Microbiological-Chemical Sourced Chondroitin Sulfates Protect Neuroblastoma SH-SY5Y Cells against Oxidative Stress and Are Suitable for Hydrogel-Based Controlled Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of CS Polysaccharides
2.2. Cell Culture
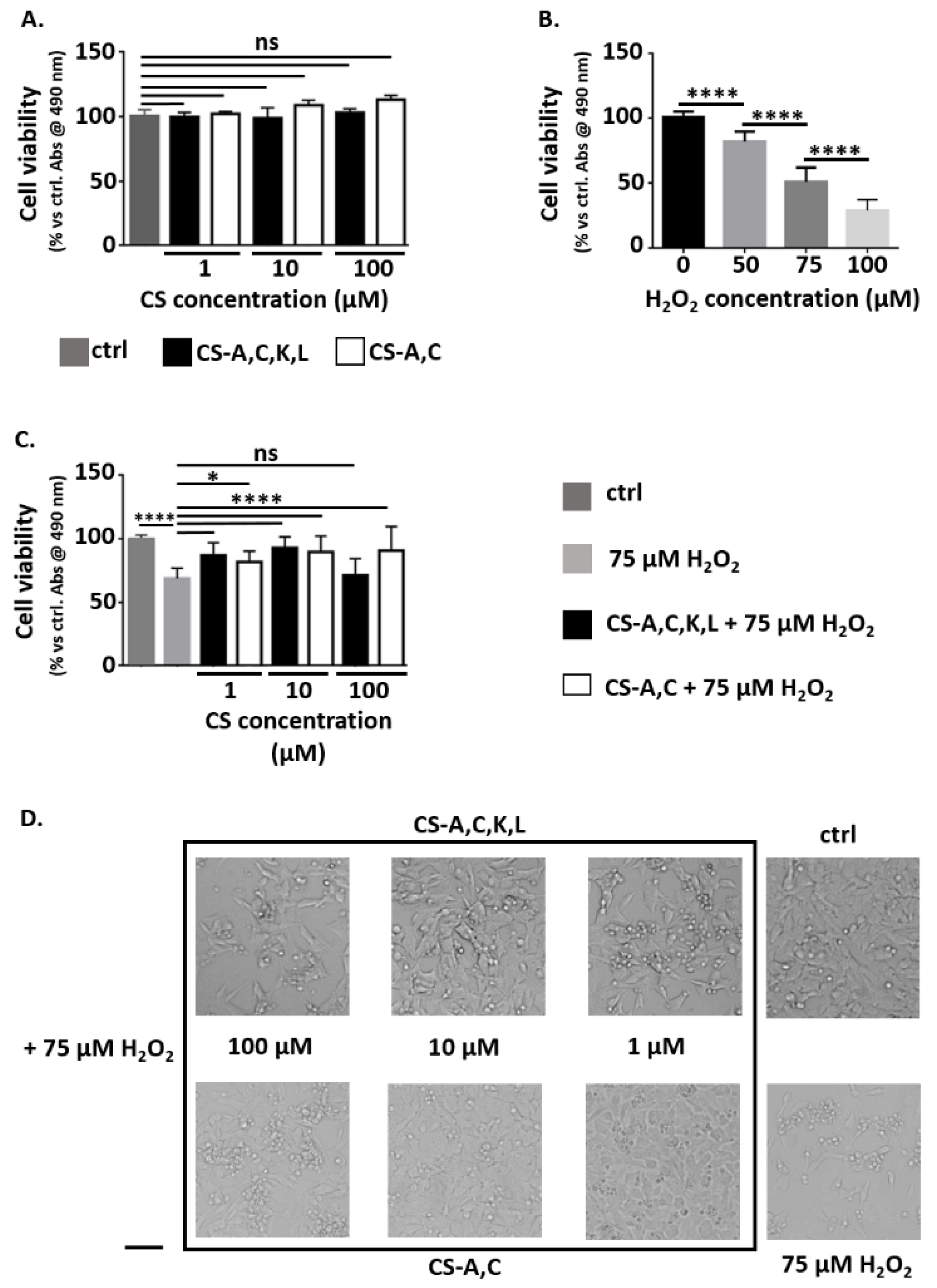
2.3. Neuroprotective Properties of CS Polysaccharides
2.4. Preparation and Characterization of Carbomer/Agarose Semi-IPNs
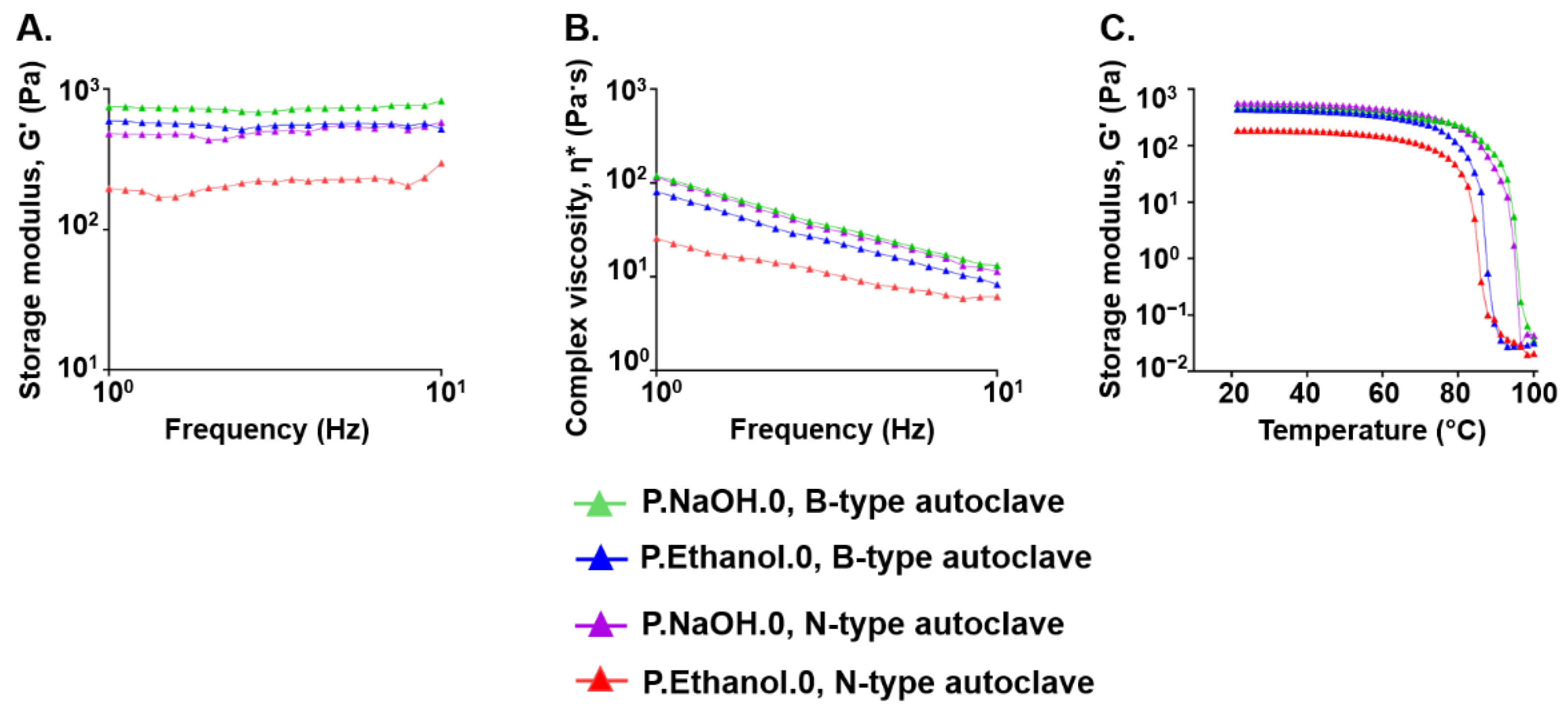
2.5. Effect of the Sterilization Process on the Viscoelastic Properties of Carbomer/Agarose Semi-IPNs
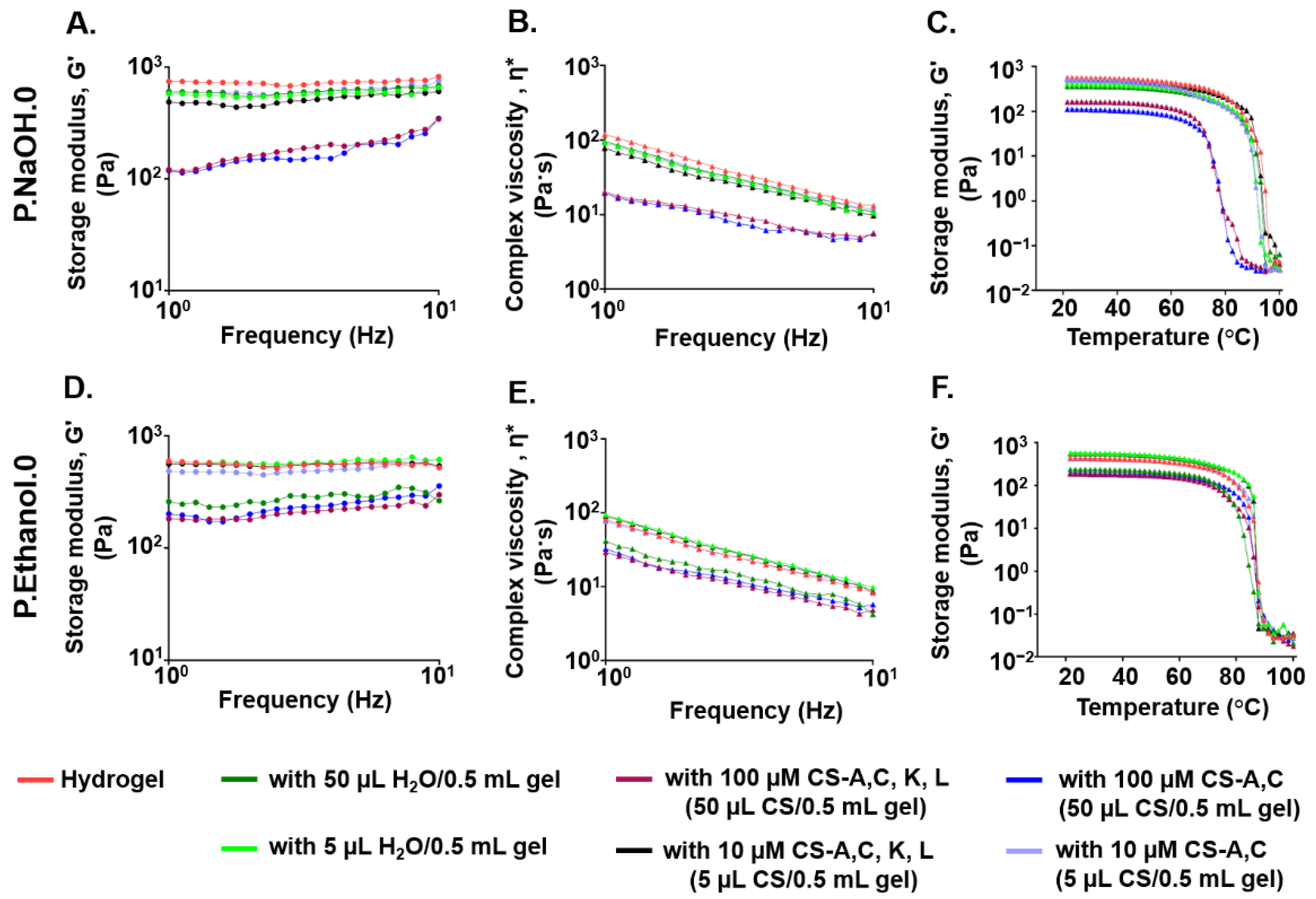
2.6. Effect of CS Polysaccharide Loading on the Viscoelastic Properties of Carbomer/Agarose Semi-IPNs
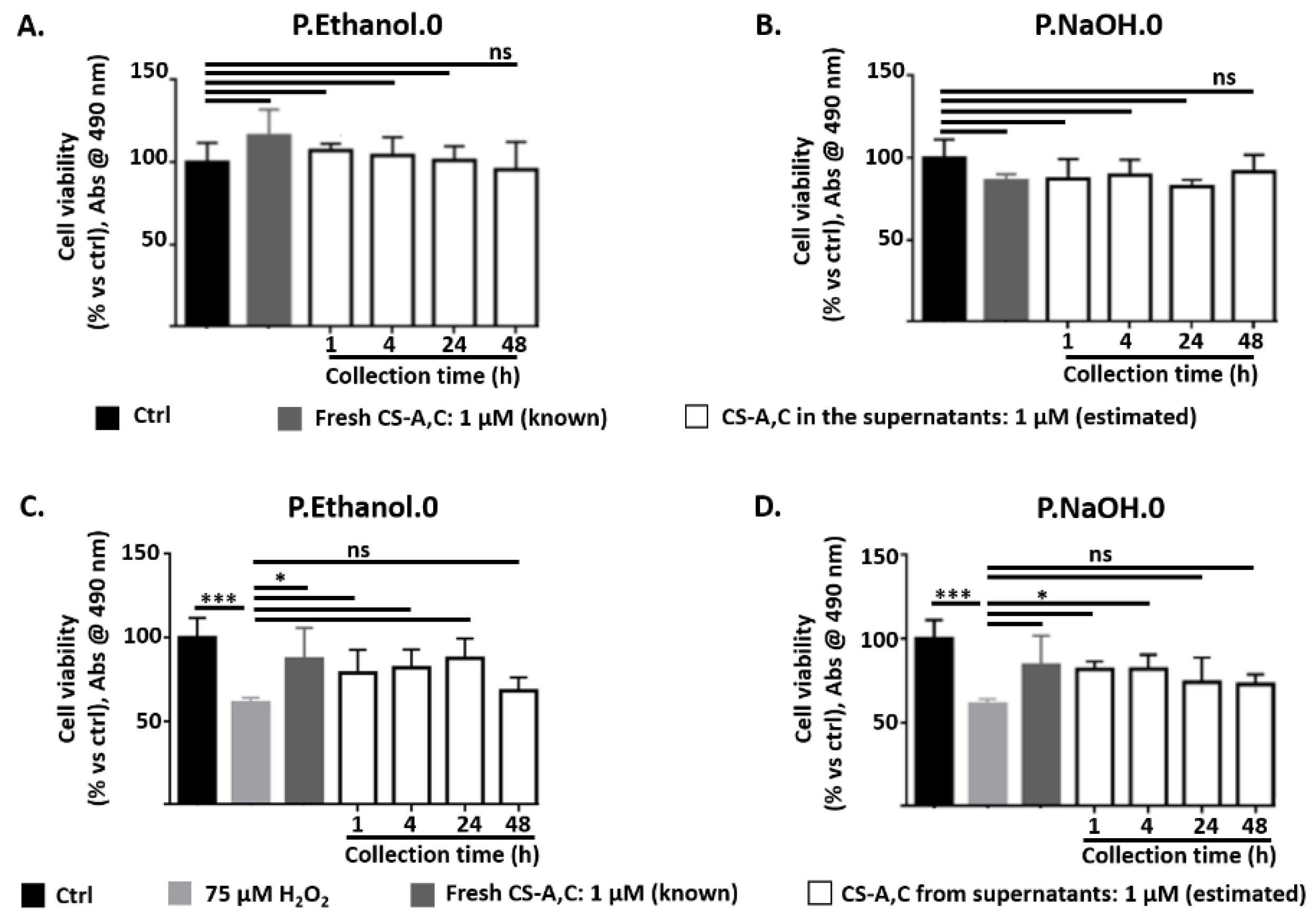
2.7. CS Polysaccharide Release from Carbomer/Agarose Semi-IPNs
2.8. Statistical Analysis
3. Results
3.1. Neuroprotective Properties of CS Polysaccharides
3.2. Effect of Sterilization Process on the Viscoelastic Properties of Carbomer/Agarose Semi-IPNs
3.3. Effect of CS Polysaccharide Loading on the Viscoelastic Properties of Carbomer/Agarose Semi-IPNs
3.4. CS Polysaccharide Release from Carbomer/Agarose Semi-IPNs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedini, E.; Corsaro, M.M.; Fernández-Mayoralas, A.; Iadonisi, A. Chondroitin, dermatan, heparan, and keratan sulfate: Structure and functions. In Extracellular Sugar-Based Biopolymers Matrices; Cohen, E., Merzendorfer, H., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 187–232. [Google Scholar]
- Soares da Costa, D.; Reis, R.L.; Pashkuleva, I. Sulfation of glycosaminoglycans and its implications in human health and disorders. Annu. Rev. Biomed. Eng. 2018, 19, 1–26. [Google Scholar] [CrossRef]
- Couchman, J.R.; Pataki, C.A. An Introduction to Proteoglycans and Their Localization. J. Histochem. Cytochem. 2012, 60, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Deepa, S.S.; Carulli, D.; Galtrey, C.; Rhodes, K.; Fukuda, J.; Mikami, T.; Sugahara, K.; Fawcett, J.W. Composition of perineuronal net extracellular matrix in rat brain: A different disaccharide composition for the net-associated proteoglycans. J. Biol. Chem. 2006, 281, 17789–17800. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Oohashi, T.; Pizzorusso, T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat. Rev. Neurosci. 2019, 20, 451–465. [Google Scholar] [CrossRef]
