Natural Extracts That Stimulate Adipocyte Browning and Their Underlying Mechanisms
Abstract
1. Introduction
2. Edible Dietary Extracts That Stimulate Adipocyte Browning
2.1. Berries
2.2. Omija Fruit
2.3. Green Tea
2.4. Cinnamon
2.5. Germinated Soybean Germ
2.6. Ganoderma Tsugae
3. Plant Extracts That Stimulate Adipocyte Browning
3.1. Panax Ginseng
3.2. Phyllostachys Pubescens and Scutellaria baicalensis
3.3. Humulus Japonicus
3.4. Immature Citrus Reticulata
3.5. Glucoraphanin from Broccoli Seeds
4. Marine Products That Induce Adipose Browning
4.1. Sargassum Serratifolium (C. Agardh)
| Extract (Part/Solvent) | Model | Conc. | Effects | Active Component | Ref. |
|---|---|---|---|---|---|
| Sargassum Serratifolium (whole/EtOH) | C57BL/6J mice | 30–120 mg/kg/day | ↑AMPK signaling pathway ↑UCP1-positive cells ↓lipogenesis (↓SREBP1c, SCD-1, FAS) ↑lipolysis (↑PLIN, CPT1, ACSL1) ↑mitochondria function | SHQA SQA | [13,62] |
| Spirulina maxima (whole/EtOH) | 3T3-L1 cells | 50, 100 μg/mL | ↑lipid accumulation ↓adipogenesis (↓C/EBPα, PPARγ, aP2) ↓lipogenesis (↓SREBP1c, ACC, FAS, LPAATβ, Lipin1, DGAT1) | chlorophyll A C-phycocyanin | [63] |
| ICR mice | 150, 450 mg/kg/day | ↑p-AMPK ↑adipose browning proteins (↑PRDM16, PGC1α, UCP1) | |||
| Phaeodactylum Tricornutum (whole/ND) | C57BL/6J mice | 0.81, 1.62, 3.25 mg/kg/day | ↓body weight, organ weight, adipocyte size ↑blood metabolic profile | fucoxanthin | [64] |
| 3T3-L1 cells | 20, 40 μM | ↑UCP1 | |||
| Nitzschia laevis (whole/EtOH) | C57BL/6J mice | 10, 50 mg/kg/day | ↓body weight↑BAT cell number ↑thermogenesis (↑UCP1) | ND | [65] |
| Undaria pinnatifida (whole/chloroform/ methanol) | Wistar rats and KK-Ay mice | 0.5 and 2% in diet | ↓WAT weight ↑BAT weight ↑UCP1 only in WAT | fucoxanthin | [66] |
4.2. Spirulina Maxima
4.3. Phaeodactylum Tricornutum
4.4. Nitzschia Laevis
4.5. Undaria Pinnatifida
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silvester, A.J.; Aseer, K.R.; Yun, J.W. Dietary polyphenols and their roles in fat browning. J. Nutr. Biochem. 2019, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaisanlahti, A.; Glumoff, T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Wang, X.; Wahl, R. Responses of the insulin signaling pathways in the brown adipose tissue of rats following cold exposure. PLoS ONE 2014, 9, e99772. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F. The beige adipocyte as a therapy for metabolic diseases. Int. J. Mol. Sci. 2019, 20, 5058. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pan, M.H.; Hung, W.L.; Tung, Y.C.; Ho, C.T. From white to beige adipocytes: Therapeutic potential of dietary molecules against obesity and their molecular mechanisms. Food Funct. 2019, 10, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Shen, M.; Yadav, H.; Thakali, K.M. Novel browning agents, mechanisms, and therapeutic potentials of brown adipose tissue. Biomed. Res. Int. 2016, 2016, 2365609. [Google Scholar] [CrossRef]
