Regulation of Phosphorylated State of NMDA Receptor by STEP61 Phosphatase after Mild-Traumatic Brain Injury: Role of Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals and Antibodies
2.3. Traumatic Brain Injury Induction Protocol
2.4. Immunohistochemical Procedures
2.5. Image Analysis
2.6. Memory Flexibility Test
2.7. Novel Object Recognition Test
2.8. Immunoblotting
2.9. Subcellular Fractionation
2.10. Slice Preparation and Electrophysiology
2.11. Statistical Analysis
3. Results
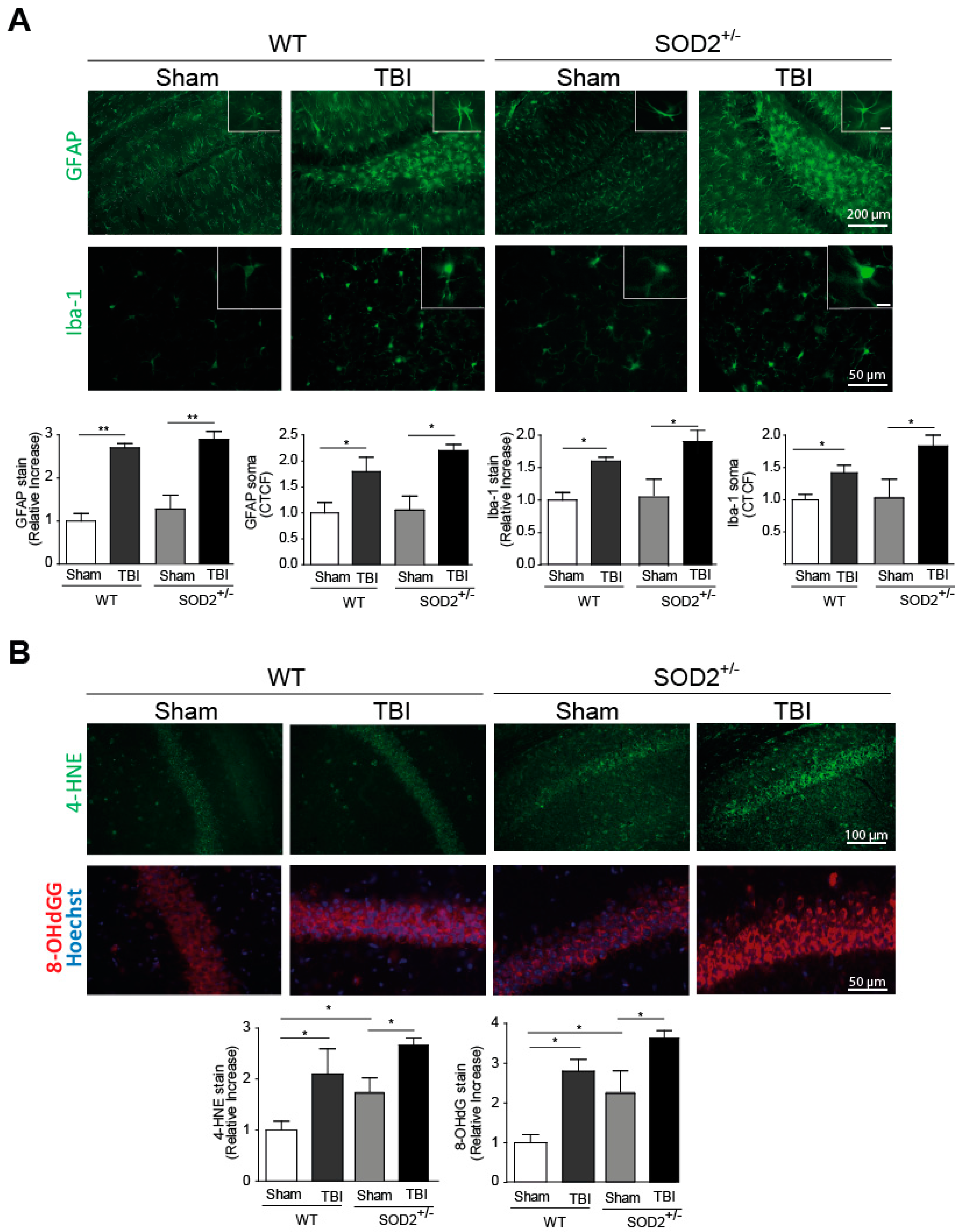
3.1. TBI Generates Neuroinflammation and Oxidative Stress in WT and SOD2+/− Animals
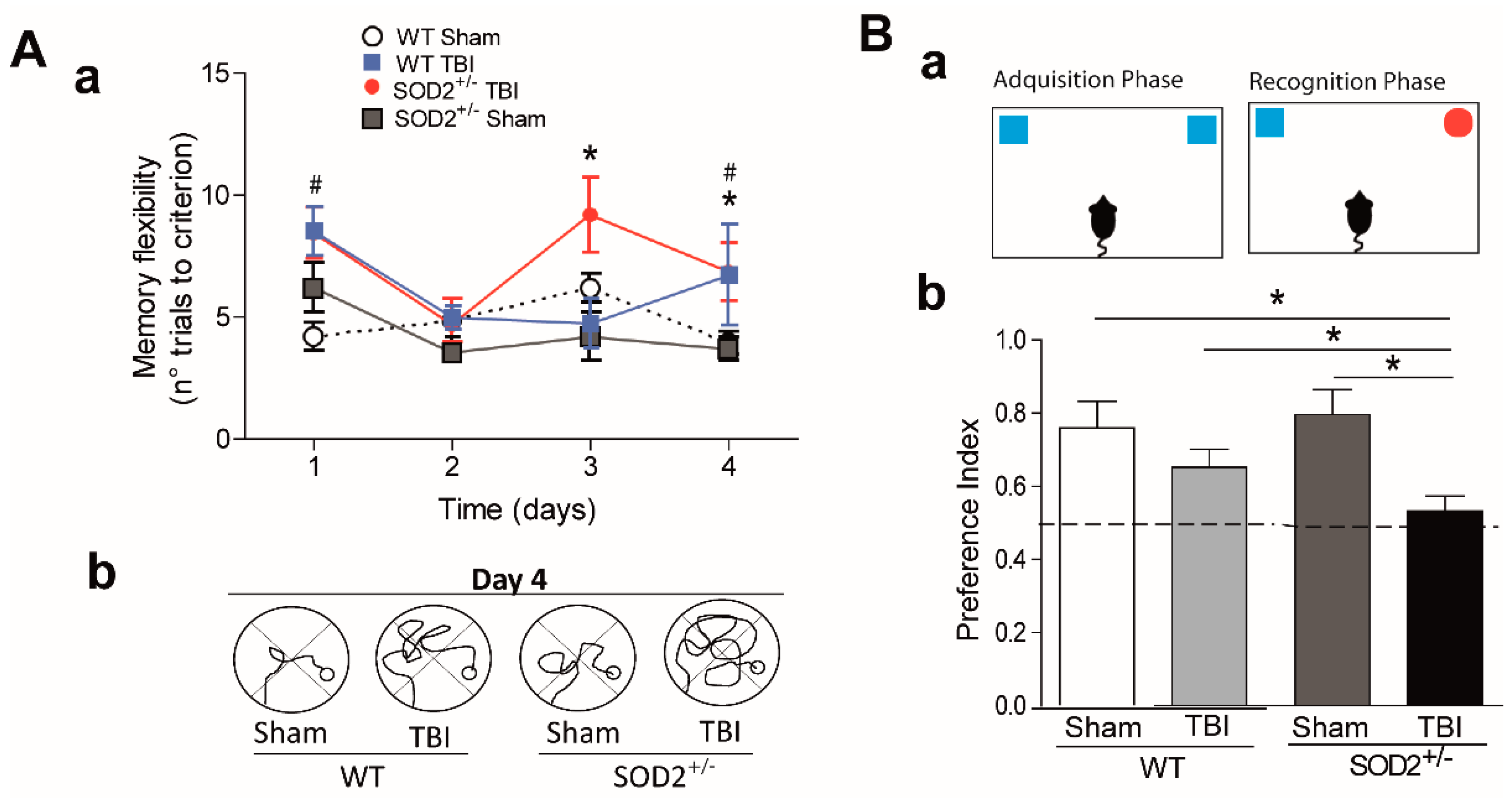
3.2. Behavioral Evaluation of WT and SOD2+/− Mice Subjected to Brain Trauma
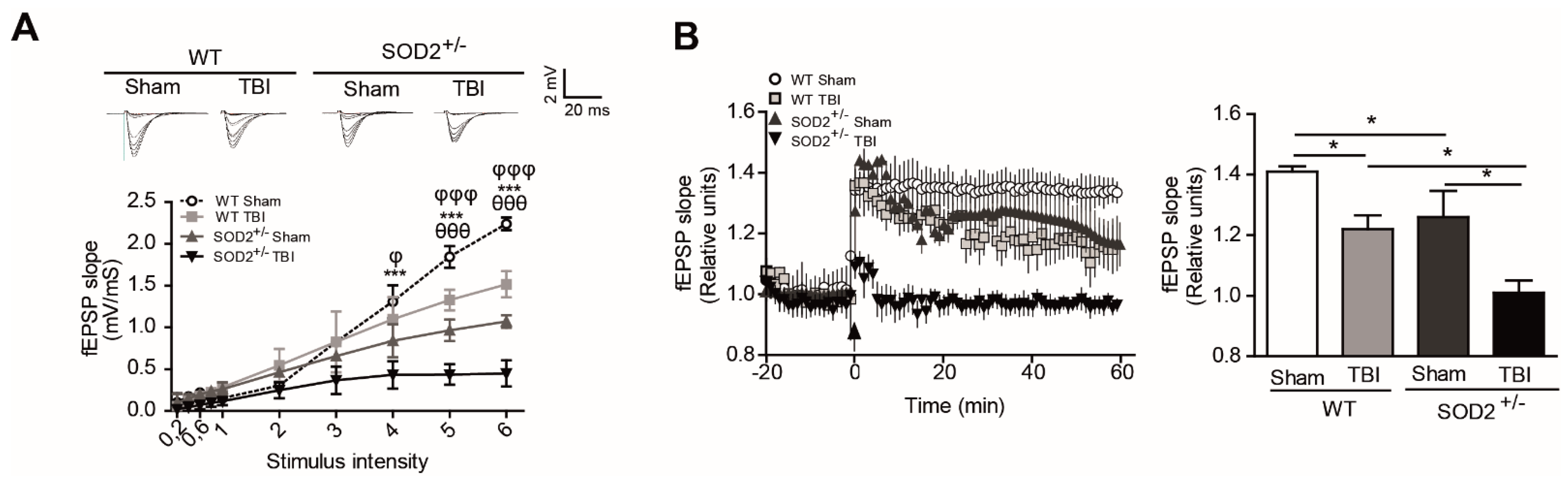
3.3. Evaluation of the Plasticity and Synaptic Response of WT and SOD2+/− Mice Subjected to Brain Trauma
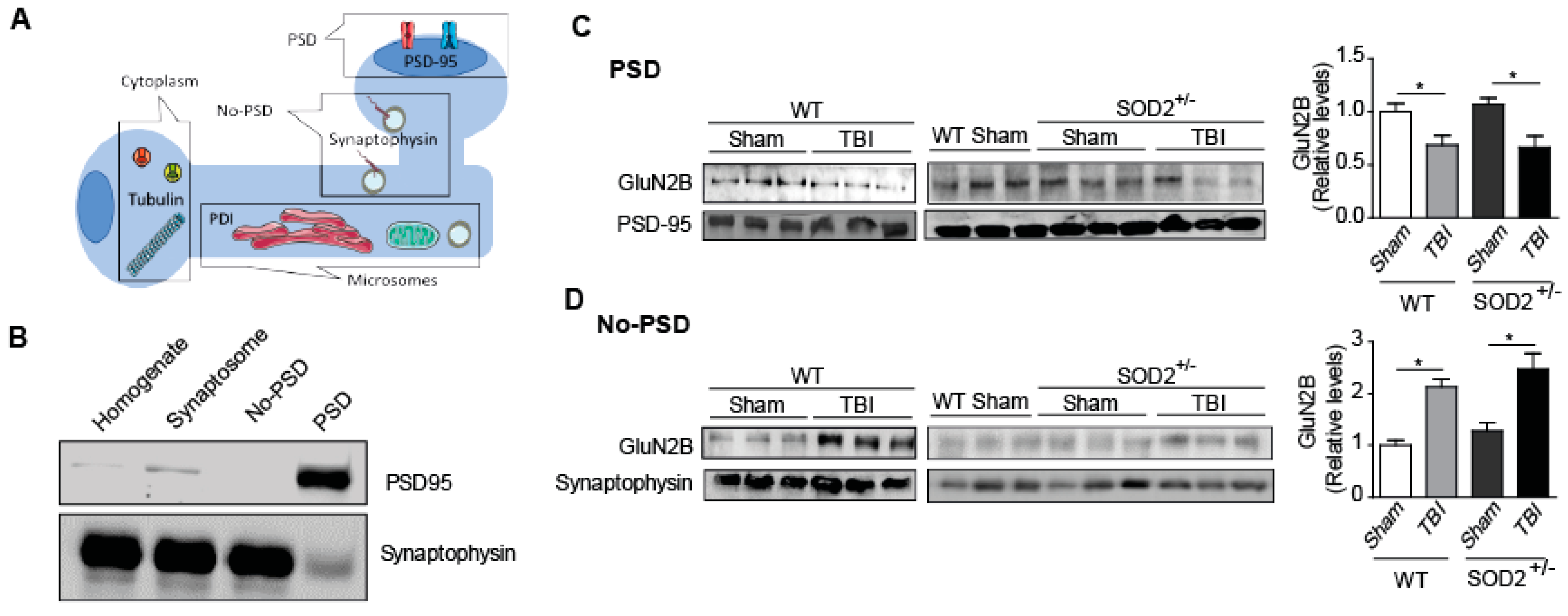
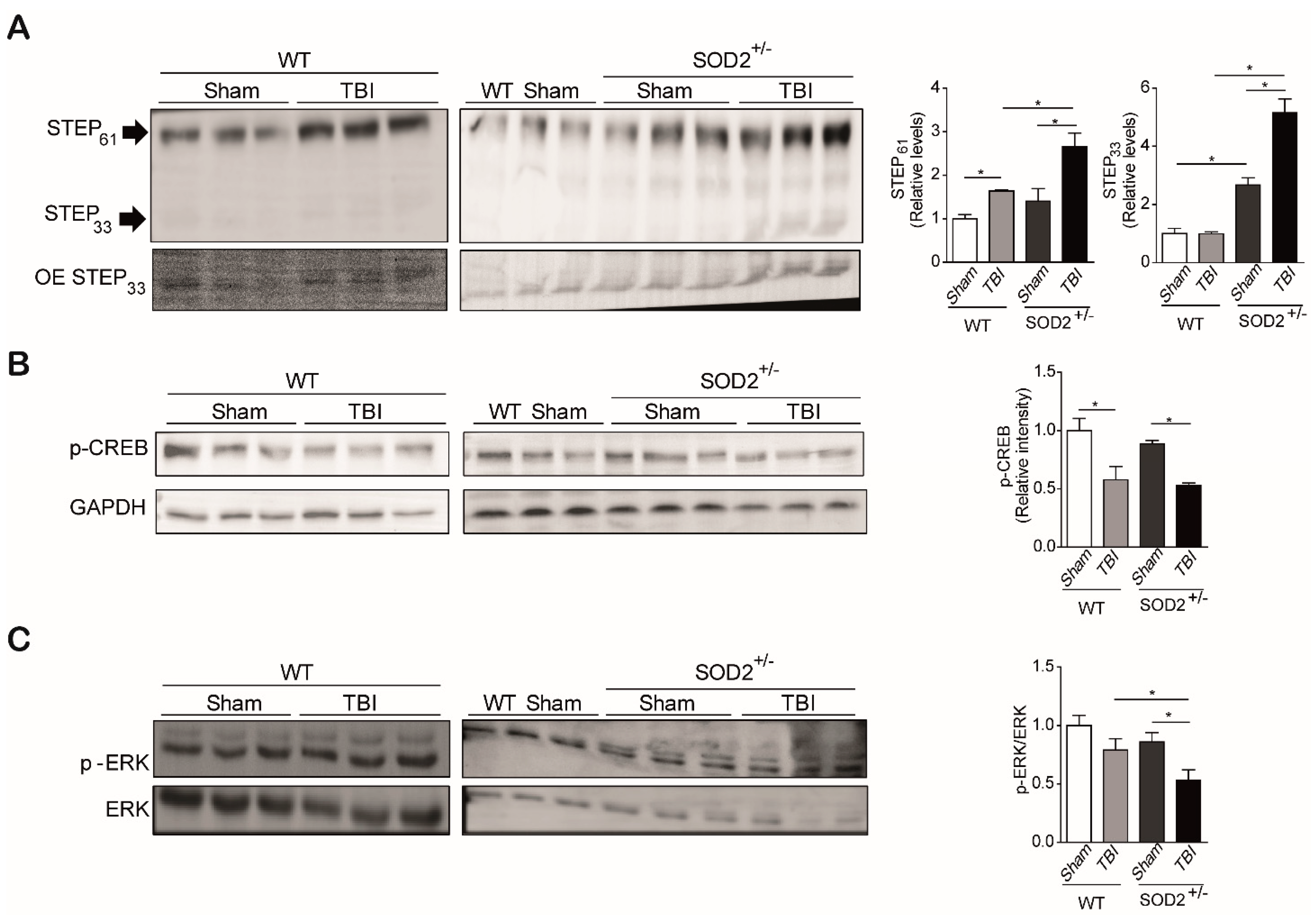
3.4. The Signaling Associated with NMDARs Is Altered in WT and SOD2+/− Mice Subjected to Brain Trauma
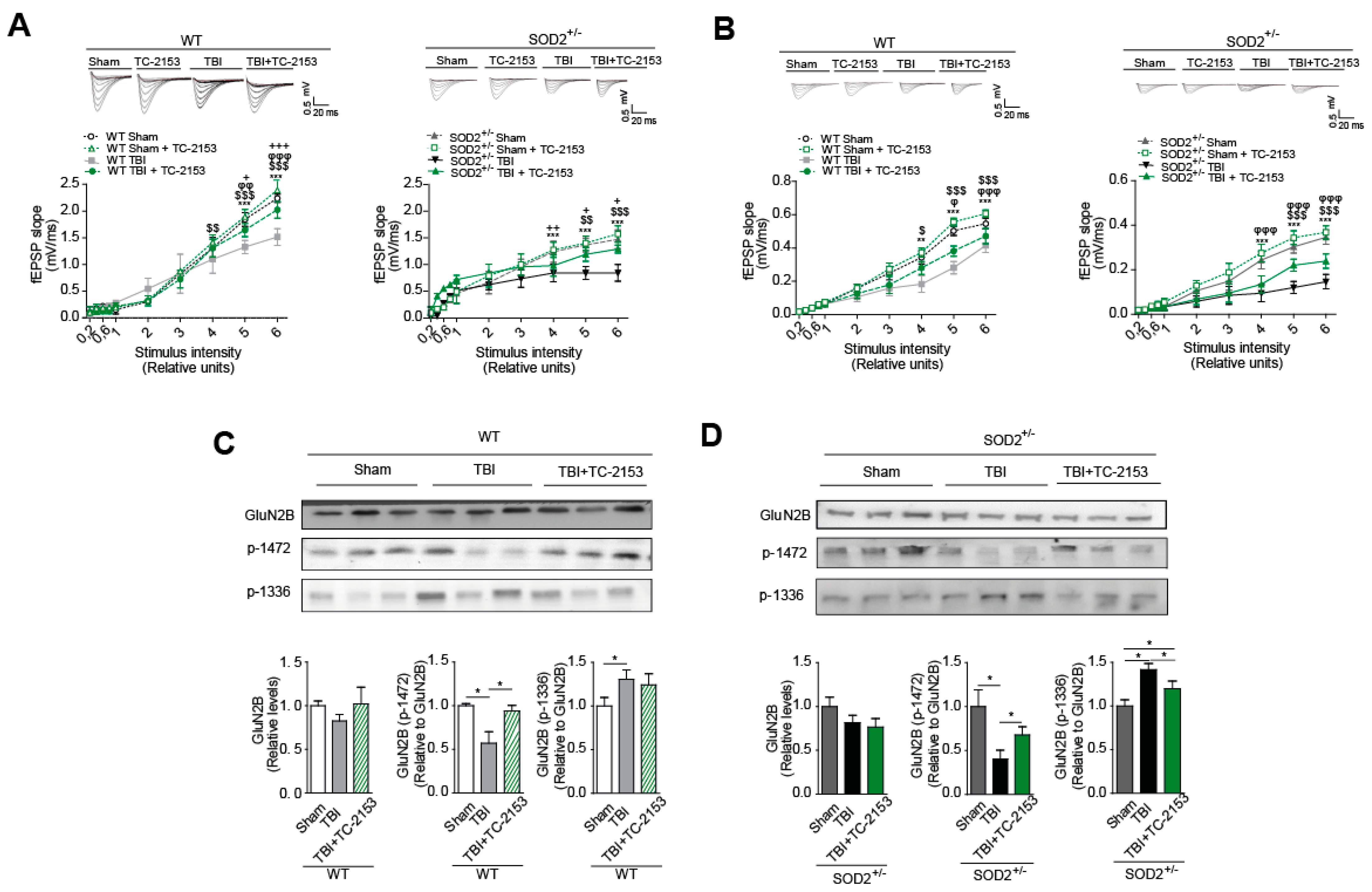
3.5. The Pharmacological Inhibition of STEP61 Restores the Damage Produced after Brain Trauma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mollayeva, T.; Mollayeva, S.; Colantonio, A. Traumatic brain injury: Sex, gender and intersecting vulnerabilities. Nat. Rev. Neurol. 2018, 14, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.E. Mechanisms of traumatic brain injury: Biomechanical, structural and cellular considerations. Crit. Care Nurs. Q. 2000, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Wong, M.D.; Samii, A.; Hovda, D.A. Evidence for energy failure following irreversible traumatic brain injury. Ann. N. Y. Acad. Sci. 1999, 893, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Masel, B.E.; DeWitt, D.S. Traumatic brain injury: A disease process, not an event. J. Neurotrauma 2010, 27, 1529–1540. [Google Scholar] [CrossRef] [Green Version]
- Jordan, B.D. Chronic traumatic encephalopathy and other long-term sequelae. Continuum 2014, 20, 1588–1604. [Google Scholar] [CrossRef]
- Chen, Y.; Constantini, S.; Trembovler, V.; Weinstock, M.; Shohami, E. An experimental model of closed head injury in mice: Pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 1996, 13, 557–568. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.E. Cognitive impairments following traumatic brain injury. Etiologies and interventions. Crit. Care Nurs. Clin. N. Am. 2000, 12, 447–456. [Google Scholar] [CrossRef]
- Carvajal, F.J.; Mattison, H.A.; Cerpa, W. Role of NMDA Receptor-Mediated Glutamatergic Signaling in Chronic and Acute Neuropathologies. Neural Plast. 2016, 2016, 2701526. [Google Scholar] [CrossRef] [PubMed]
- Mira, R.G.; Cerpa, W. Building a Bridge between NMDAR-Mediated Excitotoxicity and Mitochondrial Dysfunction in Chronic and Acute Diseases. Cell. Mol. Neurobiol. 2020. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef] [Green Version]
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef]