- Gama, C.I.; Tully, S.E.; Sotogaku, N.; Clark, P.M.; Rawat, M.; Vaidehi, N.; Goddard, W.A.; Nishi, A.; Hsieh-Wilson, L.C. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat. Chem. Biol. 2006, 2, 467–473. [Google Scholar] [CrossRef]
- Carulli, D.; Pizzorusso, T.; Kwok, J.C.F.; Putignano, E.; Poli, A.; Forostyak, S.; Andrews, M.R.; Deepa, S.S.; Glant, T.T.; Fawcett, J.W. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 2010, 133, 2331–2347. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, G.; Hausen, D.; Hartig, W.; Drlicek, M.; Arendt, T.; Brauer, K. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer’s disease. Neuroscience 1999, 92, 791–805. [Google Scholar] [CrossRef]
- Morawski, M.; Bruckner, M.K.; Riederer, P.; Bruckner, G.; Arendt, T. Perineuronal nets potentially protect against oxidative stress. Exp. Neurol. 2004, 188, 309–315. [Google Scholar] [CrossRef]
- Sato, Y.; Nakanishi, K.; Tokita, Y.; Kakizawa, H.; Ida, M.; Maeda, H.; Matsui, F.; Aono, S.; Saito, A.; Kuroda, Y.; et al. A highly sulfated chondroitin sulfate preparation, CS-E, prevents excitatory amino acid-induced neuronal cell death. J. Neurochem. 2008, 104, 1565–1576. [Google Scholar] [CrossRef]
- Morawski, M.; Brückner, G.; Jäger, C.; Seeger, G.; Matthews, R.T.; Arendt, T. Involvement of perineuronal and perisynaptic extracellular matrix in Alzheimer’s disease neuropathology. Brain Pathol. 2012, 22, 547–561. [Google Scholar] [CrossRef]
- Baig, S.; Wilcock, G.K.; Love, S. Loss of perineuronal net N-acetylgalactosamine in Alzheimer’s disease. Acta Neuropathol. 2005, 110, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; García, A.G.; Verges, J.; Montell, E.; López, M.G. Antioxidant, antiinflammatory and neuroprotective actions of chondroitin sulfate and proteoglycans. Osteoarthr. Cartil. 2010, 18, S24–S27. [Google Scholar] [CrossRef]
- Mishra, S.; Ganguli, M. Functions of, and replenishment strategies for, chondroitin sulfate in the human body. Drug Discov. Today 2021, 26, 1185–1199. [Google Scholar] [CrossRef]
- Iannuzzi, C.; Borriello, M.; D’Agostino, A.; Cimini, D.; Schiraldi, C.; Sirangelo, I. Protective effect of extractive and biotechnological chondroitin in insulin amyloid and advanced glycation end product-induced toxicity. J. Cell. Physiol. 2019, 234, 3814–3828. [Google Scholar] [CrossRef] [PubMed]
