Hyperoxidation of Peroxiredoxins and Effects on Physiology of Drosophila
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Strains and Procedures
2.2. Immunoblot Analysis
2.3. Locomotor Activity Analysis
2.4. Statistical Methods
3. Results
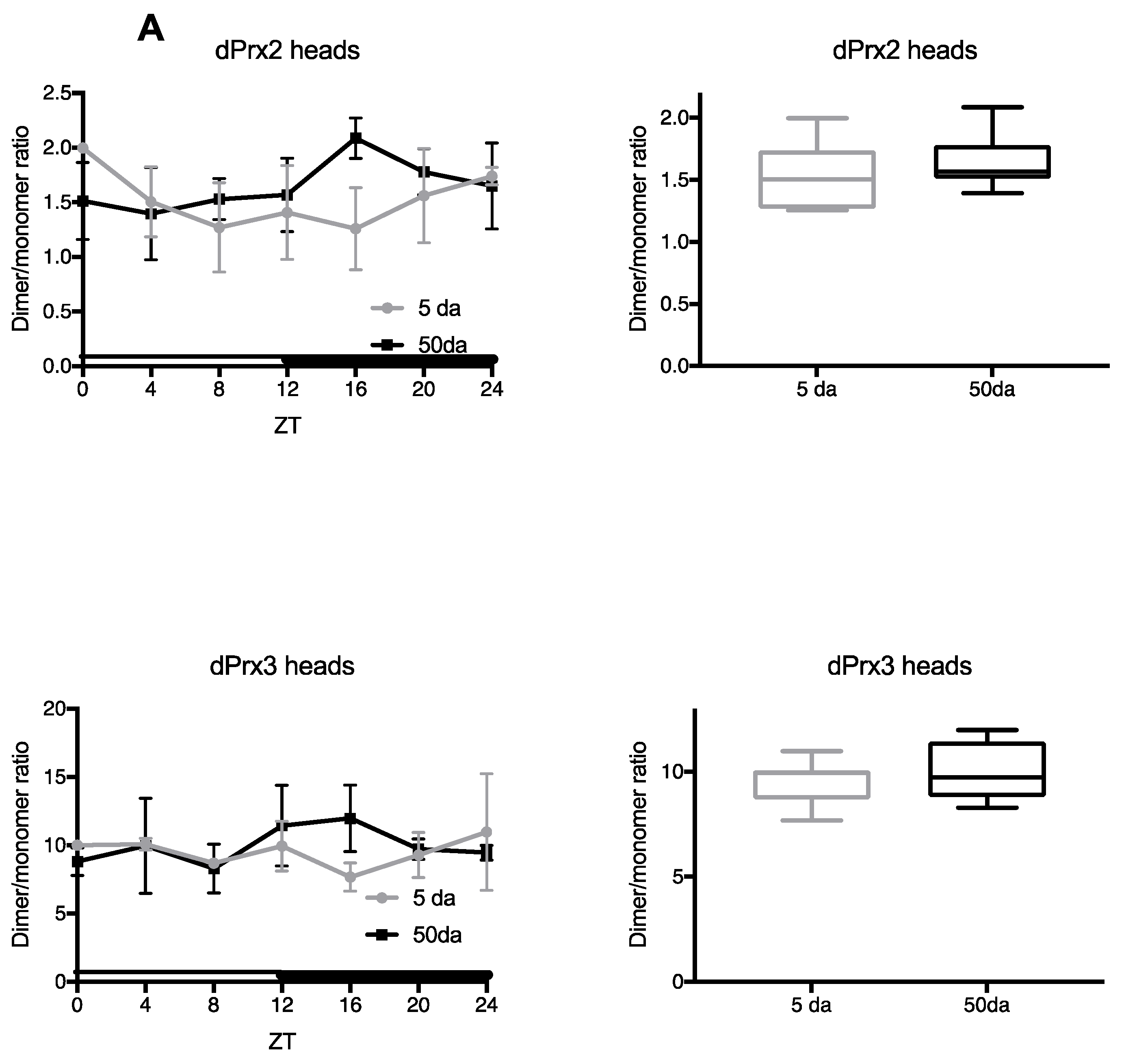
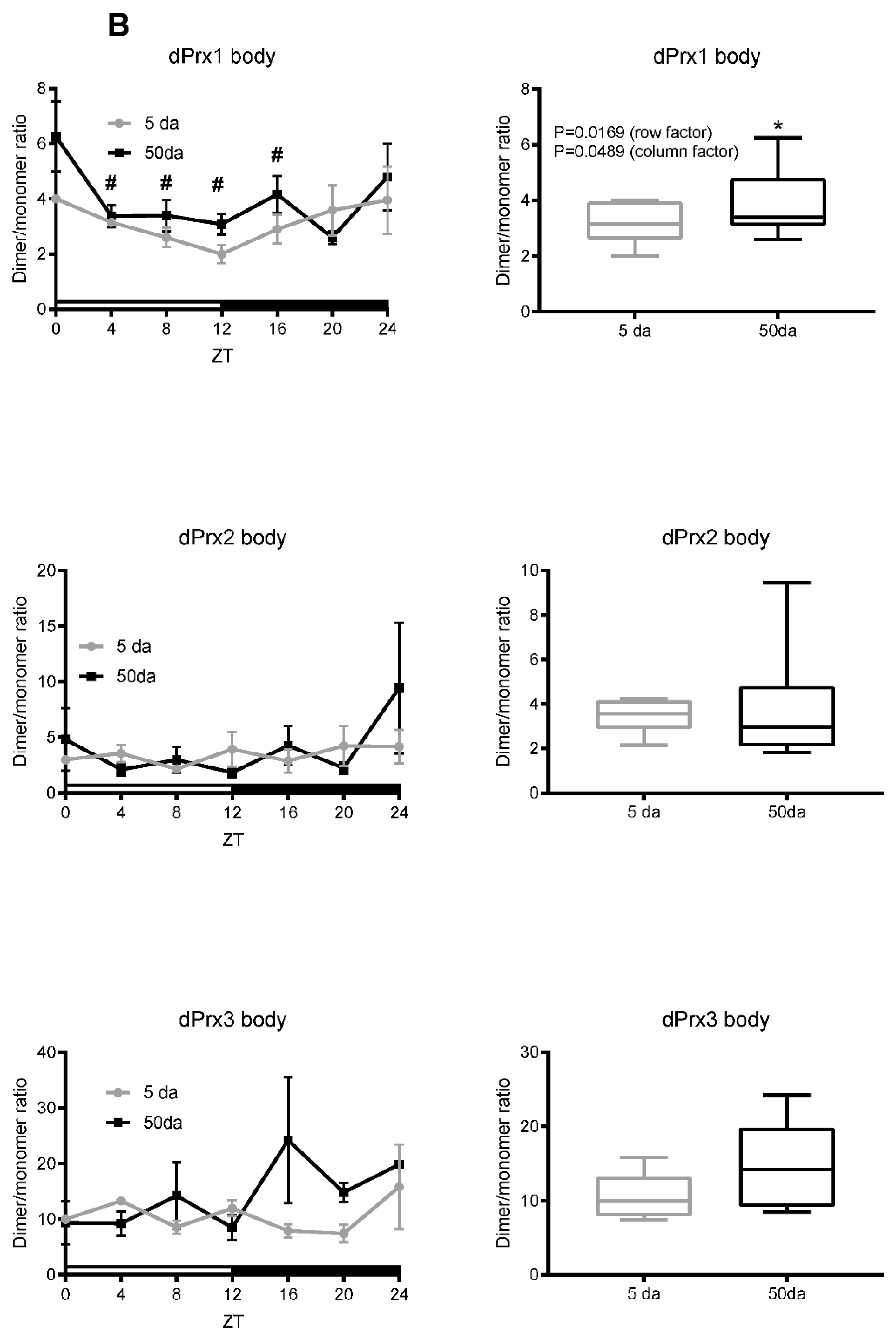
3.1. Ratios of Prx Dimer:Monomer as an Indirect Indicator of Their Activity
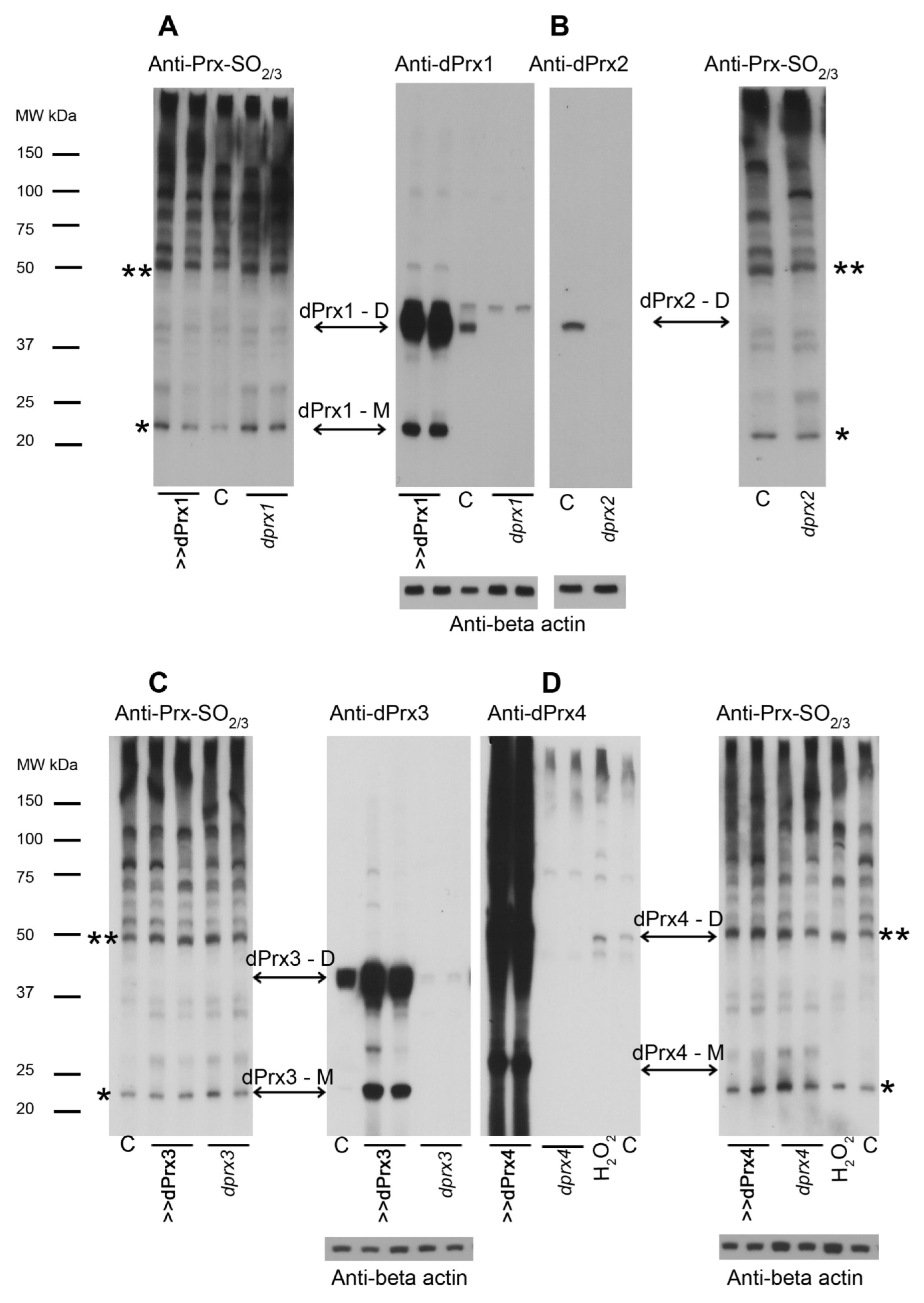
3.2. Analysis of Prx Hyperoxidation
3.3. The Effects of Prx1 and Prx2 Underexpression
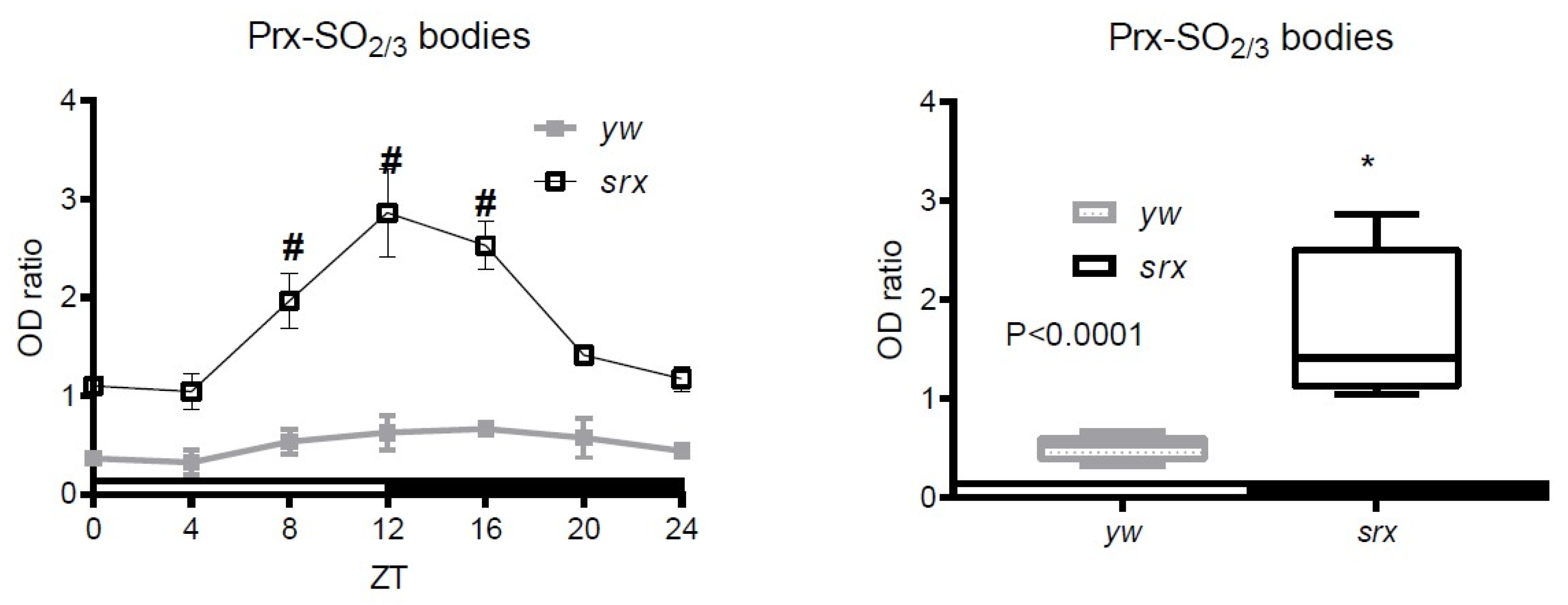
3.4. Hyperoxidation of Prxs in the Srx Mutant
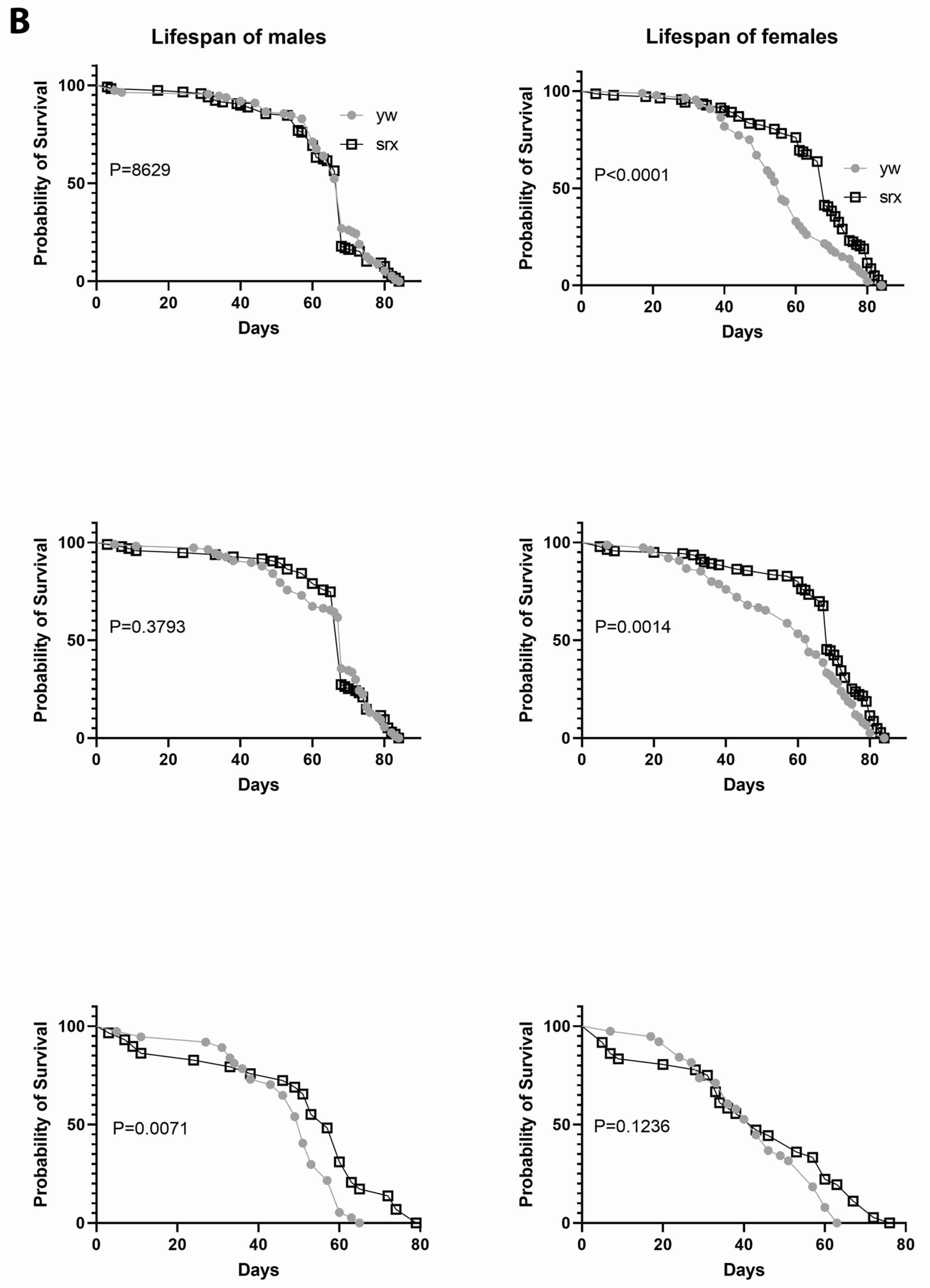
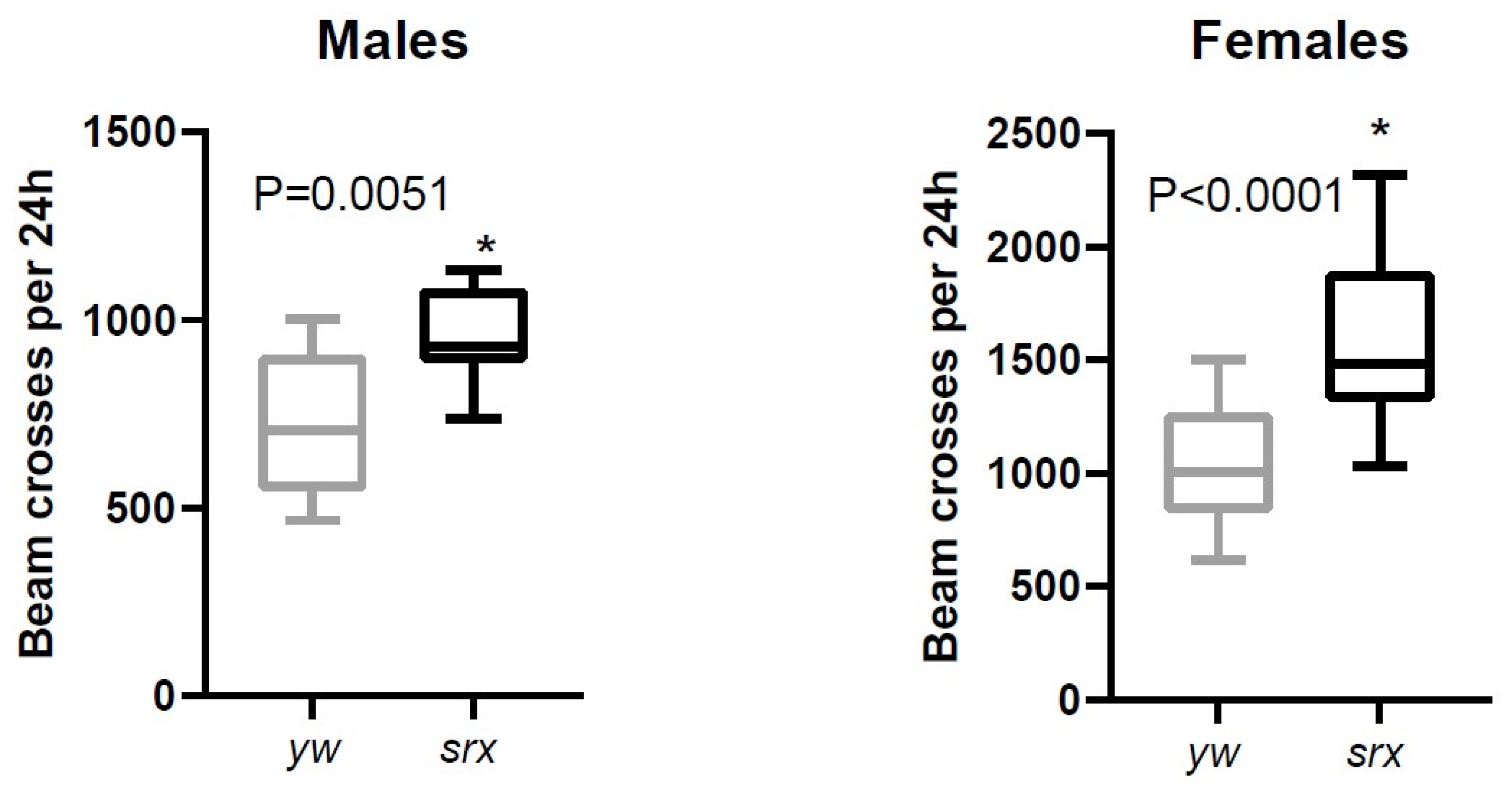
3.5. The Effect of Sulfiredoxin Underexpression on the Physiology of Drosophila
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, Z.A.; Poole, L.B.; Karplus, P.A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 2003, 300, 650–653. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A. Multiple functions of peroxiredoxins: Peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid. Redox Signal. 2011, 15, 781–794. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A. Multiple functions of 2-Cys peroxiredoxins, I and II, and their regulations via post-translational modifications. Free Radic. Biol. Med. 2020, 152, 107–115. [Google Scholar] [CrossRef]
- Poynton, R.A.; Peskin, A.V.; Haynes, A.C.; Lowther, W.T.; Hampton, M.B.; Winterbourn, C.C. Kinetic analysis of structural influences on the susceptibility of peroxiredoxins 2 and 3 to hyperoxidation. Biochem. J. 2016, 473, 411–421. [Google Scholar] [CrossRef]
- Yang, K.S.; Kang, S.W.; Woo, H.A.; Hwang, S.C.; Chae, H.Z.; Kim, K.; Rhee, S.G. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. J. Biol. Chem. 2002, 277, 38029–38036. [Google Scholar] [CrossRef]
- Cox, A.G.; Pearson, A.G.; Pullar, J.M.; Jonsson, T.J.; Lowther, W.T.; Winterbourn, C.C.; Hampton, M.B. Mitochondrial peroxiredoxin 3 is more resilient to hyperoxidation than cytoplasmic peroxiredoxins. Biochem. J. 2009, 421, 51–58. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Peskin, A.V. Kinetic Approaches to Measuring Peroxiredoxin Reactivity. Mol. Cells 2016, 39, 26–30. [Google Scholar] [CrossRef]