- Liu, D.; Bordicchia, M.; Zhang, C.; Fang, H.; Wei, W.; Li, J.L.; Guilherme, A.; Guntur, K.; Czech, M.P.; Collins, S. Activation of mtorc1 is essential for beta-adrenergic stimulation of adipose browning. J. Clin. Investig. 2016, 126, 1704–1716. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013, 123, 3404–3408. [Google Scholar] [CrossRef] [PubMed]
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Porter, C.; Annamalai, P.; Enerbäck, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014, 63, 4089–4099. [Google Scholar] [CrossRef]
- Kwon, M.; Lee, B.; Lim, S.; Kim, H.-R. Effects of sargaquinoic acid in sargassum serratifolium on inducing brown adipocyte-like phenotype in mouse adipocytes in vitro. Planta Med. 2020, 86, 45–54. [Google Scholar] [CrossRef]
- Kwon, M.; Lim, S.-J.; Joung, E.-J.; Lee, B.; Oh, C.-W.; Kim, H.-R. Meroterpenoid-rich fraction of an ethanolic extract from sargassum serratifolium alleviates obesity and non-alcoholic fatty liver disease in high fat-fed c57bl/6j mice. J. Funct. Foods 2018, 47, 288–298. [Google Scholar] [CrossRef]
- Lanzi, C.R.; Perdicaro, D.J.; Landa, M.S.; Fontana, A.; Antoniolli, A.; Miatello, R.M.; Oteiza, P.I.; Prieto, M.A.V. Grape pomace extract induced beige cells in white adipose tissue from rats and in 3t3-l1 adipocytes. J. Nutr. Biochem. 2018, 56, 224–233. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Bindels, L.B.; Geurts, L.; Van Hul, M.; Cani, P.D.; Delzenne, N.M. A polyphenolic extract from green tea leaves activates fat browning in high-fat-diet-induced obese mice. J. Nutr. Biochem. 2017, 49, 15–21. [Google Scholar] [CrossRef]
- Park, W.Y.; Choe, S.-K.; Park, J.; Um, J.-Y. Black raspberry (rubus coreanus miquel) promotes browning of preadipocytes and iinguinal white adipose tissue in cold-induced mice. Nutrients 2019, 11, 2164. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Kim, H.; Park, J.; Jung, Y.; Youn, D.; Lee, J.; Jin, J.; So, H.; Park, R.; Kim, S. Rubi fructus (rubus coreanus) activates the expression of thermogenic genes in vivo and in vitro. Int. J. Obes. 2015, 39, 456–464. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Cianciosi, D.; Ansary, J.; Mezzetti, B.; Bompadre, S.; Quiles, J.L.; Giampieri, F.; Battino, M. Strawberry (fragaria × ananassa cv. Romina) methanolic extract promotes browning in 3t3-l1 cells. Food Funct. 2020, 11, 297–304. [Google Scholar]
