Vigilance Decrement and Enhancement Techniques: A Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Strategy
2.3. Variables of Interest
3. Vigilance Enhancement Based on Unconventional Techniques
3.1. Pharmaceutical Drugs
3.2. Video Game
3.3. Transcranial Direct Current Stimulation
3.4. Music
3.5. Binaural Auditory Beats
3.6. Haptic Stimulation
3.7. Cognitive Workload Modulation
4. Vigilance Enhancement Based on Conventional Techniques
4.1. Caffeine
4.2. Fragrance Administration
4.3. Chewing Gum
5. Comprehensive Summary
6. Challenges
6.1. Safety
6.2. Health
6.3. Cost
6.4. Assessment Methods
6.5. Translation to Real-Life Practice
6.6. Response Time
6.7. Adhering to Ethical Approval
7. Recommendation
7.1. General Recommendation
7.2. Recommendations for Future Experimental Studies
7.2.1. Duration of Measurement and Enhancement Stimulations
7.2.2. Resting Period during the Acquisition
7.2.3. Time of Day, Spatial Location, and Visual and Sound Level
7.2.4. Vigilance Task Considerations
7.2.5. Pre- and Post-Test Measurements
7.2.6. Inclusion of Individual and Group Analysis
8. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Näsholm, E.; Rohlfing, S.; Sauer, J. Pirate stealth or inattentional blindness? The effects of target relevance and sustained attention on security monitoring for experienced and naïve operators. PLoS ONE 2014, 9, e86157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meuter, R.F.; Lacherez, P.F. When and why threats go undetected: Impacts of event rate and shift length on threat detection accuracy during airport baggage screening. Hum. Factors 2016, 58, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Körber, M.; Cingel, A.; Zimmermann, M.; Bengler, K. Vigilance decrement and passive fatigue caused by monotony in automated driving. Procedia Manuf. 2015, 3, 2403–2409. [Google Scholar] [CrossRef]
- Gill, G. Vigilance in cytoscreening: Looking without seeing. Adv. Med. Lab. Prof. 1996, 8, 14–15. [Google Scholar]
- Ko, L.-W.; Komarov, O.; Hairston, W.D.; Jung, T.-P.; Lin, C.-T. Sustained attention in real classroom settings: An eeg study. Front. Hum. Neurosci. 2017, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Reinerman-Jones, L.; Matthews, G.; Mercado, J.E. Detection tasks in nuclear power plant operation: Vigilance decrement and physiological workload monitoring. Saf. Sci. 2016, 88, 97–107. [Google Scholar] [CrossRef]
- Brookings, J.B.; Wilson, G.F.; Swain, C.R. Psychophysiological responses to changes in workload during simulated air traffic control. J. Biol. Psychol. 1996, 42, 361–377. [Google Scholar] [CrossRef]
- Warm, J.S.; Parasuraman, R.; Matthews, G. Vigilance requires hard mental work and is stressful. Hum. Factors 2008, 50, 433–441. [Google Scholar] [CrossRef]
- Pattyn, N.; Neyt, X.; Henderickx, D.; Soetens, E. Psychophysiological investigation of vigilance decrement: Boredom or cognitive fatigue? Physiol. Behav. 2008, 93, 369–378. [Google Scholar] [CrossRef]
- Thomson, D.R.; Besner, D.; Smilek, D. A resource-control account of sustained attention: Evidence from mind-wandering and vigilance paradigms. Perspect. Psychol. Sci. 2015, 10, 82–96. [Google Scholar] [CrossRef]
- Manly, T.; Robertson, I.H.; Galloway, M.; Hawkins, K. The absent mind: Further investigations of sustained attention to response. Neuropsychologia 1999, 37, 661–670. [Google Scholar] [CrossRef]
- Gartenberg, D.; Gunzelmann, G.; Hassanzadeh-Behbahani, S.; Trafton, J.G. Examining the role of task requirements in the magnitude of the vigilance decrement. Front. Psychol. 2018, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.; Slagter, H.A.; Rawlings, N.B.; Francis, A.D.; Greischar, L.L.; Davidson, R. Mental training enhances attentional stability: Neural and behavioral evidence. J. Neurosci. 2009, 29, 13418–13427. [Google Scholar] [CrossRef] [PubMed]
- MacLean, K.A.; Ferrer, E.; Aichele, S.R.; Bridwell, D.A.; Zanesco, A.P.; Jacobs, T.L.; King, B.G.; Rosenberg, E.L.; Sahdra, B.K.; Shaver, P.R. Intensive meditation training improves perceptual discrimination and sustained attention. Psychol. Sci. 2010, 21, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Sheela, H.R.R.N.; Ganpat, T.S. Efficacy of Yoga for sustained attention in university students. Ayu 2013, 34, 270. [Google Scholar] [PubMed]
- Telles, S.; Gupta, R.K.; Verma, S.; Kala, N.; Balkrishna, A. Changes in vigilance, self rated sleep and state anxiety in military personnel in India following yoga. J. BMC Res. Notes 2018, 11, 518. [Google Scholar] [CrossRef]
- Ballester, R.; Huertas, F.; Molina, E.; Sanabria, D. Sport participation and vigilance in children: Influence of different sport expertise. J. Sport Health Sci. 2018, 7, 497–504. [Google Scholar] [CrossRef]
- Lambourne, K.; Tomporowski, P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. J. Brain Res. 2010, 1341, 12–24. [Google Scholar] [CrossRef]
- McLellan, T.M.; Kamimori, G.H.; Voss, D.M.; Bell, D.G.; Cole, K.G.; Johnson, D. Caffeine maintains vigilance and improves run times during night operations for Special Forces. Aviat. Space. Environ. Med. 2005, 76, 647–654. [Google Scholar]
- Vangkilde, S.; Bundesen, C.; Coull, J.T. Prompt but inefficient: Nicotine differentially modulates discrete components of attention. Psychopharmacology 2011, 218, 667–680. [Google Scholar] [CrossRef]
- Lieberman, H.R.; Falco, C.M.; Slade, S.S. Carbohydrate administration during a day of sustained aerobic activity improves vigilance, as assessed by a novel ambulatory monitoring device, and mood. Am. J. Clin. Nutr. 2002, 76, 120–127. [Google Scholar] [CrossRef]
- Hirano, Y.; Onozuka, M. Chewing and attention: A positive effect on sustained attention. BioMed Res. Int. 2015, 2015, 6. [Google Scholar] [CrossRef]
- Johnson, A. Cognitive facilitation following intentional odor exposure. Sensors 2011, 11, 5469–5488. [Google Scholar] [CrossRef]
- Matsubara, E.; Fukagawa, M.; Okamoto, T.; Fukuda, A.; Hayashi, C.; Ohnuki, K.; Shimizu, K.; Kondo, R. Volatiles emitted from the leaves of Laurus nobilis L. improve vigilance performance in visual discrimination task. Biomed. Res. 2011, 32, 19–28. [Google Scholar] [CrossRef]
- Giurgea, C.E. The nootropic concept and its prospective implications. Drug Dev. Res. 1982, 2, 441–446. [Google Scholar] [CrossRef]
- Szalma, J.; Daly, T.; Teo, G.; Hancock, G.; Hancock, P. Training for vigilance on the move: A video game-based paradigm for sustained attention. J. Ergon. 2018, 61, 482–505. [Google Scholar] [CrossRef]
- Szalma, J.L.; Schmidt, T.; Teo, G.; Hancock, P.A. Vigilance on the move: Video game-based measurement of sustained attention. J. Ergon. 2014, 57, 1315–1336. [Google Scholar] [CrossRef]
- Löffler, B.S.; Stecher, H.I.; Fudickar, S.; Sordi, D.D.; Otto-Sobotka, F.; Hein, A.; Herrmann, C.S. Counteracting the Slowdown of Reaction Times in a Vigilance Experiment With 40-Hz Transcranial Alternating Current Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 2053–2061. [Google Scholar] [CrossRef]
- Polanía, R.; Nitsche, M.A.; Korman, C.; Batsikadze, G.; Paulus, W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr. Biol. 2012, 22, 1314–1318. [Google Scholar] [CrossRef]
- Santarnecchi, E.; Polizzotto, N.R.; Godone, M.; Giovannelli, F.; Feurra, M.; Matzen, L.; Rossi, A.; Rossi, S. Frequency-dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Curr. Biol. 2013, 23, 1449–1453. [Google Scholar] [CrossRef]
- Annarumma, L.; D’Atri, A.; Alfonsi, V.; De Gennaro, L. The Efficacy of Transcranial Current Stimulation Techniques to Modulate Resting-State EEG, to Affect Vigilance and to Promote Sleepiness. J. Brain Sci. 2018, 8, 137. [Google Scholar] [CrossRef]