- Cañas, N.; Valero, T.; Villarroya, M.; Montell, E.; Vergés, J.; García, A.G.; López, M.G. Chondroitin sulfate protects SH-SY5Y cells from oxidative stress by inducing Heme Oxygenase-1 via phosphatidylinositol 3-kinase/Akt. J. Pharmacol. Exp. Ther. 2007, 323, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Bravo, R.; Arimon, M.; Valle-Delgado, J.J.; García, R.; Durany, N.; Castel, S.; Cruz, M.; Ventura, S.; Fernàndez-Busquets, X. Sulfated polysaccharides promote the assembly of amyloid beta(1-42) peptide into stable fibrils of reduced cytotoxicity. J. Biol. Chem. 2008, 283, 32471–32483. [Google Scholar] [CrossRef]
- Zhao, N.; Meng, J.; Jiang, W.; Xu, W.; Liu, C.; Wang, F. Study on the relationships between molecular weights of chondroitin sulfate oligosaccharides and Aβ-induced oxidative stress and the related mechanisms. Glycobiology 2020, 31, 492–507. [Google Scholar] [CrossRef]
- Köwitsch, A.; Zhou, G.; Groth, T. Medical application of glycosaminoglycans: A review. J. Tissue Eng. Regen. Med. 2018, 12, e23–e41. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, M.; Beccati, D.; Shriver, Z.; Naggi, A.; Viswanathan, K.; Bisio, A.; Capila, I.; Lansing, J.C.; Guglieri, S.; Fraser, B.; et al. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat. Biotechnol. 2008, 6, 669–675. [Google Scholar] [CrossRef]
- Badri, A.; Williams, A.; Linhardt, R.J.; Koffas, M.A.G. The road to animal-free glycosaminoglycan production: Current efforts and bottlenecks. Curr. Opin. Biotechnol. 2018, 53, 85–92. [Google Scholar] [CrossRef]
- Badri, A.; Williams, A.; Awofiranye, A.; Datta, P.; Xia, K.; He, W.; Fraser, K.; Dordick, J.S.; Linhardt, R.J.; Koffas, M.A.G. Complete biosynthesis of a sulfated chondroitin in Escherichia coli. Nat. Commun. 2021, 12, 1389. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, W.; Wang, Y.; Sheng, J.; Xu, R.; Li, J.; Du, G.; Kang, Z. Biosynthesis of non-animal chondroitin sulfate from methanol using genetically engineered Pichia pastoris. Green Chem. 2021, 23, 4365–4374. [Google Scholar] [CrossRef]
- Bedini, E.; Laezza, A.; Iadonisi, A. Chemical derivatization of sulfated glycosaminoglycans. Eur. J. Org. Chem. 2016, 2016, 3018–3042. [Google Scholar] [CrossRef]
