Antioxidant Activity of Hemp (Cannabis sativa L.) Seed Oil in Drosophila melanogaster Larvae under Non-Stress and H2O2-Induced Oxidative Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Chemical Analysis
2.2.1. NMR
2.2.2. GC-MS Analyses
2.3. In Vivo Analysis of the Effects of Hemp Seed Oil on Oxidative Stress Markers and the Life Cycle of D. melanogaster
2.3.1. D. melanogaster Strain and Culture Maintenance
2.3.2. Experimental Design
2.3.3. Oxidative Stress Markers
MDA Assay
Glutathione Assay
Antioxidant Defense Enzymes: Superoxide Dismutase and Catalase
2.3.4. D. melanogaster Life Cycle
2.4. Statistical Analysis
3. Results
3.1. Chemical Analysis of Hemp Seed Oil
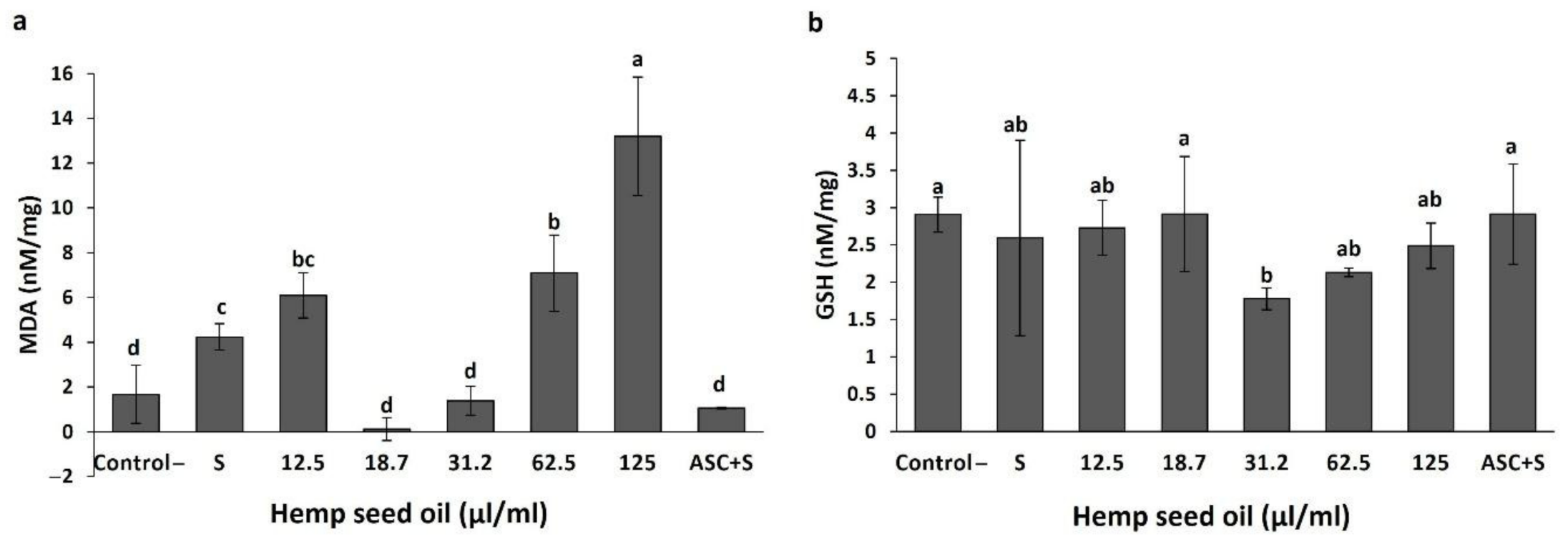
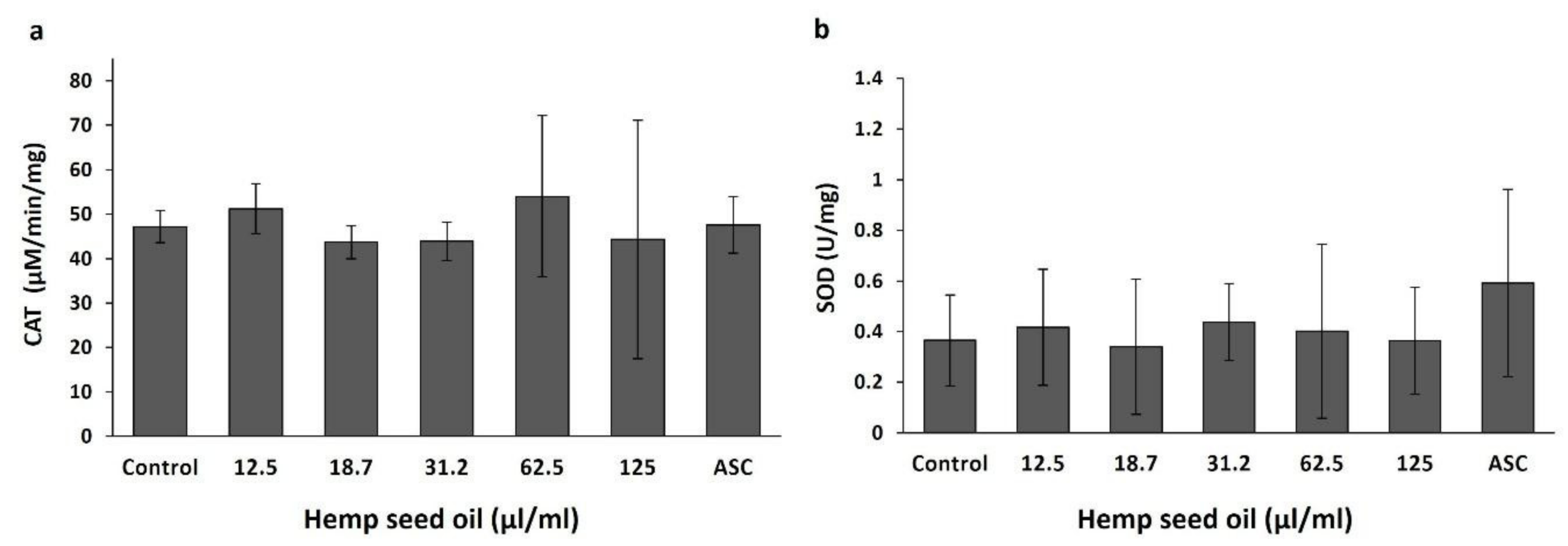
3.2. Effects of Hemp Seed Oil on Oxidative Stress Markers in D. melanogaster Larvae-Protective Function
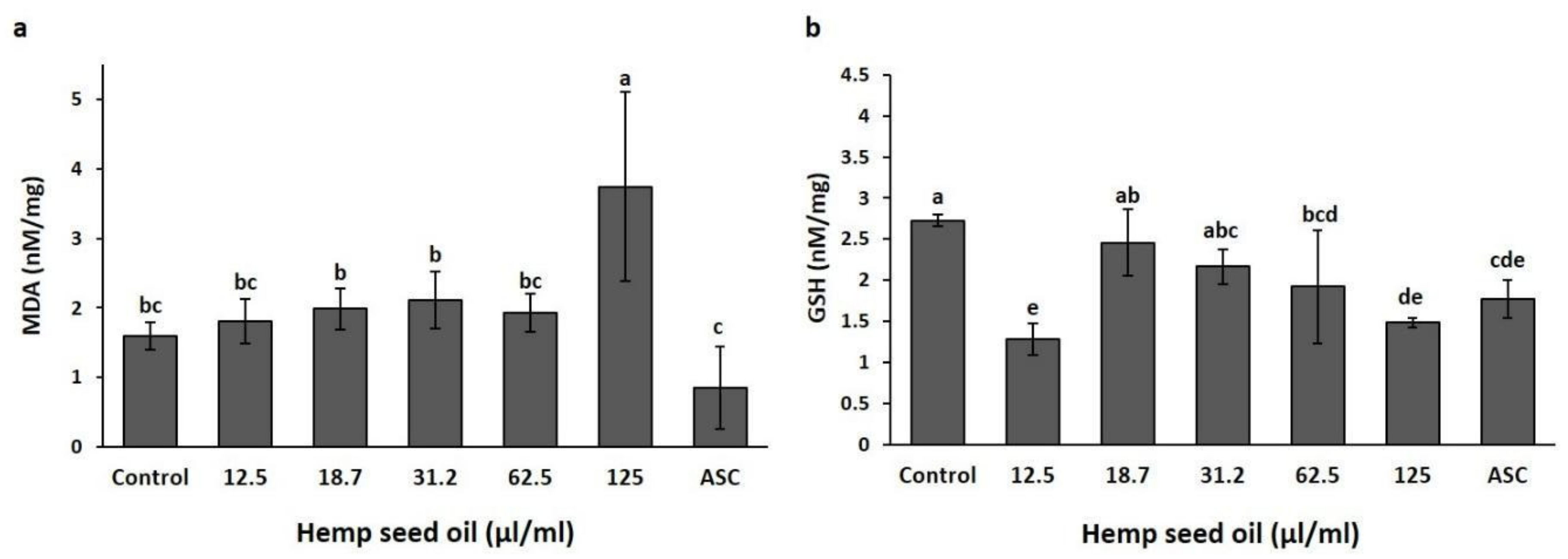
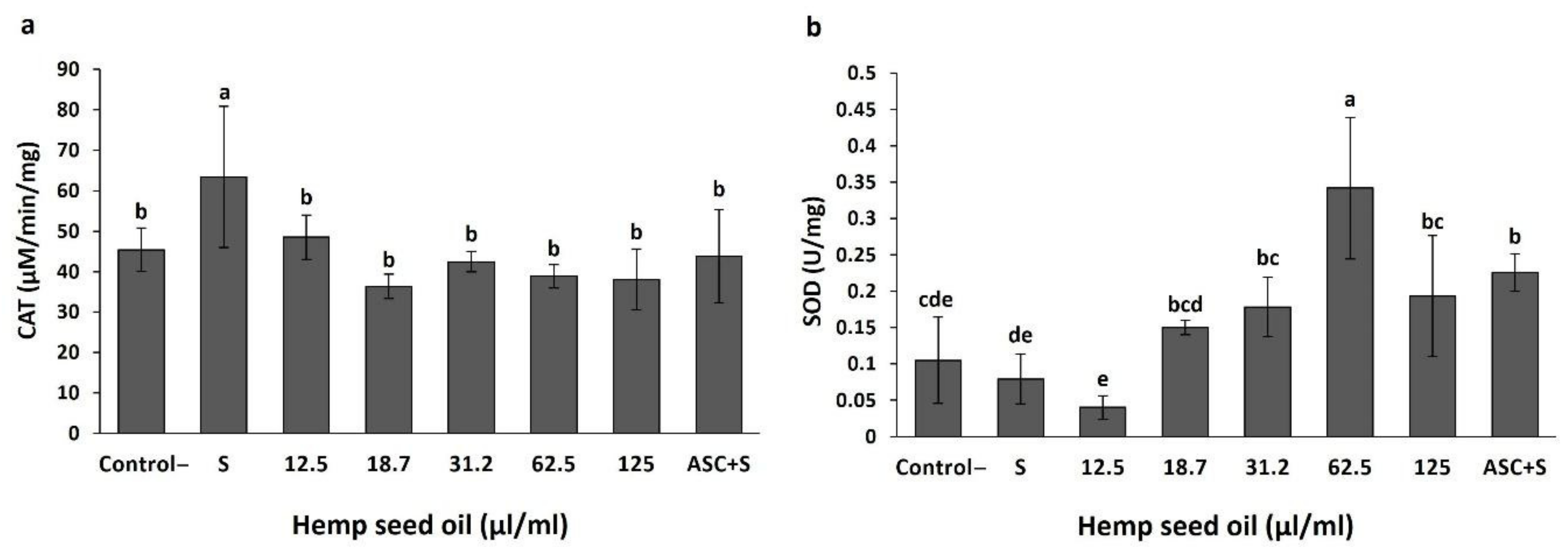
3.3. Antioxidant Activity of Hemp Seed Oil under H2O2-Induced Stress in D. melanogaster Larvae
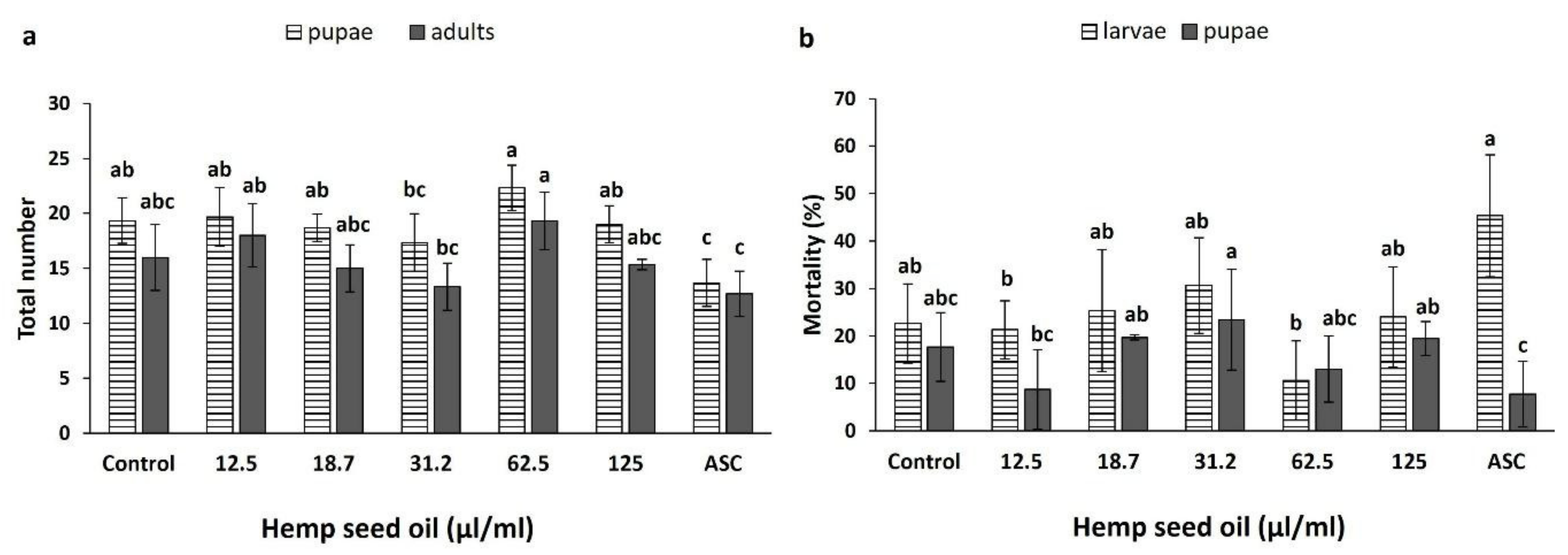
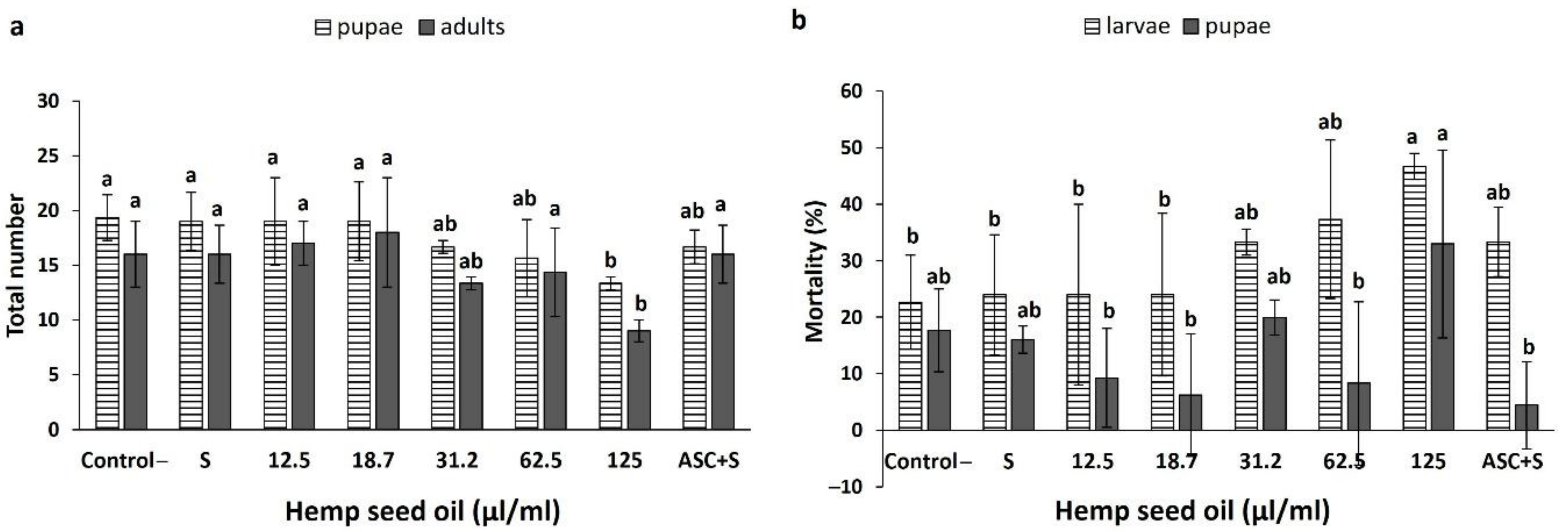
3.4. Effects of Hemp Seed Oil on the Life Cycle of D. melanogaster under Non-Stress and H2O2-Induced Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carus, M.; Sarmento, L. The European Hemp Industry: Cultivation, Processing and Applications for Fibres, Shivs, Seeds and Flowers; European Industrial Hemp Association: Brussels, Belgium, 2017; pp. 1–9. [Google Scholar]
- Ross, S.; ElSohly, M. Constituents of Cannabis sativa L. XXVIII—A review of the natural constituents: 1980–1994. Zagazig J. Pharm. Sci. 1995, 4, 1–10. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Elsohly, M.A.; Boeren, E.G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980, 43, 169–234. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Leizer, C.; Ribnicky, D.; Poulev, A.; Dushenkov, S.; Raskin, I. The Composition of Hemp Seed Oil and Its Potential as an Important Source of Nutrition. J. Nutraceuticals Funct. Med. Foods 2000, 2, 35–53. [Google Scholar] [CrossRef]