- Nelson, J.T.; McKinley, R.A.; Golob, E.J.; Warm, J.S.; Parasuraman, R. Enhancing vigilance in operators with prefrontal cortex transcranial direct current stimulation (tDCS). Neuroimage 2014, 85, 909–917. [Google Scholar] [CrossRef]
- McIntire, L.K.; McKinley, R.A.; Goodyear, C.; Nelson, J. A comparison of the effects of transcranial direct current stimulation and caffeine on vigilance and cognitive performance during extended wakefulness. Brain Stimul. 2014, 7, 499–507. [Google Scholar] [CrossRef]
- Hill, P. Tactile Based Performance Enhancement System. U.S. Patent 8,552,847, 8 October 2013. [Google Scholar]
- McBride, S.A.; Johnson, R.F.; Merullo, D.J.; Bartow, R.E., Jr. Effects of the periodic administration of odor or vibration on a 3-hr. vigilance task. Percept. Mot. Ski. 2004, 98, 307–318. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, D.; Afzal, N.; Zhang, Y.; Wu, R. Rhythmic haptic stimuli improve short-term attention. IEEE Trans. Haptics 2016, 9, 437–442. [Google Scholar] [CrossRef]
- Arrabito, G.R.; Ho, G.; Aghaei, B.; Burns, C.; Hou, M. Sustained attention in auditory and visual monitoring tasks: Evaluation of the administration of a rest break or exogenous vibrotactile signals. J. Hum. Factors 2015, 57, 1403–1416. [Google Scholar] [CrossRef]
- Bodala, I.P.; Li, J.; Thakor, N.V.; Al-Nashash, H. EEG and eye tracking demonstrate vigilance enhancement with challenge integration. Front. Hum. Neurosci. 2016, 10, 273. [Google Scholar] [CrossRef]
- Gupta, A.; Bhushan, B.; Behera, L. Short-term enhancement of cognitive functions and music: A three-channel model. Sci. Rep. 2018, 8, 15528. [Google Scholar] [CrossRef]
- Wolfe, D.E.; Noguchi, L.K. The use of music with young children to improve sustained attention during a vigilance task in the presence of auditory distractions. J. Music Ther. 2009, 46, 69–82. [Google Scholar] [CrossRef]
- Lane, J.D.; Kasian, S.J.; Owens, J.E.; Marsh, G.R. Binaural auditory beats affect vigilance performance and mood. Physiol. Behav. 1998, 63, 249–252. [Google Scholar] [CrossRef]
- Frederick, J.A.; Lubar, J.F.; Rasey, H.W.; Brim, S.A.; Blackburn, J. Effects of 18.5 Hz auditory and visual stimulation on EEG amplitude at the vertex. J. Neurother. 1999, 3, 23–28. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Wesensten, N.; Belenky, G.; Kautz, M.A.; Thorne, D.R.; Reichardt, R.M.; Balkin, T. Maintaining alertness and performance during sleep deprivation: Modafinil versus caffeine. Psychopharmacology 2002, 159, 238–247. [Google Scholar] [CrossRef]
- Turner, D.C.; Robbins, T.W.; Clark, L.; Aron, A.R.; Dowson, J.; Sahakian, B. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology 2003, 165, 260–269. [Google Scholar] [CrossRef]
- Randall, D.C.; Shneerson, J.M.; File, S.E. Cognitive effects of modafinil in student volunteers may depend on IQ. Pharmacol. Biochem. Behav. 2005, 82, 133–139. [Google Scholar] [CrossRef]
- Randall, D.C.; Viswanath, A.; Bharania, P.; Elsabagh, S.M.; Hartley, D.E.; Shneerson, J.M.; File, S.E. Does modafinil enhance cognitive performance in young volunteers who are not sleep-deprived? J. Clin. Psychopharmacol. 2005, 25, 175–179. [Google Scholar] [CrossRef]
- Dean, A.C.; Sevak, R.J.; Monterosso, J.R.; Hellemann, G.; Sugar, C.A.; London, E.D. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J. Stud. Alcohol Drugs 2011, 72, 943–953. [Google Scholar] [CrossRef]
- Green, C.S.; Bavelier, D. Action video game modifies visual selective attention. Nature 2003, 423, 534. [Google Scholar] [CrossRef]
- Dye, M.W.; Bavelier, D. Differential development of visual attention skills in school-age children. J. Vis. Res. 2010, 50, 452–459. [Google Scholar] [CrossRef]
- Teo, G.W.; Schmidt, T.N.; Szalma, J.L.; Hancock, G.M.; Hancock, P.A. The effect of knowledge of results for training vigilance in a video game-based environment. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2016, 1421–1425. [Google Scholar] [CrossRef]
- Anguera, J.A.; Boccanfuso, J.; Rintoul, J.L.; Al-Hashimi, O.; Faraji, F.; Janowich, J.; Kong, E.; Larraburo, Y.; Rolle, C.; Johnston, E. Video game training enhances cognitive control in older adults. J. Nat. 2013, 501, 97. [Google Scholar] [CrossRef]
- Schmidt, T.N.; Teo, G.W.; Hancock, G.M.; Amicarelle, Z.; Szalma, J.L.; Hancock, P.A. Action video game players and vigilance performance. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2013, 57, 1450–1454. [Google Scholar] [CrossRef]
- Plewnia, C.; Zwissler, B.; Längst, I.; Maurer, B.; Giel, K.; Krüger, R. Effects of transcranial direct current stimulation (tDCS) on executive functions: Influence of COMT Val/Met polymorphism. Cortex 2013, 49, 1801–1807. [Google Scholar] [CrossRef]
- Mauri, P.; Miniussi, C.; Balconi, M.; Brignani, D. Bursts of transcranial electrical stimulation increase arousal in a continuous performance test. Neuropsychologia 2015, 74, 127–136. [Google Scholar] [CrossRef]
- Miller, J.; Berger, B.; Sauseng, P. Anodal transcranial direct current stimulation (tDCS) increases frontal–midline theta activity in the human EEG: A preliminary investigation of non-invasive stimulation. Neurosci. Lett. 2015, 588, 114–119. [Google Scholar] [CrossRef]
- Hsu, W.-Y.; Zanto, T.P.; Anguera, J.A.; Lin, Y.-Y.; Gazzaley, A. Delayed enhancement of multitasking performance: Effects of anodal transcranial direct current stimulation on the prefrontal cortex. Cortex 2015, 69, 175–185. [Google Scholar] [CrossRef]
- Manor, B.; Zhou, J.; Harrison, R.; Lo, O.-Y.; Travison, T.G.; Hausdorff, J.M.; Pascual-Leone, A.; Lipsitz, L. Transcranial Direct Current Stimulation May Improve Cognitive-Motor Function in Functionally Limited Older Adults. Neurorehabilit. Neural Repair 2018, 32, 788–798. [Google Scholar] [CrossRef]
- Huang, R.-H.; Shih, Y.-N. Effects of background music on concentration of workers. J. Work. 2011, 38, 383–387. [Google Scholar]
- Johansson, R.; Holmqvist, K.; Mossberg, F.; Lindgren, M. Eye movements and reading comprehension while listening to preferred and non-preferred study music. Psychol. Music 2012, 40, 339–356. [Google Scholar] [CrossRef]
- Shih, Y.-N.; Huang, R.-H.; Chiang, H.-Y. Background music: Effects on attention performance. J. Work 2012, 42, 573–578. [Google Scholar]
- Mori, F.; Naghsh, F.A.; Tezuka, T. The Effect of Music on the Level of Mental Concentration and its Temporal Change. Proc. CSEDU 2014, 1, 34–42. [Google Scholar]
- Baldwin, C.L.; Lewis, B.A. Positive valence music restores executive control over sustained attention. J. PLoS ONE 2017, 12, e0186231. [Google Scholar] [CrossRef]
- Goodin, P.; Ciorciari, J.; Baker, K.; Carrey, A.-M.; Harper, M.; Kaufman, J. A high-density EEG investigation into steady state binaural beat stimulation. PLoS ONE 2012, 7, e34789. [Google Scholar] [CrossRef]
- Arrabito, G.R.; Ho, G.; Aghaei, B.; Burns, C.; Hou, M. Effects of vibrotactile stimulation for sustaining performance in a vigilance task: A pilot study. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2011, 55, 1160–1164. [Google Scholar] [CrossRef]
- Koelega, H. Stimulant drugs and vigilance performance: A review. J. Psychopharmacol. 1993, 111, 1–16. [Google Scholar] [CrossRef]
- Husain, M.; Mehta, M.A. Cognitive enhancement by drugs in health and disease. Trends Cogn. Sci. 2011, 15, 28–36. [Google Scholar] [CrossRef]
- Greely, H.; Sahakian, B.; Harris, J.; Kessler, R.C.; Gazzaniga, M.; Campbell, P.; Farah, M. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature 2008, 456, 702. [Google Scholar] [CrossRef]
- Kim, D. Practical use and risk of modafinil, a novel waking drug. Environ. Health Toxicology 2012, 27, e2012007. [Google Scholar] [CrossRef]
- Repantis, D.; Schlattmann, P.; Laisney, O.; Heuser, I. Modafinil and methylphenidate for neuroenhancement in healthy individuals: A systematic review. Pharmacol. Res. 2010, 62, 187–206. [Google Scholar] [CrossRef]
