Water-Soluble Melanoidin Pigment as a New Antioxidant Component of Fermented Willowherb Leaves (Epilobium angustifolium)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Epilobium Angustifolium Leaves Fermentation
2.3. Antioxidant Activity Assays
2.4. Chemical Composition Analysis
2.5. High-Performance Liquid Chromatography with Photodiode Array Detection and Electrospray Ionization Triple Quadrupole Mass Spectrometric Detection (HPLC-PDA-ESI-tQ-MS)
2.6. Metabolite Quantification
2.7. Melanoidin Isolation
2.8. Melanoidin Analysis
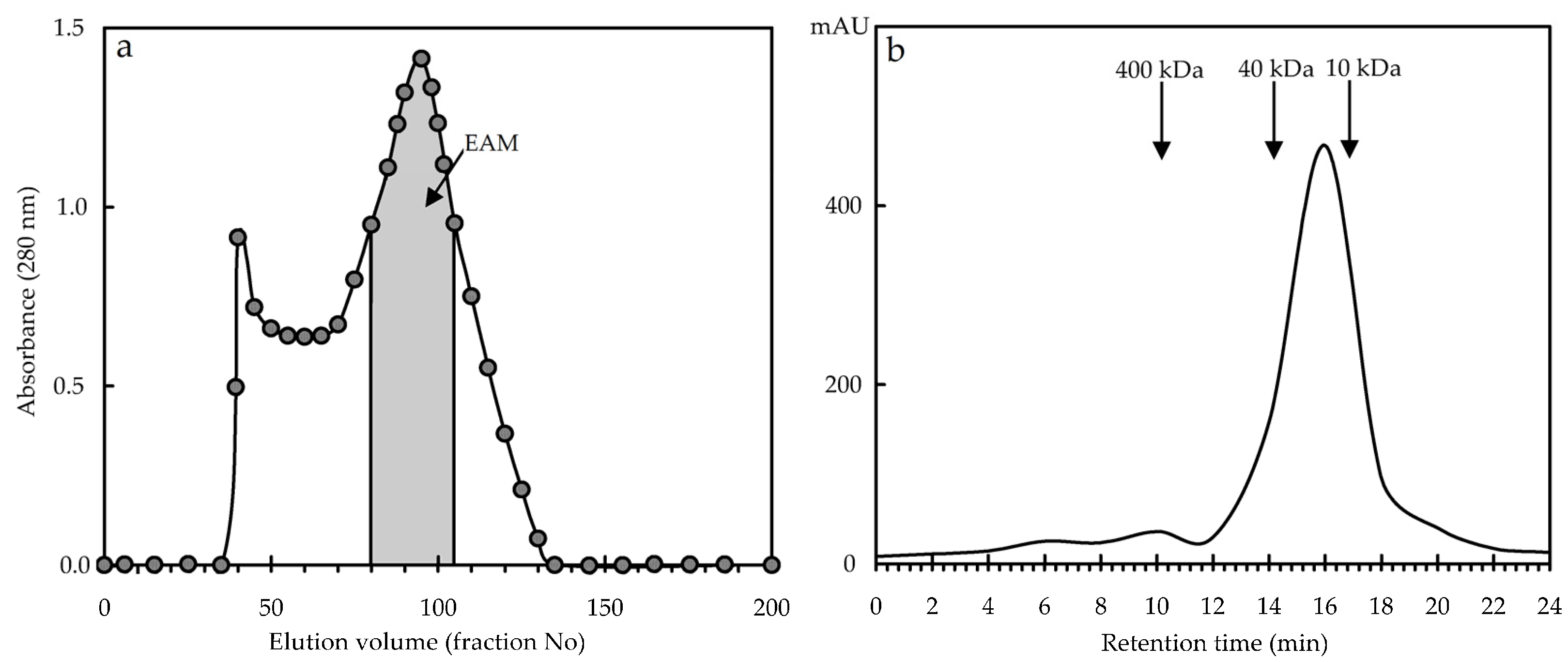
2.8.1. High-Performance Gel Permeation Chromatography with Photodiode Array Detection
2.8.2. Functional Group Quantification
2.8.3. Elemental Composition
2.8.4. Spectroscopy in Ultraviolet and Visible Region (UV-Vis)
2.8.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.8.6. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.8.7. Alkaline Destruction
2.8.8. HPLC-PDA-ESI-tQ-MS Analysis of Alkaline Destruction Products
2.9. Plant Decoction Preparation
2.10. Simulated Gastrointestinal and Colonic Digestion
2.11. Coulometric Analysis of Trolox Equivalent Content
2.12. Statistical Analysis
3. Results and Discussion
3.1. Fermentation of Epilobium angustifolium: Antioxidant Potency and Chemical Profile of Non-Fermented and Fermented Leaves
3.2. Melanoidin from Fermented E. angustifolium Leaves: Physico-Chemical and Spectral Characteristics
3.2.1. Isolation of E. angustifolium Melanoidin and General Characteristics
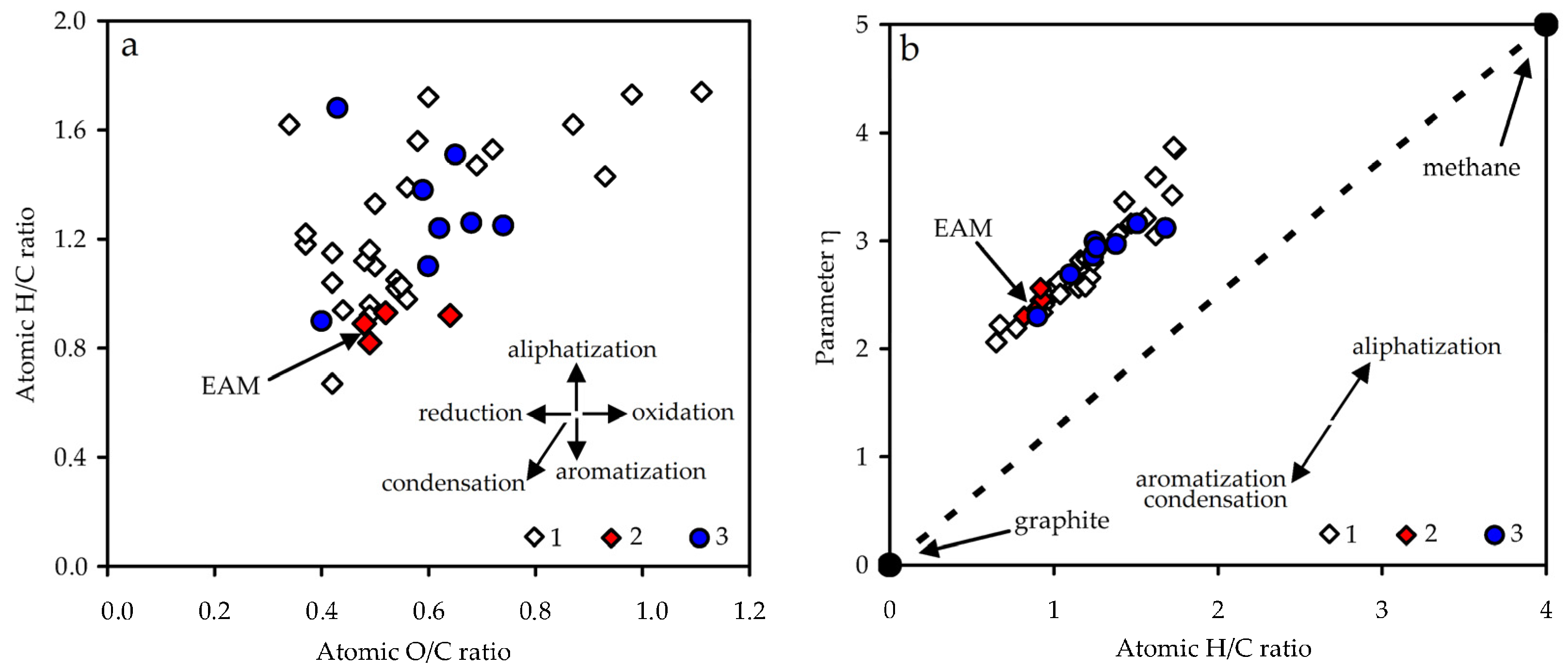
3.2.2. Elemental Composition
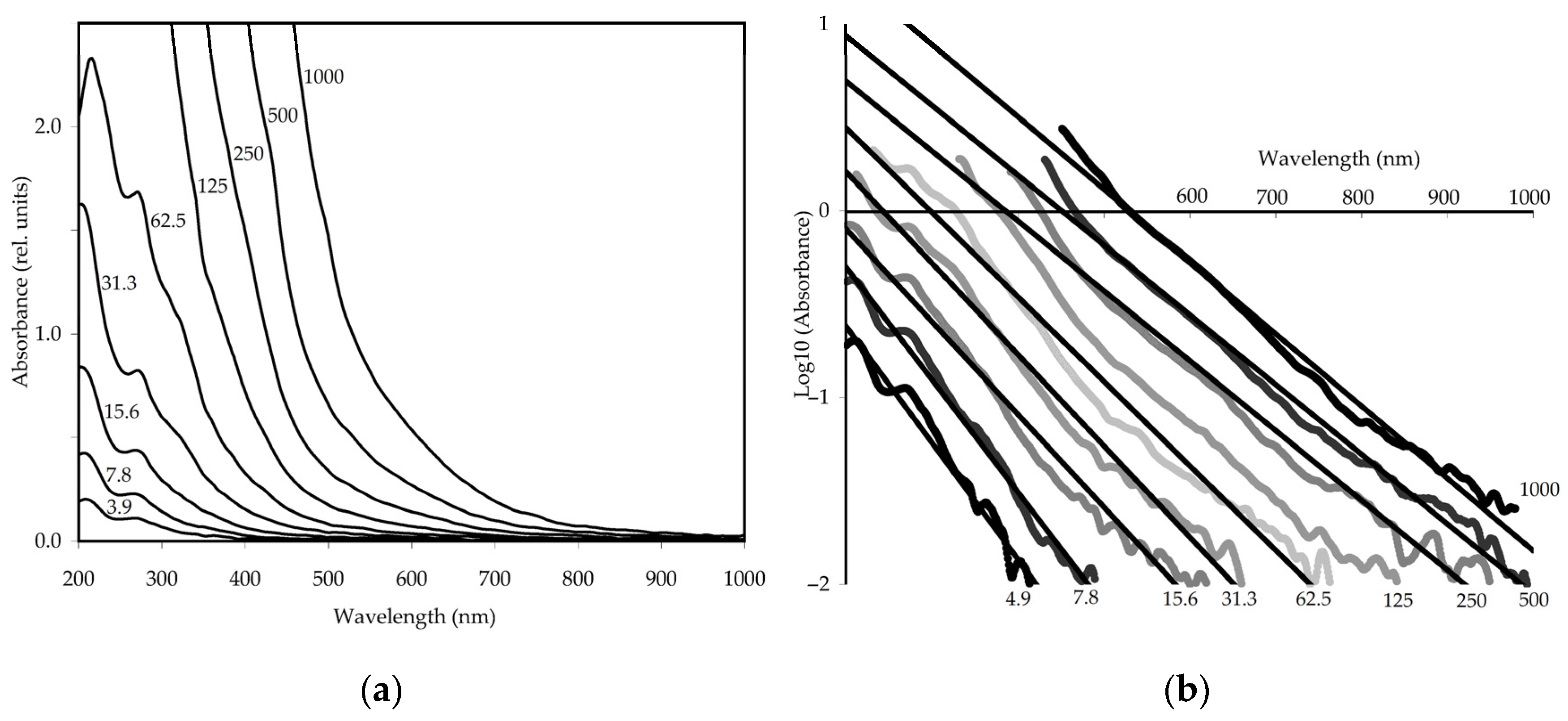
3.2.3. UV-Vis Spectroscopy
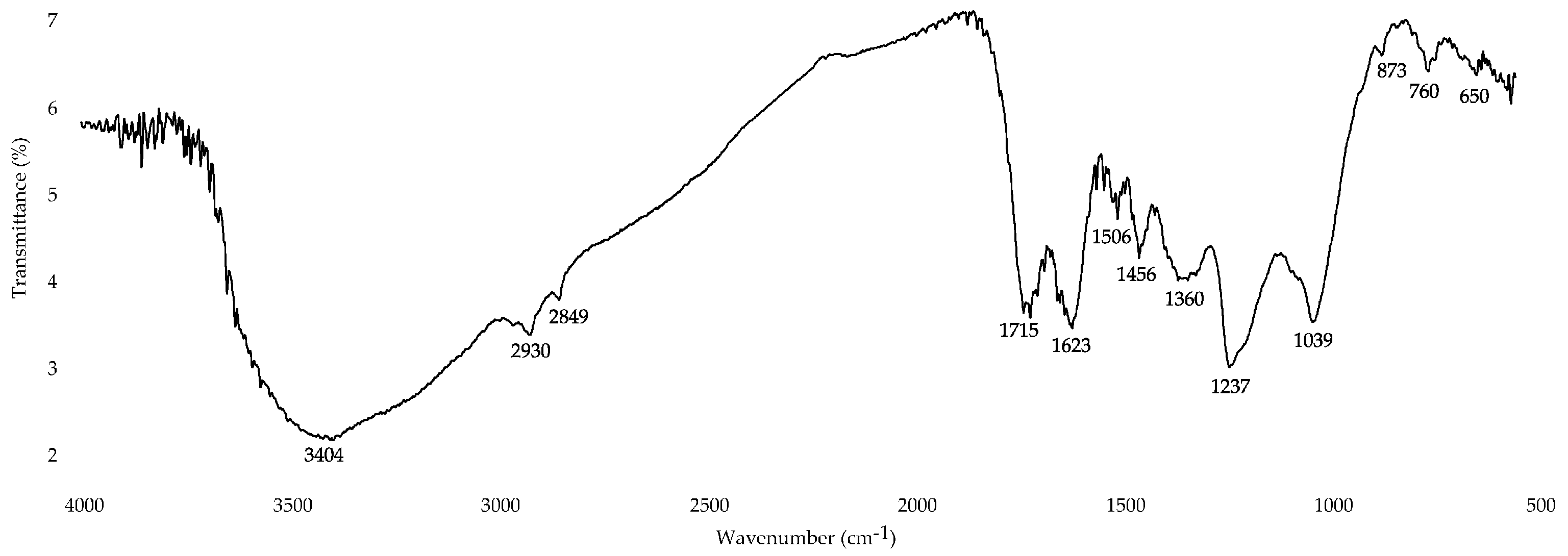
3.2.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
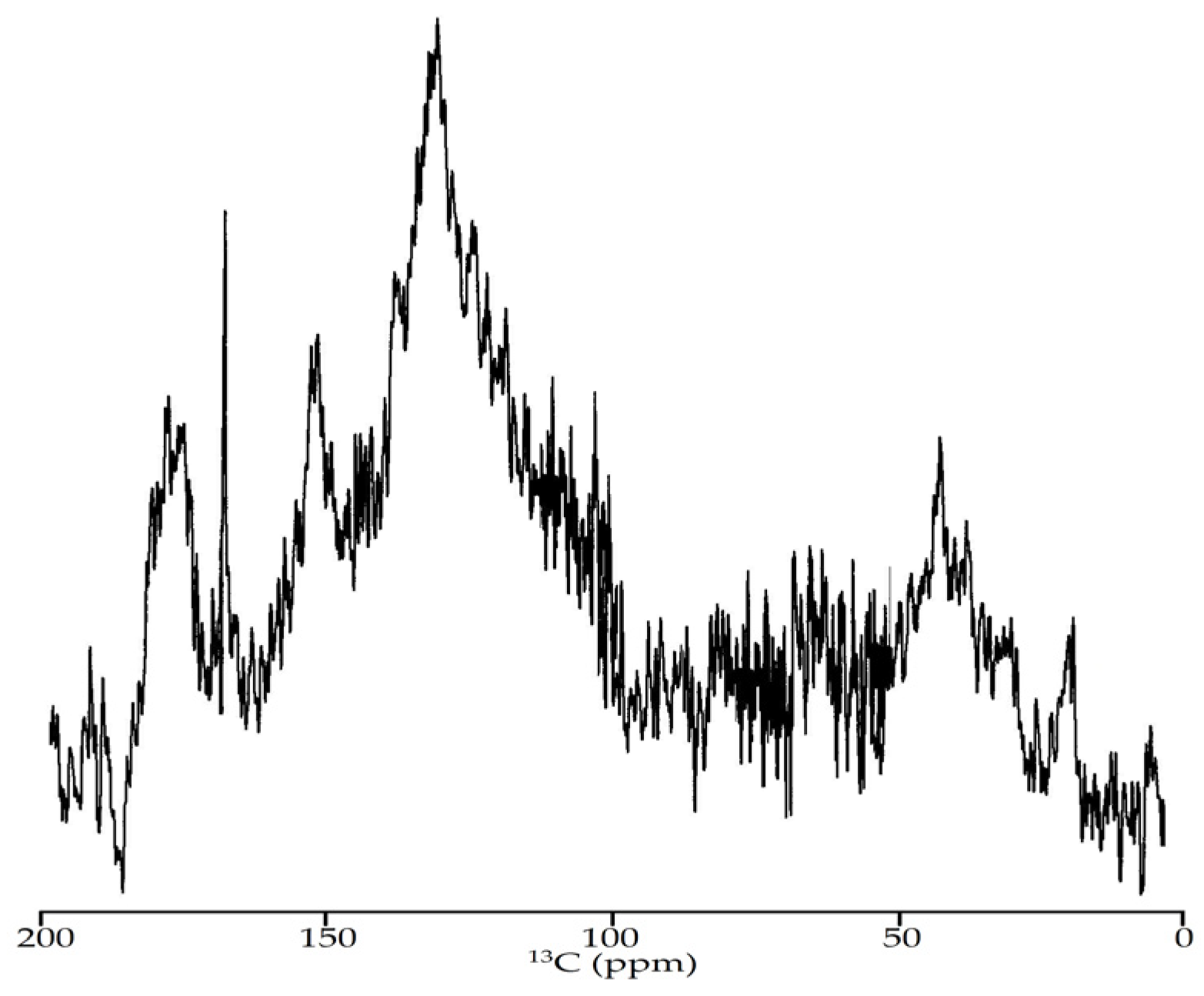
3.2.5. NMR Spectroscopy
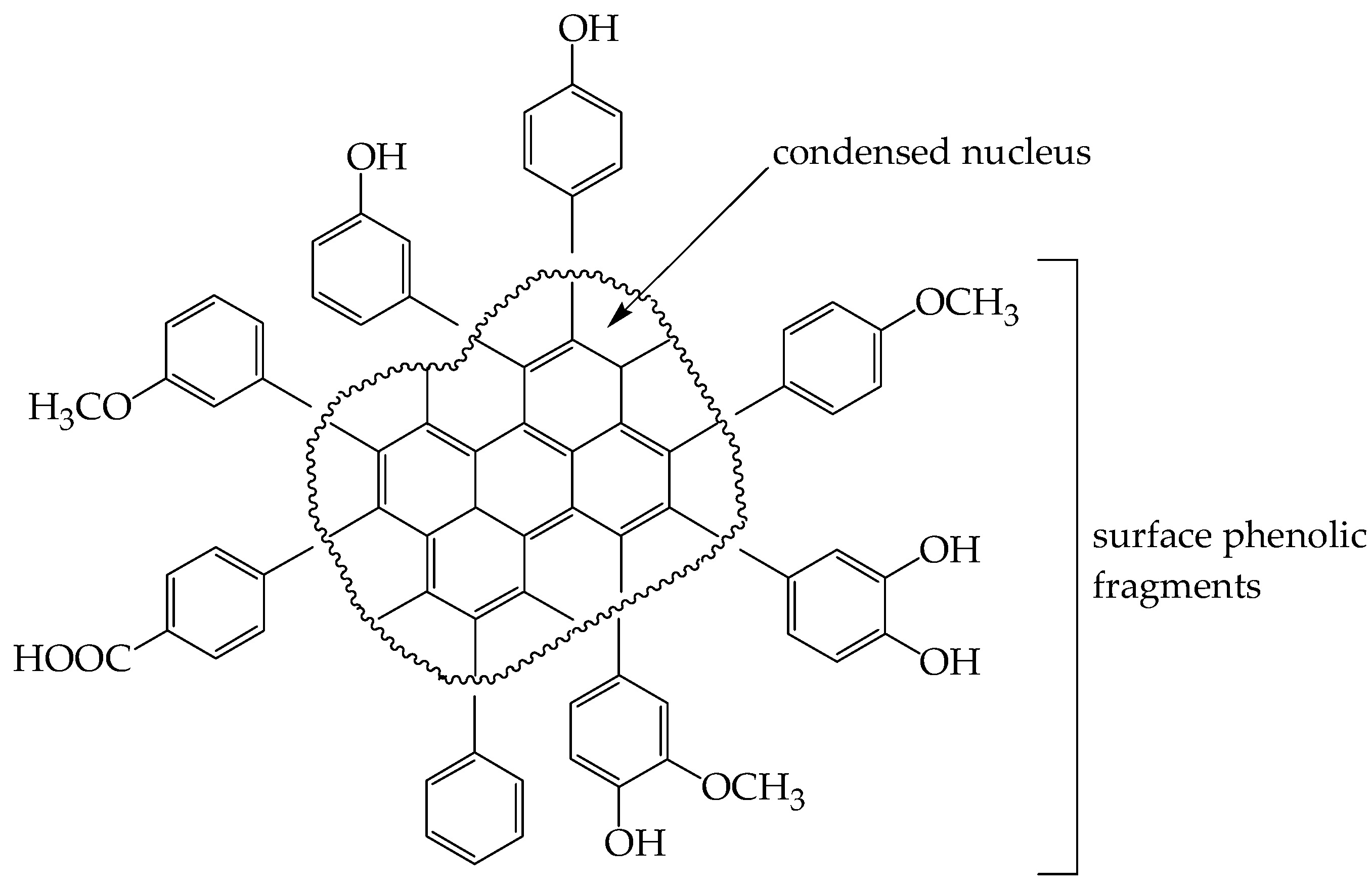
3.2.6. Alkaline Destruction
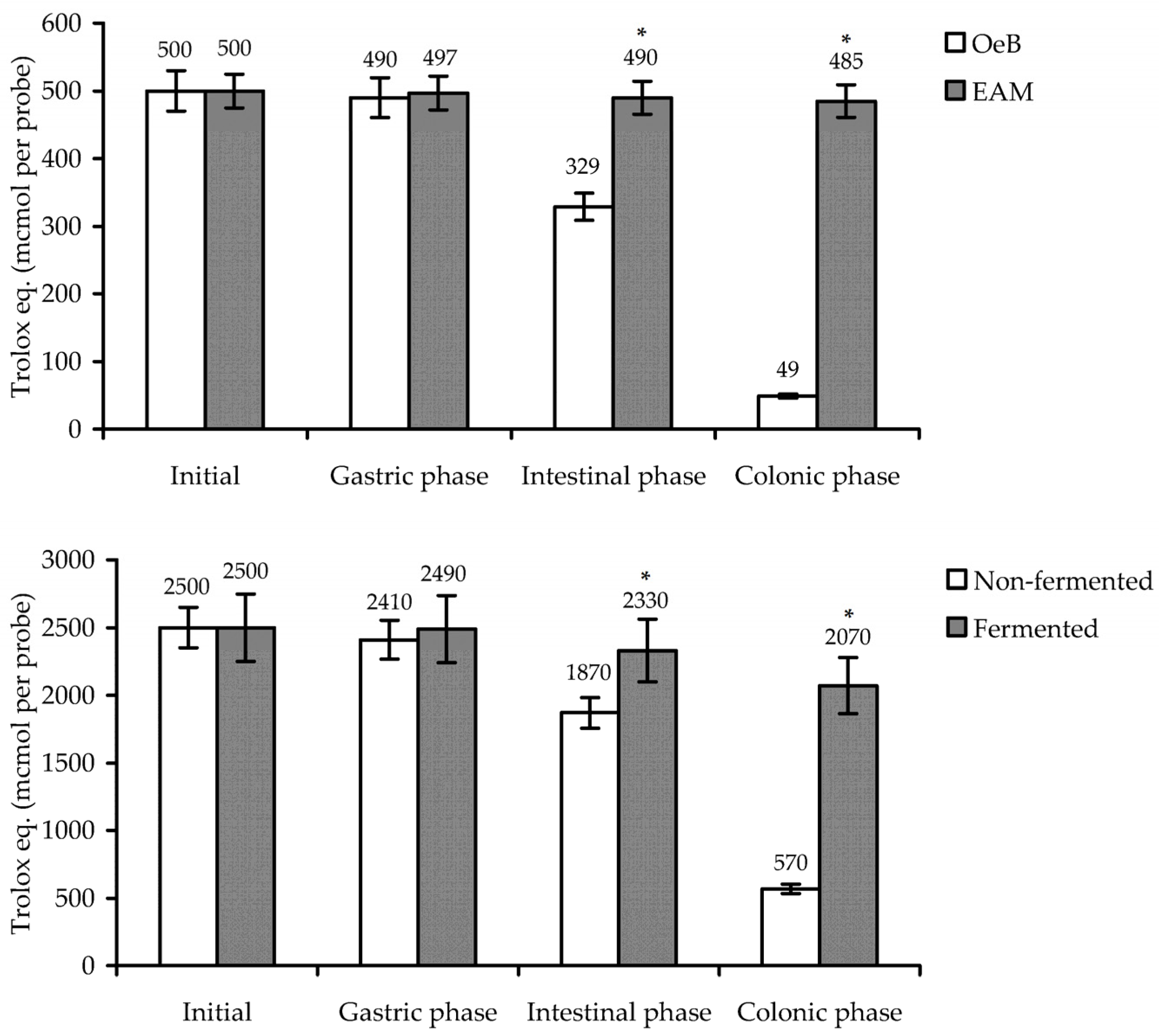
3.3. Antioxidant Potential of E. angustifolium Melanoidin: In Vitro Activity and Gastrointestinal/Colonic Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mota de Carvalho, N.; Costa, E.M.; Silva, S.; Pimentel, L.; Fernandes, T.H.; Pintado, M.E. Fermented foods and beverages in human diet and their influence on gut microbiota and health. Fermentation 2018, 4, 90. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef] [PubMed]
- Amoah, I.; Taarji, N.; Johnson, P.-N.T.; Barrett, J.; Cairncross, C.; Rush, E. Plant-based food by-products: Prospects for valorisation in functional bread development. Sustainability 2020, 12, 7785. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Cornea-Cipcigan, M.; Topal, E.; Kösoğlu, M. Impact of fermentation processes on the bioactive profile and health-promoting properties of bee bread, mead and honey vinegar. Processes 2020, 8, 1081. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Sharika, R.; Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C.; Tencomnao, T.; Prasansuklab, A. Role of herbal teas in regulating cellular homeostasis and autophagy and their implications in regulating overall health. Nutrients 2021, 13, 2162. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.L.; Kumar, L. Potential impact of the current and future climate on the yield, quality, and climate suitability for tea [Camellia sinensis (L.) O. Kuntze]: A systematic review. Agronomy 2021, 11, 619. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Sava, V.M.; Yang, S.-M.; Hong, M.-Y.; Yang, P.-C.; Huang, G.S. Isolation and characterization of melanic pigments derived from tea and tea polyphenols. Food Chem. 2001, 73, 177–184. [Google Scholar] [CrossRef]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological properties of melanin and its function in health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M. Physicochemical characteristics and antioxidant activity of melanoidin pigment from the fermented leaves of Orthosiphon stamineus. Rev. Bras. Farmacogn. 2012, 22, 284–290. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Kulaitiene, J.; Najman, K.; Hallmann, E. Studies of the variability of polyphenols and carotenoids in different methods fermented organic leaves of willowherb (Chamerion angustifolium (L.) Holub). Appl. Sci. 2020, 10, 5254. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Hallmann, E.; Najman, K. Effect of different durations of solid-phase fermentation for fireweed (Chamerion angustifolium (L.) Holub) leaves on the content of polyphenols and antioxidant activity in vitro. Molecules 2020, 25, 1011. [Google Scholar] [CrossRef] [PubMed]
