Effect of Germination on Alfalfa and Buckwheat: Phytochemical Profiling by UHPLC-ESI-QTOF-MS/MS, Bioactive Compounds, and In-Vitro Studies of Their Diabetes and Obesity-Related Functions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials Collection
2.2. Chemicals
2.3. Methods
2.3.1. Preparation of Ethanolic Extracts
2.3.2. Total Phenolic Content (TPC)
2.3.3. Total Flavonoid Content (TFC)
2.3.4. Total Saponin Content (TSC)
2.3.5. DPPH Radical Scavenging Activity
2.3.6. ABTS Radical Scavenging Activity
2.3.7. Pancreatic Lipase Inhibition Assay
2.3.8. α-Glucosidase Inhibitory Assay
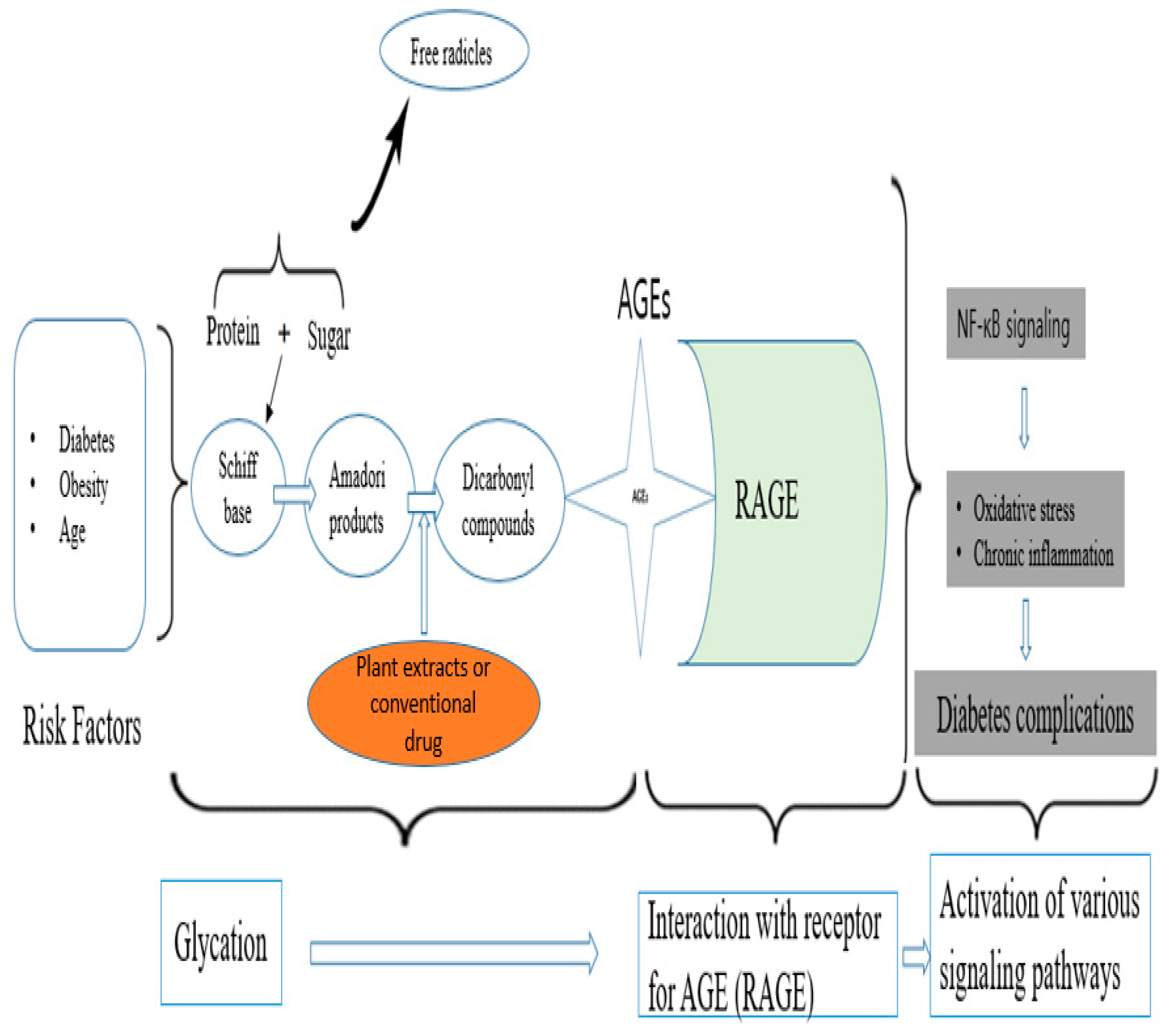
2.3.9. Inhibition of AGEs Formation
2.3.10. UHPLC-Q-TOF-MS/MS2 Phenolic Compounds Identification
2.3.11. Statistical Analysis
3. Results
3.1. Metabolites Identification
3.2. Heat Map and Principal Component Analysis (PCA)
3.3. TPC, TFC, and TSC and Antioxidant Potential of Ethanol Extracts
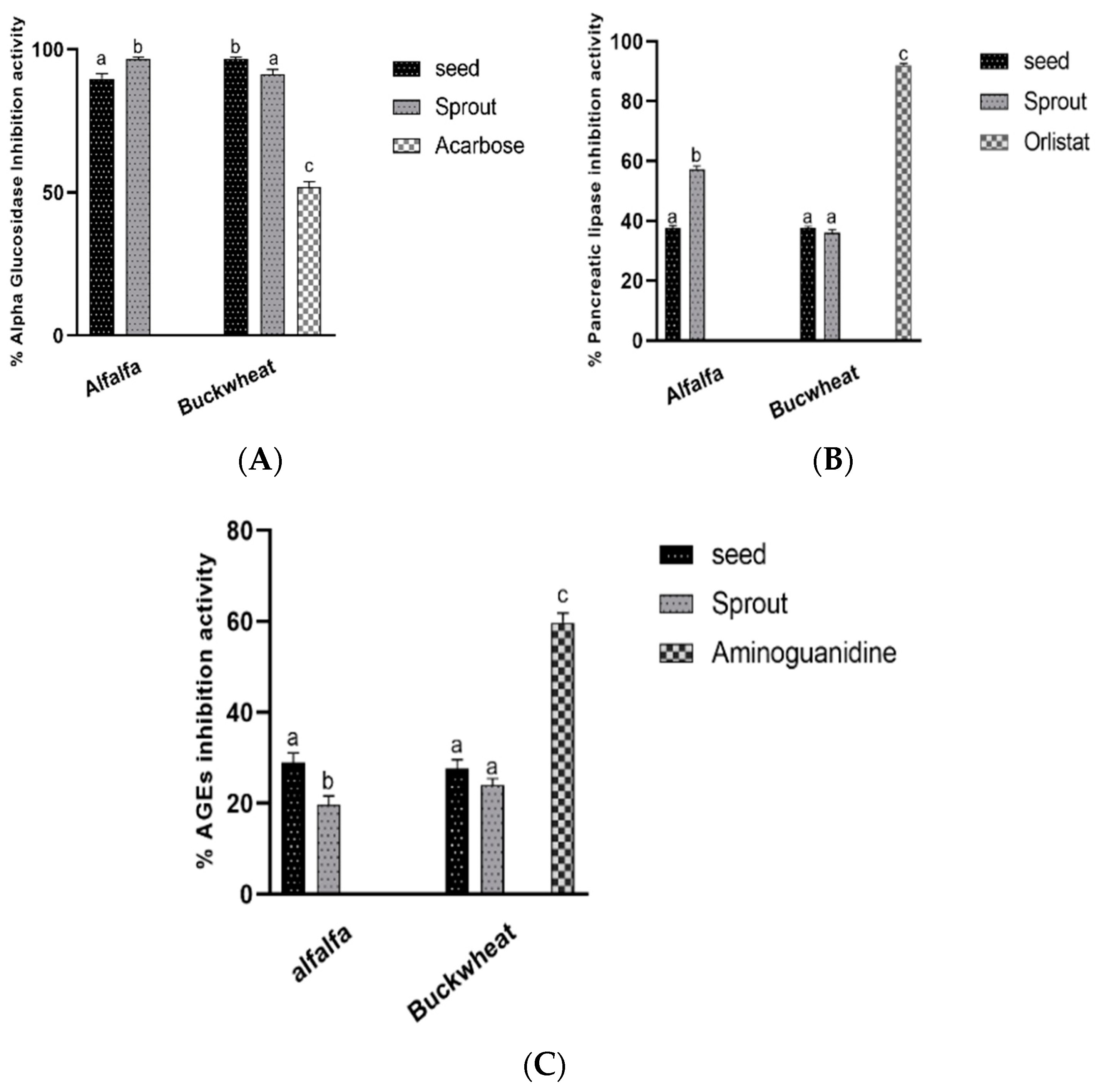
3.4. Antidiabetic and Antiobesity Activities In Vitro
Enzymes and Advanced Glycation End Products Formation Inhibitory Activities
4. Discussion
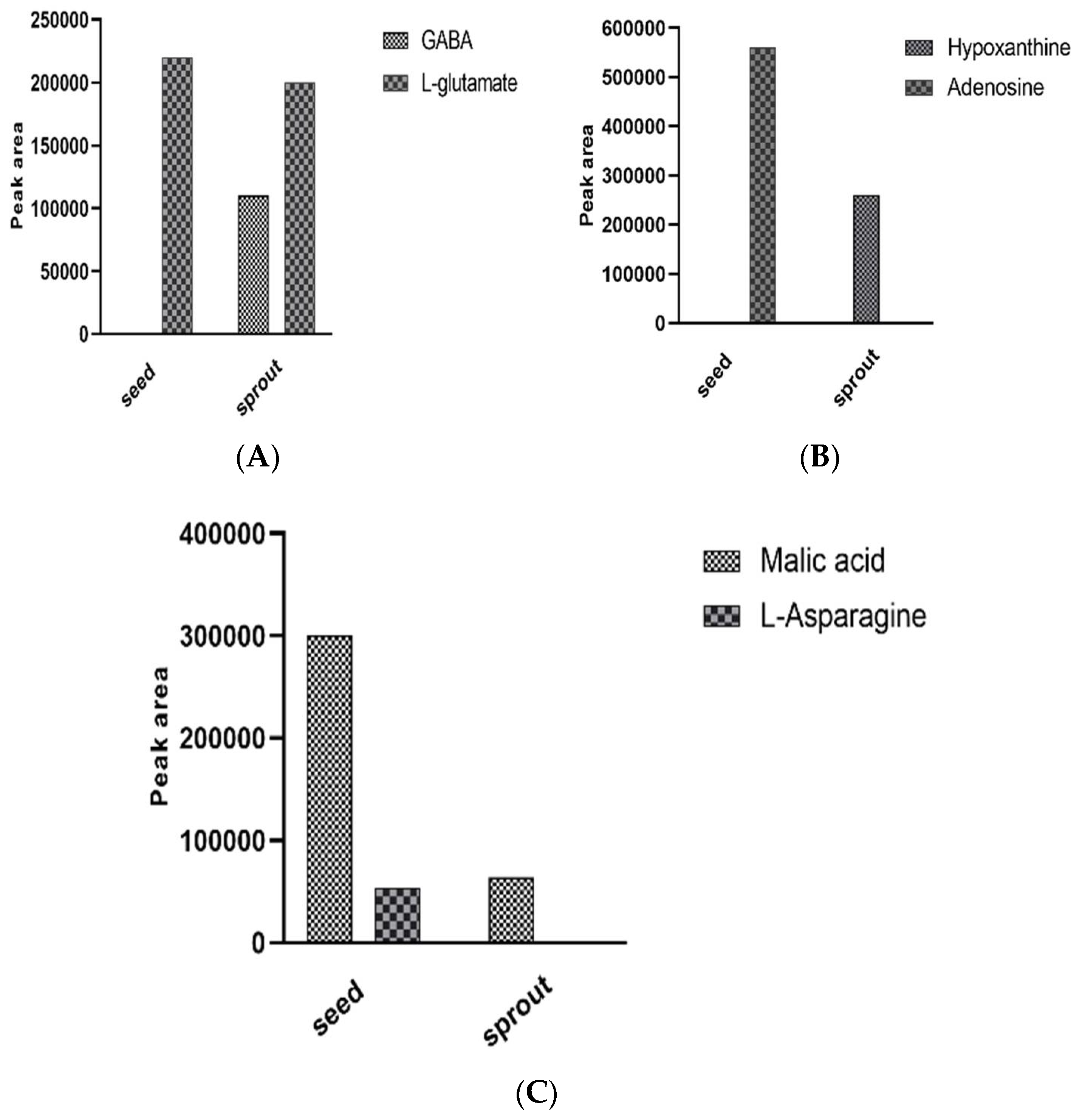
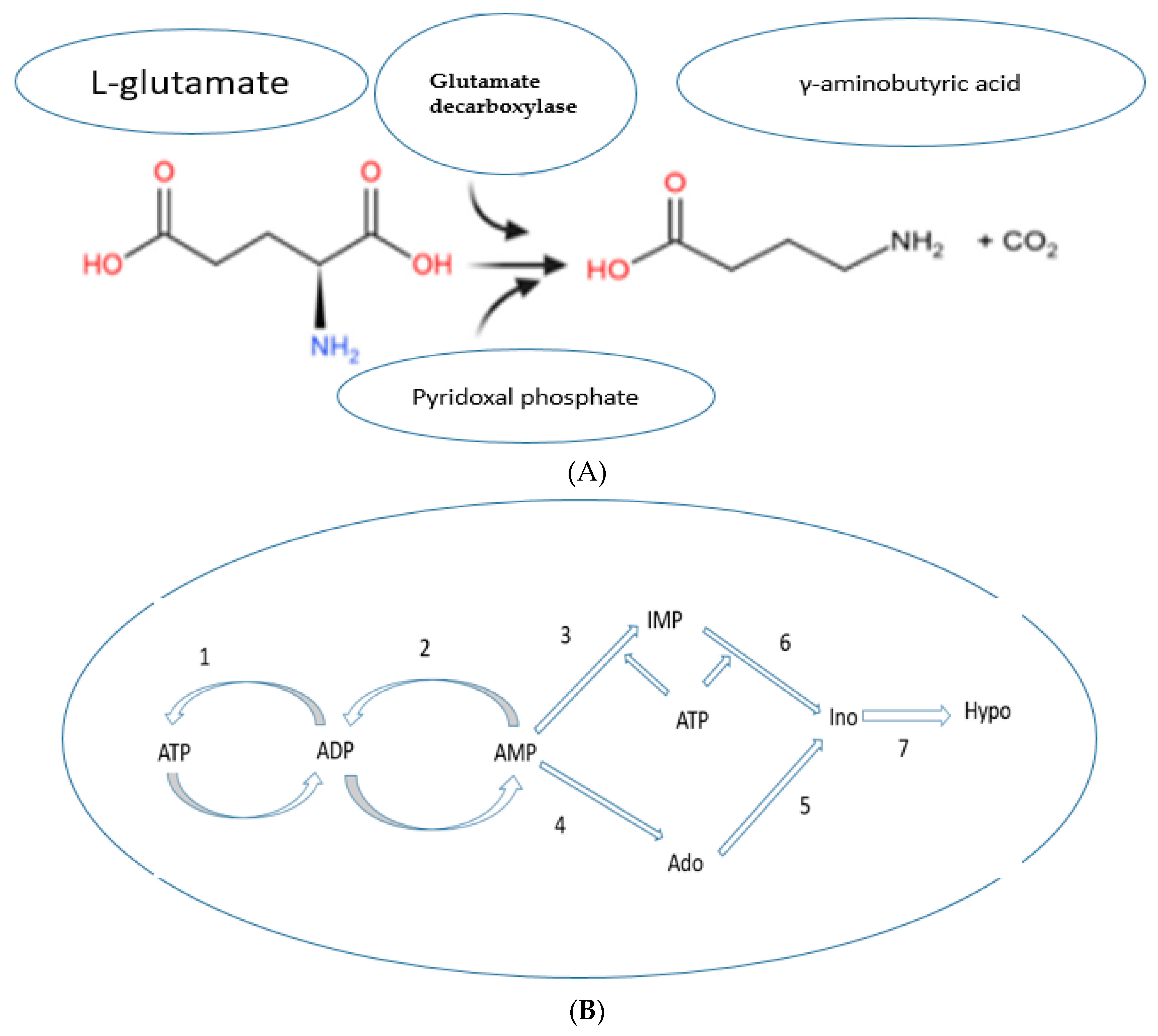
Enzymatic Bioconversion of Compounds during the Germination Process Led to the Synthesis of Potential Diabetes Biomarkers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd Allah, A.L.; Abd-Elrahman, W.M. Hypocholesterolemic and Anti-Obesity Effects of Radish Sprouts (Raphanus Sativus) in Adult Females. Egypt. J. Food Sci. 2021, 49, 19–34. [Google Scholar]
- Nanmori, T.; Kohno, A. Changes in α and β-Amylase Activities during Germination of Seeds of Alfalfa (Medicago sativa L.). Plant Cell Physiol. 1991, 32, 459–466. [Google Scholar] [CrossRef]
- Dariya, B.; Nagaraju, G.P. Advanced glycation end products in diabetes, cancer and phytochemical therapy. Drug Discov. Today 2020, 25, 1614–1623. [Google Scholar] [CrossRef]
- Zinca, G.; Vizireanu, C. Impact of germination on phenolic compounds content and antioxidant activity of alfalfa seeds (Medicago sativa L.). J. Agroaliment. Process. Technol. 2013, 19, 105–110. [Google Scholar]