- Ren, J.; Chung, S.H. Anti-inflammatory effect of α-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-κB and mitogen-activated protein kinase pathways. J. Agric. Food Chem. 2007, 55, 5073–5080. [Google Scholar] [CrossRef]
- Kelley, D. Immunomodulatory Effects of Flaxseed and Other Oils Rich in α-Linolenic Acid. In Flaxseed in Human Nutrition; Cunnane, S.C., Thompson, L.U., Eds.; AOCS Press: Champaign, IL, USA, 1995; pp. 147–163. [Google Scholar]
- Callaway, J.; Schwab, U.; Harvima, I.; Halonen, P.; Mykkänen, O.; Hyvönen, P.; Järvinen, T. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J. Dermatol. Treat. 2005, 16, 87–94. [Google Scholar] [CrossRef]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Singh, A.P.; Fathordoobady, F.; Guo, Y.; Singh, A.; Kitts, D.D. Antioxidants help favorably regulate the kinetics of lipid peroxidation, polyunsaturated fatty acids degradation and acidic cannabinoids decarboxylation in hempseed oil. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Compr. Rev. Food Sci. Food Saf. 2019, 19, 282–308. [Google Scholar] [CrossRef]
- Liang, J.; Aachary, A.A.; Thiyam-Holländer, U. Hemp seed oil: Minor components and oil quality. Lipid Technol. 2015, 27, 231–233. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J. Mitochondrial Antioxidants and the Maintenance of Cellular Hydrogen Peroxide Levels. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Kaushal, N.; Dhadwal, S.; Kaur, P. Ameliorative effects of hempseed (Cannabis sativa) against hypercholesterolemia associated cardiovascular changes. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 330–338. [Google Scholar] [CrossRef]
