Analysis of Lipophilic Antioxidants in the Leaves of Kaempferia parviflora Wall. Ex Baker Using LC–MRM–MS and GC–FID/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Chemicals
2.2. Plant Material
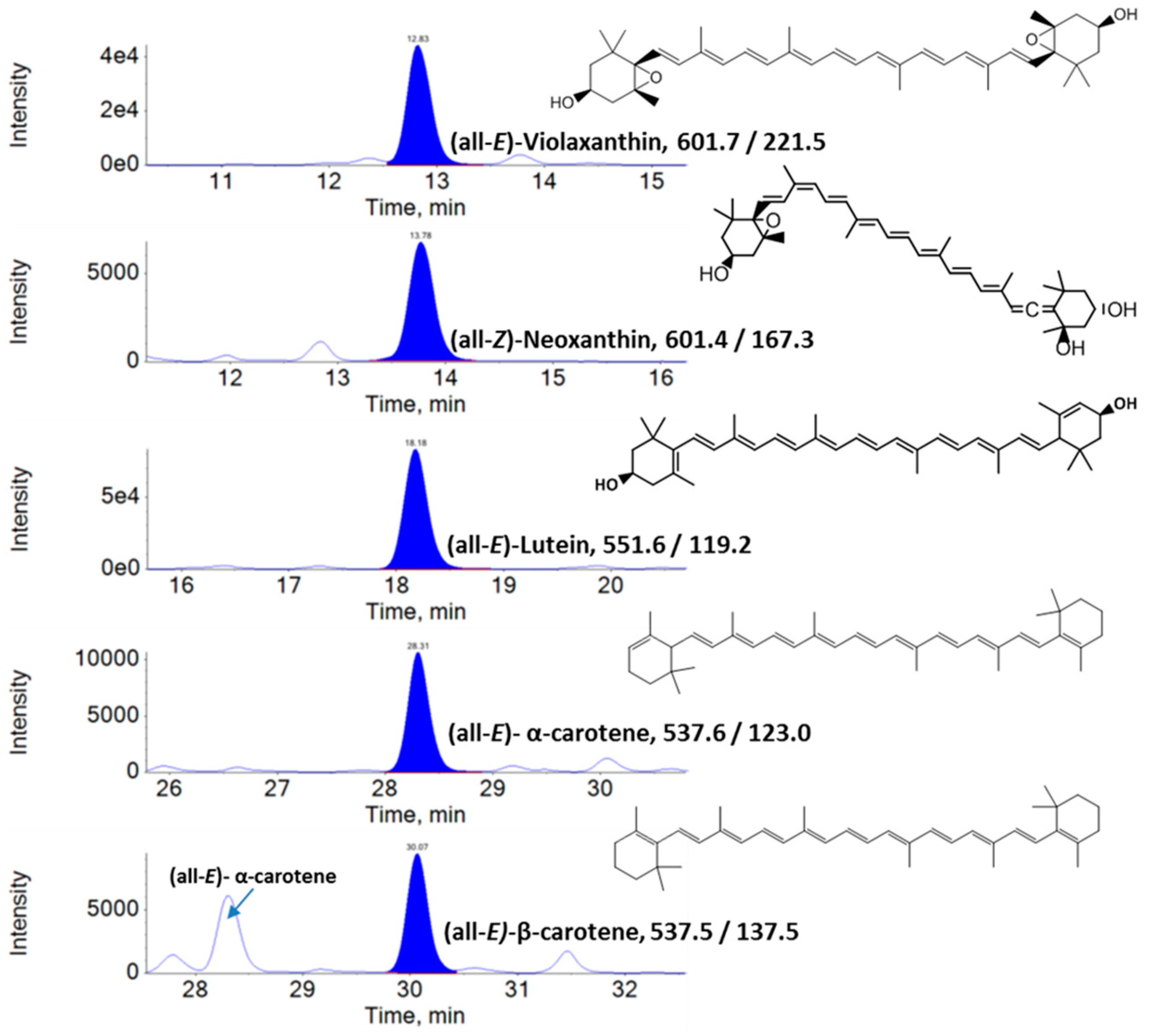
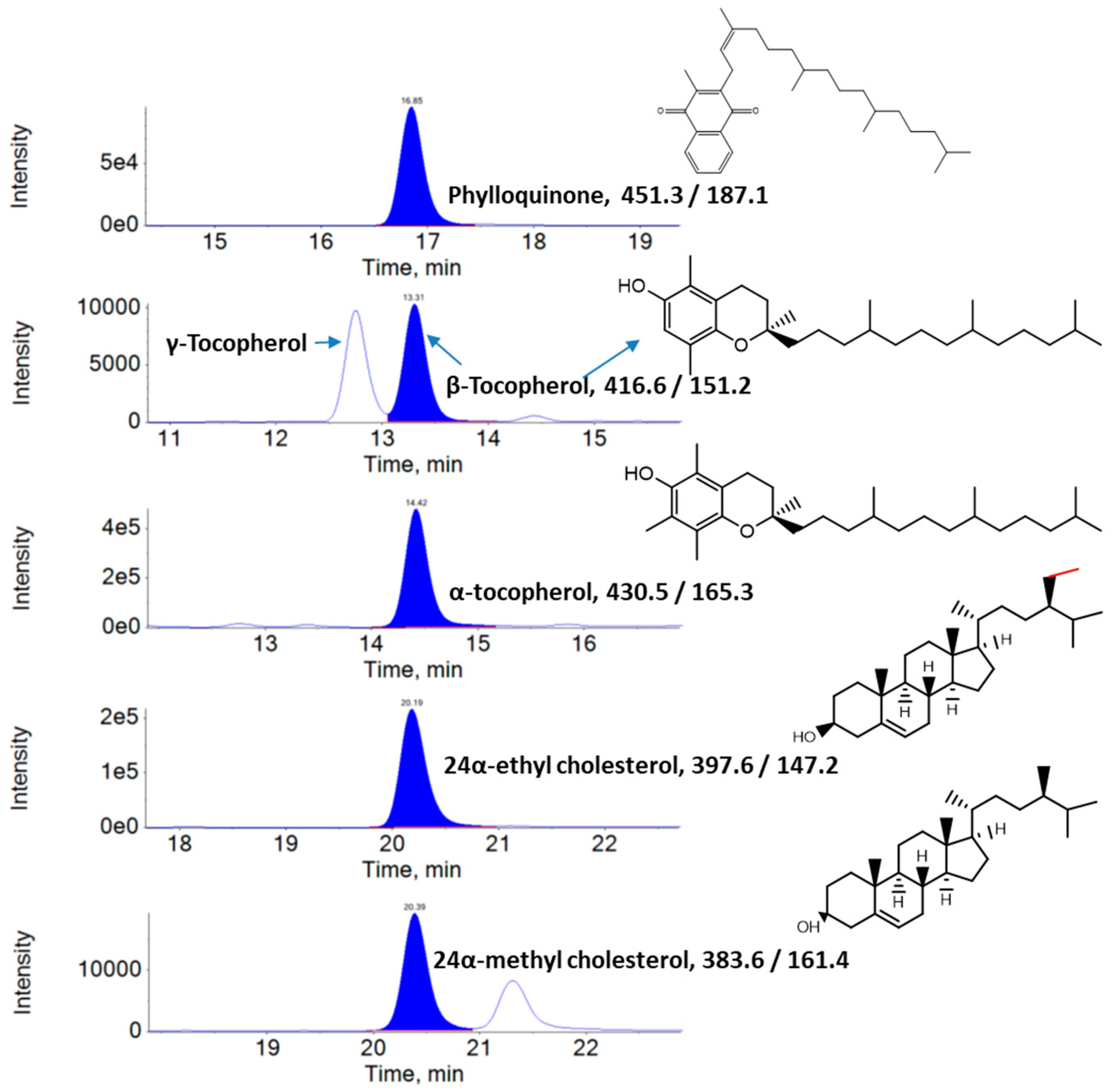
2.3. Analysis of Lipophilic Metabolites Using Liquid Chromatography–Multiple Reaction Monitoring–Mass Spectrometry (LC–MRM–MS)
Comparative High-Performance Liquid Chromatography (HPLC) Analysis of Carotenoids in Carrot, KP-BG, and Lettuce
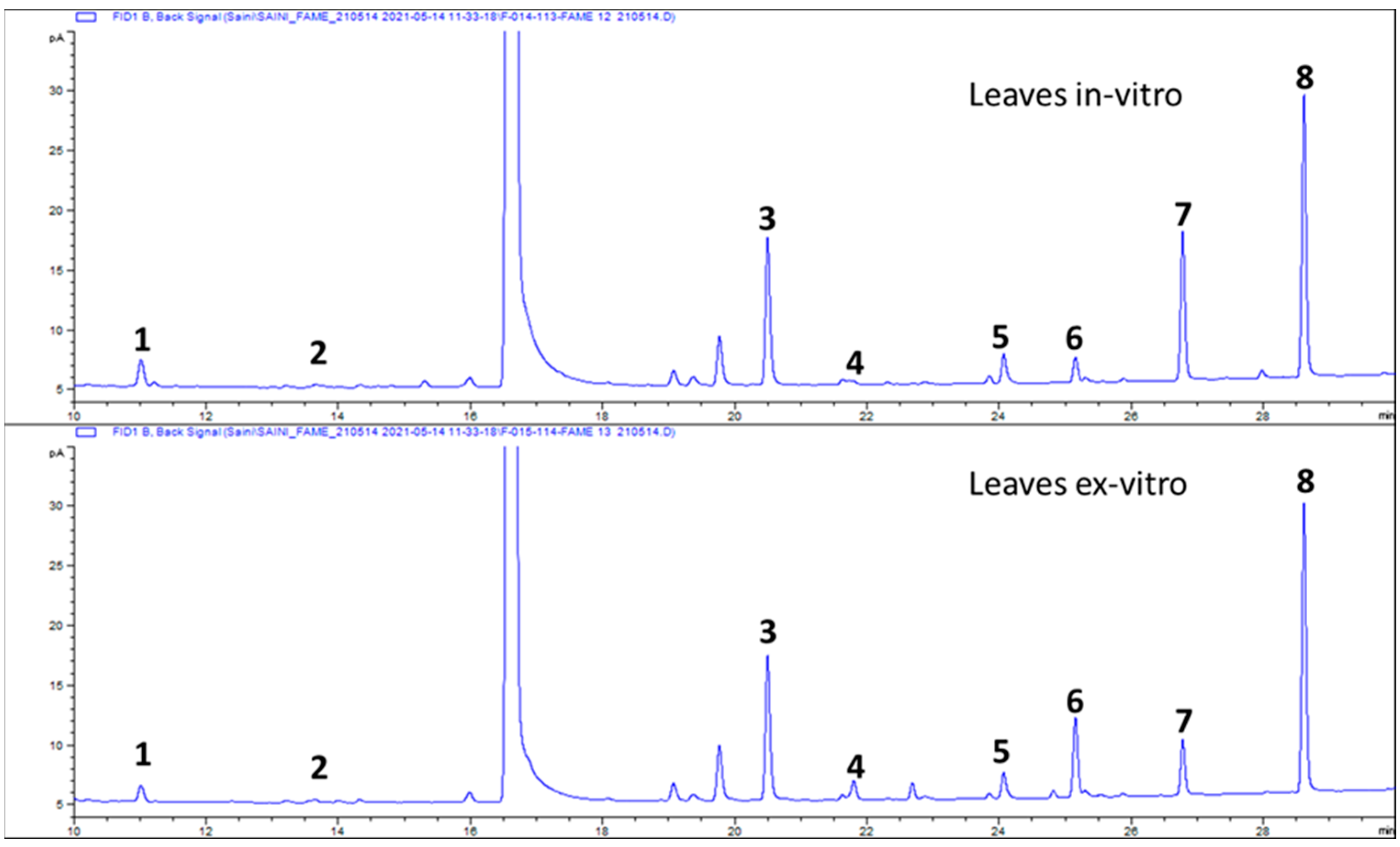
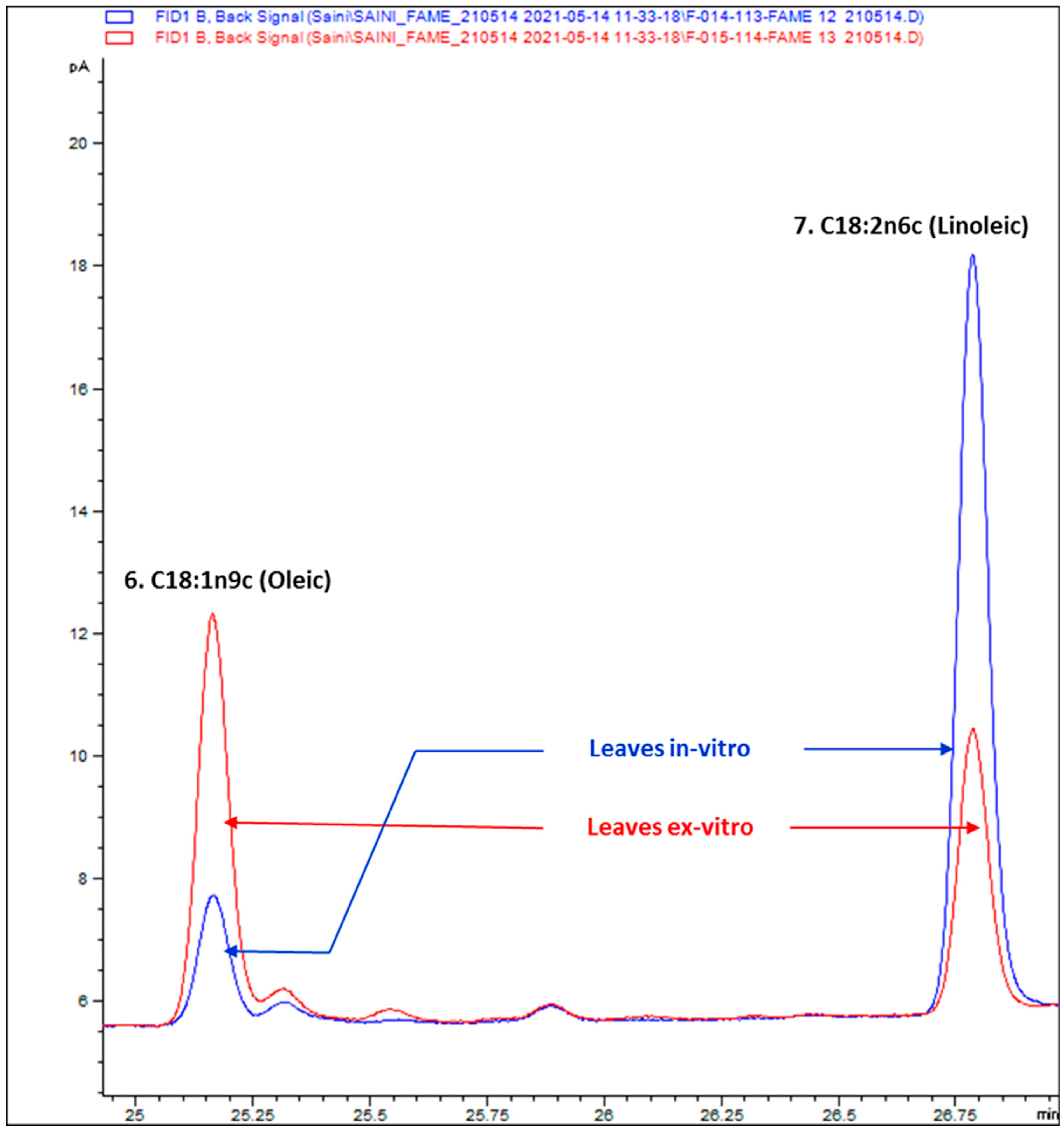
2.4. Composition of Fatty Acids
3. Results
3.1. Lipophilic Metabolite Content
3.2. Composition of Fatty Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Techaprasan, J.; Klinbunga, S.; Ngamriabsakul, C.; Jenjittikul, T. Genetic variation of Kaempferia (Zingiberaceae) in Thailand based on chloroplast DNA (psbA-trnH and petA-psbJ) sequences. Genet. Mol. Res. 2010, 9, 1957–1973. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Mohamed, T.A.; Essa, A.F.; Abd-El Gawad, A.M.; Alqahtani, A.S.; Shahat, A.A.; Yoneyama, T.; Farrag, A.R.H.; Noji, M.; El-Seedi, H.R.; et al. Recent advances in Kaempferia phytochemistry and biological activity: A comprehensive review. Nutrients 2019, 11, 2396. [Google Scholar] [CrossRef] [Green Version]
- Kochuthressia, K.; Britto, S.J.; Jaseentha, M.; Raphael, R. In vitro antimicrobial evaluation of Kaempferia galanga L. rhizome extract. Am. J. Biotechnol. Mol. Sci. 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Ridtitid, W.; Sae-Wong, C.; Reanmongkol, W.; Wongnawa, M. Antinociceptive activity of the methanolic extract of Kaempferia galanga Linn. in experimental animals. J. Ethnopharmacol. 2008, 118, 225–230. [Google Scholar] [CrossRef]
- Ahmed, F.R.S.; Amin, R.; Hasan, I.; Asaduzzaman, A.; Kabir, S.R. Antitumor properties of a methyl-β-d-galactopyranoside specific lectin from Kaempferia rotunda against Ehrlich ascites carcinoma cells. Int. J. Biol. Macromol. 2017, 102, 952–959. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kato, T.; Azuma, T.; Kikuzaki, H.; Abe, K. Anti-allergenic activity of polymethoxyflavones from Kaempferia parviflora. J. Funct. Foods 2015, 13, 100–107. [Google Scholar] [CrossRef]