- Caldwell, J.A.; Caldwell, J.L.; Smith, J.K.; Brown, D.L. Modafinil′s effects on simulator performance and mood in pilots during 37 h without sleep. J. Aviat. Space Environ. Med. 2004, 75, 777–784. [Google Scholar]
- Caldwell, J.A.; Caldwell, J.L.; Smyth, N.K.; Hall, K.K. A double-blind, placebo-controlled investigation of the efficacy of modafinil for sustaining the alertness and performance of aviators: A helicopter simulator study. J. Psychopharmacol. 2000, 150, 272–282. [Google Scholar] [CrossRef]
- Estrada, A.; Kelley, A.M.; Webb, C.M.; Athy, J.R.; Crowley, J.S. Modafinil as a replacement for dextroamphetamine for sustaining alertness in military helicopter pilots. J. Aviat. Space Environ. Med. 2012, 83, 556–567. [Google Scholar] [CrossRef]
- Talih, F.; Ajaltouni, J. Probable Nootropicinduced Psychiatric Adverse Effects: A Series of Four Cases. Innov. Clin. Neurosci. 2015, 12, 21. [Google Scholar]
- Green, C.S.; Bavelier, D. Effect of action video games on the spatial distribution of visuospatial attention. J. Exp. Psychol. 2006, 32, 1465. [Google Scholar] [CrossRef]
- Green, C.S.; Bavelier, D. Learning, attentional control, and action video games. Curr. Biol. 2012, 22, R197–R206. [Google Scholar] [CrossRef]
- Achtman, R.L.; Green, C.S.; Bavelier, D. Video games as a tool to train visual skills. J. Restor. Neurol. Neurosci. 2008, 26, 435–446. [Google Scholar]
- Oei, A.C.; Patterson, M.D. Are videogame training gains specific or general? Front. Syst. Neurosci. 2014, 8, 54. [Google Scholar] [CrossRef]
- Cardoso-Leite, P.; Bavelier, D. Video game play, attention, and learning: How to shape the development of attention and influence learning? J. Curr. Opin. Neurol. 2014, 27, 185–191. [Google Scholar] [CrossRef]
- Bediou, B.; Adams, D.M.; Mayer, R.E.; Tipton, E.; Green, C.S.; Bavelier, D. Meta-analysis of action video game impact on perceptual, attentional, and cognitive skills. J. Psychol. Bull. 2018, 144, 77. [Google Scholar] [CrossRef]
- Trick, L.M.; Jaspers-Fayer, F.; Sethi, N. Multiple-object tracking in children: The “Catch the Spies” task. Cogn. Dev. 2005, 20, 373–387. [Google Scholar] [CrossRef]
- Latham, A.J.; Patston, L.L.; Tippett, L. Just how expert are “expert” video-game players? Assessing the experience and expertise of video-game players across “action” video-game genres. Front. Psychol. 2013, 4, 941. [Google Scholar] [CrossRef][Green Version]
- Kluger, A.N.; DeNisi, A. The effects of feedback interventions on performance: A historical review, a meta-analysis, and a preliminary feedback intervention theory. Psychol. Bull. 1996, 119, 254. [Google Scholar] [CrossRef]
- Dittmar, M.L. Effects of Knowledge of Results on Performance in Successive and Simultaneous Vigilance Tasks: A Signal Detection Analysis. Ph.D. Thesis, University of Cincinnati, Cincinnati, OH, USA, 1984. [Google Scholar]
- Hitchcock, E.M.; Dember, W.N.; Warm, J.S.; Moroney, B.W.; See, J.E. Effects of cueing and knowledge of results on workload and boredom in sustained attention. Hum. Factors 1999, 41, 365–372. [Google Scholar] [CrossRef]
- Teo, G.W.; Schmidt, T.N.; Szalma, J.L.; Hancock, G.M.; Hancock, P.A. The effects of feedback in vigilance training on performance, workload, stress and coping. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2013, 57, 1119–1123. [Google Scholar] [CrossRef]
- Castel, A.D.; Pratt, J.; Drummond, E. The effects of action video game experience on the time course of inhibition of return and the efficiency of visual search. J. Acta Psychol. 2005, 119, 217–230. [Google Scholar] [CrossRef]
- Becker, A.B.; Warm, J.S.; Dember, W.N. Specific and nonspecific transfer effects in training for vigilance. In Human Performance in Automated Systems. Current Trends; Mouloua, M., Parasuraman, R., Eds.; Erlbaum: Hillsdale, MI, USA, 1994; pp. 294–299. [Google Scholar]
- Helton, W.S.; Shaw, T.; Warm, J.S.; Matthews, G.; Hancock, P. Effects of warned and unwarned demand transitions on vigilance performance and stress. Anxiety Stress Coping 2008, 21, 173–184. [Google Scholar] [CrossRef]
- Warm, J.S.; Finomore, V.; Shaw, T.H.; Funke, M.E.; Hausen, M.J.; Matthews, G.; Taylor, P.; Vidulich, M.A.; Repperger, D.W.; Szalma, J.L. Effects of training with knowledge of results on diagnosticity in vigilance performance. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2009, 53, 1066–1070. [Google Scholar] [CrossRef]
- Szalma, J.L.; Hancock, P.A.; Dember, W.N.; Warm, J.S. Training for vigilance: The effect of knowledge of results format and dispositional optimism and pessimism on performance and stress. Br. J. Psychol. 2006, 97, 115–135. [Google Scholar] [CrossRef]
- Schmidt, T.N.; Teo, G.W.; Szalma, J.L.; Hancock, G.M.; Hancock, P.A. The effect of video game play on performance in a vigilance task. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2012, 56, 1544–1547. [Google Scholar] [CrossRef]
- Narra, M.; Mathew, B. Developmental differences on cognitive inhibition in children and adults: Evidence from vigilance task. Int. J. Brain Cogn. Sci. 2012, 1, 1–5. [Google Scholar] [CrossRef]
- Levy, F. The development of sustained attention (vigilance) and inhibition in children: Some normative data. J. Child Psychol. Psychiatry 1980, 21, 77–84. [Google Scholar] [CrossRef]
- Giambra, L.M.; Quilter, R.E. Sex differences in sustained attention across the adult life span. J. Appl. Psychol. 1989, 74, 91. [Google Scholar] [CrossRef]
- Feng, J.; Spence, I.; Pratt, J. Playing an action video game reduces gender differences in spatial cognition. J. Psychol. Sci. 2007, 18, 850–855. [Google Scholar] [CrossRef]
- Bikson, M.; Datta, A.; Elwassif, M. Establishing safety limits for transcranial direct current stimulation. J. Clin. Neurophysiol. 2009, 120, 1033–1034. [Google Scholar] [CrossRef]
- Arul-Anandam, A.P.; Loo, C. Transcranial direct current stimulation: A new tool for the treatment of depression? J. Affect. Disord. 2009, 117, 137–145. [Google Scholar] [CrossRef]
- Paulus, W. Outlasting excitability shifts induced by direct current stimulation of the human brain. In Supplements to Clinical Neurophysiology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 708–714. [Google Scholar]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- McKinley, R.A.; Bridges, N.; Walters, C.M.; Nelson, J. Modulating the brain at work using noninvasive transcranial stimulation. Neuroimage 2012, 59, 129–137. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Boggio, P.S.; Fregni, F.; Pascual-Leone, A. Treatment of depression with transcranial direct current stimulation (tDCS): A review. Exp. Neurol. 2009, 219, 14–19. [Google Scholar] [CrossRef]
- Mehta, R.K.; Parasuraman, R. Neuroergonomics: A review of applications to physical and cognitive work. Front. Hum. Neurosci. 2013, 7, 889. [Google Scholar] [CrossRef]
- Schuijer, J.W.; Jong, I.M.D.; Kupper, F.; Atteveldt, N.M.V. Transcranial Electrical Stimulation to Enhance Cognitive Performance of Healthy Minors: A Complex Governance Challenge. Front. Hum. Neurosci. 2017, 11, 142. [Google Scholar] [CrossRef]
- Elmasry, J.; Loo, C.; Martin, D. A systematic review of transcranial electrical stimulation combined with cognitive training. J. Restor. Neurol. Neurosci. 2015, 33, 263–278. [Google Scholar] [CrossRef]
- Simonsmeier, B.A.; Grabner, R.H.; Hein, J.; Krenz, U.; Schneider, M. Electrical brain stimulation (tES) improves learning more than performance: A meta-analysis. Neurosci. Biobehav. Rev. 2018, 84, 171–181. [Google Scholar] [CrossRef]
