Strategies to Broaden the Applications of Olive Biophenols Oleuropein and Hydroxytyrosol in Food Products
Abstract
1. Introduction
2. Encapsulation of OLE and HT
2.1. Lipids
| Formulation | Application | Main Findings | Ref. |
|---|---|---|---|
| Liposomes | |||
| Liposomes with OLE, HT and TYR | Drug-delivery system | ↑ EE% for OLE No cytotoxic effects on human chondrocyte cells | [30] |
| DPPC liposomes with OLE | Beverages | EE: 34% Particle size: 405 nm Stable in commercial lemonade drink over 47 days at 5 °C | [33] |
| Ufasomes with OLE | Claim for food application | ↑ antioxidant activity of encapsulated OLE against oxidative stress induced by H2O2 on CaCo-2 cells | [34] |
| Liposomes with phosphatidyl-HT | Claim for food application | Particle size 85 nm; Surface charge: <−25 mV (stable liposomes) | [32] |
| Nanostructured lipid carriers | |||
| OLE-loaded NLC | Claim for food application | OLE leakage was not observed in the nanocarriers within the 3 months of storage Good stability of OLE-loaded NLC | [35] |
| Emulsions | |||
| Lipid emulsions and microemulsions | Claim for food application | Digestibility assay: ↓ Gastric lipolysis of microemulsion compared to emulsions. ↓ Effect of duodenal lipolysis by the dispersion type. | [36,37] |
| OLE-loaded W/O/W | Claim for food application | Emulsions were stabilised for + than 40 days of storage with ↑ hydrophobic emulsifier concentration and ↓ OLE concentration | [38] |
| OLE-loaded O/W | Claim for food application | Stable monodisperse oil-in-water O/W was produced when higher hydrophobic triglyceride oils are used | [39] |
| OLE-loaded O/W | Claim for food application | ↑ stability due to the surface activity of OLE | [40] |
| Nano OLE-loaded W/O/W | Claim for food application | Optimum conditions for formulation: 8% WPC, 1.97% pectin and 8.74% Span 80 EE: 91%; Droplet size: 191 nm; Surface charge: −26.8 mV | [41] |
| O/W, W/O/W and GDE with HT and perilla oil | Claim for food applications | Emulsions structurally stable at 4 °C up to 22 days. HT losses up to 24% throughout the storage of GDE → ↓ antioxidant activity of the emulsion. No lipid oxidation during storage. | [42] |
| GDE with HT | Animal fat replacing | Physical properties: ↑ formation of weaker gels; no significant loss levels until 30 days; minimal changes in colour and pH of W/O/W during storage. Oxidation: systems little prone to oxidation even at 30 days. Biological activity: ↑ antioxidant and ↑ antimicrobial activity | [43] |
| HT in W/O/W enriched in chia oil | Meat supplementation | Presence of HT: ↑ oxidative stability: ↑ DPPH free radicals scavenging; ↑ FRAP; ↓ TBARS | [44] |
2.2. Biopolymer-Based Systems
| Formulation | Application | Main Findings | Ref. |
| Cellulose microcapsules with HT | Claim for food application | EE: 82.4–88.1% Particle size: 156.6–304.0 µm Microcapsules with HT are gastro-resistant and retain > 50% of their antioxidant capacity in simulated GI fluids. | [53] |
| Starch granules with HT and probiotics | Nutraceuticals | Resistant against GI tract conditions and stable up to 6 months of storage under refrigeration. ↓ HT bioavailability by the administration of live L. plantarum bacteria with the olive phenol-containing extract, compared to the extract alone. | [54] |
| Starch nanocrystals or nanoparticles in a PVA film with HT | Active packaging | HT migrated values for all formulations ≤ migration limits for food contact materials. Gradual release of HT during 21 days. Highest gradual release for films with starch nanoparticles. ↑ antioxidant activity for all ternary formulations over time. | [55] |
| Poly(ε-caprolactone)-based NC and montmorillonite, Cloisite30B films with HT | Active packaging | HT ↑ poly(ε-caprolactone) crystallinity, ↓ thermal stability and plasticizing effect. Interaction of HT-Cloisite30B led to a prolonged release of the HT. | [56] |
| Pectin plus fish gelatin composite films with HT and DHPG | Strawberry preservation | ↑ stretching capacity and resistance to breakage. The edible film preserved strawberries with a significant delay in visible decay. | [57] |
| Meat preservation | ↓ lipid oxidation in raw beef meat during refrigerated storage. Film with adequate mechanical and oxygen barrier properties. Film with beeswax ↓ lipid oxidation and ↓ the oxygen barrier capacity. | [58] | |
| MD-OLE and IN-OLE | Claim for food application | Protection of OLE from GI conditions. | [59] |
| Eudraguard® protect with HT | Claim for food application | Spherical non-aggregate particle (particle size: 230 nm) Loading capacity of HT: 38% | [60] |
2.3. Complexation Methods
| Formulation | Application | Results | Ref. |
|---|---|---|---|
| Oleuropein | |||
| α-CD·OLE, β-CD·OLE and Ɣ-CD·OLE | Claim for food application | OLE form binary complexes (1:1) with the three types of CDs β-CD is the most effective for complexation. | [71] |
| β-LG·OLE | Claim for food application | ↑ stability of formed complexes and validity of docking results for β-LG·OLE. | [72] |
| OLE·ALA | Claim for food application | OLE binds to ALA mainly via electrostatic, van der Waals and hydrogen bonds. | [73] |
| Hydroxytyrosol and Oleuropein | |||
