Effect of Thermal Processing on Color, Phenolic Compounds, and Antioxidant Activity of Faba Bean (Vicia faba L.) Leaves and Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Processing
2.2.1. Dry Heat Treatment
2.2.2. Wet Heat Treatment (Steaming)
2.3. Measurement of Color Variations
2.4. Sample Extraction
2.4.1. For L-dopa, Total Phenolics, Total Flavonoids and Antioxidant Activity Measurements
2.4.2. For Vitamin C Content Determination
2.5. HPLC Analysis of L-dopa Content
2.6. HPLC Analysis of Vc Content
2.7. Measurement of Total Phenolics Content (TPC) and Total Flavonoids Content (TFC)
2.8. Measurement of Antioxidant Activity
2.9. Statistical Analysis
3. Results and Discussion
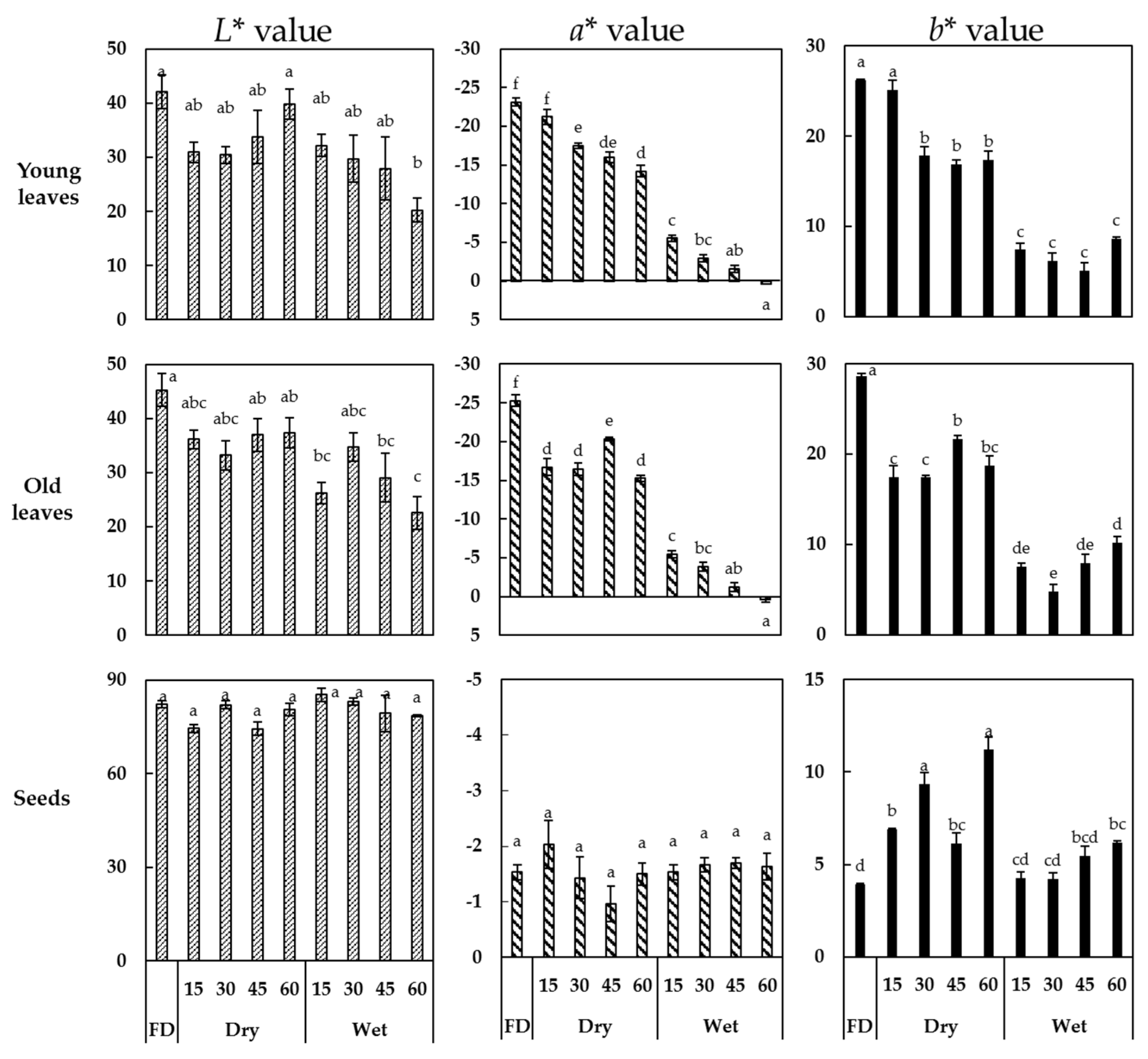
3.1. Color Parameters of Young and Old Faba Leaves, and Faba Seeds after Thermal Treatment
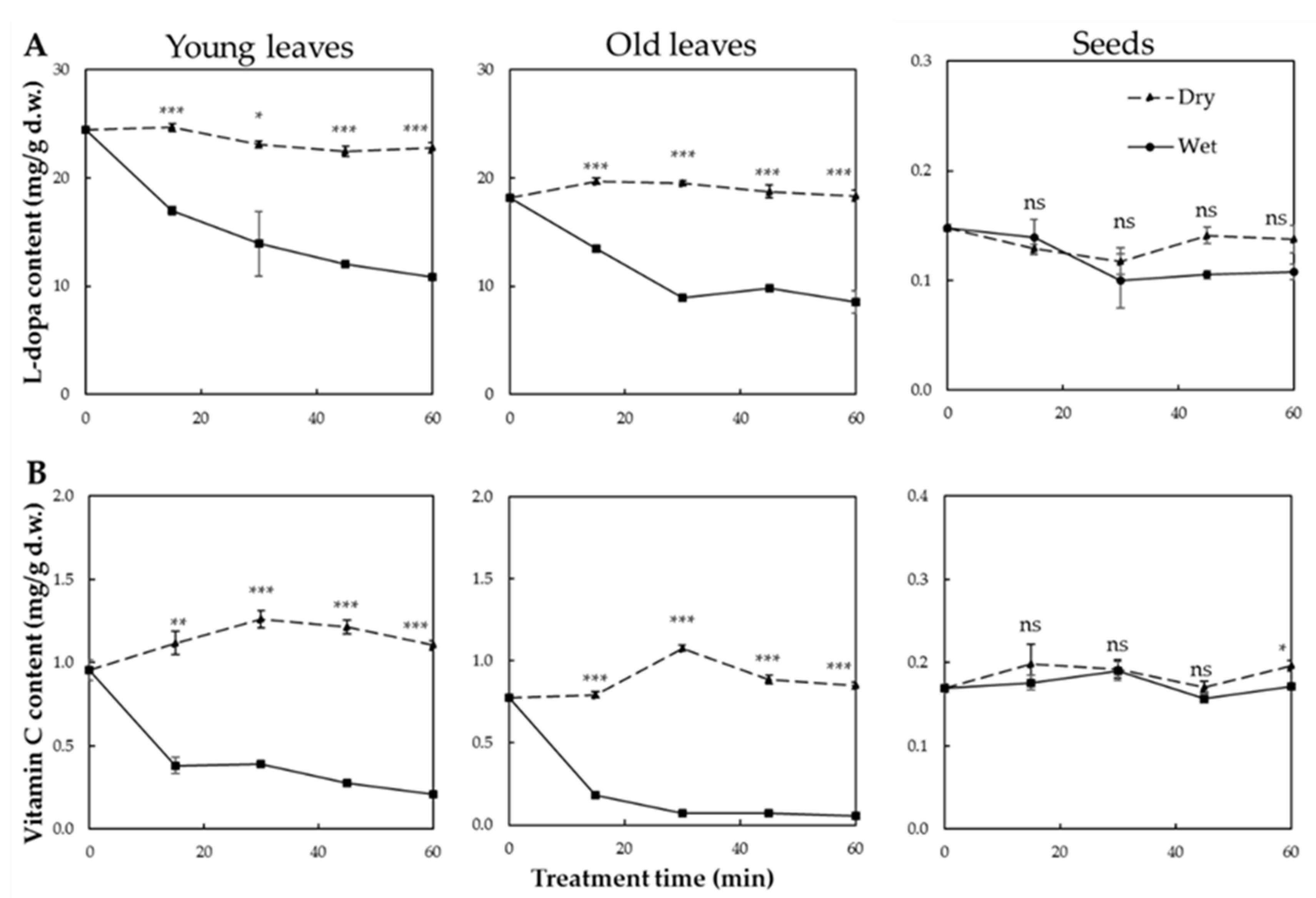
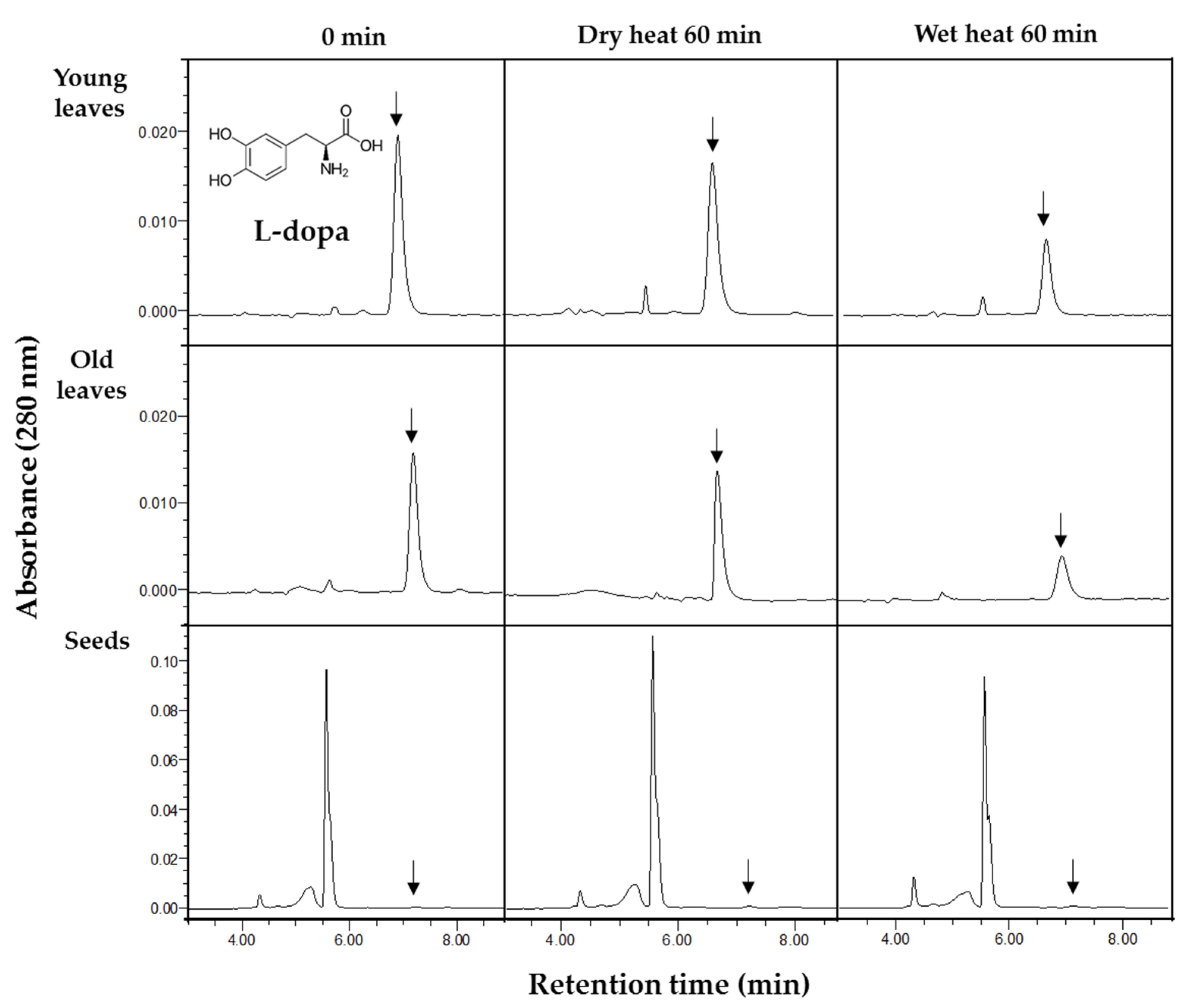
3.2. Changes in L-dopa Content after Thermal Treatment
3.3. Changes in Vc Content after Thermal Treatment
3.4. Total Phenolics Content
3.5. Changes in Total Flavonoids Content
3.6. DPPH and ABTS Radical Scavenging Abilities
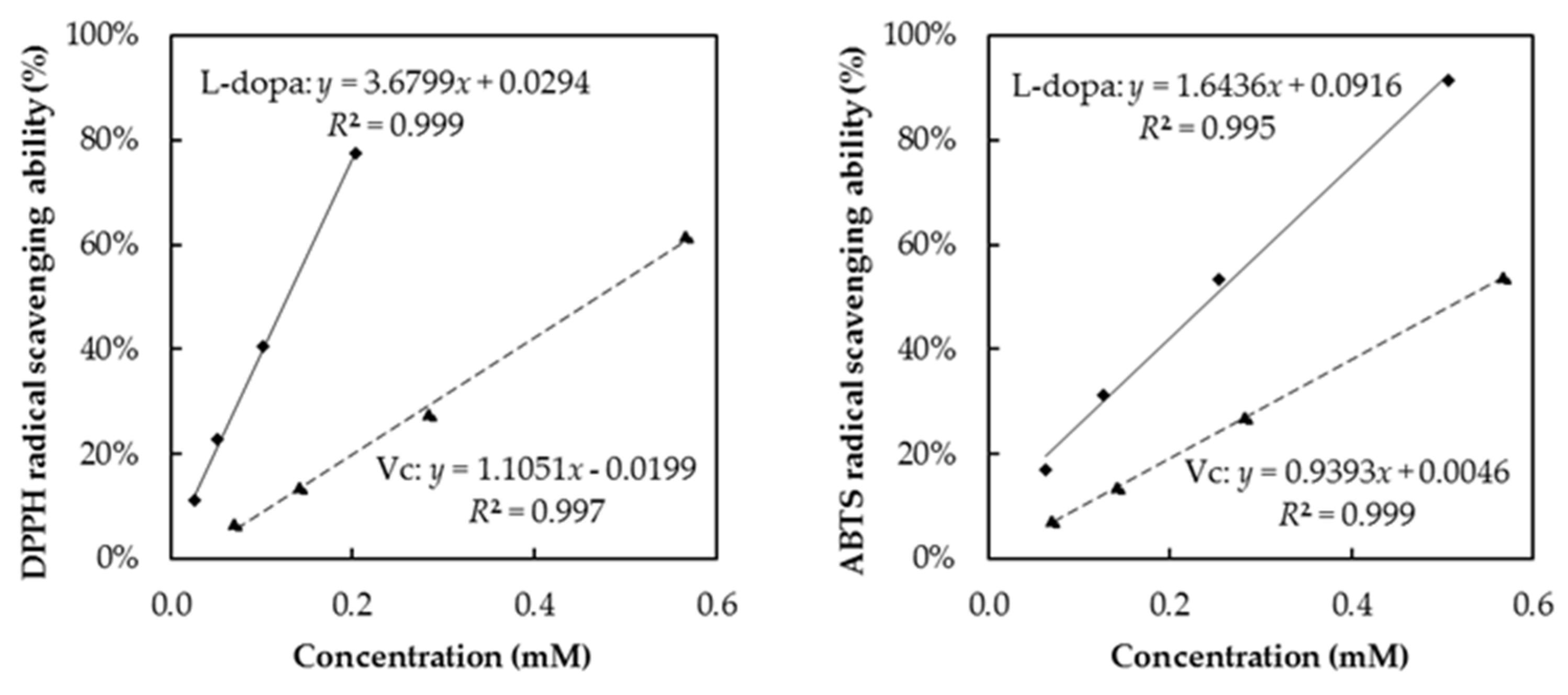
3.7. Correlation Analysis and the Antioxidant Activity of Pure Standard Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional composition, anti-nutritional factors, pretreatments-cum-processing impact and food formulation potential of faba bean (Vicia faba L.): A comprehensive review. LWT 2021, 138, 110796. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Autio, W.R.; Mangan, F.X.; Zandvakili, O. Yield and accumulation trend of biomass and L-DOPA in different parts of eight faba bean cultivars. Crop Sci. 2018, 58, 2020–2028. [Google Scholar] [CrossRef]
- Singh, A.K.; Bharati, R.C.; Manibhushan, N.C.; Pedapati, A. An assessment of faba bean (Vicia faba L.) current status and future prospect. Afr. J. Agric. Res. 2013, 8, 6634–6641. [Google Scholar] [CrossRef]
- Renna, M.; Signore, A.; Paradiso, V.M.; Santamaria, P. Faba greens, globe artichoke’s offshoots, crenate broomrape and summer squash greens: Unconventional vegetables of Puglia (southern Italy) with good quality traits. Front. Plant Sci. 2018, 9, 378. [Google Scholar] [CrossRef]