- Corsuto, L.; Rother, S.; Koehler, L.; Bedini, E.; Moeller, S.; Schnabelrauch, M.; Hintze, V.; Schiraldi, C.; Scharnweber, D. Sulfation degree not origin of chondroitin sulfate derivatives modulates keratinocyte response. Carbohydr. Polym. 2018, 191, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Vessella, G.; Vázquez, J.A.; Valcárcel, J.; Lagartera, L.; Monterrey, D.T.; Bastida, A.; García-Junceda, E.; Bedini, E.; Fernández-Mayoralas, A.; Revuelta, J. Deciphering structural determinants in chondroitin sulfate binding to FGF-2: Paving the way to enhanced predictability of their biological functions. Polymers 2021, 13, 313. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, P.; Cheng, Y.; Zhang, X.; Sheng, J.; Wang, D.; Li, J.; Zhang, Q.; Zhong, C.; Cao, R.; et al. Enhancing the intestinal absorption of low molecular weight chondroitin sulfate by conjugation with α-linolenic acid and the transport mechanism of the conjugates. Int. J. Pharm. 2014, 465, 143–158. [Google Scholar] [CrossRef]
- Kubo, M.; Ando, K.; Mimura, T.; Matsusue, Y.; Mori, K. Chondroitin sulfate for the treatment of hip and knee osteoarthritis: Current status and future trends. Life Sci. 2009, 85, 477–483. [Google Scholar] [CrossRef]
- Henrotin, Y.; Mathy, M.; Sanchez, C.; Lambert, C. Chondroitin sulfate in the treatment of osteoarthritis: From in vitro studies to clinical recommendations. Ther. Adv. Musculoskelet. Dis. 2010, 2, 335–348. [Google Scholar] [CrossRef]
- Hui, J.H.; Chan, S.-W.; Li, J.; Goh, J.C.H.; Li, L.; Ren, X.F.; Lee, E.H. Intra-articular delivery of chondroitin sulfate for the treatment of joint defects in rabbit model. J. Mol. Histol. 2007, 38, 483–489. [Google Scholar] [CrossRef]
- He, Z.; Wang, B.; Hu, C.; Zhao, J. An overview of hydrogel-based intra-articular drug delivery for the treatment of osteoarthritis. Colloids Surf. B Biointerfaces 2017, 154, 33–39. [Google Scholar] [CrossRef]
- Dragicevic, N.; Krajisnik, D.; Milic, J.; Fahr, A.; Maibach, H. Development of hydrophilic gels containing coenzyme Q10-loaded liposomes: Characterization, stability and rheology measurements. Drug Dev. Ind. Pharm. 2019, 45, 43–54. [Google Scholar] [CrossRef]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Benigni, M.; Pescina, S.; Grimaudo, M.A.; Padula, C.; Santi, P.; Nicoli, S. Development of microemulsions of suitable viscosity for cyclosporine skin delivery. Int. J. Pharm. 2018, 545, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Panda, A.K.; Asif, S.; Verma, D.; Talegaonkar, S.; Manzoor, N.; Khan, A.; Ahmed, F.J.; Dudeja, M.; Iqbal, Z. A vaginal drug delivery model. Drug Deliv. 2016, 23, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Kumria, R.; Nair, A.B.; Goomber, G.; Gupta, S. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016, 23, 471–478. [Google Scholar] [CrossRef]