| β-CD·HT, β-CD·OLE and β-CD·TYR | Claim for food application | No OH group of HT and OLE is shielded in the β-CD cavity Antioxidant activity: β-CD·HT > β-CD·OLE > β-CD·TYR. | [74] |
| Hydroxytyrosol | |||
| β-CD·olive biophenols | Claim for food application | ↓ bitter taste and preserves them against chemical and physical decomposition reactions during storage. | [75] |
| β-CD·HT, HP-β-CD·HT | Claim for food application | Insertion of the HT through the narrower face of the CDs. ↑ antioxidant capacity and photoprotection of HT. | [76] |
| β-CD·HT | Food industry | ↓ HT bioaccessibility (−20%) and absorption (−10%) in presence of foods (7 mg of HT in the meal). β-CD did not affect bioaccessibility and absorption. | [77] |
| β-CD·HT | Claim for food application | β-CD and drying processes do not affect the efficiency of HT to reduce the DPPH radical. | [78] |
| HT/DHPG-soluble and insoluble dietary fiber of apple cell wall | Dietary fiber | Non-covalent interaction between phenols and the apple cell wall fibers. Antioxidant activity of HT/DHPG was not altered after complexation with apple cell wall fibers and after a simulated gastrointestinal digestion. | [79] |
2.4. Microorganisms
3. Chemical Modifications of OLE and HT
3.1. Modifications of OLE
3.2. Modifications of HT
| Application | Results | Ref. |
|---|---|---|
| Virgin olive oil | ↑ oxidation preventive action with HT. | [94] |
| Claim for Food application | The protective effects against DNA damage of HT esters were inversely proportional to their chain length. | [86] |
| Food application | Antioxidant capacity of HT esters > TYR esters. ↓capacity in lipophilic food matrices. | [93] |
| Functional foods | HT octanoate (C8) is the most effective to inhibit the oxidation in fish O/W. | [95] |
| Claim for food application | ↓ antioxidant activity of HT esters with alkyl chain length around C8–C11 in different matrices | [96] |
| Claim for application | ↓ Antioxidant function of HT esters with chain lengths > C10, measured through ABTS (in ethanol medium) and DCF (on cultured muscle cells). | [97] |
| Claim for food application | HT esters were produced by enzymatic transesterification with cuphea oil. HT esters antioxidant activity ≈ HT decanoate (C10). | [98] |
| Therapeutic strategy | Antioxidant capacity of HT laurate (C12) > HT against H2O2 induced apoptosis in U937cells and C2C12 murine myoblasts. | [99] |
| Claim for food application | Antioxidant activity of all the HT, with exception of HT stearate (C18), >HT C10 esters in human erythrocytes. C12 were optimum in scavenging free radicals. | [100] |
| Claim for food application | HT esters were able to scavenge DPPH radical, render inhibitory effects on cupric ion-induced LDL oxidation and show protective effects against hydroxy radical- and peroxy radical-induced DNA scission. | [101] |
| Claim for food packaging application) | PHTA (up to 50 mg/mL) fully scavenged DPPH free radicals. No cytotoxic activities from polyacrylates films in RAT1 normal fibroblast cells were observed for concentrations of 0.25–1 mg/mL of polyacrylate film | [102] |
3.2.1. Esters
3.2.2. Ethers
3.2.3. Glycosides
3.2.4. Phospholipids
3.2.5. Isochromans
3.2.6. Sulphur and Selenium
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visioli, F.; Grande, S.; Bogani, P.; Galli, C.; Quiles, J.; Ramirez-Tortosa, M.; Yaqoob, P. Antioxidant properties of olive oil phenolics. In Olive Oil Health; CABI Publishing: Oxford, UK, 2006; pp. 109–118. [Google Scholar]
- Di Tommaso, D.; Calabrese, R.; Rotilio, D. Identification and Quantitation of Hydroxytyrosol in Italian Wines. J. High Resolut. Chromatogr. 1998, 21, 549–553. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Guyot, S.; Marnet, N.; Lopes-da-Silva, J.A.; Renard, C.M.G.C.; Coimbra, M.A. Characterisation of phenolic extracts from olive pulp and olive pomace by electrospray mass spectrometry. J. Sci. Food Agric. 2005, 85, 21–32. [Google Scholar] [CrossRef]
- García-García, M.I.; Hernández-García, S.; Sánchez-Ferrer, Á.; García-Carmona, F. Kinetic Study of Hydroxytyrosol Oxidation and Its Related Compounds by Red Globe Grape Polyphenol Oxidase. J. Agric. Food Chem. 2013, 61, 6050–6055. [Google Scholar] [CrossRef]
- Ramírez, E.; Brenes, M.; García, P.; Medina, E.; Romero, C. Oleuropein hydrolysis in natural green olives: Importance of the endogenous enzymes. Food Chem. 2016, 206, 204–209. [Google Scholar] [CrossRef]
- Hu, T.; He, X.-W.; Jiang, J.-G.; Xu, X.-L. Hydroxytyrosol and Its Potential Therapeutic Effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef]
- Rothwell, J.; Pérez-Jiménez, J.; Neveu, V.; Medina-Ramon, A.; M’Hiri, N.; Garcia Lobato, P.; Manach, C.; Knox, K.; Eisner, R.; Wishart, D.; et al. Food compositions: Oleuropein, Phenol-Explorer 3.6: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Visioli, F.; Caruso, D.; Plasmati, E.; Patelli, R.; Mulinacci, N.; Romani, A.; Galli, G.; Galli, C. Hydroxytyrosol, as a component of olive mill waste water, is dose-dependently absorbed and increases the antioxidant capacity of rat plasma. Free Radic. Res. 2001, 34, 301–305. [Google Scholar] [CrossRef]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef]