- Milnerwood, A.J.; Gladding, C.M.; Pouladi, M.A.; Kaufman, A.M.; Hines, R.M.; Boyd, J.D.; Ko, R.W.; Vasuta, O.C.; Graham, R.K.; Hayden, M.R.; et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron 2010, 65, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Briz, V.; Chishti, A.; Bi, X.; Baudry, M. Distinct roles for mu-calpain and m-calpain in synaptic NMDAR-mediated neuroprotection and extrasynaptic NMDAR-mediated neurodegeneration. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 18880–18892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardingham, G.E.; Arnold, F.J.; Bading, H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat. Neurosci. 2001, 4, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Gladding, C.M.; Raymond, L.A. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol. Cell. Neurosci. 2011, 48, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Gladding, C.M.; Sepers, M.D.; Xu, J.; Zhang, L.Y.; Milnerwood, A.J.; Lombroso, P.J.; Raymond, L.A. Calpain and STriatal-Enriched protein tyrosine phosphatase (STEP) activation contribute to extrasynaptic NMDA receptor localization in a Huntington’s disease mouse model. Hum. Mol. Genet. 2012, 21, 3739–3752. [Google Scholar] [CrossRef]
- Ivanov, A.; Pellegrino, C.; Rama, S.; Dumalska, I.; Salyha, Y.; Ben-Ari, Y.; Medina, I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J. Physiol. 2006, 572, 789–798. [Google Scholar] [CrossRef]
- Carvajal, F.J.; Mira, R.G.; Rovegno, M.; Minniti, A.N.; Cerpa, W. Age-related NMDA signaling alterations in SOD2 deficient mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2010–2020. [Google Scholar] [CrossRef]
- Groc, L.; Bard, L.; Choquet, D. Surface trafficking of N-methyl-D-aspartate receptors: Physiological and pathological perspectives. Neuroscience 2009, 158, 4–18. [Google Scholar] [CrossRef]
- Lau, C.G.; Zukin, R.S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007, 8, 413–426. [Google Scholar] [CrossRef]
- Xu, F.; Plummer, M.R.; Len, G.W.; Nakazawa, T.; Yamamoto, T.; Black, I.B.; Wu, K. Brain-derived neurotrophic factor rapidly increases NMDA receptor channel activity through Fyn-mediated phosphorylation. Brain Res. 2006, 1121, 22–34. [Google Scholar] [CrossRef]
- Goebel-Goody, S.M.; Davies, K.D.; Alvestad Linger, R.M.; Freund, R.K.; Browning, M.D. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience 2009, 158, 1446–1459. [Google Scholar] [CrossRef]
- Roche, K.W.; Standley, S.; McCallum, J.; Dune Ly, C.; Ehlers, M.D.; Wenthold, R.J. Molecular determinants of NMDA receptor internalization. Nat. Neurosci. 2001, 4, 794–802. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Liu, J.; Lombroso, P.J. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J. Biol. Chem. 2002, 277, 24274–24279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Kurup, P.; Zhang, Y.; Goebel-Goody, S.M.; Wu, P.H.; Hawasli, A.H.; Baum, M.L.; Bibb, J.A.; Lombroso, P.J. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 9330–9343. [Google Scholar] [CrossRef]
- Basen-Engquist, K.; Coyle, K.K.; Parcel, G.S.; Kirby, D.; Banspach, S.W.; Carvajal, S.C.; Baumler, E. Schoolwide effects of a multicomponent HIV, STD, and pregnancy prevention program for high school students. Health Educ. Behav. Off. Publ. Soc. Public Health Educ. 2001, 28, 166–185. [Google Scholar] [CrossRef] [PubMed]