- Świeca, M. Elicitation and treatment with precursors of phenolics synthesis improve low-molecular antioxidants and anti-oxidant capacity of buckwheat sprouts. Acta Sci. Pol. Technol. Aliment. 2016, 15, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Pradeep, P.; Sreerama, Y.N. Impact of processing on the phenolic profiles of small millets: Evaluation of their antioxidant and enzyme inhibitory properties associated with hyperglycemia. Food Chem. 2015, 169, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.-M.; Chelliah, R.; Ham, H.J.; Kim, J.-H.; Han, S.-I.; Hur, J.H.; Oh, D.-H. Phenolic Profile, Antioxidant, and Antidiabetic Potential Exerted by Millet Grain Varieties. Antioxidants 2020, 9, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza-Sánchez, M.; Guevara-González, R.G.; Castaño-Tostado, E.; Mercado-Silva, E.M.; Acosta-Gallegos, J.A.; Guzmán, N.E.R.; Reynoso-Camacho, R. Effect of chemical stress on germination of cv Dalia bean (Phaseolus vularis L.) as an alternative to increase antioxidant and nutraceutical compounds in sprouts. Food Chem. 2016, 212, 128–137. [Google Scholar] [CrossRef]
- Li, W.; Pickard, M.D.; Beta, T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2019, 275, 361–368. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef]
- Gülçin, I.; Gören, A.C.; Taslimi, P.; Alwasel, S.H.; Kılıc, O.; Bursal, E. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)-analysis of its polyphenol contents by LC-MS/MS. Biocatal. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar] [CrossRef]
- Basha, S.I.; Ghosh, S.; Vinothkumar, K.; Ramesh, B.; Mohan, K.M.; Sukumar, E. Fumaric acid incorporated Ag/agar-agar hybrid hydrogel: A multifunctional avenue to tackle wound healing. Mater. Sci. Eng. C 2020, 111, 110743. [Google Scholar] [CrossRef]
- Chiriac, E.R.; Chiţescu, C.L.; Borda, D.; Lupoae, M.; Gird, C.E.; Geană, E.I.; Blaga, G.V.; Boscencu, R. Comparison of the polyphenolic profile of Medicago sativa L. and Trifolium pratense L. sprouts in different germination stages using the UHPLC-Q exactive hybrid quadrupole orbitrap high-resolution mass spectrometry. Molecules 2020, 25, 2321. [Google Scholar] [CrossRef] [PubMed]