- Fki, I.; Allouche, N.; Sayadi, S. The use of polyphenolic extract, purified hydroxytyrosol and 3,4-dihydroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: A potential alternative to synthetic antioxidants. Food Chem. 2005, 93, 197–204. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, 13, 22. [Google Scholar]
- Nova Mentis. GRAS Notice (GRN) No. 876; Office of Food Additive Safety; 2019. Available online: https://www.fda.gov/media/134474/download (accessed on 17 September 2020).
- European commission. Commission Implementing Decision (EU) 2017/2373 of 14 December 2017 authorising the placing on the market of hydroxytyrosol as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. Off. J. Eur. Union 2017, 2017, 56–59. [Google Scholar]
- BioActor B.V.; BioPartner Center. Application for the Approval of Bonolive® (standardised olive leaf extract). In Advisory Committee on Novel Foods and Processes; 2016. Available online: https://acnfp.food.gov.uk/sites/default/files/bonolive.nonconf.pdf (accessed on 20 November 2020).
- Steele, E.A.; Breen, C.; Campbell, E.; Martin, R. Food Regulations and Enforcement in the USA. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–13. ISBN 9780081005965. [Google Scholar]
- Coppa, C.; Gonçalves, B.; Lee, S.; Nunes, V.; Gonçalves, C.; Rodrigues, C.; Oliveira, C. Extraction of oleuropein from olive leaves and applicability in foods. Qual. Assur. Saf. Crop. Foods 2020, 12, 50–62. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Resende, D.; Monteiro, M.; Silva, A.M.S.; Cardoso, S.M.; Coimbra, M.A. Application of Hydroxytyrosol in the Functional Foods Field: From Ingredient to Dietary Supplements. Antioxidants 2020, 9, 1246. [Google Scholar] [CrossRef]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Longo, E.; Morozova, K.; Scampicchio, M. Effect of light irradiation on the antioxidant stability of oleuropein. Food Chem. 2017, 237, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kranz, P.; Braun, N.; Schulze, N.; Kunz, B. Sensory Quality of Functional Beverages: Bitterness Perception and Bitter Masking of Olive Leaf Extract Fortified Fruit Smoothies. J. Food Sci. 2010, 75, S308–S311. [Google Scholar] [CrossRef]
- Williamson, S. Interactions Affecting the Bioavailability of Dietary Polyphenols in Vivo. Int. J. Vitam. Nutr. Res. 2007, 77, 224–235. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; McClements, D.J. Resveratrol encapsulation: Designing delivery systems to overcome solubility, stability and bioavailability issues. Trends Food Sci. Technol. 2014, 38, 88–103. [Google Scholar] [CrossRef]
- Can Karaca, A.; Low, N.H.; Nickerson, M.T. Potential use of plant proteins in the microencapsulation of lipophilic materials in foods. Trends Food Sci. Technol. 2015, 42, 5–12. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess. Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Albuquerque, J.; Moura, C.; Sarmento, B.; Reis, S. Solid Lipid Nanoparticles: A Potential Multifunctional Approach towards Rheumatoid Arthritis Theranostics. Molecules 2015, 20, 11103–11118. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical characterization of liposomes containing nutraceutical compounds: Tyrosol, hydroxytyrosol and oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-J.; Qin, F.; Tu, J.-L.; Li, B. Preparation, Characterization, and Antioxidant Activity Evaluation of Liposomes Containing Water-Soluble Hydroxytyrosol from Olive. Molecules 2017, 22, 870. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.O.; Compton, D.L. Phosphatidyl-hydroxytyrosol and phosphatidyl-tyrosol bilayer properties. Chem. Phys. Lipids 2017, 202, 69–76. [Google Scholar] [CrossRef]
- González-Ortega, R.; Šturm, L.; Skrt, M.; Di Mattia, C.D.; Pittia, P.; Poklar Ulrih, N. Liposomal Encapsulation of Oleuropein and an Olive Leaf Extract: Molecular Interactions, Antioxidant Effects and Applications in Model Food Systems. Food Biophys. 2020. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Cosco, D.; Dini, L.; Marzio, L.; Di Fresta, M.; Paolino, D. Oleuropein-laded ufasomes improve the nutraceutical efficacy. Nanomaterials 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Soleimanifard, M.; Sadeghi Mahoonak, A.; Ghorbani, M.; Heidari, R.; Sepahvand, A. The formulation optimization and properties of novel oleuropein-loaded nanocarriers. J. Food Sci. Technol. 2020, 57, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Chatzidaki, M.D.; Mateos-Diaz, E.; Leal-Calderon, F.; Xenakis, A.; Carrière, F. Water-in-oil microemulsions versus emulsions as carriers of hydroxytyrosol: An in vitro gastrointestinal lipolysis study using the pHstat technique. Food Funct. 2016, 7, 2258–2269. [Google Scholar] [CrossRef] [PubMed]
- Chatzidaki, M.D.; Arik, N.; Monteil, J.; Papadimitriou, V.; Leal-Calderon, F.; Xenakis, A. Microemulsion versus emulsion as effective carrier of hydroxytyrosol. Colloids Surf. B Biointerfaces 2016, 137, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Souilem, S.; Kobayashi, I.; Neves, M.A.; Jlaiel, L.; Isoda, H.; Sayadi, S.; Nakajima, M. Interfacial characteristics and microchannel emulsification of oleuropein-containing triglyceride oil–water systems. Food Res. Int. 2014, 62, 467–475. [Google Scholar] [CrossRef]