- Hidaka, M.; Horikawa, K.; Akase, T.; Makihara, H.; Ogami, T.; Tomozawa, H.; Tsubata, M.; Ibuki, A.; Matsumoto, Y. Efficacy of Kaempferia parviflora in a mouse model of obesity-induced dermatopathy. J. Nat. Med. 2017, 71, 59–67. [Google Scholar] [CrossRef]
- Win, N.N.; Ngwe, H.; Abe, I.; Morita, H. Naturally occurring Vpr inhibitors from medicinal plants of Myanmar. J. Nat. Med. 2017, 71, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeap, Y.S.Y.; Kassim, N.K.; Ng, R.C.; Ee, G.C.L.; Saiful Yazan, L.; Musa, K.H. Antioxidant properties of ginger (Kaempferia angustifolia Rosc.) and its chemical markers. Int. J. Food Prop. 2017, 20, 1158–1172. [Google Scholar] [CrossRef] [Green Version]
- Sawasdee, P.; Sabphon, C.; Sitthiwongwanit, D.; Kokpol, U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res. 2009, 23, 1792–1794. [Google Scholar] [CrossRef] [PubMed]
- Kaewkroek, K.; Wattanapiromsakul, C.; Matsuda, H.; Nakamura, S.; Tewtrakul, S. Anti-inflammatory activity of compounds from Kaempferia marginata rhizomes. Songklanakarin J. Sci. Technol. 2017, 39, 91–99. [Google Scholar]
- Plaingam, W.; Sangsuthum, S.; Angkhasirisap, W.; Tencomnao, T. Kaempferia parviflora rhizome extract and Myristica fragrans volatile oil increase the levels of monoamine neurotransmitters and impact the proteomic profiles in the rat hippocampus: Mechanistic insights into their neuroprotective effects. J. Tradit. Complement. Med. 2017, 7, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, T.V.; Sharma, C.; Adiga, S.; Bairy, K.; Shenoy, S.; Shenoy, G. Wound healing activity of alcoholic extract of Kaempferia galanga in Wistar rats. Indian J. Physiol. Pharmacol. 2006, 50, 384–390. [Google Scholar]
- Pripdeevech, P.; Pitija, K.; Rujjanawate, C.; Pojanagaroon, S.; Kittakoop, P.; Wongpornchai, S. Adaptogenic-active components from Kaempferia parviflora rhizomes. Food Chem. 2012, 132, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Kayano, S.-I.; Matsumura, Y.; Konishi, Y.; Tanaka, Y.; Kikuzaki, H. Antimutagenic and glucosidase inhibitory effects of constituents from Kaempferia parviflora. Food Chem. 2011, 125, 471–475. [Google Scholar] [CrossRef]