- Beitane, I.G.; Krumina–Zemture, M.; Sabovics, M. Effect of germination and extrusion on the phenolic content and antioxidant activity of raw buckwheat (Fagopyrum esculentum Moench). EMU DSpace 2018, 16, 2. [Google Scholar]
- Hu, L.; Cai, X.; Dong, S.; Zhen, Y.; Hu, J.; Wang, S.; Jiang, J.; Huang, J.; Han, Y.; Qian, Y.; et al. Synthesis and Anticancer Activity of Novel Actinonin Derivatives as HsPDF Inhibitors. J. Med. Chem. 2020, 63, 6959–6978. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, C.V. Aminoacid Support in the Prevention of Diabetes and Diabetic Complications. Curr. Protein Pept. Sci. 2009, 10, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Tseng, M.-C.; Cheng, W. Identification and cloning of the antioxidant enzyme, glutathione peroxidase, of white shrimp, Litopenaeus vannamei, and its expression following Vibrio alginolyticus infection. Fish Shellfish. Immunol. 2007, 23, 34–45. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Wang, D.; Fang, F.; Lai, J.; Wu, T.; Tsao, R. Isoflavone, γ-aminobutyric acid contents and antioxidant activities are significantly increased during germi-nation of three Chinese soybean cultivars. J. Funct. Foods 2015, 14, 596–604. [Google Scholar] [CrossRef]
- Szafrańska, K.; Szewczyk, R.; Janas, K. Involvement of melatonin applied to Vigna radiata L. seeds in plant response to chilling stress. Open Life Sci. 2014, 9, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-L.; Huang, S.-L.; Yen, G.-C. Inhibitory Effect of Phenolic Acids on the Proliferation of 3T3-L1 Preadipocytes in Relation to Their Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 4191–4197. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, C.; Abbasi, A.M.; Chen, G.; You, L.; Li, T.; Fu, X.; Wang, Y.; Guo, X.; Liu, R.H. Effect of germination on vitamin C, phenolic compounds and antioxidant activity in flaxseed (Linum usita-tissimum L.). Int. J. Food Sci. Technol. 2015, 50, 2545–2553. [Google Scholar] [CrossRef]
