Nutrigenomics of Dietary Lipids
Abstract
1. Introduction
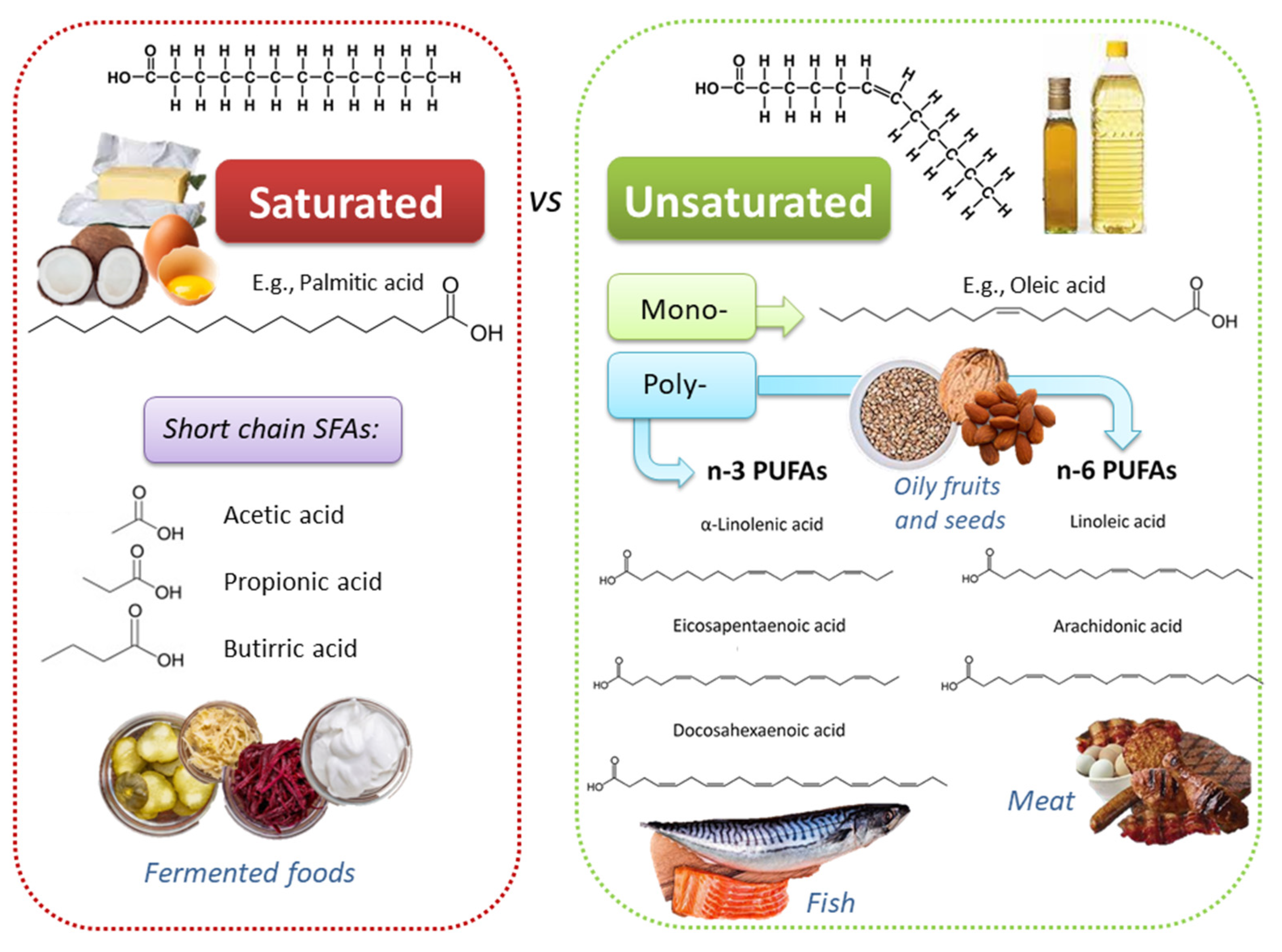
2. Dietary Lipids: Fatty Acids in Plant- and Animal-Based Food Products
3. Dietary Lipids Bioavailability, Bioaccessibility, and Toxicity
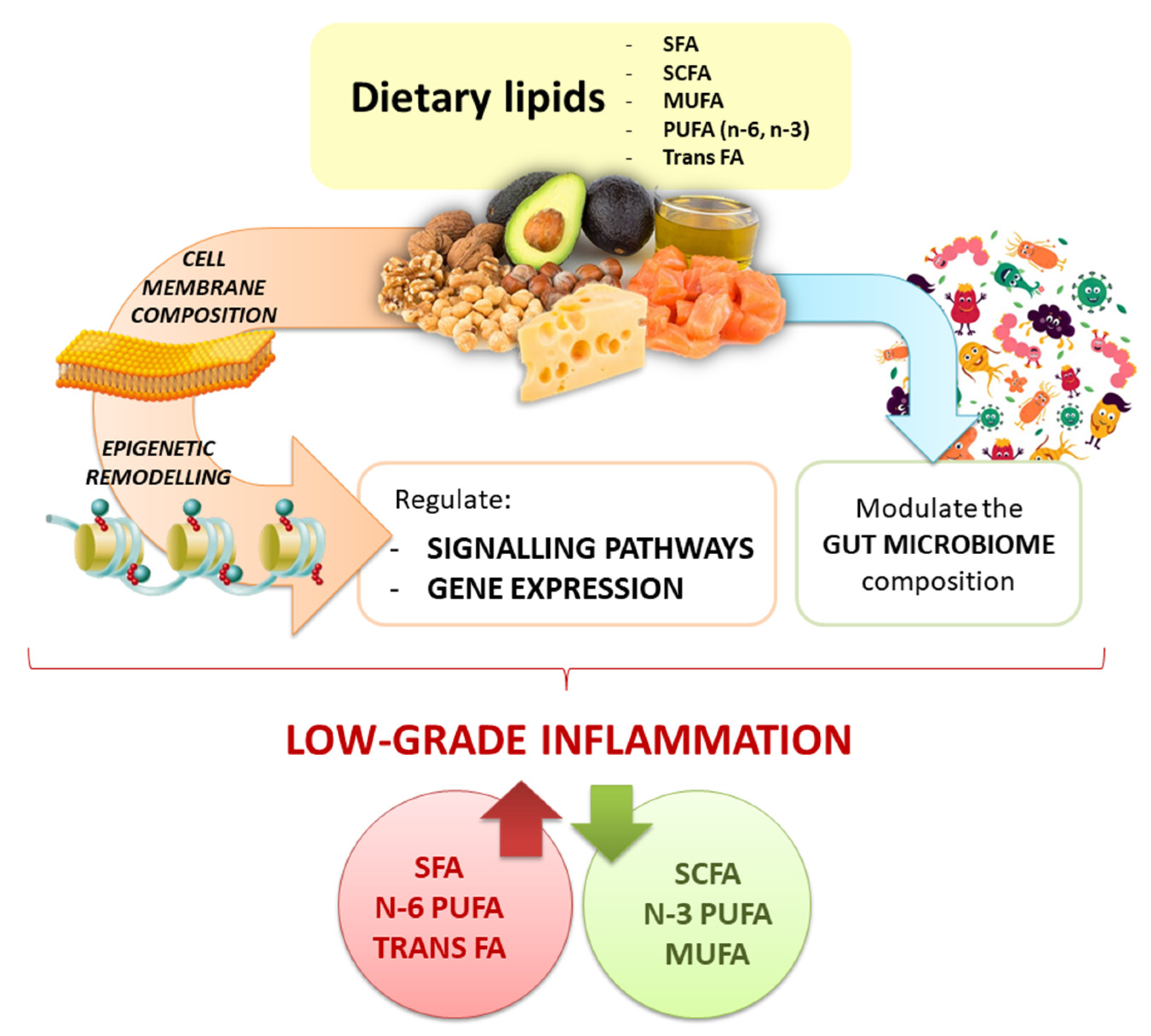
4. Crosstalk between Fatty Acids and Inflammation
5. Nutrigenomics of Fats: Evidence from Animal Studies
6. Nutrigenomics of Fats: Evidence from Human Studies
| Gene | SNP | Dietary Fat Interaction | Main Results | References |
|---|---|---|---|---|
| ADIPOQ | rs2241766, rs16861209, rs17300539 | SFA [C14:0 + C16:0 + EA], MUFA [C16:1n-7 + AO], PUFA [ω-6 PUFAs + ω-3 PUFAs], ω-6 [LA (18:2 n-6) + DGLA (20:3 n-6) + AA (20:4n-6)], ω-3-HUFA [EPA + DPA (22:5 ω-3) + DHA], and ω-3 [LA (18:3n3) + ω-3 HUFA]. | rs2241766G allele: ↑ total plasma ω-3 FA content was protective against inflammation. Gene-plasma FA profile interaction: rs2241766 and ω-3; rs16861209 and ARA and DPA; rs17300539 and SFA. | [155] |
| rs17300539, rs182052, rs16861209, rs1501299 | High-MUFA: total fat 38%, carbohydrate 45% of energy. MUFA intake of 20% of energy. | rs182052 G/G genotype: serum adiponectin levels ↑ in 3.8% after a high-MUFA diet. In these patients, a high-MUFA diet may help to ↑ adiponectin concentrations with advancing age. | [157] | |
| rs17300539, rs2241766 | ω-3 PUFA (fish oil supplementation—daily doses of 0.45, 0.9, and 1.8 g 20:5n3 and 22:6n3 (1.51:1), or placebo). | rs17300539A allele: ↑ serum adiponectin levels. rs2241766 T/T genotype: subjects aged >58y had a 22% ↑ in serum adiponectin levels compared to baseline after the highest dose of 20:5n3 and 22:6n3. | [156] | |
| APOE | rs429358, rs7412 | SFA (Food4Me Study) | APOE ε4 allele was associated with higher total cholesterol. | [161] |
| rs429358, rs7412 | Low-fat diet (24% from fat, 8% from SFA, 59% from carbohydrate), high-fat high-SFA diet (38% from fat, 18% from SFA, 45% from carbohydrate), and high-fat high-SFA diet supplemented with 3.45 g DHA/d | APOE ε4 carriers: ↑ CRP plasma levels after eight weeks of a high-SFA and high-SFA-DHA diets relative to low-fat diet. | [162] | |
| rs429358, rs7412 | SFA with MUFA or ω-6FA | Diet-genotype interaction: differential responsiveness to MUFA intake between ε3/ε3 and ε4 carriers. | [163] | |
| CRP | rs2808630, rs3093058, rs3093062 | SFA and MUFA | Presence of rs3093058 and rs3093062 minor allele: ↑ CRP levels in the presence of ↑ triglyceride or cholesterol intake. rs2808630 minor allele: ↑ intake of SFA and MUFA, ↑ CRP levels. Presence of the minor allele of these 3 SNPs: ↑ ω-6 to -3 ratio | [165] |
| rs1205, rs1417938, rs2808630 | FA | CRP SNPs modulated the risk of being in the inflammatory group depending on individual plasma FA and lipid profile. | [168] | |
| rs3093068, rs1130864, rs1205 | MedDiet | The minor allele of rs3093068 and rs1130864: ↑ CRP levels rs1205T allele: ↓ CRP concentrations. Interaction between rs3093068 and MedDiet. | [166] | |
| FADS cluster | FADS1: rs174537; FADS2: rs174575, rs2727270; FADS3: rs1000778 | ω-3 and ω-6 | The presence of rs174537, rs174575, and rs2727270 minor alleles: ↑ LA levels rs174537T and rs2727270T: ↓ DGLA and ARA levels rs1000778T allele: ↓ ARA levels | [173] |
| FADS haplotype | 28 closely linked SNPs | ω-3 and ω-6 | Two common FADS haplotype differ in their ability to generate LC-PUFAs. | [171] |
| FADS1 | rs174537 | ARA/LA | rs174537 impacts the synthesis of ARA and the overall capacity of whole blood to synthesize 5-lipoxygenase products. | [172] |
| rs174537 | PUFA | rs174537T allele carriers: ↓ in 20:4 ω-6 levels, ↓ delta-5 desaturase enzyme activity, and ↓ FADS1 gene expression. | [174] | |
| rs174550 | Habitual diet with a supplement of 30, 40, or 50 mL (27–45 g) sunflower oil (62% of LA) daily depending on BMI | In men carrying the T/T genotype, plasma eicosanoid concentrations correlated with the ARA proportion and with hsCRP. No correlations were found for C/C genotype. | [175] |
7. Dietary Lipids Modulate Gut Microbiota Composition and Metabolites Production
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ADIPOQ | Adiponectin, C1Q and collagen domain containing |
| ALA | α-Linolenic acid |
| AMPK | 5′AMP-activated protein kinase |
| ARA | Arachidonic acid |
| CO | Coconut oil |
| ChREBP | Carbohydrate response element binding protein |
| COX | Cyclooxygenase |
| CRC | Colorectal cancer |
| CRP | C-reactive protein |
| DHA | Docosahexaenoic acid |
| DGLA | Dihomo gamma-linolenic acid |
| EPA | Eicosapentaenoic acid |
| EA | Estearic acid |
| EVOO | Extra virgin olive oil |
| FAs | Fatty acids |
| FADS | Fatty acid desaturase |
| FFAs | Free fatty acids |
| GFAP | Glial fibrillary acidic protein |
| GPCRs/GPRz | G protein-coupled receptors |
| HDACs | Histone deacetylases |
| HFD | High-fat diet |
| HPETE | Hydroperoxyeicosatetraenoic acid |
| HUFA | Highly unsaturated fatty acid |
| IFNγ | Interferon γ |
| IL | Interleukine |
| IRF3 | Interferon regulatory factor 3 |
| LA | Linoleic acid |
| LDLR | Low density lipoprotein receptor |
| LFD | Low-fat diet |
| LOX | Lipoxygenase |
| LTs | Leukotrienes |
| LXRA | Liver X receptor-alpha |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MCFAs | Medium chain fatty acids |
| MedDiet | Mediterranean diet |
| MUFAs | Monounsaturated fatty acids |
| MyD88 | Myeloid differentiation primary response 88 |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NAFLD | Non-alcoholic fatty liver disease |
| NF-κB | Nuclear factor kappa B |
| NLRP3 | Nucleotide-binding and oligomerization domain–like receptor, leucine-rich repeat and pyrin domain–containing 3 inflammasome |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| OA | Oleic acid |
| PBMCs | Peripheral blood mononuclear cells |
| PGs | Prostaglandins |
| PL | Phospholipids |
| PO | Palm oil |
| PPAR-γ | Peroxisome proliferator-activated receptor γ |
| PUFAs | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| SCFAs | Short chain fatty acids |
| SCI | Systemic chronic inflammation |
| SFAs | Saturated fatty acids |
| SNPs | Single nucleotide polymorphisms |
| SOD | Superoxide dismutase |
| SREBPs | Sterol regulatory element binding proteins |
| T2DM | Type 2 diabetes mellitus |
| TG | Triglycerides |
| TLRs | Toll-like receptors |
| TNFα | Tumor necrosis factor α |
| TXs | Thromboxanes |
| ω-3 | Omega-3 fatty acids |
| ω-6 | Omega-6 fatty acids |
| USF1 | Upstream transcription factor 1 |
| UCP2 | Uncoupling protein 2 |
| VEGF | Vascular endothelial growth factor |
References
- Springmann, M.; Wiebe, K.; Mason-D’Croz, D.; Sulser, T.B.; Rayner, M.; Scarborough, P. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: A global modelling analysis with country-level detail. Lancet Planet. Health 2018, 2, e451–e461. [Google Scholar] [CrossRef]
