Comprehensive Analysis of Secondary Metabolites in the Extracts from Different Lily Bulbs and Their Antioxidant Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Collection and Preparation
2.2. Preparation of Methanol Extracts
2.3. Total Phenolic Acid and Total Flavonoid Content Determination
2.3.1. Total Phenolic Acids
2.3.2. Total Flavonoids
2.4. Determination of Antioxidant Activity
2.4.1. DPPH Radical Scavenging
2.4.2. Ferric Reducing Antioxidant Power (FRAP)
2.4.3. Cupricion Reducing Capacity (CUPRAC)
2.5. Metabolite Profiling
2.5.1. UPLC Conditions
2.5.2. ESI-Q TRAP-MS/MS
2.6. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Acid and Flavonoid Content
3.2. Antioxidant Activity
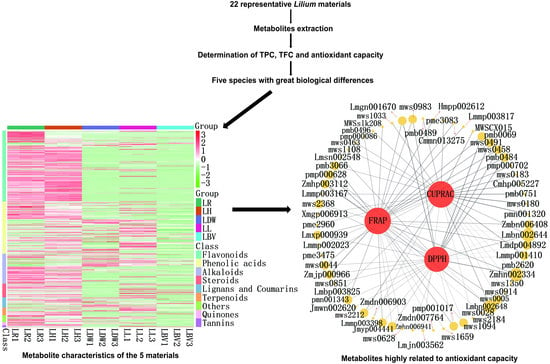
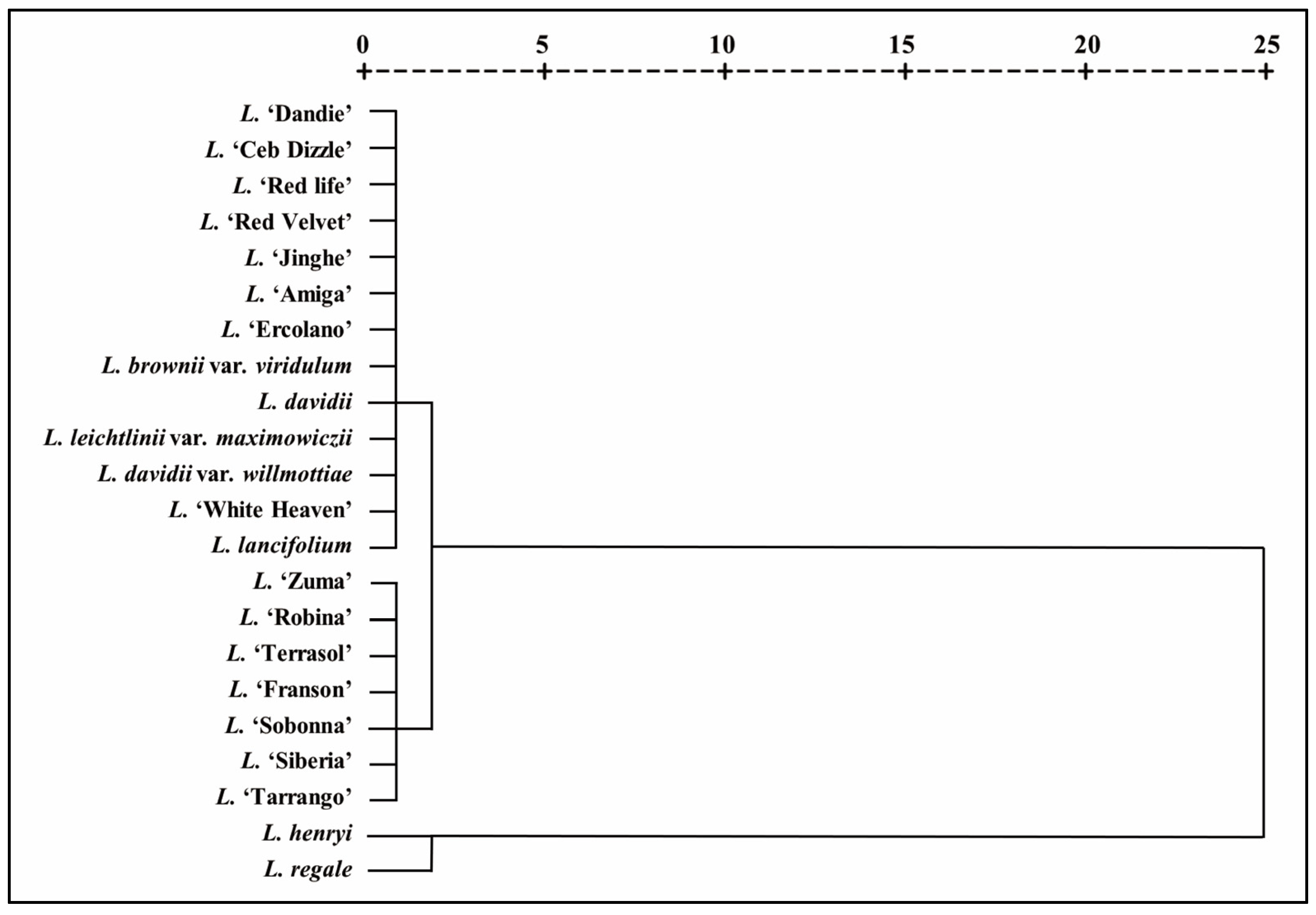
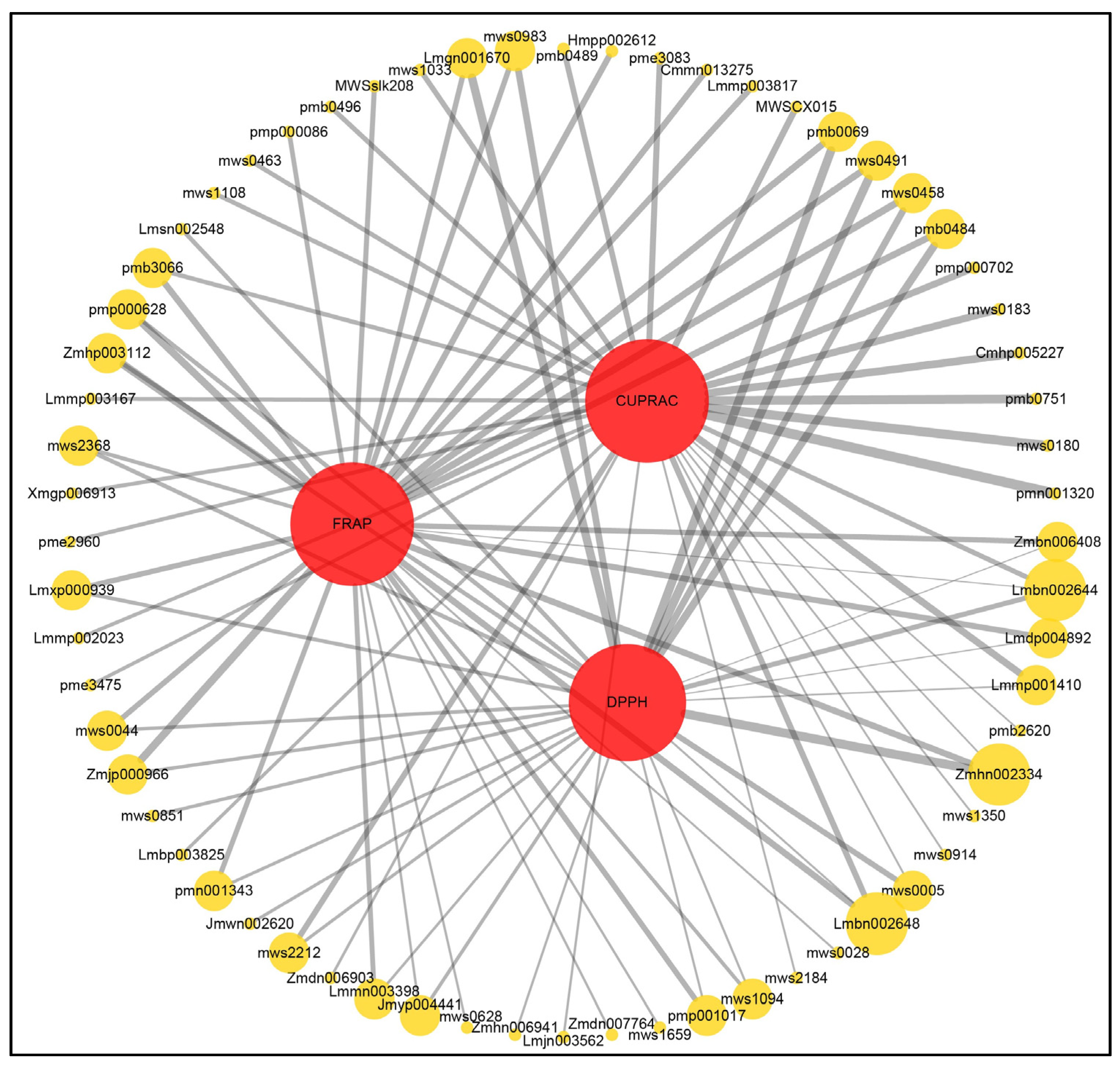
3.3. Correlation and Hierarchical Cluster Analysis of TPC, TFC and Antioxidant Capacity
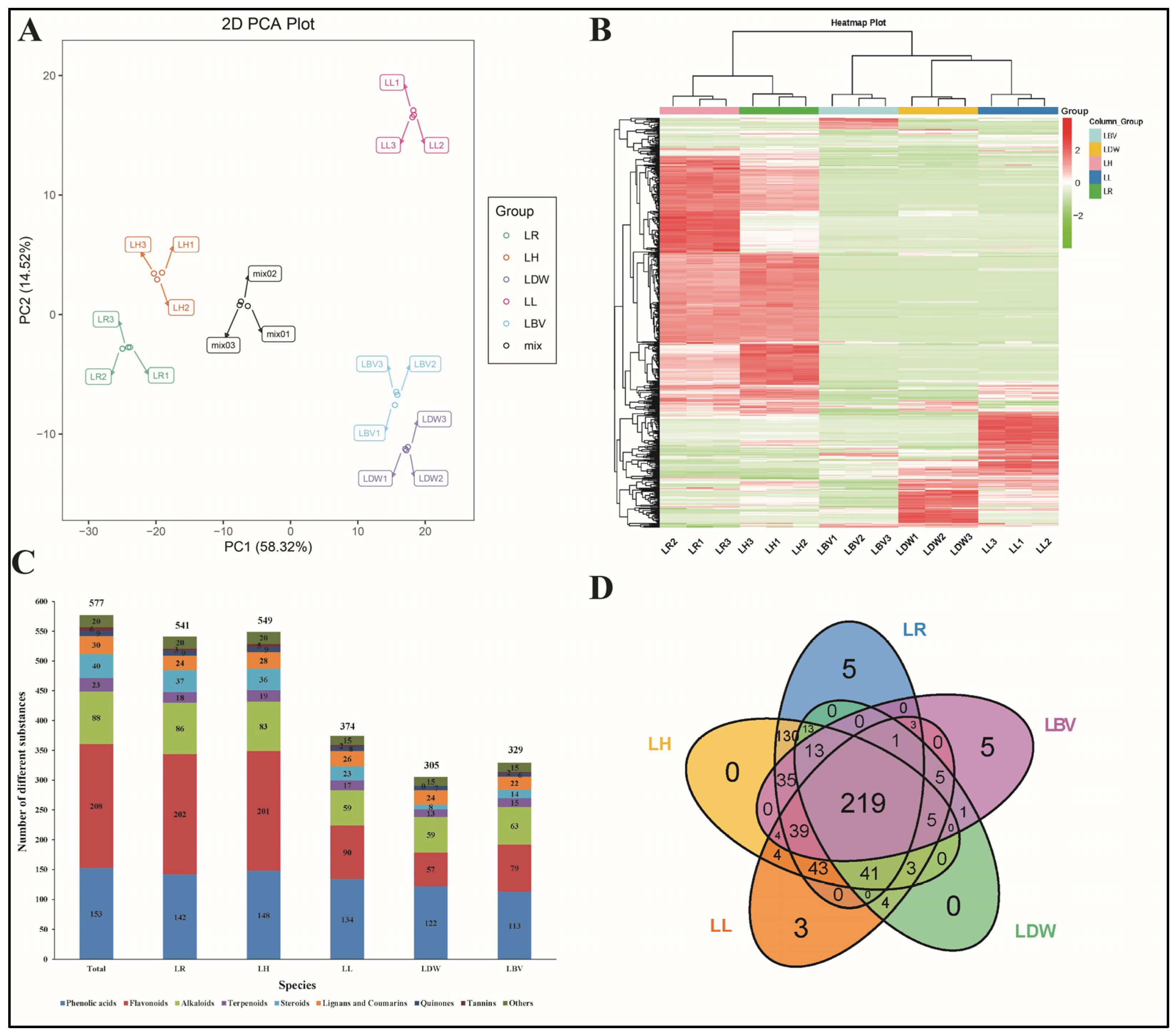
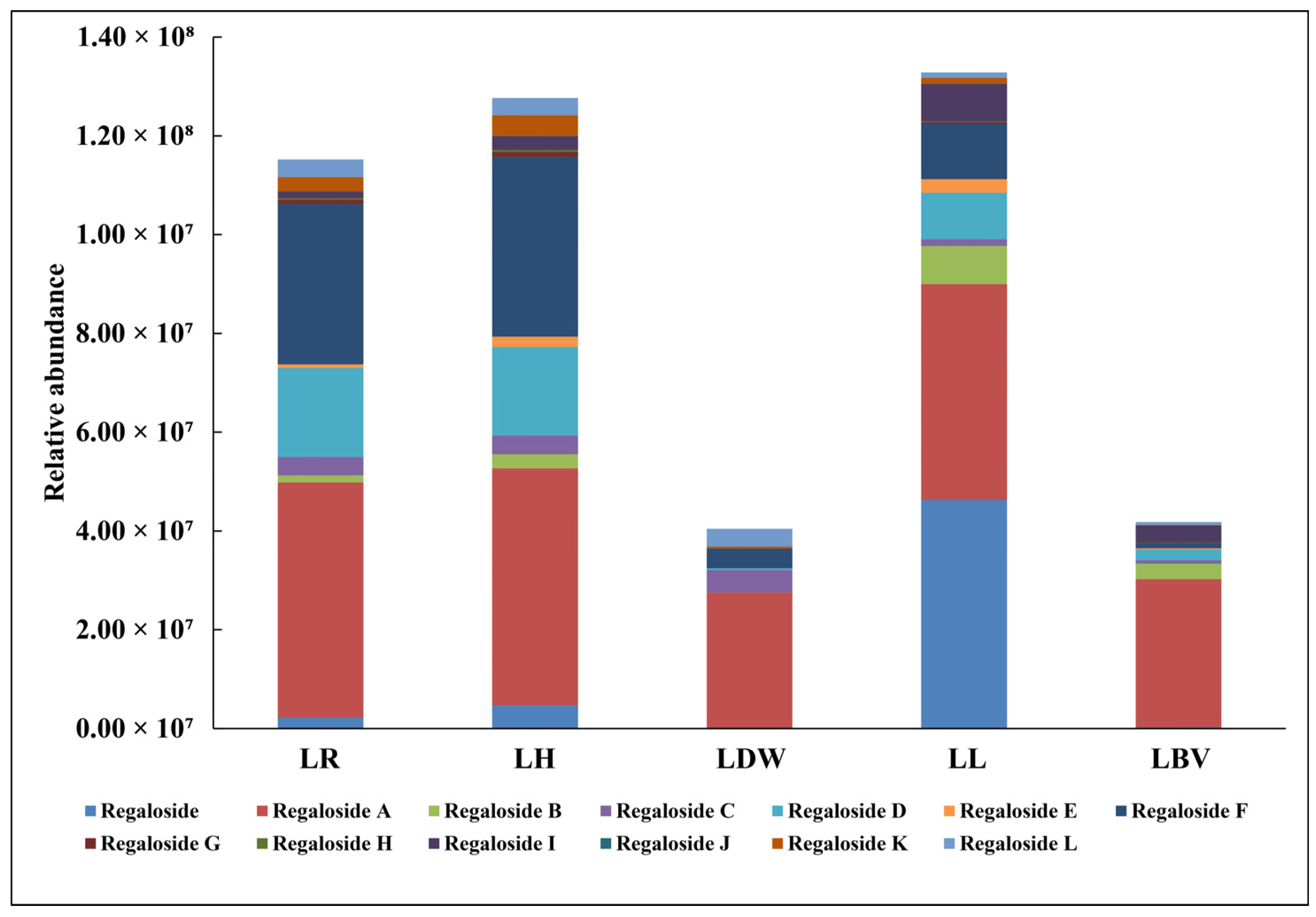
3.4. Profiling of Secondary Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; An, R.; Huang, X. Genus Lilium: A review on traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2021, 270, 113852. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, S.-C.; Yeau, S.H.; Lee, N.S. Major lineages of the genus Lilium (Liliaceae) based on nrDNA ITS sequences, with special emphasis on the Korean species. J. Plant Biol. 2011, 54, 159–171. [Google Scholar] [CrossRef]
- Chung, M.Y.; López-Pujol, J.; Chung, J.M.; Kim, K.-J.; Park, S.J.; Chung, M.G. Polyploidy in Lilium lancifolium: Evidence of autotriploidy and no niche divergence between diploid and triploid cytotypes in their native ranges. Flora 2015, 213, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Fang, C.; Luo, J.; Wang, S. The Diversity of Nutritional Metabolites: Origin, Dissection, and Application in Crop Breeding. Front. Plant Sci. 2019, 10, 1028. [Google Scholar] [CrossRef]
