Feeding Broiler Chickens with Grape Seed and Skin Meals to Enhance α- and γ-Tocopherol Content and Meat Oxidative Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Byproducts
2.2. Solvents and Reagents
2.3. Birds and Diets
2.4. Collection of Samples and Measurements
2.5. Chemical Analyses
2.5.1. Extractable and Non-Extractable Polyphenol Contents
2.5.2. Plasma and Meat α-Tocopherol and γ-Tocopherol Assessment
2.5.3. Meat Lipid Oxidation
2.6. Calculations and Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Ileal and Excreta Extractable and Non-Extractable Polyphenol Contents
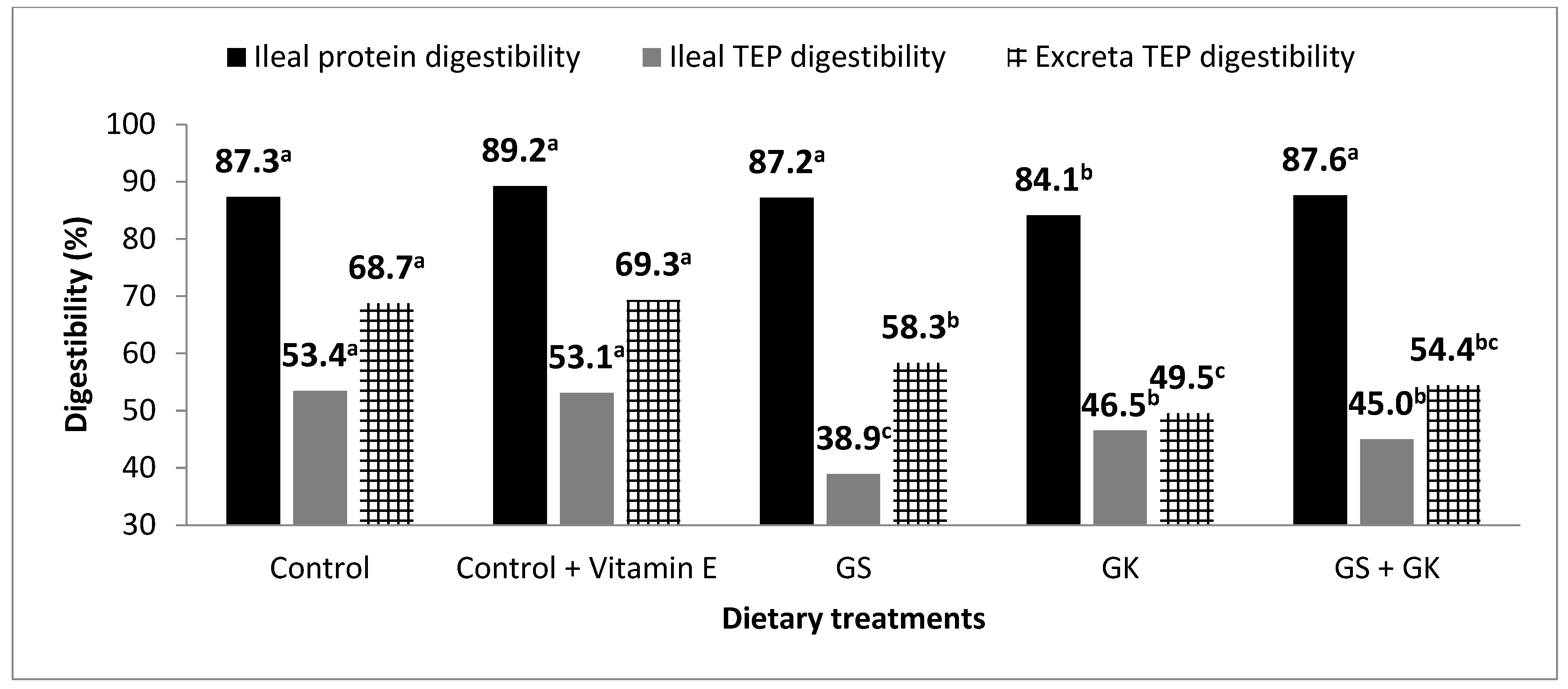
3.3. Protein and Polyphenol Digestibility
3.4. Plasma α- and γ-Tocopherol Concentrations
3.5. Meat α- and γ-Tocopherol Concentrations and Lipid Oxidation
4. Discussion
4.1. Chemical Composition and Polyphenolic Content of Grape Seed and Skin Meals
4.2. Growth Performance and Protein and Polyphenol Intestinal Utilization
4.3. Plasma and Meat α- and γ-Tocopherol Concentrations and MDA Values
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO: Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/ (accessed on 3 March 2021).