- Shanghai Scientific & Technical Publishers. Chinese Materia Medica, (Translated from the Chinese Name “Zhong Hua Ben Cao”); Shanghai Scientific & Technical Publishers: Shanghai, China, 1999. [Google Scholar]
- Lu, Y.-H.; Tian, C.-R.; Gao, C.-Y.; Wang, B.-N.; Yang, W.-Y.; Kong, X.; Chai, L.-Q.; Chen, G.-C.; Yin, X.-F.; He, Y.-H. Phenolic composition, antioxidant capacity and inhibitory effects on α-glucosidase and lipase of immature faba bean seeds. Int. J. Food Prop. 2018, 21, 2366–2377. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Barker, A.V.; Zandvakili, O.R.; Liu, X. Agronomy, nutritional value, and medicinal application of faba bean (Vicia faba L.). Hortic. Plant J. 2019, 5, 170–182. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Rajeswari, D.V. Nutritional and biological properties of Vicia faba L.: A perspective review. Int. Food Res. J. 2018, 25, 1332–1340. [Google Scholar]
- Hitoshi, M. Some Properties of the arginine decarboxylase in Vicia faba Leaves. Plant Cell Physiol. 1984, 25, 523–530. [Google Scholar] [CrossRef]
- Neugart, S.; Rohn, S.; Schreiner, M. Identification of complex, naturally occurring flavonoid glycosides in Vicia Faba and Pisum Sativum leaves by HPLC-DAD-ESI-MSn and the genotypic effect on their flavonoid profile. Food Res. Int. 2015, 76, 114–121. [Google Scholar] [CrossRef]
- Duan, S.; Kwon, S.-J.; Lim, Y.J.; Gil, C.S.; Jin, C.; Eom, S.H. L-3,4-dihydroxyphenylalanine accumulation in faba bean (Vicia faba L.) tissues during different growth stages. Agronomy 2021, 11, 502. [Google Scholar] [CrossRef]
- Pathan, A.; Alshahrani, A.M. Gold standard of symptomatic treatment in Parkinson disease: Carbidopa/Levodopa. Neuro Pharmac J. 2018, 3, 63–68. [Google Scholar] [CrossRef]
- Büttner, T.; Kuhn, W.; Patzold, T.; Przuntek, H. L-dopa improves colour vision in Parkinson’s disease. J. Neural Transm. 1994, 7, 13–19. [Google Scholar] [CrossRef]
- Worth, D.; Harvey, J.; Brown, J.; Lee, M. The effects of intravenous L-dopa on plasma renin activity, renal function, and blood pressure in man. Eur. J. Clin. Pharmacol. 1988, 35, 137–141. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, D.-G.; Lee, M.-K.; Kim, J.M.; Hong, M.J.; Kang, K.-Y.; Eom, S.H.; Kang, S.-Y.; Kim, J.-B.; Kwon, S.-J. Fatty acid composition, isoflavone and L-3,4-dihydroxyphenylalanine (L-dopa) contents in different parts of faba bean (Vicia faba) genotypes. Plant Breed. Biotech. 