- Gabbianelli, R.; Damiani, E. Epigenetics and neurodegeneration: Role of early-life nutrition. J. Nutr. Biochem. 2018, 57, 1–13. [Google Scholar] [CrossRef]
- Bordoni, L.; Gabbianelli, R. Primers on nutrigenetics and nutri(epi)genomics: Origins and development of precision nutrition. Biochimie 2019, 160, 156–171. [Google Scholar] [CrossRef]
- Petracci, I.; Gabbianelli, R.; Bordoni, L. The Role of Nutri(epi)genomics in Achieving the Body’s Full Potential in Physical Activity. Antioxidants 2020, 9, 498. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Barabási, A.-L.; Menichetti, G.; Loscalzo, J. The unmapped chemical complexity of our diet. Nat. Food 2020, 1, 33–37. [Google Scholar] [CrossRef]
- Finucane, O.M.; Lyons, C.L.; Murphy, A.M.; Reynolds, C.M.; Klinger, R.; Healy, N.P.; Cooke, A.A.; Coll, R.C.; McAllan, L.; Nilaweera, K.N.; et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes 2015, 64, 2116–2128. [Google Scholar] [CrossRef]
- Nestel, P.J. Dietary Fat and Blood Pressure. Curr. Hypertens. Rep. 2019, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Paszczyk, B.; Polak-Śliwińska, M.; Łuczyńska, J. Fatty Acids Profile, Trans Isomers, and Lipid Quality Indices in Smoked and Unsmoked Cheeses and Cheese-Like Products. Int. J. Environ. Res. Public Health 2019, 17, 71. [Google Scholar] [CrossRef]
- Al-Khalaifah, H. Modulatory Effect of Dietary Polyunsaturated Fatty Acids on Immunity, Represented by Phagocytic Activity. Front. Vet. Sci. 2020, 7, 569939. [Google Scholar] [CrossRef]
- van der Gaag, E.J.; Wieffer, R.; van der Kraats, J. Advising Consumption of Green Vegetables, Beef, and Full-Fat Dairy Products Has No Adverse Effects on the Lipid Profiles in Children. Nutrients 2017, 9, 518. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Hopkins, D.L.; Jacobs, J.L. Increasing omega-3 levels in meat from ruminants under pasture-based systems. Rev. Sci. Tech. 2018, 37, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Kolarič, L.; Šimko, P. Determination of Cholesterol Content in Butter by HPLC: Up-to-Date Optimization, and In-House Validation Using Reference Materials. Foods 2020, 9, 1378. [Google Scholar] [CrossRef] [PubMed]
- Wilczek, M.M.; Olszewski, R.; Krupienicz, A. Trans-Fatty Acids and Cardiovascular Disease: Urgent Need for Legislation. Cardiology 2017, 138, 254–258. [Google Scholar] [CrossRef]
- Hu, F.B.; Willett, W.C. Optimal diets for prevention of coronary heart disease. JAMA 2002, 288, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Ginter, E.; Simko, V. New data on harmful effects of trans-fatty acids. Bratisl. Lek. Listy 2016, 117, 251–253. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, W.; Lai, Y.; Zhang, B.; Zhang, D. Edible Plant Oil: Global Status, Health Issues, and Perspectives. Front. Plant Sci. 2020, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, L.; Liu, X.; Luo, R.; Liao, G.; Li, L.; Liu, J.; Cheng, J.; Lu, Y.; Chen, Y. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018, 203, 291–304. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, M.; Liu, X.; Chen, X.; Yuan, Y.; Li, L.; Liu, J.; Lu, Y.; Cheng, J.; Chen, Y. Oleic acid ameliorates palmitic acid induced hepatocellular lipotoxicity by inhibition of ER stress and pyroptosis. Nutr. Metab. 2020, 17, 11. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Casillas, R.; Bulló, M.; Castañer, O.; Ros, E.; Sáez, G.T.; Toledo, E.; Estruch, R.; Ruiz-Gutiérrez, V.; Fitó, M.; et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur. J. Nutr. 2017, 56, 89–97. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Arranz, S.; Andres-Lacueva, C.; Llorach, R.; Medina-Remón, A.; Lamuela-Raventos, R.M.; et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacol. Res. 2012, 65, 577–583. [Google Scholar] [CrossRef]
- Pacetti, D.; Boarelli, M.C.; Giovannetti, R.; Ferraro, S.; Conti, P.; Alfei, B.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Fedeli, D.; et al. Chemical and Sensory Profiling of Monovarietal Extra Virgin Olive Oils from the Italian Marche Region. Antioxidants 2020, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Fedeli, D.; Fiorini, D.; Gabbianelli, R. Extra Virgin Olive Oil and Nigella sativa Oil Produced in Central Italy: A Comparison of the Nutrigenomic Effects of Two Mediterranean Oils in a Low-Grade Inflammation Model. Antioxidants 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Nasuti, C.; Fedeli, D.; Bordoni, L.; Piangerelli, M.; Servili, M.; Selvaggini, R.; Gabbianelli, R. Anti-Inflammatory, Anti-Arthritic and Anti-Nociceptive Activities of Nigella sativa Oil in a Rat Model of Arthritis. Antioxidants 2019, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and Anti-Inflammatory Properties of Nigella sativa Oil in Human Pre-Adipocytes. Antioxidants 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Storage stability of egg sticks fortified with omega-3 fatty acids. J. Sci. Food Agric. 2018, 98, 3452–3461. [Google Scholar] [CrossRef]
- Stupin, A.; Rasic, L.; Matic, A.; Stupin, M.; Kralik, Z.; Kralik, G.; Grcevic, M.; Drenjancevic, I. Omega-3 polyunsaturated fatty acids-enriched hen eggs consumption enhances microvascular reactivity in young healthy individuals. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Metab. 2018, 43, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Veena, N.; Surendra Nath, B.; Srinivas, B.; Balasubramanyam, B. V Quality attributes of dahi prepared from milk fortified with omega-3 fatty acids, phytosterols and polydetxrose. J. Food Sci. Technol. 2017, 54, 1765–1775. [Google Scholar] [CrossRef]
- Stanton, A.V.; James, K.; Brennan, M.M.; O’Donovan, F.; Buskandar, F.; Shortall, K.; El-Sayed, T.; Kennedy, J.; Hayes, H.; Fahey, A.G.; et al. Omega-3 index and blood pressure responses to eating foods naturally enriched with omega-3 polyunsaturated fatty acids: A randomized controlled trial. Sci. Rep. 2020, 10, 15444. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Malau-Aduli, B.S.; Cavalieri, J.; Malau-Aduli, A.E.O.; Nichols, P.D. Enhancing Omega-3 Long-Chain Polyunsaturated Fatty Acid Content of Dairy-Derived Foods for Human Consumption. Nutrients 2019, 11, 743. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, F.; Wu, Y.; Zhang, Y.; Gao, B.; Yu, L. Triacylglycerols and Fatty Acid Compositions of Cucumber, Tomato, Pumpkin, and Carrot Seed Oils by Ultra-Performance Convergence Chromatography Combined with Quadrupole Time-of-Flight Mass Spectrometry. Foods 2020, 9, 970. [Google Scholar] [CrossRef]
- Yun, J.-M.; Surh, J. Fatty Acid Composition as a Predictor for the Oxidation Stability of Korean Vegetable Oils with or without Induced Oxidative Stress. Prev. Nutr. Food Sci. 2012, 17, 158–165. [Google Scholar] [CrossRef]