- Souilem, S.; Kobayashi, I.; Neves, M.A.; Sayadi, S.; Ichikawa, S.; Nakajima, M. Preparation of Monodisperse Food-Grade Oleuropein-Loaded W/O/W Emulsions Using Microchannel Emulsification and Evaluation of Their Storage Stability. Food Bioprocess. Technol. 2014, 7, 2014–2027. [Google Scholar] [CrossRef]
- Souilem, S.; Treesuwan, W.; Kobayashi, I.; Khalid, N.; Bouallagui, Z.; Neves, M.A.; Uemura, K.; Isoda, H.; Sayadi, S.; Nakajima, M. Simulation of oleuropein structural conformation in vacuum, water and triolein–water systems using molecular dynamics. Food Res. Int. 2016, 88, 79–90. [Google Scholar] [CrossRef]
- Gharehbeglou, P.; Jafari, S.M.; Homayouni, A.; Hamishekar, H.; Mirzaei, H. Fabrication of double W1/O/W2 nano-emulsions loaded with oleuropein in the internal phase (W1) and evaluation of their release rate. Food Hydrocoll. 2019, 89, 44–55. [Google Scholar] [CrossRef]
- Flaiz, L.; Freire, M.; Cofrades, S.; Mateos, R.; Weiss, J.; Jiménez-Colmenero, F.; Bou, R. Comparison of simple, double and gelled double emulsions as hydroxytyrosol and n-3 fatty acid delivery systems. Food Chem. 2016, 213, 49–57. [Google Scholar] [CrossRef]
- Freire, M.; Bou, R.; Cofrades, S.; Jiménez-Colmenero, F. Technological characteristics of cold-set gelled double emulsion enriched with n-3 fatty acids: Effect of hydroxytyrosol addition and chilling storage. Food Res. Int. 2017, 100, 298–305. [Google Scholar] [CrossRef]
- Cofrades, S.; Santos-López, J.A.; Freire, M.; Benedí, J.; Sánchez-Muniz, F.J.; Jiménez-Colmenero, F. Oxidative stability of meat systems made with W1/O/W2 emulsions prepared with hydroxytyrosol and chia oil as lipid phase. LWT Food Sci. Technol. 2014, 59, 941–947. [Google Scholar] [CrossRef]
- Salvi, V.R.; Pawar, P. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Fanun, M. Microemulsions as delivery systems. Curr. Opin. Colloid Interface Sci. 2012, 17, 306–313. [Google Scholar] [CrossRef]
- Chaves, L.L.; Lima, S.; Vieira, A.C.C.; Ferreira, D.; Sarmento, B.; Reis, S. Overcoming clofazimine intrinsic toxicity: Statistical modelling and characterization of solid lipid nanoparticles. J. R. Soc. Interface 2018, 15, 20170932. [Google Scholar] [CrossRef] [PubMed]
- Seabra, C.L.; Nunes, C.; Brás, M.; Gomez-Lazaro, M.; Reis, C.A.; Gonçalves, I.C.; Reis, S.; Martins, M.C.L. Lipid nanoparticles to counteract gastric infection without affecting gut microbiota. Eur. J. Pharm. Biopharm. 2018, 127, 378–386. [Google Scholar] [CrossRef]
- Matos, M.; Laca, A.; Rea, F.; Iglesias, O.; Rayner, M.; Gutiérrez, G. O/W emulsions stabilized by OSA-modified starch granules versus non-ionic surfactant: Stability, rheological behaviour and resveratrol encapsulation. J. Food Eng. 2018, 222, 207–217. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef]
- Lopes-de-Campos, D.M.; Pinto, R.; Costa Lima, S.A.; Santos, T.; Sarmento, B.; Nunes, C.; Reis, S. Delivering amoxicillin at the infection site—A rational design through lipid nanoparticles. Int. J. Nanomed. 2019, 14, 2781–2795. [Google Scholar] [CrossRef]
- Matalanis, A.; Jones, O.G.; McClements, D.J. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll. 2011, 25, 1865–1880. [Google Scholar] [CrossRef]
- Elumalai, R.; Patil, S.; Maliyakkal, N.; Rangarajan, A.; Kondaiah, P.; Raichur, A.M. Protamine-carboxymethyl cellulose magnetic nanocapsules for enhanced delivery of anticancer drugs against drug resistant cancers. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 969–981. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Dutil, I.; Gauquelin, L.; Yan, N.; Farnood, R.R. Preparation of self-healing acrylic latex coatings using novel oil-filled ethyl cellulose microcapsules. Prog. Org. Coat. 2015, 85, 168–177. [Google Scholar] [CrossRef]
- Liakos, I.L.; Iordache, F.; Carzino, R.; Scarpellini, A.; Oneto, M.; Bianchini, P.; Grumezescu, A.M.; Holban, A.M. Cellulose—Essential oil nanocapsules with antimicrobial activity for biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 471–479. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, M.; Gabriel, M.S.; Teixeira Neto, Â.A.; da Silva Bernardes, J.; Berry, R.; Tam, K.C. Polymeric hollow microcapsules (PHM) via cellulose nanocrystal stabilized Pickering emulsion polymerization. J. Colloid Interface Sci. 2019, 555, 489–497. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Inclusion of hydroxytyrosol in ethyl cellulose microparticles: In vitro release studies under digestion conditions. Food Hydrocoll. 2018, 84, 104–116. [Google Scholar] [CrossRef]
- Aponte, M.; Ungaro, F.; d’Angelo, I.; De Caro, C.; Russo, R.; Blaiotta, G.; Dal Piaz, F.; Calignano, A.; Miro, A. Improving in vivo conversion of oleuropein into hydroxytyrosol by oral granules containing probiotic Lactobacillus plantarum 299v and an Olea europaea standardized extract. Int. J. Pharm. 2018, 543, 73–82. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Di Michele, A.; Pannucci, E.; Botticella, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Nanostructured starch combined with hydroxytyrosol in poly(vinyl alcohol) based ternary films as active packaging system. Carbohydr. Polym. 2018, 193, 239–248. [Google Scholar] [CrossRef]
