Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans Regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Walnut Flower Extract
Preparation of the Extracts
2.3. Experimental Design
2.4. Determination of Total Bioactive Compounds
2.4.1. Total Phenolic Content
2.4.2. Total Flavonoid Content
2.4.3. Condensed Tannin Content
2.5. Determination of the Antioxidant Activity
2.5.1. DPPH Radical Scavenging Activity
2.5.2. FRAP Assay
2.5.3. TEAC Assay
2.6. Analyses of the WMF Extracts
2.6.1. Individual Phenolic Investigation
2.6.2. Tocopherol Quantification
2.7. Biological Activities
2.7.1. Enzyme Inhibitory Assays
Tyrosinase Inhibitory Assay
α-Glucosidase Inhibitory Assay
2.7.2. Biological Activities of WMF Extract on Cell Lines
Cell Culture
Preparation of Extract Solutions
Viability Assays
Dichloro-Fluorescein Diacetate (DCFH-DA) Assay
2.8. Statistical Analysis
3. Results and Discussion
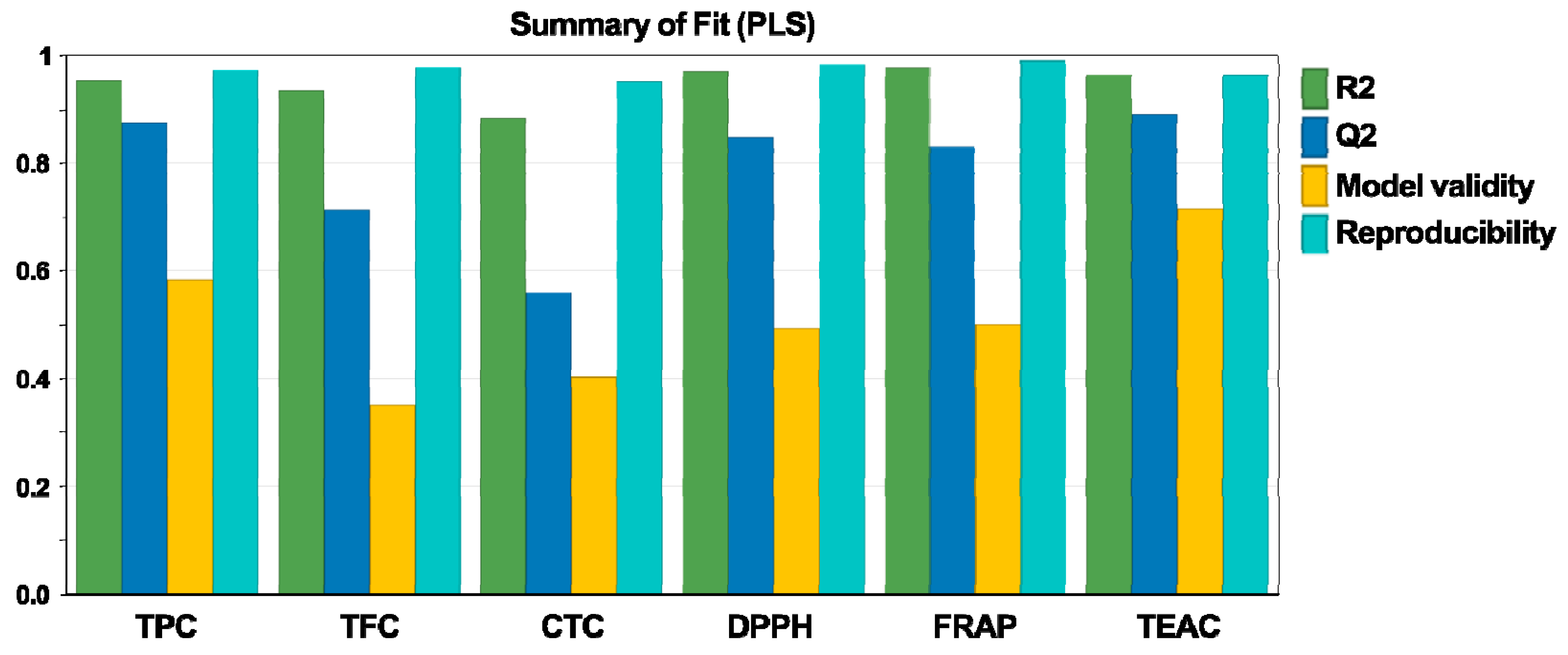
3.1. Fitting the Experimental Data
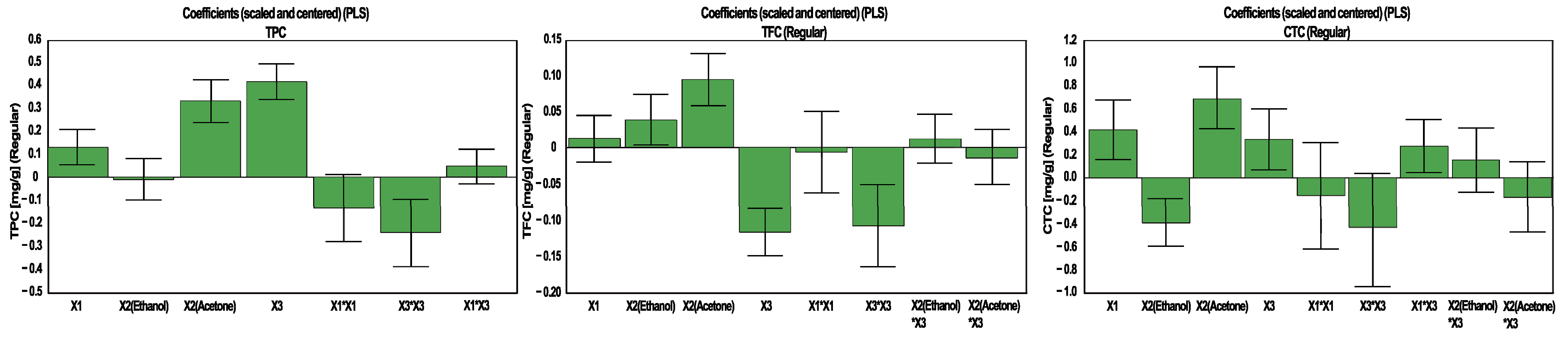
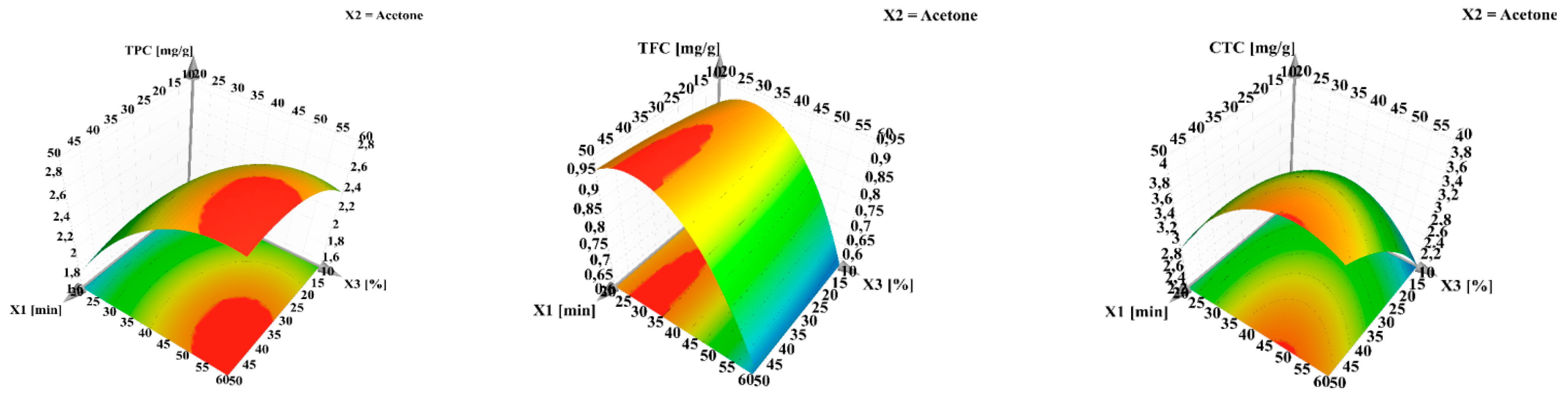
3.2. The Influence of Experimental Conditions
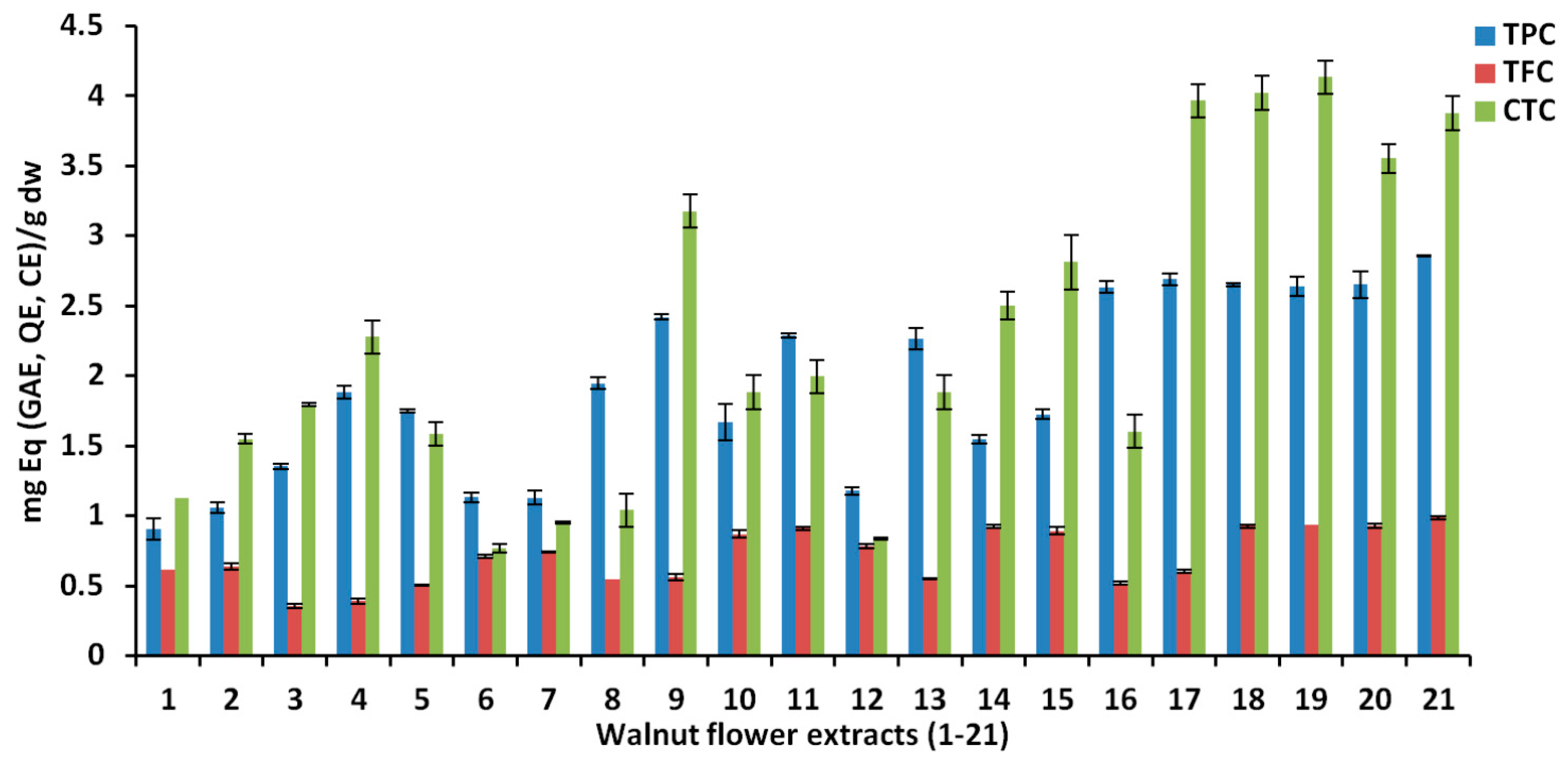
3.2.1. The Influence of Experimental Conditions on TPC, TFC and CTC
3.2.2. The Influence of Experimental Conditions on Antioxidant Activity
3.3. Phenolic Content and Antioxidant Activity
3.4. Individual Bioactive Compounds
3.5. Biological Activities
3.5.1. Enzyme Inhibitory Assays
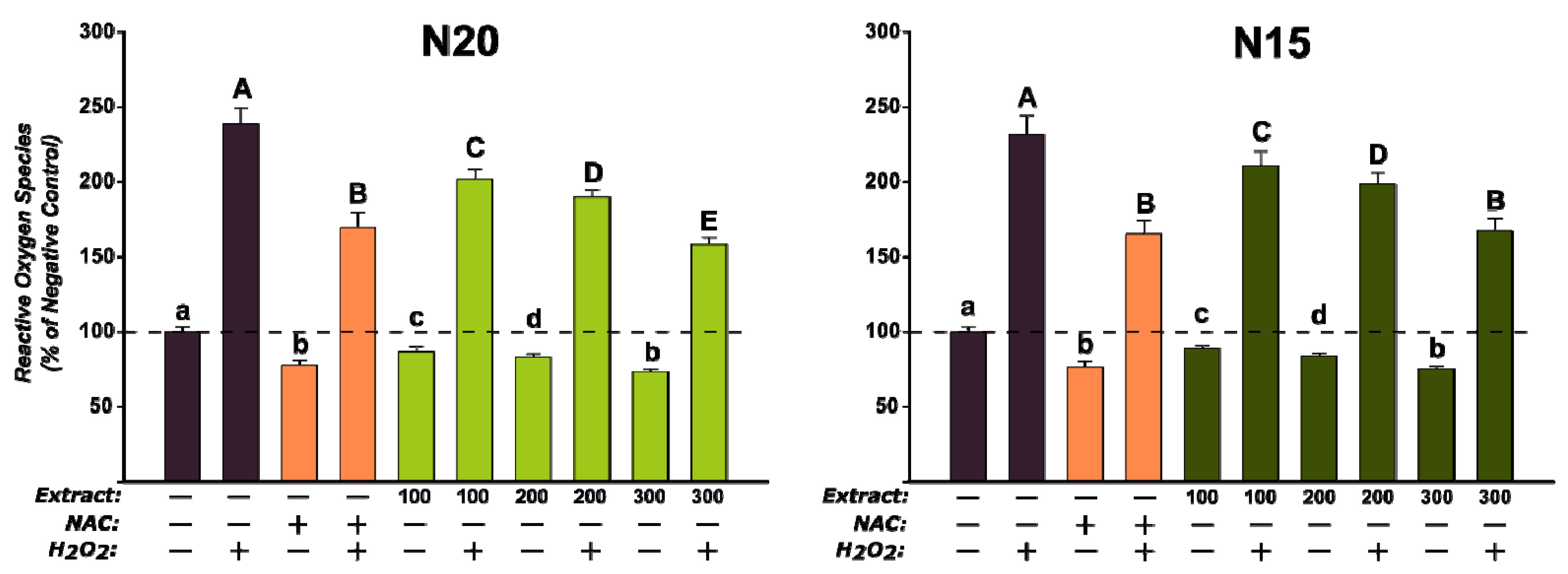
3.5.2. Biological Activities on Cell Lines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Panth, N.; Paudel, K.R.; Karki, R. Phytochemical profile and biological activity of Juglans regia. J. Integr. Med. 2016, 14, 359–373. [Google Scholar] [CrossRef]
- Delaviz, H.; Mohammadi, J.; Ghalamfarsa, G.; Mohammadi, B.; Farhadi, N. A review study on phytochemistry and pharmacology applications of Juglans regia plant. Pharmacogn. Rev. 2017, 11, 145. [Google Scholar]
- Rusu, M.E.; Mocan, A.; Ferreira, I.C.F.R.; Popa, D.-S. Health Benefits of Nut Consumption in Middle-Aged and Elderly Population. Antioxidants 2019, 8, 302. [Google Scholar] [CrossRef]
- Yan, M.; Chen, M.; Zhou, F.; Cai, D.; Bai, H.; Wang, P.; Lei, H.; Ma, Q. Separation and analysis of flavonoid chemical constituents in flowers of Juglans regia L. by ultra-high-performance liquid chromatography-hybrid quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2019, 164, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Georgiu, C.; Pop, A.; Mocan, A.; Kiss, B.; Vostinaru, O.; Fizesan, I.; Stefan, M.-G.; Gheldiu, A.-M.; Mates, L.; et al. Antioxidant Effects of Walnut (Juglans regia L.) Kernel and Walnut Septum Extract in a D-Galactose-Induced Aging Model and in Naturally Aged Rats. Antioxidants 2020, 9, 424. [Google Scholar] [CrossRef]
- Santos, A.; Barros, L.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Leaves and decoction of Juglans regia L.: Different performances regarding bioactive compounds and in vitro antioxidant and antitumor effects. Ind. Crop. Prod. 2013, 51, 430–436. [Google Scholar] [CrossRef]
- Vieira, V.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Coutinho, J.A.P.; Ferreira, O.; Barros, L.; Ferreira, I.C.F.R. Hydroethanolic extract of Juglans regia L. green husks: A source of bioactive phytochemicals. Food Chem. Toxicol. 2020, 137, 111189. [Google Scholar] [CrossRef]