- Tena, N.; Aparicio, R.; García-González, D.L. Virgin olive oil stability study by mesh cell-FTIR spectroscopy. Talanta 2017, 167, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Imran, M.; Ahmad, N.; Hussain, A.I. Fatty acids characterization and oxidative stability of spray dried designer egg powder. Lipids Health Dis. 2018, 17, 282. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Manzoor, M.F.; Javed, A.; Ali, Z.; Akhtar, M.N.; Ali, M.; Hussain, Y. Fatty acids characterization, oxidative perspectives and consumer acceptability of oil extracted from pre-treated chia (Salvia hispanica L.) seeds. Lipids Health Dis. 2016, 15, 162. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-Based Diets Are Associated With a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef]
- Martínez-Ramírez, H.R.; Kramer, J.K.G.; de Lange, C.F.M. Ileal flows and apparent ileal digestibility of fatty acids in growing gilts fed flaxseed containing diets. J. Anim. Sci. 2013, 91, 2729–2739. [Google Scholar] [CrossRef][Green Version]
- Just, A.; Andersen, J.O.; Jørgensen, H. The influence of diet composition on the apparent digestibility of crude fat and fatty acids at the terminal ileum and overall in pigs. Zeitschrift Tierphysiologie Tierernährung Futtermittelkd. 1980, 44, 82–92. [Google Scholar] [CrossRef]
- Plourde, M.; Cunnane, S.C. Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as supplements. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Metab. 2007, 32, 619–634. [Google Scholar] [CrossRef]
- Goyens, P.L.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef]
- Gerster, H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int. J. Vitam. Nutr. Res. Int. Zeitschrift fur Vitamin- und Ernahrungsforschung. J. Int. Vitaminol. Nutr. 1998, 68, 159–173. [Google Scholar]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins. Leukot. Essent. Fatty Acids 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Lawson, L.D.; Hughes, B.G. Absorption of eicosapentaenoic acid and docosahexaenoic acid from fish oil triacylglycerols or fish oil ethyl esters co-ingested with a high-fat meal. Biochem. Biophys. Res. Commun. 1988, 156, 960–963. [Google Scholar] [CrossRef]
- Davidson, M.H.; Johnson, J.; Rooney, M.W.; Kyle, M.L.; Kling, D.F. A novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: The ECLIPSE (Epanova(®) compared to Lovaza(®) in a pharmacokinetic single-dose evaluation) study. J. Clin. Lipidol. 2012, 6, 573–584. [Google Scholar] [CrossRef]
- Kling, D.F.; Johnson, J.; Rooney, M.; Davidson, M. Omega-3 Free Fatty Acids Demonstrate More Than 4-Fold Greater Bioavailability for EPA and DHA Compared with Omega-3-acid Ethyl Esters in Conjunction with a Low-Fat Diet: The ECLIPSE Study†. J. Clin. Lipidol. 2011, 5, 231. [Google Scholar] [CrossRef]
- Offman, E.; Marenco, T.; Ferber, S.; Johnson, J.; Kling, D.; Curcio, D.; Davidson, M. Steady-state bioavailability of prescription omega-3 on a low-fat diet is significantly improved with a free fatty acid formulation compared with an ethyl ester formulation: The ECLIPSE II study. Vasc. Health Risk Manag. 2013, 9, 563–573. [Google Scholar] [CrossRef]
- Shen, Z.; Apriani, C.; Weerakkody, R.; Sanguansri, L.; Augustin, M.A. Food matrix effects on in vitro digestion of microencapsulated tuna oil powder. J. Agric. Food Chem. 2011, 59, 8442–8449. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Guérin-Dubiard, C.; Bourlieu, C.; Capozzi, F.; Bordoni, A.; Dupont, D. In Vivo Digestion of Egg Products Enriched with DHA: Effect of the Food Matrix on DHA Bioavailability. Foods 2020, 10, 6. [Google Scholar] [CrossRef]
- Lamothe, S.; Rémillard, N.; Tremblay, J.; Britten, M. Influence of dairy matrices on nutrient release in a simulated gastrointestinal environment. Food Res. Int. 2017, 92, 138–146. [Google Scholar] [CrossRef]
- Fardet, A.; Dupont, D.; Rioux, L.-E.; Turgeon, S.L. Influence of food structure on dairy protein, lipid and calcium bioavailability: A narrative review of evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1987–2010. [Google Scholar] [CrossRef]
- Lamothe, S.; Corbeil, M.-M.; Turgeon, S.L.; Britten, M. Influence of cheese matrix on lipid digestion in a simulated gastro-intestinal environment. Food Funct. 2012, 3, 724–731. [Google Scholar] [CrossRef]
- Michalski, M.C.; Genot, C.; Gayet, C.; Lopez, C.; Fine, F.; Joffre, F.; Vendeuvre, J.L.; Bouvier, J.; Chardigny, J.M.; Raynal-Ljutovac, K. Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Prog. Lipid Res. 2013, 52, 354–373. [Google Scholar] [CrossRef]
- Kerr, B.J.; Kellner, T.A.; Shurson, G.C. Characteristics of lipids and their feeding value in swine diets. J. Anim. Sci. Biotechnol. 2015, 6, 30. [Google Scholar] [CrossRef]
- Huang, C.; Chiba, L.I.; Bergen, W.G. Bioavailability and metabolism of omega-3 polyunsaturated fatty acids in pigs and omega-3 polyunsaturated fatty acid-enriched pork: A review. Livest. Sci. 2021, 243, 104370. [Google Scholar] [CrossRef]
- Neubronner, J.; Schuchardt, J.P.; Kressel, G.; Merkel, M.; von Schacky, C.; Hahn, A. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur. J. Clin. Nutr. 2011, 65, 247–254. [Google Scholar] [CrossRef]
- Ghasemifard, S.; Turchini, G.M.; Sinclair, A.J. Omega-3 long chain fatty acid “bioavailability”: A review of evidence and methodological considerations. Prog. Lipid Res. 2014, 56, 92–108. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations--a comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Wakil, A.; Mir, M.; Mellor, D.D.; Mellor, S.F.; Atkin, S.L. The bioavailability of eicosapentaenoic acid from reconstituted triglyceride fish oil is higher than that obtained from the triglyceride and monoglyceride forms. Asia Pac. J. Clin. Nutr. 2010, 19, 499–505. [Google Scholar]
- Mozaffarian, D.; Lemaitre, R.N.; King, I.B.; Song, X.; Huang, H.; Sacks, F.M.; Rimm, E.B.; Wang, M.; Siscovick, D.S. Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: A cohort study. Ann. Intern. Med. 2013, 158, 515–525. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Laidlaw, M.; Cockerline, C.A.; Rowe, W.J. A randomized clinical trial to determine the efficacy of manufacturers’ recommended doses of omega-3 fatty acids from different sources in facilitating cardiovascular disease risk reduction. Lipids Health Dis. 2014, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.M.; Hallaråker, H.; Sæbø, P.C.; Innis, S.M.; Kelley, K.M.; Sanoshy, K.D.; Berger, A.; Maki, K.C. Bioavailability of long chain omega-3 polyunsaturated fatty acids from phospholipid-rich herring roe oil in men and women with mildly elevated triacylglycerols. Prostaglandins. Leukot. Essent. Fatty Acids 2016, 111, 17–24. [Google Scholar] [CrossRef]
- Yamada, S.; Kamada, N.; Amiya, T.; Nakamoto, N.; Nakaoka, T.; Kimura, M.; Saito, Y.; Ejima, C.; Kanai, T.; Saito, H. Gut microbiota-mediated generation of saturated fatty acids elicits inflammation in the liver in murine high-fat diet-induced steatohepatitis. BMC Gastroenterol. 2017, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Hirabara, S.M.; Curi, R.; Maechler, P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J. Cell. Physiol. 2010, 222, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Csala, M. [Hyper-free fatty acidemia—Insulin resistance and β-cell death]. Orv. Hetil. 2016, 157, 733–739. [Google Scholar] [CrossRef]