- Sui, Y.; Yang, J.; Ye, Q.; Li, H.; Wang, H. Infrared, convective, and sequential infrared and convective drying of wine grape pomace. Dry Technol. 2014, 32, 686–694. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Souquet, J.M.; Cheynier, V.; Brossaud, F.; Moutounet, M. Polymeric proanthocyanidins from grape skins. Phytochemistry 1996, 43, 509–512. [Google Scholar] [CrossRef]
- Vivas, N.; Nonier, M.F.; Vivas de Gaulejac, N.; Absalon, C.; Bertrand, A.; Mirabel, M. Differentiation of proanthocyanidin tannins from seeds, skins and stems of grapes (Vitis vinifera) and heartwood of Quebracho (Schinopsis balansae) by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and thioacidolysis/liquid chromatography/electrospray ionization mass spectrometry. Anal. Chim. Acta 2004, 513, 247–256. [Google Scholar]
- Yilmaz, Y.; Goksel, Z.; Erdogan, S.S.; Ozturk, A.; Atak, A.; Ozer, C. Antioxidant activity and phenolic content of seed, skin and pulp parts of 22 grape (Vitis vinifera L.) cultivars (4 common and 18 registered or candidate for registration). J. Food Process. Preserv. 2015, 39, 1682–1691. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Brannan, R.G.; Mah, E. Grape seed extract inhibits lipid oxidation in muscle from different species during refrigerated and frozen storage and oxidation catalyzed by peroxynitrite and iron/ascorbate in a pyrogallol red model system. Meat Sci. 2007, 77, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Viveros, A.; Rebolé, A.; Rica, B.D.; Arija, I.; Brenes, A. Influence of dietary enzyme addition on polyphenol utilization and meat lipid oxidation of chicks fed grape pomace. Food Res. Int. 2015, 73, 197–203. [Google Scholar] [CrossRef]
- Nardoia, M.; Ruiz-Capillas, C.; Casamassima, D.; Herrero, A.M.; Pintado, T.; Jiménez-Colmenero, F.; Chamorro, S.; Brenes, A. Effect of polyphenols dietary grape by-products on chicken patties. Eur. Food Res. Technol. 2018, 244, 367–377. [Google Scholar] [CrossRef]
- Aditya, S.; Sang-Jip, O.; Ahammed, M.; Lohakare, J. Supplementation of grape pomace (Vitis vinifera) in broiler diets and its effect on growth performance, apparent total tract digestibility of nutrients, blood profile, and meat quality. Anim. Nutr 2018, 4, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.; O’Grady, M.N.; O’Callaghan, Y.C.; O’Brien, N.M.; Kerry, J.P. Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 2007, 76, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Manso, T.; Gallardo, T.; Guerra-Rivas, C. Modifying milk and meat fat quality through feed changes. Small Rumin. Res. 2016, 142, 31–37. [Google Scholar] [CrossRef]
- Pazos, M.; Gallardo, J.M.; Torres, J.L.; Medina, I. Activity of grape polyphenols as inhibitors of the oxidation of fish lipids and frozen fish muscle. Food Chem. 2005, 92, 547–557. [Google Scholar] [CrossRef]
- Zdunczyk, Z.; Jankowski, J. Poultry meat as functional food: Modification of the fatty acid profile review. Ann. Anim. Sci. 2013, 13, 463–480. [Google Scholar] [CrossRef]
- Cortinas, L.; Barroeta, A.; Villaverde, C.; Galobart, J.; Guardiola, F.; Baucells, M.D. Influence of the dietary polyunsaturation level on chicken meat quality: Lipid oxidation. Poult. Sci. 2005, 84, 48–55. [Google Scholar] [CrossRef]
- Frank, J.; Budek, A.; Lundh, T.; Parker, R.S.; Swanson, J.E.; Lourenço, C.F.; Gago, B.; Laranjinha, J.; Vessby, B.; Kamal-Eldin, A. Dietary flavonoids with a catechol structure increase alpha tocopherol in rats and protect the vitamin from oxidation in vitro. J. Lipid Res. 2006, 47, 2718–2725. [Google Scholar] [CrossRef] [PubMed]