- Konate, K.; Yomalan, K.; Sytar, O.; Zerbo, P.; Brestic, M.; Patrick, V.D.; Gagniuc, P.; Barro, N. Free Radicals Scavenging Capacity, Antidiabetic and Antihypertensive Activities of Flavonoid-Rich Fractions from Leaves of Trichilia emetica and Opilia amentacea in an Animal Model of Type 2 Diabetes Mellitus. Evid. Based Complement Altern. Med. 2014, 2014, 867075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakaya, S.; Koca, M.; Sytar, O.; Dursunoglu, B.; Ozbek, H.; Duman, H.; Guvenalp, Z.; Kılıc, C.S. Antioxidant and anticholinesterase potential of Ferulago cassia with farther bio-guided isolation of active coumarin constituents. South Afr. J. Bot. 2019, 121, 536–542. [Google Scholar] [CrossRef]
- Abiri, R.; Abdul-Hamid, H.; Sytar, O.; Abiri, R.; Bezerra de Almeida, E., Jr.; Sharma, S.K.; Bulgakov, V.P.; Arroo, R.R.J.; Malik, S. A Brief Overview of Potential Treatments for Viral Diseases Using Natural Plant Compounds: The Case of SARS-Cov. Molecules 2021, 26, 3868. [Google Scholar] [CrossRef] [PubMed]
- Munafo Jr, J.P.; Gianfagna, T. Chemistry and biological activity of steroidal glycosides from the Lilium genus. Nat. Prod. Rep. 2015, 32, 454–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, J.; Attia, F.A.K.; Kang, W.; Wei, J.; Liu, Z.; Li, C. A critical review on chemical constituents and pharmacological effects of Lilium. Food Sci. Hum. Wellness 2019, 8, 330–336. [Google Scholar] [CrossRef]
- Bruni, A.; Ballero, M.; Poli, F. Quantitative ethnopharmacological study of the Campidano Valley and Urzulei district, Sardinia, Italy. J. Ethnopharmacol. 1997, 57, 97–124. [Google Scholar] [CrossRef]
- Mimaki, Y.; Satou, T.; Kuroda, M.; Sashida, Y.; Hatakeyama, Y. Steroidal saponins from the bulbs of Lilium candidum. Phytochemistry 1999, 51, 567–573. [Google Scholar] [CrossRef]
- Pieroni, A.; Dibra, B.; Grishaj, G.; Grishaj, I.; Maçai, S.G. Traditional phytotherapy of the Albanians of Lepushe, northern Albanian alps. Fitoterapia 2005, 76, 379–399. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission: Pharmacopoeia of the People’s Republic of China; China Medical Science Publisher: Beijing, China, 2020; Volume 1, p. 138.