- Chaurasia, B.; Summers, S.A. Ceramides—Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol. Metab. 2015, 26, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Capurso, C.; Capurso, A. From excess adiposity to insulin resistance: The role of free fatty acids. Vascul. Pharmacol. 2012, 57, 91–97. [Google Scholar] [CrossRef]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.-Y.; Jun, H.-S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 384. [Google Scholar] [CrossRef]
- Nishi, H.; Higashihara, T.; Inagi, R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients 2019, 11, 1664. [Google Scholar] [CrossRef]
- Tovar, A.R.; Torres, N. The role of dietary protein on lipotoxicity. Biochim. Biophys. Acta 2010, 1801, 367–371. [Google Scholar] [CrossRef]
- Inoue, N.; Nagao, K.; Sakata, K.; Yamano, N.; Gunawardena, P.E.R.; Han, S.-Y.; Matsui, T.; Nakamori, T.; Furuta, H.; Takamatsu, K.; et al. Screening of soy protein-derived hypotriglyceridemic di-peptides in vitro and in vivo. Lipids Health Dis. 2011, 10, 85. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Butterfield, D.A. Brain lipid peroxidation and alzheimer disease: Synergy between the Butterfield and Mattson laboratories. Ageing Res. Rev. 2020, 64, 101049. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Free radicals in Alzheimer’s disease: Lipid peroxidation biomarkers. Clin. Chim. Acta 2019, 491, 85–90. [Google Scholar] [CrossRef]
- Nishiyama, K.; Fujimoto, Y.; Takeuchi, T.; Azuma, Y.-T. Aggressive Crosstalk Between Fatty Acids and Inflammation in Macrophages and Their Influence on Metabolic Homeostasis. Neurochem. Res. 2018, 43, 19–26. [Google Scholar] [CrossRef]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Polyunsaturated fatty acids and inflammation. IUBMB Life 2015, 67, 659–667. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.T.; Jönsson, L.S.; Latulippe, M.E.; Marcos, A.; et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br. J. Nutr. 2013, 109, S1–S34. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin. Pharmacol. 2013, 13, 632–640. [Google Scholar] [CrossRef]
- Bannenberg, G.; Serhan, C.N. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim. Biophys. Acta 2010, 1801, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Curto, E.; Milligan, G. Metabolism meets immunity: The role of free fatty acid receptors in the immune system. Biochem. Pharmacol. 2016, 114, 3–13. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Oh, D.Y.; Walenta, E.; Akiyama, T.E.; Lagakos, W.S.; Lackey, D.; Pessentheiner, A.R.; Sasik, R.; Hah, N.; Chi, T.J.; Cox, J.M.; et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 2014, 20, 942–947. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. The Omega-6:Omega-3 ratio: A critical appraisal and possible successor. Prostaglandins. Leukot. Essent. Fatty Acids 2018, 132, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sibille, K.T.; King, C.; Garrett, T.J.; Glover, T.L.; Zhang, H.; Chen, H.; Reddy, D.; Goodin, B.R.; Sotolongo, A.; Petrov, M.E.; et al. Omega-6: Omega-3 PUFA Ratio, Pain, Functioning, and Distress in Adults With Knee Pain. Clin. J. Pain 2018, 34, 182–189. [Google Scholar] [CrossRef]
- Murphy, A.M.; Lyons, C.L.; Finucane, O.M.; Roche, H.M. Interactions between differential fatty acids and inflammatory stressors-impact on metabolic health. Prostaglandins. Leukot. Essent. Fatty Acids 2015, 92, 49–55. [Google Scholar] [CrossRef]
- Chan, K.L.; Pillon, N.J.; Sivaloganathan, D.M.; Costford, S.R.; Liu, Z.; Théret, M.; Chazaud, B.; Klip, A. Palmitoleate Reverses High Fat-induced Proinflammatory Macrophage Polarization via AMP-activated Protein Kinase (AMPK). J. Biol. Chem. 2015, 290, 16979–16988. [Google Scholar] [CrossRef]
- Hwang, D.H.; Kim, J.-A.; Lee, J.Y. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur. J. Pharmacol. 2016, 785, 24–35. [Google Scholar] [CrossRef]
- Li, B.; Leung, J.C.K.; Chan, L.Y.Y.; Yiu, W.H.; Tang, S.C.W. A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog. Lipid Res. 2020, 77, 101020. [Google Scholar] [CrossRef]
- Suganami, T.; Ogawa, Y. Adipose tissue macrophages: Their role in adipose tissue remodeling. J. Leukoc. Biol. 2010, 88, 33–39. [Google Scholar] [CrossRef]
- Dasu, M.R.; Jialal, I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E145–E154. [Google Scholar] [CrossRef]
- Tsutsui, H.; Imamura, M.; Fujimoto, J.; Nakanishi, K. The TLR4/TRIF-Mediated Activation of NLRP3 Inflammasome Underlies Endotoxin-Induced Liver Injury in Mice. Gastroenterol. Res. Pract. 2010, 2010, 641865. [Google Scholar] [CrossRef] [PubMed]
- Lyons, C.L.; Kennedy, E.B.; Roche, H.M. Metabolic Inflammation-Differential Modulation by Dietary Constituents. Nutrients 2016, 8, 247. [Google Scholar] [CrossRef]
- De Boer, A.A.; Monk, J.M.; Liddle, D.M.; Hutchinson, A.L.; Power, K.A.; Ma, D.W.L.; Robinson, L.E. Fish-oil-derived n-3 polyunsaturated fatty acids reduce NLRP3 inflammasome activity and obesity-related inflammatory cross-talk between adipocytes and CD11b(+) macrophages. J. Nutr. Biochem. 2016, 34, 61–72. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Aoyama, M.; Kotani, J.; Usami, M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 2010, 26, 653–661. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 310–396. [Google Scholar] [CrossRef] [PubMed]
- Mirmonsef, P.; Zariffard, M.R.; Gilbert, D.; Makinde, H.; Landay, A.L.; Spear, G.T. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. Am. J. Reprod. Immunol. 2012, 67, 391–400. [Google Scholar] [CrossRef]
- Mao, L.; Hochstetter, D.; Yao, L.; Zhao, Y.; Zhou, J.; Wang, Y.; Xu, P. Green Tea Polyphenol (-)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2019, 20, 5081. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Zhao, H.; Dong, L.; Zhen, Y.-F.; Xing, H.-Y.; Ma, H.-J.; Song, G.-Y. Resveratrol ameliorates high-fat diet-induced insulin resistance and fatty acid oxidation via ATM-AMPK axis in skeletal muscle. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9117–9125. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, A.; Kishimoto, Y.; Mabashi-Asazuma, H.; Kondo, K.; Iida, K. Gallic Acid Inhibits Lipid Accumulation via AMPK Pathway and Suppresses Apoptosis and Macrophage-Mediated Inflammation in Hepatocytes. Nutrients 2020, 12, 1479. [Google Scholar] [CrossRef]
- Sokolova, M.; Yang, K.; Hansen, S.H.; Louwe, M.C.; Kummen, M.; Hov, J.E.R.; Sjaastad, I.; Berge, R.K.; Halvorsen, B.; Aukrust, P.; et al. NLRP3 inflammasome deficiency attenuates metabolic disturbances involving alterations in the gut microbial profile in mice exposed to high fat diet. Sci. Rep. 2020, 10, 21006. [Google Scholar] [CrossRef]