- Girgih, A.T.; Alashi, A.M.; He, R.; Malomo, S.A.; Raj, P.; Netticadan, T.; Aluko, R.E. A Novel Hemp Seed Meal Protein Hydrolysate Reduces Oxidative Stress Factors in Spontaneously Hypertensive Rats. Nutrients 2014, 6, 5652–5666. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Liu, Y.-H.; Wang, B.; Chen, C.-Y.; Zhang, H.-M.; Kang, J.X. Identification of a sustainable two-plant diet that effectively prevents age-related metabolic syndrome and extends lifespan in aged mice. J. Nutr. Biochem. 2018, 51, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Jurgoński, A.; Opyd, P.M.; Fotschki, B. Effects of native or partially defatted hemp seeds on hindgut function, antioxidant status and lipid metabolism in diet-induced obese rats. J. Funct. Foods 2020, 72, 104071. [Google Scholar] [CrossRef]
- Smeriglio, A.; Galati, E.M.; Monforte, M.T.; Lanuzza, F.; D’Angelo, V.; Circosta, C. Polyphenolic Compounds and Antioxidant Activity of Cold-Pressed Seed Oil from Finola Cultivar of Cannabis sativa L. Phytother. Res. 2016, 30, 1298–1307. [Google Scholar] [CrossRef]
- Kenari, R.E.; Dehghan, B. Optimization of ultrasound-assisted solvent extraction of hemp (Cannabis sativa L.) seed oil using RSM: Evaluation of oxidative stability and physicochemical properties of oil. Food Sci. Nutr. 2020, 8, 4976–4986. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhou, K.K.; Parry, J. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem. 2005, 91, 723–729. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Moersel, J.-T. Screening of the antiradical action of vegetable oils. J. Food Compos. Anal. 2006, 19, 838–842. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, M.; Zhou, Q.; Huang, F.; Li, W.; Liu, C. Bioactive compounds and antioxidant activities of cold-pressed seed oils. Oil Crop Sci. 2018, 3, 191. [Google Scholar]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. THE content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Uluata, S.; Özdemir, N. Antioxidant Activities and Oxidative Stabilities of Some Unconventional Oilseeds. J. Am. Oil Chem. Soc. 2011, 89, 551–559. [Google Scholar] [CrossRef]