- Cruz Gonzalez, P.; Fong, K.N.; Brown, T. The Effects of Transcranial Direct Current Stimulation on the Cognitive Functions in Older Adults with Mild Cognitive Impairment: A Pilot Study. J. Behav. Neurol. 2018, 2018, 14. [Google Scholar] [CrossRef]
- Axelrod, V.; Rees, G.; Lavidor, M.; Bar, M. Increasing propensity to mind-wander with transcranial direct current stimulation. Proc. Natl. Acad. Sci. USA 2015, 112, 3314–3319. [Google Scholar] [CrossRef]
- Cohen, R.K.; Soskic, S.; Iuculano, T.; Kanai, R.; Walsh, V. Modulating neuronal activity produces specific and long-lasting changes in numerical competence. J. Curr. Biol. Cell Press 2010, 20, 2016–2020. [Google Scholar] [CrossRef]
- Li, L.M.; Leech, R.; Scott, G.; Malhotra, P.; Seemungal, B.; Sharp, D. The effect of oppositional parietal transcranial direct current stimulation on lateralized brain functions. Eur. J. Neurosci. 2015, 42, 2904–2914. [Google Scholar] [CrossRef]
- Nieratschker, V.; Kiefer, C.; Giel, K.; Krüger, R.; Plewnia, C. The COMT Val/Met polymorphism modulates effects of tDCS on response inhibition. Brain Stimul. 2015, 8, 283–288. [Google Scholar] [CrossRef]
- Matthews, G.; Warm, J.S.; Reinerman, L.E.; Langheim, L.K.; Saxby, D.J. Task engagement, attention, and executive control. In Handbook of Individual Differences in Cognition; Springer: Berlin/Heidelberg, Germany, 2010; pp. 205–230. [Google Scholar]
- Reteig, L.; Talsma, L.; van Schouwenburg, M.; Slagter, H. Transcranial electrical stimulation as a tool to enhance attention. J. Cogn. Enhanc. 2017, 1, 10–25. [Google Scholar] [CrossRef]
- Hsu, T.-Y.; Juan, C.-H.; Tseng, P. Individual differences and state-dependent responses in transcranial direct current stimulation. Front. Hum. Neurosci. 2016, 10, 643. [Google Scholar] [CrossRef]
- Sloboda, J.A.; O’Neill, S.A.; Ivaldi, A. Functions of music in everyday life: An exploratory study using the Experience Sampling Method. Musicae Sci. 2001, 5, 9–32. [Google Scholar] [CrossRef]
- Rickard, N.S.; Toukhsati, S.R.; Field, S.E. The effect of music on cognitive performance: Insight from neurobiological and animal studies. Behav. Cogn. Neurosci. Rev. 2005, 4, 235–261. [Google Scholar] [CrossRef]
- Wokoun, W. Vigilance with Background Music; Human Engineering Lab: Aberdeen Proving Ground, MD, USA, 1963. [Google Scholar]
- Davies, D.; Lang, L.; Shackleton, V. The effects of music and task difficulty on performance at a visual vigilance task. J. Br. J. Psychol. 1973, 64, 383–389. [Google Scholar] [CrossRef]
- Scheufele, P.M. Effects of progressive relaxation and classical music on measurements of attention, relaxation, and stress responses. J. Behav. Med. 2000, 23, 207–228. [Google Scholar] [CrossRef]
- Corhan, C.M.; Gounard, B.R. Types of music, schedules of background stimulation, and visual vigilance performance. J. Percept. Mot. Ski. 1976, 42, 662. [Google Scholar] [CrossRef]
- Wolf, R.H.; Weiner, F.F. Effects of four noise conditions on arithmetic performance. Percept. Mot. Ski. 1972, 35, 928–930. [Google Scholar] [CrossRef]
- Fontaine, C.W.; Schwalm, N.D. Effects of familiarity of music on vigilant performance. J. Percept. Mot. Ski. 1979, 49, 71–74. [Google Scholar] [CrossRef]
- Dorney, L.; Goh, E.K.M.; Lee, C. The impact of music and imagery on physical performance and arousal: Studies of coordination and endurance. J. Sport Behav. 1992, 15, 21. [Google Scholar]
- Ünal, A.B.; Steg, L.; Epstude, K. The influence of music on mental effort and driving performance. Accid. Anal. Prev. 2012, 48, 271–278. [Google Scholar] [CrossRef]
- Konz, S.; Mcdougal, D. The effect of background music on the control activity of an automobile driver. Hum. Factors 1968, 10, 233–243. [Google Scholar] [CrossRef]
- Soto, D.; Funes, M.J.; Guzmán-García, A.; Warbrick, T.; Rotshtein, P.; Humphreys, G.W. Pleasant music overcomes the loss of awareness in patients with visual neglect. Proc. Natl. Acad. Sci. USA 2009, 106, 6011–6016. [Google Scholar] [CrossRef]
- Perham, N.; Withey, T. Liked music increases spatial rotation performance regardless of tempo. Curr. Psychol. 2012, 31, 168–181. [Google Scholar] [CrossRef]
- Dalton, B.H.; Behm, D.G.; Kibele, A. Effects of sound types and volumes on simulated driving, vigilance tasks and heart rate. J. Occup. Ergon. 2007, 7, 153–168. [Google Scholar]
- Brodsky, W. The effects of music tempo on simulated driving performance and vehicular control. J. Transp. Res. Part F Traffic Psychol. Behav. 2001, 4, 219–241. [Google Scholar] [CrossRef]
- Beh, H.C.; Hirst, R. Performance on driving-related tasks during music. J. Ergon. 1999, 42, 1087–1098. [Google Scholar] [CrossRef]
- Nittono, H.; Tsuda, A.; Akai, S.; Nakajima, Y. Tempo of background sound and performance speed. Percept. Mot. Ski. 2000, 90, 1122. [Google Scholar] [CrossRef]
- Moris, D.N.; Linos, D. Music meets surgery: Two sides to the art of “healing”. Surg. Endosc. 2013, 27, 719–723. [Google Scholar] [CrossRef]
- Jaušovec, N.; Habe, K. The “Mozart effect”: An electroencephalographic analysis employing the methods of induced event-related desynchronization/synchronization and event-related coherence. Brain Topogr. 2003, 16, 73–84. [Google Scholar] [CrossRef]
- Verrusio, W.; Ettorre, E.; Vicenzini, E.; Vanacore, N.; Cacciafesta, M.; Mecarelli, O. The Mozart effect: A quantitative EEG study. Conscious. Cogn. 2015, 35, 150–155. [Google Scholar] [CrossRef]
- Penn, P.E.; Bootzin, R. Behavioural techniques for enhancing alertness and performance in shift work. J. Work 1990, 4, 213–226. [Google Scholar] [CrossRef]
- Lesiuk, T. The effect of music listening on work performance. Psychol. Music 2005, 33, 173–191. [Google Scholar] [CrossRef]
- Alikonis, C.R.; Warm, J.S.; Matthews, G.; Dember, W.N.; Hitchcock, E.M.; Kellaris, J.J. Vigilance, workload, and boredom: Two competing models. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2002, 46, 1531–1535. [Google Scholar] [CrossRef]
- Furnham, A.; Strbac, L. Music is as distracting as noise: The differential distraction of background music and noise on the cognitive test performance of introverts and extraverts. J. Ergon. 2002, 45, 203–217. [Google Scholar] [CrossRef]
- Miller, L.K.; Schyb, M. Facilitation and interference by background music. J. Music Ther. 1989, 26, 42–54. [Google Scholar] [CrossRef]
- Kämpfe, J.; Sedlmeier, P.; Renkewitz, F. The impact of background music on adult listeners: A meta-analysis. Psychol. Music 2011, 39, 424–448. [Google Scholar] [CrossRef]
- Treisman, A. How the deployment of attention determines what we see. Vis. Cogn. 2006, 14, 411–443. [Google Scholar] [CrossRef]
- Lesiuk, T. The effect of preferred music listening on stress levels of air traffic controllers. Arts Psychother. 2008, 35, 1–10. [Google Scholar] [CrossRef]
- Vernon, D. Human Potential: Exploring Techniques Used to Enhance Human Performance; Routledge: London, UK, 2009. [Google Scholar]
- Reedijk, S.A.; Bolders, A.; Colzato, L.S.; Hommel, B. Eliminating the attentional blink through binaural beats: A case for tailored cognitive enhancement. Front. Psychiatry 2015, 6, 82. [Google Scholar] [CrossRef]
- Beauchene, C.; Abaid, N.; Moran, R.; Diana, R.A.; Leonessa, A. The effect of binaural beats on visuospatial working memory and cortical connectivity. PLoS ONE 2016, 11, e0166630. [Google Scholar] [CrossRef]
- Draganova, R.; Ross, B.; Wollbrink, A.; Pantev, C. Cortical steady-state responses to central and peripheral auditory beats. J. Cereb. Cortex 2007, 18, 1193–1200. [Google Scholar] [CrossRef]
- Jirakittayakorn, N.; Wongsawat, Y. Brain responses to 40-Hz binaural beat and effects on emotion and memory. Int. J. Psychophysiol. 2017, 120, 96–107. [Google Scholar] [CrossRef]
- Colzato, L.S.; Barone, H.; Sellaro, R.; Hommel, B. More attentional focusing through binaural beats: Evidence from the global–local task. J. Psychol. Res. 2017, 81, 271–277. [Google Scholar] [CrossRef]