- Mun, Y.C.; Ahn, J.Y.; Yoo, E.S.; Lee, K.E.; Nam, E.M.; Huh, J.; Woo, H.A.; Rhee, S.G.; Seong, C.M. Peroxiredoxin 3 Has Important Roles on Arsenic Trioxide Induced Apoptosis in Human Acute Promyelocytic Leukemia Cell Line via Hyperoxidation of Mitochondrial Specific Reactive Oxygen Species. Mol. Cells 2020, 43, 813–820. [Google Scholar] [CrossRef]
- Knoops, B.; Goemaere, J.; Van der Eecken, V.; Declercq, J.P. Peroxiredoxin 5: Structure, mechanism, and function of the mammalian atypical 2-Cys peroxiredoxin. Antioxid. Redox Signal. 2011, 15, 817–829. [Google Scholar] [CrossRef]
- Cao, Z.; Subramaniam, S.; Bulleid, N.J. Lack of an efficient endoplasmic reticulum-localized recycling system protects peroxiredoxin IV from hyperoxidation. J. Biol. Chem. 2014, 289, 5490–5498. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Jeong, W.; Chang, T.S.; Woo, H.A. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: Its discovery, mechanism of action, and biological significance. Kidney Int. Suppl. 2007, 72, S3–S8. [Google Scholar] [CrossRef]
- Radyuk, S.N.; Rebrin, I.; Klichko, V.I.; Sohal, B.H.; Michalak, K.; Benes, J.; Sohal, R.S.; Orr, W.C. Mitochondrial peroxiredoxins are critical for the maintenance of redox state and the survival of adult Drosophila. Free Radic. Biol. Med. 2010, 49, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Radyuk, S.N.; Klichko, V.I.; Michalak, K.; Orr, W.C. The effect of peroxiredoxin 4 on fly physiology is a complex interplay of antioxidant and signaling functions. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1426–1438. [Google Scholar] [CrossRef]
- Lee, W.; Choi, K.S.; Riddell, J.; Ip, C.; Ghosh, D.; Park, J.H.; Park, Y.M. Human peroxiredoxin 1 and 2 are not duplicate proteins: The unique presence of CYS83 in Prx1 underscores the structural and functional differences between Prx1 and Prx2. J. Biol. Chem. 2007, 282, 22011–22022. [Google Scholar] [CrossRef]
- Beaver, L.M.; Klichko, V.I.; Chow, E.S.; Kotwica-Rolinska, J.; Williamson, M.; Orr, W.C.; Radyuk, S.N.; Giebultowicz, J.M. Circadian regulation of glutathione levels and biosynthesis in Drosophila melanogaster. PLoS ONE 2012, 7, e50454. [Google Scholar] [CrossRef] [PubMed]