- Tokgun, O.; Akca, H.; Mammadov, R.; Aykurt, C.; Deniz, G. Convolvulus galaticus, Crocus antalyensis, and Lilium candidum extracts show their antitumor activity through induction of p53-mediated apoptosis on human breast cancer cell line MCF-7 cells. J. Med. Food 2012, 15, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Jin, H.; Li, X.; Yang, X.; Cui, H.; Xin, A.; Zhao, R.; Qin, B. Purification, characterization and antioxidant activities of a polysaccharide from the roots of Lilium davidii var. unicolor Cotton. Int. J. Biol. Macromol. 2019, 135, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Yun, N.; Jang, Y.P.; Kim, J. Lilium lancifolium Thunb. extract attenuates pulmonary inflammation and air space enlargement in a cigarette smoke-exposed mouse model. J. Ethnopharmacol. 2013, 149, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Extraction Technology and Anti-depression Activity of Saponins from Lilium brownii. Med. Plant 2014, 5, 46–48. [Google Scholar]
- Zhu, M.; Luo, J.; Lv, H.; Kong, L. Determination of anti-hyperglycaemic activity in steroidal glycoside rich fraction of lily bulbs and characterization of the chemical profiles by LC-Q-TOF-MS/MS. J. Funct. Foods 2014, 6, 585–597. [Google Scholar] [CrossRef]
- Sim, W.S.; Choi, S.I.; Jung, T.D.; Cho, B.Y.; Choi, S.H.; Park, S.M.; Lee, O.H. Antioxidant and anti-inflammatory effects of Lilium lancifolium bulbs extract. J. Food Biochem. 2020, 44, e13176. [Google Scholar] [CrossRef]
- Rubin, N.; Huleihel, M.; Zaccai, M. Stress conditions during plant growth increase the anti-herpetic properties of Lilium candidum leaf extracts and fractions. J. Med. Plants Res. 2015, 9, 954–961. [Google Scholar]
- Drobnik, J.; Wełna, K. Identification of plants in an early cosmetic formulary from 1864. Pol. J. Cosmetol. 2016, 19, 176–181. [Google Scholar]
- Cesar, F.; Carnevale Neto, F.; Porto, G.S.; Campos, P.M. Patent analysis: A look at the innovative nature of plant-based cosmetics. Quim. Nova 2017, 40, 840–847. [Google Scholar]