- Rusu, M.E.; Gheldiu, A.-M.; Mocan, A.; Moldovan, C.; Popa, D.-S.; Tomuta, I.; Vlase, L. Process Optimization for Improved Phenolic Compounds Recovery from Walnut (Juglans regia L.) Septum: Phytochemical Profile and Biological Activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Gheldiu, A.-M.; Mocan, A.; Vlase, L.; Popa, D.-S. Anti-aging potential of tree nuts with a focus on phytochemical composition, molecular mechanisms and thermal stability of major bioactive compounds. Food Funct. 2018, 9, 2554–2575. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Fizesan, I.; Pop, A.; Mocan, A.; Gheldiu, A.-M.; Babota, M.; Vodnar, D.C.; Jurj, A.; Berindan-Neagoe, I.; Vlase, L.; et al. Walnut (Juglans regia L.) Septum: Assessment of Bioactive Molecules and In Vitro Biological Effects. Molecules 2020, 25, 2187. [Google Scholar] [CrossRef] [PubMed]
- Fizeșan, I.; Rusu, M.E.; Georgiu, C.; Pop, A.; Ștefan, M.-G.; Muntean, D.-M.; Mirel, S.; Vostinaru, O.; Kiss, B.; Popa, D.-S. Antitussive, Antioxidant, and Anti-Inflammatory Effects of a Walnut (Juglans regia L.) Septum Extract Rich in Bioactive Compounds. Antioxidants 2021, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Mashhoody, T.; Panjehshahin, M. Effect of aqueous extract of walnut septum on blood glucose and pancreatic structure in streptozotocin-induced diabetic mouse. Iran. J. Pharmacol. Ther. 2012, 11, 10–14. [Google Scholar]
- Dzidziguri, D.; Rukhadze, M.; Modebadze, I.; Bakuradze, E.; Kurtanidze, M.; Giqoshvili, V. The study of the immune corrective properties of greek walnut (Juglans regia L.) septa on the experimental model of leukopenia. Georg. Med. News 2016, 252, 84–89. [Google Scholar]
- Ramishvili, L.; Gordeziani, M.; Tavdishvili, E.; Bedineishvili, N.; Dzidziguri, D.; Kotrikadze, N. The effect of extract of greek walnut (Juglans regia L.) septa on some functional characteristics of erythrocytes. Georg. Med. News 2016, 261, 51–57. [Google Scholar]
- Wang, C.; Zhang, W.; Pan, X. Nutritional Quality of the Walnut Male Inflorescences at Four Flowering Stages. J. Food Nutr. Res. 2014, 2, 457–464. [Google Scholar] [CrossRef]
- Zhang, W.E.; Wang, C.L.; Shi, B.B.; Pan, X.J. Effect of storage temperature and time on the nutritional quality of walnut male inflorescences. J. Food Drug Anal. 2017, 25, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.; Nabavi, S.; Nabavi, S. Antihemolytic activity and mineral contents of Juglans regia L. flowers. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1881–1883. [Google Scholar]
- Nabavi, S.F.; Ebrahimzadeh, M.A.; Nabavi, S.M.; Mahmoudi, M.; Rad, S.K. Biological activities of Juglans regia flowers. Braz. J. Pharmacogn. 2011, 21, 465–470. [Google Scholar] [CrossRef]
- Hosseini, E.; Karimzadeh, K.; Vessal, M. Effects of a hydroalcoholic extract of walnut male flowers on diabetic rats. Zahedan J. Res. Med. Sci. 2013, 15, 55–58. [Google Scholar]
- Muzaffer, U.; Paul, V. Phytochemical analysis, in vitro antioxidant and antimicrobial activities of male flower of Juglans regia L. Int. J. Food Prop. 2018, 21, 345–356. [Google Scholar] [CrossRef]
- Gavan, A.; Iurian, S.; Casian, T.; Porfire, A.; Porav, S.; Voina, I.; Oprea, A.; Tomuta, I. Fluidised bed granulation of two APIs: QbD approach and development of a NIR in-line monitoring method. Asian J. Pharm. Sci. 2020, 15, 506–517. [Google Scholar] [CrossRef]
- Casian, T.; Iurian, S.; Bogdan, C.; Rus, L.; Moldovan, M.; Tomuta, I. QbD for Pediatric Oral Lyophilisates Development: Risk assessment followed by Screening and Optimization. Drug Dev. Ind. Pharm. 2017, 43, 1932–1944. [Google Scholar] [CrossRef]
- Babotă, M.; Mocan, A.; Vlase, L.; Crisan, O.; Ielciu, I.; Gheldiu, A.M.; Vodnar, D.C.; Crişan, G.; Păltinean, R. Phytochemical analysis, antioxidant and antimicrobial activities of Helichrysum arenarium (L.) Moench. and Antennaria dioica (L.) Gaertn. Flowers. Molecules 2018, 23, 409. [Google Scholar] [CrossRef]