- Radyuk, S.N.; Sohal, R.S.; Orr, W.C. Thioredoxin peroxidases can foster cytoprotection or cell death in response to different stressors: Over- and under-expression of thioredoxin peroxidase in Drosophila cells. Biochem. J. 2003, 371, 743–752. [Google Scholar] [CrossRef]
- Kang, S.W.; Rhee, S.G.; Chang, T.S.; Jeong, W.; Choi, M.H. 2-Cys peroxiredoxin function in intracellular signal transduction: Therapeutic implications. Trends Mol. Med. 2005, 11, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Radyuk, S.N.; Klichko, V.I.; Spinola, B.; Sohal, R.S.; Orr, W.C. The peroxiredoxin gene family in Drosophila melanogaster. Free Radic. Biol. Med. 2001, 31, 1090–1100. [Google Scholar] [CrossRef]
- Radyuk, S.N.; Orr, W.C. The Multifaceted Impact of Peroxiredoxins on Aging and Disease. Antioxid. Redox Signal. 2018, 29, 1293–1311. [Google Scholar] [CrossRef]
- Rhee, S.G.; Kang, S.W.; Chang, T.S.; Jeong, W.; Kim, K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 2001, 52, 35–41. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef]
- Peskin, A.V.; Dickerhof, N.; Poynton, R.A.; Paton, L.N.; Pace, P.E.; Hampton, M.B.; Winterbourn, C.C. Hyperoxidation of peroxiredoxins 2 and 3: Rate constants for the reactions of the sulfenic acid of the peroxidatic cysteine. J. Biol. Chem. 2013, 288, 14170–14177. [Google Scholar] [CrossRef]
- Pei, J.F.; Li, X.K.; Li, W.Q.; Gao, Q.; Zhang, Y.; Wang, X.M.; Fu, J.Q.; Cui, S.S.; Qu, J.H.; Zhao, X.; et al. Diurnal oscillations of endogenous H2O2 sustained by p66(Shc) regulate circadian clocks. Nat. Cell Biol. 2019, 21, 1553–1564. [Google Scholar] [CrossRef]
- Sohal, R.S.; Ku, H.H.; Agarwal, S.; Forster, M.J.; Lal, H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994, 74, 121–133. [Google Scholar] [CrossRef]
- Sohal, R.S.; Sohal, B.H.; Orr, W.C. Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative damage, and longevity in different species of flies. Free Radic. Biol. Med. 1995, 19, 499–504. [Google Scholar] [CrossRef]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef]
- Woo, H.A.; Kang, S.W.; Kim, H.K.; Yang, K.S.; Chae, H.Z.; Rhee, S.G. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J. Biol. Chem. 2003, 278, 47361–47364. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.S.; Reddy, A.B. Circadian clocks in human red blood cells. Nature 2011, 469, 498–503. [Google Scholar] [CrossRef]
