Association of Particulate Matter from Cooking Oil Fumes with Heart Rate Variability and Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Continuous Holter Monitoring
2.3. Particulate Matter
2.4. Particulate PAHs
2.5. Urinary 8-OHdG and MDA
2.6. Statistical Methods
3. Results
3.1. Personal Characteristics and Environmental Exposures
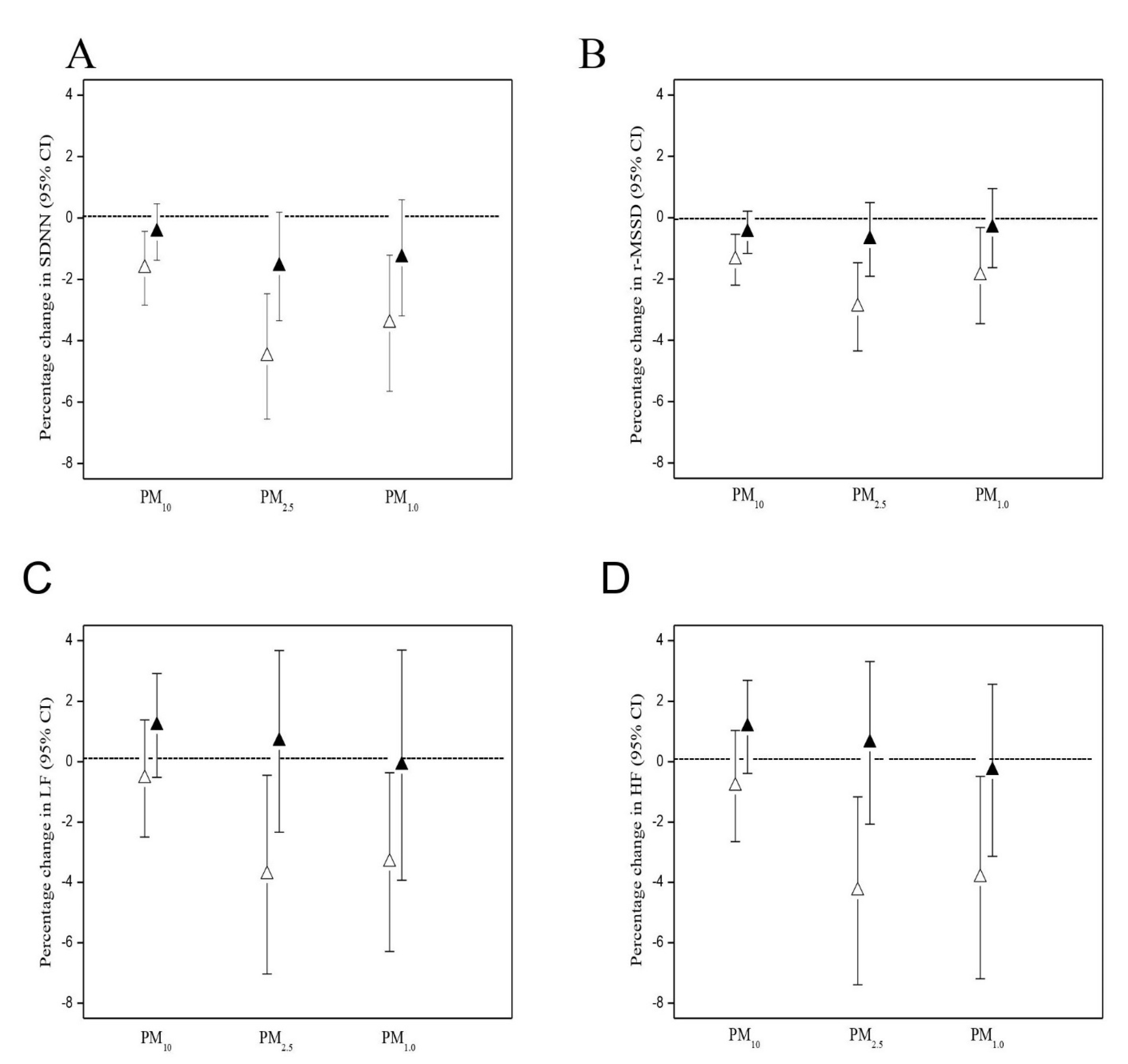
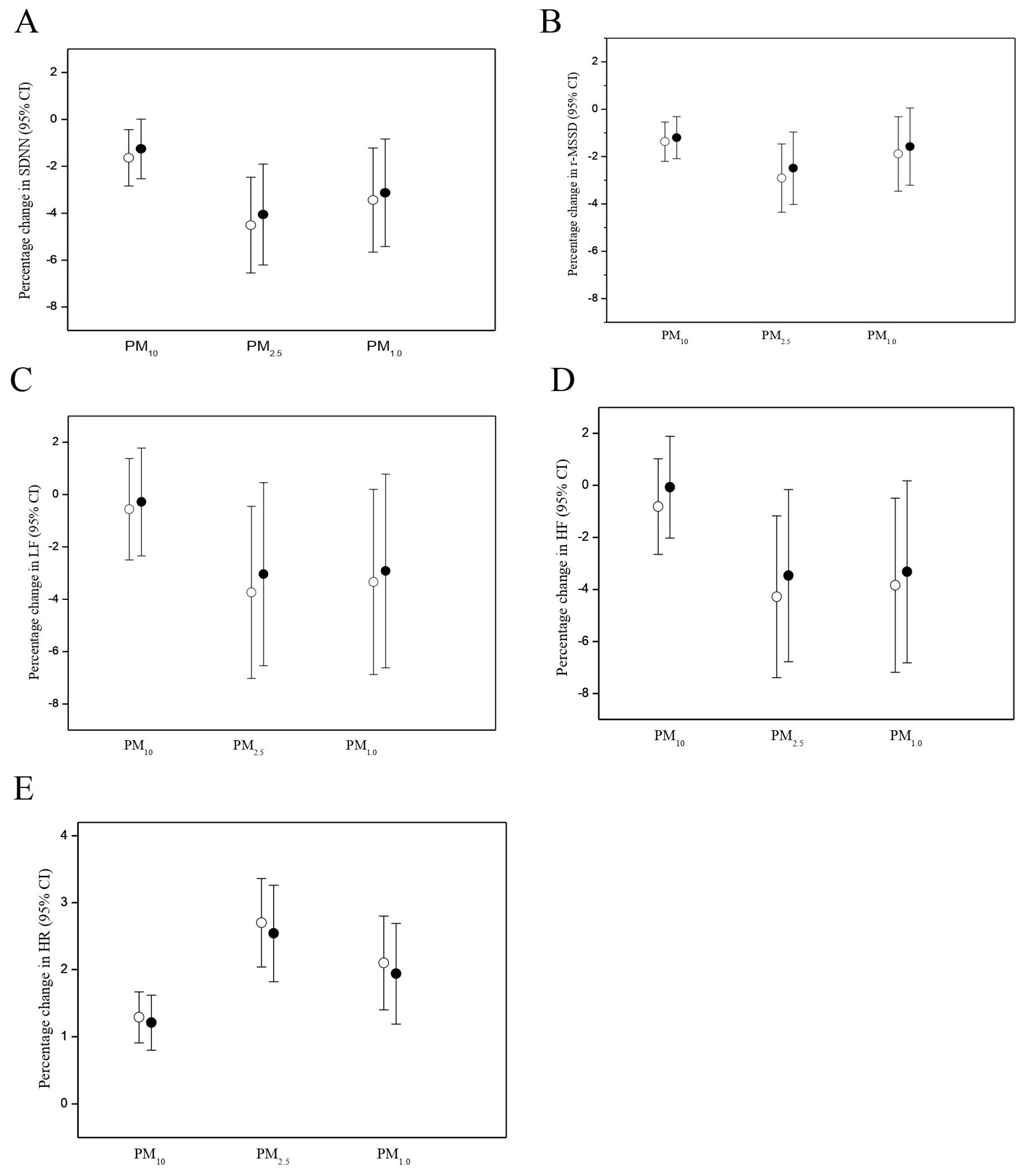
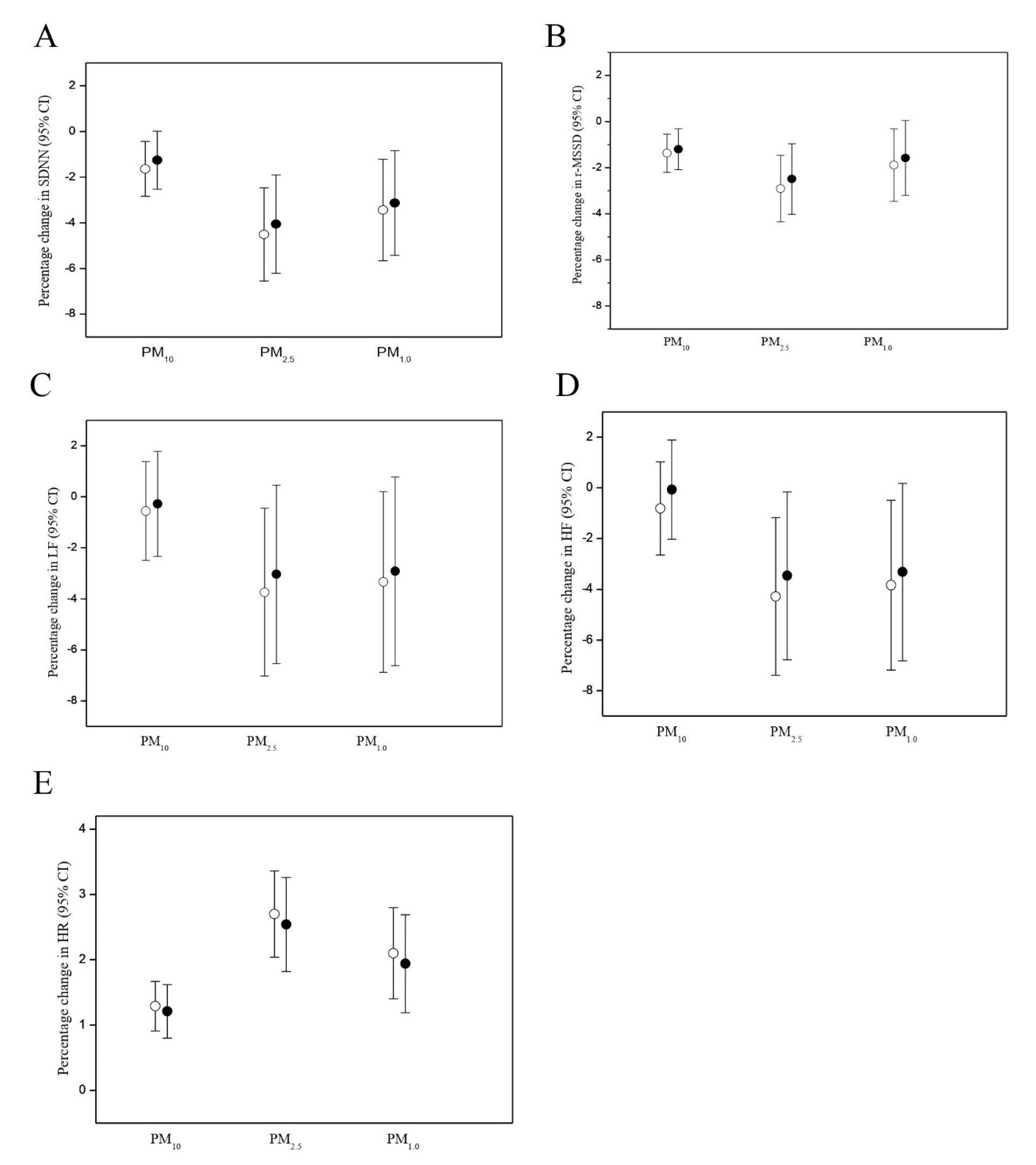
3.2. PM Effects on HR and HRV
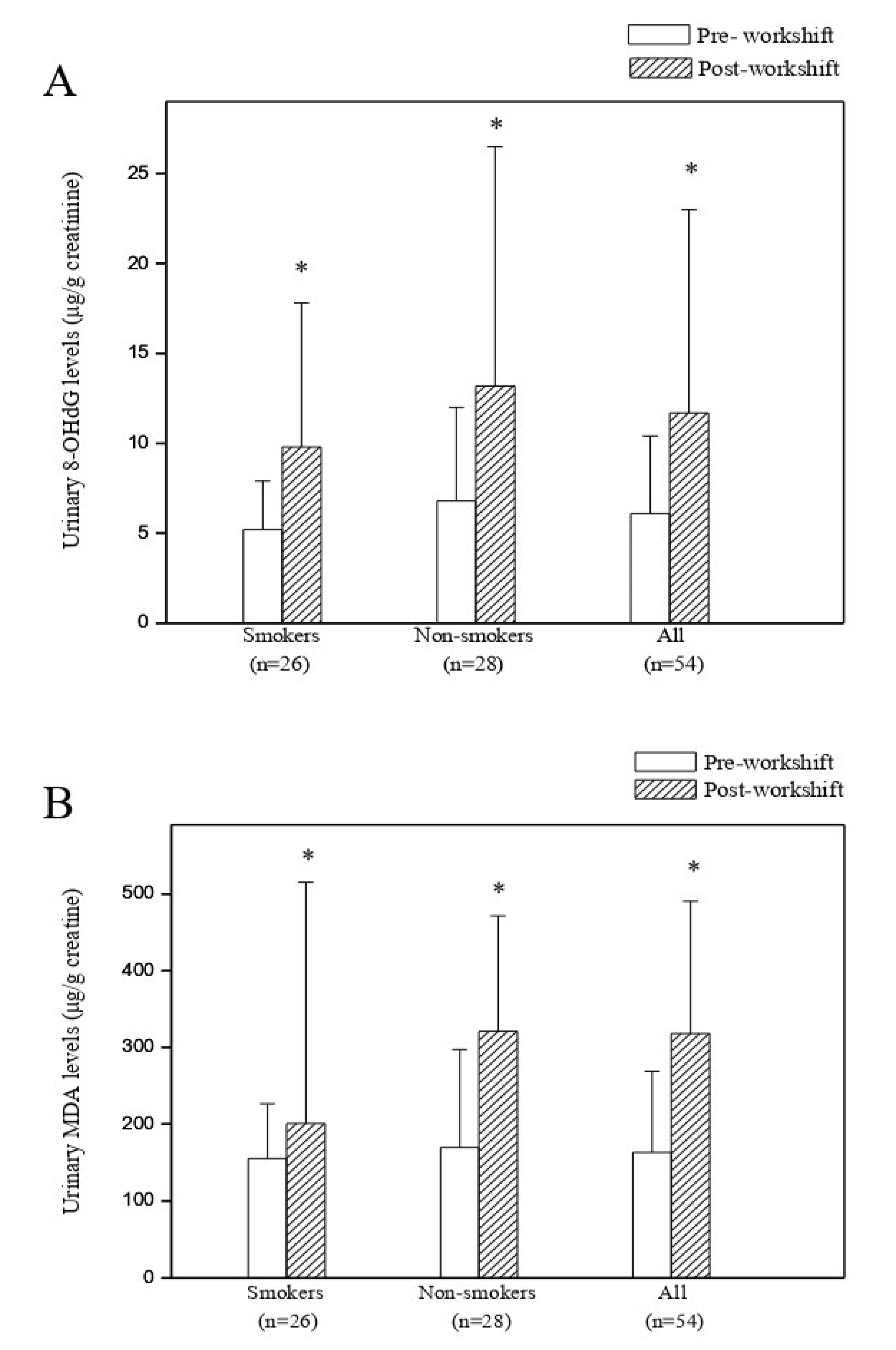
3.3. Predictors of Cross-Shift Changes in Urinary 8-OHdG and MDA Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hung, C.S.; Huang, C.C.; Pan, S.C.; Ma, H.P.; Huang, C.C.; Guo, Y.L.L.; Ho, Y.L. Acute Particulate Matter Exposure Is Associated with Disturbances in Heart Rate Complexity in Patients with Prior Myocardial Infarction. Sci. Total Environ. 2020, 733, 138842. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.; Ruiz, S.; Hermosillo, A.G.; Borja-Aburto, V.H.; Cárdenas, M. Ambient Fine Particles Modify Heart Rate Variability in Young Healthy Adults. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Magari, S.R.; Schwartz, J.; Williams, P.L.; Hauser, R.; Smith, T.J.; Christiani, D.C. The Association Between Personal Measurements of Environmental Exposure to Particulates and Heart Rate Variability. Epidemiology 2002, 13, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; O’Neill, M.S.; Vokonas, P.S.; Sparrow, D.; Schwartz, J. Effects of Air Pollution on Heart Rate Variability: The VA Normative Aging Study. Environ. Health Perspect. 2005, 113, 304–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adar, S.D.; Gold, D.R.; Coull, B.A.; Schwartz, J.; Stone, P.H.; Suh, H. Focused Exposures to Airborne Traffic Particles and Heart Rate Variability in the Elderly. Epidemiology 2007, 18, 95–103. [Google Scholar] [CrossRef]