- Chaipech, S.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O. Structures of two new phenolic glycosides, kaempferiaosides A and B, and hepatoprotective constituents from the rhizomes of Kaempferia parviflora. Chem. Pharm. Bull. 2012, 60, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Thao, N.P.; Luyen, B.T.T.; Lee, S.H.; Jang, H.D.; Kim, Y.H. Anti-osteoporotic and antioxidant activities by rhizomes of Kaempferia parviflora wall. Ex Baker. Nat. Prod. Sci. 2016, 22, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Tewtrakul, S.; Subhadhirasakul, S.; Karalai, C.; Ponglimanont, C.; Cheenpracha, S. Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurata. Food Chem. 2009, 115, 534–538. [Google Scholar] [CrossRef]
- Nakao, K.; Murata, K.; Deguchi, T.; Itoh, K.; Fujita, T.; Higashino, M.; Yoshioka, Y.; Matsumura, S.-I.; Tanaka, R.; Shinada, T. Xanthine oxidase inhibitory activities and crystal structures of methoxyflavones from Kaempferia parviflora rhizome. Biol. Pharm. Bull. 2011, 34, 1143–1146. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, M.R.; Zakaria, Z.A.; Duad, I.A.; Hidayat, M.T. Antinociceptive and anti-inflammatory activities of the aqueous extract of Kaempferia galanga leaves in animal models. J. Nat. Med. 2008, 62, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Dash, P.R.; Nasrin, M. Study of sedative activity of different extracts of Kaempferia galanga in Swiss albino mice. BMC Complement. Altern. Med. 2015, 15, 158. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K.; Lim, K.K.; Joe, C.E.; Lim, T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, K.-S.; Ak, G.; Zengin, G.; Cziáky, Z.; Jekő, J.; Adaikalam, K.; Song, K.; Kim, D.H.; Sivanesan, I. Establishment of a rapid micropropagation system for Kaempferia parviflora wall. Ex Baker: Phytochemical analysis of leaf extracts and evaluation of biological activities. Plants 2021, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Preetha, T.S.; Hemanthakumar, A.S.; Krishnan, P.N. A comprehensive review of Kaempferia galanga L. (Zingiberaceae): A high sought medicinal plant in tropical Asia. J. Med. Plants Stud. 2016, 4, 270–276. [Google Scholar]