- Fotschki, B.; Opyd, P.; Juśkiewicz, J.; Wiczkowski, W.; Jurgoński, A. Comparative Effects of Dietary Hemp and Poppy Seed Oil on Lipid Metabolism and the Antioxidant Status in Lean and Obese Zucker Rats. Molecules 2020, 25, 2921. [Google Scholar] [CrossRef]
- Pasqua, T.; Rocca, C.; Lupi, F.R.; Baldino, N.; Amelio, D.; Parisi, O.I.; Granieri, M.C.; De Bartolo, A.; Lauria, A.; Dattilo, M.; et al. Cardiac and Metabolic Impact of Functional Foods with Antioxidant Properties Based on Whey Derived Proteins Enriched with Hemp Seed Oil. Antioxidants 2020, 9, 1066. [Google Scholar] [CrossRef]
- Afridi, A.J.; Zuberi, A.; Yousafzai, A.M.; Kamran, M.; Ullah, S. Hemp (Marijuana) reverted Copper-induced toxic effects on the essential fatty acid profile of Labeo rohita and Cirrhinus mrigala. Mol. Biol. Rep. 2018, 46, 391–401. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Berg, J.J.V.D.; De Fouw, N.J.; Kuypers, F.A.; Roelofsen, B.; Houtsmuller, U.M.; Kamp, J.A.D. Increased n-3 polyunsaturated fatty acid content of red blood cells from fish oil-fed rabbits increases in vitro lipid peroxidation, but decreases hemolysis. Free Radic. Biol. Med. 1991, 11, 393–399. [Google Scholar] [CrossRef]
- Tasset-Cuevas, I.; Fernández-Bedmar, Z.; Lozano-Baena, M.D.; Campos-Sánchez, J.; De Haro-Bailón, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Protective Effect of Borage Seed Oil and Gamma Linolenic Acid on DNA: In Vivo and In Vitro Studies. PLoS ONE 2013, 8, e56986. [Google Scholar] [CrossRef]
- Subedi, R.P.; Vartak, R.R.; Kale, P.G. Management of stress exerted by hydrogen peroxide in Drosophila melanogaster using Abhrak bhasma. J. Appl. Pharm. Sci. 2017, 7, 65–71. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Beutler, E. Glutathione in Red Cell Metabolism: A Manual of Biochemical Methods, 2nd ed.; Grune and Stratton: New York, NY, USA, 1974; pp. 112–114. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Elsevier BV: Amsterdam, The Netherlands, 1974; pp. 673–684. [Google Scholar]
