives; Springer: Singapore, 2020; pp. 251–263. [Google Scholar]
- Misra, R.; Mangi, S.; Joshi, S.; Mittal, S.; Gupta, S.K.; Pandey, R.M. LycoRed as an alternative to hormone replacement therapy in lowering serum lipids and oxidative stress markers: A randomized controlled clinical trial. J. Obstet. Gynaecol. Res. 2006, 32, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.; Periago, M.J.; Böhm, V.; Berruezo, G.R. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br. J. Nutr. 2008, 99, 137–146. [Google Scholar] [CrossRef]
- Wong, N.D. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat. Rev. Cardiol. 2014, 11, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Shin, S.; Park, H.B.; Chang, H.J.; Arsanjani, R.; Min, J.K.; Kim, Y.J.; Lee, B.K.; Choi, J.H.; Hong, G.R.; Chung, N. Impact of intensive LDL cholesterol lowering on coronary artery atherosclerosis progression: A serial CT angiography study. JACC Cardiovasc. Imaging 2017, 10, 437–446. [Google Scholar] [CrossRef]
- Asanuma, T.; Higashikuni, Y.; Yamashita, H.; Nagai, R.; Hisada, T.; Sugiura, S. Discordance of the Areas of Peak Wall Shear Stress and Tissue Stress in Coronary Artery Plaques as Revealed by Fluid-Structure Interaction Finite Element Analysis. Int. Heart J. 2013, 54, 54–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusis, A.J.; Mar, R.; Pajukanta, P. Genetics of atherosclerosis. Annu. Rev. Genomics Hum. Genet. 2004, 5, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Sanz, J.; Moreno, P.; Fuster, V. The year in atherothrombosis. J. Am. Coll. Cardiol. 2013, 62, 1131–1143. [Google Scholar] [CrossRef] [Green Version]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Ghattas, A.; Griffiths, H.R.; Devitt, A.; Lip, G.Y.; Shantsila, E. Monocytes in coronary artery disease and atherosclerosis: Where are we now? J. Am. Coll. Cardiol. 2013, 62, 1541–1551. [Google Scholar] [CrossRef] [Green Version]
- Battes, L.C.; Cheng, J.M.; Oemrawsingh, R.M.; Boersma, E.; Garcia-Garcia, H.M.; De Boer, S.P.; Buljubasic, N.; Van Mieghem, N.A.; Regar, E.; Van Geuns, R.-J.; et al. Circulating cytokines in relation to the extent and composition of coronary atherosclerosis: Results from the ATHEROREMO-IVUS study. Atherosclerosis 2014, 236, 18–24. [Google Scholar] [CrossRef]
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008, 79, 360–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabas, I. Macrophage death and defective infammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010, 10, 36–46. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of Vulnerable/Unstable Plaque. Arter. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef] [Green Version]
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of Atherosclerosis Plaque Progression. Heart Lung Circ. 2013, 22, 399–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Doyle, B.; Caplice, N. Plaque Neovascularization and Antiangiogenic Therapy for Atherosclerosis. J. Am. Coll. Cardiol. 2007, 49, 2073–2080. [Google Scholar] [CrossRef] [Green Version]
- Mehilli, J.; Presbitero, P. Coronary artery disease and acute coronary syndrome in women. Heart 2020, 106, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Libby, P.; Gersh, B.; Yellon, D.; Böhm, M.; Lopaschuk, G.; Opie, L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014, 383, 1933–1943. [Google Scholar] [CrossRef] [Green Version]
- Fox, K.M.; Ferrari, R. Heart rate: A forgotten link in coronary artery disease? Nat. Rev. Cardiol. 2011, 8, 369–379. [Google Scholar] [CrossRef]
- Panza, J.A.; Holly, T.A.; Asch, F.M.; She, L.; Pellikka, P.A.; Velazquez, E.J.; Lee, K.L.; Borges-Neto, S.; Farsky, P.S.; Jones, R.H.; et al. Inducible Myocardial Ischemia and Outcomes in Patients with Coronary Artery Disease and Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2013, 61, 1860–1870. [Google Scholar] [CrossRef] [Green Version]
- Hajar, R. Risk factors for coronary artery disease: Historical perspectives. Heart Views 2017, 18, 109. [Google Scholar] [CrossRef]