- Jariene, E.; Lasinskas, M.; Danilcenko, H.; Vaitkeviciene, N.; Slepetiene, A.; Najman, K.; Hallmann, E. Polyphenols, antioxidant activity and volatile compounds in fermented leaves of medicinal plant rosebay willowherb (Chamerion angustifolium (L.) Holub). Plants 2020, 9, 1683. [Google Scholar] [CrossRef] [PubMed]
- Nadson, G.A. About Substitution of Chinese Tea with Other Plants; Ginsburg’s Publishing House: Peterhof, Russia, 1918; pp. 5–6. [Google Scholar]
- Anonymous. About Counterfeiting and Mixing Teas and Deceptions in the Tea Trade; University Publishing House: Moscow, Russia, 1860; pp. 9–10. [Google Scholar]
- Tserevitinov, F.V. Tea Substituents; Pischepromizdat: Moscow, Russia, 1942; pp. 15–16. [Google Scholar]
- Adamczak, A.; Dreger, M.; Seidler-Łożykowska, K.; Wielgus, K. Fireweed (Epilobium angustifolium L.): Botany, phytochemistry and traditional uses. A review. Herba Pol. 2019, 65, 51–63. [Google Scholar] [CrossRef]
- Kadam, P.; Patil, M.; Yadav, K. A review on phytopharmacopial potential of Epilobium angustifolium. Pharmacogn. J. 2018, 10, 1076–1078. [Google Scholar] [CrossRef]
- Vitalone, A.; Allkanjari, O. Epilobium spp.: Pharmacology and phytochemistry. Phytother. Res. 2018, 32, 1229–1240. [Google Scholar] [CrossRef]
- Granica, S.; Piwowarski, J.P.; Czerwińska, M.E.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Vasil’eva, A.G.; Gadimli, A.I.; Isaev, J.I.; Vennos, C. Caffeoylquinic acids and flavonoids of fringed sagewort (Artemisia frigida Willd.): HPLC-DAD-ESI-QQQ-MS profile, HPLC-DAD quantification, in vitro digestion stability, and antioxidant capacity. Antioxidants 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Okhlopkova, Z.M.; Zulfugarov, I.S. Chemical composition and antioxidant activity of Tánara Ótó (Dracocephalum palmatum Stephan), a medicinal plant used by the North-Yakutian nomads. Molecules 2013, 18, 14105–14121. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Gornostai, T.G.; Selyutina, I.Y.; Zilfikarov, I.N. Effect of low temperature cultivation on the phytochemical profile and bioactivity of Arctic plants: A case of Dracocephalum palmatum. Int. J. Mol. Sci. 2017, 18, 2579. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kidawara, M.; Iseki, M.; Umegaki, C.; Kishi, T. A simple fluorometric determination of vitamin C. Chem. Pharm. Bull. 1998, 46, 1474–1476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olennikov, D.N.; Chirikova, N.K.; Vasilieva, A.G.; Fedorov, I.A. LC-MS profile, gastrointestinal and gut microbiota stability and antioxidant activity of Rhodiola rosea herb metabolites: A comparative study with subterranean organs. Antioxidants 2020, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Vasilieva, A.G.; Chirikova, N.K. Fragaria viridis fruit metabolites: Variation of LC-MS profile and antioxidant potential during ripening and storage. Pharmaceuticals 2020, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, D.; Jusha, M.; Saroha, K.; Singif, N.; Vashishta, B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm. 2008, 58, 215–220. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules 2014, 19, 18296–18316. [Google Scholar] [CrossRef]
- Chandel, H.S.; Pathak, A.K.; Tailang, M. Standardization of some herbal antidiabetic drugs in polyherbal formulation. Pharmacogn. Res. 2011, 3, 49–56. [Google Scholar] [CrossRef]
- Iverson, S.J.; Lang, S.L.C.; Cooper, M.H. Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 2001, 36, 1283–1287. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Samuelsen, A.B. Quantitative analysis of polysaccharides from Plantago major leaves using the Dreywood method. Chem. Nat. Comp. 2006, 42, 265–268. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M. Quantitative determination of phenolic compounds in Mentha piperita leaves. Chem. Nat. Comp. 2010, 46, 22–27. [Google Scholar] [CrossRef]