- Mocan, A.; Moldovan, C.; Zengin, G.; Bender, O.; Locatelli, M.; Simirgiotis, M.; Atalay, A.; Vodnar, D.C.; Rohn, S.; Crișan, G. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food Chem. Toxicol. 2018, 115, 414–424. [Google Scholar] [CrossRef]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A Critical Evaluation of the Vanillin Reaction as an Assay for Tannin in Sorghum Grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Mocan, A.; Fernandes, A.; Barros, L.; Crişan, G.; Smiljković, M.; Soković, M.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of the wild mushroom: Polyporus squamosus (Huds.) Fr: A study with samples from Romania. Food Funct. 2018, 9, 160–170. [Google Scholar] [CrossRef]
- Mocan, A.; Babota, M.; Pop, A.; Fizesan, I.; Diuzheva, A.; Locatteli, M.; Carradori, S.; Campestre, C.; Menghini, L.; Sisea, C.R.; et al. Chemical Constituents and Biologic Activities of Sage Species: A Comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (Schur ex Griseb. & Schenk) Schur. Antioxidants 2020, 9, 480. [Google Scholar]
- Mocan, A.; Schafberg, M.; Crisan, G.; Rohn, S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Foods. 2016, 24, 579–594. [Google Scholar] [CrossRef]
- Păltinean, R.; Mocan, A.; Vlase, L.; Gheldiu, A.; Crișan, G.; Ielciu, I.; Voștinaru, O.; Crișan, O. Evaluation of Polyphenolic Content, Antioxidant and Diuretic Activities of Six Fumaria Species. Molecules 2017, 22, 639. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.C.; Crişan, G.; Rohn, S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzym. Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef]
- Tanase, C.; Mocan, A.; Cosarca, S.; Gavan, A.; Nicolescu, A.; Gheldiu, A.-M.; Vodnar, D.; Muntean, D.-L.; Crisan, O. Biological and Chemical Insights of Beech (Fagus sylvatica L.) Bark: A Source of Bioactive Compounds with Functional Properties. Antioxidants 2019, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.L.; Carpa, R.; Fizeșan, I.; Vlase, L.; Bogdan, C.; Iurian, S.M.; Benedec, D.; Pop, A. Phytochemical Profile and Biological Activities of Tendrils and Leaves Extracts from a Variety of Vitis vinifera L. Antioxidants 2020, 9, 373. [Google Scholar] [CrossRef]
- Tefas, L.R.; Sylvester, B.; Tomuta, I.; Sesarman, A.; Licarete, E.; Banciu, M.; Porfire, A. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des. Devel. Ther. 2017, 11, 1605–1621. [Google Scholar] [CrossRef]
- Gavan, A.; Porfire, A.; Marina, C.; Tomuta, I. Formulation and pharmaceutical development of quetiapine fumarate sustained release matrix tablets using a QbD approach. Acta Pharm. 2017, 67, 53–70. [Google Scholar] [CrossRef][Green Version]
- Iurian, S.; Turdean, L.; Tomuta, I. Risk assessment and experimental design in the development of a prolonged release drug delivery system with paliperidone. Drug Des. Devel. Ther. 2017, 11, 733–746. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salvadó, J.-S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comprehensive Review on the Chemical Constituents and Functional Uses of Walnut (Juglans spp.) Husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef]