- Gaffary, Y.; Lécuyer, A. The Use of Haptic and Tactile Information in the Car to Improve Driving Safety: A Review of Current Technologies. J. Front. ICT 2018, 5, 5. [Google Scholar] [CrossRef]
- Kalisch, T.; Kattenstroth, J.-C.; Kowalewski, R.; Tegenthoff, M.; Dinse, H.R. Cognitive and tactile factors affecting human haptic performance in later life. PLoS ONE 2012, 7, e30420. [Google Scholar] [CrossRef]
- Barone, J.J.; Roberts, H.R. Caffeine consumption. J. Food Chem. Toxicol. 1996, 34, 119–129. [Google Scholar] [CrossRef]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and physical performance enhancer or psychoactive drug? J. Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef]
- Porciúncula, L.; Ardais, A.; Espinosa, J.; Mioranzza, S.; Rocha, A.; Sallaberry, C. The Janus face of caffeine: s04–02. J. Neurochem. 2013, 125, 17. [Google Scholar]
- Kamimori, G.H.; Johnson, D.; Thorne, D.; Belenky, G. Multiple caffeine doses maintain vigilance during early morning operations. Aviat. Space Environ. Med. 2005, 76, 1046–1050. [Google Scholar]
- Frewer, L.; Lader, M. The effects of caffeine on two computerized tests of attention and vigilance. Hum. Psychopharmacol. Clin. Exp. 1991, 6, 119–128. [Google Scholar] [CrossRef]
- Lane, J.D.; Phillips-Bute, B.G. Caffeine deprivation affects vigilance performance and mood. Physiol. Behav. 1998, 65, 171–175. [Google Scholar] [CrossRef]
- Lieberman, H.; Wurtman, R.; Emde, G.; Roberts, C.; Coviella, I. The effects of low doses of caffeine on human performance and mood. Psychopharmacology 1987, 92, 308–312. [Google Scholar] [CrossRef]
- Clubley, M.; Bye, C.; Henson, T.; Peck, A.; Riddington, C. Effects of caffeine and cyclizine alone and in combination on human performance, subjective effects and EEG activity. Br. J. Clin. Pharm. 1979, 7, U57–U63. [Google Scholar] [CrossRef][Green Version]
- Kamimori, G.H.; McLellan, T.M.; Tate, C.M.; Voss, D.M.; Niro, P.; Lieberman, H.R. Caffeine improves reaction time, vigilance and logical reasoning during extended periods with restricted opportunities for sleep. J. Psychopharmacol. 2015, 232, 2031–2042. [Google Scholar] [CrossRef]
- Kawamura, N.; Maeda, H.; Nakamura, J.; Morita, K.; Nakazawa, Y. Effects of caffeine on event-related potentials: Comparison of oddball with single-tone paradigms. Psychiatry Clin. Neurosci. 1996, 50, 217–221. [Google Scholar] [CrossRef]
- Fine, B.J.; Kobrick, J.L.; Lieberman, H.R.; Marlowe, B.; Riley, R.H.; Tharion, W. Effects of caffeine or diphenhydramine on visual vigilance. J. Psychopharmacol. 1994, 114, 233–238. [Google Scholar] [CrossRef]
- Amendola, C.; Gabrieli, J.; Lieberman, H. Caffeine’s effects on performance and mood are independent of age and gender. J. Nutr. Neurosci. 1998, 1, 269–280. [Google Scholar] [CrossRef]
- Lanini, J.; Galduróz, J.C.F.; Pompéia, S. Acute personalized habitual caffeine doses improve attention and have selective effects when considering the fractionation of executive functions. Hum. Psychopharmacol. Clin. Exp. 2016, 31, 29–43. [Google Scholar] [CrossRef]
- Reyner, L.A.; Horne, J.A. Early morning driver sleepiness: Effectiveness of 200 mg caffeine. J. Psychophysiol. 2000, 37, 251–256. [Google Scholar] [CrossRef]
- Kilpeläinen, A.A.; Huttunen, K.H.; Lohi, J.J.; Lyytinen, H. Effect of caffeine on vigilance and cognitive performance during extended wakefulness. Int. J. Aviat. Psychol. 2010, 20, 144–159. [Google Scholar] [CrossRef]
- Doan, B.K.; Hickey, P.A.; Lieberman, H.R.; Fischer, J.R. Caffeinated tube food effect on pilot performance during a 9-hour, simulated nighttime U-2 mission. J. Aviat. Space Environ. Med. 2006, 77, 1034–1040. [Google Scholar]
- McLellan, T.M.; Kamimori, G.H.; Bell, D.G.; Smith, I.F.; Johnson, D.; Belenky, G. Caffeine maintains vigilance and marksmanship in simulated urban operations with sleep deprivation. Aviat. Space Environ. Med. 2005, 76, 39–45. [Google Scholar]
- Nikić, P.M.; Andrić, B.R.; Stojimirović, B.B.; Trbojevic-Stanković, J.; Bukumirić, Z. Habitual coffee consumption enhances attention and vigilance in hemodialysis patients. BioMed Res. Int. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The safety of ingested caffeine: A comprehensive review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. J. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar] [CrossRef]
- Lee, D.R.; Lee, J.; Rota, M.; Lee, J.; Ahn, H.S.; Park, S.M.; Shin, D. Coffee consumption and risk of fractures: A systematic review and dose–response meta-analysis. J. Bone 2014, 63, 20–28. [Google Scholar] [CrossRef]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef]
- Ruxton, C. The impact of caffeine on mood, cognitive function, performance and hydration: A review of benefits and risks. Nutr. Bull. 2008, 33, 15–25. [Google Scholar] [CrossRef]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef]
- Nehlig, A. Is caffeine a cognitive enhancer? J. Alzheimer’s Dis. 2010, 20, S85–S94. [Google Scholar] [CrossRef]
- Snel, J.; Lorist, M.M.; Tieges, Z. Coffee, caffeine, and cognitive performance. Coffee Tea Choc. Brain 2004, 2, 53–67. [Google Scholar]
- Fillmore, M.T. Invistigating the behavioral effects of caffeine: The contribution of drug-related expectancies. Pharmacopsychoecologia. 1994, 7, 63–73. [Google Scholar]
- Nehlig, A.; Daval, J.-L.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef]
- Goldstein, A.; Warren, R.; Kaizer, S. Psychotropic effects of caffeine in man. I. Individual differences in sensitivity to caffeine-induced wakefulness. J. Pharmacol. Exp. Ther. 1965, 149, 156–159. [Google Scholar]
- Evans, S.M.; Griffiths, R.R. Caffeine tolerance and choice in humans. Psychopharmacology 1992, 108, 51–59. [Google Scholar] [CrossRef]
- Fillmore, M.T.; Vogel-Sprott, M. Psychomotor performance under alcohol and under caffeine: Expectancy and pharmacological effects. Exp. Clin. Psychopharmacol. 1994, 2, 319. [Google Scholar] [CrossRef]
- Warm, J.S.; Dember, W.N.; Parasuraman, R. Effects of olfactory stimulation on performance and stress. J. Soc. Cosmet. Chem. 1991, 42, 199–210. [Google Scholar]
- Baron, R.A.; Thomley, J. A whiff of reality: Positive affect as a potential mediator of the effects of pleasant fragrances on task performance and helping. J. Environ. Behav. 1994, 26, 766–784. [Google Scholar] [CrossRef]
- Baron, R.A.; Kalsher, M. Effects of a pleasant ambient fragrance on simulated driving performance: The sweet smell of safety? J. Environ. Behav. 1998, 30, 535–552. [Google Scholar] [CrossRef]
- Milotic, D. The impact of fragrance on consumer choice. J. Consum. Behav. Int. Res. Rev. 2003, 3, 179–191. [Google Scholar] [CrossRef]
- Field, T.; Diego, M.; Hernandez-Reif, M.; Cisneros, W.; Feijo, L.; Vera, Y.; Gil, K.; Grina, D.; Claire He, Q. Lavender fragrance cleansing gel effects on relaxation. Int. J. Neurosci. 2005, 115, 207–222. [Google Scholar] [CrossRef]
- Heuberger, E.; Ilmberger, J. The influence of essential oils on human vigilance. Nat. Prod. Commun. 2010, 5, 1441–1446. [Google Scholar] [CrossRef]
- Sullivan, T.E.; Warm, J.S.; Schefft, B.K.; Dember, W.N.; O’Dell, M.W.; Peterson, S. Effects of olfactory stimulation on the vigilance performance of individuals with brain injury. J. Clin. Exp. Neuropsychol. 1998, 20, 227–236. [Google Scholar] [CrossRef]
- Parasuraman, R.; Warm, J.; Dember, W. Effects of Olfactory Stimulation on Skin Conductance and Event-Related Potentials during Visual Sustained Attention; Progress Report No. 6. Submitted to the Fragrance Research Fund, Ltd.; Fragrance Research Fund, Ltd.: New York, NY, USA, 1992. [Google Scholar]