- Abu-Reidah, I.M.; Arráez-Román, D.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phytochemical characterisation of green beans (Phaseolus vulgaris L.) by using high-performance liquid chromatography coupled with time-of-flight mass spectrometry. Phytochem. Anal. 2013, 24, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Atanasova-Penichon, V.; Chéreau, S.; Richard-Forget, F. Metabolomics to decipher the chemical defense of cereals against Fusarium graminearum and deoxynivalenol accumulation. Int. J. Mol. Sci. 2015, 16, 24839–24872. [Google Scholar] [CrossRef]
- Dowom, S.A.; Abrishamchi, P.; Radjabian, T.; Salami, S.A. Enhanced phenolic acids production in regenerated shoot cultures of Salvia virgata Jacq. after elicitation with Ag+ ions, methyl jasmonate and yeast extract. Ind. Crop. Prod. 2017, 103, 81–88. [Google Scholar] [CrossRef]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Đurović, S.; Nikolić, B.; Luković, N.; Jovanović, J.; Stefanović, A.; Šekuljica, N.; Mijin, D.; Knežević-Jugović, Z. The impact of high-power ultrasound and microwave on the phenolic acid profile and antioxidant activity of the extract from yellow soybean seeds. Ind. Crop. Prod. 2018, 122, 223–231. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Y.; Yan, L.; Guo, Y.; Niu, L. Phenolic compounds and antioxidant activity of bulb extracts of six Lilium species native to China. Molecules 2012, 17, 9361–9378. [Google Scholar] [CrossRef]
- Dong, R.; Liu, S.; Zheng, Y.; Zhang, X.; He, Z.; Wang, Z.; Wang, Y.; Xie, J.; Chen, Y.; Yu, Q. Release and metabolism of bound polyphenols from carrot dietary fiber and their potential activity in in vitro digestion and colonic fermentation. Food Funct. 2020, 11, 6652–6665. [Google Scholar] [CrossRef]