- Park, H.J.; Cho, J.-Y.; Kim, M.K.; Koh, P.-O.; Cho, K.-W.; Kim, C.H.; Lee, K.-S.; Chung, B.Y.; Kim, G.-S.; Cho, J.-H. Anti-obesity effect of schisandra chinensis in 3t3-l1 cells and high fat diet-induced obese rats. Food Chem. 2012, 134, 227–234. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, H.-J.; Kim, S.R.; Choi, M.-S.; Jung, U.J. Omija fruit ethanol extract improves adiposity and related metabolic disturbances in mice fed a high-fat diet. J. Nutr. Biochem. 2017, 41, 137–141. [Google Scholar] [CrossRef]
- Kwan, H.Y.; Wu, J.; Su, T.; Chao, X.-J.; Liu, B.; Fu, X.; Chan, C.L.; Lau, R.H.Y.; Tse, A.K.W.; Han, Q.B. Cinnamon induces browning in subcutaneous adipocytes. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.-Y.; Jiang, X.-W.; Yang, Y.; Xing, B.; Yao, D.; Wu, Q.; Xu, Z.-H.; Zhao, Q.-C. Cinnamomum cassia extract promotes thermogenesis during exposure to cold via activation of brown adipose tissue. J. Ethnopharmacol. 2021, 266, 113413. [Google Scholar] [CrossRef]
- Kim, H.-J.; Choi, E.-J.; Kim, H.S.; Choi, C.-W.; Choi, S.-W.; Kim, S.-L.; Seo, W.-D.; Do, S.H. Germinated soy germ extract ameliorates obesity through beige fat activation. Food Funct. 2019, 10, 836–848. [Google Scholar] [CrossRef]
- Tseng, H.H.; Yeh, W.C.; Tu, Y.C.; Yang, B.F.; Lai, Y.T.; Lee, H.K.; Yang, Y.C.; Huang, H.C.; Lee, Y.J.; Ou, C.C. Proteomic profiling of ganoderma tsugae ethanol extract-induced adipogenesis displaying browning features. FEBS Lett. 2018, 592, 1643–1666. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef]
- Han, H.J.; Jung, U.J.; Kim, H.-J.; Cho, S.-J.; Kim, A.H.; Han, Y.; Choi, M.-S. Combined supplementation with grape pomace and omija fruit ethanol extracts dose-dependently improves body composition, plasma lipid profiles, inflammatory status, and antioxidant capacity in overweight and obese subjects. J. Med. Food 2016, 19, 170–180. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef]
- Singletary, K. Cinnamon: Update of potential health benefits. Nutr. Today 2019, 54, 42–52. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Lui, W.-Y.; Wu, K.; Chan, C.-L.; Dai, S.-H.; Sui, Z.-Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Chin, S.K.; Law, C.; Supramaniam, C.; Cheng, P.-G.; Mujumdar, A. Convective drying of ganoderma tsugae murrill and effect of temperature on basidiospores. Dry. Technol. 2008, 26, 1524–1533. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar]
- Mau, J.-L.; Tsai, S.-Y.; Tseng, Y.-H.; Huang, S.-J. Antioxidant properties of hot water extracts from ganoderma tsugae murill. LWT-Food Sci. Technol. 2005, 38, 589–597. [Google Scholar] [CrossRef]
- Paterson, R.R. Ganoderma-a therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef]