- Chirikova, N.K.; Olennikov, D.N.; Tankhaeva, L.M. Quantitative determination of flavonoid content in the aerial part of Baical scullcap (Scutellaria baicalensis Georgi). Russ. J. Bioorg. Chem. 2010, 36, 915–922. [Google Scholar] [CrossRef]
- Rajković, M.B.; Novaković, I.D.; Petrović, A. Determination of titratable acidity in white wine. J. Agricult. Sci. 2007, 52, 169–184. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Nikolaev, V.M.; Kim, S.-W.; Vennos, C. Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the Siberian species and their inhibitory potential against α-amylase and α-glucosidase. Front. Pharmacol. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, O.V.; Kulikova, N.A.; Alekseeva, T.N.; Stepanova, E.V.; Davidchuk, V.N.; Belyaeva, E.Y.; Tsvetkova, E.A. A comparative characterization of fungal melanin and the humin-like substances synthesized by Cerrena maxima 0275. Appl. Biochem. Microbiol. 2007, 43, 61–67. [Google Scholar] [CrossRef]
- Falkehag, S.I.; Marton, J.; Adler, E. Lignin structure and reactions. In Advances in Chemistry; Marton, J., Ed.; American Chemical Society: Washington, DC, USA, 1966; Volume 59, pp. 79–89. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. In vitro bioaccessibility, human gut microbiota metabolites and hepatoprotective potential of chebulic ellagitannins: A case of Padma Hepaten® formulation. Nutrients 2015, 7, 8735–8749. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, C.; Liu, S.; Yang, L. The galloyl catechins contributing to main antioxidant capacity of tea made from Camellia sinensis in China. Sci. World J. 2014, 2014, 863984. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Agafonova, S.V. Antioxidant components of Laetiporus sulphureus (Bull.: Fr.) Murr. fruit bodies. Appl. Biochem. Microbiol. 2011, 47, 419–425. [Google Scholar] [CrossRef]
- Stolarczyk, M.; Naruszewicz, M.; Kiss, A.K. Extracts from Epilobium sp. herbs induce apoptosis in human hormone-dependent prostate cancer cells by activating the mitochondrial pathway. J. Pharm. Pharmacol. 2013, 65, 1044–1054. [Google Scholar] [CrossRef]
- Duckstein, S.M.; Lotter, E.M.; Meyer, U.; Lindequist, U.; Stintzing, F.C. Phenolic constituents from Alchemilla vulgaris L. and Alchemilla mollis (Buser) Rothm. at different dates of harvest. Z. Naturforsch. C 2012, 67, 529−540. [Google Scholar] [CrossRef]
- Baert, N.; Kim, J.; Karonen, M.; Salminen, J.-P. Inter-population and inter-organ distribution of the main polyphenolic compounds of Epilobium angustifolium. Phytochemistry 2017, 134, 54–63. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Olennikov, D.N. Phenolome of Asian agrimony tea (Agrimonia asiatica Juz., Rosaceae): LC-MS profile, α-glucosidase inhibitory potential and stability. Foods 2020, 9, 1348. [Google Scholar] [CrossRef]
- Baert, N.; Karonen, M.; Salminen, J.-P. Isolation, characterisation and quantification of the main oligomeric macrocyclic ellagitannins in Epilobium angustifolium by ultra-high performance chromatography with diode array detection and electrospray tandem mass spectrometry. J. Chromatogr. A 2015, 1419, 26–36. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Vennos, C. New ellagic acid glycosides from Punica granatum. Chem. Nat. Comp. 2019, 55, 878–882. [Google Scholar] [CrossRef]
- Olennikov, D.N. Acylglycosides and acylquinic acids from roots of Panax ginseng. Chem. Nat. Comp. 2020, 56, 1044–1047. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Nikolaev, V.M.; Chirikova, N.K. Sagan Dalya tea, a new “old” probable adaptogenic drug: Metabolic characterization and bioactivity potentials of Rhododendron adamsii leaves. Antioxidants 2021, 10, 863. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Gadimli, A.I.; Isaev, J.I.; Kashchenko, N.I.; Prokopyev, A.S.; Katayeva, T.N.; Chirikova, N.K.; Vennos, C. Caucasian Gentiana species: Untargeted LC-MS metabolic profiling, antioxidant and digestive enzyme inhibiting activity of six plants. Metabolites 2019, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Wubshet, S.G.; Moresco, H.H.; Tahtah, Y.; Brighente, I.M.C.; Staerk, D. High-resolution bioactivity profiling combined with HPLC-HRMS-SPE-NMR: α-Glucosidase inhibitors and acetylated ellagic acid rhamnosides from Myrcia palustris DC. (Myrtaceae). Phytochemistry 2015, 116, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I. Spireasalicin, a new acylated glycoside of quercetin from Spiraea salicicfolia. Chem. Nat. Comp. 2017, 53, 1038–1044. [Google Scholar] [CrossRef]