- Carty, N.C.; Xu, J.; Kurup, P.; Brouillette, J.; Goebel-Goody, S.M.; Austin, D.R.; Yuan, P.; Chen, G.; Correa, P.R.; Haroutunian, V.; et al. The tyrosine phosphatase STEP: Implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl. Psychiatry 2012, 2, e137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Chatterjee, M.; Baguley, T.D.; Brouillette, J.; Kurup, P.; Ghosh, D.; Kanyo, J.; Zhang, Y.; Seyb, K.; Ononenyi, C.; et al. Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of Alzheimer’s disease. PLoS Biol. 2014, 12, e1001923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deb, I.; Poddar, R.; Paul, S. Oxidative stress-induced oligomerization inhibits the activity of the non-receptor tyrosine phosphatase STEP61. J. Neurochem. 2011, 116, 1097–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Remmen, H.; Salvador, C.; Yang, H.; Huang, T.T.; Epstein, C.J.; Richardson, A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch. Biochem. Biophys. 1999, 363, 91–97. [Google Scholar] [CrossRef]
- Kilbourne, M.; Kuehn, R.; Tosun, C.; Caridi, J.; Keledjian, K.; Bochicchio, G.; Scalea, T.; Gerzanich, V.; Simard, J.M. Novel model of frontal impact closed head injury in the rat. J. Neurotrauma 2009, 26, 2233–2243. [Google Scholar] [CrossRef] [Green Version]
- Mira, R.G.; Lira, M.; Quintanilla, R.A.; Cerpa, W. Alcohol consumption during adolescence alters the hippocampal response to traumatic brain injury. Biochem. Biophys. Res. Commun. 2020, 528, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Lira, M.; Zamorano, P.; Cerpa, W. Exo70 intracellular redistribution after repeated mild traumatic brain injury. Biol. Res. 2021, 54, 5. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, F.J.; Zolezzi, J.M.; Tapia-Rojas, C.; Godoy, J.A.; Inestrosa, N.C. Tetrahydrohyperforin decreases cholinergic markers associated with amyloid-beta plaques, 4-hydroxynonenal formation, and caspase-3 activation in AbetaPP/PS1 mice. J. Alzheimer’s Dis. 2013, 36, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Serrano, F.G.; Tapia-Rojas, C.; Carvajal, F.J.; Hancke, J.; Cerpa, W.; Inestrosa, N.C. Andrographolide reduces cognitive impairment in young and mature AbetaPPswe/PS-1 mice. Mol. Neurodegener. 2014, 9, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inestrosa, N.C.; Carvajal, F.J.; Zolezzi, J.M.; Tapia-Rojas, C.; Serrano, F.; Karmelic, D.; Toledo, E.M.; Toro, A.; Toro, J.; Santos, M.J. Peroxisome proliferators reduce spatial memory impairment, synaptic failure, and neurodegeneration in brains of a double transgenic mice model of Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 33, 941–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadbent, N.J.; Gaskin, S.; Squire, L.R.; Clark, R.E. Object recognition memory and the rodent hippocampus. Learn. Mem. 2010, 17, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Ennaceur, A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav. Brain Res. 2010, 215, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Waldo Cerpa, E.L.-E.A.B. RoR2 functions as a noncanonical Wnt receptor thatregulates NMDAR-mediated synaptic transmission. Proc. Natl. Acad. Sci. USA 2015, 112, 4797–4802. [Google Scholar] [CrossRef] [Green Version]