- Saba, E.; Jeon, B.R.; Jeong, D.H.; Lee, K.; Goo, Y.K.; Kim, S.H.; Sung, C.K.; Roh, S.S.; Kim, S.D.; Kim, H.K.; et al. Black ginseng extract ameliorates hypercholesterolemia in rats. J. Ginseng. Res. 2016, 40, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.S.; Gu, L.J.; Fang, Z.M.; Wang, C.Y.; Wang, Z.; Lee, M.R.; Li, Z.; Li, J.J.; Sung, C.K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from panax ginseng by hplc-elsd. J. Pharm. Biomed. Anal. 2009, 50, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.N.; Liu, Z.; Wang, Z.; Li, X.D.; Zhang, L.X.; Li, W.; Wang, Y.P. Ameliorative effects and possible molecular mechanism of action of black ginseng (panax ginseng) on acetaminophen-mediated liver injury. Molecules 2017, 22, 664. [Google Scholar]
- Hong, Y.; Lin, Y.; Si, Q.; Yang, L.; Dong, W.; Gu, X. Ginsenoside rb2 alleviates obesity by activation of brown fat and induction of browning of white fat. Front. Endocrinol. 2019, 10, 153. [Google Scholar] [CrossRef]
- Kim, K.; Nam, K.H.; Yi, S.A.; Park, J.W.; Han, J.W.; Lee, J. Ginsenoside rg3 induces browning of 3t3-l1 adipocytes by activating ampk signaling. Nutrients 2020, 12, 427. [Google Scholar] [CrossRef]
- Lee, K.; Seo, Y.J.; Song, J.H.; Chei, S.; Lee, B.Y. Ginsenoside rg1 promotes browning by inducing ucp1 expression and mitochondrial activity in 3t3-l1 and subcutaneous white adipocytes. J. Ginseng. Res. 2019, 43, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Fang, X.; Li, X.; Zhao, D.; Mo, F.; Jiang, G.; Yu, N.; Zhang, Y.; Guo, Y.; Fu, M.; et al. Ginsenoside rb1 promotes browning through regulation of ppargamma in 3t3-l1 adipocytes. Biochem. Biophys. Res. Commun. 2015, 466, 530–535. [Google Scholar] [CrossRef]
- Park, S.-J.; Park, M.; Sharma, A.; Kim, K.; Lee, H.-J. Black ginseng and ginsenoside rb1 promote browning by inducing ucp1 expression in 3t3-l1 and primary white adipocytes. Nutrients 2019, 11, 2747. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Kim, H.C.; Shin, Y.K.; Min, H.; Cho, S.W.; Kim, Z.S.; Han, S.M.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Jeong, J.H. Humulus japonicus stimulates thermogenesis and ameliorates oxidative stress in mouse adipocytes. J. Mol. Endocrinol. 2019, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-C.; Ho, C.-T.; Pan, M.-H. Immature citrus reticulata extract promotes browning of beige adipocytes in high-fat diet-induced c57bl/6 mice. J. Agric. Food Chem. 2018, 66, 9697–9703. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Xu, L.; Kohno, S.; Ushida, Y.; Aoki, Y.; Umeda, R.; Fuke, N.; Zhuge, F.; Ni, Y.; Nagashimada, M.; et al. Glucoraphanin ameliorates obesity and insulin resistance through adipose tissue browning and reduction of metabolic endotoxemia in mice. Diabetes 2017, 66, 1222–1236. [Google Scholar] [CrossRef]