- Kiss, A.; Kowalski, J.; Melzig, M.F. Compounds from Epilobium angustifolium inhibit the specific metallopeptidases ACE, NEP and APN. Planta Med. 2004, 70, 919–923. [Google Scholar] [CrossRef]
- Seshadri, T.R.; Vasishta, K. Polyphenols of the leaves of Psidium guava–quercetin, guaijaverin, leucocyanidin and amritoside. Phytochemistry 1965, 4, 989–992. [Google Scholar] [CrossRef]
- Olennikov, D.N. Synanthropic plants as an underestimated source of bioactive phytochemicals: A case of Galeopsis bifida (Lamiaceae). Plants 2020, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K. New flavonol glycosides from Rhodiola quadrifida. Chem. Nat. Comp. 2020, 56, 1048–1054. [Google Scholar] [CrossRef]
- Chirikova, N.K.; Olennikov, D.N. Phenolic compounds from Siberian species Thymus baicalensis and T. sibiricus. Chem. Nat. Comp. 2018, 54, 572–576. [Google Scholar] [CrossRef]
- Sójka, M.; Janowski, M.; Grzelak-Błaszczyk, K. Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur. Food Res. Technol. 2019, 245, 1113–1122. [Google Scholar] [CrossRef]
- Macierzyński, J.; Sójka, M.; Kosmala, M.; Karlińska, E. Transformation of oligomeric ellagitannins, typical for Rubus and Fragaria genus, during strong acid hydrolysis. J. Agric. Food Chem. 2020, 68, 8212–8222. [Google Scholar] [CrossRef]
- Aguilera-Carbo, A.; Augur, C.; Prado-Barragan, L.A. Microbial production of ellagic acid and biodegradation of ellagitannins. Appl. Microbiol. Biotechnol. 2008, 78, 189–199. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Rokhin, A.V.; Agafonova, S.V. Physicochemical properties and antioxidant activity of melanin fractions from Inonotus obliquus sclerotia. Chem. Nat. Comp. 2012, 48, 396–403. [Google Scholar] [CrossRef]
- Metner, J.M.; Townsel, M.E.; Moore, C.L.; Williamson, G.D.; Soteres, B.J.; Fisher, M.S.; Willis, I. Melanin accelerates the tyrosinase-catalyzed oxygenation of p-hydroxyanisole (MMEH). Pigm. Cell Res. 1990, 3, 90–97. [Google Scholar] [CrossRef]
- Wang, H.; Pan, Y.; Tang, X.; Huang, Z. Isolation and characterization of melanin from Osmanthus fragrans’ seeds. LWT 2006, 39, 496–502. [Google Scholar] [CrossRef]
- Yao, Z.; Qi, J.; Wang, L. Isolation, fractionation and characterization of melanin-like pigments from chestnut (Castanea mollissima) shells. J. Food Sci. 2012, 77, C671–C676. [Google Scholar] [CrossRef] [PubMed]
- Pugh, N.D.; Balachandran, P.; Lata, H.; Dayan, F.E.; Joshi, V.; Bedir, E.; Makino, T.; Moraes, R.; Khan, I.; Pasco, D.S. Melanin: Dietary mucosal immune modulator from Echinacea and other botanical supplements. Int. Immunopharmacol. 2005, 5, 637–647. [Google Scholar] [CrossRef] [PubMed]
- El-Obeid, A.; Al-Harbi, S.; Al-Jomah, N.; Hassib, A. Herbal melanin modulates tumor necrosis factor alpha (TNF-a), interleukin 6 (IL-6) and vascular endothelial growth factor (VEGF) production. Phytomedicine 2006, 13, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Gil-Avilés, M.D.R.; Montes-Avila, J.; Díaz-Camacho, S.P.; Osuna-Martínez, L.U.; Delgado-Vargas, F. Soluble melanins of the Randia echinocarpa fruit—Structural characteristics and toxicity. J. Food Biochem. 2019, 43, e13077. [Google Scholar] [CrossRef]
- Li, J.; Jia, X.; Zhang, R.; Liu, L.; Chi, J.; Huang, F.; Dong, L.; Zhang, M. Isolation, structural characterization and antioxidant activity of black sesame melanin. Sci. Agricult. Sin. 2020, 53, 2477–2492. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Tang, B. Physicochemical properties and biological activities of melanin extracted from sunflower testae. Food Sci. Technol. Res. 2018, 24, 1029–1038. [Google Scholar] [CrossRef]