- Morohashi, Y.; Shimokoriyama, M. Physiological studies on germination of Phaseolus mungo seeds: II. Glucose and organic-acid metabolisms in the early phases of germination. J. Exp. Bot. 1972, 23, 54–61. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, H.; Zhang, C.; Lu, Y.; Zhu, X.; Lu, Z. γ-Aminobutyric acid-rich yogurt fermented by Streptococcus salivarius subsp. thermophiles fmb5 apprars to have anti-diabetic effect on streptozotocin-induced diabetic mice. J. Funct. Foods 2016, 20, 267–275. [Google Scholar] [CrossRef]
- Roohinejad, S.; Omidizadeh, A.; Mirhosseini, H.; Saari, N.; Mustafa, S.; Hussin, A.S.M.; Hamid, A.; Manap, M.Y.A. Effect of Pre-Germination Time on Amino Acid Profile and Gamma Amino Butyric Acid (GABA) Contents in Different Varieties of Malaysian Brown Rice. Int. J. Food Prop. 2011, 14, 1386–1399. [Google Scholar] [CrossRef]
- Sharma, S.; Saxena, D.C.; Riar, C.S. Analysing the effect of germination on phenolics, dietary fibres, minerals and γ-amino butyric acid contents of barnyard millet (Echinochloa frumentaceae). Food Biosci. 2016, 13, 60–68. [Google Scholar] [CrossRef]
- Barsotti, C.; Ipata, P.L. Metabolic regulation of ATP breakdown and of adenosine production in rat brain extracts. Int. J. Biochem. Cell Biol. 2004, 36, 2214–2225. [Google Scholar] [CrossRef]
- Varadaiah, Y.G.C.; Sivanesan, S.; Nayak, S.B.; Thirumalarao, K.R. Purine metabolites can indicate diabetes progression. Arch. Physiol. Biochem. 2019, 1–5. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kamata, K. Effect of chronic insulin treatment on NO production and endothelium-dependent relaxation in aortae from established STZ-induced diabetic rats. Atherosclerosis 2001, 155, 313–320. [Google Scholar] [CrossRef]
- Dakin, H. The formation of l-malic acid as a product of alcoholic fermentation by yeast. J. Biol. Chem. 1924, 61, 139–145. [Google Scholar] [CrossRef]
- Gou, L.; Zhan, Y.; Lee, J.; Li, X.; Lü, Z.R.; Zhou, H.M.; Lu, H.; Wang, X.Y.; Park, Y.D.; Yang, J.M. Effects of L-malic acid on alpha-glucosidase: Inhibition kinetics and computational molecular dynamics simulations. Appl. Biochem. Biotechnol. 2015, 175, 2232–2245. [Google Scholar] [CrossRef]