- Cho, E.-A.; Kim, S.-Y.; Na, I.-H.; Kim, D.-C.; In, M.-J.; Chae, H.-J. Antioxidant and anticoagulant activities of water and ethanol extracts of phyllostachys pubescence leaf produced in geoje. J. Appl. Biol. Chem. 2010, 53, 170–173. [Google Scholar] [CrossRef]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of scutellaria baicalensis water extract on lps-activated raw 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Son, E.; Im, G.; Kim, D.S. Herbal combination of phyllostachys pubescens and scutellaria baicalensis inhibits adipogenesis and promotes browning via ampk activation in 3t3-l1 adipocytes. Plants 2020, 9, 1422. [Google Scholar] [CrossRef]
- Park, T.S.; Ryu, Y.K.; Park, H.Y.; Kim, J.Y.; Go, J.; Noh, J.R.; Kim, Y.H.; Hwang, J.H.; Choi, D.H.; Oh, W.K.; et al. Humulus japonicus inhibits the progression of alzheimer’s disease in a app/ps1 transgenic mouse model. Int. J. Mol. Med. 2017, 39, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Kim, K.-Y.; Lee, S.-H.; Kim, M.-Y.; Park, H.-J.; Jeong, H.-S. Antioxidant and antitumor activities of methanolic extracts from humulus japonicus. Korean J. Food Nutr. 2012, 25, 357–361. [Google Scholar] [CrossRef]
- Hong, M.-S.; Son, E.-S.; Lee, S.-J.; Lee, S.-K.; Lee, Y.-J.; Song, S.-D.; Cho, S.-N.; Barry, C.; Eum, S.-Y. Anti-mycobacterial effects of the extract of humulus japonicus. Korean J. Food Sci. Technol. 2014, 46, 94–99. [Google Scholar] [CrossRef]
- Sung, B.; Chung, J.W.; Bae, H.R.; Choi, J.S.; Kim, C.M.; Kim, N.D. Humulus japonicus extract exhibits antioxidative and anti-aging effects via modulation of the ampk-sirt1 pathway. Exp. Ther. Med. 2015, 9, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Noh, J.R.; Kim, Y.H.; Hwang, J.H.; Kim, K.S.; Choi, D.H.; Go, M.J.; Han, S.S.; Oh, W.K.; Lee, C.H. Anti-atherogenic effect of humulus japonicus in apolipoprotein e-deficient mice. Int. J. Mol. Med. 2016, 38, 1101–1110. [Google Scholar] [CrossRef]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef]
- Dutt, M.; Vasconcellos, M.; Song, K.; Gmitter, F.; Grosser, J. In vitro production of autotetraploid ponkan mandarin (citrus reticulata blanco) using cell suspension cultures. Euphytica 2009, 173, 235–242. [Google Scholar] [CrossRef]
- Sun, Y.; Qiao, L.; Shen, Y.; Jiang, P.; Chen, J.; Ye, X. Phytochemical profile and antioxidant activity of physiological drop of citrus fruits. J. Food Sci. 2013, 78, C37–C42. [Google Scholar] [CrossRef]
- Lv, X.; Meng, G.; Li, W.; Fan, D.; Wang, X.; Espinoza-Pinochet, C.A.; Cespedes-Acuna, C.L. Sulforaphane and its antioxidative effects in broccoli seeds and sprouts of different cultivars. Food Chem. 2020, 316, 126216. [Google Scholar] [CrossRef]
- Garcia-Saldana, J.S.; Campas-Baypoli, O.N.; Lopez-Cervantes, J.; Sanchez-Machado, D.I.; Cantu-Soto, E.U.; Rodriguez-Ramirez, R. Microencapsulation of sulforaphane from broccoli seed extracts by gelatin/gum arabic and gelatin/pectin complexes. Food Chem. 2016, 201, 94–100. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. Keap1 and done? Targeting the nrf2 pathway with sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Trepiana, J.; González-Arceo, M.; Aguirre, L.; Milton-Laskibar, I.; González, M.; Eseberri, I.; Fernández-Quintela, A.; Portillo, M.P. Anti-obesity effects of microalgae. Int. J. Mol. Sci. 2020, 21, 41. [Google Scholar] [CrossRef]