2017, 5, 314–324. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—a review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–919. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Eun, J.-B. Flavonoids in fruits and vegetables after thermal and nonthermal processing: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 3159–3188. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Sheehy, T.; O’Neill, C.M. Is vitamin D deficiency a public health concern for low middle income countries? A systematic literature review. Eur. J. Nutr. 2019, 58, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.H.S.; Silva, M.A. Retention of vitamin C in drying processes of fruits and vegetables—A review. Dry. Technol. 2008, 26, 1421–1437. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of processing on the in vitro and in vivo protein quality of beans (Phaseolus vulgaris and Vicia Faba). Nutrients 2018, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Ragaee, S.; Seetharaman, K.; Abdel-Aal, E.-S.M. The impact of milling and thermal processing on phenolic compounds in cereal grains. Crit. Rev. Food Sci. Nutr. 2014, 54, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-W.; Xie, W.-H. Effect of different processing methods on certain antinutritional factors and protein digestibility in green and white faba bean (Vicia fabaL.). CYTA J. Food 2013, 11, 43–49. [Google Scholar] [CrossRef]
- Luo, Y.; Gu, Z.; Han, Y.; Chen, Z. The impact of processing on phytic acid, in vitro soluble iron and Phy/Fe molar ratio of faba bean (Vicia faba L.). J. Sci. Food Agric. 2009, 89, 861–866. [Google Scholar] [CrossRef]
- Polanowska, K.; Łukasik, R.M.; Kuligowski, M.; Nowak, J. Development of a sustainable, simple, and robust method for efficient L-DOPA extraction. Molecules 2019, 24, 2325. [Google Scholar] [CrossRef]
- Nishiyama, I.; Yamashita, Y.; Yamanaka, M.; Shimohashi, A.; Fukuda, T.; Oota, T. Varietal difference in vitamin C content in the fruit of kiwifruit and other Actinidia species. J. Agric. Food Chem. 2004, 52, 5472–5475. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Oh, C.-S.; Park, Y.-D.; Eom, S.H.; Kim, D.-O.; Kim, U.-J.; Cho, Y.-S. Physiological components of kiwifruits with in vitro antioxidant and acetylcholinesterase inhibitory activities. Food Sci. Biotechnol. 2014, 23, 943–949. [Google Scholar] [CrossRef]