- Baccarelli, A.; Cassano, P.A.; Litonjua, A.; Park, S.K.; Suh, H.; Sparrow, D.; Vokonas, P.; Schwartz, J. Cardiac Autonomic Dysfunction: Effects from Particulate Air Pollution and Protection by Dietary Methyl Nutrients and Metabolic Polymorphisms. Circulation 2008, 117, 1802–1809. [Google Scholar] [CrossRef] [Green Version]
- Pope, C.A., III; Verrier, R.L.; Lovett, E.G.; Larson, A.C.; Raizenne, M.E.; Kanner, R.E.; Schwartz, J.; Villegas, G.M.; Gold, D.R.; Dockery, D.W. Heart Rate Variability Associated with Particulate Air Pollution. Am. Heart J. 1999, 138, 890–899. [Google Scholar] [CrossRef]
- Cavallari, J.M.; Eisen, E.A.; Chen, J.C.; Fang, S.C.; Dobson, C.B.; Schwartz, J.; Christiani, D.C. Night Heart Rate Variability and Particulate Exposures Among Boilermaker Construction Workers. Environ. Health Perspect. 2007, 115, 1046–1051. [Google Scholar] [CrossRef] [Green Version]
- Riediker, M. Cardiovascular Effects of Fine Particulate Matter Components in Highway Patrol Officers. Inhal. Toxicol. 2007, 19, 99–105. [Google Scholar] [CrossRef]
- Chuang, K.J.; Chan, C.C.; Su, T.C.; Lee, C.T.; Tang, C.S. The Effect of Urban Air Pollution on Inflammation, Oxidative Stress, Coagulation, and Autonomic Desfunction in Young Adults. Am. J. Respir. Crit. Care Med. 2007, 176, 370–376. [Google Scholar] [CrossRef]
- Chiang, T.A.; Wu, P.F.; Wang, L.F.; Lee, H.; Lee, C.H.; Ko, Y.C. Mutagenicity and Polycyclic Aromatic Hydrocarbon Content of Fumes from Heated Cooking Oils Produced in Taiwan. Mutat. Res. 1997, 381, 157–161. [Google Scholar] [CrossRef]
- Tung, Y.H.; Ko, J.L.; Liang, Y.F.; Yin, L.; Pu, Y.; Lin, P. Cooking Oil Fume-Induced Cytokin Expression and Oxidative Stress in Human Lung Epithelial Cells. Environ. Res. 2001, 87, 47–54. [Google Scholar] [CrossRef]