- Jeong, W.; Park, S.J.; Chang, T.S.; Lee, D.Y.; Rhee, S.G. Molecular mechanism of the reduction of cysteine sulfinic acid of peroxiredoxin to cysteine by mammalian sulfiredoxin. J. Biol. Chem. 2006, 281, 14400–14407. [Google Scholar] [CrossRef] [PubMed]
- Lowther, W.T.; Haynes, A.C. Reduction of cysteine sulfinic acid in eukaryotic, typical 2-Cys peroxiredoxins by sulfiredoxin. Antioxid. Redox Signal. 2011, 15, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Jeong, W.; Woo, H.A.; Lee, S.M.; Park, S.; Rhee, S.G. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J. Biol. Chem. 2004, 279, 50994–51001. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.S.; Yoon, H.J.; Kim, J.Y.; Woo, H.A.; Rhee, S.G. Circadian rhythm of hyperoxidized peroxiredoxin II is determined by hemoglobin autoxidation and the 20S proteasome in red blood cells. Proc. Natl. Acad. Sci. USA 2014, 111, 12043–12048. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Han, S.H.; Sung, S.H.; Lee, H.E.; Kim, Y.M.; Noh, Y.H.; Bae, S.H.; Rhee, S.G.; Chang, T.S. Sulfiredoxin protein is critical for redox balance and survival of cells exposed to low steady-state levels of H2O2. J. Biol. Chem. 2012, 287, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Sung, S.H.; Lee, H.E.; Kang, H.T.; Lee, S.K.; Oh, S.Y.; Woo, H.A.; Kil, I.S.; Rhee, S.G. Peroxiredoxin III and sulfiredoxin together protect mice from pyrazole-induced oxidative liver injury. Antioxid. Redox Signal. 2012, 17, 1351–1361. [Google Scholar] [CrossRef]
- Bae, S.H.; Sung, S.H.; Cho, E.J.; Lee, S.K.; Lee, H.E.; Woo, H.A.; Yu, D.Y.; Kil, I.S.; Rhee, S.G. Concerted action of sulfiredoxin and peroxiredoxin I protects against alcohol-induced oxidative injury in mouse liver. Hepatology 2011, 53, 945–953. [Google Scholar] [CrossRef]
- Woo, H.A.; Jeong, W.; Chang, T.S.; Park, K.J.; Park, S.J.; Yang, J.S.; Rhee, S.G. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-cys peroxiredoxins. J. Biol. Chem. 2005, 280, 3125–3128. [Google Scholar] [CrossRef]