- Masullo, M.; Mari, A.; Cerulli, A.; Bottone, A.; Kontek, B.; Olas, B.; Pizza, C.; Piacente, S. Quali-quantitative analysis of the phenolic fraction of the flowers of Corylus avellana, source of the Italian PGI product “Nocciola di Giffoni”: Isolation of antioxidant diarylheptanoids. Phytochemistry 2016, 130, 273–281. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizeșan, I.; Pop, A.; Gheldiu, A.-M.; Mocan, A.; Crișan, G.; Vlase, L.; Loghin, F.; Popa, D.-S.; Tomuta, I. Enhanced Recovery of Antioxidant Compounds from Hazelnut (Corylus avellana L.) Involucre Based on Extraction Optimization: Phytochemical Profile and Biological Activities. Antioxidants 2019, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; Tassi, L.; Orteca, G.; Saladini, M.; Villa, C.; Veronesi, P.; Leonelli, C.; Ferrari, E. Process Intensification by Experimental Design Application to Microwave-Assisted Extraction of Phenolic Compounds from Juglans regia L. Food Anal Method. 2017, 10, 575–586. [Google Scholar] [CrossRef]
- Catanzaro, E.; Greco, G.; Potenza, L.; Calcabrini, C.; Fimognari, C. Natural products to fight cancer: A focus on Juglans regia. Toxins 2018, 10, 469. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.O.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Kan, H.; Chen, S.X.; Thakur, K.; Wang, S.; Zhang, J.G.; Shang, Y.F.; Wei, Z.J. Comparison of phenolic compounds extracted from Diaphragma juglandis fructus, walnut pellicle, and flowers of Juglans regia using methanol, ultrasonic wave, and enzyme assisted-extraction. Food Chem. 2020, 321, 126672. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jerónimo, C.; Silva, B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010, 48, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordoñez, M.; Knox, C.; Llorach, R.; Eisner, R.; Cruz, J.; Neveu, V.; Wishart, D.; Manach, C.; et al. Database update Phenol-Explorer 2.0: A major update of the Phenol-Explorer database integrating data on polyphenol metabolism and pharmacokinetics in humans and experimental animals. Database 2012, 2012, bas031. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Bolling, B. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef]
- Pycia, K.; Kapusta, I.; Jaworska, G. Impact of the Degree of Maturity of Walnuts (Juglans regia L.) and Their Variety on the Antioxidant Potential and the Content of Tocopherols and Polyphenols. Molecules 2019, 24, 2936. [Google Scholar] [CrossRef]
- Akbari, V.; Jamei, R.; Heidari, R.; Esfahlan, J.A. Antiradical activity of different parts of Walnut (Juglans regia L.) fruit as a function of genotype. Food Chem. 2012, 135, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.N.; Mir, J.I.; Ahmed, N.; Jan, S.; Fazili, K.M. Bioefficacy potential of different genotypes of walnut Juglans regia L. J. Food Sci. Technol. 2018, 55, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Trandafir, I.; Cosmulescu, S.; Botu, M.; Nour, V. Antioxidant activity, and phenolic and mineral contents of the walnut kernel (Juglans regia L.) as a function of the pellicle color. Fruits 2016, 71, 177–184. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Bolling, B.W.; Shahidi, F. Nuts and their co-products: The impact of processing (roasting) on phenolics, bioavailability, and health benefits—A comprehensive review. J. Funct. Foods 2016, 26, 88–122. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- de la Rosa, L.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis). J. Agric. Food Chem. 2011, 59, 152–162. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, L.; Moore, J.; Wu, T.; Wang, Z. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chem. 2009, 113, 160–165. [Google Scholar] [CrossRef]
- Pycia, K.; Kapusta, I.; Jaworska, G.; Jankowska, A. Antioxidant properties, profile of polyphenolic compounds and tocopherol content in various walnut (Juglans regia L.) varieties. Eur. Food Res. Technol. 2019, 245, 607–616. [Google Scholar] [CrossRef]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.-B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A Systematic Screening of Total Antioxidants in Dietary Plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef]