- Beltrán, A.; Valente, A.J.M.; Jiménez, A.; Garrigós, M.C. Characterization of poly(ε-caprolactone)-based nanocomposites containing hydroxytyrosol for active food packaging. J. Agric. Food Chem. 2014, 62, 2244–2252. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Vioque, B.; Rubio-Senent, F.; Fernández-Bolaños, J. Physical and functional properties of pectin-fish gelatin films containing the olive phenols hydroxytyrosol and 3,4-dihydroxyphenylglycol. Carbohydr. Polym. 2017, 178, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of edible pectin-fish gelatin films containing the olive antioxidants hydroxytyrosol and 3,4-dihydroxyphenylglycol on beef meat during refrigerated storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Gómez-Caravaca, A.M.; Giménez, B.; Cebrián, R.; Maqueda, M.; Parada, J.; Martínez-Férez, A.; Segura-Carretero, A.; Robert, P. Role of maltodextrin and inulin as encapsulating agents on the protection of oleuropein during in vitro gastrointestinal digestion. Food Chem. 2020, 310, 125976. [Google Scholar] [CrossRef] [PubMed]
- Tirado, D.F.; Latini, A.; Calvo, L. The encapsulation of hydroxytyrosol-rich olive oil in Eudraguard® protect via supercritical fluid extraction of emulsions. J. Food Eng. 2021, 290, 110215. [Google Scholar] [CrossRef]
- Cian, R.E.; Campos-Soldini, A.; Chel-Guerrero, L.; Drago, S.R.; Betancur-Ancona, D. Bioactive Phaseolus lunatus peptides release from maltodextrin/gum arabic microcapsules obtained by spray drying after simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2019, 54, 2002–2009. [Google Scholar] [CrossRef]
- De Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Ratnasooriya, C.C.; Rupasinghe, H.P.V. Extraction of phenolic compounds from grapes and their pomace using β-cyclodextrin. Food Chem. 2012, 134, 625–631. [Google Scholar] [CrossRef]
- Saenger, W. Cyclodextrin Inclusion Compounds in Research and Industry. Angew. Chemie Int. Ed. Engl. 1980, 19, 344–362. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Efmorfopoulou, E.; Rodis, P. Complexation of oleuropein and trans-cinnamic acid with cyclodextrins. Chem. Nat. Compd. 2004, 40, 362–366. [Google Scholar] [CrossRef]
- Vanaei, S.; Parizi, M.S.; Abdolhosseini, S.; Katouzian, I. Spectroscopic, molecular docking and molecular dynamic simulation studies on the complexes of β-lactoglobulin, safranal and oleuropein. Int. J. Biol. Macromol. 2020, 165, 2326–2337. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M.; Maghsoudlou, Y.; Karami, L.; Eikani, M.H. Experimental and molecular docking study of the binding interactions between bovine α-lactalbumin and oleuropein. Food Hydrocoll. 2020, 105, 105859. [Google Scholar] [CrossRef]
- Aree, T.; Jongrungruangchok, S. Structure–antioxidant activity relationship of β-cyclodextrin inclusion complexes with olive tyrosol, hydroxytyrosol and oleuropein: Deep insights from X-ray analysis, DFT calculation and DPPH assay. Carbohydr. Polym. 2018, 199, 661–669. [Google Scholar] [CrossRef]
- Rescifina, A.; Chiacchio, U.; Iannazzo, D.; Piperno, A.; Romeo, G. β-cyclodextrin and caffeine complexes with natural polyphenols from olive and olive oils: NMR, thermodynamic, and molecular modeling studies. J. Agric. Food Chem. 2010, 58, 11876–11882. [Google Scholar] [CrossRef]
- López-García, M.Á.; López, Ó.; Maya, I.; Fernández-Bolaños, J.G. Complexation of hydroxytyrosol with β-cyclodextrins. An efficient photoprotection. Tetrahedron 2010, 66, 8006–8011. [Google Scholar] [CrossRef]
- Malapert, A.; Tomao, V.; Dangles, O.; Reboul, E. Effect of Foods and β-Cyclodextrin on the Bioaccessibility and the Uptake by Caco-2 Cells of Hydroxytyrosol from Either a Pure Standard or Alperujo. J. Agric. Food Chem. 2018, 66, 4614–4620. [Google Scholar] [CrossRef]
- Malapert, A.; Reboul, E.; Tourbin, M.; Dangles, O.; Thiéry, A.; Ziarelli, F.; Tomao, V. Characterization of hydroxytyrosol-β-cyclodextrin complexes in solution and in the solid state, a potential bioactive ingredient. LWT 2019, 102, 317–323. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Knicker, H.; Fernández-Bolaños, J. Confirmation by solid-state NMR spectroscopy of a strong complex phenol-dietary fiber with retention of antioxidant activity in vitro. Food Hydrocoll. 2020, 102, 105584. [Google Scholar] [CrossRef]
- De Oliveira, A.L.M.S.; Maciel, G.M.; Rossetto, R.; de Liz, M.V.; Rampazzo Ribeiro, V.; Haminiuk, C.W.I. Saccharomyces cerevisiae biosorbed with grape pomace flavonoids: Adsorption studies and in vitro simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2019, 54, 1413–1422. [Google Scholar] [CrossRef]
- Jilani, H.; Cilla, A.; Barberá, R.; Hamdi, M. Biosorption of green and black tea polyphenols into Saccharomyces cerevisiae improves their bioaccessibility. J. Funct. Foods 2015, 17, 11–21. [Google Scholar] [CrossRef]
- Jilani, H.; Cilla, A.; Barberá, R.; Hamdi, M. Improved bioaccessibility and antioxidant capacity of olive leaf (Olea europaea L.) polyphenols through biosorption on Saccharomyces cerevisiae. Ind. Crops Prod. 2016, 84, 131–138. [Google Scholar] [CrossRef]
- Liu, L.; Jin, C.; Zhang, Y. Lipophilic phenolic compounds (Lipo-PCs): Emerging antioxidants applied in lipid systems. RSC Adv. 2014, 4, 2879–2891. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive Profile, Dehydration, Extraction and Application of the Bioactive Components of Olive Leaves; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 42, ISBN 9190864174. [Google Scholar]