- Burnham, A.K. Van Krevelen diagrams. In Encyclopedia of Petroleum Geoscience. Encyclopedia of Earth Sciences Series; Sorkhabi, R., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Gyul’maliev, A.M.; Golovin, G.S.; Gagarin, S.G. Classification of fossil fuels according to structural-chemical characteristics. Solid Fuel Chem. 2007, 41, 257–266. [Google Scholar] [CrossRef]
- Ungnade, H.E.; Lamb, R.W. The absorption spectra of benzoic acid and esters. J. Am. Chem. Soc. 1952, 74, 3789–3794. [Google Scholar] [CrossRef]
- Dearden, J.C.; Forbes, W.F. Light absorption studies. XIV. Ultraviolet absorption spectra of phenols. Can. J. Chem. 1959, 37, 1294–1304. [Google Scholar] [CrossRef]
- Karimova, N.V.; Luo, M.; Grassian, V.; Gerber, R.B. Absorption spectra of benzoic acid in water at different pH and in the presence of salts: Insights from the integration of experimental data and theoretical cluster models. Phys. Chem. Chem. Phys. 2020, 22, 5046–5056. [Google Scholar] [CrossRef]
- Price, W.C.; Walsh, A.D. The absorption spectra of benzene derivatives in the vacuum utra-violet. Proc. R. Soc. A 1947, 191, 22–31. [Google Scholar] [CrossRef]
- Savel’eva, A.V.; Preis, Y.I. Indicator significance of the extinction coefficients of the alkaline extracts of weakly decomposed peats for reconstructing the hydrothermal conditions of Western Siberian bogs. Solid Fuel Chem. 2019, 53, 214–220. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. Evaluation of humic acid photocatalytic degradation by UV–vis and fluorescence spectroscopy. Catal. Today 2005, 101, 267–274. [Google Scholar] [CrossRef]
- Zhang, M.W.; Sun, L.; Chi, J.W.; Lai, L.Z.; Wang, Z.J. Comparative study on natural pigments in black rice, black soy bean and black sesame. J. Chin. Cereals Oils Ass. 1998, 13, 6–9. [Google Scholar]
- Bilińska, B. Progress in infrared investigations of melanin structures. Spectrochim. Acta A 1996, 52, 1157–1162. [Google Scholar] [CrossRef]
- Lüdemann, H.-D.; Lentz, H.; Martin, J.P. Carbon-13 nuclear magnetic resonance spectra of some fungal melanins and humic acids. Soil Sci. Soc. Am. J. 1982, 46, 957–962. [Google Scholar] [CrossRef]
- Duff, G.A.; Roberts, J.E.; Foster, N. Analysis of the structure of synthetic and natural melanins by solid-phase NMR. Biochemistry 1988, 27, 7112–7116. [Google Scholar] [CrossRef] [PubMed]
- Knicker, H.; Almendros, G.; González-Vila, F.J.; Lüdemann, H.-D.; Martin, F. 13C and 15N NMR analysis of some fungal melanins in comparison with soil organic matter. Org. Geochem. 1995, 23, 1023–1028. [Google Scholar] [CrossRef]
- d’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigm. Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin pigment in plants: Current knowledge and future perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef]
- Mason, H.S.; Ingram, D.J.E.; Allen, B. The free radical property of melanins. Arch. Biochem. Biophys. 1960, 86, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.; Bredeston, L.; Malanga, G.; Mordoh, J. Role of melanin as a scavenger of active oxygen species. Pigm. Cell Res. 1993, 6, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Gan, E.V.; Haberman, H.F.; Menon, I.A. Oxidation of NADH by melanin and melanoproteins. Biochim. Biophys. Acta 1974, 370, 62–69. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.G.; Szpoganicz, B.; Caramori, G.F.; de Almeida, V.R.; Mangrich, A.S.; Mangoni, A.P. Spectroscopy and theoretical studies of natural melanin (eumelanin) and its complexation by iron (III). J. Coord. Chem. 2014, 67, 986–1001. [Google Scholar] [CrossRef]
- Sarna, T.; Duleba, A.; Korytowski, W.; Swartz, H. Interaction of melanin with oxygen. Arch. Biochem. Biophys. 1980, 200, 140–148. [Google Scholar] [CrossRef]
- Dunford, H.B. Free radicals in iron containing systems. Free Radic. Biol. Med. 1987, 3, 405–421. [Google Scholar] [CrossRef]
- Schwabe, K.; Lassmann, G.; Damereau, W.; Naundorf, H. Protection of melanoma cells against superoxide radicals by melanins. J. Cancer Res. Clin. Oncol. 1989, 115, 597–600. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Lipińska, L.; Klewicka, E.; Sójka, M. The structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Eidenberger, T.; Napolitano, A.; d’Ischia, M. Black sesame pigment: DPPH assay-guided purification, antioxidant/antinitrosating properties, and identification of a degradative structural marker. J. Agric. Food Chem. 2012, 60, 8895–8901. [Google Scholar] [CrossRef]