- Holvoet, P.; Collen, D. Oxidation of Low Density Lipoproteins in the Pathogenesis of Atherosclerosis. Atherosclerosis 1998, 137, S33–S38. [Google Scholar] [CrossRef]
- Borek, C. Antioxidant Health Effects of Aged Garlic Extract. J. Nutr. 2001, 131, 1010S–1015S. [Google Scholar] [CrossRef] [Green Version]
- Henrotin, Y.; Deby-Dupont, G.; Deby, C.; Franchimont, P.; Emerit, I. Active Oxygen Species, Articular Inflammation and Cartilage Damage. EXS 1992, 62, 308–322. [Google Scholar]
- Kumar, A.; Kaundal, R.K.; Iyer, S.; Sharma, S.S. Effects of Resveratrol on Nerve Functions, Oxidative Stress and DNA Fragmentation in Experimental Diabetic Neuropathy. Life Sci. 2007, 80, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H. Studies on Biomarkers in Cancer Etiology and Prevention: A Summary and Challenge of 20 Years of Interdisciplinary Research. Mutat. Res. 2000, 462, 255–279. [Google Scholar] [CrossRef]
- Guichardant, M.; Vallette-Talbi, L.; Cavadini, C.; Crozier, G.; Berger, G. Malondialdehyde Measurement in Urine. J. Chromatogr. 1994, 655, 112–116. [Google Scholar] [CrossRef]
- Kosugi, H.; Enomoto, H.; Ishizuka, Y.; Kikigawa, K. Variations in the Level of Thiobarbituric Acid Reactant in Health Humans under Different Physiological Conditions. Biol. Pharm. Bull. 1994, 17, 1645–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burczyniski, M.E.; Penning, T.M. Genotoxic Polycyclic Aromatic Hydrocarbon Ortho-Quinones Generated by Aldo-Keto Reductases Induce CYP1A1 via Nuclear Translocation of the Aryl Hydrocarbon Receptor. Cancer Res. 2000, 60, 908–915. [Google Scholar]
- Erhola, M.; Toyokuni, S.; Okada, K.; Tanaka, T.; Hiai, H.; Ochi, H.; Uchida, K.; Osawa, T.; Nieminen, M.M.; Alho, H.; et al. Biomarker Evidence of DNA Oxidation in Lung Cancer Patients: Association of Urinary 8-Hydroxy-2-Deoxyguanosine Excretion with Radiotherapy, Chemotherapy, and Response to Treatment. FEBS