- Jin, C.W.; Ghimeray, A.K.; Wang, L.; Xu, M.L.; Piao, J.P.; Cho, D.H. Far infrared assisted kenaf leaf tea preparation and its effect on phenolic compounds, antioxidant and ACE inhibitory activity. J. Med. Plants Res. 2013, 7, 1121–1138. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, D.; Gao, H. Kinetics, physicochemical properties, and antioxidant activities of Angelica keiskei processed under four drying conditions. LWT 2018, 98, 349–357. [Google Scholar] [CrossRef]
- Echavarría, A.P.; Pagán, J.; Ibarz, A. Melanoidins formed by Maillard reaction in food and their biological activity. Food Eng. Rev. 2012, 4, 203–223. [Google Scholar] [CrossRef]
- Carabasa-Giribet, M.; Ibarz-Ribas, A. Kinetics of colour development in aqueous glucose systems at high temperatures. J. Sci. Food Agric. 2000, 44, 181–189. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Randhir, R.; ZandVakili, O.; Ebadi, A. Accumulation of L-DOPA in various organs of faba bean and influence of drought, nitrogen stress, and processing methods on L-DOPA yield. Crop J. 2018, 6, 426–434. [Google Scholar] [CrossRef]
- Mugendi, J.B.; Njagi, E.N.M.; Kuria, E.N.; Mwasaru, M.A.; Mureithi, J.G.; Apostolides, Z. Effects of processing technique on the nutritional composition and anti-nutrient content of mucuna bean (Mucuna pruriens L.). Afr. J. Food Sci. 2010, 4, 156–166. [Google Scholar]
- Nyirenda, D.; Musukwa, M.; Jonsson, L.O. The effects of different processing methods of velvet beans (Mucuna pruriens) on L-dopa content, proximate composition and broiler chicken performance. Trop. Subtrop. Agroecosyst. 2003, 1, 253–260. [Google Scholar]
- Kocabiyik, H.; Kayisoglu, B.; Tezer, D. Effect of moisture content on thermal properties of pumpkin seed. Int. J. Food Prop. 2009, 12, 277–285. [Google Scholar] [CrossRef]
- Pappert, E.J.; Buhrfiend, C.; Lipton, J.W.; Carvey, P.M.; Stebbins, G.T.; Goetz, C.G. Levodopa stability in solution: Time course, environmental effects, and practical recommendations for clinical use. Mov. Disord. 1996, 11, 24–26. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Alany, R.G.; Chuang, V.; Wen, J. Studies of the rate constant of L-DOPA oxidation and decarboxylation by HPLC. Chromatographia 2012, 75, 597–606. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci. Biotechnol. 2017, 27, 333–342. [Google Scholar] [CrossRef] [PubMed]