- Yogeswara, I.; Maneerat, S.; Haltrich, D. Glutamate Decarboxylase from Lactic Acid Bacteria—A Key Enzyme in GABA Synthesis. Microorganisms 2020, 8, 1923. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Z.; Gao, Y.; Huang, X.; Zou, Y.; Yang, T. Effects of Germination on the Nutritional Properties, Phenolic Profiles, and Antioxidant Activities of Buckwheat. J. Food Sci. 2015, 80, H1111–H1119. [Google Scholar] [CrossRef]
- Liu, H.; Kang, Y.; Zhao, X.; Liu, Y.; Zhang, X.; Zhang, S. Effects of elicitation on bioactive compounds and biological activities of sprouts. J. Funct. Foods 2019, 53, 136–145. [Google Scholar] [CrossRef]
- Ruiz, R.G.; Price, K.; Rose, M.; Rhodes, M.; Fenwick, R. A preliminary study on the effect of germination on saponin content and composition of lentils and chick-peas. Z. Lebensm.-Unters. Forsch. 1996, 203, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Padilla-Zakour, O.; Zhao, Y.; Tao, S. Influences of High Hydrostatic Pressure, Microwave Heating, and Boiling on Chemical Compositions, Antinutritional Factors, Fatty Acids, In Vitro Protein Digestibility, and Microstructure of Buckwheat. Food Bioprocess Technol. 2015, 8, 2235–2245. [Google Scholar] [CrossRef]
- Story, J.; Lepage, S.L.; Petro, M.S.; West, L.G.; Cassidy, M.M.; Lightfoot, F.G.; Vahouny, G.V. Interactions of alfalfa plant and sprout saponins with cholesterol in vitro and in cholesterol-fed rats. Am. J. Clin. Nutr. 1984, 39, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Ghimeray, A.K.; Gurung, A.; Jin, C.W.; Rho, H.S.; Cho, D.H. Phenolic contents, antioxidant and α-glucosidase inhibition properties of Nepalese strain buckwheat vegetables. Afr. J. Biotechnol. 2012, 11, 184–190. [Google Scholar]
- Zielinska, D.; Szawara-Nowak, D.; Zieliński, H. Antioxidative and Anti-Glycation Activity of Buckwheat Hull Tea Infusion. Int. J. Food Prop. 2013, 16, 228–239. [Google Scholar] [CrossRef]