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Begum, J.; Anwar, M.N. Essential oils of leaves and rhizomes of Kaempferia galanga Linn. Chittagong Univ. J. Biol. Sci. 2008, 3, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Norhayati, Y.; Nurulhidayah, A.; Rini Zunnurni, M.J.; Norliana, A.R.; Norliana, W.; Mohd Ifwat, I. Antioxidative constituents of selected Malaysian ‘Ulam’. Sci. Int. 2018, 30, 273–278. [Google Scholar]
- Sandmann, G. Antioxidant protection from UV- and light-stress related to carotenoid structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef] [Green Version]
- Lim, G.H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty acid- and lipid-mediated signaling in plant defense. Annu. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef]
- Cabianca, A.; Müller, L.; Pawlowski, K.; Dahlin, P. Changes in the plant β-sitosterol/stigmasterol ratio caused by the plant parasitic nematode Meloidogyne incognita. Plants 2021, 10, 292. [Google Scholar] [CrossRef]
- Ma, J.; Qiu, D.; Pang, Y.; Gao, H.; Wang, X.; Qin, Y. Diverse roles of tocopherols in response to abiotic and biotic stresses and strategies for genetic biofortification in plants. Mol. Breed. 2020, 40, 18. [Google Scholar] [CrossRef]
- Chang, K.H.; Cheng, M.L.; Chiang, M.C.; Chen, C.M. Lipophilic antioxidants in neurodegenerative diseases. Clin. Chim. Acta Int. J. Clin. Chem. 2018, 485, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Pogačnik, L.; Ota, A.; Ulrih, N.P. An overview of crucial dietary substances and their modes of action for prevention of neurodegenerative diseases. Cells 2020, 9, 576. [Google Scholar] [CrossRef] [Green Version]
- Shin, G.H.; Kim, J.T.; Park, H.J. Recent developments in nanoformulations of lipophilic functional foods. Trends Food Sci. Technol. 2015, 46, 144–157. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: Their role in cardiovascular protection. Biomedicines 2020, 25, 306. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Moon, S.H.; Gansukh, E.; Keum, Y.S. An efficient one-step scheme for the purification of major xanthophyll carotenoids from lettuce, and assessment of their comparative anticancer potential. Food Chem. 2018, 266, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Shang, X.; Assefa, A.D.; Keum, Y.S.; Saini, R.K. Metabolite profiling of green, green/red, and red lettuce cultivars: Variation in health beneficial compounds and antioxidant potential. Food Res. Int. 2018, 105, 361–370. [Google Scholar] [CrossRef]