- Varga, M.; Berkesi, O.; Darula, Z.; May, N.V.; Palagyi, A. Structural characterization of allomelanin from black oat. Phytochemistry 2016, 130, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Arnoldi, A. Food-derived antioxidants and COVID-19. J. Food Biochem. 2021, 45, e13557. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Y.; Kani, Y.A.; Iliya, S.; Muhammad, J.B.; Binji, A.; El-Fulaty Ahmad, A.; Kabir, M.B.; Bindawa, K.U.; Ahmed, A. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria. SAGE Open Med. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Klouda, C.B.; Stone, W.L. Oxidative stress, proton fluxes, and chloroquine/hydroxychloroquine treatment for COVID-19. Antioxidants 2020, 9, 894. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef]
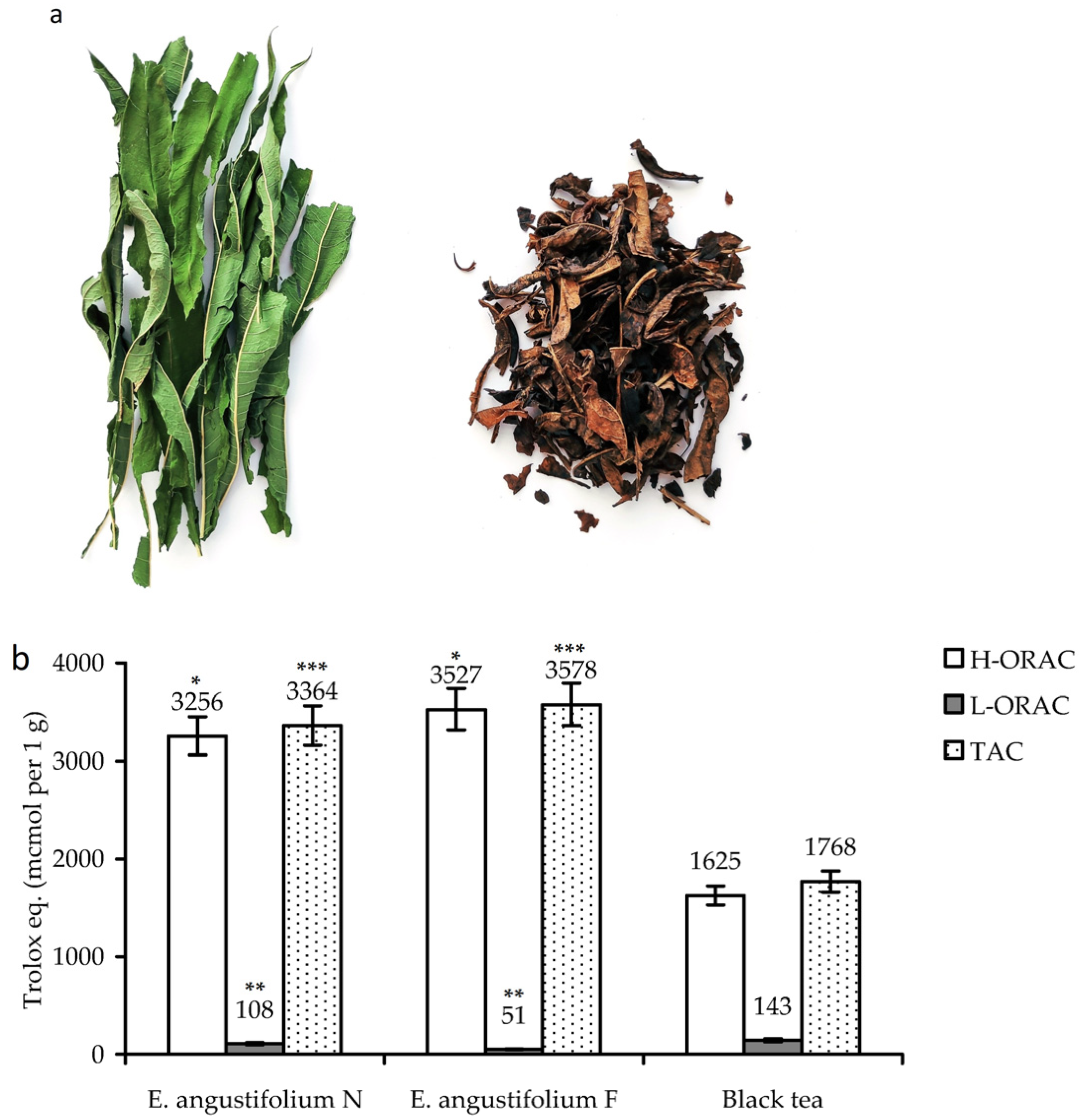
| Compound Group | E. angustifolium before Fermentation | E. angustifolium after Fermentation |
|---|---|---|
| Extractives (solvent water), % a | 28.42 ± 0.84 | 38.53 ± 1.15 |
| Extractives (solvent methanol), % a | 41.20 ± 1.38 | 49.63 ± 1.47 |
| Lipids, mg/g b | 43.70 ± 1.29 | 41.61 ± 1.21 |
| Carotenoids, mg/g b | 0.41 ± 0.01 | 0.39 ± 0.01 |
| Chlorophylls, mg/g b | 8.65 ± 0.17 | trace |
| Proteins, mg/g b | 22.14 ± 0.45 | 14.63 ± 0.28 |
| Free amino acids, mg/g b | 20.10 ± 0.40 | 37.82 ± 0.74 |
| Water-soluble polysaccharides, mg/g b | 28.93 ± 0.84 | 19.11 ± 0.57 |
| Mono-/disaccharides, mg/g b | 35.18 ± 1.05 | 56.63 ± 1.12 |
| Titratable acidity, mg/g b | 18.59 ± 0.37 | 25.61 ± 0.51 |
| Total phenolics, mg/g b | 277.15 ± 6.73 | 252.11 ± 6.04 |
| Flavonoids, mg/g b | 52.73 ± 1.21 | 50.61 ± 1.19 |
| Tannins, mg/g b | 183.11 ± 3.66 | 12.52 ± 0.32 |
| Melanoidin, mg/g b | not detected | 145.31 ± 4.35 |
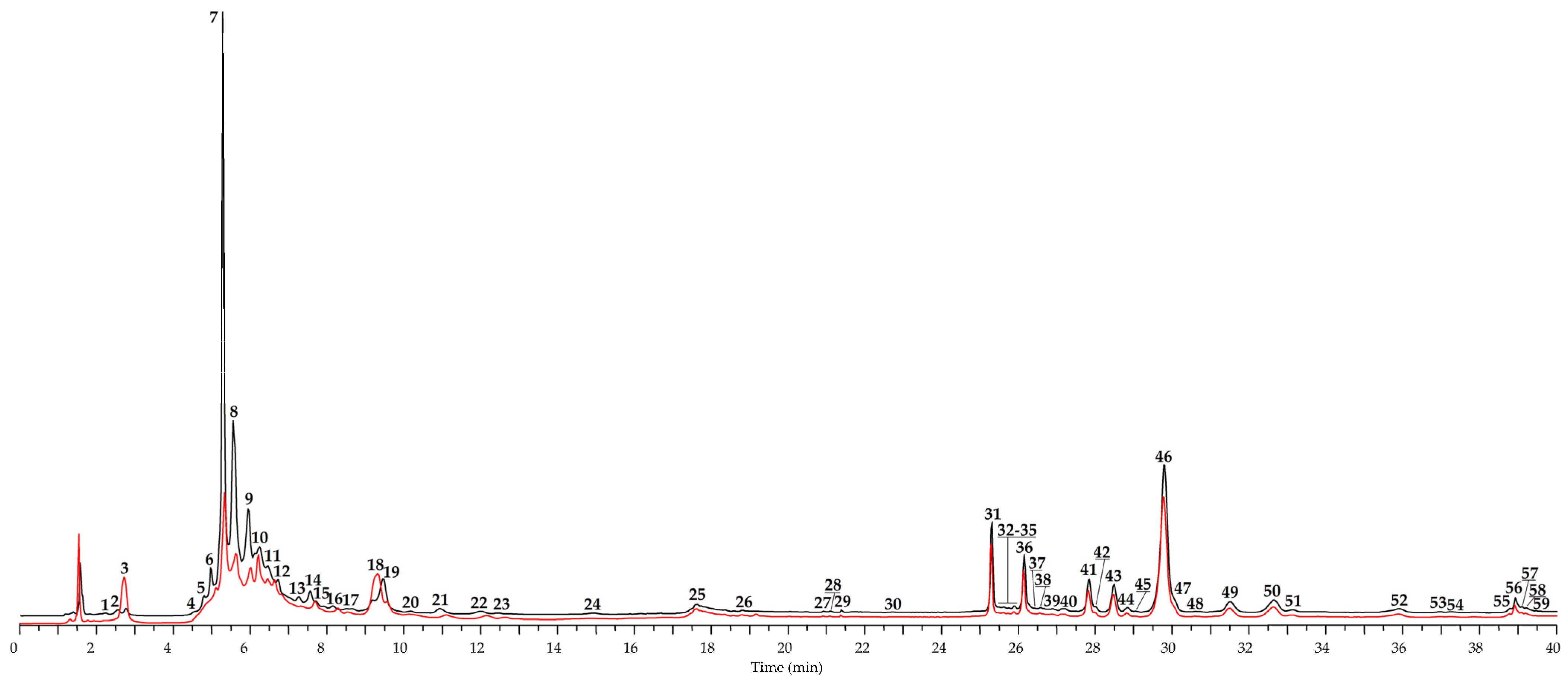
| No | tR, min | Compound a | Previous Data b | [M − H]−, m/z c | MS/MS, m/z | Content in Leaves, mg/g of Dry Plant Weight ± S.D. | |
|---|---|---|---|---|---|---|---|
| before Fermentation | after Fermentation | ||||||
| 1 | 2.27 | 1-O-Galloyl-βDGlcp S (glucogallin) [27] | 331 | 169 | 0.83 ± 0.02 | traces | |
| 2 | 2.53 | Gallic acid O-Hex L [27] | 331 | 169 | 0.96 ± 0.02 | traces | |
| 3 | 3.28 | Gallic acid S [42] | [43] | 169 | 2.37 ± 0.04 | 15.27 ± 0.30 | |
| 4 | 4.51 | Gall-HHDP-Hex2 L [43,44,45] | 795 | 633, 481, 463, 301 | 0.57 ± 0.01 | traces | |
| 5 | 4.77 | Gall-HHDP-Hex2 L [43,44,45] | 795 | 633, 481, 463, 301 | 0.73 ± 0.02 | traces | |
| 6 | 5.01 | Gall-HHDP-Hex L [43,44,45] | [43] | 633 | 481, 463, 301 | 1.67 ± 0.03 | 0.22 ± 0.00 |
| 7 | 5.32 | Oenothein B S [43,46] | [43] | 1567, 783 * | 765, 301 | 112.62 ± 2.36 | 6.53 ± 0.12 |
| 8 | 5.57 | Oenothein A L [43,46] | [45] | 1175 * | 765, 301 | 29.63 ± 0.56 | 2.77 ± 0.05 |
| 9 | 5.91 | Tetrameric tellimagrandin I L [45,47] | [45] | 1044 ** | 765, 301, 275 | 10.56 ± 0.20 | 0.93 ± 0.02 |
| 10 | 6.24 | Pentameric tellimagrandin I L [45,47] | [45] | 1035 ** | 765, 301, 275 | 6.73 ± 0.11 | 4.22 ± 0.09 |
| 11 | 6.49 | Hexameric tellimagrandin I L [45,47] | [45] | 1567 ** | 765, 301, 275 | 3.25 ± 0.06 | 1.20 ± 0.02 |
| 12 | 6.68 | Heptameric tellimagrandin I L [45,47] | [45] | 1828 ** | 765, 301, 275 | 1.04 ± 0.02 | 0.83 ± 0.02 |
| 13 | 7.37 | Ellagic acid O-Hex3 L [48] | 787 | 625, 463, 301 | 0.43 ± 0.01 | traces | |
| 14 | 7.51 | 1-O-Ellagoyl-gentiobiose S (amritoside) [48] | 625 | 463, 301 | 1.12 ± 0.02 | 0.37 ± 0.01 | |
| 15 | 7.93 | Di-ellagoyl O-Hex3 L [48] | 1071 | 909, 787, 625, 463, 301 | 0.20 ± 0.00 | traces | |
| 16 | 8.17 | 1,6′-Di-O-ellagoyl-gentiobiose S (granatoside A) [48] | 909 | 463, 341, 301 | 0.43 ± 0.01 | traces | |
| 17 | 8.53 | 1-O-Ellagoyl-βDGlcp S [48] | 463 | 301 | 0.18 ± 0.00 | traces | |
| 18 | 9.36 | Ellagic acid S [43] | [43] | 301 | 2.83 ± 0.05 | 10.91 ± 0.20 | |
| 19 | 9.56 | 1,6′-Di-O-ellagoyl-βDGlcp S (granatoside B) [48] | 747 | 463, 301 | 2.07 ± 0.05 | 0.31 ± 0.00 | |
| 20 | 10.21 | Ellagic acid O-Hex3-O-Ac L [28,44] | 829 | 787, 625, 463, 301 | traces | traces | |