- Mollica, A.; Zengin, G.; Stefanucci, A.; Ferrante, C.; Menghini, L.; Orlando, G.; Brunetti, L.; Locatelli, M.; Dimmito, M.; Novellino, E.; et al. Nutraceutical potential of Corylus avellana daily supplements for obesity and related dysmetabolism. J. Funct. Foods 2018, 47, 562–574. [Google Scholar] [CrossRef]
- Montella, R.; Coisson, J.D.; Travaglia, F.; Locatelli, M.; Malfa, P.; Martelli, A.; Arlorio, M. Bioactive compounds from hazelnut skin (Corylus avellana L.): Effects on Lactobacillus plantarum P17630 and Lactobacillus crispatus P17631. J. Funct. Foods 2013, 5, 306–315. [Google Scholar] [CrossRef]
- Jakopic, J.; Petkovsek, M.M.; Likozar, A.; Solar, A.; Stampar, F.; Veberic, R. HPLC-MS identification of phenols in hazelnut (Corylus avellana L.) kernels. Food Chem. 2011, 124, 1100–1106. [Google Scholar] [CrossRef]
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. J. Agric. Food Chem. 2007, 55, 1212–1220. [Google Scholar] [CrossRef]
- Rusu, M.E.; Simedrea, R.; Gheldiu, A.-M.; Mocan, A.; Vlase, L.; Popa, D.-S.; Ferreira, I.C.F.R. Benefits of tree nut consumption on aging and age-related diseases: Mechanisms of actions. Trends Food Sci. Technol. 2019, 88, 104–120. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of health claims related to vitamin E and protection of DNA, proteins and lipids from oxidative damage, maintenance of the normal function of the immune system. EFSA J. 2010, 8, 1816. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Becerra-Tomás, N.; Papandreou, C.; Bulló, M. Dietary Patterns Emphasizing the Consumption of Plant Foods in the Management of Type 2 Diabetes: A Narrative Review. Adv. Nutr. 2019, 1, S320–S331. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Lee, M.-J.; Liu, A.B.; Yang, Z.; Lin, Y.; Shih, J.W.; Yang, C.S. δ-tocopherol is more active than α- or γ -tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev. Res. 2011, 4, 404–413. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Khan, M.T.H.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Sabourye, A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Song, Y.H.; Park, C.; Lee, K.W.; Kim, J.Y.; Kim, D.W.; Kim, K.D.; Lee, K.W.; Curtis-Long, M.J.; Park, K.H. Highly potent tyrosinase inhibitor, neorauflavane from Campylotropis hirtella and inhibitory mechanism with molecular docking. Bioorg. Med. Chem. 2016, 24, 153–159. [Google Scholar] [CrossRef]
- Negi, A.S.; Luqman, S.; Srivastava, S.; Krishna, V.; Gupta, N.; Darokar, M.P. Antiproliferative and antioxidant activities of Juglans regia fruit extracts. Pharm. Biol. 2011, 49, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Muzaffer, U.; Paul, V.I.; Prasad, N.R.; Karthikeyan, R.; Agilan, B. Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomedicine 2018, 42, 100–111. [Google Scholar] [CrossRef]
- Muthaiyah, B.; Essa, M.M.; Chauhan, V.; Chauhan, A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem. Res. 2011, 36, 2096–2103. [Google Scholar] [CrossRef]
- Anjum, S.; Gani, A.; Ahmad, M.; Shah, A.; Masoodi, F.A.; Shah, Y.; Gani, A. Antioxidant and antiproliferative activity of walnut extract (Juglans regia L.) processed by different methods and identification of compounds using GC/MS and LC/MS technique. J. Food Process. Preserv. 2017, 41, e12756. [Google Scholar] [CrossRef]