- Zhong, Y.; Shahidi, F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef]
- Grasso, S.; Siracusa, L.; Spatafora, C.; Renis, M.; Tringali, C. Hydroxytyrosol lipophilic analogues: Enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorg. Chem. 2007, 35, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yong, Q.; Lian, Z.; Huang, C.; Yu, S. Continuous Bioconversion of Oleuropein from Olive Leaf Extract to Produce the Bioactive Product Hydroxytyrosol Using Carrier-Immobilized Enzyme. Appl. Biochem. Biotechnol. 2020, 190, 148–165. [Google Scholar] [CrossRef]
- Bonacci, S.; Paonessa, R.; Costanzo, P.; Salerno, R.; Maiuolo, J.; Nardi, M.; Procopio, A.; Manuela, O. Peracetylation as a strategy to improve oleuropein stability and its affinity to fatty foods. Food Funct. 2018, 9, 5759–5767. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Pereira-Caro, G.; Saha, S.; Cert, R.; Redondo-Horcajo, M.; Bravo, L.; Kroon, P.A. Acetylation of hydroxytyrosol enhances its transport across differentiated Caco-2 cell monolayers. Food Chem. 2011, 125, 865–872. [Google Scholar] [CrossRef]
- Mateos, R.; Goya, L.; Bravo, L. Metabolism of the olive oil phenols hydroxytyrosol, tyrosol, and hydroxytyrosyl acetate by human hepatoma HepG2 cells. J. Agric. Food Chem. 2005, 53, 9897–9905. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; D’Agostino, M.; Maiuolo, J.; Oliverio, M.; Procopio, A.; Iannone, M.; Rotiroti, D.; Russo, D. Antiproliferative and antioxidant effects on breast cancer cells of oleuropein and its semisynthetic peracetylated derivatives. Food Chem. 2011, 127, 1609–1614. [Google Scholar] [CrossRef]
- Jerbi, A.; Mosset, P.; Grée, R.; Kammoun, M. Selective modification of oleuropein, a multifunctional bioactive natural product. J. Saudi Chem. Soc. 2019, 23, 1049–1059. [Google Scholar] [CrossRef]
- Mateos, R.; Trujillo, M.; Pereira-Caro, G.; Madrona, A.; Cert, A.; Espartero, J.L. New lipophilic tyrosyl esters. Comparative antioxidant evaluation with hydroxytyrosyl esters. J. Agric. Food Chem. 2008, 56, 10960–10966. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Mateos, R.; De Teran, L.C.; Espartero, J.L.; Cert, R.; Jover, M.; Alcudia, F.; Bautista, J.; Cert, A.; Parrado, J. Lipophilic hydroxytyrosyl esters. Antioxidant activity in lipid matrices and biological systems. J. Agric. Food Chem. 2006, 54, 3779–3785. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.L.; Black, E.D.; Drolet, A.M. Use of Polyamide Oxidative Fluorescence Test on Lipid Emulsions: Contrast in Relative Effectiveness of Antioxidants in Bulk Versus Dispersed Systems. J. Agric. Food Chem. 1989, 37, 615–624. [Google Scholar] [CrossRef]
- Medina, I.; Lois, S.; Alcantara, D.; Lucas, R.; Morales, J.C. Effect of lipophilization of hydroxytyrosol on its antioxidant activity in fish oils and fish oil-in-water emulsions. J. Agric. Food Chem. 2009, 57, 9773–9779. [Google Scholar] [CrossRef]
- Lucas, R.; Comelles, F.; Alcántara, D.; Maldonado, O.S.; Curcuroze, M.; Parra, J.L.; Morales, J.C. Surface-active properties of lipophilic antioxidants tyrosol and hydroxytyrosol fatty acid esters: A potential explanation for the nonlinear hypothesis of the antioxidant activity in oil-in-water emulsions. J. Agric. Food Chem. 2010, 58, 8021–8026. [Google Scholar] [CrossRef]
- Tofani, D.; Balducci, V.; Gasperi, T.; Incerpi, S.; Gambacorta, A. Fatty acid hydroxytyrosyl esters: Structure/antioxidant activity relationship by abts and in cell-culture DCF assays. J. Agric. Food Chem. 2010, 58, 5292–5299. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, J.A.; Cermak, S.C.; Evans, K.O.; Compton, D.L.; Evangelista, R.; Berhow, M.A. Medium-chain alkyl esters of tyrosol and hydroxytyrosol antioxidants by cuphea oil transesterification. Eur. J. Lipid Sci. Technol. 2013, 115, 363–371. [Google Scholar] [CrossRef]
- Burattini, S.; Salucci, S.; Baldassarri, V.; Accorsi, A.; Piatti, E.; Madrona, A.; Espartero, J.L.; Candiracci, M.; Zappia, G.; Falcieri, E. Anti-apoptotic activity of hydroxytyrosol and hydroxytyrosyl laurate. Food Chem. Toxicol. 2013, 55, 248–256. [Google Scholar] [CrossRef]
- Candiracci, M.; Madrona, A.; Espartero, J.L.; Zappia, G.; Piatti, E. Lipophilic hydroxytyrosol esters significantly improve the oxidative state of human red blood cells. J. Funct. Foods 2016, 23, 339–347. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, D.; Shahidi, F. Antioxidant properties of tyrosol and hydroxytyrosol saturated fatty acid esters. Food Chem. 2018, 245, 1262–1268. [Google Scholar] [CrossRef]
- Fazio, A.; Caroleo, M.C.; Cione, E.; Plastina, P. Novel acrylic polymers for food packaging: Synthesis and antioxidant properties. Food Packag. Shelf Life 2017, 11, 84–90. [Google Scholar] [CrossRef]
- Arrua, D.; Strumia, M.C.; Nazareno, M.A. Immobilization of caffeic acid on a polypropylene film: Synthesis and antioxidant properties. J. Agric. Food Chem. 2010, 58, 9228–9234. [Google Scholar] [CrossRef]
- Tian, F.; Decker, E.A.; Goddard, J.M. Controlling lipid oxidation of food by active packaging technologies. Food Funct. 2013, 4, 669–680. [Google Scholar] [CrossRef]