- Hosseini, M.; Asgary, S.; Najafi, S. Inhibitory potential of pure isoflavonoids, red clover, and alfalfa extracts on hemoglobin glycosylation. ARYA Atheroscler. 2015, 11, 133. [Google Scholar] [PubMed]

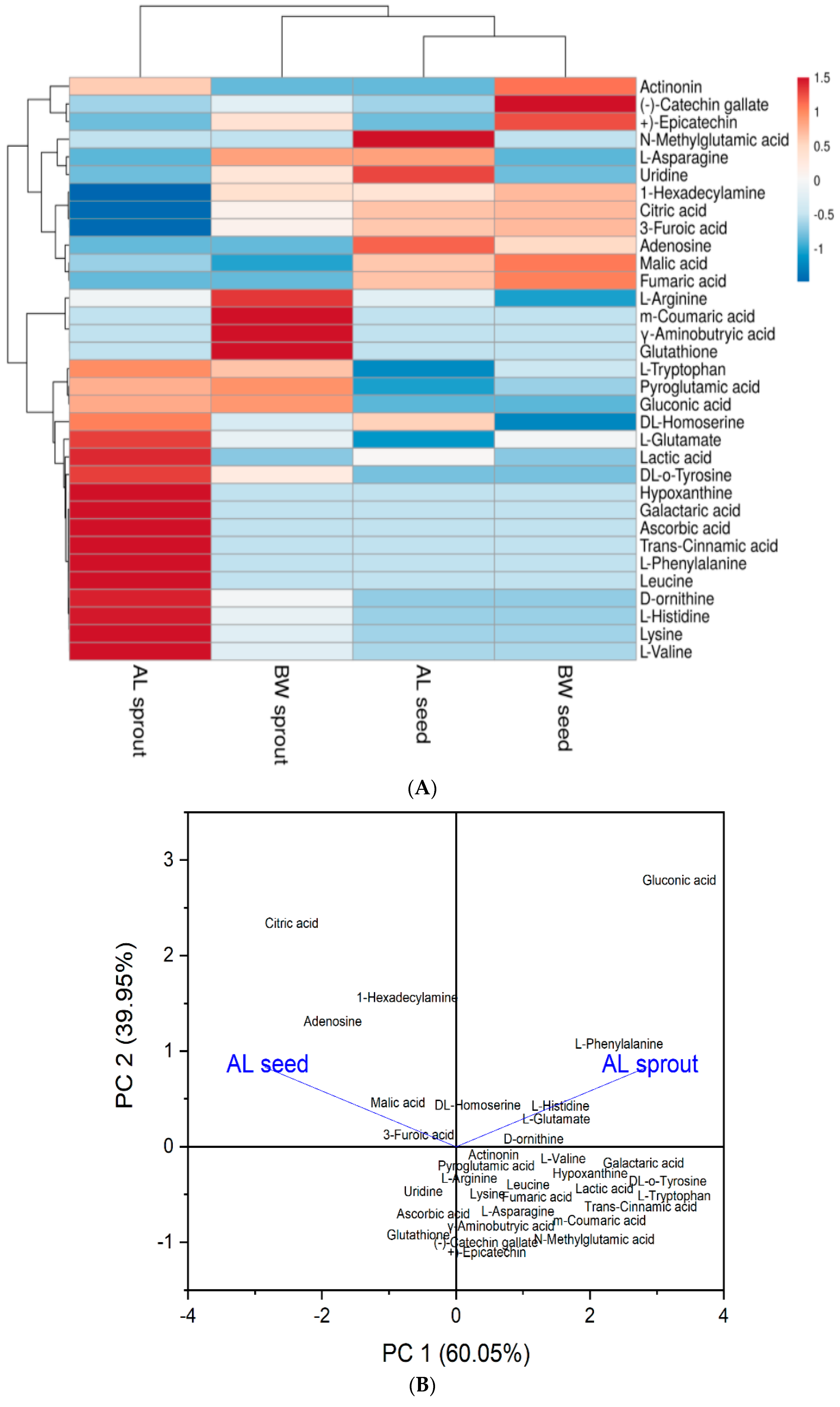
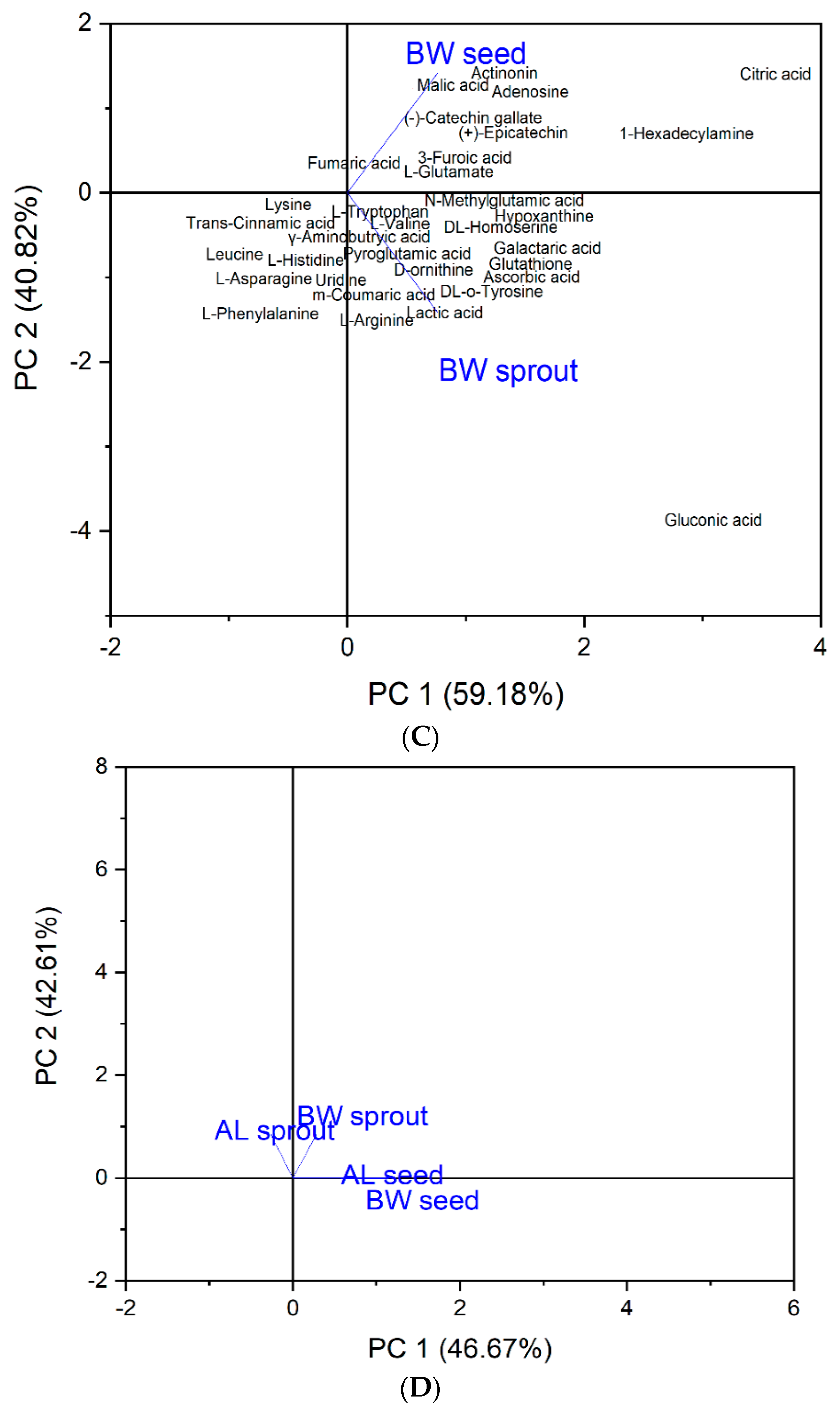
| Peak No. | RT Per Min. | Molecular Weight | [M−H]-(m/z) | Molecular Formula | MS/MS (% Abundance) | Compound Identified |
|---|---|---|---|---|---|---|
| 1. | 0.70 | 174.11 | 173.10 | C6H14N4O2 | 131 (100%), 173 (38%) | L-Arginine |
| 2. | 0.78 | 132.05 | 131.04 | C4H8N2O3 | 131 (100%) | L-Asparagine |
| 3. | 0.82 | 147.05 | 146.04 | C5H9NO4 | 102 (100%), 146 (100%) | L-Glutamate |
| 4. | 1.04 | 116.01 | 115.00 | C4H4O4 | 115 (100%) | Fumaric acid |
| 5. | 1.05 | 134.02 | 133.01 | C4H6O5 | 71 (100%), 133 (45%), 59 (30%) | Malic acid |
| 6. | 1.22 | 244.07 | 243.06 | C9H12N2O6 | 243 (100%), 82 (45%) | Uridine |
| 7. | 1.22 | 112.02 | 111.01 | C5H4O3 | 111(100%), 67 (100%) | 3-Furoic acid |
| 8. | 1.22 | 192.03 | 191.02 | C6H8O7 | 191 ((15%) | Citric acid |
| 9. | 1.22 | 129.04 | 128.03 | C5H7NO3 | 129 (10%) | Pyroglutamic acid |
| 10. | 1.23 | 267.10 | 268.10 | C10H13N5O4 | 136 (100%), 268 (10%) | Adenosine |
| 11. | 1.18 | 161.07 | 160.06 | C6H11NO4 | 58 (100%), 142 (50%) | N-Methylglutamic acid |
| 12. | 5.95 | 204.09 | 203.08 | C11H12N2O2 | 116 (40%), 142 (18%), 74 (8%), 186 (6%) | L-Tryptophan |
| 13. | 9.68 | 290.08 | 289.07 | C15H14O6 | 289 (20%) | (+)-Epicatechin |
| 14. | 13.36 | 385.26 | 384.25 | C19H35N3O5 | 112 (70%), 113 (18%), 224 (15%), | Actinonin |
| 15. | 16.24 | 442.09 | 441.08 | C22H18O10 | 125 (80%), 124 (15%), 145 (23%), 303 (2%) | (−)-Catechin gallate |
| Peak No. | RT Per Min. | Molecular Weight | [M−H]-(m/z) | Molecular Formula | MS/MS (% Abundance) | Compound Identified |
|---|---|---|---|---|---|---|
| 1. | 0.69 | 146.11 | 145.10 | C6H14N2O2 | 145 (100%) | Lysine |
| 2. | 0.72 | 174.11 | 173.10 | C6H14N4O2 | 173 (100%) | L-Arginine |
| 3. | 0.72 | 155.07 | 154.06 | C6H9N3O2 | 93 (100%), 67 (23%), 137 (15%) | L-Histidine |
| 4. | 0.79 | 132.05 | 131.04 | C4H8N2O3 | 131 (100%) | L-Asparagine |