- Herbert, D.; Franz, S.; Popkova, Y.; Anderegg, U.; Schiller, J.; Schwede, K.; Lorz, A.; Simon, J.C.; Saalbach, A. High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J. Investig. Dermatol. 2018, 138, 1999–2009. [Google Scholar] [CrossRef]
- Hernández, E.Á.; Kahl, S.; Seelig, A.; Begovatz, P.; Irmler, M.; Kupriyanova, Y.; Nowotny, B.; Nowotny, P.; Herder, C.; Barosa, C.; et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J. Clin. Investig. 2017, 127, 695–708. [Google Scholar] [CrossRef]
- Russo, S.B.; Baicu, C.F.; Van Laer, A.; Geng, T.; Kasiganesan, H.; Zile, M.R.; Cowart, L.A. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Investig. 2012, 122, 3919–3930. [Google Scholar] [CrossRef]
- Muthuramu, I.; Amin, R.; Postnov, A.; Mishra, M.; Jacobs, F.; Gheysens, O.; Van Veldhoven, P.P.; De Geest, B. Coconut Oil Aggravates Pressure Overload-Induced Cardiomyopathy without Inducing Obesity, Systemic Insulin Resistance, or Cardiac Steatosis. Int. J. Mol. Sci. 2017, 18, 1565. [Google Scholar] [CrossRef] [PubMed]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010, 219, 25–32. [Google Scholar] [CrossRef]
- Ralston, J.C.; Nguyen-Tu, M.-S.; Lyons, C.L.; Cooke, A.A.; Murphy, A.M.; Falvey, A.; Finucane, O.M.; McGillicuddy, F.C.; Rutter, G.A.; Roche, H.M. Dietary substitution of SFA with MUFA within high-fat diets attenuates hyperinsulinaemia and pancreatic islet dysfunction. Br. J. Nutr. 2020, 124, 247–255. [Google Scholar] [CrossRef] [PubMed]
- McLean, F.H.; Campbell, F.M.; Sergi, D.; Grant, C.; Morris, A.C.; Hay, E.A.; MacKenzie, A.; Mayer, C.D.; Langston, R.F.; Williams, L.M. Early and reversible changes to the hippocampal proteome in mice on a high-fat diet. Nutr. Metab. 2019, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Macartney, M.J.; Peoples, G.E.; McLennan, P.L. Cardiac Arrhythmia Prevention in Ischemia and Reperfusion by Low-Dose Dietary Fish Oil Supplementation in Rats. J. Nutr. 2020, 150, 3086–3093. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, L.; Wen, M.; Che, H.; Du, L.; Wang, J.; Xue, C.; Xu, J.; Wang, Y. Eicosapentaenoic acid-enriched phospholipids improve atherosclerosis by mediating cholesterol metabolism. J. Funct. Foods 2017, 32, 90–97. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Ding, L.; Shi, H.-H.; Xu, J.; Xue, C.-H.; Zhang, T.-T.; Wang, Y.-M. Eicosapentaenoic acid in the form of phospholipids exerts superior anti-atherosclerosis effects to its triglyceride form in ApoE(-/-) mice. Food Funct. 2019, 10, 4177–4188. [Google Scholar] [CrossRef]
- Wang, R.; Chai, Q.; Lu, T.; Lee, H.-C. Activation of vascular BK channels by docosahexaenoic acid is dependent on cytochrome P450 epoxygenase activity. Cardiovasc. Res. 2011, 90, 344–352. [Google Scholar] [CrossRef]
- Börjesson, S.I.; Elinder, F. An electrostatic potassium channel opener targeting the final voltage sensor transition. J. Gen. Physiol. 2011, 137, 563–577. [Google Scholar] [CrossRef]
- Takashima, A.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Nishimoto, S.; Yagi, S.; Yamada, H.; Soeki, T.; Wakatsuki, T.; et al. Combination of n-3 polyunsaturated fatty acids reduces atherogenesis in apolipoprotein E-deficient mice by inhibiting macrophage activation. Atherosclerosis 2016, 254, 142–150. [Google Scholar] [CrossRef]
- Chang, C.L.; Torrejon, C.; Jung, U.J.; Graf, K.; Deckelbaum, R.J. Incremental replacement of saturated fats by n-3 fatty acids in high-fat, high-cholesterol diets reduces elevated plasma lipid levels and arterial lipoprotein lipase, macrophages and atherosclerosis in LDLR-/- mice. Atherosclerosis 2014, 234, 401–409. [Google Scholar] [CrossRef]
- Deyama, S.; Ishikawa, Y.; Yoshikawa, K.; Shimoda, K.; Ide, S.; Satoh, M.; Minami, M. Resolvin D1 and D2 Reverse Lipopolysaccharide-Induced Depression-Like Behaviors Through the mTORC1 Signaling Pathway. Int. J. Neuropsychopharmacol. 2017, 20, 575–584. [Google Scholar] [CrossRef]
- Wen, M.; Xu, J.; Ding, L.; Zhang, L.; Du, L.; Wang, J.; Wang, Y.; Xue, C. Eicosapentaenoic acid-enriched phospholipids improve Aβ1–40-induced cognitive deficiency in a rat model of Alzheimer’s disease. J. Funct. Foods 2016, 24, 537–548. [Google Scholar] [CrossRef]
- Liao, K.; Yan, J.; Mai, K.; Ai, Q. Dietary Olive and Perilla Oils Affect Liver Mitochondrial DNA Methylation in Large Yellow Croakers. J. Nutr. 2015, 145, 2479–2485. [Google Scholar] [CrossRef]
- Boddicker, R.L.; Koltes, J.E.; Fritz-Waters, E.R.; Koesterke, L.; Weeks, N.; Yin, T.; Mani, V.; Nettleton, D.; Reecy, J.M.; Baumgard, L.H.; et al. Genome-wide methylation profile following prenatal and postnatal dietary omega-3 fatty acid supplementation in pigs. Anim. Genet. 2016, 47, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wen, J.; Chen, G.; Ge, M.; Gao, Y.; Ye, X.; Liu, C.; Cai, C. Omega-3 Polyunsaturated Fatty Acids Inhibited Tumor Growth via Preventing the Decrease of Genomic DNA Methylation in Colorectal Cancer Rats. Nutr. Cancer 2016, 68, 113–119. [Google Scholar] [CrossRef]
- Lomba, A.; Martínez, J.A.; García-Díaz, D.F.; Paternain, L.; Marti, A.; Campión, J.; Milagro, F.I. Weight gain induced by an isocaloric pair-fed high fat diet: A nutriepigenetic study on FASN and NDUFB6 gene promoters. Mol. Genet. Metab. 2010, 101, 273–278. [Google Scholar] [CrossRef]
- Samblas, M.; Carraro, J.C.; Martínez, J.A.; Milagro, F.I. The regulation of inflammation-related genes after palmitic acid and DHA treatments is not mediated by DNA methylation. J. Physiol. Biochem. 2019, 75, 341–349. [Google Scholar] [CrossRef]
- Cordero, P.; Campion, J.; Milagro, F.I.; Martinez, J.A. Transcriptomic and epigenetic changes in early liver steatosis associated to obesity: Effect of dietary methyl donor supplementation. Mol. Genet. Metab. 2013, 110, 388–395. [Google Scholar] [CrossRef]
- Uriarte, G.; Paternain, L.; Milagro, F.I.; Martínez, J.A.; Campion, J. Shifting to a control diet after a high-fat, high-sucrose diet intake induces epigenetic changes in retroperitoneal adipocytes of Wistar rats. J. Physiol. Biochem. 2013, 69, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Lottenberg, A.M.; Afonso, M.d.S.; Lavrador, M.S.F.; Machado, R.M.; Nakandakare, E.R. The role of dietary fatty acids in the pathology of metabolic syndrome. J. Nutr. Biochem. 2012, 23, 1027–1040. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, M.; Serna, D.S. Nutrigenomics of ω-3 fatty acids: Regulators of the master transcription factors. Nutrition 2017, 41, 90–96. [Google Scholar] [CrossRef]
- Naeini, Z.; Toupchian, O.; Vatannejad, A.; Sotoudeh, G.; Teimouri, M.; Ghorbani, M.; Nasli-Esfahani, E.; Koohdani, F. Effects of DHA-enriched fish oil on gene expression levels of p53 and NF-κB and PPAR-γ activity in PBMCs of patients with T2DM: A randomized, double-blind, clinical trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 441–447. [Google Scholar] [CrossRef]