- De Cillis, F.; Leoni, B.; Massaro, M.; Renna, M.; Santamaria, P. Yield and quality of faba bean (Vicia faba L. var. major) genotypes as a vegetable for fresh consumption: A comparison between italian landraces and commercial varieties. Agriculture 2019, 9, 253. [Google Scholar] [CrossRef]
- Zhang, M.; Hettiarachchy, N.S.; Horax, R.; Chen, P.; Over, K.F. Effect of maturity stages and drying methods on the retention of selected nutrients and phytochemicals in bitter melon (Momordica charantia) leaf. J. Food Sci. 2009, 74, 441–448. [Google Scholar] [CrossRef]
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Pak Dek, M.S.; Hairuddin, M.R. Effect of freeze-drying on the antioxidant compounds and antioxidant activity of selected tropical fruits. Int. J. Mol. Sci. 2011, 12, 4678–4692. [Google Scholar] [CrossRef]
- Moriyama, M.; Oba, K. Comparative study on the vitamin C contents of the food legume seeds. J. Nutr. Sci. Vitaminol. 2008, 54, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villota, R.; Karel, M. Prediction of ascorbic acid retention during drying: II. Simulation of retention in a model system. J. Food Process. Preserv. 1980, 4, 141–159. [Google Scholar] [CrossRef]
- M’Rabet, Y.; Rokbeni, N.; Cluzet, S.; Boulila, A.; Richard, T.; Krisa, S.; Marzouki, L.; Casabianca, H.; Hosni, K. Profiling of phenolic compounds and antioxidant activity of Melia azedarach L. leaves and fruits at two stages of maturity. Ind. Crops Prod. 2017, 107, 232–243. [Google Scholar] [CrossRef]
- Ngamsuk, S.; Huang, T.-C.; Hsu, J.-L. Determination of phenolic compounds, procyanidins, and antioxidant activity in processed Coffea arabica L. leaves. Foods 2019, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xu, Y.; Wang, B.; Li, S.; Guo, F.; Hua, H.; Zhao, Y.; Yu, Z. Comparison of phenolic compounds, antioxidant and antidiabetic activities between selected edible beans and their different growth periods leaves. J. Funct. Foods 2017, 35, 694–702. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, P.; Shetty, K. L-DOPA and total phenolic stimulation in dark germinated fava bean in response to peptide and phytochemical elicitors. Process. Biochem. 2002, 37, 1247–1256. [Google Scholar] [CrossRef]