Lett. 1997, 409, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Toraason, M.; Hayden, C.; Marlow, D.; Rinehart, R.; Mathias, P.; Werren, D.; Olsen, L.D.; Neumeister, C.E.; Mathews, E.S.; Cheever, K.L.; et al. DNA Strand Breaks, Oxidative Damage, and 1-OH Pyrene in Roofers with Coal-Tar Pitch Dust and/or Asphalt Fume Exposure. Int. Arch. Occup. Environ. Health 2001, 74, 396–404. [Google Scholar] [CrossRef]
- Cherng, S.H.; Huang, K.H.; Yang, S.C.; Wu, T.C.; Yang, J.L.; Lee, H. Human 8-Oxoguanine DNA Glycosylase 1 mRNA Expression as an Oxidative Stress Exposure Biomarker of Cooking Oil Fumes. J. Toxicol. Environ. Health A 2002, 65, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Chuang, K.J.; Chan, C.C.; Chen, N.T.; Su, T.C.; Lin, L.Y. Effects of Particle Size Fractions on Reducing Heart Rate Variability in Cardiac and Hypertensive Patients. Environ. Health Perspect. 2005, 113, 1693–1697. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.H.; Chan, C.C.; Huang, Y.L.; Wu, K.Y. Urinary 1-Hydroxypyrene and Malondialdehyde in Male Workers in Chinese Restaurants. Occup. Environ. Med. 2008, 65, 732–735. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.W.; Wang, C.J.; Chang, L.W.; Chao, M.R. Clinical-Scale High-Throughput Analysis of Urinary 8-Oxo-7,8-Dihydro-2-Deoxyguanosine by Isotope-Dilution Liquid Chroma-Tography-Tandem Mass Spectrometry with On-Line Solid Phase Extraction. Clin. Chem. 2006, 52, 1381–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.L.; Chuang, I.C.; Pan, C.H.; Hsiech, C.; Shi, T.S.; Lin, T.H. Determination of Chromium in Whole Blood and Urine by Graphite Furnace AAS. At. Spectrosc. 2000, 21, 10–16. [Google Scholar]
- Liao, D.; Creason, J.; Shy, C.; Williams, R.; Waters, R.; Zweidinger, R. Daily Variation of Particulate Air Pollution and Door Cardiac Autonomic Control in the Elderly. Environ. Health Perspect. 1999, 107, 521–525. [Google Scholar] [CrossRef]