| 5. | 0.81 | 132.09 | 131.08 | C5H12N2O2 | 88 (100%), 131 (52%) | D-ornithine |
| 6. | 0.81 | 119.06 | 118.05 | C4H9NO3 | 74 (100%) 100 (100%) | DL-Homoserine |
| 7. | 0.82 | 103.06 | 102.05 | C4H9NO2 | 102 (100%) | γ-Aminobutryic acid |
| 8. | 0.82 | 147.05 | 146.04 | C5H9NO4 | 102 (39%), 128 (30%) | L-glutamate |
| 9. | 0.85 | 196.06 | 195.05 | C6H12O7 | 75 (100%), 59 (62%), 85 (37%), 129 (10%), 99 (5%) | Gluconic acid |
| 10. | 0.86 | 210.04 | 209.03 | C6H10O8 | 71 (100%), 57 (96%), 85 (60%), 129 (15%) | Galactaric acid |
| 11. | 0.82 | 129.04 | 128.03 | C5H7NO3 | 128 (100%) | Pyroglutamic acid |
| 12. | 0.92 | 176.03 | 175.02 | C6H8O6 | 129 (100%), 57 (100%) | L-ascorbic acid |
| 13. | 1.01 | 117.08 | 116.07 | C5H11NO2 | 116 (100%) | L-Valine |
| 14. | 1.06 | 134.02 | 133.01 | C4H6O5 | 71 (100%), 133 (30%) | Malic acid |
| 15. | 1.08 | 307.08 | 306.07 | C10H17N3O6S | 143 (100%), 128 (98%), 167 (50%), 74 (49%) | Glutathione |
| 16. | 1.21 | 90.03 | 89.02 | C3H6O3 | 89 (100%) | Lactic acid |
| 17. | 1.23 | 112.02 | 111.01 | C5H4O3 | 111 (100%), 67 (100%) | 3-Furoic acid |
| 18. | 1.23 | 244.07 | 243.06 | C9H12N2O6 | 243 (80%), 110 (72%), 84 (50%), | Uridine |
| 19. | 1.23 | 136.04 | 135.03 | C5H4N4O | 65 (100%), 66 (40%), 106 (9%), 92 (8%) | Hypoxanthine |
| 20. | 1.24 | 181.07 | 180.06 | C9H11NO3 | 119 (100%), 180 (99%), 93 (45%), 163 (40%) | DL-o-Tyrosine |
| 21. | 1.24 | 192.03 | 191.02 | C6H8O7 | 191 ((15%) | Citric acid |
| 22. | 1.26 | 131.09 | 130.08 | C6H13NO2 | 130 (100%) | Leucine |
| 23. | 3 | 165.08 | 164.07 | C9H11NO2 | 103 (100%), 147 (50%) 77 (50%) | L-Phenylalanine |
| 24. | 3 | 148.05 | 147.04 | C9H8O2 | 61 (52%) | Trans-Cinnamic acid |
| 25. | 5.96 | 204.09 | 203.08 | C11H12N2O2 | 142 (40%), 74 (28%), 116 (20%) | L-Tryptophan |
| 26. | 7.92 | 164.05 | 163.04 | C9H8O3 | 91 (50%), 119 (100%), 163 (5%) | m-Coumaric acid |
| 27. | 9.68 | 290.08 | 289.07 | C15H14O6 | 109 (100%), 137 (83%), 151 (50%) | (+)-Epicatechin |
| 28. | 13.36 | 385.26 | 384.25 | C19H35N3O5 | 224 (39%), 111(37%), 180 (20%) | Actinonin |