- Raudenbush, B.; Grayhem, R.; Sears, T.; Wilson, I. Effects of Peppermint and Cinnamon Odor Administration on Simulated Driving Alertness, Mood and Workload. N. Am. J. Psychol. 2009, 11, 245–256. [Google Scholar]
- Herz, R. Influences of odors on mood and affective cognition. Olfaction Tast. Cogn. 2002, 160, 177. [Google Scholar]
- Barker, S.; Grayhem, P.; Koon, J.; Perkins, J.; Whalen, A.; Raudenbush, B. Improved performance on clerical tasks associated with administration of peppermint odor. J. Percept. Mot. Ski. 2003, 97, 1007–1010. [Google Scholar] [CrossRef]
- Jones, K.; Ruhl, R.; Warm, J.; Dember, W. Olfaction and vigilance: The role of hedonic value. Autom. Technol. Hum. Perform. Curr. Res. Trends 1999, 6, 193. [Google Scholar]
- Moss, M.; Hewitt, S.; Moss, L.; Wesnes, K. Modulation of cognitive performance and mood by aromas of peppermint and ylang-ylang. Int. J. Neurosci. 2008, 118, 59–77. [Google Scholar] [CrossRef]
- Gould, A.; Martin, G.N. A good odour to breathe?’The effect of pleasant ambient odour on human visual vigilance. Appl. Cogn. Psychol. 2001, 15, 225–232. [Google Scholar] [CrossRef]
- Ilmberger, J.; Heuberger, E.; Mahrhofer, C.; Dessovic, H.; Kowarik, D.; Buchbauer, G. The influence of essential oils on human attention. I: Alertness. Chem. Senses 2001, 26, 239–245. [Google Scholar] [CrossRef]
- Cross, S.N.; Lin, M.-H.; Childers, T.L. Sensory identity: The impact of olfaction on consumption. In Consumer Culture Theory; Emerald Group Publishing Limited: Bingley, UK, 2015; pp. 331–347. [Google Scholar]
- Aron, E.N. The Highly Sensitive Person: How to Thrive When the World Overwhelms You; Kensington Publishing Corp.: New York, NY, USA, 2013. [Google Scholar]
- Lin, M.-H.; Cross, S.N.; Childers, T.L. Understanding olfaction and emotions and the moderating role of individual differences. Eur. J. Mark. 2018, 52, 811–836. [Google Scholar] [CrossRef]
- Wrzesniewski, A.; McCauley, C.; Rozin, P. Odor and affect: Individual differences in the impact of odor on liking for places, things and people. Chem. Senses 1999, 24, 713–721. [Google Scholar] [CrossRef]
- Lin, M.-H.J.; Cross, S.N.; Childers, T.L. Olfactory imagery and emotions: Neuroscientific evidence. In Ideas in Marketing: Finding the New and Polishing the Old; Springer: Berlin/Heidelberg, Germany, 2015; pp. 617–620. [Google Scholar]
- Allen, A.P.; Smith, A.P. Effects of chewing gum and time-on-task on alertness and attention. J. Nutr. Neurosci. 2012, 15, 176–185. [Google Scholar] [CrossRef]
- Tucha, O.; Mecklinger, L.; Maier, K.; Hammerl, M.; Lange, K. Chewing gum differentially affects aspects of attention in healthy subjects. J. Appet. 2004, 42, 327–329. [Google Scholar] [CrossRef]
- Johnson, A.J.; Muneem, M.; Miles, C. Chewing gum benefits sustained attention in the absence of task degradation. Nutr. Neurosci. 2013, 16, 153–159. [Google Scholar] [CrossRef]
- Allen, A.P.; Jacob, T.J.; Smith, A.P. Effects and after-effects of chewing gum on vigilance, heart rate, EEG and mood. J. Physiol. Behav. 2014, 133, 244–251. [Google Scholar] [CrossRef]
- Allen, A.P.; Smith, A.P. A review of the evidence that chewing gum affects stress, alertness and cognition. J. Behav. Neurosci. Res. 2011, 9, 7–23. [Google Scholar]
- Morgan, K.; Johnson, A.J.; Miles, C. Chewing gum moderates the vigilance decrement. Br. J. Psychol. 2014, 105, 214–225. [Google Scholar] [CrossRef]
- Allen, A.P.; Smith, A.P. Chewing gum: Cognitive performance, mood, well-being, and associated physiology. J. BioMed Res. Int. 2015, 1, 16. [Google Scholar] [CrossRef]
- Johnson, A.J.; Jenks, R.; Miles, C.; Albert, M.; Cox, M. Chewing gum moderates multi-task induced shifts in stress, mood, and alertness. A re-examination. J. Appet. 2011, 56, 408–411. [Google Scholar] [CrossRef]
- Tucha, L.; Simpson, W. The role of time on task performance in modifying the effects of gum chewing on attention. J. Appet. 2011, 56, 299–301. [Google Scholar] [CrossRef]
- Allen, A.P. Chewing Gum’s Effects on Alertness, Performance and Stress. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2013. [Google Scholar]
- Stephens, R.; Tunney, R. Role of glucose in chewing gum-related facilitation of cognitive function. J. Appet. 2004, 43, 211–213. [Google Scholar] [CrossRef]
- Onyper, S.V.; Carr, T.L.; Farrar, J.S.; Floyd, B.R. Cognitive advantages of chewing gum. Now you see them, now you don’t. J. Appet. 2011, 57, 321–328. [Google Scholar] [CrossRef]
- Gong, D.; He, H.; Liu, D.; Ma, W.; Dong, L.; Luo, C.; Yao, D. Enhanced functional connectivity and increased gray matter volume of insula related to action video game playing. Sci. Rep. 2015, 5, 9763. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; Stock, A.-K.; Beste, C.; Colzato, L.S. Action video gaming and cognitive control: Playing first person shooter games is associated with improved action cascading but not inhibition. PLoS ONE 2015, 10, e0144364. [Google Scholar] [CrossRef]
- Li, R.; Polat, U.; Makous, W.; Bavelier, D. Enhancing the contrast sensitivity function through action video game training. Nat. Neurosci. 2009, 12, 549. [Google Scholar] [CrossRef]
- Chen, R.; Chen, J.; Li, L. Action videogame play improves visual motor control. J. Vis. 2015, 15, e42. [Google Scholar] [CrossRef]
- Green, C.S.; Sugarman, M.A.; Medford, K.; Klobusicky, E.; Bavelier, D. The effect of action video game experience on task-switching. Comput. Hum. Behav. 2012, 28, 984–994. [Google Scholar] [CrossRef]
- Colzato, L.S.; van den Wildenberg, W.P.; Zmigrod, S.; Hommel, B. Action video gaming and cognitive control: Playing first person shooter games is associated with improvement in working memory but not action inhibition. Psychol. Res. 2013, 77, 234–239. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Meinzer, M.; Antonenko, D.; Lindenberg, R.; Hetzer, S.; Ulm, L.; Avirame, K.; Flaisch, T.; Flöel, A. Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation. J. Neurosci. 2012, 32, 1859–1866. [Google Scholar] [CrossRef]
- Battleday, R.M.; Brem, A.-K. Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review. Eur. Neuropsychopharmacol. 2015, 25, 1865–1881. [Google Scholar] [CrossRef]
- Tavakoli, A.V.; Yun, K. Transcranial alternating current stimulation (tACS) mechanisms and protocols. Front. Cell. Neurosci. 2017, 11, 214. [Google Scholar] [CrossRef]
- Smith, A. Effects of chewing gum on mood, learning, memory and performance of an intelligence test. Nutr. Neurosci. 2009, 12, 81–88. [Google Scholar] [CrossRef]
- Dibben, N.; Williamson, V. An exploratory survey of in-vehicle music listening. Psychol. Music 2007, 35, 571–589. [Google Scholar] [CrossRef]
- Kozlov, M.D.; Hughes, R.W.; Jones, D.M. Gummed-up memory: Chewing gum impairs short-term recall. Q. J. Exp. Psychol. 2012, 65, 501–513. [Google Scholar] [CrossRef]
- Allen, A.; Smith, A. Demand characteristics, pre-test attitudes and time-on-task trends in the effects of chewing gum on attention and reported mood in healthy volunteers. Appetite 2012, 59, 349–356. [Google Scholar] [CrossRef]
- Weis, R.; Cerankosky, B.C. Effects of video-game ownership on young boys’ academic and behavioral functioning: A randomized, controlled study. Psychol. Sci. 2010, 21, 463–470. [Google Scholar] [CrossRef]
- Wright, J. The effects of video game play on academic performance. Mod. Psychol. Stud. 2011, 17, 6. [Google Scholar]
- An, S.A.; Tillman, D.A.; Boren, R.; Wang, J. Fostering Elementary Students’ Mathematics Disposition through Music-Mathematics Integrated Lessons. Int. J. Math. Teach. Learn. 2014, 14, 19. [Google Scholar]
- Van der Heijden, G.A.; Schepers, J.J.; Nijssen, E. Understanding workplace boredom among white collar employees: Temporary reactions and individual differences. Eur. J. Work Organ. Psychol. 2012, 21, 349–375. [Google Scholar] [CrossRef]