| 21 | 10.47 | Ellagic acid2 O-Hex3-O-Ac L [28,44] | 1113 | 1071, 909, 787, 625, 463, 301 | 0.37 ± 0.01 | traces | |
| 22 | 12.01 | Ellagic acid O-methyl ester O-Hex L [28,44] | 477 | 315, 301 | traces | traces | |
| 23 | 12.52 | Ellagic acid di-O-methyl ester O-Hex L [28,44] | 491 | 329, 301 | traces | traces | |
| 24 | 14.90 | Ellagic acid2 O-methyl ester O-Hex L [28,44] | 761 | 477, 315, 301 | traces | traces | |
| 25 | 17.54 | Ellagic acid2 di-O-methyl ester O-Hex L [28,44] | 775 | 477, 315, 301 | 0.29 ± 0.00 | 0.14 ± 0.00 | |
| 26 | 18.95 | Ellagic acid tri-O-methyl ester O-Hex L [28,44] | 505 | 343, 301 | traces | traces | |
| 27 | 20.99 | 1-O-Sinapoyl-βDGlcp S [49] | 385 | 223 | traces | traces | |
| 28 | 21.14 | 6-O-Sinapoyl-βDGlcp S [49] | 385 | 223 | traces | traces | |
| 29 | 21.47 | 1-O-Caffeoyl-βDGlcp S [49] | 341 | 179, 135 | traces | traces | |
| 30 | 22.72 | 1-O-Caffeoylquinic acid S [50] | 353 | 191, 179, 173, 135 | traces | traces | |
| 31 | 25.27 | 4-O-Caffeoylquinic acid S [50] | 353 | 191, 179, 173, 135 | 8.68 ± 0.16 | 8.21 ± 0.16 | |
| 32 | 25.48 | Caffeic acid2 O-Hex L [51] | 503 | 341, 179, 135 | traces | traces | |
| 33 | 25.53 | Myricetin O-HexA-O-Hex-O-Gall2 L [52] | 959 | 807, 655, 493, 317 | traces | traces | |
| 34 | 25.72 | Quercetin O-HexA-O-Hex-O-Gall2 L [52] | 943 | 791, 639, 477, 301 | traces | traces | |
| 35 | 25.93 | Quercetin O-HexA-O-Hex2-O-Gall L [52] | 953 | 801, 639, 477, 301 | traces | traces | |
| 36 | 26.14 | 5-O-Caffeoylquinic acid S [50] | [43] | 353 | 191, 179, 165 | 5.27 ± 0.11 | 4.63 ± 0.10 |
| 37 | 26.45 | Myricetin O-HexA-O-Hex-O-Gall L [52] | 807 | 655, 493, 317 | traces | traces | |
| 38 | 26.58 | Quercetin O-HexA-O-Hex-O-Gall L [52] | 791 | 639, 477, 301 | traces | traces | |
| 39 | 26.87 | Myricetin O-Hex-O-Gall L [52] | [43] | 631 | 479, 317 | traces | traces |
| 40 | 27.21 | Quercetin O-Hex-O-Gall2 L [52] | 767 | 615, 463, 301 | traces | traces | |
| 41 | 27.80 | Myricetin O-HexA L [45] | [45] | 493 | 317 | 5.69 ± 0.11 | 5.43 ± 0.11 |
| 42 | 27.98 | Myricetin-3-O-βDGalp S [43] | [43] | 479 | 317 | 0.36 ± 0.00 | 0.22 ± 0.00 |
| 43 | 28.49 | Quercetin-3-O-(6″-O-Gall)-βDGalp S [43] | [43] | 615 | 463, 301 | 4.56 ± 0.09 | 4.02 ± 0.08 |
| 44 | 28.81 | Myricetin-3-O-αLRhap S (myricitrin) [43] | [43] | 463 | 317 | 0.46 ± 0.01 | 0.31 ± 0.00 |
| 45 | 29.11 | Quercetin-O-Rut S (rutin) [46] | 609 | 463, 301 | traces | traces | |
| 46 | 29.79 | Quercetin-3-O-βDGlcAp S (miquelianin) [43] | [43] | 477 | 301 | 31.65 ± 0.65 | 30.81 ± 0.62 |
| 47 | 30.02 | Quercetin-3-O-βDGalp S (hyperoside) [43] | [43] | 463 | 301 | 0.93 ± 0.02 | 0.86 ± 0.02 |
| 48 | 30.54 | Kaempferol O-Hex-O-Gall L [43] | [43] | 599 | 447, 285 | traces | traces |
| 49 | 31.51 | Quercetin-3-O-αLAraf S (avicularin) [43] | [43] | 433 | 301 | 1.98 ± 0.04 | 1.53 ± 0.03 |
| 50 | 32.63 | Kaempferol-3-O-βDGlcAp S [43] | [43] | 461 | 285 | 2.35 ± 0.05 | 2.21 ± 0.04 |
| 51 | 33.12 | Quercetin-3-O-αLRhap S (quercitrin) [43] | [43] | 447 | 301 | 0.53 ± 0.01 | 0.50 ± 0.01 |
| 52 | 35.87 | Kaempferol-3-O-αLRhap S (afzelin) [43] | [43] | 431 | 285 | 0.39 ± 0.00 | 0.26 ± 0.00 |
| 53 | 36.95 | Ellagic acid 3-O-methyl ester S [28,46] | 315 | 301 | traces | traces | |
| 54 | 37.32 | Ellagic acid 3,3′-di-O-methyl ether S [28,46] | 329 | 301 | traces | traces | |
| 55 | 38.73 | Myricetin O-Hex-O-pCou L [53] | 625 | 479, 317 | traces | traces | |
| 56 | 38.98 | Quercetin-3-O-(6″-O-pCou)-βDGlcp S (helichrysoside) [53] | 609 | 463, 301 | 1.14 ± 0.02 | 1.01 ± 0.02 | |
| 57 | 39.08 | Kaempferol-3-O-(6″-O-pCou)-βDGlcp S (tiliroside) [53] | [54] | 593 | 447, 285 | traces | traces |
| 58 | 39.22 | Quercetin O-Hex-O-pCou-O-Ac [53] | 651 | 609, 463, 301 | traces | Traces | |
| 59 | 39.48 | Kaempferol O-Hex-O-pCou-O-Ac [53] | 635 | 593, 447, 285 | traces | traces | |
| Total content: | |||||||
| gallic acid/gallic acid glycosides | 4.16 | 15.27 | |||||
| ellagitannins | 166.80 | 16.70 | |||||
| ellagic acid/ellagic acid glycosides | 7.92 | 11.73 | |||||
| hydroxycinnamates | 14.13 | 12.84 | |||||
| myricetin glycosides | 6.51 | 5.96 | |||||
| quercetin glycosides | 40.79 | 38.73 | |||||
| kaempferol glycosides | 2.79 | 2.47 | |||||