- Woo, H.A.; Chae, H.Z.; Hwang, S.C.; Yang, K.S.; Kang, S.W.; Kim, K.; Rhee, S.G. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science 2003, 300, 653–656. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, H.; Chawsheen, H.A.; Mishra, M.; Young, M.R.; Gerard, M.; Toledano, M.B.; Colburn, N.H.; Wei, Q. Tumor promoter-induced sulfiredoxin is required for mouse skin tumorigenesis. Carcinogenesis 2014, 35, 1177–1184. [Google Scholar] [CrossRef]
- Liu, X.P.; Liu, X.Y.; Zhang, J.; Xia, Z.L.; Liu, X.; Qin, H.J.; Wang, D.W. Molecular and functional characterization of sulfiredoxin homologs from higher plants. Cell Res. 2006, 16, 287–296. [Google Scholar] [CrossRef]
- Li, L.; Lin, G.; Gu, H.; Yu, L.; Ni, C. Effects of Srxn1 on growth and Notch signalling of astrocyte induced by hydrogen peroxide. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1917–1923. [Google Scholar] [CrossRef]
- Kil, I.S.; Ryu, K.W.; Lee, S.K.; Kim, J.Y.; Chu, S.Y.; Kim, J.H.; Park, S.; Rhee, S.G. Circadian Oscillation of Sulfiredoxin in the Mitochondria. Mol. Cell 2015, 59, 651–663. [Google Scholar] [CrossRef]
- Lan, W.; Lin, J.; Liu, W.; Wang, F.; Xie, Y. Sulfiredoxin-1 protects spinal cord neurons against oxidative stress in the oxygen-glucose deprivation/reoxygenation model through the bax/cytochrome c/caspase 3 apoptosis pathway. Neurosci. Lett. 2021, 744, 135615. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, Z.; Ou, J.; Su, X.; Liu, J. Inflammatory response and oxidative stress attenuated by sulfiredoxin1 in neuronlike cells depends on nuclear factor erythroid2related factor 2. Mol. Med. Rep. 2020, 22, 4734–4742. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Yu, S.; Li, L.; Zhao, X.; Li, Q.; Zhao, J.; Zhao, Y. Neuroprotective effects of sulfiredoxin-1 during cerebral ischemia/reperfusion oxidative stress injury in rats. Brain Res. Bull. 2017, 132, 99–108. [Google Scholar] [CrossRef]
- Zhang, J.; He, Z.; Guo, J.; Li, Z.; Wang, X.; Yang, C.; Cui, X. Sulfiredoxin-1 protects against simulated ischaemia/reperfusion injury in cardiomyocyte by inhibiting PI3K/AKT-regulated mitochondrial apoptotic pathways. Biosci. Rep. 2016, 36. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, S.; Zhao, Y. Sulfiredoxin-1 alleviates high glucose-induced podocyte injury though promoting Nrf2/ARE signaling via inactivation of GSK-3beta. Biochem. Biophys. Res. Commun. 2019, 516, 1137–1144. [Google Scholar] [CrossRef]