- Chan, C.C.; Chuang, K.J.; Shiao, G.M.; Lin, L.Y. Personal Exposure to Submicrometer Particles and Heart Rate Variability in Human Subjects. Environ. Health Perspect. 2004, 112, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Bigger, J.T., Jr.; Steinman, R.C.; Rolnitzky, L.M.; Kleiger, R.E.; Rottman, J.N. Frequency Domain Measures of Heart Period Variability and Mortality After Myocardial Infarction. Circulation 1992, 85, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T., Jr.; Moss, A.J. Decreased Heart Rate Variability and Its Association with Mortality After Myocardial Infarction. Am. J. Cardiol. 1987, 113, 256–262. [Google Scholar] [CrossRef]
- Schwartz, J.; Dockery, D.W.; Neas, L.M. Is Daily Mortality Associated Specifically with Fine Particles? J. Air Waste Manag. Assoc. 1996, 46, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Cavallari, J.M.; Stone, P.H.; Christiani, D.C. Obesity Is a Modifier of Autonomic Cardiac Responses to Fine Metal. Environ. Health Perspect. 2007, 115, 1002–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.B.; Lee, B.M. Oxidative Stress to DNA, Protein, and Antioxidant Enzymes (Superoxide Dismutase and Catalase) in Rats Treated with Ben-Zo(a)pyrene. Cancer Lett. 1997, 113, 205e12. [Google Scholar] [CrossRef]
- Pilger, A.; Rüdiger, H.W. 8-Hydroxy-2-Deoxyguanosine as a Marker of Oxidative DNA Damage Related to Occupational and Environmental Exposures. Int. Arch. Occup. Environ. Health 2006, 80, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.H.; Shih, T.S.; Chen, C.J.; Hsu, J.H.; Wang, S.C.; Huang, C.P.; Kuo, C.T.; Wu, K.Y.; Hu, H.; Chan, C.C. Reduction of Cooking Oil Fume Exposure Following an Engineering Intervention in Chinese Restaurants. Occup. Environ. Med. 2011, 68, 10–15. [Google Scholar] [CrossRef]