- Kim, D.H.; Enkhtaivan, G.; Saini, R.K.; Keum, Y.S.; Kang, K.W.; Sivanesan, I. Production of bioactive compounds in cladode culture of Turbinicarpus valdezianus (H. Moeller) Glass & R. C. Foster. Ind. Crop. Prod. 2019, 138, 111491. [Google Scholar]
- Park, H.Y.; Kim, D.H.; Saini, R.K.; Gopal, J.; Keum, Y.S.; Sivanesan, I. Micropropagation and quantification of bioactive compounds in Mertensia maritima (L.) gray. Int. J. Mol. Sci. 2019, 20, 2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.K.; Assefa, A.D.; Keum, Y.-S. Spices in the Apiaceae family represent the healthiest fatty acid profile: A aystematic comparison of 34 widely used spices and herbs. Foods 2021, 10, 854. [Google Scholar] [CrossRef]
- Saini, R.K.; Rauf, A.; Khalil, A.A.; Ko, E.-Y.; Keum, Y.-S.; Anwar, S.; Alamri, A.; Rengasamy, K.R.R. Edible mushrooms show significant differences in sterols and fatty acid compositions. S. Afr. J. Bot. 2021, 141, 344–356. [Google Scholar] [CrossRef]
- Cruz, R.; Casal, S.; Mendes, E.; Costa, A.; Santos, C.; Morais, S. Validation of a single-extraction procedure for sequential analysis of vitamin E, cholesterol, fatty acids, and total fat in seafood. Food Anal. Methods 2012, 6, 1196–1204. [Google Scholar] [CrossRef] [Green Version]
- Dima, Ş.; Dima, C.; Iordăchescu, G. Encapsulation of functional lipophilic food and drug biocomponents. Food Eng. Rev. 2015, 7, 417–438. [Google Scholar] [CrossRef]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From plants to food and feed industries. Methods Mol. Biol. Clifton NJ 2018, 1852, 57–71. [Google Scholar]
- Sivanesan, I.; Saini, R.K.; Kim, D.H. Bioactive compounds in hyperhydric and normal micropropagated shoots of Aronia melanocarpa (michx.) Elliott. Ind. Crops Prod. 2016, 83, 31–38. [Google Scholar] [CrossRef]