- Jamilian, M.; Tabassi, Z.; Reiner, Ž.; Panahandeh, I.; Naderi, F.; Aghadavod, E.; Amirani, E.; Taghizadeh, M.; Shafabakhsh, R.; Satari, M.; et al. The effects of n-3 fatty acids from flaxseed oil on genetic and metabolic profiles in patients with gestational diabetes mellitus: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2020, 123, 792–799. [Google Scholar] [CrossRef]
- Golpour, P.; Nourbakhsh, M.; Mazaherioun, M.; Janani, L.; Nourbakhsh, M.; Yaghmaei, P. Improvement of NRF2 gene expression and antioxidant status in patients with type 2 diabetes mellitus after supplementation with omega-3 polyunsaturated fatty acids: A double-blind randomised placebo-controlled clinical trial. Diabetes Res. Clin. Pract. 2020, 162, 108120. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Taghizadeh, M.; Aghadavod, E.; Mafi, A.; Dadgostar, E.; Daneshvar Kakhaki, R.; Abolhassani, J.; Asemi, Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Neurol. Neurosurg. 2019, 176, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Samimi, M.; Mirhosseini, N.; Afshar Ebrahimi, F.; Aghadavod, E.; Talaee, R.; Jafarnejad, S.; Hashemi Dizaji, S.; Asemi, Z. The influences of vitamin D and omega-3 co-supplementation on clinical, metabolic and genetic parameters in women with polycystic ovary syndrome. J. Affect. Disord. 2018, 238, 32–38. [Google Scholar] [CrossRef]
- Larsen, S.V.; Holven, K.B.; Ottestad, I.; Dagsland, K.N.; Myhrstad, M.C.W.; Ulven, S.M. Plasma fatty acid levels and gene expression related to lipid metabolism in peripheral blood mononuclear cells: A cross-sectional study in healthy subjects. Genes Nutr. 2018, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Matualatupauw, J.C.; Bohl, M.; Gregersen, S.; Hermansen, K.; Afman, L.A. Dietary medium-chain saturated fatty acids induce gene expression of energy metabolism-related pathways in adipose tissue of abdominally obese subjects. Int. J. Obes. 2017, 41, 1348–1354. [Google Scholar] [CrossRef]
- Ulven, S.M.; Christensen, J.J.; Nygård, O.; Svardal, A.; Leder, L.; Ottestad, I.; Lysne, V.; Laupsa-Borge, J.; Ueland, P.M.; Midttun, Ø.; et al. Using metabolic profiling and gene expression analyses to explore molecular effects of replacing saturated fat with polyunsaturated fat-a randomized controlled dietary intervention study. Am. J. Clin. Nutr. 2019, 109, 1239–1250. [Google Scholar] [CrossRef]
- Castañer, O.; Corella, D.; Covas, M.-I.; Sorlí, J.V.; Subirana, I.; Flores-Mateo, G.; Nonell, L.; Bulló, M.; de la Torre, R.; Portolés, O.; et al. In vivo transcriptomic profile after a Mediterranean diet in high-cardiovascular risk patients: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 98, 845–853. [Google Scholar] [CrossRef]
- Camargo, A.; Delgado-Lista, J.; Garcia-Rios, A.; Cruz-Teno, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Gutierrez-Mariscal, F.M.; Lora-Aguilar, P.; Rodriguez-Cantalejo, F.; Fuentes-Jimenez, F.; et al. Expression of proinflammatory, proatherogenic genes is reduced by the Mediterranean diet in elderly people. Br. J. Nutr. 2012, 108, 500–508. [Google Scholar] [CrossRef]
- Perrone, M.A.; Gualtieri, P.; Gratteri, S.; Ali, W.; Sergi, D.; Muscoli, S.; Cammarano, A.; Bernardini, S.; Di Renzo, L.; Romeo, F. Effects of postprandial hydroxytyrosol and derivates on oxidation of LDL, cardiometabolic state and gene expression: A nutrigenomic approach for cardiovascular prevention. J. Cardiovasc. Med. 2019, 20, 419–426. [Google Scholar] [CrossRef]
- Farràs, M.; Arranz, S.; Carrión, S.; Subirana, I.; Muñoz-Aguayo, D.; Blanchart, G.; Kool, M.; Solà, R.; Motilva, M.J.; Escolà-Gil, J.C.; et al. A Functional Virgin Olive Oil Enriched with Olive Oil and Thyme Phenolic Compounds Improves the Expression of Cholesterol Efflux-Related Genes: A Randomized, Crossover, Controlled Trial. Nutrients 2019, 11, 1732. [Google Scholar] [CrossRef]
- Arpón, A.; Milagro, F.I.; Razquin, C.; Corella, D.; Estruch, R.; Fitó, M.; Marti, A.; Martínez-González, M.A.; Ros, E.; Salas-Salvadó, J.; et al. Impact of Consuming Extra-Virgin Olive Oil or Nuts within a Mediterranean Diet on DNA Methylation in Peripheral White Blood Cells within the PREDIMED-Navarra Randomized Controlled Trial: A Role for Dietary Lipids. Nutrients 2017, 10, 15. [Google Scholar] [CrossRef]
- González-Becerra, K.; Ramos-Lopez, O.; Barrón-Cabrera, E.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez-López, E.; Martínez, J.A. Fatty acids, epigenetic mechanisms and chronic diseases: A systematic review. Lipids Health Dis. 2019, 18, 178. [Google Scholar] [CrossRef]
- Ghadge, A.A.; Khaire, A.A.; Kuvalekar, A.A. Adiponectin: A potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018, 39, 151–158. [Google Scholar] [CrossRef]
- Maintinguer Norde, M.; Oki, É.; de Castro, I.A.; Pacheco Souza, J.M.; Teixeira Damasceno, N.R.; Mara Fisberg, R.; Lobo Marchioni, D.M.; Macedo Rogero, M. Influence of adiponectin gene variants and plasma fatty acids on systemic inflammation state association-A cross-sectional population-based study, São Paulo, Brazil. Mol. Nutr. Food Res. 2016, 60, 278–286. [Google Scholar] [CrossRef]
- Alsaleh, A.; Crepostnaia, D.; Maniou, Z.; Lewis, F.J.; Hall, W.L.; Sanders, T.A.B.; O’Dell, S.D. Adiponectin gene variant interacts with fish oil supplementation to influence serum adiponectin in older individuals. J. Nutr. 2013, 143, 1021–1027. [Google Scholar] [CrossRef]
- AlSaleh, A.; O’Dell, S.D.; Frost, G.S.; Griffin, B.A.; Lovegrove, J.A.; Jebb, S.A.; Sanders, T.A.B. Single nucleotide polymorphisms at the ADIPOQ gene locus interact with age and dietary intake of fat to determine serum adiponectin in subjects at risk of the metabolic syndrome. Am. J. Clin. Nutr. 2011, 94, 262–269. [Google Scholar] [CrossRef]
- Marais, A.D. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology 2019, 51, 165–176. [Google Scholar] [CrossRef]
- Ordovas, J.M.; Corella, D. Genetic variation and lipid metabolism: Modulation by dietary factors. Curr. Cardiol. Rep. 2005, 7, 480–486. [Google Scholar] [CrossRef]
- Corella, D.; Ordovás, J.M. Aging and cardiovascular diseases: The role of gene-diet interactions. Ageing Res. Rev. 2014, 18, 53–73. [Google Scholar] [CrossRef]
- Fallaize, R.; Celis-Morales, C.; Macready, A.L.; Marsaux, C.F.; Forster, H.; O’Donovan, C.; Woolhead, C.; San-Cristobal, R.; Kolossa, S.; Hallmann, J.; et al. The effect of the apolipoprotein E genotype on response to personalized dietary advice intervention: Findings from the Food4Me randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 827–836. [Google Scholar] [CrossRef]