- Flowers-Geary, L.; Bleczinki, W.; Harvey, R.G.; Penning, T.M. Cytotoxicity and Mutagenicity of Polycyclic Aromatic Hydrocarbon O-Quinones Produced by Dihydrodiol Dehydrogenase. Chem. Biol. Interact. 1996, 99, 55–72. [Google Scholar] [CrossRef]
- Eckberg, D.L. Human Sinus Arrhythmia as an Index of Vagal Cardiac Outflow. J. Appl. Physiol. 1983, 54, 961–966. [Google Scholar] [CrossRef]
- Yoshida, R.; Ogawa, Y.; Mori, I.; Nakata, A.; Wang, R.; Ueno, S.; Shioji, I.; Hisanaga, N. Associations Between Oxidative Stress Levels and Total Duration of Engagement in Jobs with Exposure to Fly Ash Among Workers at Municipal Solid Waste Incinerators. Mutagenesis 2003, 18, 533–537. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Mean ± SD | Median | Range |
|---|---|---|---|
| Age (years) | 33.6 ± 10.5 | 34.5 | 15–56 |
| Body mass index (kg/m2) | 23.2 ± 3.7 | 22.1 | 17.6–32.9 |
| Years as a cook (years) | 13.4 ± 10.5 | 14.0 | 1–40 |
| Obese subjects † | 10 (20.8) * | ─ | ─ |
| Cigarette smokers | 26 (48.1%) * | ─ | ─ |
| Heart rate (5-min mean, beats/minute) | 91.3 ± 10.2 | 90.4 | 64–115 |
| PM10 (5-min mean, μg/m3) | 72.8 ± 134.7 | 45.6 | 1.9–2481.9 |
| PM2.5 (5-min mean, μg/m3) | 49.7 ± 56.2 | 35.9 | 1.5–1168.3 |
| PM1.0 (5-min mean, μg/m3) | 37.3 ± 36.2 | 28.4 | 1.2–532.3 |
| Pyrene (ng/m3) | 4.5 ± 8.9 | 3.8 | ND–58.7 |
| Benzo(k)fluroranhene (ng/m3) | 1.7 ± 3.0 | 1.4 | ND–25.3 |
| Benzo(a)pyrene (ng/m3) | 11.4 ± 20.1 | 6.9 | 0.1–154.4 |
| Benzo(ghi)perylene (ng/m3) | 8.8 ± 20.8 | 5.5 | ND–177.7 |
| Dibenzo(a,e)pyrene (ng/m3) | 9.3 ± 24.6 | 6.1 | ND–179.2 |
| Ambient temperature (5-min mean, °C) | 25.8 ± 3.9 | 26.4 | 16.0–36.9 |
| Outcome | Moving Average Period | PM10 | PM2.5 | PM1.0 |
|---|---|---|---|---|
| HR | ||||
| 15-min | 0.71 (0.42 to 1.01) * | 1.37 (0.91 to 1.82) * | 1.26 (0.72 to 1.80) * | |