- Anderson, W.W.; Collingridge, G.L. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J. Neurosci. Methods 2007, 162, 346–356. [Google Scholar] [CrossRef]
- Chen, M.; Lu, T.J.; Chen, X.J.; Zhou, Y.; Chen, Q.; Feng, X.Y.; Xu, L.; Duan, W.H.; Xiong, Z.Q. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke 2008, 39, 3042–3048. [Google Scholar] [CrossRef]
- Chen, G.; Chen, K.S.; Knox, J.; Inglis, J.; Bernard, A.; Martin, S.J.; Justice, A.; McConlogue, L.; Games, D.; Freedman, S.B.; et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature 2000, 408, 975–979. [Google Scholar] [CrossRef]
- Toledo, E.M.; Inestrosa, N.C. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol. Psychiatry 2010, 15, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Dosemeci, A.; Tao-Cheng, J.H.; Vinade, L.; Jaffe, H. Preparation of postsynaptic density fraction from hippocampal slices and proteomic analysis. Biochem. Biophys. Res. Commun. 2006, 339, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Bading, H. Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochim. Biophys. Acta 2002, 1600, 148–153. [Google Scholar] [CrossRef]
- Pelkey, K.A.; Askalan, R.; Paul, S.; Kalia, L.V.; Nguyen, T.H.; Pitcher, G.M.; Salter, M.W.; Lombroso, P.J. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron 2002, 34, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Xie, D.D.; Dong, J.H.; Li, H.; Li, K.S.; Su, J.; Chen, L.Z.; Xu, Y.F.; Wang, H.M.; Gong, Z.; et al. Molecular mechanism of ERK dephosphorylation by striatal-enriched protein tyrosine phosphatase. J. Neurochem. 2014, 128, 315–329. [Google Scholar] [CrossRef] [Green Version]
- Goebel-Goody, S.M.; Baum, M.; Paspalas, C.D.; Fernandez, S.M.; Carty, N.C.; Kurup, P.; Lombroso, P.J. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 65–87. [Google Scholar] [CrossRef]
- Hermel, E.; Gafni, J.; Propp, S.S.; Leavitt, B.R.; Wellington, C.L.; Young, J.E.; Hackam, A.S.; Logvinova, A.V.; Peel, A.L.; Chen, S.F.; et al. Specific caspase interactions and amplification are involved in selective neuronal vulnerability in Huntington’s disease. Cell Death Differ. 2004, 11, 424–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castonguay, D.; Dufort-Gervais, J.; Menard, C.; Chatterjee, M.; Quirion, R.; Bontempi, B.; Schneider, J.S.; Arnsten, A.F.T.; Nairn, A.C.; Norris, C.M.; et al. The Tyrosine Phosphatase STEP Is Involved in Age-Related Memory Decline. Curr. Biol. 2018, 28, 1079–1089. [Google Scholar] [CrossRef] [Green Version]
- Munoz, J.J.; Tarrega, C.; Blanco-Aparicio, C.; Pulido, R. Differential interaction of the tyrosine phosphatases PTP-SL, STEP and HePTP with the mitogen-activated protein kinases ERK1/2 and p38alpha is determined by a kinase specificity sequence and influenced by reducing agents. Biochem. J. 2003, 372, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Nairn, A.C.; Wang, P.; Lombroso, P.J. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat. Neurosci. 2003, 6, 34–42. [Google Scholar] [CrossRef]