- Neaton, J.; Wentworth, D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and difference by age for 316.099 white man. Multiple Risk Factor Intervention Trial Research Group. Arch. Intern. Med. 1992, 152, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Chang, C.C.; Hadley, T. Genetic risk stratification: A paradigm shift in prevention of coronary artery disease. JACC Basic Transl. Sci. 2021, 6, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.M.; de Vries, L.; Rensen, P.C.; van Dijk, K.W.; Blauw, G.J.; van Heemst, D.; Noordam, R. Apolipoprotein E genotype, lifestyle and coronary artery disease: Gene-environment interaction analyses in the UK Biobank population. Atherosclerosis 2021. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar]
- Ra, L.M.; Franch, A.A.; Gil-Campos, M.; Trabazo, R.L.; Moreno Villares, J.M.; Suarez, M.V.; Giner, P.M.C.; Nutrition Committee of the Spanish Association of Pediatrics. Childhood obesity. Recommendations of the nutrition committee of the Spanish association of pediatrics. Part, I. Prevention. Early detection. Role of the pediatrician. An. Pediatr. 2006, 65, 607–615. [Google Scholar]
- Li-Ping, C.H.; Shu-Ying, H.E.; Zheng, H.; Yu-Lu, D.A. Effects and mechanisms of lycopene on the proliferation of vascular smooth muscle cells. Chin. J. Nat. Med. 2010, 8, 218–222. [Google Scholar]
- Kim, J.-W.; Covel, N.; Guess, P.; Rekow, E.; Zhang, Y. Concerns of Hydrothermal Degradation in CAD/CAM Zirconia. J. Dent. Res. 2010, 89, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.S.; Tong, Y.; Ling, L.; Chen, D.; Sun, H.J.; and Zhou, H. NLRP3 gene deletion attenuates angiotensin ii-induced phenotypic transformation of vascular smooth muscle cells and vascular remodeling. Cell. Physiol. Biochem. 2017, 44, 2269–2280. [Google Scholar] [CrossRef]
- Chen, S.; Huang, L.; Hu, C. Antioxidative reaction of carotenes against peroxidation of fatty acids initiated by nitrogen dioxide: A theoretical study. J. Phys. Chem. B 2015, 119, 9640–9650. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-M.; Hung, C.-F.; Tseng, Y.-L.; Chen, B.-H.; Jian, J.-S.; Wu, W.-B. Lycopene binds PDGF-BB and inhibits PDGF-BB-induced intracellular signaling transduction pathway in rat smooth muscle cells. Biochem. Pharmacol. 2007, 74, 54–63. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Vascular smooth muscle cell in atherosclerosis. Acta Physiol. 2015, 214, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Sung, H.J.; Eskin, S.G.; Sakurai, Y.; Yee, A.; Kataoka, N.; McIntire, L.V. Oxidative stress produced with cell migration increases synthetic phenotype of vascular smooth muscle cells. Ann. Biomed. Eng. 2005, 33, 1546–1554. [Google Scholar] [CrossRef]
- Bae, J.W.; Bae, J.-S. Barrier protective effects of lycopene in human endothelial cells. Inflamm. Res. 2011, 60, 751–758. [Google Scholar] [CrossRef]
- Tang, X.; Yang, X.; Peng, Y.; Lin, J. Protective Effects of Lycopene against H2O2-Induced Oxidative Injury and Apoptosis in Human Endothelial Cells. Cardiovasc. Drugs Ther. 2009, 23, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Klipstein-Grobusch, K.; Launer, L.J.; Geleijnse, J.M.; Boeing, H.; Hofman, A.; Witteman, J.C.M. Serum carotenoids and atherosclerosis The Rotterdam Study. Atherosclerosis 2000, 148, 49–56. [Google Scholar] [CrossRef]
- Rissanen, T.H.; Voutilainen, S.; Nyyssönen, K.; Salonen, J.T. Lycopene, atherosclerosis, and coronary heart disease. Exp. Biol. Med. 2002, 227, 900–907. [Google Scholar] [CrossRef]
- Hu, M.-Y.; Li, Y.-L.; Jiang, C.-H.; Liu, Z.-Q.; Qu, S.-L.; Huang, Y.-M. Comparison of lycopene and fluvastatin effects on atherosclerosis induced by a high-fat diet in rabbits. Nutrition 2008, 24, 1030–1038. [Google Scholar] [CrossRef]
- Rissanen, T.H.; Voutilainen, S.; Nyyssönen, K.; Salonen, R.; Kaplan, G.A.; Salonen, J.T. Serum lycopene concentrations and carotid atherosclerosis: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2003, 77, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Engelhard, Y.N.; Gazer, B.; Paran, E.; Sheva, B. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: A double-blind, placebo-controlled pilot study. Am. Heart J. 2006, 151, 100. [Google Scholar] [CrossRef]