| 30-min | 1.55 (0.80 to 1.51) * | 2.39 (1.79 to 3.01) * | 1.92 (1.26 to 2.59) * | |
| 1-h | 1.29 (0.91 to 1.67) * | 2.70 (0.53 to 4.91) * | 2.10 (1.41 to 2.81) * | |
| 2-h | 1.16 (0.41 to 1.92) * | 2.17 (1.12 to 3.23) * | 1.26 (0.17 to 2.36) * | |
| SDNN | ||||
| 15-min | −0.90 (−1.78 to −0.02) * | −2.19 (−3.52 to −0.85) * | −1.78 (−3.36 to −0.16) * | |
| 30-min | −1.41 (−2.46 to −0.35) * | −4.27 (−5.99 to −2.51) * | −3.20 (−5.10 to −1.27) * | |
| 1-h | −1.63 (−2.83 to −0.49) * | −4.51 (−6.53 to −2.45) * | −3.44 (−5.63 to −1.20) * | |
| 2-h | −0.14 (−2.85 to 2.63) | −3.85 (−7.41 to −0.15) * | −3.92 (−7.52 to −0.18) * | |
| r-MSSD | ||||
| 15-min | −0.80 (−1.42 to −0.18) * | −0.76 (−1.29 to −0.23) * | −0.72 (−1.18 to −0.26) * | |
| 30-min | −1.24 (−1.85 to −0.63) * | −2.33 (−3.60 to −1.05) * | −1.72 (−3.01 to −0.43) * | |
| 1-h | −1.37 (−2.21 to −0.53) * | −2.91 (−4.34 to −1.46) * | −1.89 (−3.45 to −0.31) * | |
| 2-h | −0.61 (−2.53 to 1.34) | −3.26 (−5.34 to −1.13) * | −3.45 (−6.04 to −0.79) * |
| Outcome | Moving Average Period | PM10 | PM2.5 | PM1.0 |
|---|---|---|---|---|
| LF | ||||
| 15-min | −0.27 (−1.63 to 1.12) | −0.71 (−0.98 to −0.44) * | −1.58 (−2.84 to −0.30) * | |
| 30-min | −0.45 (−2.27 to 1.41) | −2.66 (−4.86 to −0.41) * | −2.73 (−4.87 to −0.54) * | |
| 1-h | −0.56 (−2.48 to 1.40) | −3.69 (−6.92 to −0.34) * | −3.34 (−6.25 to −0.34) * | |
| 2-h | −1.45 (−5.64 to 2.94) | −6.51 (−11.94 to −0.74) * | −6.05 (−11.58 to −0.18) * | |
| HF | ||||
| 15-min | −0.49 (−1.56 to 0.60) | −1.31 (−2.30 to −0.31) * | −1.47 (−2.69 to −0.23) * | |
| 30-min | −0.62 (−2.21 to 1.00) | −3.00 (−5.35 to −0.58) * | −2.77 (−5.04 to −0.44) * | |
| 1-h | −0.81 (−2.63 to 1.05) | −4.28 (−7.34 to −1.17) * | −3.84 (−7.13 to −0.43) * | |
| 2-h | −1.20 (−5.27 to 3.03) | −6.86 (−12.09 to −1.33) * | −6.64 (−11.22 to −1.82) * |
| Log10 8-OHdG (μg/g Creatinine) | Log10 MDA (μg/g Creatinine) | |