- Aizenman, E.; Lipton, S.A.; Loring, R.H. Selective modulation of NMDA responses by reduction and oxidation. Neuron 1989, 2, 1257–1263. [Google Scholar] [CrossRef]
- Patel, R.; Sesti, F. Oxidation of ion channels in the aging nervous system. Brain Res. 2016, 1639, 174–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paula-Lima, A.C.; Adasme, T.; Hidalgo, C. Contribution of Ca2+ release channels to hippocampal synaptic plasticity and spatial memory: Potential redox modulation. Antioxid. Redox Signal. 2014, 21, 892–914. [Google Scholar] [CrossRef] [PubMed]
- Aizenman, E. Modulation of N-methyl-D-aspartate receptors by hydroxyl radicals in rat cortical neurons in vitro. Neurosci. Lett. 1995, 189, 57–59. [Google Scholar] [CrossRef]
- Aizenman, E.; Hartnett, K.A.; Reynolds, I.J. Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron 1990, 5, 841–846. [Google Scholar] [CrossRef]
- Carroll, R.C.; Zukin, R.S. NMDA-receptor trafficking and targeting: Implications for synaptic transmission and plasticity. Trends Neurosci. 2002, 25, 571–577. [Google Scholar] [CrossRef]
- Cahill-Smith, S.; Li, J.M. Oxidative stress, redox signalling and endothelial dysfunction in ageing-related neurodegenerative diseases: A role of NADPH oxidase 2. Br. J. Clin. Pharmacol. 2014, 78, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, N.; Ghosh, R.; Mandal, S.C. Antioxidant protection: A promising therapeutic intervention in neurodegenerative disease. Free Radic. Res. 2011, 45, 888–905. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Control of redox state and redox signaling by neural antioxidant systems. Antioxid. Redox Signal. 2011, 14, 1449–1465. [Google Scholar] [CrossRef]
- Von Bernhardi, R.; Eugenin, J. Alzheimer’s disease: Redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antioxid. Redox Signal. 2012, 16, 974–1031. [Google Scholar] [CrossRef]
- Huang, Y.H.; Lin, Y.; Brown, T.E.; Han, M.H.; Saal, D.B.; Neve, R.L.; Zukin, R.S.; Sorg, B.A.; Nestler, E.J.; Malenka, R.C.; et al. CREB modulates the functional output of nucleus accumbens neurons: A critical role of N-methyl-D-aspartate glutamate receptor (NMDAR) receptors. J. Biol. Chem. 2008, 283, 2751–2760. [Google Scholar] [CrossRef] [Green Version]
- Meyer, D.A.; Torres-Altoro, M.I.; Tan, Z.; Tozzi, A.; Di Filippo, M.; DiNapoli, V.; Plattner, F.; Kansy, J.W.; Benkovic, S.A.; Huber, J.D.; et al. Ischemic stroke injury is mediated by aberrant Cdk5. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 8259–8267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plattner, F.; Hernandez, A.; Kistler, T.M.; Pozo, K.; Zhong, P.; Yuen, E.Y.; Tan, C.; Hawasli, A.H.; Cooke, S.F.; Nishi, A.; et al. Memory enhancement by targeting Cdk5 regulation of NR2B. Neuron 2014, 81, 1070–1083. [Google Scholar] [CrossRef] [Green Version]