- Kwon, M.; Lee, B.; Lim, S.-J.; Choi, J.S.; Kim, H.-R. Sargahydroquinoic acid, a major compound in sargassum serratifolium (c. Agardh) c. Agardh, widely activates lipid catabolic pathways, contributing to the formation of beige-like adipocytes. J. Funct. Foods 2019, 58, 355–366. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Kim, K.-J.; Choi, J.; Koh, E.-J.; Lee, B.-Y. Spirulina maxima extract reduces obesity through suppression of adipogenesis and activation of browning in 3t3-l1 cells and high-fat diet-induced obese mice. Nutrients 2018, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, J.-H.; Yang, S.-H.; Um, J.-I.; Hong, K.W.; Kang, K.; Pan, C.-H.; Hwang, K.T.; Kim, S.M. Anti-obesity effect of standardized extract of microalga phaeodactylum tricornutum containing fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Wei, H.; Cheng, K.-W.; Chen, F. Extract of the microalga nitzschia laevis prevents high-fat-diet-induced obesity in mice by modulating the composition of gut microbiota. Mol. Nutr. Food Res. 2019, 63, 1800808. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, undaria pinnatifida, shows antiobesity effect through ucp1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Baky, H.H.; El-Baroty, G.S. Characterization and bioactivity of phycocyanin isolated from spirulina maxima grown under salt stress. Food Funct. 2012, 3, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.Y.; Lee, H.Y. Enhancement of chlorophyll a production from marine spirulina maxima by an optimized ultrasonic extraction process. Appl. Sci. 2018, 8, 26. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, B.; Sun, P.; Lu, X.; Liu, J.; Chen, F. Screening of diatom strains and characterization of cyclotella cryptica as a potential fucoxanthin producer. Mar. Drugs 2016, 14, 125. [Google Scholar] [CrossRef]
- Wen, Z.-Y.; Chen, F. Production potential of eicosapentaenoic acid by the diatom nitzschia laevis. Biotechnol. Lett. 2000, 22, 727–733. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Johnson, W.D.; Ribnicky, D.; Poulev, A.; Stadler, K.; Coulter, A.A. Fucoxanthin and its metabolite fucoxanthinol do not induce browning in human adipocytes. J. Agric. Food Chem. 2017, 65, 10915–10924. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Takahashi, N.; Kawada, T.; Miyashita, K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3t3-l1 cells. Int. J. Mol. Med. 2006, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
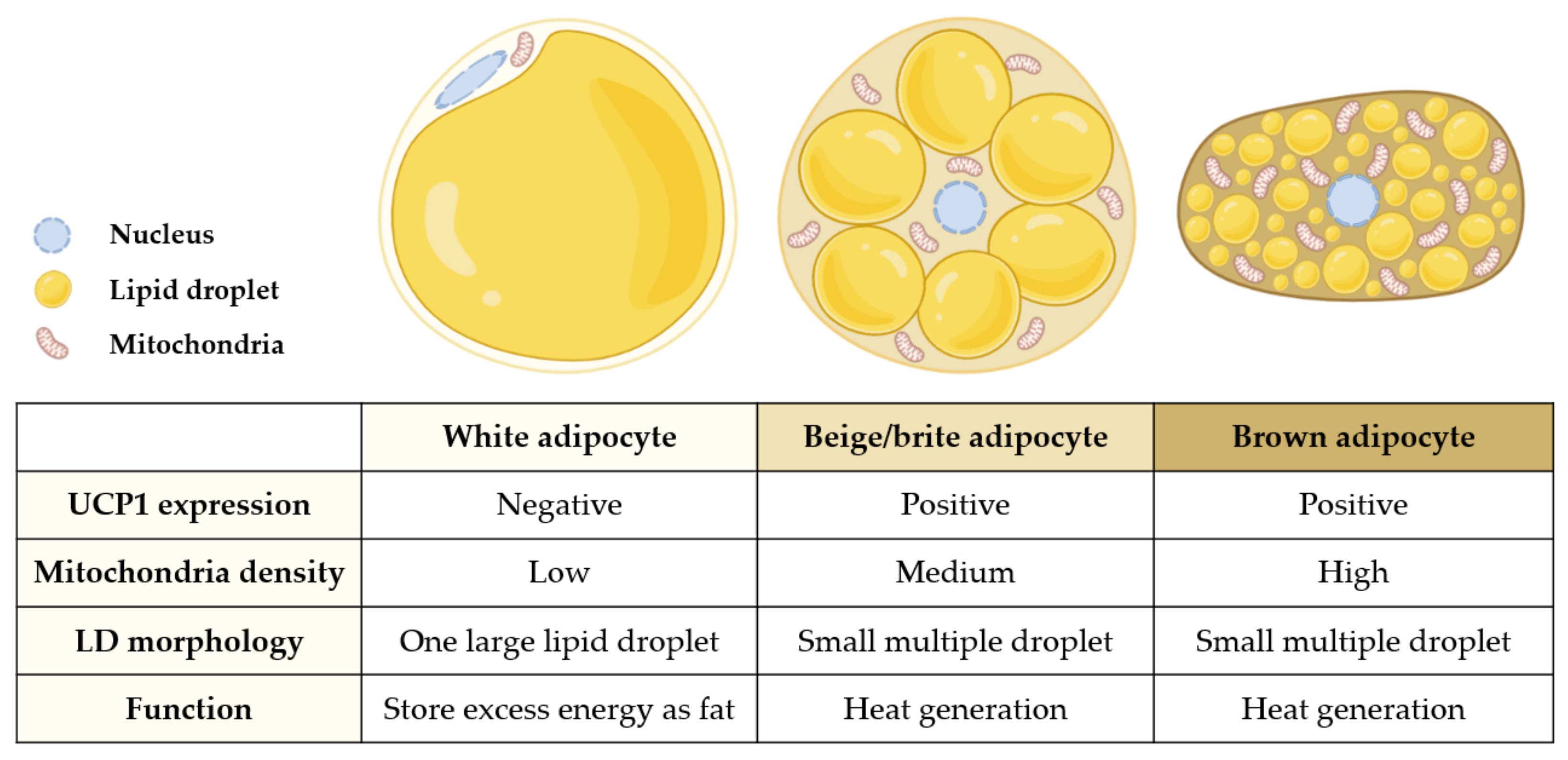
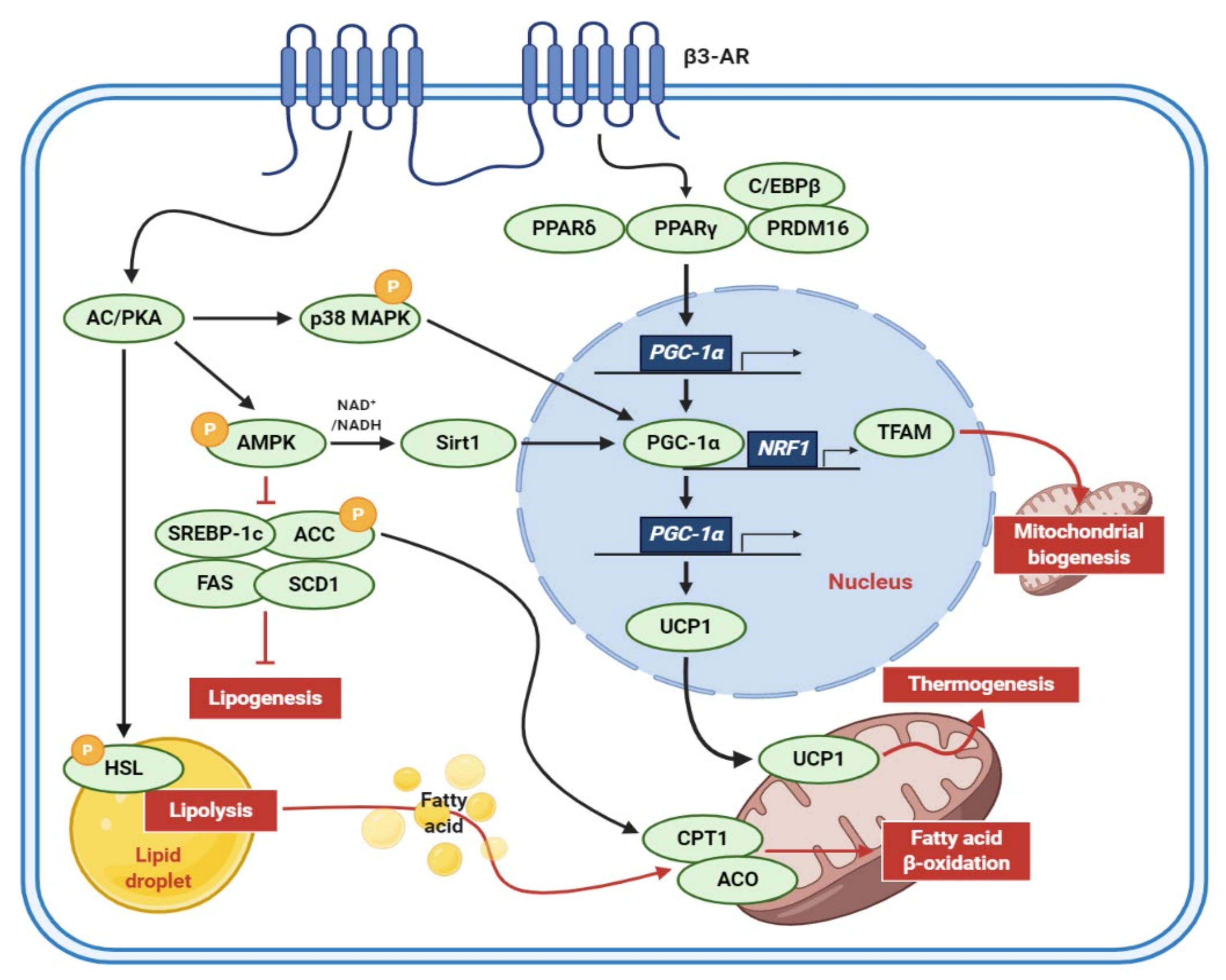