- Chaieb, N.; González, J.L.; López-Mesas, M.; Bouslama, M.; Valiente, M. Polyphenols content and antioxidant capacity of thirteen faba bean (Vicia faba L.) genotypes cultivated in Tunisia. Food Res. Int. 2011, 44, 970–977. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.; De Mello, J. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Hwang, I.G.; Shin, Y.J.; Lee, S.; Lee, J.; Yoo, S.M. Effects of different cooking methods on the antioxidant properties of red pepper (Capsicum annuum L.). Prev. Nutr. Food Sci. 2012, 17, 286–292. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Effect of different cooking methods on polyphenols, carotenoids and antioxidant activities of selected edible leaves. Antioxidants 2018, 7, 117. [Google Scholar] [CrossRef]
- Siah, S.; Wood, J.A.; Agboola, S.; Konczak, I.; Blanchard, C.L. Effects of soaking, boiling and autoclaving on the phenolic contents and antioxidant activities of faba beans (Vicia faba L.) differing in seed coat colours. Food Chem. 2014, 142, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Siah, S.; Konczak, I.; Wood, J.A.; Agboola, S.; Blanchard, C.L. Effects of roasting on phenolic composition and in vitro antioxidant capacity of Australian grown faba beans (Vicia faba L.). Plant Foods Hum. Nutr. 2014, 69, 85–91. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoid functions in plants and their interactions with other organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Ozga, J.A.; Lopes-Lutz, D.; Schieber, A.; Reinecke, D.M. Characterization of proanthocyanidins in pea (Pisum sativum L.), lentil (Lens culinaris L.), and faba bean (Vicia faba L.) seeds. Food Res. Int. 2012, 46, 528–535. [Google Scholar] [CrossRef]
- Agbo, E.A.; Kouassi, K.; Gouekou, D.A.; Méité, S.; Gbogouri, A.G.; Brou, K. Antioxidant activities in sweet potatoes leaves steamed with spices. J. Food Res. 2020, 9, 41–49. [Google Scholar] [CrossRef]
- Okumura, K.; Hosoya, T.; Kawarazaki, K.; Izawa, N.; Kumazawa, S. Antioxidant activity of phenolic compounds from fava bean sprouts. J. Food Sci. 2016, 81, 1394–1398. [Google Scholar] [CrossRef]