- Girgih, A.T.; Udenigwe, C.C.; Aluko, R.E. In Vitro Antioxidant Properties of Hemp Seed (Cannabis sativa L.) Protein Hydrolysate Fractions. J. Am. Oil Chem. Soc. 2011, 88, 381–389. [Google Scholar] [CrossRef]
- Moccia, S.; Siano, F.; Russo, G.L.; Volpe, M.G.; La Cara, F.; Pacifico, S.; Piccolella, S.; Picariello, G. Antiproliferative and antioxidant effect of polar hemp extracts (Cannabis sativa L., Fedora cv.) in human colorectal cell lines. Int. J. Food Sci. Nutr. 2019, 71, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Sowndhararajan, K.; Joo, T.; Lim, C.; Cho, H.; Kim, S.; Kim, G.-Y.; Jhoo, J.-W. Ethanol and supercritical fluid extracts of hemp seed (Cannabis sativa L.) increase gene expression of antioxidant enzymes in HepG2 cells. Asian Pac. J. Reprod. 2015, 4, 147–152. [Google Scholar] [CrossRef]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect οf genotype and growing year on the nutritional, phytochemical, and antioxidant properties of in-dustrial hemp (Cannabis sativa L.) seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef]
- Siano, F.; Moccia, S.; Picariello, G.; Russo, G.L.; Sorrentino, G.; Di Stasio, M.; La Cara, F.; Volpe, M.G. Comparative Study of Chemical, Biochemical Characteristic and ATR-FTIR Analysis of Seeds, Oil and Flour of the Edible Fedora Cultivar Hemp (Cannabis sativa L.). Molecules 2018, 24, 83. [Google Scholar] [CrossRef]
- Konca, Y.; Cimen, B.; Yalcin, H.; Kaliber, M.; Beyzi, S.B. Effect of Hempseed (Cannabis sativa sp.) Inclusion to the Diet on Performance, Carcass and Antioxidative Activity in Japanese Quail (Coturnix japonica). Food Sci. Anim. Resour. 2014, 34, 141–150. [Google Scholar] [CrossRef]
- Teh, S.-S.; Birch, J. Physicochemical and quality characteristics of cold-pressed hemp, flax and canola seed oils. J. Food Compos. Anal. 2013, 30, 26–31. [Google Scholar] [CrossRef]
- USDA. National Nutrient Database for Standard Reference; Release 28 (Slightly revised); USDA: Beltsville, MD, USA, 2016.