- Carvalho-Wells, A.L.; Jackson, K.G.; Lockyer, S.; Lovegrove, J.A.; Minihane, A.M. APOE genotype influences triglyceride and C-reactive protein responses to altered dietary fat intake in UK adults. Am. J. Clin. Nutr. 2012, 96, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, K.M.; Weech, M.; Jackson, K.G.; Lovegrove, J.A. Impact of the Apolipoprotein E (epsilon) Genotype on Cardiometabolic Risk Markers and Responsiveness to Acute and Chronic Dietary Fat Manipulation. Nutrients 2019, 11, 2044. [Google Scholar] [CrossRef]
- Kettunen, T.; Eklund, C.; Kähönen, M.; Jula, A.; Päivä, H.; Lyytikäinen, L.-P.; Hurme, M.; Lehtimäki, T. Polymorphism in the C-reactive protein (CRP) gene affects CRP levels in plasma and one early marker of atherosclerosis in men: The Health 2000 Survey. Scand. J. Clin. Lab. Investig. 2011, 71, 353–361. [Google Scholar] [CrossRef]
- Nienaber-Rousseau, C.; Swanepoel, B.; Dolman, R.C.; Pieters, M.; Conradie, K.R.; Towers, G.W. Interactions between C-reactive protein genotypes with markers of nutritional status in relation to inflammation. Nutrients 2014, 6, 5034–5050. [Google Scholar] [CrossRef] [PubMed]
- Arouca, A.B.; Meirhaeghe, A.; Dallongeville, J.; Moreno, L.A.; Lourenço, G.J.; Marcos, A.; Huybrechts, I.; Manios, Y.; Lambrinou, C.-P.; Gottrand, F.; et al. Interplay between the Mediterranean diet and C-reactive protein genetic polymorphisms towards inflammation in adolescents. Clin. Nutr. 2020, 39, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Kiefte-de Jong, J.C.; Hofman, A.; Dehghan, A.; Rivadeneira, F.; Franco, O.H. Polyunsaturated fatty acids and serum C-reactive protein: The Rotterdam study. Am. J. Epidemiol. 2015, 181, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Oki, E.; Norde, M.M.; Carioca, A.A.F.; Ikeda, R.E.; Souza, J.M.P.; Castro, I.A.; Marchioni, D.M.L.; Fisberg, R.M.; Rogero, M.M. Interaction of SNP in the CRP gene and plasma fatty acid profile in inflammatory pattern: A cross-sectional population-based study. Nutrition 2016, 32, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Reischl, E.; Tanjung, C.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Meldrum, S.; Simmer, K.; Heinrich, J.; Demmelmair, H. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid Metabolism and Dietary Impact on Health. Annu. Rev. Nutr. 2019, 39, 21–44. [Google Scholar] [CrossRef]
- Brayner, B.; Kaur, G.; Keske, M.A.; Livingstone, K.M. FADS Polymorphism, Omega-3 Fatty Acids and Diabetes Risk: A Systematic Review. Nutrients 2018, 10, 758. [Google Scholar] [CrossRef]
- Ameur, A.; Enroth, S.; Johansson, A.; Zaboli, G.; Igl, W.; Johansson, A.C.V.; Rivas, M.A.; Daly, M.J.; Schmitz, G.; Hicks, A.A.; et al. Genetic adaptation of fatty-acid metabolism: A human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am. J. Hum. Genet. 2012, 90, 809–820. [Google Scholar] [CrossRef]
- Hester, A.G.; Murphy, R.C.; Uhlson, C.J.; Ivester, P.; Lee, T.C.; Sergeant, S.; Miller, L.R.; Howard, T.D.; Mathias, R.A.; Chilton, F.H. Relationship between a common variant in the fatty acid desaturase (FADS) cluster and eicosanoid generation in humans. J. Biol. Chem. 2014, 289, 22482–22489. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.Y.; Lim, H.H.; Yang, L.I.; Chae, J.S.; Lee, J.H. Fatty acid desaturase (FADS) gene polymorphisms and insulin resistance in association with serum phospholipid polyunsaturated fatty acid composition in healthy Korean men: Cross-sectional study. Nutr. Metab. 2011, 8, 24. [Google Scholar] [CrossRef]
- Klingel, S.L.; Valsesia, A.; Astrup, A.; Kunesova, M.; Saris, W.H.M.; Langin, D.; Viguerie, N.; Mutch, D.M. FADS1 genotype is distinguished by human subcutaneous adipose tissue fatty acids, but not inflammatory gene expression. Int. J. Obes. 2019, 43, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, M.A.; Fauland, A.; Shimizu, B.-I.; Ågren, J.; Wheelock, C.E.; Laakso, M.; Schwab, U.; Pihlajamäki, J. Inflammatory response to dietary linoleic acid depends on FADS1 genotype. Am. J. Clin. Nutr. 2019, 109, 165–175. [Google Scholar] [CrossRef]
- Mullins, V.A.; Bresette, W.; Johnstone, L.; Hallmark, B.; Chilton, F.H. Genomics in Personalized Nutrition: Can You “Eat for Your Genes”? Nutrients 2020, 12, 3118. [Google Scholar] [CrossRef]
- Rudkowska, I.; Guénard, F.; Julien, P.; Couture, P.; Lemieux, S.; Barbier, O.; Calder, P.C.; Minihane, A.M.; Vohl, M.-C. Genome-wide association study of the plasma triglyceride response to an n-3 polyunsaturated fatty acid supplementation. J. Lipid Res. 2014, 55, 1245–1253. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Milagro, F.I.; Allayee, H.; Chmurzynska, A.; Choi, M.S.; Curi, R.; De Caterina, R.; Ferguson, L.R.; Goni, L.; Kang, J.X.; et al. Guide for Current Nutrigenetic, Nutrigenomic, and Nutriepigenetic Approaches for Precision Nutrition Involving the Prevention and Management of Chronic Diseases Associated with Obesity. J. Nutrigenet. Nutr. 2017, 10, 43–62. [Google Scholar] [CrossRef]
- Ferguson, L.R.; De Caterina, R.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Choi, M.S.; Curi, R.; de Luis, D.A.; Gil, Á.; et al. Guide and Position of the International Society of Nutrigenetics/Nutrigenomics on Personalised Nutrition: Part 1—Fields of Precision Nutrition. J. Nutrigenet. Nutr. 2016, 9, 12–27. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe 2019, 26, 314–324. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Agans, R.; Gordon, A.; Kramer, D.L.; Perez-Burillo, S.; Rufián-Henares, J.A.; Paliy, O. Dietary Fatty Acids Sustain the Growth of the Human Gut Microbiota. Appl. Environ. Microbiol. 2018, 84, e01525-18. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Yoon, B.K.; Li, D.; Cho, N.-J. Nanotechnology Formulations for Antibacterial Free Fatty Acids and Monoglycerides. Molecules 2016, 21, 305. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Rock, C.O. Exogenous fatty acid metabolism in bacteria. Biochimie 2017, 141, 30–39. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5, 5-3. [Google Scholar] [CrossRef]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.-L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef]
- Kim, K.-A.; Gu, W.; Lee, I.-A.; Joh, E.-H.; Kim, D.-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.Y.; Ha, C.W.Y.; Hoffmann, J.M.A.; Oscarsson, J.; Dinudom, A.; Mather, T.J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity 2015, 23, 1429–1439. [Google Scholar] [CrossRef]
- Huang, E.Y.; Leone, V.A.; Devkota, S.; Wang, Y.; Brady, M.J.; Chang, E.B. Composition of dietary fat source shapes gut microbiota architecture and alters host inflammatory mediators in mouse adipose tissue. JPEN J. Parenter. Enter. Nutr. 2013, 37, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Djurasevic, S.; Bojic, S.; Nikolic, B.; Dimkic, I.; Todorovic, Z.; Djordjevic, J.; Mitic-Culafic, D. Beneficial Effect of Virgin Coconut Oil on Alloxan-Induced Diabetes and Microbiota Composition in Rats. Plant Foods Hum. Nutr. 2018, 73, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, Y.; Jacoby, J.J.; Jiang, Y.; Zhang, Y.; Yu, L.L. Effects of Medium- and Long-Chain Triacylglycerols on Lipid Metabolism and Gut Microbiota Composition in C57BL/6J Mice. J. Agric. Food Chem. 2017, 65, 6599–6607. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, W.; Tao, H.; Zhang, Y.; Liu, L.; Liu, Z.; Qiu, B.; Xu, T. Effect of industrial trans-fatty acids-enriched diet on gut microbiota of C57BL/6 mice. Eur. J. Nutr. 2019, 58, 2625–2638. [Google Scholar] [CrossRef]