- Luehring, M.; Blanck, R.; Wolffram, S. Vitamin E-sparing effect and vitamin E-independent antioxidative effects of the flavonol quercetin in growing pigs. Anim. Feed Sci. Technol. 2011, 169, 199–207. [Google Scholar] [CrossRef]
- Goñi, I.; Brenes, A.; Centeno, C.; Viveros, A.; Saura-Calixto, F.; Rebolé, A.; Arija, I.; Esteve, R. Effect of dietary grape pomace and vitamin E on growth performance, nutrient digestibility, and susceptibility to meat lipid oxidation. Poult. Sci. 2007, 86, 508–516. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Rebolé, A.; Arija, I.; Romero, C.; Álvarez, I.; Rey, A.; Brenes, A. Addition of exogenous enzymes to diets containing grape pomace: Effects on intestinal utilization of catechins and antioxidant status of chickens. Food Res. Int. 2017, 96, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, I.; Chamorro, S.; Pérez-Jiménez, J.; López-Andrés, P.; Álvarez-Acero, I.; Herrero, A.M.; Nardoia, M.; Brenes, A.; Viveros, A.; Arija, I.; et al. Phenolic Metabolites in Plasma and Thigh Meat of Chickens Supplemented with Grape Byproducts. J. Agric. Food Chem. 2019, 67, 4463–4471. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Goñi, I.; Centeno, C.; Sayago-Ayerdi, S.G.; Arija, I. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult. Sci. 2008, 87, 307–316. [Google Scholar] [CrossRef]
- Nardoia, M.; Romero, C.; Brenes, A.; Arija, I.; Viveros, A.; Ruiz-Capillas, C.; Chamorro, S. Addition of fermented and unfermented grape skin in broilers’ diets: Effect on digestion, growth performance, intestinal microbiota and oxidative stability of meat. Animal 2020, 14, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Vrhovsek, U.; Masuero, D.; Trainotti, D. Differences in the amount and structure of extractable skin and seed tannins amongst red grape varieties. Aust. J. Grape Wine R 2009, 15, 27–35. [Google Scholar] [CrossRef]
- Labarbe, B.; Cheynier, V.; Brossaud, F.; Souquet, J.M.; Moutounet, M. Quantitative fractionation of grape proanthocyanidins according to their degree of polymerization. J. Agric. Food Chem. 1999, 47, 2719–2723. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Romero, C.; Brenes, A.; Sánchez-Patán, F.; Bartolomé, B.; Viveros, A.; Arija, I. Impact of a sustained consumption of grape extract on digestion, gut microbial metabolism and intestinal barrier in broiler chickens. Food Funct. 2019, 10, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists International: Arlington, VA, USA, 1995. [Google Scholar]
- Van Soest, J.P.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Wiseman, J.; Edmundo, B.K.; Shepperson, N. The apparent metabolizable energy of sunflower oil and sunflower acid oil for broiler chickens. Anim. Feed Sci. Technol. 1992, 36, 41–51. [Google Scholar] [CrossRef]
- Siriwan, P.; Bryden, W.L.; Mollah, Y.; Annison, E.F. Measurements of endogenous amino acid losses in poultry. Brit. Poult. Sci. 1993, 34, 939–949. [Google Scholar] [CrossRef]
- Montreau, F.R. Sur le dosage des composés phénoliques totaux dans les vins par la méthode Folin-Ciocalteu. J. Int. Sci. Vigne Vin. 1972, 6, 397–404. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of phenolic plant metabolites. In Methods in Ecology; Lawton, J.H., Likens, G.E., Eds.; Blackwell: Oxford, UK, 1994; pp. 83–85. [Google Scholar]