- Pereira-caro, G.; Madrona, A.; Bravo, L.; Luis, J.; Alcudia, F.; Cert, A.; Mateos, R. Antioxidant activity evaluation of alkyl hydroxytyrosyl ethers, a new class of hydroxytyrosol derivatives. Food Chem. 2009, 115, 86–91. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Revisiting the polar paradox theory: A critical overview. J. Agric. Food Chem. 2011, 59, 3499–3504. [Google Scholar] [CrossRef]
- Sørensen, A.D.M.; Durand, E.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; Jacobsen, C. Antioxidant properties and efficacies of synthesized alkyl caffeates, ferulates, and coumarates. J. Agric. Food Chem. 2014, 62, 12553–12562. [Google Scholar] [CrossRef]
- Griffith, B.; Langenhan, J.; Thorson, J. ‘Sweetening’ natural products via glycorandomization. Curr. Opin. Biotechnol. 2005, 16, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Khymenets, O.; Joglar, J.; Clapés, P.; Parella, T.; Covas, M.I.; De La Torre, R. Biocatalyzed synthesis and structural characterization of monoglucuronides of hydroxytyrosol, tyrosol, homovanillic alcohol, and 3-(4′-hydroxyphenyl) propanol. Adv. Synth. Catal. 2006, 348, 2155–2162. [Google Scholar] [CrossRef]
- Trincone, A.; Pagnotta, E.; Tramice, A. Enzymatic routes for the production of mono-and di-glucosylated derivatives of hydroxytyrosol. Bioresour. Technol. 2012, 115, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Karnišová Potocká, E.; Mastihubová, M.; Mastihuba, V. Enzymatic synthesis of tyrosol and hydroxytyrosol β-d-fructofuranosides. Biocatal. Biotransf. 2019, 37, 18–24. [Google Scholar] [CrossRef]
- Espinosa-Salinas, I.; Rodriguez-Casado, A.; Molina, S.; Rodriguez-Gonzalez, A.; Ordovas, J.M.; Ramirez de Molina, A. Beneficial Effects of Bioactive Phospholipids: Genomic Bases. Curr. Nutr. Food Sci. 2011, 7, 145–154. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Casado, V.; Reglero, G.; Torres, C.F. Novel and efficient solid to solid transphosphatidylation of two phenylalkanols in a biphasic GRAS medium. J. Mol. Catal. B Enzym. 2014, 99, 14–19. [Google Scholar] [CrossRef]
- Casado, V.; Reglero, G.; Torres, C.F. Production and Scale-up of phosphatidyl-tyrosol catalyzed by a food grade phospholipase D. Food Bioprod. Process. 2013, 91, 599–608. [Google Scholar] [CrossRef]
- Martin, D.; Garcia-Serrano, A.; Casado, V.; Vázquez, L.; Reglero, G.; Torres, C.F. Antioxidant activity of phosphatidyl derivatives of hydroxytyrosol in edible oils. Eur. J. Lipid Sci. Technol. 2014, 116, 1035–1043. [Google Scholar] [CrossRef]
- Martin, D.; Moran-Valero, M.I.; Casado, V.; Reglero, G.; Torres, C.F. Phosphatidyl derivative of hydroxytyrosol. in vitro intestinal digestion, bioaccessibility, and its effect on antioxidant activity. J. Agric. Food Chem. 2014, 62, 9751–9759. [Google Scholar] [CrossRef]
- Martínez, M.A.; Ares, I.; Martínez-Larrañaga, M.R.; Anadón, A.; Casado, V.; Vazquez, L.; Martin, D.; Reglero, G.; Torres, C. Acute and repeated dose (28 days) oral safety studies of phosphatidyl-hydroxytyrosol. Food Chem. Toxicol. 2018, 120, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Coccioli, F.; Guiso, M.; Marra, C. The occurrence in olive oil of a new class of phenolic compounds: Hydroxy-isochromans. Food Chem. 2002, 77, 405–411. [Google Scholar] [CrossRef]
- Guiso, M.; Marra, C.; Cavarischia, C. Isochromans from 2-(3′,4′-dihydroxy)phenylethanol. Tetrahedron Lett. 2001, 42, 6531–6534. [Google Scholar] [CrossRef]
- Togna, G.I.; Togna, A.R.; Franconi, M.; Marra, C.; Guiso, M. Olive Oil Isochromans Inhibit Human Platelet Reactivity. J. Nutr. 2003, 133, 2532–2536. [Google Scholar] [CrossRef]
- Trefiletti, G.; Rita Togna, A.; Latina, V.; Marra, C.; Guiso, M.; Togna, G.I. 1-Phenyl-6,7-dihydroxy-isochroman suppresses lipopolysaccharide-induced pro-inflammatory mediator production in human monocytes. Br. J. Nutr. 2011, 106, 33–36. [Google Scholar] [CrossRef]
- Togna, A.R.; Latina, V.; Trefiletti, G.; Guiso, M.; Moschini, S.; Togna, G.I. 1-Phenil-6,7-dihydroxy-isochroman inhibits inflammatory activation of microglia. Brain Res. Bull. 2013, 95, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zeh, M.; Lorenz, P.; Kreutzmann, P.; Schönfeld, P. Hydroxy-1-aryl-isochromans: Protective compounds against lipid peroxidation and cellular nitrosative stress. Redox Rep. 2008, 13, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Kruska, N.; Reiser, G. Antioxidative activity of the olive oil constituent hydroxy-1-aryl-isochromans in cells and cell-free systems. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Madrona, A.; Pereira-Caro, G.; Domínguez, V.; Cert, R.M.; Parrado, J.; Sarriá, B.; Bravo, L.; Espartero, J.L. Synthesis and antioxidant evaluation of isochroman-derivatives of hydroxytyrosol: Structure–activity relationship. Food Chem. 2015, 173, 313–320. [Google Scholar] [CrossRef]
- Vazquez-Prieto, M.A.; Miatello, R.M. Organosulfur compounds and cardiovascular disease. Mol. Aspects Med. 2010, 31, 540–545. [Google Scholar] [CrossRef]
- Gomes, V.P.M.; Torres, C.; Rodríguez-Borges, J.E.; Paiva-Martins, F. A Convenient Synthesis of Hydroxytyrosol Monosulfate Metabolites. J. Agric. Food Chem. 2015, 63, 9565–9571. [Google Scholar] [CrossRef]