| flavonol glycosides | 50.09 | 47.16 | |||||
| phenolics | 243.10 | 103.70 | |||||
| Plant Source [Ref.] | Element Content, % | Atomic Ratio | Parameter η | Structural Parameter δ | ||||
|---|---|---|---|---|---|---|---|---|
| Carbon | Hydrogen | Oxygen | Nitrogen | Hydrogen/Carbon | Oxygen/Carbon | |||
| E. angustifolium (EAM) | 58.3 | 4.3 | 37.1 | 0.2 | 0.89 | 0.48 | 2.36 | 5.4 |
| Camellia sinensis [10] | 54.2 | 4.2 | 38.3 | 3.2 | 0.93 | 0.52 | 2.46 | 4.8 |
| 49.8 | 3.8 | 42.5 | 3.8 | 0.92 | 0.64 | 2.56 | 4.5 | |
| Orthosiphon stamineus [12] | 57.1 | 3.9 | 36.9 | 2.0 | 0.82 | 0.49 | 2.30 | 5.6 |
| Castanea mollissima [65] | 47.0 | 4.9 | 46.5 | 1.5 | 1.25 | 0.74 | 2.99 | 2.9 |
| 52.5 | 4.8 | 41.8 | 0.9 | 1.10 | 0.60 | 2.69 | 4.0 | |
| 51.4 | 5.3 | 42.5 | 0.8 | 1.24 | 0.62 | 2.86 | 3.3 | |
| Echinacea purpurea [66] | 50.0 | 7.0 | 29.1 | 13.0 | 1.68 | 0.43 | 3.12 | 1.3 |
| Nigella sativa [67] | 50.3 | 5.8 | 39.4 | 4.4 | 1.38 | 0.59 | 2.97 | 2.6 |
| Randia echinocarpa [68] | 48.3 | 6.1 | 41.8 | 3.7 | 1.51 | 0.65 | 3.16 | 2.0 |
| Sesamum indicum [69] | 47.6 | 5.0 | 43.4 | 3.7 | 1.26 | 0.68 | 2.94 | 3.1 |
| Helianthus anuus [70] | 61.1 | 4.6 | 32.5 | 1.7 | 0.90 | 0.40 | 2.30 | 5.6 |
| Melanoidin Source [Ref.] | NMR 13C Spectrum Region, ppm | ||||||
|---|---|---|---|---|---|---|---|
| 220–160 | 160–140 | 140–110 | 110–90 | 90–60 | 60–45 | 45–0 | |
| E. angustifolium (EAM) | 16 | 17 | 35 | 8 | 5 | 5 | 14 |
| Castanea mollissima [65] | 10–13 | 6–21 | 14–35 | 39–68 | 8–13 | ||
| Inonotus obliquus [62] | 27 | 14 | 43 | 3 | 1 | 1 | 11 |
| Hendersonula toruloidea [83] | 15 | 8 | 16 | 9 | 18 | 11 | 20 |
| Trichoderma harzianum [83] | 15 | 3 | 9 | 5 | 17 | 12 | 39 |
| Ulocladium atrium [83] | 7 | 2 | 5 | 11 | 43 | 7 | 25 |
| Compound | t, min | UV Spectrum, λmax, nm | ESI-MS Data, m/z | Relative Content, % |
|---|---|---|---|---|
| 3,5-Dihydroxybenzoic acid | 4.92 | 203, 249, 307 | 153 | traces |
| 3,4-Dihydroxybenzoic acid | 5.22 | 203, 259, 293 | 153 | 82 |
| 2,6-Dihydroxybenzoic acid | 6.34 | 203, 307 | 153 | traces |
| 4-Hydroxybenzoic acid | 6.70 | 201, 255 | 137 | 6 |
| 3-Methoxy-4-hydroxybenzoic acid | 7.57 | 202, 260, 288 | 167, 153 | 7 |
| 3-Hydroxybenzoic acid | 7.93 | 202, 297 | 137 | 1 |
| 4-Methoxybenzoic acid | 10.22 | 205, 268 | 151, 137 | 3 |
| 3,4-Dimethoxybenzoic acid | 10.56 | 204, 258, 289 | 181, 167, 153 | traces |
| Benzoic acid | 11.42 | 225, 271 | 121 | traces |
| 3-Methoxybenzoic acid | 12.81 | 200, 291 | 151, 137 | traces |
| Assay a | EAM | Oenothein B | Trolox |
|---|---|---|---|
| DPPH• b | 6.14 ± 0.14 i | 10.53 ± 0.21 iii | 8.50 ± 0.15 ii |
| ABTS•+ b | 5.29 ± 0.11 v | 8.61 ± 0.15 vi | 3.46 ± 0.07 iv |
| DMPD•+ b | 14.86 ± 0.30 vii | 25.33 ± 0.52 viii | 50.89 ± 0.99 ix |
| O2•− b | 12.69 ± 0.24 x | 37.18 ± 0.75 xi | 89.12 ± 1.62 xii |
| •OH b | 15.26 ± 0.28 xiv | 28.63 ± 0.53 xv | 10.37 ± 0.18 xiii |
| Cl• c | 1293.73 ± 19.39 xvii | 1108.67 ± 22.10 xvii | 1000 xvi |
| NO d | 27.20 ± 0.52 xviii | 35.11 ± 0.71 xix | 893.14 ± 26.79 xx |
| FeCA e | 396.15 ± 9.91 xxiii | 293.15 ± 7.11 xxii | 40.27 ± 0.85 xxi |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olennikov, D.N.; Kirillina, C.S.; Chirikova, N.K. Water-Soluble Melanoidin Pigment as a New Antioxidant Component of Fermented Willowherb Leaves (Epilobium angustifolium). Antioxidants 2021, 10, 1300. https://doi.org/10.3390/antiox10081300
Olennikov DN, Kirillina CS, Chirikova NK. Water-Soluble Melanoidin Pigment as a New Antioxidant Component of Fermented Willowherb Leaves (Epilobium angustifolium). Antioxidants. 2021; 10(8):1300. https://doi.org/10.3390/antiox10081300
Chicago/Turabian StyleOlennikov, Daniil N., Christina S. Kirillina, and Nadezhda K. Chirikova. 2021. "Water-Soluble Melanoidin Pigment as a New Antioxidant Component of Fermented Willowherb Leaves (Epilobium angustifolium)" Antioxidants 10, no. 8: 1300. https://doi.org/10.3390/antiox10081300
APA StyleOlennikov, D. N., Kirillina, C. S., & Chirikova, N. K. (2021). Water-Soluble Melanoidin Pigment as a New Antioxidant Component of Fermented Willowherb Leaves (Epilobium angustifolium). Antioxidants, 10(8), 1300. https://doi.org/10.3390/antiox10081300