- Brito, T.; Lima, L.; Santos, M.; Moreira, R.; Cameron, L.; Fai, A.; Ferreira, M. Antimicrobial, antioxidant, volatile and phenolic profiles of cabbage-stalk and pineapple-crown flour revealed by GC-MS and UPLC-MSE. Food Chem. 2021, 339, 127882. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Quequezana, L.; Vuorinen, A.L.; Kallio, H.; Yang, B. Impact of cultivar, growth temperature and developmental stage on phenolic compounds and ascorbic acid in purple and yellow potato tubers. Food Chem. 2020, 326, 126966. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Luo, J.; Li, L.; Kong, L. Preparative separation of phenylpropenoid glycerides from the bulbs of Lilium lancifolium by high-speed counter-current chromatography and evaluation of their antioxidant activities. Food Chem. 2012, 131, 1056–1062. [Google Scholar] [CrossRef]
- Rocchetti, G.; Miras-Moreno, M.B.; Zengin, G.; Senkardes, I.; Sadeer, N.B.; Mahomoodally, M.F.; Lucini, L. UHPLC-QTOF-MS phytochemical profiling and in vitro biological properties of Rhamnus petiolaris (Rhamnaceae). Ind. Crop. Prod. 2019, 142, 111856. [Google Scholar] [CrossRef]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Shi, M.; Huang, F.; Deng, C.; Wang, Y.; Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2019, 59, 953–964. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, X.; Gong, Q.; Cao, J.; Shen, W.; Yin, X.; Grierson, D.; Zhang, B.; Xu, C.; Li, X. Three AP2/ERF family members modulate flavonoid synthesis by regulating type IV chalcone isomerase in citrus. Plant Biotechnol. J. 2021, 19, 671. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Tech. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Vastag, D. Flavonoids and phenolic acids as potential natural antioxidants. In Antioxidants; IntechOpen: London, UK, 2019; pp. 1–20. [Google Scholar]
- Shimomura, H.; Sashida, Y.; Mimaki, Y.I. Regaloside A and B acylated glycerol glucosides from Lilium regale. Phytochemistry 1988, 27, 451–454. [Google Scholar] [CrossRef]
- Murray, A.F.; Palatini, K.; Komarnytsky, S.; Gianfagna, T.J.; Munafo, J.P., Jr. Phenylpropanoid Glycerol Glucosides Attenuate Glucose Production in Hepatocytes. ACS Omega 2019, 4, 10670–10676. [Google Scholar] [CrossRef]