- USDA. Seeds, Hemp Seed, Hulled; Basic Report 12012; USDA National Nutrient Database: Beltsville, MD, USA, 2018.
- Panchal, K.; Tiwari, A.K. Drosophila melanogaster “a potential model organism” for identification of pharmacological properties of plants/plant-derived components. Biomed. Pharmacother. 2017, 89, 1331–1345. [Google Scholar] [CrossRef]
- De Lazzari, F.; Sandrelli, F.; Whitworth, A.J.; Bisaglia, M. Antioxidant Therapy in Parkinson’s Disease: Insights from Drosophila melanogaster. Antioxidants 2020, 9, 52. [Google Scholar] [CrossRef]
- Wangler, M.F.; Yamamoto, S.; Bellen, H.J. Fruit Flies in Biomedical Research. Genetics 2015, 199, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Orr, W.; Sohal, R. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 1994, 263, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Kok, T.; Vaarwerk, F.; Zwingman, I.; Van Maanen, J.; Kleinjans, J. Peroxidation of linoleic, arachidonic and oleic acid in relation to the induction of oxidative DNA damage and cytogenetic effects. Carcinogenesis 1994, 15, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Lu, M.; Dudek, E.; Reddan, J.; Taylor, A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free Radic. Biol. Med. 2003, 34, 521–530. [Google Scholar] [CrossRef]
- Valls, V.; Goicoechea, M.; Muñiz, P.; Sáez, G.; Cabo, J. Effect of corn oil and vitamin E on the oxidative status of adipose tissues and liver in rat. Food Chem. 2003, 81, 281–286. [Google Scholar] [CrossRef]
- Vrailas-Mortimer, A.; Gomez, R.; Dowse, H.; Sanyal, S. A survey of the protective effects of some commercially available antioxidant supplements in genetically and chemically induced models of oxidative stress in Drosophila melanogaster. Exp. Gerontol. 2012, 47, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Davis, A.J.; Giebultowicz, J.M. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2008, 374, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Emamgholipour, S.; Hossein-Nezhad, A.; Ansari, M. Can Melatonin Act as an Antioxidant in Hydrogen Peroxide-Induced Oxidative Stress Model in Human Peripheral Blood Mononuclear Cells? Biochem. Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tangade, A.; Sinha, S. Cytoprotective properties of Hemidesmus indicus against H2O2 on Drosophila melanogaster. Int. Conf. Front. Life Earth Sci. 2018, 5, 135–142. [Google Scholar]
- Yang, X.; Zhang, D.; Song, L.-M.; Xu, Q.; Li, H.; Xu, H. Chemical Profile and Antioxidant Activity of the Oil from Peony Seeds (Paeonia suffruticosa Andr.). Oxidative Med. Cell. Longev. 2017, 2017, 9164905–11. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Sun, J.; Tower, J. FLP Recombinase—Mediated Induction of Cu/Zn-Superoxide Dismutase Transgene Expression Can Extend the Life Span of Adult Drosophila melanogaster Flies. Mol. Cell. Biol. 1999, 19, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Kaneuchi, T.; Togawa, T.; Matsuo, T.; Fuyama, Y.; Aigaki, T. Efficient measurement of H2O2 resistance in Drosophila using an activity monitor. Biogerontology 2003, 4, 157–165. [Google Scholar] [CrossRef]
- Missirlis, F.; Rahlfs, S.; Dimopoulos, N.; Bauer, H.; Becker, K.; Hilliker, A.; Phillips, J.P.; Jäckle, H. A Putative Glutathione Peroxidase of Drosophila Encodes a Thioredoxin Peroxidase That Provides Resistance against Oxidative Stress but Fails to Complement a Lack of Catalase Activity. Biol. Chem. 2003, 384, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Radyuk, S.N.; Klichko, V.I.; Spinola, B.; Sohal, R.S.; Orr, W.C. The peroxiredoxin gene family in Drosophila melanogaster. Free Radic. Biol. Med. 2001, 31, 1090–1100. [Google Scholar] [CrossRef]
- Bauer, H.; Kanzok, S.M.; Schirmer, R.H. Thioredoxin-2 but not thioredoxin-1 is a substrate of thioredoxin peroxidase-1 from Drosophila melanogaster: Isolation and characterization of a second thioredoxin in D. melanogaster and evidence for distinct biological functions of Trx-1 and Trx-2. J. Biol. Chem. 2002, 277, 17457–17463. [Google Scholar] [CrossRef] [PubMed]