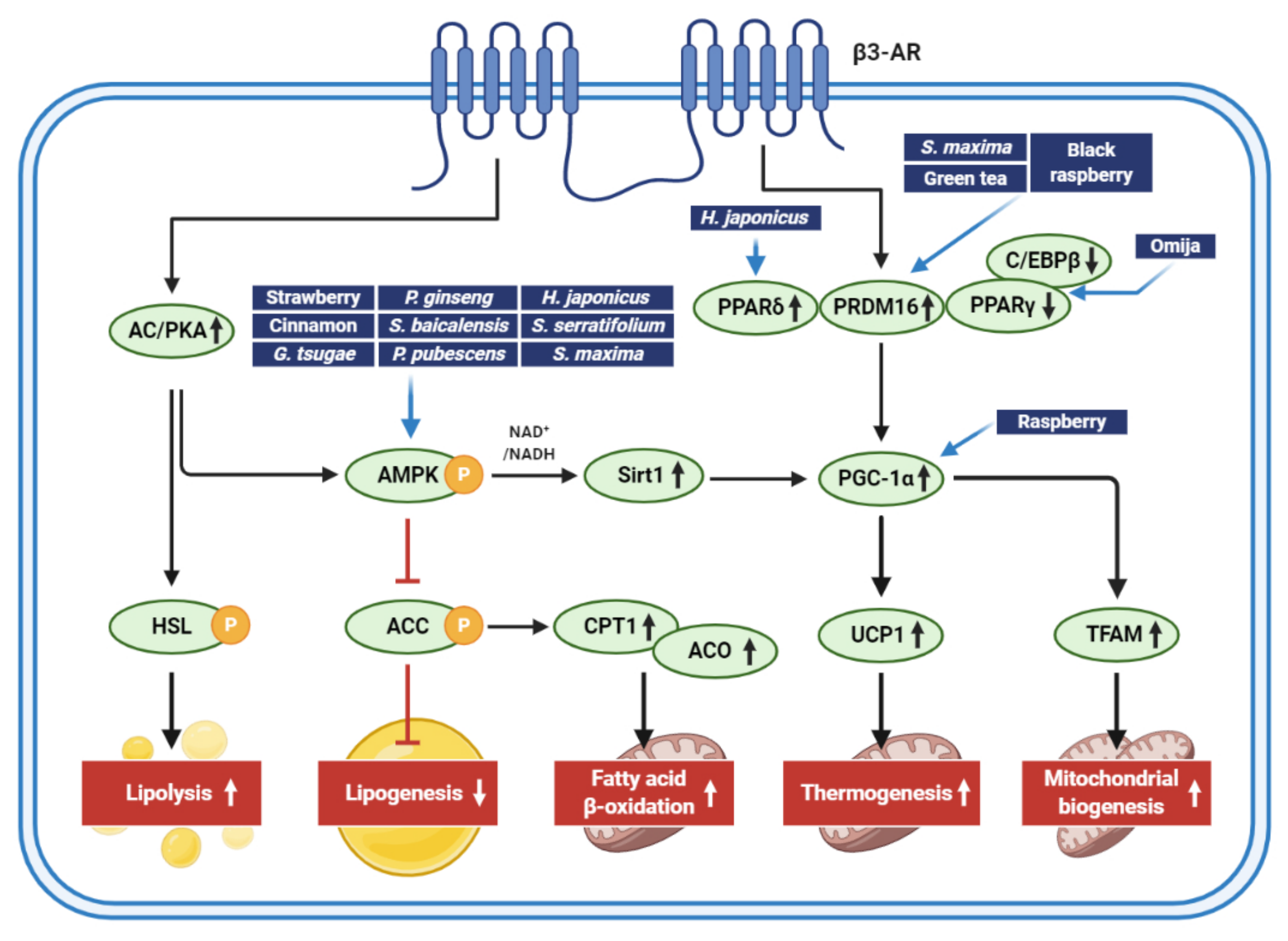
| Extract (Part/Solvent) | Model | Conc. | Effects | Active Component | Ref. |
|---|---|---|---|---|---|
| Red raspberry (fruit/hot water) | C57BL/6J mice | 100 mg/kg/day | ↓obesity (↓body/ WAT weights) ↑thermogenesis (↑ucp1, pgc1α, Cidea) | ellagic acid | [19] |
| ↑mitochondrial biogenesis (↑Sirt3, Nrf1) | |||||
| Primary brown preadipocytes | 100 μg/mL | ↑mitochondrial activity ↑brown adipogenesis (ap2, adiponectin, resistin) ↑thermogenesis (↑ucp1, pgc1α, Cidea) ↑p-AMPK, p-ACC | |||
| Black raspberry (fruit/hot water) | hMSCs | 5–10 μg/mL | ↓adipogenesis | ellagic acid | [17] |
| zebrafish | 100 μg/mL | (↓PPARγ, C/EBPα) | |||
| 3T3-L1 cells | 5–10 μg/mL | ↓adipogenesis (↓PPARγ, C/EBPα) ↑thermogenesis (↑UCP1, PGC1α) ↑mitochondrial function (↑CIDEA, Nrf1, CPT1B) | |||
| C57BL/6J mice | 100 mg/kg/day | ↑thermogenesis (↑body temperature in cold-exposure, ↑UCP1, PGC1α, PRDM16, TBX1) | |||
| Strawberry (fruit/80% MeOH) | 3T3-L1 cells | 5–10 μg/mL | ↓adipogenesis (↓PPARγ, C/EBPα, resistin) ↑thermogenesis (↑UCP1, PDK4) ↑mitochondrial biogenesis (↑AMPK, Sirt1, PGC1α) | ND | [20] |
| Omija fruit (fruit/50% EtOH) | 3T3-L1 cells Sprague–Dawley rats | 20, 150 μg/mL 5, 200 mg/kg/day | ↓adipogenesis (↓C/EBPβ, PPARγ, C/EBPα) ↓obesity (↓body/ WAT weights) | ND | [21] |
| C57BL/6J mice | 500 mg/kg/day | ↓WAT /↑BAT weights ↑energy expenditure ↑thermogenesis (↑Pparα, Cidea, and COX8β.) | ND | [22] | |
| Green Tea (leaf/water) | C57BL/6J mice | 0.5% | ↓obesity (↓body/ WAT/ BAT weights) ↑brown-specific markers (↑Ucp1, Prdm16, and CIDEA) ↑mitochondrial activity (↑Pgc1α and Cpt1B) | Catechin EGCG | [16] |
| Cinnamon (bark/70–80% EtOH) | 3T3-L1 cells | 80 μg/mL | ↑brown-specific markers (↑Cidea, Prdm16, Pgc, Cpt-1 and ↑PRDM16), cAMP ↓white adipocyte markers (↓Dpt and Igf) | Catechin Quercetin Icariin Chlorogenic acid Protocatechuic acid asculetin | [23] |
| ex vivo adipocytes isolated from db/db mice | 80 μg/mL | ↑brown-specific markers (↑Ucp-1, Cidea, Prdm16 and ↑UCP1) | |||
| C57BL/6J mice fed with high fat diet | 500 mg/kg/day | ↑brown-specific markers (↑Ucp-1, Cidea, Prdm16 and ↑UCP1) ↓white adipocyte markers (↓Dpt) | |||
| Kunming mice | 90, 180, 360 mg/kg/day | ↑thermogenesis (↑body temperature in cold-exposure, ↑Ucp1, Ppargc1 α, Prdm16 in BAT) ↑energy expenditure (↑VO2 and VCO2) Uncoupling ATP production, AMPK-SIRT1 pathway in BAT | Cinnamaldehyde Cinnamic acid 2-methoxycinnamaldehyde coumarin | [24] | |
| Geminated soy germ(germ/EtOH) | 3T3-L1 cells | 0.1–10 μg/mL | ↓adipogenesis, lipogenesis ↑Lipolysis, β-oxidation | Soya saponin Ab | [25] |
| C57BL/6J mice | 1 mg/kg | ↓obesity (↓body/ WAT weights) ↑thermogenesis ↑mitochondrial biogenesis | |||