- Kim, B.R.; Thapa, P.; Kim, H.M.; Jin, C.H.; Kim, S.H.; Kim, J.B.; Choi, H.; Han, A.R.; Nam, J.W. Purification of Phenylpropanoids from the Scaly Bulbs of Lilium Longiflorum by CPC and Determination of Their DPP-IV Inhibitory Potentials. ACS Omega 2020, 5, 4050–4057. [Google Scholar] [CrossRef] [Green Version]
- Yamaba, H.; Haba, M.; Kunita, M.; Sakaida, T.; Tanaka, H.; Yashiro, Y.; Nakata, S. Morphological change of skin fibroblasts induced by UV Irradiation is involved in photoaging. Exp. Derm. 2016, 25 (Suppl. 3), 45–51. [Google Scholar] [CrossRef] [Green Version]

| Number | Species/Cultivar | Genotype |
|---|---|---|
| 1 | L. davidii | Wild species (W) |
| 2 | L. leichtlinii var. maximowiczii | Wild species (W) |
| 3 | L. davidii var. willmottiae | Wild species (W) |
| 4 | L. henryi | Wild species (W) |
| 5 | L. regale | Wild species (W) |
| 6 | L. lancifolium | Wild species (W) |
| 7 | L. brownii var. viridulum | Wild species (W) |
| 8 | L. ‘Red Velvet’ | Asiatic hybrids (A) |
| 9 | L. ‘Red life’ | Asiatic hybrids (A) |
| 10 | L. ‘Dandie’ | Asiatic hybrids (A) |
| 11 | L. ‘Jinghe’ | Asiatic hybrids (A) |
| 12 | L. ‘White Heaven’ | L. longiflorum hybrids (L) |
| 13 | L. ‘Amiga’ | L. longiflorum and Asiatic hybrids (LA) |
| 14 | L. ‘Ceb Dizzle’ | L. longiflorum and Asiatic hybrids (LA) |
| 15 | L. ‘Ercolano’ | L. longiflorum and Asiatic hybrids (LA) |
| 16 | L. ‘Siberia’ | Oriental hybrids (O) |
| 17 | L. ‘Sobonna’ | Oriental hybrids (O) |
| 18 | L. ‘Tarrango’ | Oriental hybrids (O) |
| 19 | L. ‘Zuma’ | Oriental hybrids (O) |
| 20 | L. ‘Terrasol’ | Trumpet and Oriental hybrids (OT) |
| 21 | L. ‘Robina’ | Trumpet and Oriental hybrids (OT) |
| 22 | L. ‘Franson’ | Trumpet and Oriental hybrids (OT) |
| No. | Materials | Genotype | TPC (GAE mg/g DW) | TFC (QE mg/g DW) | DPPH (TE μg/g DW) | FRAP (TE μg/g DW) | CUPRAC (TE μg/g DW) |
|---|---|---|---|---|---|---|---|
| 1 | L. davidii | W | 0.87 ± 0.04 jk | 2.49 ± 0.70 efg | 1047.84 ± 70.19 j | 730.01 ± 53.82 i | 2107.78 ± 416.78 h |