- Atzeri, A.; Lucas, R.; Incani, A.; Peñalver, P.; Zafra-Gómez, A.; Melis, M.P.; Pizzala, R.; Morales, J.C.; Deiana, M. Hydroxytyrosol and tyrosol sulfate metabolites protect against the oxidized cholesterol pro-oxidant effect in Caco-2 human enterocyte-like cells. Food Funct. 2016, 7, 337–346. [Google Scholar] [CrossRef]
- Begines, P.; Biedermann, D.; Valentová, K.; Petrásková, L.; Pelantová, H.; Maya, I.; Fernández-Bolaños, J.G.; Křen, V. Chemoenzymatic Synthesis and Radical Scavenging of Sulfated Hydroxytyrosol, Tyrosol, and Acetylated Derivatives. J. Agric. Food Chem. 2019, 67, 7281–7288. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Mugesh, G. Synthesis, characterization, and antioxidant activity of some ebselen analogues. Chem. A Eur. J. 2007, 13, 4594–4601. [Google Scholar] [CrossRef]
- Arnér, E.S.J.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Gómez-Carretero, A.; Maya, I.; Fernández-Bolaños, J.; Duthie, G.G.; de Roos, B. Selenium and sulphur derivatives of hydroxytyrosol: Inhibition of lipid peroxidation in liver microsomes of vitamin E-deficient rats. Eur. J. Nutr. 2018, 58, 1847–1851. [Google Scholar] [CrossRef]
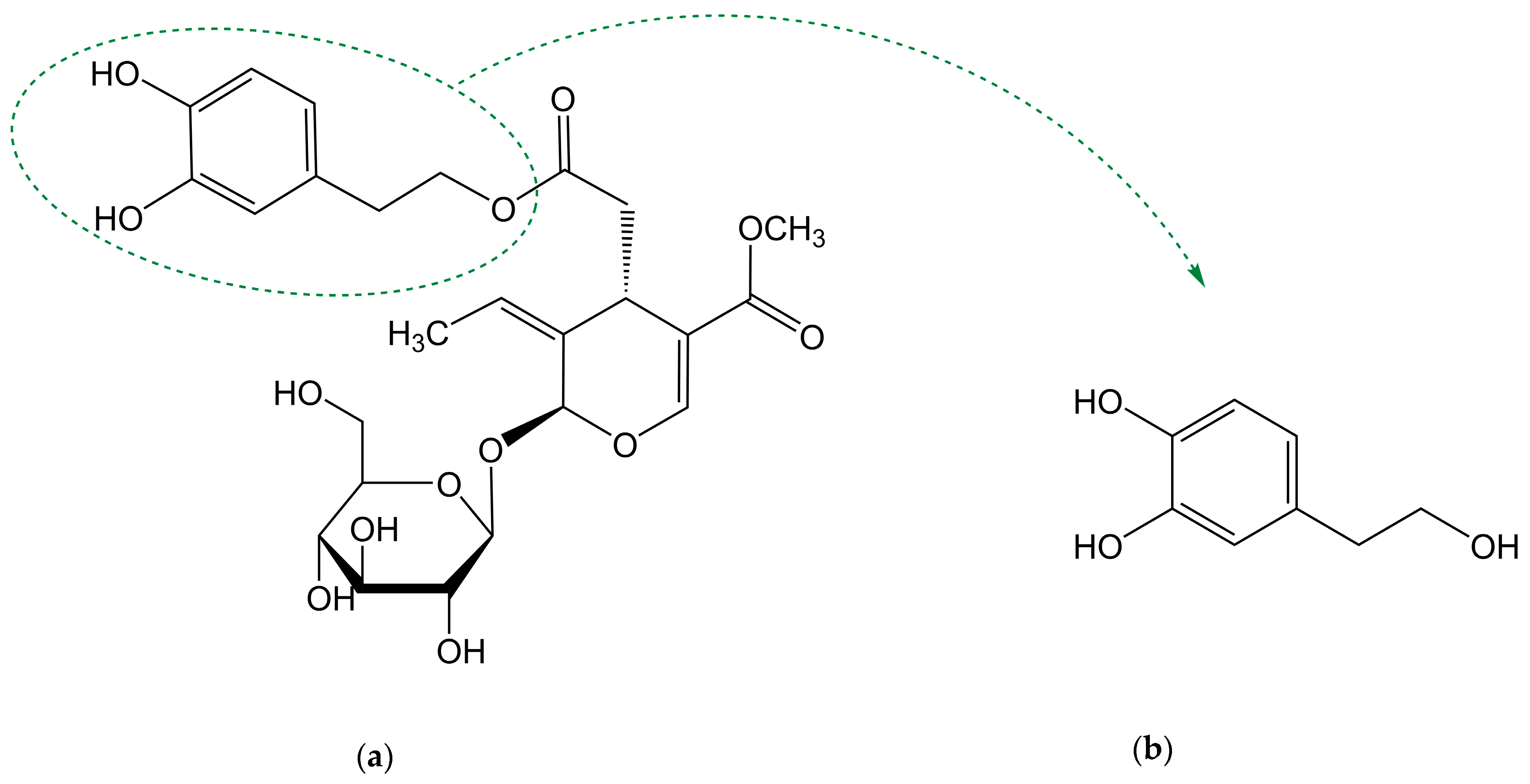
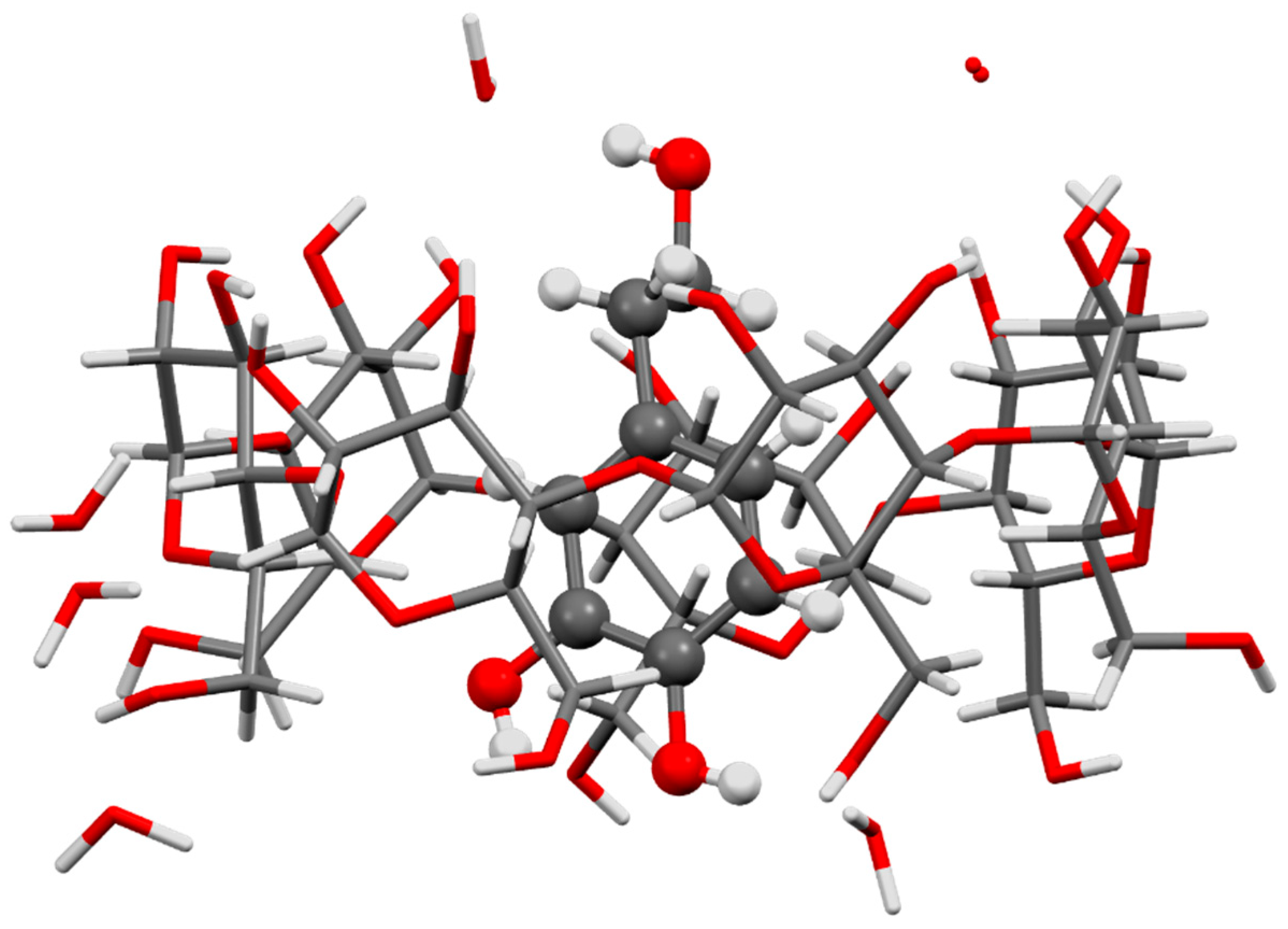
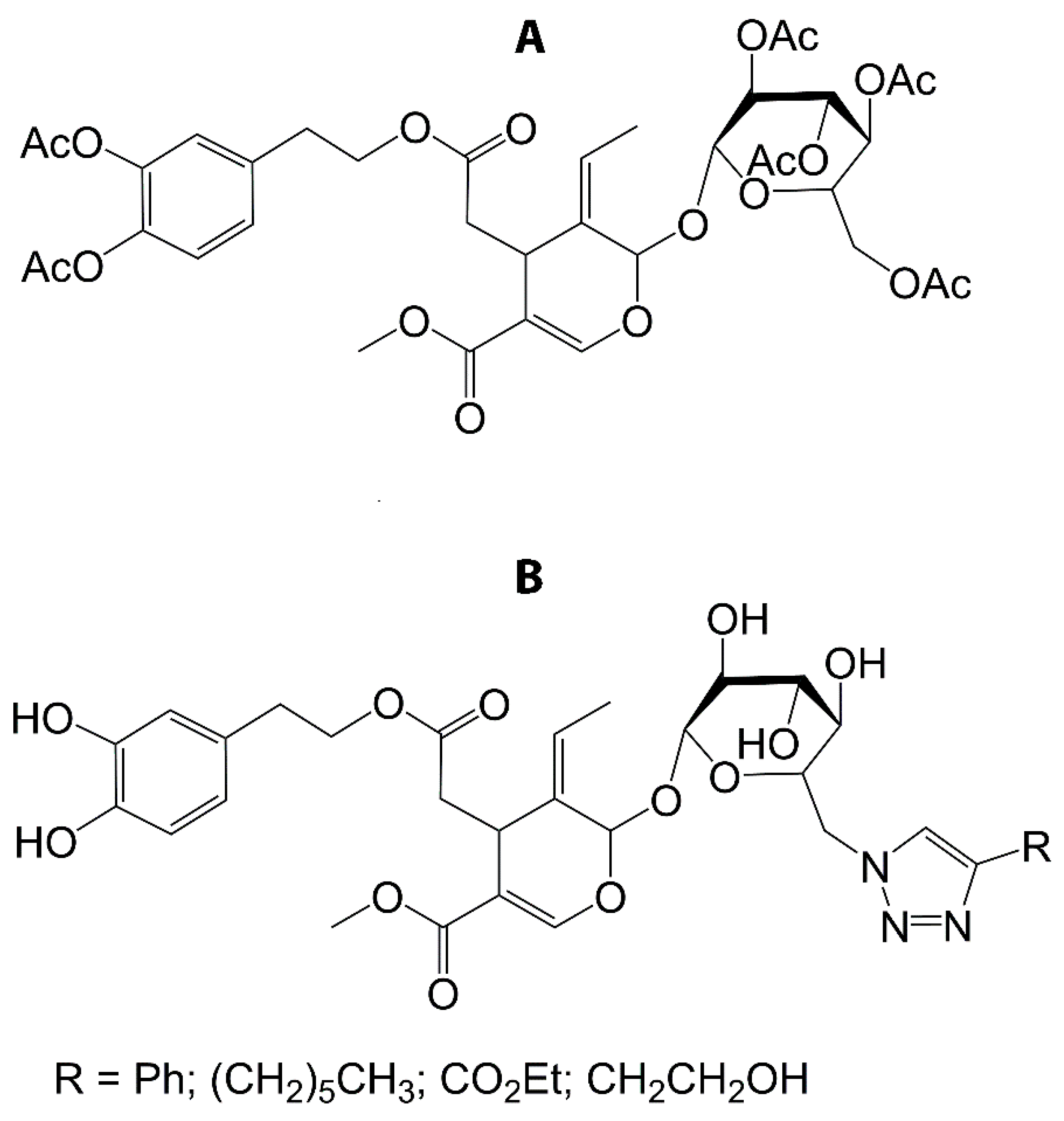
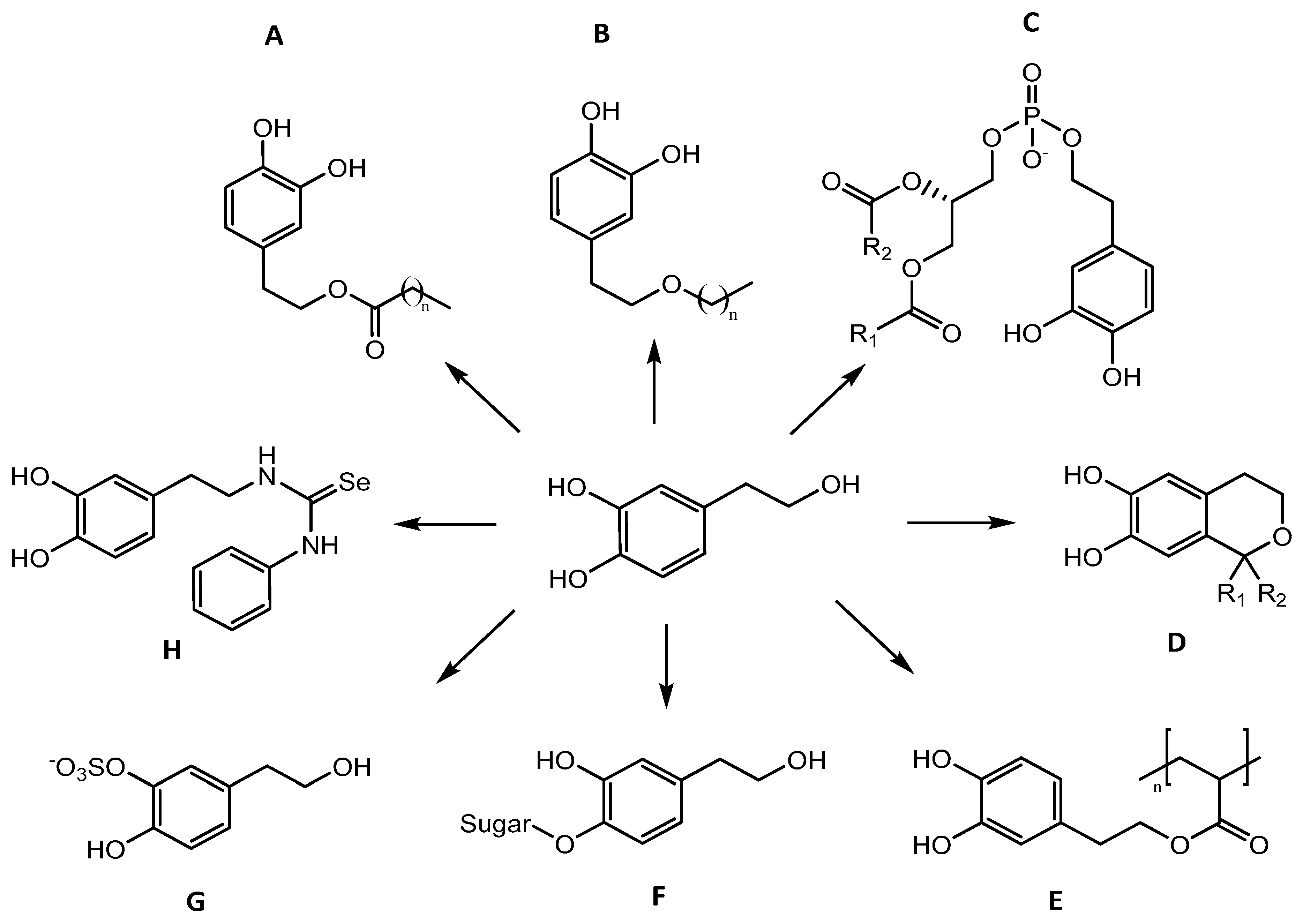
| Formulation | Application | Results | Ref. |
| Ethers | |||
| HT ethers | Sunflower oil | ↑ antioxidant activity for HT and its ethers than the commonly used antioxidants. Antioxidant activity of HT in different matrices is in agreement with the polar paradox and it is dependent of the length of the alkyls chains. | [106] |
| Glycosides | |||
| HT 4′or 3′-O-β-glucuronide | Claim for food application | Methodology developed for the biocatalysed syntheses of glucuronides with a single step product isolation and a high yield. | [110] |
| HT α-glycosidic derivatives | Claim for food application | It was possible to glycosylate regioselectively only the alcoholic primary position (total reaction yield: 20%). | [111] |
| HT β-fructofuranosides | Claim for food application | Yield of fructose-transglycosylation reaction was 19.5%. The reaction was regioselective (fructosylation only on primary hydroxy group of the phenolic acceptors). | [112] |
| Phospholipids | |||
| PHT | Claim for food application | Solid to solid reaction system for transphosphatidylation of phosphatidylcholine with HT. | [115,116] |
| PHT | Functional food | PHT antioxidant activity in diverse edible oils ≥ HT. | [117] |
| PHT | Claim for food application | After intestinal digestion, a closer value of EC50 between digested PHT and HT was achieved (0.6 and 0.5 mM respectively). | [118] |
| PHT | Claim for food application | PHT safe for rats and no toxicity was detected even at higher doses in both acute and repeated dose oral toxicity studies (2000 mg PHT/kg body mass). | [119] |
| Isochromans | |||
| Hydroxy-1-aryl-isochromans | Claim for food application | Suppression of ROS release from mitochondria from rat brain and liver (EC50 of 20 µM). | [126] |
| Isochromans | Food preparations | Antioxidant capacity for isochromans and HT > α-tocopherol and BHT. The results partially agreed with the polar paradox. | [127] |
| Selenium and Sulphur | |||
| Mono-O-sulfate HT | Claim for food application | Ten monosulfates (good yields) synthesized in 1 or 2 steps using simple, cheap and fast procedures with good yield. | [129] |
| Sulfate metabolites of HT | Claim for food application | Protection of intestinal cells against the pro-oxidant effect of oxidised cholesterol | [130] |
| Selenium and Sulphur derivatives of HT | Claim for application | Five thioureas, a disulfide, a thiol, three selenoureas, a diselenide and a selenium showed higher inhibition of lipid peroxidation than HT in vitamin E-deficient microsomes. | [134] |
| Sulfated HT | Claim for application | AST allowed the preparation of respective metabolites in a single step. ↓ anti-lipoperoxidant, radical scavenging and reducing properties of HT and ↑ hydrophilicity. | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, M.; Silva, A.F.R.; Resende, D.; Braga, S.S.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Strategies to Broaden the Applications of Olive Biophenols Oleuropein and Hydroxytyrosol in Food Products. Antioxidants 2021, 10, 444. https://doi.org/10.3390/antiox10030444
Monteiro M, Silva AFR, Resende D, Braga SS, Coimbra MA, Silva AMS, Cardoso SM. Strategies to Broaden the Applications of Olive Biophenols Oleuropein and Hydroxytyrosol in Food Products. Antioxidants. 2021; 10(3):444. https://doi.org/10.3390/antiox10030444
Chicago/Turabian StyleMonteiro, Mariana, Andreia F. R. Silva, Daniela Resende, Susana S. Braga, Manuel A. Coimbra, Artur M. S. Silva, and Susana M. Cardoso. 2021. "Strategies to Broaden the Applications of Olive Biophenols Oleuropein and Hydroxytyrosol in Food Products" Antioxidants 10, no. 3: 444. https://doi.org/10.3390/antiox10030444
APA StyleMonteiro, M., Silva, A. F. R., Resende, D., Braga, S. S., Coimbra, M. A., Silva, A. M. S., & Cardoso, S. M. (2021). Strategies to Broaden the Applications of Olive Biophenols Oleuropein and Hydroxytyrosol in Food Products. Antioxidants, 10(3), 444. https://doi.org/10.3390/antiox10030444