| Ganoderma tsugae (fruiting body/EtOH) | 3T3-L1 cells | 0.2 mg/mL | ↑small lipid droplets formation ↑intracellular lipid metabolism flux/flexibility ↑UCP1, Cidea, HSP60, cyto c ↓NADH/NAD+ ratio, NADH content | Triterpenoid | [26] |
| C57BL/6Narl | 150, 300 mg/kg/day | ↑WAT browning (↑UCP1)↓glucose/lipid disorders ↑SIRT1, p-AMPK |
| Extract (Part/Solvent) | Model | Conc. | Effects | Active Component | Ref. |
|---|---|---|---|---|---|
| Panax ginseng (root and leaf/ND) | 3T3-L1 cells | 25–100 μg/mL | ↓adipogenesis (↓C/EBPα, SREBP1-c) ↑mitochondrial activity ↑brown-adipocyte-specific markers (↑UCP1, PRDM16, PGC-1 α) ↑p-AMPK | Gensenoside Rb1 Rb2 Rg1 Rg3 | [39,40,41,42,43] |
| primary white adipocytes | |||||
| Phyllostachys pubescens and Scutellaria baicalensis (root and leaf/70% EtOH) | 3T3-L1 cells | 60–480 μg/mL | ↓adipogenesis (↓PPARγ, C/EBPα) ↓lipogenesis (↓SREBP-1c, FAS) ↑fatty acid oxidation, lipolysis (↑p-ACC, CPT1) ↑BAT markers (↑UCP1, PRDM16, PGC1α) ↑thermogenesis (↑UCP2) ↑p-AMPK | Chlorogenic acid Orientin Isoorientin Baicalin Wogonoside Baicalein Tricin Wogonin Chrysin | [26] |
| Humulus japonicas (leaf/water) | 3T3-L1 cells | 20, 100 μg/mL | ↑thermogenesis, browning (↑UCP1, PRDM16, PGC-1 α) ↑fatty acid oxidation, lipolysis ↓lipogenesis, lipid accumulation ↓oxidative stress/↑SOD1, catalase, GPx1 ↑AMPK/PPARδ signaling pathway | ND | [44] |
| Immature Citrus reticulate (fruit/hot water) | C57BL/6 mice | 1% | ↓body/visceral fat weights, adipocyte size ↓fatty livers, insulin resistance, dyslipidemia ↑cold tolerance in cold exposure ↑thermogenesis (↑UCP1, PRDM16, NRF1) ↑beige adipocyte-selective markers (↑TEME26, CD137, Cidea) | Synephrine Narirutin Hesperidin Nobiletin tangeretin | [45] |
| Broccoli Seeds (sprout/water) | C57BL/6JSlc mice | 0.3% | ↓body weight gain, fat mass ↑energy expenditure ↑major browning marker (↑UCP1) ↓insulin resistance, glucose tolerance ↓plasma lipopolysaccharide ↓relative abundance of Desulfovibrionaceae bacteria ↓oxidative stress, inflammation | Glucoraphanin | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-K.; Lee, B.; Kim, C.Y. Natural Extracts That Stimulate Adipocyte Browning and Their Underlying Mechanisms. Antioxidants 2021, 10, 308. https://doi.org/10.3390/antiox10020308
Lee M-K, Lee B, Kim CY. Natural Extracts That Stimulate Adipocyte Browning and Their Underlying Mechanisms. Antioxidants. 2021; 10(2):308. https://doi.org/10.3390/antiox10020308
Chicago/Turabian StyleLee, Min-Kyeong, Bonggi Lee, and Choon Young Kim. 2021. "Natural Extracts That Stimulate Adipocyte Browning and Their Underlying Mechanisms" Antioxidants 10, no. 2: 308. https://doi.org/10.3390/antiox10020308
APA StyleLee, M.-K., Lee, B., & Kim, C. Y. (2021). Natural Extracts That Stimulate Adipocyte Browning and Their Underlying Mechanisms. Antioxidants, 10(2), 308. https://doi.org/10.3390/antiox10020308