- Das Gupta, S.; Suh, N. Tocopherols in cancer: An update. Mol. Nutr. Food Res. 2016, 60, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Finkelstein, E.I.; Reddy, S.; Valacchi, G.; Traber, M.; Cross, C.E.; Van der Vliet, A. Acrolein-induced cytotoxicity in cultured human bronchial epithelial cells. Modulation by alpha-tocopherol and ascorbic acid. Toxicology 2002, 170, 173–185. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Cheva, A.; Zarampouka, K.; Huang, H.; Li, C.; Huang, Y.; Katsikogiannis, N.; Zarogoulidis, K. Tocopherols and tocotrienols as anticancer treatment for lung cancer: Future nutrition. J. Thorac. Dis. 2013, 5, 349. [Google Scholar]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Shirai, M.; Yamanishi, R.; Moon, J.-H.; MUROTA, K.; TERAO, J. Effect of quercetin and its conjugated metabolite on the hydrogen peroxide-induced intracellular production of reactive oxygen species in mouse fibroblasts. Biosci. Biotechnol. Biochem. 2002, 66, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Tai, B.H.; Cuong, N.M.; Kim, Y.-H.; Jang, H.-D. Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from mampat (Cratoxylum formosum). Food Sci. Biotechnol. 2012, 21, 587–595. [Google Scholar] [CrossRef]
- Tanigawa, S.; Fujii, M.; Hou, D.-X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Nussler, A.; Liu, L.; Hao, L.; Song, F.; Schirmeier, A.; Nussler, N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007, 47, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Han, X.; Piao, M.; Oh, M.; Fernando, P.; Kang, K.; Ryu, Y.; Jung, U.; Kim, I.; Hyun, J. Hyperoside Induces Endogenous Antioxidant System to Alleviate Oxidative Stress. J. Cancer Prev. 2016, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Basak, P.; Sadhukhan, P.; Sarkar, P.; Sil, P.C. Perspectives of the Nrf-2 signaling pathway in cancer progression and therapy. Toxicol. Rep. 2017, 4, 306–318. [Google Scholar] [CrossRef] [PubMed]

| Variables | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Independent Variables (Factors) | |||
| X1—Extraction time (min) | 10 | 30 | 50 |
| X2—Solvent | Methanol | Ethanol | Acetone |
| X3—Water in solvent (%, v/v) | 20 | 40 | 60 |
| Dependent variables (responses) | |||
| Y1—Total phenolic content (TPC), mg GAE/g 1 | |||
| Y2—Total flavonoid content (TFC), mg QE/g 2 | |||
| Y3—Condensed tannin content (CTC), mg CE/g 3 | |||
| Y4—DPPH antioxidant activity, mg TE/g 4 | |||
| Y5—FRAP antioxidant activity, mg TE/g | |||
| Y6—TEAC antioxidant activity, mg TE/g | |||
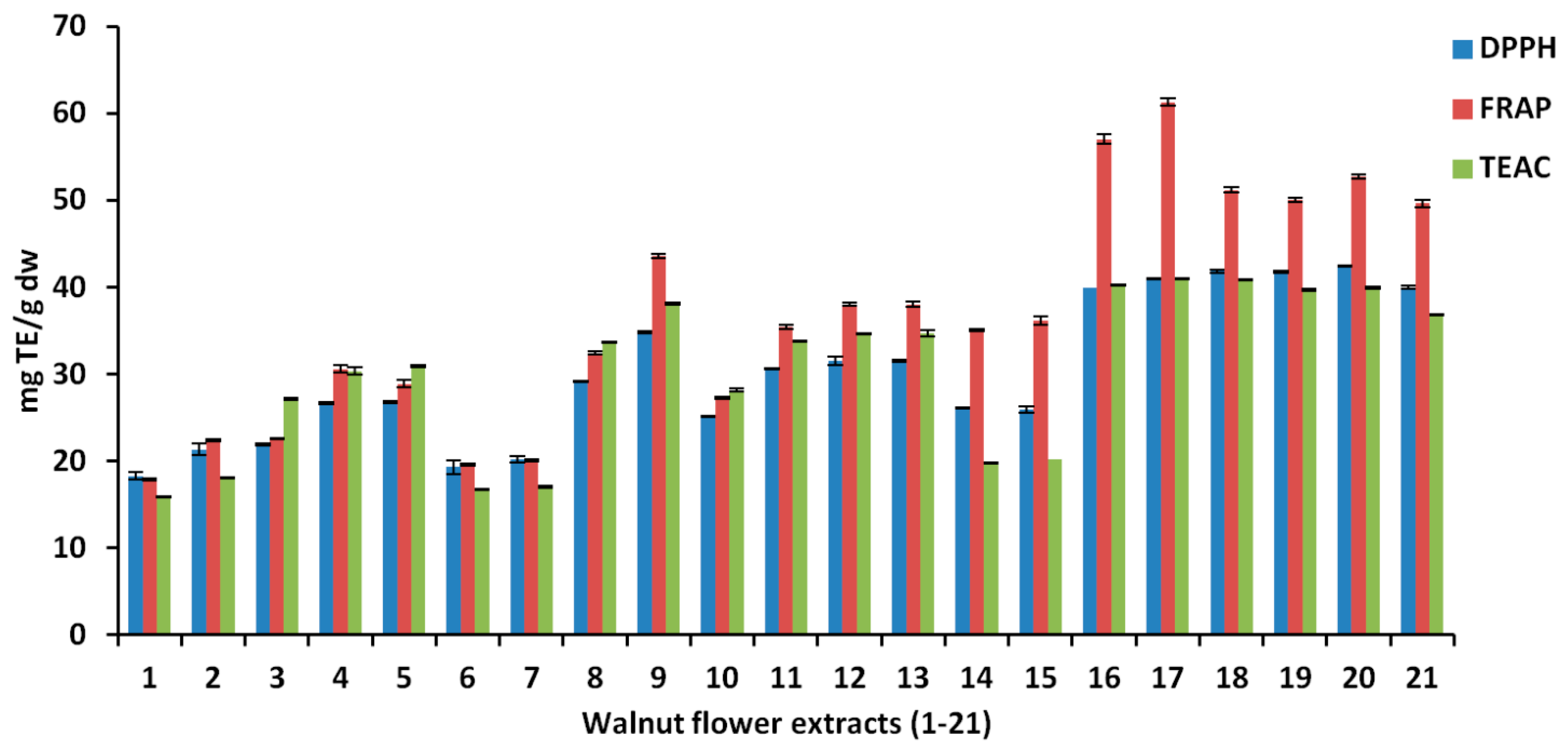
| Exp. | Run | X1 | X2 | X3 | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 |
|---|---|---|---|---|---|---|---|---|---|---|
| Extraction Time (Min) | Solvent | Water% (v/v) | TPC | TFC | CTC | DPPH | FRAP | TEAC | ||
| N1 | 1 | 10 | Methanol | 20 | 0.9049 | 0.6155 | 1.1252 | 18.311 | 17.861 | 15.914 |
| N2 | 18 | 50 | Methanol | 20 | 1.0593 | 0.6396 | 1.5492 | 21.381 | 22.437 | 18.127 |
| N3 | 21 | 10 | Methanol | 60 | 1.3529 | 0.3574 | 1.7938 | 21.885 | 22.546 | 27.165 |
| N4 | 6 | 50 | Methanol | 60 | 1.8835 | 0.3923 | 2.2773 | 26.679 | 30.615 | 30.377 |
| N5 | 10 | 30 | Methanol | 40 | 1.7495 | 0.5055 | 1.5885 | 26.808 | 28.941 | 30.949 |
| N6 | 17 | 10 | Ethanol | 20 | 1.1322 | 0.7088 | 0.7704 | 19.344 | 19.643 | 16.771 |
| N7 | 12 | 50 | Ethanol | 20 | 1.1301 | 0.7436 | 0.9503 | 20.211 | 20.125 | 17.080 |
| N8 | 9 | 10 | Ethanol | 60 | 1.9479 | 0.5502 | 1.0403 | 29.218 | 32.446 | 33.685 |
| N9 | 5 | 50 | Ethanol | 60 | 2.4204 | 0.5639 | 3.1770 | 34.897 | 43.597 | 38.158 |
| N10 | 14 | 10 | Ethanol | 40 | 1.6673 | 0.8688 | 1.8837 | 25.205 | 27.303 | 28.164 |
| N11 | 20 | 50 | Ethanol | 40 | 2.2891 | 0.9115 | 1.9962 | 30.678 | 35.420 | 33.828 |
| N12 | 16 | 30 | Ethanol | 20 | 1.1794 | 0.7828 | 0.8378 | 31.576 | 38.070 | 34.708 |
| N13 | 8 | 30 | Ethanol | 60 | 2.2677 | 0.5500 | 1.8837 | 31.576 | 38.070 | 34.708 |