- Enya, S.; Yamamoto, C.; Mizuno, H.; Esaki, T.; Lin, H.-K.; Iga, M.; Morohashi, K.; Hirano, Y.; Kataoka, H.; Masujima, T.; et al. Dual Roles of Glutathione in Ecdysone Biosynthesis and Antioxidant Function During Larval Development in Drosophila. Genetics 2017, 207, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.S.; Allen, T.; Singh, R. Inevitable glutathione, then and now. Indian J. Exp. Boil. 2002, 40, 706–716. [Google Scholar]
- Porter, N.A. A Perspective on Free Radical Autoxidation: The Physical Organic Chemistry of Polyunsaturated Fatty Acid and Sterol Peroxidation. J. Org. Chem. 2013, 78, 3511–3524. [Google Scholar] [CrossRef] [PubMed]
- Fagali, N.; Catalá, A. Antioxidant activity of conjugated linoleic acid isomers, linoleic acid and its methyl ester determined by photoemission and DPPH techniques. Biophys. Chem. 2008, 137, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. Free radical scavenging properties of conjugated linoleic acids. J. Agric. Food Chem. 2001, 49, 3452–3456. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Valli, V.; Bordoni, A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Basiricò, L.; Morera, P.; DiPasquale, D.; Tröscher, A.; Bernabucci, U. Comparison between conjugated linoleic acid and essential fatty acids in preventing oxidative stress in bovine mammary epithelial cells. J. Dairy Sci. 2017, 100, 2299–2309. [Google Scholar] [CrossRef]
- Cervera, M.A.R.; Valenzuela, R.; Hernandez-Rodas, M.C.; Barrera, C.; Espinosa, A.; Marambio, M.; Valenzuela, A. Vegetable oils rich in alpha linolenic acid increment hepatic n-3 LCPUFA, modulating the fatty acid metabolism and antioxidant response in rats. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Wang, R.; Kern, J.T.; Goodfriend, T.L.; Ball, D.L.; Luesch, H. Activation of the antioxidant response element by specific oxidized metabolites of linoleic acid. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhou, Y.; Wu, M.; Li, X.; Mai, K.; Ai, Q. ω-6 Polyunsaturated fatty acids (linoleic acid) activate both autophagy and antioxidation in a synergistic feedback loop via TOR-dependent and TOR-independent signaling pathways. Cell Death Dis. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.V.; Doh, S.T.; Chan, J.Y.; Kong, A.-N.; Cai, L. Regulatory potential for concerted modulation of Nrf2- and Nfkb1-mediated gene expression in inflammation and carcinogenesis. Br. J. Cancer 2008, 99, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Sykiotis, G.P.; Bohmann, D. Keap1/Nrf2 Signaling Regulates Oxidative Stress Tolerance and Lifespan in Drosophila. Dev. Cell 2008, 14, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Park, S.H.; Han, J.H.; Hong, Y.K.; Hwang, S.; Lee, S.; Kim, D.; Han, S.Y.; Kim, E.S.; Cho, K.S. The effects of hempseed meal intake and linoleic acid on Drosophila models of neurodegenerative diseases and hypercholesterolemia. Mol. Cells 2011, 31, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Champigny, C.M.; Cormier, R.P.J.; Simard, C.J.; St-Coeur, P.-D.; Fortin, S.; Pichaud, N. Omega-3 Monoacylglyceride Effects on Longevity, Mitochondrial Metabolism and Oxidative Stress: Insights from Drosophila melanogaster. Mar. Drugs 2018, 16, 453. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.D.O.; Couto-Lima, C.A.; Machado, M.C.R.; Espreafico, E.M.; Ramos, R.G.P.; Alberici, L.C. Protective action of Omega-3 on paraquat intoxication in Drosophila melanogaster. J. Toxicol. Environ. Health Part A 2017, 80, 1050–1063. [Google Scholar] [CrossRef]
- Fougeron, A.-S.; Farine, J.-P.; Flaven-Pouchon, J.; Everaerts, C.; Ferveur, J.-F. Fatty-Acid Preference Changes during Development in Drosophila melanogaster. PLoS ONE 2011, 6, e26899. [Google Scholar] [CrossRef]
- Shen, L.R.; Lai, C.Q.; Feng, X.; Parnell, L.D.; Wan, J.B.; Wang, J.D.; Li, D.; Ordovas, J.M.; Kang, J.X. Drosophila lacks C20 and C22 PUFAs. J. Lipid Res. 2010, 51, 2985–2992. [Google Scholar] [CrossRef]
- Tortoriello, G.; Rhodes, B.P.; Takacs, S.M.; Stuart, J.M.; Basnet, A.; Raboune, S.; Widlanski, T.S.; Doherty, P.; Harkany, T.; Bradshaw, H.B. Targeted Lipidomics in Drosophila melanogaster Identifies Novel 2-Monoacylglycerols and N-acyl Amides. PLoS ONE 2013, 8, e67865. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, J.; Liu, J.; Peng, C.; Suen, Y.L.; Wang, M.; Jiang, Y.; Chen, Z.-Y.; Chen, F. DHA-rich marine microalga Schizochytrium mangrovei possesses anti-ageing effects on Drosophila melanogaster. J. Funct. Foods 2013, 5, 888–896. [Google Scholar] [CrossRef]