| 29. | 16.25 | 442.09 | 441.08 | C22H18O10 | 125 (80%), 145 (23%), 124 (15%), 303 (2%) | (−)-Catechin gallate |
| 30. | 17 | 241.28 | 242.29 | C16H35N | 136 (100%), 268 (10%) | 1-Hexadecylamine |
| Extracts | TPC (mg Ferulic Acid Equivalent/100 g, DW) | TFC (mg Catechin Equivalent/100 g, DW | TSC (mg Soy Saponin B Equivalent/100 g, DW) | DPPH (µmol Trolox Equivalent/g, DW) | ABTS (µmol Trolox Equivalent/g, DW) |
|---|---|---|---|---|---|
| AL seed | 425.7 ± 5.1 a | 208.4 ± 0.3 a | 24.5 ± 1.3 a | 2.73 ± 0.22 a | 12.42 ± 0.18 a |
| AL sprout | 406.1 ± 0.2 b | 198.8 ± 0.4 b | 41.9 ± 1.6 b | 5.23 ± 0.20 b | 14.85 ± 0.20 b |
| BW Seed | 352.1 ± 11.1 c | 220.7 ± 0.8 c | 80.8 ± 5.5 c | 3.85± 0.33 b | 15.28 ± 0.43 b |
| BW sprout | 315.8 ± 4.9 d | 208.1 ± 0.9 a | 25.4 ± 1.9 a | 12.20 ± 0.61 c | 19.73 ± 0.10 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloo, S.-O.; Ofosu, F.-K.; Oh, D.-H. Effect of Germination on Alfalfa and Buckwheat: Phytochemical Profiling by UHPLC-ESI-QTOF-MS/MS, Bioactive Compounds, and In-Vitro Studies of Their Diabetes and Obesity-Related Functions. Antioxidants 2021, 10, 1613. https://doi.org/10.3390/antiox10101613
Aloo S-O, Ofosu F-K, Oh D-H. Effect of Germination on Alfalfa and Buckwheat: Phytochemical Profiling by UHPLC-ESI-QTOF-MS/MS, Bioactive Compounds, and In-Vitro Studies of Their Diabetes and Obesity-Related Functions. Antioxidants. 2021; 10(10):1613. https://doi.org/10.3390/antiox10101613
Chicago/Turabian StyleAloo, Simon-Okomo, Fred-Kwame Ofosu, and Deog-Hwan Oh. 2021. "Effect of Germination on Alfalfa and Buckwheat: Phytochemical Profiling by UHPLC-ESI-QTOF-MS/MS, Bioactive Compounds, and In-Vitro Studies of Their Diabetes and Obesity-Related Functions" Antioxidants 10, no. 10: 1613. https://doi.org/10.3390/antiox10101613
APA StyleAloo, S.-O., Ofosu, F.-K., & Oh, D.-H. (2021). Effect of Germination on Alfalfa and Buckwheat: Phytochemical Profiling by UHPLC-ESI-QTOF-MS/MS, Bioactive Compounds, and In-Vitro Studies of Their Diabetes and Obesity-Related Functions. Antioxidants, 10(10), 1613. https://doi.org/10.3390/antiox10101613