- Hua, Y.; Fan, R.; Zhao, L.; Tong, C.; Qian, X.; Zhang, M.; Xiao, R.; Ma, W. Trans-fatty acids alter the gut microbiota in high-fat-diet-induced obese rats. Br. J. Nutr. 2020, 124, 1251–1263. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; O’ Doherty, R.M.; Murphy, E.F.; Wall, R.; O’ Sullivan, O.; Nilaweera, K.; Fitzgerald, G.F.; Cotter, P.D.; Ross, R.P.; Stanton, C. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br. J. Nutr. 2014, 111, 1905–1917. [Google Scholar] [CrossRef]
- Yu, H.-N.; Zhu, J.; Pan, W.; Shen, S.-R.; Shan, W.-G.; Das, U.N. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch. Med. Res. 2014, 45, 195–202. [Google Scholar] [CrossRef]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef]
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Paul Ross, R.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain. Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef]
- Patrone, V.; Ferrari, S.; Lizier, M.; Lucchini, F.; Minuti, A.; Tondelli, B.; Trevisi, E.; Rossi, F.; Callegari, M.L. Short-term modifications in the distal gut microbiota of weaning mice induced by a high-fat diet. Microbiology 2012, 158, 983–992. [Google Scholar] [CrossRef]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Milagro, F.I.; Martinez, J.A. Shifts in microbiota species and fermentation products in a dietary model enriched in fat and sucrose. Benef. Microbes 2015, 6, 97–111. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef]
- Mazidi, M.; Katsiki, N.; Shekoohi, N.; Banach, M. Monounsaturated Fatty Acid Levels May Not Affect Cardiovascular Events: Results From a Mendelian Randomization Analysis. Front. Nutr. 2020, 7, 123. [Google Scholar] [CrossRef]
- Parchem, K.; Sasson, S.; Ferreri, C.; Bartoszek, A. Qualitative analysis of phospholipids and their oxidised derivatives—Used techniques and examples of their applications related to lipidomic research and food analysis. Free Radic. Res. 2019, 53, 1068–1100. [Google Scholar] [CrossRef]

| Fatty Acids | Pro-Inflammatory Effect | Anti-Inflammatory Effect | Other Effects | References |
|---|---|---|---|---|
| SFAs | TLR2/TLR4 signaling pathways | [96,97,98] | ||
| MyD88-dependent NF-κB and MAPK activation | [97] | |||
| IL-1α, IL-1β, IL-6, IL-8, IL-12, TNFα, IFNγ release | ||||
| MyD88-independent IRF3, and NF-κB activation | ||||
| NADPH oxidase activation and ROS release | [99] | |||
| NLRP3 inflammasome assembly and activation | [100,101] | |||
| Reduced pro-inflammatory response in combination with polyphenols (i.e., epigallocatechin gallate, resveratrol) | [110,111,112] | |||
| SCFAs | GPR41/GPR43-mediated signaling pathways | [103] | ||
| Inhibition of NF-κB activation | [104] | |||
| Anti-inflammatory IL-10 release via HDACs inhibition | [105,106,107] | |||
| GPCRs-mediated inflammatory responses | [108,109] | |||
| MUFAs | Downregulation of IL-1β and IL-18 expression via NLRP3 inflammasome inhibition | [7] | ||
| AMPK-mediated anti-inflammatory response | [95] | |||
| Do not activate TLR2/TLR4 signaling pathways | [96] | |||
| ω-6 PUFAs | ARA-derived eicosanoids, such as HPETE, PG, TX, LT, and lipoxins, induce inflammatory response via GPCRs | [81] | ||
| Promote obesity, T2DM, arthritis | [91,92,93] | |||
| ω-3 UFAs | EPA/DHA-derived eicosanoids, such as resolvins, protectins, and maresins, induce a milder inflammatory response and accelerate resolution of inflammation | [82,83,84,85] | ||
| NF-κB signaling suppression via PPAR-γ activation, impairment of TLRs activation, and GPR40 and GPR120-mediated anti-inflammatory cascade | [86,87,88,89,90] | |||
| NLRP3 inflammasome inhibition | [102] | |||
| Contrast obesity, type 2 diabetes, arthritis | [91,92,93] |
| Fatty Acids | Pro-Inflammatory Effect | Anti-Inflammatory Effect | Other Effects | References |
|---|---|---|---|---|
| PO (2 g/kg body weight) | increase LOX and insulin resistance | [115] | ||
| HFD (29.64% SFAs, and 4.86% PUFAs) | IL-1β, IL-6, TNF-α, OP, Cox2, SA8, SA9, CXCL1, CCL3 | [114] | ||
| SFAs (99.8% fat) | induce cardiac hypertrophy, left ventricular systolic, and diastolic dysfunction, and autophagy | [116] | ||
| SFAs (0.2% cholesterol and 10% CO) | reduce oxidative stress and myocardial fibrosis | [117] | ||
| HFD (60% pork lard) | IL-6, TNF-α, MCP-1 | disrupt cognition | [118] | |
| HFD (60% kcal) | metabolism, cellular stress responses, cyto-skeletal organization, cell signaling, and the immune system | [120] | ||
| MUFA-HFD (45% kcal (OA)) | NLRP3, IL-1β, IL-18 | improve insulin sensitivity | [7] | |
| MUFA-HFD (45% kcal sunflower oil) | IL-1β, IL-6 | attenuate hyperinsulinemia | [119] | |
| PUFAs (0.31% or 1.25% of DHA) | reduce heart rate and arrhythmia vulnerability | [121] | ||
| DHA-PL, EPA-PL (1% dietary DHA or EPA incorporated into phospholipids) | TNF-α, IL-6, IL-1β, CD68 | reduce atherosclerotic lesions | [122] | |
| EPA-PL (1% EPA-PL) | CRP, TNF-α, IL-6, MCP-1 | regulate cholesterolmetabolism | [123] | |
| resolvin D1 (10 ng) and D2 (10 ng) | antidepressant, activation of mTORC1 signaling | [128] | ||
| EPA-PL (150 or 300 mg/kg body weight) | CD11b, GFAP, IL-1β, TNF-α | alleviate oxidative stress, and hyper-phosphorylated tau | [129] | |
| ω-3 PUFAs (12% fish iol) | mtDNA methylation | [130] | ||
| ω-3 PUFAs (0.5% EPA and DHA) | DNA methylation | [131] | ||
| ω-3 PUFAs (1 g/kg body weight) | DNA methylation | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordoni, L.; Petracci, I.; Zhao, F.; Min, W.; Pierella, E.; Assmann, T.S.; Martinez, J.A.; Gabbianelli, R. Nutrigenomics of Dietary Lipids. Antioxidants 2021, 10, 994. https://doi.org/10.3390/antiox10070994
Bordoni L, Petracci I, Zhao F, Min W, Pierella E, Assmann TS, Martinez JA, Gabbianelli R. Nutrigenomics of Dietary Lipids. Antioxidants. 2021; 10(7):994. https://doi.org/10.3390/antiox10070994
Chicago/Turabian StyleBordoni, Laura, Irene Petracci, Fanrui Zhao, Weihong Min, Elisa Pierella, Taís Silveira Assmann, J Alfredo Martinez, and Rosita Gabbianelli. 2021. "Nutrigenomics of Dietary Lipids" Antioxidants 10, no. 7: 994. https://doi.org/10.3390/antiox10070994
APA StyleBordoni, L., Petracci, I., Zhao, F., Min, W., Pierella, E., Assmann, T. S., Martinez, J. A., & Gabbianelli, R. (2021). Nutrigenomics of Dietary Lipids. Antioxidants, 10(7), 994. https://doi.org/10.3390/antiox10070994