- Cos, P.; Rajan, P.; Vedernikova, I.; Calomme, M.; Pieters, L.; Vlietinck, A.J.; Augustyns, K.; Haemers, A.; Berghe, D.V. In Vitro antioxidant profile of phenolic acid derivatives. Free Radic. Res. 2002, 36, 711–716. [Google Scholar] [CrossRef]
- Gülçin, I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 2007, 32, 431–438. [Google Scholar] [CrossRef]

| Freeze Dried (0 Min) | Dry Heat | Wet Heat | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15 Min | 30 Min | 45 Min | 60 Min | 15 Min | 30 Min | 45 Min | 60 Min | |||
| TPC (mg GAE/g d.w.) | Young leaves | 54.31 ± 0.72 a | 55.60 ±2.11 a | 52.87 ±1.03 a | 52.91 ± 1.72 a | 53.09 ± 3.02 a | 35.24 ± 1.14 b | 30.96 ± 6.35 b | 26.30 ± 0.27 b | 24.21 ± 0.53 b |
| Old leaves | 43.18 ± 0.50 a | 44.92 ± 1.00 a | 46.78 ± 0.74 a | 46.20 ± 1.29 a | 47.29 ± 1.79 a | 31.29 ± 0.60 b | 23.28 ± 0.83 c | 25.58 ± 0.31 c | 21.59 ± 0.35 c | |
| Seeds | 3.86 ± 0.11 a | 4.13 ± 0.15 a | 4.08 ± 0.23 a | 4.31 ± 0.22 a | 4.11 ± 0.21 a | 3.76 ± 0.20 a | 3.67 ± 0.10 a | 3.67 ± 0.21 a | 3.74 ± 0.28 a | |
| TFC (mg CE/g d.w.) | Young leaves | 63.67 ± 1.26 a | 58.87 ± 2.97 a | 57.92 ± 1.27 a | 53.22 ± 1.63 ab | 56.64 ± 1.71 a | 37.84 ± 0.09 bc | 30.80 ± 8.19 c | 28.85 ± 0.35 c | 29.99 ± 1.09 c |
| Old leaves | 43.68 ± 0.22 a | 43.76 ± 1.55 a | 45.98 ± 1.90 a | 44.12 ± 0.53 a | 43.64 ± 2.13 a | 29.58 ± 0.74 b | 20.36 ± 1.43 c | 22.48 ± 0.53 c | 23.12 ± 1.73 c | |
| Seeds | 1.00 ± 0.06 a | 1.02 ± 0.03 a | 0.94 ± 0.05 a | 1.06 ± 0.01 a | 0.97 ± 0.06 a | 1.20 ± 0.06 a | 1.05 ± 0.11 a | 1.18 ± 0.09 a | 1.12 ± 0.24 a | |
| DPPH (mg VCE/g d.w.) | Young leaves | 73.70 ± 0.47 a | 71.81 ± 0.84 a | 70.89 ± 0.87 a | 68.40 ± 1.39 a | 69.62 ± 1.22 a | 51.94 ± 0.96 b | 43.31 ± 9.40 b | 41.71 ± 0.57 b | 40.54 ± 0.67 b |
| Old leaves | 54.55 ± 0.72 a | 56.96 ± 0.57 a | 58.87 ± 0.59 a | 58.67 ± 0.88 a | 56.86 ± 1.39 a | 41.19 ± 0.52 b | 27.48 ± 1.37 d | 33.12 ± 0.44 c | 30.21 ± 1.96 cd | |
| Seeds | 1.47 ± 0.06 a | 1.29 ± 0.27 a | 1.15 ± 0.13 a | 1.49 ± 0.08 a | 1.30 ± 0.07 a | 1.19 ± 0.04 a | 1.15 ± 0.12 a | 1.41 ± 0.08 a | 1.36 ± 0.25 a | |
| ABTS (mg VCE/g d.w.) | Young leaves | 52.96 ± 3.92 a | 54.67 ± 0.76 a | 50.74 ± 1.18 a | 52.57 ± 1.48 a | 53.10 ± 0.29 a | 30.03 ± 3.93 b | 26.87 ± 0.96 b | 26.10 ± 2.29 b | 22.95 ± 2.67 b |