- Park, H.Y.; Saini, R.K.; Gopal, J.; Keum, Y.-S.; Kim, D.H.; Lee, O.; Sivanesan, I. Micropropagation and subsequent enrichment of carotenoids, fatty acids, and tocopherol contents in Sedum dasyphyllum L. Front. Chem. 2017, 5, 77. [Google Scholar] [CrossRef] [Green Version]
- Yoon, G.-A.; Yeum, K.-J.; Cho, Y.-S.; Chen, C.-Y.O.; Tang, G.; Blumberg, J.B.; Russell, R.M.; Yoon, S.; Lee-Kim, Y.C. Carotenoids and total phenolic contents in plant foods commonly consumed in Korea. Nutr. Res. Pr. 2012, 6, 481–490. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, Y.; Zhang, F.; Guo, X.; Liao, Z. Effect of thermal processing on carotenoids and folate changes in six varieties of sweet potato (Ipomoes batata L.). Foods 2019, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Ponder, A.; Hallmann, E. Phenolics and carotenoid contents in the leaves of different organic and conventional raspberry (Rubus idaeus L.) cultivars and their in vitro activity. Antioxidants 2019, 8, 458. [Google Scholar] [CrossRef] [Green Version]
- Lakshminarayana, R.; Raju, M.; Krishnakantha, T.P.; Baskaran, V. Determination of major carotenoids in a few Indian leafy vegetables by high-performance liquid chromatography. J. Agric. Food Chem. 2005, 53, 2838–2842. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Shang, X.M.; Ko, E.Y.; Choi, J.H.; Kim, D.; Keum, Y.-S. Characterization of nutritionally important phytoconstituents in minimally processed ready-to-eat baby-leaf vegetables using HPLC–DAD and GC–MS. J. Food Meas. Charact. 2016, 10, 341–349. [Google Scholar] [CrossRef]
- Purkiewicz, A.; Ciborska, J.; Tańska, M.; Narwojsz, A.; Starowicz, M.; Przybyłowicz, K.E.; Sawicki, T. The impact of the method extraction and different carrot variety on the carotenoid profile, total phenolic content and antioxidant properties of juices. Plants 2020, 9, 1759. [Google Scholar] [CrossRef]
- Basset, G.J.; Latimer, S.; Fatihi, A.; Soubeyrand, E.; Block, A. Phylloquinone (vitamin K1): Occurrence, biosynthesis and functions. Mini Rev. Med. Chem. 2017, 17, 1028–1038. [Google Scholar] [CrossRef]