- Varum, F.J.O.; Veiga, F.; Sousa, J.S.; Basit, A.W. Mucoadhesive platforms for targeted delivery to the colon. Int. J. Pharm. 2011, 420, 11–19. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L.; Wang, X.; Tang, L.; Ma, J. The application of mucoadhesive polymers in nasal drug delivery. Drug Dev. Ind. Pharm. 2010, 36, 323–336. [Google Scholar] [CrossRef]
- Brady, J.; Dürig, T.; Lee, P.I.; Li, J.-X. Polymer Properties and Characterization. In Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice; Qiu, Y., Chen, Y., Zhang, G.G., Yu, L., Mantri, R.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 181–223. [Google Scholar]
- Liu, J.; Li, L. SDS-aided immobilization and controlled release of camptothecin from agarose hydrogel. Eur. J. Pharm. Sci. 2005, 25, 237–244. [Google Scholar] [CrossRef]
- Gustavsson, E.; Larsson, O. Chapter 6-Monolithic Polysaccharide Materials. In Monolithic Materials-Preparation, Properties and Applications; Švec, F., Tennikova, T.B., Deyl, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 121–141. [Google Scholar]
- Tunesi, M.; Prina, E.; Munarin, F.; Rodilossi, S.; Albani, D.; Petrini, P.; Giordano, C. Cross-linked poly(acrylic acids) microgels and agarose as semi-interpenetrating networks for resveratrol release. J. Mater. Sci. Mater. Electron. 2015, 26, 5328. [Google Scholar] [CrossRef]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef]
- Tunesi, M.; Raimondi, I.; Russo, T.; Colombo, L.; Micotti, E.; Brandi, E.; Cappelletti, P.; Cigada, A.; Negro, A.; Ambrosio, L.; et al. Hydrogel-based delivery of Tat-fused protein Hsp70 protects dopaminergic cells in vitro and in a mouse model of Parkinson’s disease. NPG Asia Mater. 2019, 11, 28. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Runge, M.B.; Wang, H.; Spinner, R.J.; Windebank, A.J.; Yaszemski, M.J. Reformulating polycaprolactone fumarate to eliminate toxic diethylene glycol: Effects of polymeric branching andautoclave sterilization on material properties. Acta Biomater. 2012, 8, 133–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cimini, D.; De Rosa, M.; Carlino, E.; Ruggiero, A.; Schiraldi, C. Homologous overexpression of RfaH in E. coli K4 improves the production of chondroitin-like capsular polysaccharide. Microb. Cell Factories 2013, 12, 46. [Google Scholar] [CrossRef]