| N14 | 3 | 10 | Acetone | 20 | 1.5474 | 0.9248 | 2.5023 | 26.169 | 35.118 | 19.769 |
| N15 | 13 | 50 | Acetone | 20 | 1.7240 | 0.8917 | 2.8115 | 25.980 | 36.184 | 20.245 |
| N16 | 4 | 10 | Acetone | 60 | 2.6333 | 0.5194 | 1.6026 | 39.924 | 57.074 | 40.276 |
| N17 | 15 | 50 | Acetone | 60 | 2.6908 | 0.6025 | 3.9642 | 41.013 | 61.349 | 40.990 |
| N18 | 11 | 30 | Acetone | 40 | 2.6505 | 0.9259 | 4.0205 | 41.870 | 51.221 | 40.871 |
| N19 | 19 | 30 | Acetone | 40 | 2.6419 | 0.9354 | 4.1329 | 41.798 | 50.077 | 39.729 |
| N20 | 7 | 30 | Acetone | 40 | 2.6505 | 0.9276 | 3.5542 | 42.456 | 52.798 | 40.015 |
| N21 | 2 | 30 | Acetone | 40 | 2.8591 | 0.9865 | 3.8767 | 40.022 | 49.667 | 36.872 |
| Quantifiable Responses | Source | DF | SS | MS | F-Value | p |
|---|---|---|---|---|---|---|
| Total phenolic content (Y1) (R2 = 0.95, Q2 = 0.88) | Regression | 7 | 7.85 | 1.12 | 38.05 | 0.01 |
| Lack of fit | 10 | 0.35 | 0.035 | 3.12 | 0.189 | |
| Pure error | 3 | 0.03 | 0.01 | |||
| Total flavonoid content (Y2) (R2 = 0.93, Q2 = 0.71) | Regression | 8 | 0.72 | 0.09 | 21.20 | 0.01 |
| Lack of fit | 9 | 0.05 | 0.01 | 6.54 | 0.075 | |
| Pure error | 3 | 0.01 | 0.00 | |||
| Condensed tannin content (Y3) (R2 = 0.88, Q2 = 0.56) | Regression | 9 | 22.68 | 2.52 | 9.21 | 0.01 |
| Lack of fit | 8 | 2.82 | 0.35 | 5.60 | 0.092 | |
| Pure error | 3 | 0.19 | 0.06 | |||
| DPPH antioxidant activity (Y4) (R2 = 0.97, Q2 = 0.85) | Regression | 8 | 1302.18 | 162.77 | 44.14 | 0.01 |
| Lack of fit | 8 | 37.24 | 4.65 | 4.21 | 0.132 | |
| Pure error | 3 | 3.31 | 1.11 | |||
| FRAP antioxidant activity (Y5) (R2 = 0.98, Q2 = 0.83) | Regression | 7 | 3342.93 | 477.56 | 72.98 | 0.01 |
| Lack of fit | 9 | 72.617 | 8.06 | 4.10 | 0.136 | |
| Pure error | 3 | 5.89 | 1.96 | |||
| TEAC antioxidant activity (Y6) (R2 = 0.96, Q2 = 0.89) | Regression | 8 | 1541.38 | 192.67 | 38.41 | 0.01 |
| Lack of fit | 8 | 46.13 | 5.76 | 1.91 | 0.321 | |
| Pure error | 3 | 9.03 | 3.01 |
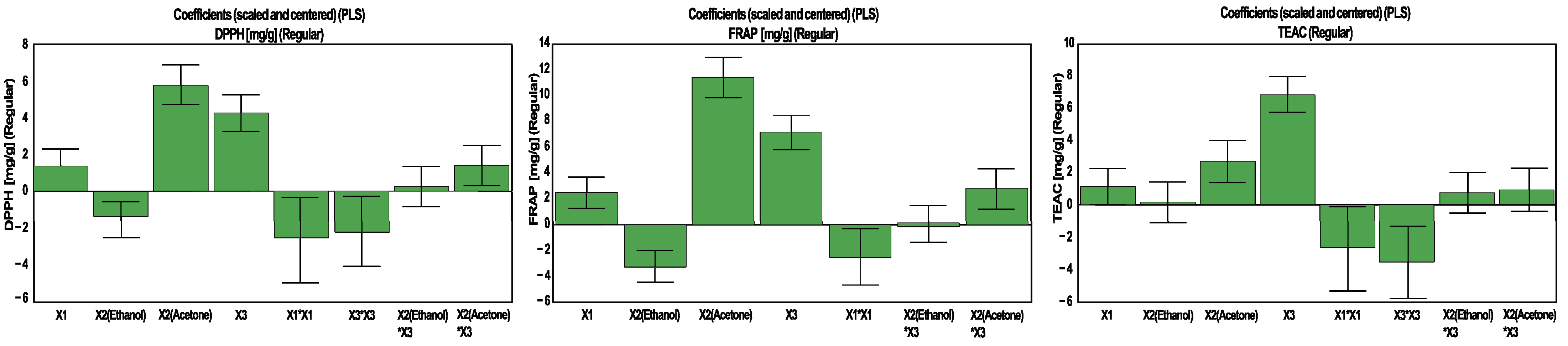
| Effect | Responses | |||||
|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | |
| TPC | TFC | CTC | DPPH | FRAP | TEAC | |
| Constant | 2.281 | 0.817 | 2.808 | 34.763 | 39.001 | 36.124 |
| Extraction time | 0.126 | 0.013 | 0.417 | 1.327 | 2.414 | 1.141 |
| X2(Ethanol) | −0.009 | 0.038 | −0.392 | −1.390 | −3.254 | 0.141 |
| X2(Acetone) | 0.329 | 0.094 | 0.681 | 5.781 | 11.379 | 2.679 |
| % water in solvent | 0.411 | −0.116 | 0.339 | 4.290 | 7.144 | 6.741 |
| X1 × X1 | −0.134 | −0.005 | −0.151 | −2.537 | −2.473 | −2.707 |
| X3 × X3 | −0.242 | −0.107 | -0.431 | −2.195 | - | −3.586 |
| X1 × X3 | 0.045 | - | 0.276 | - | - | - |
| X2(Ethanol) × X3 | - | 0.012 | 0.160 | 0.276 | 0.0556 | 0.725 |
| X2(Acetone) × X3 | - | −0.013 | −0.165 | 1.390 | 2.787 | 0.924 |
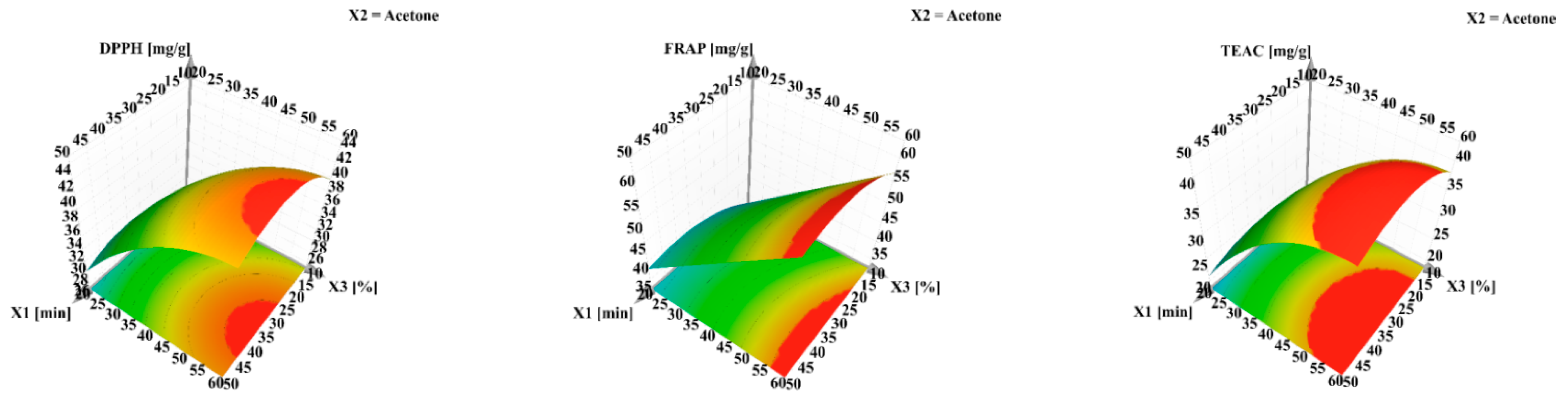
| Parameters | TPC | TFC | CTC | DPPH | FRAP | TEAC |
|---|---|---|---|---|---|---|
| Extraction time (min) | 30 | 30 | 30 | 30 | 50 | 50 |
| Solvent | Acetone | Acetone | Acetone | Acetone | Acetone | Acetone |
| Water in solvent (%) | 40 | 40 | 40 | 40 | 60 | 60 |
| Predicted | 2.92 | 0.96 | 4.03 | 43.92 | 64.45 | 42.73 |
| Determined | 2.86 | 0.98 | 4.13 | 42.46 | 61.35 | 40.99 |
| Bias (%) | 2.05 | 2.08 | 2.48 | 3.32 | 4.81 | 4.07 |
| Area | Extraction Conditions | TPC (GAE) | TFC | CTC | DPPH | FRAP | TEAC | Ref |
|---|---|---|---|---|---|---|---|---|
| Iran | Percolation in methanol (24 h) | 71.7 ± 3.2 mg/g extract | 61.7 ± 2.7 mg QE/g extract | - | IC50 = 674 ± 27.6 μg/mL | - | - | [17,18] |