| Old leaves | 37.70 ± 1.46 a | 39.47 ± 2.20 a | 42.71 ± 0.65 a | 43.20 ± 1.46 a | 42.55 ± 0.89 a | 19.25 ± 2.49 b | 15.76 ± 1.58 b | 19.13 ± 1.80 b | 17.00 ± 1.95 b | |
| Seeds | 6.61 ± 0.09 bc | 6.97 ± 0.08 abc | 6.64 ± 0.22 bc | 7.55 ± 0.24 a | 7.17 ± 0.16 ab | 6.27 ± 0.13 c | 6.39 ± 0.12 bc | 6.32 ± 0.19 c | 6.31 ± 0.14 c | |
| L-dopa | Vitamin C | TPC | TFC | DPPH | ABTS | ||
|---|---|---|---|---|---|---|---|
| Young leaf | L-dopa | 1 | |||||
| vitamin C | 0.890 *** | 1 | |||||
| TPC | 0.977 *** | 0.918 *** | 1 | ||||
| TFC | 0.861 *** | 0.888 *** | 0.882 *** | 1 | |||
| DPPH | 0.826 *** | 0.891 *** | 0.833 *** | 0.948 *** | 1 | ||
| ABTS | 0.907 *** | 0.923 *** | 0.919 *** | 0.910 *** | 0.919 *** | 1 | |
| Old leaf | L-dopa | 1 | |||||
| vitamin C | 0.954 *** | 1 | |||||
| TPC | 0.971 *** | 0.969 *** | 1 | ||||
| TFC | 0.960 *** | 0.963 *** | 0.980 *** | 1 | |||
| DPPH | 0.984 *** | 0.967 *** | 0.985 *** | 0.973 *** | 1 | ||
| ABTS | 0.933 *** | 0.967 *** | 0.960 *** | 0.941 *** | 0.956 *** | 1 | |
| Seeds | L-dopa | 1 | |||||
| vitamin C | 0.229 ns | 1 | |||||
| TPC | 0.256 ns | 0.103 ns | 1 | ||||
| TFC | 0.079 ns | −0.340 ns | 0.043 ns | 1 | |||
| DPPH | 0.495 ** | 0.079 ns | 0.179 ns | 0.309 ns | 1 | ||
| ABTS | 0.566 ** | 0.228 ns | 0.480 * | −0.252 ns | 0.396 * | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, S.-C.; Kwon, S.-J.; Eom, S.-H. Effect of Thermal Processing on Color, Phenolic Compounds, and Antioxidant Activity of Faba Bean (Vicia faba L.) Leaves and Seeds. Antioxidants 2021, 10, 1207. https://doi.org/10.3390/antiox10081207
Duan S-C, Kwon S-J, Eom S-H. Effect of Thermal Processing on Color, Phenolic Compounds, and Antioxidant Activity of Faba Bean (Vicia faba L.) Leaves and Seeds. Antioxidants. 2021; 10(8):1207. https://doi.org/10.3390/antiox10081207
Chicago/Turabian StyleDuan, Shu-Cheng, Soon-Jae Kwon, and Seok-Hyun Eom. 2021. "Effect of Thermal Processing on Color, Phenolic Compounds, and Antioxidant Activity of Faba Bean (Vicia faba L.) Leaves and Seeds" Antioxidants 10, no. 8: 1207. https://doi.org/10.3390/antiox10081207
APA StyleDuan, S.-C., Kwon, S.-J., & Eom, S.-H. (2021). Effect of Thermal Processing on Color, Phenolic Compounds, and Antioxidant Activity of Faba Bean (Vicia faba L.) Leaves and Seeds. Antioxidants, 10(8), 1207. https://doi.org/10.3390/antiox10081207