- Bedini, E.; De Castro, C.; De Rosa, M.; Di Nola, A.; Iadonisi, A.; Restaino, O.F.; Schiraldi, C.; Parrilli, M. microbiological-chemical strategy to produce chondroitin sulfate A,C. Angew. Chem. Int. Ed. 2011, 50, 6160–6163. [Google Scholar] [CrossRef]
- Laezza, A.; De Castro, C.; Parrilli, M.; Bedini, E. Inter vs. intraglycosidic acetal linkages control sulfation pattern in semi-synthetic chondroitin sulfate. Carbohydr. Polym. 2014, 112, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Disinfection, Sterilization, and Control of Hospital Waste. In Principles and Practice of Infectious Diseases; Mandell, G.L., Bennett, J.E., Eds.; Churchill Livingstone Elsevier: London, UK, 2015; pp. 3294–3309. [Google Scholar]
- Winter, S.; Smith, A.; Lappin, D.; McDonagh, G.; Kirk, B. Investigating steam penetration using thermometric methods in dental handpieces with narrow internal lumens during sterilizing processes with non-vacuum or vacuum processes. J. Hosp. Infect. 2017, 97, 338–342. [Google Scholar] [CrossRef]
- Sasaki, J.-I.; Imazato, S. Autoclave sterilization of dental handpieces: A literature review. J. Prosthodont. Res. 2020, 64, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta BBA Gen. Subj. 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to Cause Oxidative Stress, the Catalase Issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Farndale, R.W.; Sayers, C.A.; Barrett, A.J. A Direct Spectrophotometric Microassay for Sulfated Glycosaminoglycans in Cartilage Cultures. Connect. Tissue Res. 1982, 9, 247–248. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Thalla, P.K.; Fadlallah, H.; Liberelle, B.; Lequoy, P.; De Crescenzo, G.; Merhi, Y.; Lerouge, S. Chondroitin sulfate coatings display low platelet but high endothelial cell adhesive properties favorable for vascular implants. Biomacromolecules 2014, 15, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Förster, Y.; Schulze, S.; Penk, A.; Neuber, C.; Möller, S.; Hintze, V.; Scharnweber, D.; Schnabelrauch, M.; Pietzsch, J.; Huster, D.; et al. The influence of different artificial extracellular matrix implant coatings on the regeneration of a critical size femur defect in rats. Mater. Sci. Eng. C 2020, 116, 111157. [Google Scholar] [CrossRef]