| 2 | L. leichtlinii var. maximowiczii | W | 0.94 ± 0.07 jk | 2.51 ± 0.88 efg | 939.11 ± 141.34 jk | 694.25 ± 17.56 ij | 2018.89 ± 476.48 h |
| 3 | L. davidii var. willmottiae | W | 1.88 ± 0.10 i | 2.58 ± 0.49 efg | 2057.96 ± 88.19 i | 1071.01 ± 91.82 h | 3818.89 ± 501.48 gh |
| 4 | L. henryi | W | 11.63 ± 0.30 b | 12.22 ± 0.99 b | 13,249.75 ± 390.47 b | 8736.93 ± 645.45 b | 26,593.33 ± 1205.54 b |
| 5 | L. regale | W | 13.73 ± 0.35 a | 15.91 ± 2.78 a | 16,707.07 ± 847.51 a | 11,622.55 ± 344.81 a | 32,304.44 ± 1878.93 a |
| 6 | L. lancifolium | W | 2.25 ± 0.07 h | 4.36 ± 0.29 cd | 2242.67 ± 258.44 h i | 1228.10 ± 58.15 gh | 3227.22 ± 171.66 g |
| 7 | L. brownii var. viridulum | W | 0.70 ± 0.09 kl | 0.88 ± 0.18 h | 439.53 ± 171.03 k | 368.58 ± 37.74 j | 1330.00 ± 57.74 h |
| 8 | L. ‘Red Velvet’ | A | 0.95 ± 0.07 jk | 1.71 ± 0.60 fgh | 1110.87 ± 44.55 j | 691.70 ± 87.40 ij | 1696.67 ± 624.50 h |
| 9 | L. ‘Red Life’ | A | 0.81 ± 0.02 kl | 1.65 ± 0.34 fgh | 947.55 ± 69.74 jk | 579.31 ± 23.3 1 ij | 1507.78 ± 157.53 h |
| 10 | L. ‘Dandie’ | A | 0.72 ± 0.08 kl | 2.17 ± 0.57 fgh | 1067.91 ± 289.86 j | 676.37 ± 26.08 ij | 1430.00 ± 272.85 h |
| 11 | L. ‘Jinghe’ | A | 0.60 ± 0.07 l | 1.80 ± 0.39 fgh | 696.17 ± 330.2 1 jk | 428.61 ± 15.95 ij | 1307.78 ± 221.94 h |
| 12 | L. ‘White Heaven’ | L | 1.82 ± 0.12 i | 3.13 ± 0.25 def | 2233.21 ± 255.68 hi | 1054.41 ± 36.55 h | 3585.56 ± 50.92 g |
| 13 | L. ‘Amiga’ | LA | 0.57 ± 0.07 l | 1.65 ± 0.18 fgh | 775.67 ± 19.11 jk | 470.75 ± 11.71 ij | 1374.44 ± 183.59 h |
| 14 | L. ‘Ceb Dizzle’ | LA | 1.11 ± 0.05 j | 1.85 ± 0.55 fgh | 1056.82 ± 291.92 j | 584.42 ± 32.13 ij | 1463.33 ± 317.98 h |
| 15 | L. ‘Ercolano’ | LA | 0.59 ± 0.06 l | 1.31 ± 0.48 gh | 692.71 ± 153.18 jk | 415.84 ± 29.76 ij | 1085.56 ± 320.3 h |
| 16 | L. ‘Siberia’ | O | 3.95 ± 0.41 d | 4.73 ± 0.26 c | 4721.04 ± 435.55 cd | 3132.35 ± 89.85 c | 8063.33 ± 416.33 d |
| 17 | L. ‘Sobonna’ | O | 3.21 ± 0.11 f | 3.79 ± 0.38 cde | 3459.84 ± 73.17 f | 2106.21 ± 103.91 e | 8118.89 ± 963.98 d |
| 18 | L. ‘Tarrango’ | O | 4.21 ± 0.07 c | 5.19 ± 0.46 c | 4971.99 ± 402.33 c | 3119.28 ± 57.45 c | 10,596.67 ± 352.77 c |