- Tassin, T.C.; Benavides, D.R.; Plattner, F.; Nishi, A.; Bibb, J.A. Regulation of ERK Kinase by MEK1 Kinase Inhibition in the Brain. J. Biol. Chem. 2015, 290, 16319–16329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousuf, M.A.; Tan, C.; Torres-Altoro, M.I.; Lu, F.M.; Plautz, E.; Zhang, S.; Takahashi, M.; Hernandez, A.; Kernie, S.G.; Plattner, F.; et al. Involvement of aberrant Cdk5/p25 activity in experimental traumatic brain injury. J. Neurochem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Gladding, C.M.; Wang, L.; Zhang, L.Y.; Kaufman, A.M.; Milnerwood, A.J.; Raymond, L.A. P38 MAPK is involved in enhanced NMDA receptor-dependent excitotoxicity in YAC transgenic mouse model of Huntington disease. Neurobiol. Dis. 2012, 45, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Barria, A.; Malinow, R. Subunit-specific NMDA receptor trafficking to synapses. Neuron 2002, 35, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Barria, A.; Malinow, R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 2005, 48, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Groc, L.; Heine, M.; Cousins, S.L.; Stephenson, F.A.; Lounis, B.; Cognet, L.; Choquet, D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc. Natl. Acad. Sci. USA 2006, 103, 18769–18774. [Google Scholar] [CrossRef] [Green Version]
- Goebel, S.M.; Alvestad, R.M.; Coultrap, S.J.; Browning, M.D. Tyrosine phosphorylation of the N-methyl-D-aspartate receptor is enhanced in synaptic membrane fractions of the adult rat hippocampus. Brain Res. Mol. Brain Res. 2005, 142, 65–79. [Google Scholar] [CrossRef]
- Schumann, J.; Michaeli, A.; Yaka, R. Src-protein tyrosine kinases are required for cocaine-induced increase in the expression and function of the NMDA receptor in the ventral tegmental area. J. Neurochem. 2009, 108, 697–706. [Google Scholar] [CrossRef]
- Braithwaite, S.P.; Adkisson, M.; Leung, J.; Nava, A.; Masterson, B.; Urfer, R.; Oksenberg, D.; Nikolich, K. Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP). Eur. J. Neurosci. 2006, 23, 2847–2856. [Google Scholar] [CrossRef]
- Saavedra, A.; Ballesteros, J.J.; Tyebji, S.; Martinez-Torres, S.; Blazquez, G.; Lopez-Hidalgo, R.; Azkona, G.; Alberch, J.; Martin, E.D.; Perez-Navarro, E. Proteolytic Degradation of Hippocampal STEP61 in LTP and Learning. Mol. Neurobiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cases, S.; Saavedra, A.; Tyebji, S.; Giralt, A.; Alberch, J.; Perez-Navarro, E. Age-related changes in STriatal-Enriched protein tyrosine Phosphatase levels: Regulation by BDNF. Mol. Cell. Neurosci. 2018, 86, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Goebel-Goody, S.M.; Wilson-Wallis, E.D.; Royston, S.; Tagliatela, S.M.; Naegele, J.R.; Lombroso, P.J. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 2012, 11, 586–600. [Google Scholar] [CrossRef] [Green Version]
- Kabadi, S.V.; Faden, A.I. Neuroprotective strategies for traumatic brain injury: Improving clinical translation. Int. J. Mol. Sci. 2014, 15, 1216–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.H.; Johnson, V.E.; Uryu, K.; Trojanowski, J.Q.; Smith, D.H. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009, 19, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Nakamura, M.; McIntosh, T.K.; Rodriguez, A.; Berlin, J.A.; Smith, D.H.; Saatman, K.E.; Raghupathi, R.; Clemens, J.; Saido, T.C.; et al. Traumatic brain injury in young, amyloid-beta peptide overexpressing transgenic mice induces marked ipsilateral hippocampal atrophy and diminished Abeta deposition during aging. J. Comp. Neurol. 1999, 411, 390–398. [Google Scholar] [CrossRef]