- Ye, J.; Huang, B.; Gong, P. Nerve growth factor-chondroitin sulfate/hydroxyapatite-coating composite implant induces early osseointegration and nerve regeneration of peri-implant tissues in Beagle dogs. J. Orthop. Surg. Res. 2021, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Betancur, M.I.; Mason, H.D.; Alvarado-Velez, M.; Holmes, P.V.; Bellamkonda, R.V.; Karumbaiah, L. Chondroitin Sulfate Glycosaminoglycan Matrices Promote Neural Stem Cell Maintenance and Neuroprotection Post-Traumatic Brain Injury. ACS Biomater. Sci. Eng. 2017, 3, 420–430. [Google Scholar] [CrossRef]
- Altgärde, N.; Nilebäck, E.; de Battice, L.; Pashkuleva, I.; Reis, R.L.; Becher, J.; Möller, S.; Schnabelrauch, M.; Svedhem, S. Probing the biofunctionality of biotinylated hyaluronan and chondroitin sulfate by hyaluronidase degradation and aggrecan interaction. Acta Biomater. 2013, 9, 8158–8166. [Google Scholar] [CrossRef]
- Soleman, S.; Filippov, M.A.; Dityatev, A.; Fawcett, J.W. Targeting the neural extracellular matrix in neurological disorders. Neuroscience 2013, 253, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Kitagawa, H. Chondroitin sulfate and neuronal disorders. Front. Biosci. 2016, 21, 1330–1340. [Google Scholar] [CrossRef]
- Ju, C.; Gao, J.; Hou, L.; Wang, L.; Zhang, F.; Sun, F.; Zhang, T.; Xu, P.; Shi, Z.; Hu, F.; et al. Neuroprotective effect of chondroitin sulfate on SH-SY5Y cells overexpressing wild-type or A53T mutant α-synuclein. Mol. Med. Rep. 2017, 16, 8721–8728. [Google Scholar] [CrossRef]
- Yang, X. Chondroitin sulfate proteoglycans: Key modulators of neuronal plasticity, long-term memory, neurodegenerative, and psychiatric disorders. Rev. Neurosci. 2020, 31, 555–568. [Google Scholar] [CrossRef]
- McKeon, R.J.; Schreiber, R.C.; Rudge, J.S.; Silver, J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991, 11, 3398–3411. [Google Scholar] [CrossRef] [PubMed]
- Muir, E.; De Winter, F.; Verhaagen, J.; Fawcett, J. Recent advances in the therapeutic uses of chondroitinase ABC. Exp. Neurol. 2019, 321, 113032. [Google Scholar] [CrossRef]