| 19 | L. ‘Zuma’ | O | 2.83 ± 0.01 g | 2.83 ± 0.40 ef | 3073.67 ± 184.01 fg | 1707.52 ± 84.53 f | 6041.11 ± 221.94 f |
| 20 | L. ‘Terrasol’ | OT | 3.65 ± 0.04 e | 3.85 ± 0.06 cde | 4238.72 ± 171.28 de | 2426.47 ± 80.25 d | 7996.67 ± 88.19 d |
| 21 | L. ‘Robina’ | OT | 3.16 ± 0.08 f | 2.91 ± 0.27 ef | 2738.94 ± 265.84 gh | 1436.27 ± 93.52 fg | 6674.44 ± 478.81 ef |
| 22 | L. ‘Franson’ | OT | 3.60 ± 0.16 e | 2.48 ± 0.88 efg | 4066.62 ± 234.24 e | 2318.63 ± 191.36 de | 7349.44 ± 359.62 de |
| TPC | TFC | DPPH | FRAP | CUPRAC | |
|---|---|---|---|---|---|
| TPC | 1 | 0.977 ** | 0.997 ** | 0.992 ** | 0.997 ** |
| TFC | 1 | 0.983 ** | 0.986 ** | 0.977 ** | |
| DPPH | 1 | 0.997 ** | 0.996 ** | ||
| FRAP | 1 | 0.993 ** | |||
| CUPRAC | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.-C.; Liu, Y.-J.; He, G.-R.; Cao, Y.-W.; Bi, M.-M.; Song, M.; Yang, P.-P.; Xu, L.-F.; Ming, J. Comprehensive Analysis of Secondary Metabolites in the Extracts from Different Lily Bulbs and Their Antioxidant Ability. Antioxidants 2021, 10, 1634. https://doi.org/10.3390/antiox10101634
Tang Y-C, Liu Y-J, He G-R, Cao Y-W, Bi M-M, Song M, Yang P-P, Xu L-F, Ming J. Comprehensive Analysis of Secondary Metabolites in the Extracts from Different Lily Bulbs and Their Antioxidant Ability. Antioxidants. 2021; 10(10):1634. https://doi.org/10.3390/antiox10101634
Chicago/Turabian StyleTang, Yu-Chao, Yi-Jie Liu, Guo-Ren He, Yu-Wei Cao, Meng-Meng Bi, Meng Song, Pan-Pan Yang, Lei-Feng Xu, and Jun Ming. 2021. "Comprehensive Analysis of Secondary Metabolites in the Extracts from Different Lily Bulbs and Their Antioxidant Ability" Antioxidants 10, no. 10: 1634. https://doi.org/10.3390/antiox10101634
APA StyleTang, Y.-C., Liu, Y.-J., He, G.-R., Cao, Y.-W., Bi, M.-M., Song, M., Yang, P.-P., Xu, L.-F., & Ming, J. (2021). Comprehensive Analysis of Secondary Metabolites in the Extracts from Different Lily Bulbs and Their Antioxidant Ability. Antioxidants, 10(10), 1634. https://doi.org/10.3390/antiox10101634