- Kim, T.; Li, D.; Terasaka, T.; Nicholas, D.A.; Knight, V.S.; Yang, J.J.; Lawson, M.A. SRXN1 Is Necessary for Resolution of GnRH-Induced Oxidative Stress and Induction of Gonadotropin Gene Expression. Endocrinology 2019, 160, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Jiang, H.; Chawsheen, H.A.; Gerard, M.; Toledano, M.B.; Wei, Q. Nrf2-activated expression of sulfiredoxin contributes to urethane-induced lung tumorigenesis. Cancer Lett. 2018, 432, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Chawsheen, H.A.; Jiang, H.; Ying, Q.; Ding, N.; Thapa, P.; Wei, Q. The redox regulator sulfiredoxin forms a complex with thioredoxin domain-containing 5 protein in response to ER stress in lung cancer cells. J. Biol. Chem. 2019, 294, 8991–9006. [Google Scholar] [CrossRef] [PubMed]
- Barquilha, C.N.; Santos, N.J.; Moncao, C.C.D.; Barbosa, I.C.; Lima, F.O.; Justulin, L.A.; Pertega-Gomes, N.; Felisbino, S.L. Sulfiredoxin as a Potential Therapeutic Target for Advanced and Metastatic Prostate Cancer. Oxid. Med. Cell Longev. 2020, 2020, 2148562. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yu, H.; Zhang, Q.; Huang, Q.; Hong, X.; Yu, T.; Lan, H.; Mei, C.; Zhang, W.; Luo, H.; et al. SRXN1 stimulates hepatocellular carcinoma tumorigenesis and metastasis through modulating ROS/p65/BTG2 signalling. J. Cell. Mol. Med. 2020, 24, 10714–10729. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Jiang, H.; Baker, A.; Dodge, L.K.; Gerard, M.; Young, M.R.; Toledano, M.B.; Colburn, N.H. Loss of sulfiredoxin renders mice resistant to azoxymethane/dextran sulfate sodium-induced colon carcinogenesis. Carcinogenesis 2013, 34, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Jiang, H.; Xiao, Z.; Baker, A.; Young, M.R.; Veenstra, T.D.; Colburn, N.H. Sulfiredoxin-Peroxiredoxin IV axis promotes human lung cancer progression through modulation of specific phosphokinase signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 7004–7009. [Google Scholar] [CrossRef] [PubMed]
- Biteau, B.; Labarre, J.; Toledano, M.B. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 2003, 425, 980–984. [Google Scholar] [CrossRef]