- Buttriss, J.L.; Diplock, A.T. High-performance liquid chromatography methods for vitamin E in tissues. Method Enzymol 1984, 105, 131–138. [Google Scholar]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiu, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Busse-Valverde, N.; Gómez-Plaza, E.; López-Roca, J.M.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B. Effect of different enological practices on skin and seed proanthocyanidins in three varietal wines. J. Agric. Food Chem. 2010, 58, 11333–11339. [Google Scholar] [CrossRef]
- Kelm, M.A.; Versari, A.; Parpinello, G.A.; Thorngate, J.H. Mass spectral characterization of Uva Longanesi seed and skin extracts. Am. J. Enol. Vitic. 2012, 63, 402–406. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, E.; Saura-Calixto, F. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Viveros, A.; Centeno, C.; Romero, C.; Arija, I.; Brenes, A. Effects of dietary grape seed extract on growth performance, amino acid digestibility and plasma lipids and mineral content in broiler chicks. Animal 2013, 7, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Travaglia, F.; Bordiga, M.; Locatelli, M.; Coïson, J.D.; Arlorio, M. Polymeric proanthocyanidins in skins and seeds of 37 Vitis vinifera L. cultivars: A methodological comparative study. J. Food Sci. 2011, 76, C742–C749. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.T.; Centeno, C.; Treviño, J. Tannins in faba bean seeds: Effects on the digestion of protein and amino acids in growing chicks. Anim. Feed Sci. Technol. 1993, 41, 271–278. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Atkinson, J.L.; Leeson, S. Sorghum tannins: A review. World Poult. Sci. J. 1997, 53, 5–21. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Goñi, I.; Centeno, C.; Saura, C.F.; Arija, I. Effect of grape seed extract on growth performance, protein and polyphenol digestibilities and antioxidant activity in chicken. Span. J. Agric. Res. 2010, 8, 326–333. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine by-products: Phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different French grape varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef]
- Tsang, C.; Auger, C.; Mullen, W.; Bornet, A.; Rouanet, J.M.; Crozier, A.; Teissedre, P.L. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Br. J. Nutr. 2005, 94, 170–181. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.P.; Aura, A.M.; Hollman, P.C.; Gruppen, H. Procyanidins dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenil) acetic acid and 5-(3,4-dihydroxyphenil)-?-valerolactone as the major metabolites. J. Agric. Food Chem. 2009, 57, 1084–1092. [Google Scholar] [CrossRef]
- Stoupi, S.; Williamson, G.; Drynan, J.W.; Barron, D.; Clifford, M.N. Procyanidin B2 catabolism by human fecal microflora: Partial characterization of dimeric intermediates. Arch. Biochem. Biophys. 2010, 501, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.I.; López-Bote, C.J.; Sanz Arias, R. Effect of extensive feeding on a-tocopherol concentration and oxidative stability of muscle microsomes from Iberian pigs. Anim. Sci. 1997, 65, 515–520. [Google Scholar] [CrossRef]
- Daza, A.; Rey, A.I.; Ruiz, J.; López-Bote, C.J. Effects of feeding in free-range conditions or in confinement with different dietary MUFA/PUFA ratios and α-tocopheryl acetate, on antioxidants accumulation and oxidative stability in Iberian pigs. Meat Sci. 2005, 69, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Wie, M.; Sung, J.; Choi, Y.; Kim, Y.; Sang Jeong, H.; Lee, Y. Tocopherols and tocotrienols in grape seeds from 14 cultivars grown in Korea. Eur. J. Lipid Sci. Technol. 2009, 111, 1255–1258. [Google Scholar] [CrossRef]