- Ranjbar, F.; Akbarzade, F.; Khaneshi, M. The study oftype A personality in essential Hypertensionpatient. Med. J. Kordestan Univ. Med. Sci. 2006, 11, 26–31. [Google Scholar]
- Kooshki, A.; Yaghoubifar, M.A.; Behnam, V.H.R. Relation between drinking water and blood pressure. Sabzevar J. Med. Sci. 2004, 10, 23–28. [Google Scholar]
- Kooshki, A.; Mohajeri, N.; Movahedi, A. Prevalence of CVD Risk Factors related to diet in Patients Referring to Modarres Hospital in Tehran in 1999. Sabzevar J. Med. Sci. 2004, 10, 17–22. [Google Scholar]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L. Montezano AC. Oxidative stress: A unifying paradigm in hypertension. Can. J. Cardiol. 2020, 36, 659–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.I.; Ungvari, Z. Role of mitochondrial oxidative stress in hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H1417–H1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, M.; Wilcox, C.S. Oxidative stress in hypertension: Role of the kidney. Antioxid. Redox Signal. 2014, 20, 74–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prysyazhna, O.; Wolhuter, K.; Switzer, C.; Santos, C.; Yang, X.; Lynham, S.; Shah, A.M.; Eaton, P.; Burgoyne, J.R. Blood pressure–lowering by the antioxidant resveratrol is counterintuitively mediated by oxidation of cGMP-dependent protein kinase. Circulation 2019, 140, 126–137. [Google Scholar] [CrossRef]
- Navar, L.G.; Kobori, H.; Prieto, M.C.; Gonzalez-Villalobos, R.A. Intratubular Renin-Angiotensin System in Hypertension. Hypertension 2011, 57, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Whitworth, J.A. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003, 21, 1983–1992. [Google Scholar]
- Assersen, K.B.; Sumners, C.; Steckelings, U.M. The Renin-Angiotensin System in Hypertension, a Constantly Renewing Classic: Focus on the Angiotensin AT2-Receptor. Can. J. Cardiol. 2020, 36, 683–693. [Google Scholar] [CrossRef]
- Hasan, A.; Paray, B.A.; Hussain, A.; Qadir, F.A.; Attar, F.; Aziz, F.M.; Sharifi, M.; Derakhshankhah, H.; Rasti, B.; Mehrabi, M.; et al. A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. J. Biomol. Struct. Dyn. 2021, 39, 3025–3033. [Google Scholar] [CrossRef] [Green Version]
- Santos, P.C.J.; Krieger, J.; Pereira, A.C. Renin-angiotensin system, hypertension, and chronic kidney disease: Pharmacogenetic implications. J. Pharmacol. Sci. 2012, 120, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R.; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [PubMed]
- Li, X.; Xu, J. Lycopene Supplement and Blood Pressure: An Updated Meta-Analysis of Intervention Trials. Nutrients 2013, 5, 3696–3712. [Google Scholar] [CrossRef] [PubMed]
- Paran, E.; Novack, V.; Engelhard, Y.N.; Hazan-Halevy, I. The Effects of Natural Antioxidants from Tomato Extract in Treated but Uncontrolled Hypertensive Patients. Cardiovasc. Drugs Ther. 2008, 23, 145–151. [Google Scholar] [CrossRef]
- Aman, U.; Vaibhav, P.; Balaraman, R. Tomato lycopene attenuates myocardial infarction induced by isoproterenol: Electrocardiographic, biochemical and anti-apoptotic study. Asian Pac. J. Trop. Biomed. 2012, 2, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Wong, Z.W.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of the protective role of natural products on isoproterenol-induced myocardial infarction: A review. Biomed. Pharmacother. 2017, 94, 1145–1166. [Google Scholar] [CrossRef]
- Yeo, H.Y.; Kim, O.Y.; Lim, H.H.; Kim, J.Y.; Lee, J.H. Association of serum lycopene and brachial-ankle pulse wave velocity with metabolic syndrome. Metabolism 2011, 60, 537–543. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, Y.-S.; Ahn, S.; Chun, Y.H.; Lim, K.S. The Usefulness of Procalcitonin and C-Reactive Protein as Early Diagnostic Markers of Bacteremia in Cancer Patients with Febrile Neutropenia. Cancer Res. Treat. 2011, 43, 176–180. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).