- Smith, D.H.; Uryu, K.; Saatman, K.E.; Trojanowski, J.Q.; McIntosh, T.K. Protein accumulation in traumatic brain injury. Neuromol. Med. 2003, 4, 59–72. [Google Scholar] [CrossRef]
- Yang, S.T.; Hsiao, I.T.; Hsieh, C.J.; Chiang, Y.H.; Yen, T.C.; Chiu, W.T.; Lin, K.J.; Hu, C.J. Accumulation of amyloid in cognitive impairment after mild traumatic brain injury. J. Neurol. Sci. 2015, 349, 99–104. [Google Scholar] [CrossRef]
- Wolf, M.S.; Bayir, H.; Kochanek, P.M.; Clark, R.S.B. The role of autophagy in acute brain injury: A state of flux? Neurobiol. Dis. 2018, 122, 9–15. [Google Scholar] [CrossRef]
- DeWalt, G.J.; Mahajan, B.; Foster, A.R.; Thompson, L.D.E.; Marttini, A.A.; Schmidt, E.V.; Mansuri, S.; D’Souza, D.; Patel, S.B.; Tenenbaum, M.; et al. Region-specific alterations in astrocyte and microglia morphology following exposure to blasts in the mouse hippocampus. Neurosci. Lett. 2018, 664, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Wang, J.A.; Miller, D.M. Relationship of nitric oxide synthase induction to peroxynitrite-mediated oxidative damage during the first week after experimental traumatic brain injury. Exp. Neurol. 2012, 238, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witcher, K.G.; Bray, C.E.; Dziabis, J.E.; McKim, D.B.; Benner, B.N.; Rowe, R.K.; Kokiko-Cochran, O.N.; Popovich, P.G.; Lifshitz, J.; Eiferman, D.S.; et al. Traumatic brain injury-induced neuronal damage in the somatosensory cortex causes formation of rod-shaped microglia that promote astrogliosis and persistent neuroinflammation. Glia 2018, 66, 2719–2736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.G.; Laird, M.D.; Han, D.; Nguyen, K.; Scott, E.; Dong, Y.; Dhandapani, K.M.; Brann, D.W. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS ONE 2012, 7, e34504. [Google Scholar] [CrossRef] [PubMed]
- Brun, V.H.; Otnass, M.K.; Molden, S.; Steffenach, H.A.; Witter, M.P.; Moser, M.B.; Moser, E.I. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science 2002, 296, 2243–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsien, J.Z.; Huerta, P.T.; Tonegawa, S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 1996, 87, 1327–1338. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, A.; Fujimura, M.; Kato, K.; Okuyama, H.; Hashimoto, T.; Takayama, K.; Tominaga, T. Shock wave-induced brain injury in rat: Novel traumatic brain injury animal model. Acta Neurochir. Suppl. 2008, 102, 421–424. [Google Scholar]
- Gao, X.; Deng, P.; Xu, Z.C.; Chen, J. Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS ONE 2011, 6, e24566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegde, A.N. The ubiquitin-proteasome pathway and synaptic plasticity. Learn. Mem. 2010, 17, 314–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Taghibiglou, C.; Girling, K.; Dong, Z.; Lin, S.Z.; Lee, W.; Shyu, W.C.; Wang, Y.T. Critical role of increased PTEN nuclear translocation in excitotoxic and ischemic neuronal injuries. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 7997–8008. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.S.; Royston, S.E.; Xu, J.; Cavaretta, J.P.; Vest, M.O.; Lee, K.Y.; Lee, S.; Jeong, H.G.; Lombroso, P.J.; Chung, H.J. Regulation of STEP61 and tyrosine-phosphorylation of NMDA and AMPA receptors during homeostatic synaptic plasticity. Mol. Brain 2015, 8, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrup, K.; Yang, Y. Cell cycle regulation in the postmitotic neuron: Oxymoron or new biology? Nat. Rev. Neurosci. 2007, 8, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Inata, Y.; Kikuchi, S.; Samraj, R.S.; Hake, P.W.; O’Connor, M.; Ledford, J.R.; O’Connor, J.; Lahni, P.; Wolfe, V.; Piraino, G.; et al. Autophagy and mitochondrial biogenesis impairment contribute to age-dependent liver injury in experimental sepsis: Dysregulation of AMP-activated protein kinase pathway. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Hegde, A.N.; Upadhya, S.C. Role of ubiquitin-proteasome-mediated proteolysis in nervous system disease. Biochim. Biophys. Acta 2011, 1809, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Cenini, G.; Voos, W. Role of Mitochondrial Protein Quality Control in Oxidative Stress-induced Neurodegenerative Diseases. Curr. Alzheimer Res. 2016, 13, 164–173. [Google Scholar] [CrossRef]
- Bezprozvanny, I.; Hayden, M.R. Deranged neuronal calcium signaling and Huntington disease. Biochem. Biophys. Res. Commun. 2004, 322, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.; Seica, R.M.; Moreira, P.I. Mitochondria as a target for neuroprotection: Implications for Alzheimer s disease. Expert Rev. Neurother. 2017, 17, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Kokiko-Cochran, O.N.; Godbout, J.P. The Inflammatory Continuum of Traumatic Brain Injury and Alzheimer’s Disease. Front. Immunol. 2018, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012, 22, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.H.; Chen, X.H.; Nonaka, M.; Trojanowski, J.Q.; Lee, V.M.; Saatman, K.E.; Leoni, M.J.; Xu, B.N.; Wolf, J.A.; Meaney, D.F. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J. Neuropathol. Exp. Neurol. 1999, 58, 982–992. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvajal, F.J.; Cerpa, W. Regulation of Phosphorylated State of NMDA Receptor by STEP61 Phosphatase after Mild-Traumatic Brain Injury: Role of Oxidative Stress. Antioxidants 2021, 10, 1575. https://doi.org/10.3390/antiox10101575
Carvajal FJ, Cerpa W. Regulation of Phosphorylated State of NMDA Receptor by STEP61 Phosphatase after Mild-Traumatic Brain Injury: Role of Oxidative Stress. Antioxidants. 2021; 10(10):1575. https://doi.org/10.3390/antiox10101575
Chicago/Turabian StyleCarvajal, Francisco J., and Waldo Cerpa. 2021. "Regulation of Phosphorylated State of NMDA Receptor by STEP61 Phosphatase after Mild-Traumatic Brain Injury: Role of Oxidative Stress" Antioxidants 10, no. 10: 1575. https://doi.org/10.3390/antiox10101575
APA StyleCarvajal, F. J., & Cerpa, W. (2021). Regulation of Phosphorylated State of NMDA Receptor by STEP61 Phosphatase after Mild-Traumatic Brain Injury: Role of Oxidative Stress. Antioxidants, 10(10), 1575. https://doi.org/10.3390/antiox10101575