- Iglesias, J.; Pazos, M.; Torres, J.L.; Medina, I. Antioxidant mechanism of grape procyanidins in muscle tissues: Redox interactions with endogenous ascorbic acid and α-tocopherol. Food Chem. 2012, 134, 1767–1774. [Google Scholar] [CrossRef]
- Simonetti, P.; Ciappellano, S.; Gardana, C.; Bramati, L.; Pietta, P. Procyanidins from Vitis vinifera seeds: In vivo effects on oxidative stress. J. Agric. Food Chem. 2002, 50, 6217–6221. [Google Scholar] [CrossRef]
- Nishida, T.; Eruden, B.; Hosoda, K.; Matsuyama, H.; Nakagawa, K.; Miyazawa, T.; Shioya, S. Effects of green tea (Camellia sinensis) waste silage and polyethylene glycol on ruminal fermentation and blood components in cattle. Asian Aust. J. Anim. 2006, 19, 1728–1736. [Google Scholar] [CrossRef]
| Nutrients | g/100 g 1 | |
|---|---|---|
| GS Meal | GK Meal | |
| Crude protein (CP) | 19.6 ± 0.41 | 16.3 ± 0.00 |
| Crude fibre | 25.4 ± 1.87 | 14.5 ± 1.33 |
| Neutral detergent fibre (NDF) | 52.9 ± 2.36 | 49.7 ± 0.85 |
| Acid detergent fibre (ADF) | 44.6 ± 2.10 | 41.2 ± 1.08 |
| Acid detergent lignin (ADL) | 38.0 ± 1.83 | 28.3 ± 1.40 |
| Ether extract | 7.17 ± 0.31 | 7.06 ± 0.33 |
| Total extractable polyphenols | 7.93 ± 0.60 | 2.35 ± 0.14 |
| Non-extractable polyphenols | 1.15 ± 0.20 | 1.24 ± 0.10 |
| Gross energy (cal/g) | 5062 ± 13.6 | 4500 ± 19.8 |
| Phenolic Compounds | GS Meal | GK Meal | |
|---|---|---|---|
| Flavanol monomers | Catechin | 108.0 ± 5.1 | 11.6 ± 0.60 |
| Epicatechin | 93.5 ± 7.0 | 9.68 ± 0.73 | |
| Epicatechin 3-O-gallate | 5.21 ± 0.10 | 0.71 ± 0.01 | |
| Flavanol dimers | Procyanidin B1 | 32.0 ± 1.11 | 3.81 ± 0.14 |
| Procyanidin B2 | 32.9 ± 1.14 | 4.26 ± 0.16 | |
| Procyanidin B3 | 30.2 ± 1.82 | 1.51 ± 0.29 | |
| Procyanidin gallate 1 1 | 8.72 ± 0.84 | 3.04 ± 0.15 | |
| Procyanidin gallate 2 1 | 29.4 ± 3.13 | 4.67 ± 0.32 | |
| Flavanol trimers | Procyanidin C1 | 19.7 ± 1.52 | 2.92 ± 0.23 |
| Procyanidin trimer 2 2 | 6.99 ± 0.52 | 1.16 ± 0.09 | |
| Procyanidin trimer 3 2 | 16.7 ± 1.61 | 2.29 ± 0.23 | |
| Procyanidin trimer 4 2 | 8.43 ± 0.58 | 1.25 ± 0.09 | |
| Flavanol tetramers | Procyanidin cinnamtannin A2 | 25.7 ± 0.69 | 5.20 ± 0.14 |
| Procyanidin tetramer 3 | 9.78 ± 0.10 | 3.55 ± 0.20 | |
| Phenolic acids | Gallic acid | 72.2 ± 5.60 | 39.1 ± 3.80 |
| Caftaric acid | nd 4 | 25.2 ± 2.50 | |
| Fertaric acid | nd | 14.1 ± 0.50 | |
| Coutaric acid | nd | 9.78 ± 0.30 | |
| Total extractable polyphenols, g GAE 5/100 g DM | 7.93 ± 0.60 | 2.35 ± 0.14 | |
| Ingredients | Control | Control + Vit E 1 | GS 2 | GK 3 | GS + GK |
|---|---|---|---|---|---|
| Corn (8.1% CP) | 410 | 410 | 415 | 364 | 409.3 |
| Soybean (48% CP) | 383 | 383 | 373 | 359 | 371 |
| Sunflower oil | 100 | 100 | 100 | 100 | 100 |
| Salt | 3 | 3 | 3 | 3 | 3 |
| Monocalcium phosphate | 17.8 | 17.8 | 17.8 | 17.8 | 17.8 |
| Calcium carbonate | 14.2 | 14.2 | 14.2 | 14.2 | 14.2 |
| Vitamin-mineral premix 4 | 5 | 5 | 5 | 5 | 5 |
| DL-Methionine | 2 | 2 | 2 | 2 | 2.2 |
| Straw | 55 | 55 | 30 | 15 | 30 |
| Grape seed meal | 0 | 0 | 30 | 0 | 24.4 |
| Grape skin meal | 0 | 0 | 0 | 110 | 13.1 |
| Celite 5 | 10 | 10 | 10 | 10 | 10 |
| Analysed composition | |||||
| Total extractable polyphenols | 1.7 | 1.7 | 4.09 | 4.18 | 3.97 |