| China | US in methanol (40%, v/v), 50 min at 50 °C for TPC and AA; ethanol (30%, v/v), 1 h at 70 °C in water bath for TFC | 24.3 ± 0.5 mg/g dw WMF (in EFS) | 21.5 ± 0.4 mg RE/g WMF (in FS) | - | 84.3% ± 0.1% inhibition (in EFS) | 2.1 ± 0.1/100 g dw WMF (in EFS) | - | [15] |
| China | US in methanol (40%, v/v), 50 min at 50 °C for TPC and AA; ethanol (30%, v/v), 1 h at 70 °C in water bath for TFC | 3.6 mg/100 g fresh WMF; 2.1 mg/100 g dried WMF | 3.2 mg RE/100 g fresh WMF; 1.8 mg RE/100 g dried WMF | - | 86% for fresh WMF; 78%–79% for dried WMF | 3.8/100 g fresh WMF; 2.1–2.2/100 g dried WMF | - | [16] |
| China | UE in 70% methanol | 1350.8 ± 44.6 mg/g | 385.0±16.5 mg CE/g | 38.3 ± 1.2 mg CE/g | IC50 = 57.3 ± 1.4 μg/mL | IC50 = 56.3 ± 1.5 μg/mL | IC50 = 42.4 ± 1.2 μg/mL | [43] |
| India | 95% methanol (1:6, w/v) for 48 h at 20 °C | 129.8 ± 3.1 mg/g dried material | 144.6 ± 2.4 QE mg/g dried material | - | IC50 = 66.8 ± 2.1 μg/mL | 46.6 ± 4.8 g extract | IC50 = 53.9 ± 6.5 μg/mL | [20] |
| Italy | MAE, DoE 50% ethanol in water (v/v), for 30 min at 60 °C | 3.2 ± 0.5 mg/g fresh WF (one cycle); 5.9 ± 0.2 mg/g fresh WF (3 cycles) | - | - | - | - | - | [40] |
| Exp. | |||||||
|---|---|---|---|---|---|---|---|
| N2 | N4 | N7 | N9 | N15 | N20 | ||
| X1 | Extraction time (min) | 50 | 50 | 50 | 50 | 50 | 30 |
| X2 | Solvent * | M | M | E | E | A | A |
| X3 | Water in solvent (%, v/v) | 20 | 60 | 20 | 60 | 20 | 40 |
| Y1 | Catechin | 9.8 | 5.3 | 8.5 | 7.4 | 12.6 | 15.1 |
| Y2 | Syringic acid | ND | ND | ND | 0.4 | 0.4 | 0.9 |
| Y3 | Gallic acid | 4.4 | 5.9 | ND | 6.3 | 5.2 | 8.2 |
| Y4 | Protocatechuic acid | 1.4 | 1.4 | 0.9 | 1.6 | 1.1 | 1.6 |
| Y5 | p-Coumaric acid | 90.5 | 108.5 | ND | 122.9 | 272.8 | 440.8 |
| Y6 | Ferulic acid | ND | 12.6 | ND | ND | 32.9 | 49.1 |
| Y7 | Hyperoside | 332.6 | 575.2 | 544.1 | 556.5 | 1487.4 | 2662.9 |
| Y8 | Isoquercitrin | 94.4 | 348.7 | 91.3 | 148.3 | 217.7 | 405.7 |
| Y9 | Quercitrin | 196.2 | 314.1 | 284.1 | 289.7 | 714.1 | 1293.7 |
| Y10 | Quercetin | ND | 7.8 | 11.6 | 12.7 | 18.8 | 101.9 |
| Y11 | α-Tocopherol | 0.6 | 0.5 | 0.9 | ND | 1.6 | ND |
| Y12 | γ/β-Tocopherol | 0.3 | ND | 1.8 | ND | 3.1 | ND |
| Y13 | δ-Tocopherol | 4.3 | 0.6 | 14.4 | ND | 24.7 | 0.2 |
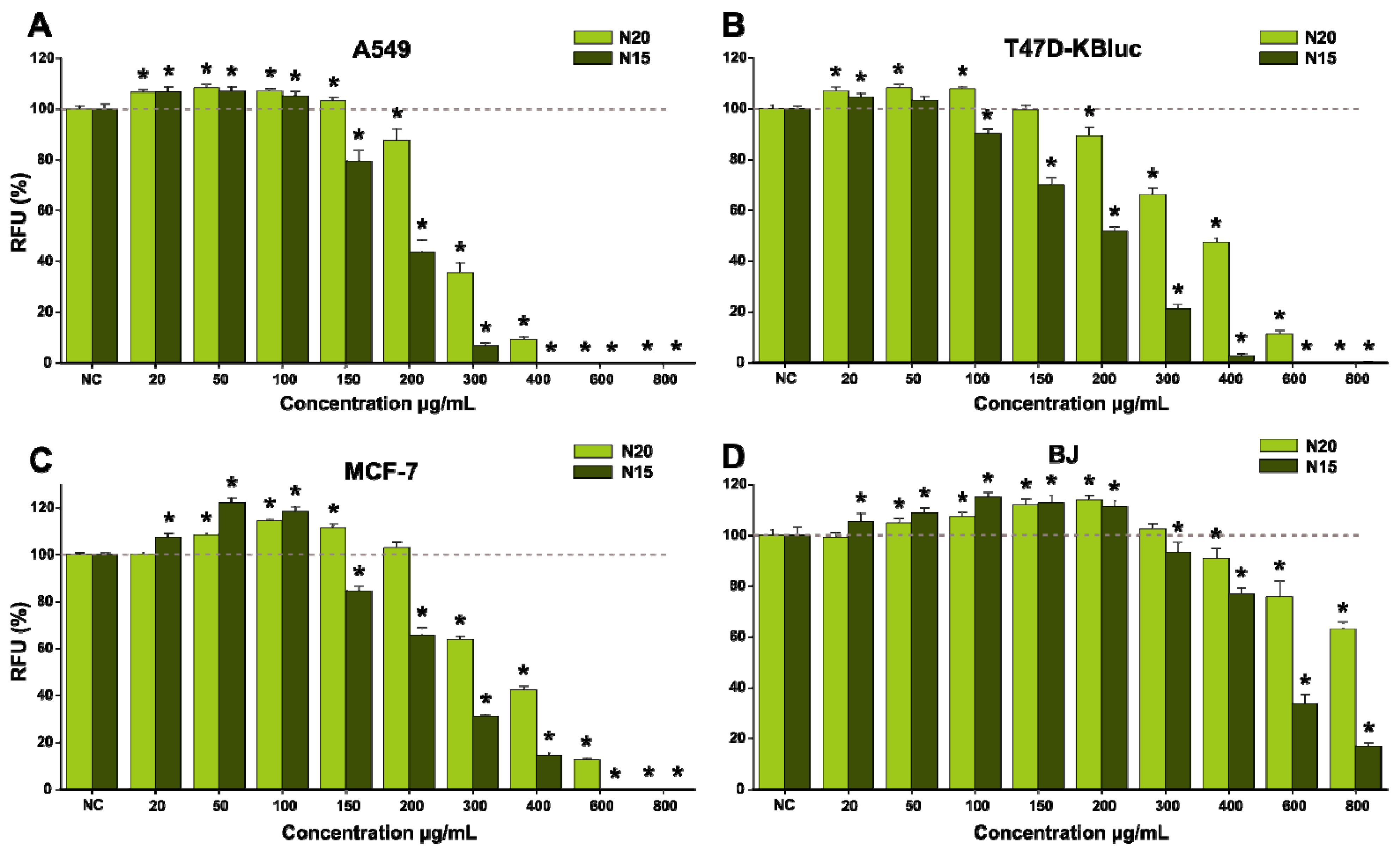
| Samples | IC50 (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | |||||||
| A549 | T47D | MCF-7 | BJ | A549 | T47D | MCF-7 | BJ | |
| N20 | 440.4 | 680.4 | 574.2 | >800 | 266.6 | 399.3 | 349.7 | >800 |
| N15 | 260.8 | 322.1 | 319.7 | 466 | 187.1 | 199.9 | 225.2 | 469 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.-S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans Regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants 2021, 10, 607. https://doi.org/10.3390/antiox10040607
Pop A, Fizeșan I, Vlase L, Rusu ME, Cherfan J, Babota M, Gheldiu A-M, Tomuta I, Popa D-S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans Regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants. 2021; 10(4):607. https://doi.org/10.3390/antiox10040607
Chicago/Turabian StylePop, Anca, Ionel Fizeșan, Laurian Vlase, Marius Emil Rusu, Julien Cherfan, Mihai Babota, Ana-Maria Gheldiu, Ioan Tomuta, and Daniela-Saveta Popa. 2021. "Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans Regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities" Antioxidants 10, no. 4: 607. https://doi.org/10.3390/antiox10040607
APA StylePop, A., Fizeșan, I., Vlase, L., Rusu, M. E., Cherfan, J., Babota, M., Gheldiu, A.-M., Tomuta, I., & Popa, D.-S. (2021). Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans Regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants, 10(4), 607. https://doi.org/10.3390/antiox10040607



_Cherfan.png)