- Holmbeck, M.A.; Rand, D.M. Dietary Fatty Acids and Temperature Modulate Mitochondrial Function and Longevity inDrosophila. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2015, 70, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
| Experiment 1 (Non-Stress Conditions) | Experiment 2 (H2O2-Induced Oxidative Stress Conditions) |
|---|---|
| Hemp seed oil (μL/mL) | Hemp seed oil (μL/mL) |
| - | 0-H2O2 (unstressed negative control; (control-)) |
| 0 (control) | 0 (stressed control; (S)) |
| 12.5 | 12.5 |
| 18.7 | 18.7 |
| 31.2 | 31.2 |
| 62.5 | 62.5 |
| 125 | 125 |
| 0 + ASC (positive control, (ASC)) | 0+ ASC (positive control; )ASC + S)) |
| RI | Rel. Amount (%) | Compound |
|---|---|---|
| 1724 | tr | Methyl tetradecanoate (methyl myristate) |
| 1824 | tr | Methyl pentadecanoate |
| 1895 | 0.1 | Methyl (9Z)-9-hexadecenoate (methyl palmitoleate) |
| 1924 | 7.6 | Methyl hexadecanoate (methyl palmitate) |
| 2014 | tr | Methyl (9Z)-9-heptadecenoate |
| 2024 | tr | Methyl heptadecanoate (methyl margarate) |
| 2083 | 3.2 | Methyl (6Z,9Z,12Z)-6,9,12-octadecatrienoate (methyl γ-linolenate) |
| 2090 | 1.0 | Methyl (6Z,9Z,12Z,15Z)-6,9,12,15-octadecatetraenoate (methyl stearidonate) |
| 2108 | 54.5 | Methyl (9Z,12Z)-9,12-octadecadienoate (methyl linoleate) |
| 2116 | 27.6 | Methyl (9Z)-9-octadecenoate (methyl oleate) * |
| 2116 | Methyl (9Z,12Z,15Z)-octadeca-9,12,15-trienoate (methyl α-linolenate) * | |
| 2124 | 3.6 | Methyl octadecanoate (methyl stearate) |
| 2311 | 0.7 | Methyl (11Z)-11-eicosenoate |
| 2324 | 1.1 | Methyl eicosanoate (methyl arachate) |
| 2424 | tr | Methyl heneicosanoate |
| 2506 | tr | Methyl (13Z,16Z)-13,16-docosadienoate |
| 2524 | 0.4 | Methyl docosanoate (methyl behenate) |
| 2624 | tr | Methyl tricosanoate |
| 2724 | 0.2 | Methyl tetracosanoate (methyl lignocerate) |
| 100 | Total | |
| 87.1 | Unsaturated | |
| 12.9 | Saturated |
| RI | Rel. Amount (%) | Compound |
|---|---|---|
| 2050 | 6.4 | Palmitic acid, trimethylsilyl ester |
| 2214 | 18.1 | Linoleic acid, trimethylsilyl ester |
| 2229 | 11.6 | Oleic acid, trimethylsilyl ester |
| 2255 | 0.9 | Octadecanoic acid, trimethylsilyl ester |
| 3014 | 13.2 | γ-Tocopherol, trimethylsilyl ether |
| 3136 | 1.1 | α-Tocopherol, trimethylsilyl ether |
| 3263 | 7.9 | Campesterol, trimethylsilyl ether |
| 3286 | 2.3 | Stigmasterol, trimethylsilyl ether |
| 3344 | 29.7 | β-Sitosterol, trimethylsilyl ether |
| 3359 | 5.7 | (24Z)-3-[(Trimethylsilyl)oxy]stigmasta-5,24(28)-diene |
| 97.0 | Total |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitorović, J.; Joković, N.; Radulović, N.; Mihajilov-Krstev, T.; Cvetković, V.J.; Jovanović, N.; Mitrović, T.; Aleksić, A.; Stanković, N.; Bernstein, N. Antioxidant Activity of Hemp (Cannabis sativa L.) Seed Oil in Drosophila melanogaster Larvae under Non-Stress and H2O2-Induced Oxidative Stress Conditions. Antioxidants 2021, 10, 830. https://doi.org/10.3390/antiox10060830
Vitorović J, Joković N, Radulović N, Mihajilov-Krstev T, Cvetković VJ, Jovanović N, Mitrović T, Aleksić A, Stanković N, Bernstein N. Antioxidant Activity of Hemp (Cannabis sativa L.) Seed Oil in Drosophila melanogaster Larvae under Non-Stress and H2O2-Induced Oxidative Stress Conditions. Antioxidants. 2021; 10(6):830. https://doi.org/10.3390/antiox10060830
Chicago/Turabian StyleVitorović, Jelena, Nataša Joković, Niko Radulović, Tatjana Mihajilov-Krstev, Vladimir J. Cvetković, Nikola Jovanović, Tatjana Mitrović, Ana Aleksić, Nemanja Stanković, and Nirit Bernstein. 2021. "Antioxidant Activity of Hemp (Cannabis sativa L.) Seed Oil in Drosophila melanogaster Larvae under Non-Stress and H2O2-Induced Oxidative Stress Conditions" Antioxidants 10, no. 6: 830. https://doi.org/10.3390/antiox10060830
APA StyleVitorović, J., Joković, N., Radulović, N., Mihajilov-Krstev, T., Cvetković, V. J., Jovanović, N., Mitrović, T., Aleksić, A., Stanković, N., & Bernstein, N. (2021). Antioxidant Activity of Hemp (Cannabis sativa L.) Seed Oil in Drosophila melanogaster Larvae under Non-Stress and H2O2-Induced Oxidative Stress Conditions. Antioxidants, 10(6), 830. https://doi.org/10.3390/antiox10060830