- Hanzen, S.; Vielfort, K.; Yang, J.; Roger, F.; Andersson, V.; Zamarbide-Fores, S.; Andersson, R.; Malm, L.; Palais, G.; Biteau, B.; et al. Lifespan Control by Redox-Dependent Recruitment of Chaperones to Misfolded Proteins. Cell 2016, 166, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Veal, E.A.; Underwood, Z.E.; Tomalin, L.E.; Morgan, B.A.; Pillay, C.S. Hyperoxidation of Peroxiredoxins: Gain or Loss of Function? Antioxid. Redox Signal. 2018, 1, 574–590. [Google Scholar] [CrossRef]
- Bischoff, L.J.M.; Kuijper, I.A.; Schimming, J.P.; Wolters, L.; Braak, B.T.; Langenberg, J.P.; Noort, D.; Beltman, J.B.; van de Water, B. A systematic analysis of Nrf2 pathway activation dynamics during repeated xenobiotic exposure. Arch. Toxicol. 2019, 93, 435–451. [Google Scholar] [CrossRef]
- Soriano, F.X.; Leveille, F.; Papadia, S.; Higgins, L.G.; Varley, J.; Baxter, P.; Hayes, J.D.; Hardingham, G.E. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J. Neurochem. 2008, 107, 533–543. [Google Scholar] [CrossRef]
- Kwak, M.S.; Kim, H.S.; Lee, B.; Kim, Y.H.; Son, M.; Shin, J.S. Immunological Significance of HMGB1 Post-Translational Modification and Redox Biology. Front. Immunol. 2020, 11, 1189. [Google Scholar] [CrossRef]
- He, Y.; Li, S.; Tang, D.; Peng, Y.; Meng, J.; Peng, S.; Deng, Z.; Qiu, S.; Liao, X.; Chen, H.; et al. Circulating Peroxiredoxin-1 is a novel damage-associated molecular pattern and aggravates acute liver injury via promoting inflammation. Free Radic. Biol. Med. 2019, 137, 24–36. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.S.; Zhang, Z.H.; Zhou, X.M.; Gao, Y.Y.; Liu, G.J.; Wang, H.; Wu, L.Y.; Li, W.; Hang, C.H. Peroxiredoxin 2 activates microglia by interacting with Toll-like receptor 4 after subarachnoid hemorrhage. J. Neuroinflamm. 2018, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Sunico, C.R.; Sultan, A.; Nakamura, T.; Dolatabadi, N.; Parker, J.; Shan, B.; Han, X.; Yates, J.R., 3rd; Masliah, E.; Ambasudhan, R.; et al. Role of sulfiredoxin as a peroxiredoxin-2 denitrosylase in human iPSC-derived dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E7564–E7571. [Google Scholar] [CrossRef]