- Damon, M.; Zhang, N.Z.; Haytowitz, D.B.; Booth, S.L. Phylloquinone (vitamin K1) content of vegetables. J. Food Compos. Anal. 2005, 18, 751–758. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Ser, S.; Cumming, J.; Ku, K. Comparative phytonutrient analysis of broccoli byproducts: The potentials for broccoli byproduct utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef] [Green Version]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and tocotrienols—Bioactive dietary compounds; what is certain, what is doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Mihaylova, D.; Vrancheva, R.; Petkova, N.; Ognyanov, M.; Desseva, I.; Ivanov, I.; Popova, M.; Popova, A. Carotenoids, tocopherols, organic acids, charbohydrate and mineral content in different medicinal plant extracts. Z. Nat. C. J. Biosci. 2018, 73, 439–448. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef]
- Sivanesan, I.; Saini, R.K.; Noorzai, R.; Zamany, A.J.; Kim, D.H. In vitro propagation, carotenoid, fatty acid and tocopherol content of Ajuga multiflora Bunge. 3 Biotech 2016, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.-Y.; Hsieh, M.-J.; Tsai, Y.-T.; Chung, H.-H.; Shyur, L.-F.; Hsieh, C.-H.; To, K.-Y. Transformation and characterization of Δ12-fatty acid acetylenase and Δ12-oleate desaturase potentially involved in the polyacetylene biosynthetic pathway from Bidens pilosa. Plants 2020, 9, 1483. [Google Scholar] [CrossRef] [PubMed]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koufan, M.; Belkoura, I.; Mazri, M.A.; Amarraque, A.; Essatte, A.; Elhorri, H.; Zaddoug, F.; Alaoui, T. Determination of antioxidant activity, total phenolics and fatty acids in essential oils and other extracts from callus culture, seeds and leaves of Argania spinosa (L.) Skeels. Plant Cell Tissue Organ Cult. 2020, 141, 217–227. [Google Scholar] [CrossRef]
- Semenova, N.V.; Makarenko, S.P.; Shmakov, V.N.; Konstantinov, Y.M.; Dudareva, L.V. Fatty acid composition of total lipids from needles and cultured calluses of conifers Pinus sylvestris L., Picea pungens Engelm., Pinus koraiensis Siebold & Zucc., and Larix sibirica Ledeb. Biochem. Suppl. Ser. A Membr. Cell Biol. 2017, 11, 287–295. [Google Scholar]


| S/No | Analyte | In Vitro Leaves | Ex Vitro Leaves |
|---|---|---|---|
| 1 | (all-E)-Violaxanthin | 9.42 | 11.03 |
| 2 | (all-Z)-Neoxanthin | 10.10 | 12.30 |
| 3 | (all-E)-Lutein | 39.42 | 44.38 |
| 4 | (all-E)-α-carotene | 10.85 | 14.79 |