- Shaw, T.H.; Matthews, G.; Warm, J.S.; Finomore, V.S.; Silverman, L.; Costa, P.T., Jr. Individual differences in vigilance: Personality, ability and states of stress. J. Res. Personal. 2010, 44, 297–308. [Google Scholar] [CrossRef]
- Rose, N.S.; Rendell, P.G.; McDaniel, M.A.; Aberle, I.; Kliegel, M. Age and individual differences in prospective memory during a “Virtual Week”: The roles of working memory, vigilance, task regularity, and cue focality. Psychol. Aging 2010, 25, 595. [Google Scholar] [CrossRef]
- Bostrom, N.; Sandberg, A. Cognitive enhancement: Methods, ethics, regulatory challenges. J. Sci. Eng. Ethics 2009, 15, 311–341. [Google Scholar] [CrossRef]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Logan, J.; Alexoff, D.; Zhu, W.; Telang, F.; Wang, G.-J.; Jayne, M.; Hooker, J.M.; Wong, C. Effects of modafinil on dopamine and dopamine transporters in the male human brain: Clinical implications. J. Am. Med Assoc. 2009, 301, 1148–1154. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ugawa, Y. Adverse events of tDCS and tACS: A review. Clin. Neurophysiol. Pract. 2017, 2, 19–25. [Google Scholar] [CrossRef]
- Carlson, E.; Saarikallio, S.; Toiviainen, P.; Bogert, B.; Kliuchko, M.; Brattico, E. Maladaptive and adaptive emotion regulation through music: A behavioral and neuroimaging study of males and females. Front. Hum. Neurosci. 2015, 9, 466. [Google Scholar] [CrossRef]
- Winocur, E.; Gavish, A.; Finkelshtein, T.; Halachmi, M.; Gazit, E. Oral habits among adolescent girls and their association with symptoms of temporomandibular disorders. J. Oral Rehabil. 2001, 28, 624–629. [Google Scholar] [CrossRef]
- Hwang, J.; Lu, A.S. Narrative and active video game in separate and additive effects of physical activity and cognitive function among young adults. Sci. Rep. 2018, 8, 11020. [Google Scholar] [CrossRef]
- Nitsche, M.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef]
- Ala, T.S.; Ahmadi-Pajouh, M.A.; Nasrabadi, A.M. Cumulative effects of theta binaural beats on brain power and functional connectivity. J. Biomed. Signal Process. Control 2018, 42, 242–252. [Google Scholar]
- Wang, D.; Xu, M.; Zhanq, Y.; Xiao, J. Preliminary study on haptic-stimulation based brainwave entrainment. In Proceedings of the 2013 World Haptics Conference (WHC), Daejeon, Korea, 14–17 April 2013. [Google Scholar]
- Sowndhararajan, K.; Kim, S. Influence of fragrances on human psychophysiological activity: With special reference to human electroencephalographic response. Sci. Pharm. 2016, 84, 724–751. [Google Scholar] [CrossRef]
- Tachtsidis, I.; Scholkmann, F. False positives and false negatives in functional near-infrared spectroscopy: Issues, challenges, and the way forward. J. Neurophotonics 2016, 3, 031405. [Google Scholar] [CrossRef]
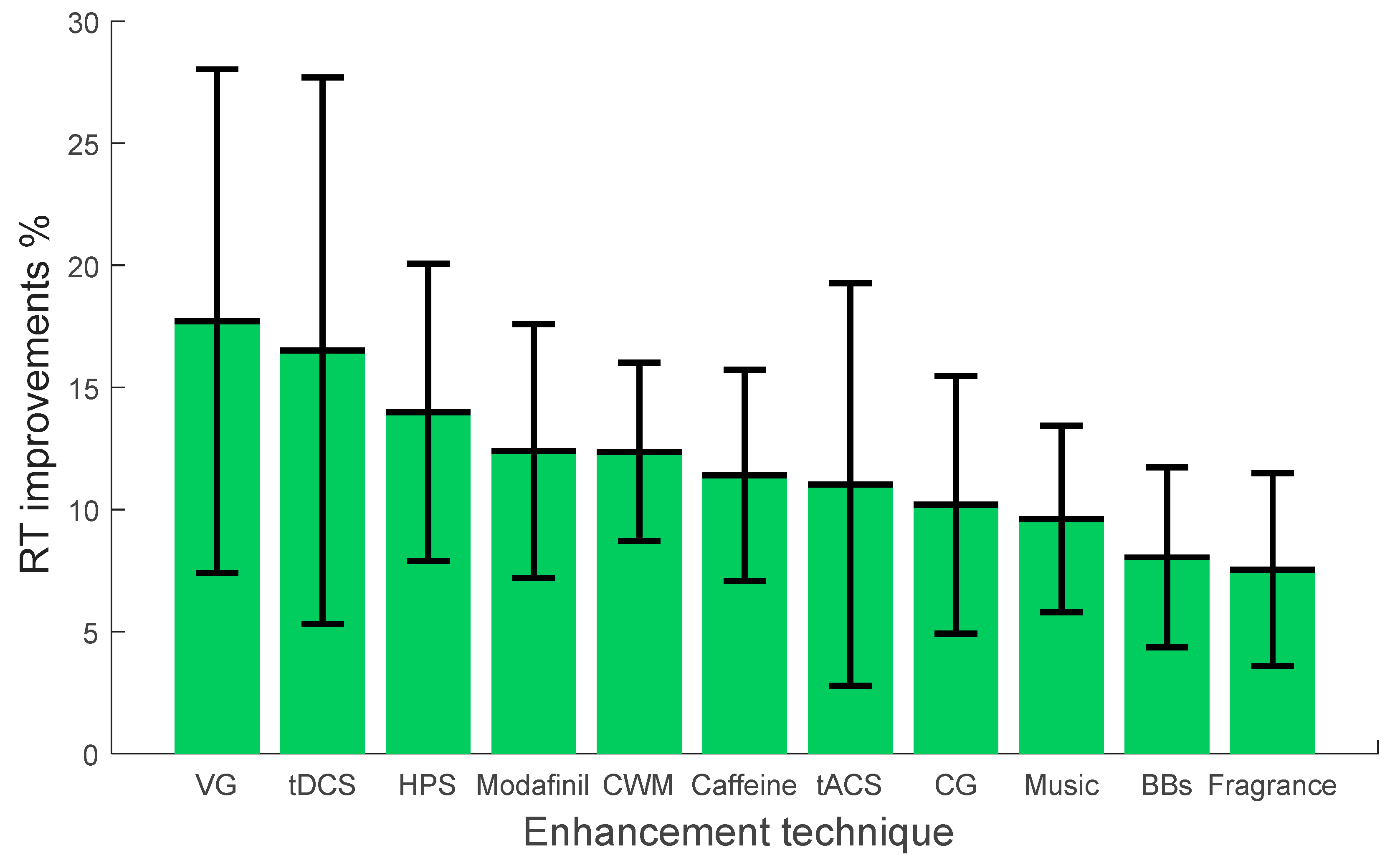
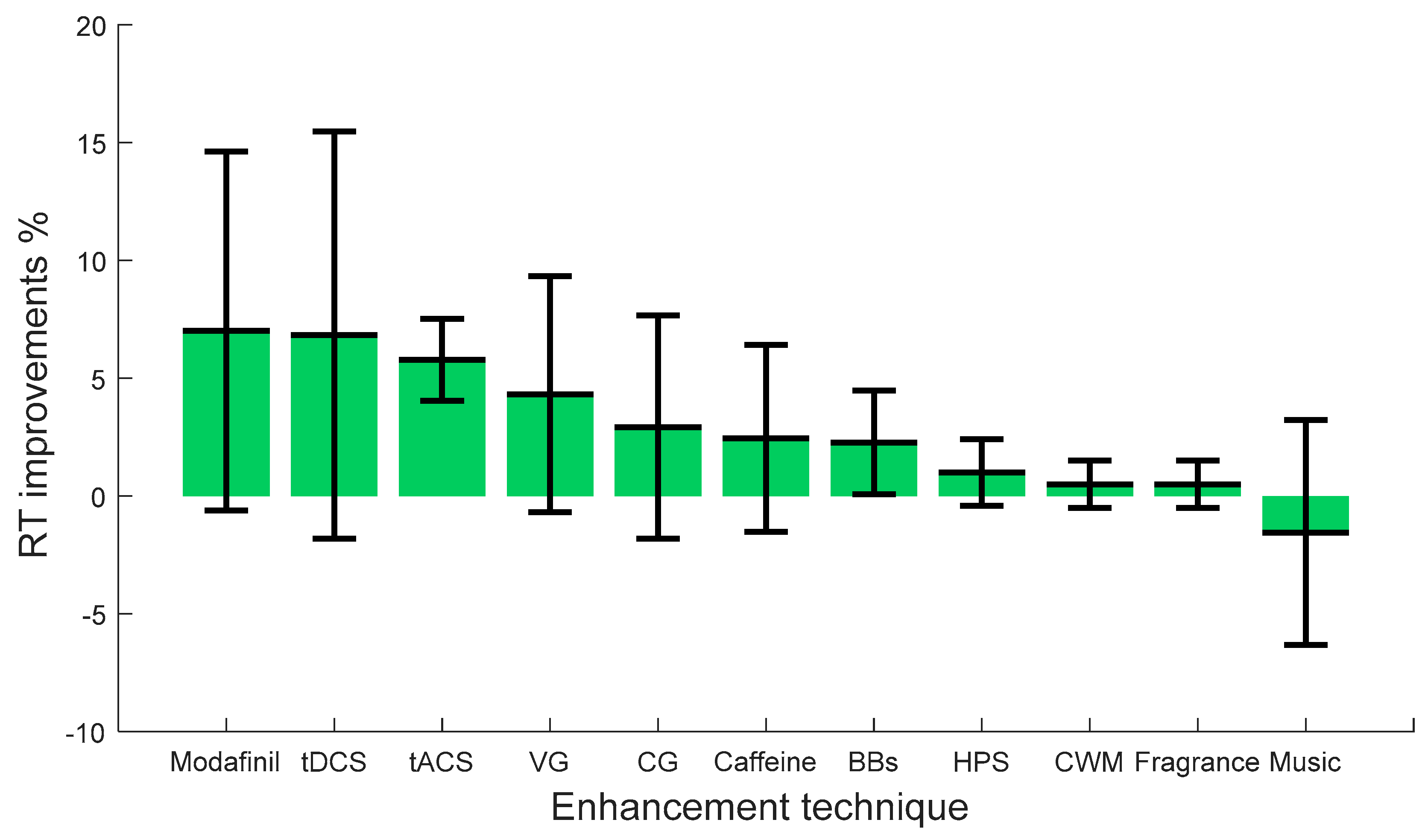
| Study | Enhancement Technique | Vigilance Task | No of Subjects | Summary of Results (Compare before and after Intervention) | Attributes of Effects on Vigilance | Comment |
|---|---|---|---|---|---|---|
| [44] | Modafinil (200, 400 mg) | Working memory and mathematical ability | 50 | Performance and alertness significantly improved compared to placebo. | Positive | Modafinil did not appear to offer advantages over caffeine. |
| [45] | Modafinil (100, 200 mg) | Memory and attention | 60 | Enhanced performance on tests of digit span, visual pattern, and spatial planning. Subjects reported to be more alert, attentive and energetic when on the drug. | Positive | Selectively improved neuropsychological task performance. |
| [46] | Modafinil (100, 200 mg) | Rapid visual information processing | 89 | Improved target detection, performance and reaction time. | Positive | The high IQ may limit the detection of Modafinil’s positive effects. |
| [47] | Modafinil (100, 200 mg) | Digit span, color naming and rapid visual information processing | 60 | Improved performance in all tasks and enhanced reaction time. | Positive | The benefits of Modafinil were dose-related. |
| [48] | Modafinil (200 mg) | Working memory, processing, and speed/attention | 41 | Improved performance on a test of sustained attention but no improvements on other cognitive tests. | Positive | Modafinil may be helpful in methamphetamine-dependent subjects who frequently used the drugs. |
| [49] | Video game (1 hour) | Visual target and random letters | 30 | Significant improvements on accuracy, reaction time and attention. | Positive | Video game playing enhanced the capacity of visual attention and its spatial distribution. |
| [50] | Video game (1 hour) | Multiple object tracking | 114 | Participants who played action games showed enhanced performance on all aspects of attention tested compared to non-gamers. | Positive | Suggested careful control of video game usage when assessing gender differences in attentional tasks. |
| [51] | Video game (40 min) | Target detection | 77 | Improved vigilance during training and during subsequent test phase in which no feedback was provided. | Positive | Video game-based methods may be useful for training sustained attention. |
| [52] | Video game (1 hour) | Multitasking, sign and drive | 174 | Enhanced sustained attention and working memory. | Positive | Suggest video game as a powerful tool for cognitive enhancement. |
| [53] | Video game (25 min) | Target detection | 294 | Enhanced accuracy and reduced response time and false alarms. | Positive | Active video game players (AVGPs) rate the task lower than NVGPs in terms of total or global perceived workload. |
| [27] | Video game (1.5 hours) | Target detection | 28 | Significant increase in correct detections. Improved overall performance for soldiers and students. | Positive | Video game did not induce the decrement function compared to vigilance task. |
| [26] | Video game (15 min) | Target detection | 32 | Sustained attention could be trained using the knowledge of results (KR) via video-game platform. The results indicated that KR enhanced sustained attention. | Positive | Video game environment can support effective sustained attention training in professional military and general population. |
| [29] | 6 Hz Theta tACS (15 min) | Delayed letter discrimination task | 18 | Significantly improved visual memory matching and reaction times compared to placebo stimulation. | Positive | Revealed the suitability of the technique to induce coupling or decoupling of behaviour between brain regions. |
| [30] | 40 Hz gamma tACS (12 min) | Raven’s matrices | 20 | Improved target detection and increased performance. | Positive | Selectively accelerated logical reasoning on the prefrontal cortex. |
| [28] | 40 Hz Gamma tACS (30 min) | Visual target | 24 | Enhanced performance in vigilance tasks and significantly decreased slowdown of reaction times. | Positive | Error rate did not differ between groups. |
| [54] | tDCS (20 min) | go/no-go paradigm | 46 | No effect on performance and reaction time. | No difference | The increase of dopaminergic activity led to a deterioration of the executive function. |
| [32] | tDCS (10 min) | Simulated air traffic controller task | 19 | Behavioral measures showed a significant improvement in target detection performance compared to the sham stimulation. | Positive | The technique suggested that enhancing performance in work settings required sustained attention. |
| [33] | tDCS and caffeine (30 min) | Psychomotor vigilance task (PVT) | 30 | Enhanced vigilance and better subjective ratings for fatigue, drowsiness, energy, and composite mood. | Positive | tDCS worked better than caffeine on vigilance and mood state in which its effects lasted several hours. |