|---|---|---|
| Predictors | Regression coefficient (95% Confidence interval) | Regression coefficient (95% Confidence interval) |
| Log PM10 | 2.842 (−2.672 to 8.356) | 0.539 (−5.581 to 6.658) |
| Log PM2.5 | 2.080 (0.869 to 3.291) b | 2.305 (−10.565 to 15.175) |
| Log PM1.0 | 2.574 (1.162 to 3.986) b | 1.114 (−6.249 to 8.477) |
| Log10 Pyrene | 0.168 (−0.107 to 0.442) | 0.138 (−0.167 to 0.443) |
| Log10 Benzo(k)fluoranthene | 1.480 (0.245 to 2.715) | 0.403 (−0.968 to 1.774) |
| Log10 Benzo(a)pyrene | 0.088 (0.055 to 0.122) b | 0.078 (0.044 to 0.113) b |
| Log10 Benzo(ghi)perylene | 0.077 (−0.816 to 0.970) | 0.057 (−0.934 to 1.048) |
| Log10 Dibenzo(a,e)pyrene | 0.089 (−0.025 to 0.203) | 0.111 (−0.016 to 0.237) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.-C.; Lin, L.-Y.; Lai, C.-H.; Chuang, K.-J.; Wu, M.-T.; Pan, C.-H. Association of Particulate Matter from Cooking Oil Fumes with Heart Rate Variability and Oxidative Stress. Antioxidants 2021, 10, 1323. https://doi.org/10.3390/antiox10081323
Chan C-C, Lin L-Y, Lai C-H, Chuang K-J, Wu M-T, Pan C-H. Association of Particulate Matter from Cooking Oil Fumes with Heart Rate Variability and Oxidative Stress. Antioxidants. 2021; 10(8):1323. https://doi.org/10.3390/antiox10081323
Chicago/Turabian StyleChan, Chang-Chuan, Lian-Yu Lin, Ching-Huang Lai, Kai-Jen Chuang, Ming-Tsang Wu, and Chih-Hong Pan. 2021. "Association of Particulate Matter from Cooking Oil Fumes with Heart Rate Variability and Oxidative Stress" Antioxidants 10, no. 8: 1323. https://doi.org/10.3390/antiox10081323
APA StyleChan, C.-C., Lin, L.-Y., Lai, C.-H., Chuang, K.-J., Wu, M.-T., & Pan, C.-H. (2021). Association of Particulate Matter from Cooking Oil Fumes with Heart Rate Variability and Oxidative Stress. Antioxidants, 10(8), 1323. https://doi.org/10.3390/antiox10081323