| Non-extractable polyphenols | 0.075 | 0.07 | 0.324 | 1.46 | 0.413 |
| Crude protein | 208 | 207 | 205 | 207 | 209 |
| Ether extract | 122 | 122 | 124 | 126 | 122 |
| Crude fibre | 48 | 48 | 46.6 | 45.8 | 46.5 |
| Calculated composition | |||||
| AME 6 (kcal/kg) | 3106 | 3106 | 3119 | 2963 | 3099 |
| Lysine | 12.4 | 12.4 | 12.2 | 12.2 | 12.2 |
| Met + Cys | 8.8 | 8.8 | 8.82 | 8.77 | 9 |
| Ca | 10.5 | 10.5 | 10.6 | 11.1 | 10.8 |
| Available P | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 |
| Dietary Treatments | Daily Weight Gain (g/d) | Daily Feed Intake (g/d) | Feed Conversion Ratio |
|---|---|---|---|
| Control | 35.8 a | 50.4 | 1.41 b |
| Control + Vitamin E | 38.2 a | 52.6 | 1.38 b |
| GS | 34.3 a | 48.6 | 1.42 b |
| GK | 29.3 b | 47.8 | 1.63 a |
| GS + GK | 35.5 a | 49.9 | 1.41 b |
| SEM 1 | 1.25 | 1.86 | 0.02 |
| p-value 2 | *** | ns | *** |
| Dietary Treatments | Total Extractable Polyphenols (g GAE 1/100 g) | Non-Extractable Polyphenols (mg Cyanidin/100 g) | ||
|---|---|---|---|---|
| Ileal | Excreta | Ileal | Excreta | |
| Control | 0.467 c | 0.372 c | 0.032 b | 0.023 c,d |
| Control + Vitamin E | 0.457 c | 0.336 c | 0.032 b | 0.016 d |
| GS | 0.624 a | 0.461 b | 0.060 b | 0.045 b,c |
| GK | 0.564 a,b | 0.551 a | 0.092 a | 0.119 a |
| GS + GK | 0.517 b,c | 0.488 b | 0.047 b | 0.072 b |
| SEM 2 | 0.03 | 0.015 | 0.008 | 0.009 |
| p-value 3 | ** | *** | *** | *** |
| Dietary Treatments | α-Tocopherol | γ-Tocopherol |
|---|---|---|
| Control | 3.84 c | 0.533 b |
| Control + Vitamin E | 36.1 a | 0.662 b |
| GS | 4.69 b,c | 0.563 b |
| GK | 5.89 b,c | 0.501 b |
| GS + GK | 9.28 b | 0.867 a |
| SEM 1 | 1.31 | 0.065 |
| p-value2 | *** | ** |
| Dietary Treatments | α-Tocopherol | γ-Tocopherol | TBARS 1 | |||
|---|---|---|---|---|---|---|
| 1 d | 7 d | 1 d | 7 d | 1 d | 7 d | |
| Control | 9.89 b | 1.64 c | 2.24 b | 0.414 b | 0.288 | 1.88 a |
| Control + Vitamin E | 71.1 a | 10.6 a | 4.02 a | 0.487 b | 0.283 | 0.870 b |
| GS | 8.25 b | 1.46 c | 2.11 b | 0.336 b | 0.282 | 1.70 a |
| GK | 4.69 b | 3.50 b,c | 1.97 b | 0.449 b | 0.267 | 1.61 a |
| GS + GK | 12.0 b | 4.56 b | 2.16 b | 0.810 a | 0.255 | 0.581 b |
| SEM 2 | 2.89 | 0.678 | 0.224 | 0.073 | 0.011 | 0.171 |
| p-value 3 | *** | *** | *** | ** | ns | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, C.; Nardoia, M.; Arija, I.; Viveros, A.; Rey, A.I.; Prodanov, M.; Chamorro, S. Feeding Broiler Chickens with Grape Seed and Skin Meals to Enhance α- and γ-Tocopherol Content and Meat Oxidative Stability. Antioxidants 2021, 10, 699. https://doi.org/10.3390/antiox10050699
Romero C, Nardoia M, Arija I, Viveros A, Rey AI, Prodanov M, Chamorro S. Feeding Broiler Chickens with Grape Seed and Skin Meals to Enhance α- and γ-Tocopherol Content and Meat Oxidative Stability. Antioxidants. 2021; 10(5):699. https://doi.org/10.3390/antiox10050699
Chicago/Turabian StyleRomero, Carlos, Maria Nardoia, Ignacio Arija, Agustín Viveros, Ana I. Rey, Marin Prodanov, and Susana Chamorro. 2021. "Feeding Broiler Chickens with Grape Seed and Skin Meals to Enhance α- and γ-Tocopherol Content and Meat Oxidative Stability" Antioxidants 10, no. 5: 699. https://doi.org/10.3390/antiox10050699
APA StyleRomero, C., Nardoia, M., Arija, I., Viveros, A., Rey, A. I., Prodanov, M., & Chamorro, S. (2021). Feeding Broiler Chickens with Grape Seed and Skin Meals to Enhance α- and γ-Tocopherol Content and Meat Oxidative Stability. Antioxidants, 10(5), 699. https://doi.org/10.3390/antiox10050699