| [55] | tDCS (9 min) | Visual digit stimuli | 23 | Stimulation decreased the reaction time and increased skin conductance and arousal. | Positive | Very sensitive to arousal. |
| [56] | tDCS (15 min) | go/no-go paradigm | 8 | No effect on the accuracy or reaction time. | No difference | Could be used to enhance Theta amplitude over the frontal midline. |
| [57] | tDCS (10 min) | Video game/multitasking paradigm | 41 | Enhanced performance on multitasking paradigm and reduced its cost by 20%. | Positive | The result suggested left prefrontal cortex (PFC) in facilitating the performance of more than one task or multitasking. |
| [58] | tDCS (20 min) | Visuospatial task | 18 | Improved executive function and dual tasking in older adults with functional limitations. | Positive | Improved gait markers. |
| [40] | Music (1 hour) | Auditory target detection | 76 | Improved accuracy and detection rate. | Positive | Supported the use of music to improve vigilance in educational and clinical settings. |
| [59] | Music (30 min) | Attention test | 89 | Preferred music improved the concentration and attention level. | Positive | It is important to select music that workers strongly like to avoid negatively affecting their concentration. |
| [60] | Music (30 min) | Reading texts | 24 | Participants scored significantly lower after listening to nonpreferred music while reading. | Negative | Participants disrupted by a nonpreferred musical background. |
| [61] | Music (10 min) | Attention test | 102 | Background music with lyrics had significant negative effects on concentration and attention level. | Negative | Music with lyrics caused distractions and reduced worker attention and performance. |
| [62] | Music (1 hour) | Conjunction search task | 12 | Listening to preferred music increased performance level. Different temporal patterns were depicted in the change of performance. | Positive | Music effected emotions and mood states. |
| [63] | Music (21 min) | Attention to response task | 158 | Positive valence music significantly decreased the miss rates relative to negative valence music or silence. | Positive | Results supported the attentional restoration theory of the impact of music on sustained attention. |
| [39] | Music (10 min) | Attention to audio | 20 | Enhanced global efficiency of brain, enhanced local neural efficiency at the prefrontal lobe, and increased sustained attention. | Positive | Music directly affected cognitive system and led to improved brain efficiency. |
| [41] | Binaural beat (1.5, 4, 16, 24 Hz; 30 min) | Target detection | 29 | Beta binaural beat yielded more correct target detections and fewer false alarms than the presentation of theta/delta binaural beats. | Positive | The study’s assessment was conducted using questionnaires, which was a subjective method. |
| [42] | Binaural beat (18.5 Hz; 5 min) | Audio-visual light | 15 | Beta binaural beat enhanced in the range of 13–21 Hz and a high increase in the 18.5 Hz amplitude. | Positive | Binaural beats may potentially enhance attention. |
| [64] | Binaural beat (7, 16 Hz; 13 min) | Reading texts | 31 | No changes detected before and after binaural beat stimulation at Beta and Theta frequencies. | No difference | Short recording time of 13 minutes. |
| [35] | Vibrotactile (5 Hz; 3 hours) | Target detection | 11 | Reduced reaction time to stimuli. | Positive | No significant difference in accuracy. |
| [65] | Vibrotactile (250 Hz; 40 min) | Auditory or a visual display | 98 | The audio results showed greater performance improvement compared to the visual modality. | Positive | Visual modality posed no benefit for sustaining the performance. |
| [37] | Vibrotactile (250 Hz; 40 min) | Auditory or a visual display | 150 | Detection accuracy was significantly greater in the auditory modality compared to the visual modality. Reduced mental workload. | Positive | A rest break can restore the performance in auditory and visual vigilance tasks. |
| [36] | Vibrotactile (15 Hz; 16 min) | Target detection | 20 | Participants performed better in perceptual sensitivity and sustaining attention level. | Positive | Haptic-based brainwave entrainment poses the potential for cognitive training. |
| Enhancer Type | Response Time | After Effect Duration of Action | Translation to Real-Life Practice | Cost Per Dose | Adhering to Ethical Approval | Advantages | Dis-Advantages |
|---|---|---|---|---|---|---|---|
| Modafinil [69] | ~20 min (slow) | 11.5 hours (400 mg) | Difficult | Moderate | Difficult | Highly effective for higher cognitive functions. Can be used for enhancement and therapeutic purposes. | May generate possible abuse and addiction and the waking mechanism has not been fully elucidated. |
| Gaming [241] | ~30 min (slow) | To be elucidated | Difficult | Moderate | Easy | Easily integrated into technology. Easily accessible. Real-time stimulation. | Long-time playing increases the risk of depression, aggressive behaviors, addiction and musculoskeletal pain. Requires full attention of users. |
| tDCS [179,242] | ~10 min (fast) | 6 hours | Difficult | Moderate | Easy | Portable, and tolerable User-friendly. Can easily be combined with pharmacotherapy. Can be applied to specific brain region. | Needs to be done in a quiet environment, such as the lab or clinic. May not be safe for long-term monitoring. |
| Music [129] | ~ minutes (fast) | To be elucidated | Easy | Low | Easy | Real-time. Long-term. stimulation Used in therapy. | Affects wide cognition domain, emotions and mood state. |
| Binaural Beats [243] | ~6 min (fast) | To be elucidated | Easy | Low | Easy | Real time. Long-term stimulation. Can enhance certain cognitive function. | Sensitive to age variations. Depends on binaural frequency. |
| Haptic/ tactile Stimulation [244] | ~11 min (fast) | To be elucidated | Easy | Low | Easy | Real-time stimulation. Long-term stimulation. Can enhance certain cognitive functions. | Affected by skin diseases. |
| Challenging integration [38] | ~5 min (fast) | To be elucidated | Moderate | Low | Easy | Real-time stimulation. Long-term stimulation. | Sensitive to individual difference. |
| Chewing Gum [211] | ~minutes (fast) | 20 min | Easy | Low | Easy | Real-time stimulation. | Has slow response. May cause jaw problem. |
| Caffeine [25,44] | ~ minutes (fast) | 2 hours (200 mg) | Easy | Low | Easy | Widely accepted by society. Has faster response. Doses intake can be easily controlled. | Reported symptoms of nervousness, excitation, pain, dry mouth, tremor, nausea, and jitteriness. |
| Fragrance/ Odour [245] | ~ minutes (fast) | To be elucidated | Easy | Low | Easy | Real-time stimulation. Has faster response. | May affect skin. Causes headache. Have poisonous effects on the brain and nervous system. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shargie, F.; Tariq, U.; Mir, H.; Alawar, H.; Babiloni, F.; Al-Nashash, H. Vigilance Decrement and Enhancement Techniques: A Review. Brain Sci. 2019, 9, 178. https://doi.org/10.3390/brainsci9080178
Al-Shargie F, Tariq U, Mir H, Alawar H, Babiloni F, Al-Nashash H. Vigilance Decrement and Enhancement Techniques: A Review. Brain Sciences. 2019; 9(8):178. https://doi.org/10.3390/brainsci9080178
Chicago/Turabian StyleAl-Shargie, Fares, Usman Tariq, Hasan Mir, Hamad Alawar, Fabio Babiloni, and Hasan Al-Nashash. 2019. "Vigilance Decrement and Enhancement Techniques: A Review" Brain Sciences 9, no. 8: 178. https://doi.org/10.3390/brainsci9080178
APA StyleAl-Shargie, F., Tariq, U., Mir, H., Alawar, H., Babiloni, F., & Al-Nashash, H. (2019). Vigilance Decrement and Enhancement Techniques: A Review. Brain Sciences, 9(8), 178. https://doi.org/10.3390/brainsci9080178