- Park, J.W.; Mieyal, J.J.; Rhee, S.G.; Chock, P.B. Deglutathionylation of 2-Cys peroxiredoxin is specifically catalyzed by sulfiredoxin. J. Biol. Chem. 2009, 284, 23364–23374. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Fu, L.; Jung, Y.; Conte, M.L.; Lawson, J.R.; Lowther, W.T.; Sun, R.; Liu, K.; Yang, J.; Carroll, K.S. Chemical proteomics reveals new targets of cysteine sulfinic acid reductase. Nat. Chem. Biol. 2018, 14, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Low, F.M.; Hampton, M.B.; Peskin, A.V.; Winterbourn, C.C. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood 2007, 109, 2611–2617. [Google Scholar] [CrossRef]
- Cox, A.G.; Pullar, J.M.; Hughes, G.; Ledgerwood, E.C.; Hampton, M.B. Oxidation of mitochondrial peroxiredoxin 3 during the initiation of receptor-mediated apoptosis. Free Radic. Biol. Med. 2008, 44, 1001–1009. [Google Scholar] [CrossRef]
- Stacey, M.M.; Vissers, M.C.; Winterbourn, C.C. Oxidation of 2-cys peroxiredoxins in human endothelial cells by hydrogen peroxide, hypochlorous acid, and chloramines. Antioxid. Redox Signal. 2012, 17, 411–421. [Google Scholar] [CrossRef]


| Under-Expressors | Resistance to Oxidative Stress | Lifespan |
|---|---|---|
| Prx1 (CG6888) | similar to control | similar to control |
| Prx2 (CG1633) | n/a | short lived, few progeny |
| Prx3 (CG5826) | slight decrease | similar to control |
| Prx4 (CG1274) | significant decrease | similar to control |
| Srx (CG6762) | similar to control or slight increase | similar to control or slight increase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGinnis, A.; Klichko, V.I.; Orr, W.C.; Radyuk, S.N. Hyperoxidation of Peroxiredoxins and Effects on Physiology of Drosophila. Antioxidants 2021, 10, 606. https://doi.org/10.3390/antiox10040606
McGinnis A, Klichko VI, Orr WC, Radyuk SN. Hyperoxidation of Peroxiredoxins and Effects on Physiology of Drosophila. Antioxidants. 2021; 10(4):606. https://doi.org/10.3390/antiox10040606
Chicago/Turabian StyleMcGinnis, Austin, Vladimir I. Klichko, William C. Orr, and Svetlana N. Radyuk. 2021. "Hyperoxidation of Peroxiredoxins and Effects on Physiology of Drosophila" Antioxidants 10, no. 4: 606. https://doi.org/10.3390/antiox10040606
APA StyleMcGinnis, A., Klichko, V. I., Orr, W. C., & Radyuk, S. N. (2021). Hyperoxidation of Peroxiredoxins and Effects on Physiology of Drosophila. Antioxidants, 10(4), 606. https://doi.org/10.3390/antiox10040606