- Vessella, G.; Traboni, S.; Cimini, D.; Iadonisi, A.; Schiraldi, C.; Bedini, E. Development of semisynthetic, regioselective pathways for accessing the missing sulfation patterns of chondroitin sulfate. Biomacromolecules 2019, 20, 3021–3030. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Dudas, B.; Hejna, M.; Cornelli, U.; Lee, J.M.; Lorens, S.; Mervis, R.; Hanin, I.; Fareed, J. The blood-brain barrier accessibility of a heparin-derived oligosaccharides C3. Thromb. Res. 2002, 105, 447–453. [Google Scholar] [CrossRef]
- Galante, R.; Pinto, T.J.A.; Colaço, R.; Serro, A.P. Sterilization of hydrogels for biomedical applications: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2472–2492. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A.; Toresco, M.; McNamara, D.; Iuga, D.; Abraham, A.; Tobyn, A.; Hawarden, L.E.; Blanc, F. Drug-Polymer Interactions in Acetaminophen/Hydroxypropylmethylcellulose Acetyl Succinate Amorphous Solid Dispersions Revealed by Multidimensional Multinuclear Solid-State NMR Spectroscopy. Mol. Pharm. 2021, 18, 3519–3531. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedini, E.; Iadonisi, A.; Schiraldi, C.; Colombo, L.; Albani, D.; Petrini, P.; Giordano, C.; Tunesi, M. Microbiological-Chemical Sourced Chondroitin Sulfates Protect Neuroblastoma SH-SY5Y Cells against Oxidative Stress and Are Suitable for Hydrogel-Based Controlled Release. Antioxidants 2021, 10, 1816. https://doi.org/10.3390/antiox10111816
Bedini E, Iadonisi A, Schiraldi C, Colombo L, Albani D, Petrini P, Giordano C, Tunesi M. Microbiological-Chemical Sourced Chondroitin Sulfates Protect Neuroblastoma SH-SY5Y Cells against Oxidative Stress and Are Suitable for Hydrogel-Based Controlled Release. Antioxidants. 2021; 10(11):1816. https://doi.org/10.3390/antiox10111816
Chicago/Turabian StyleBedini, Emiliano, Alfonso Iadonisi, Chiara Schiraldi, Laura Colombo, Diego Albani, Paola Petrini, Carmen Giordano, and Marta Tunesi. 2021. "Microbiological-Chemical Sourced Chondroitin Sulfates Protect Neuroblastoma SH-SY5Y Cells against Oxidative Stress and Are Suitable for Hydrogel-Based Controlled Release" Antioxidants 10, no. 11: 1816. https://doi.org/10.3390/antiox10111816
APA StyleBedini, E., Iadonisi, A., Schiraldi, C., Colombo, L., Albani, D., Petrini, P., Giordano, C., & Tunesi, M. (2021). Microbiological-Chemical Sourced Chondroitin Sulfates Protect Neuroblastoma SH-SY5Y Cells against Oxidative Stress and Are Suitable for Hydrogel-Based Controlled Release. Antioxidants, 10(11), 1816. https://doi.org/10.3390/antiox10111816