| 5 | (all-E)-β-carotene | 10.61 | 11.33 |
| 6 | Total carotenoids (S/No. 1+2+3+4+5) | 80.40 | 93.84 |
| 7 | Phylloquinone (Vitamin K1) | 7.31 | 7.25 |
| 8 | γ-Tocopherol | 5.49 | 3.08 |
| 9 | β-Tocopherol | 5.66 | 3.44 |
| 10 | α-Tocopherol | 31.08 | 39.70 |
| 11 | Total tocopherols (S/No. 8+9+10) | 42.23 | 46.22 |
| 12 | 24α-ethyl cholesterol | 36.62 | 30.55 |
| 13 | 24α-methyl cholesterol | 7.78 | 7.14 |
| 14 | Total phytosterols | 44.40 | 37.69 |
| S/No | FAME | RT (Minute) | In Vitro Leaves | Ex Vitro Leaves |
|---|---|---|---|---|
| 1 | C10:0 (Capric) | 11.029 | 5.47 | 3.03 |
| 2 | C12:0 (Lauric) | 13.666 | 1.03 | 1.14 |
| 3 | C16:0 (Palmitic) | 20.509 | 22.53 | 23.78 |
| 4 | C16:1 (Palmitoleic) | 21.744 | 1.37 | 3.64 |
| 5 | C18:0 (Stearic) | 24.082 | 4.86 | 4.85 |
| 6 | C18:1n9c (Oleic) | 25.169 | 3.71 | 12.28 |
| 7 | C18:2n6c (Linoleic) | 26.789 | 21.35 | 8.17 |
| 8 | C18:3n3 (α-Linolenic) | 28.625 | 39.68 | 43.12 |
| Oleic: linoleic acid | 0.17 | 1.50 | ||
| Total SFAs | 33.89 | 32.80 | ||
| Total MUFAs | 5.08 | 15.91 | ||
| Total PUFAs | 61.03 | 51.29 | ||
| PUFAs: SFAs | 1.80 | 1.56 | ||
| PUFAs: MUFAs | 12.00 | 3.22 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Saini, R.K.; Keum, Y.-S.; Sivanesan, I. Analysis of Lipophilic Antioxidants in the Leaves of Kaempferia parviflora Wall. Ex Baker Using LC–MRM–MS and GC–FID/MS. Antioxidants 2021, 10, 1573. https://doi.org/10.3390/antiox10101573
Song K, Saini RK, Keum Y-S, Sivanesan I. Analysis of Lipophilic Antioxidants in the Leaves of Kaempferia parviflora Wall. Ex Baker Using LC–MRM–MS and GC–FID/MS. Antioxidants. 2021; 10(10):1573. https://doi.org/10.3390/antiox10101573
Chicago/Turabian StyleSong, Kihwan, Ramesh Kumar Saini, Young-Soo Keum, and Iyyakkannu Sivanesan. 2021. "Analysis of Lipophilic Antioxidants in the Leaves of Kaempferia parviflora Wall. Ex Baker Using LC–MRM–MS and GC–FID/MS" Antioxidants 10, no. 10: 1573. https://doi.org/10.3390/antiox10101573
APA StyleSong, K., Saini, R. K., Keum, Y.-S., & Sivanesan, I. (2021). Analysis of Lipophilic Antioxidants in the Leaves of Kaempferia parviflora Wall. Ex Baker Using LC–MRM–MS and GC–FID/MS. Antioxidants, 10(10), 1573. https://doi.org/10.3390/antiox10101573








