Computational Modeling of the Photon Transport, Tissue Heating, and Cytochrome C Oxidase Absorption during Transcranial Near-Infrared Stimulation
Abstract
1. Introduction
2. Methods
2.1. Head Model Selection
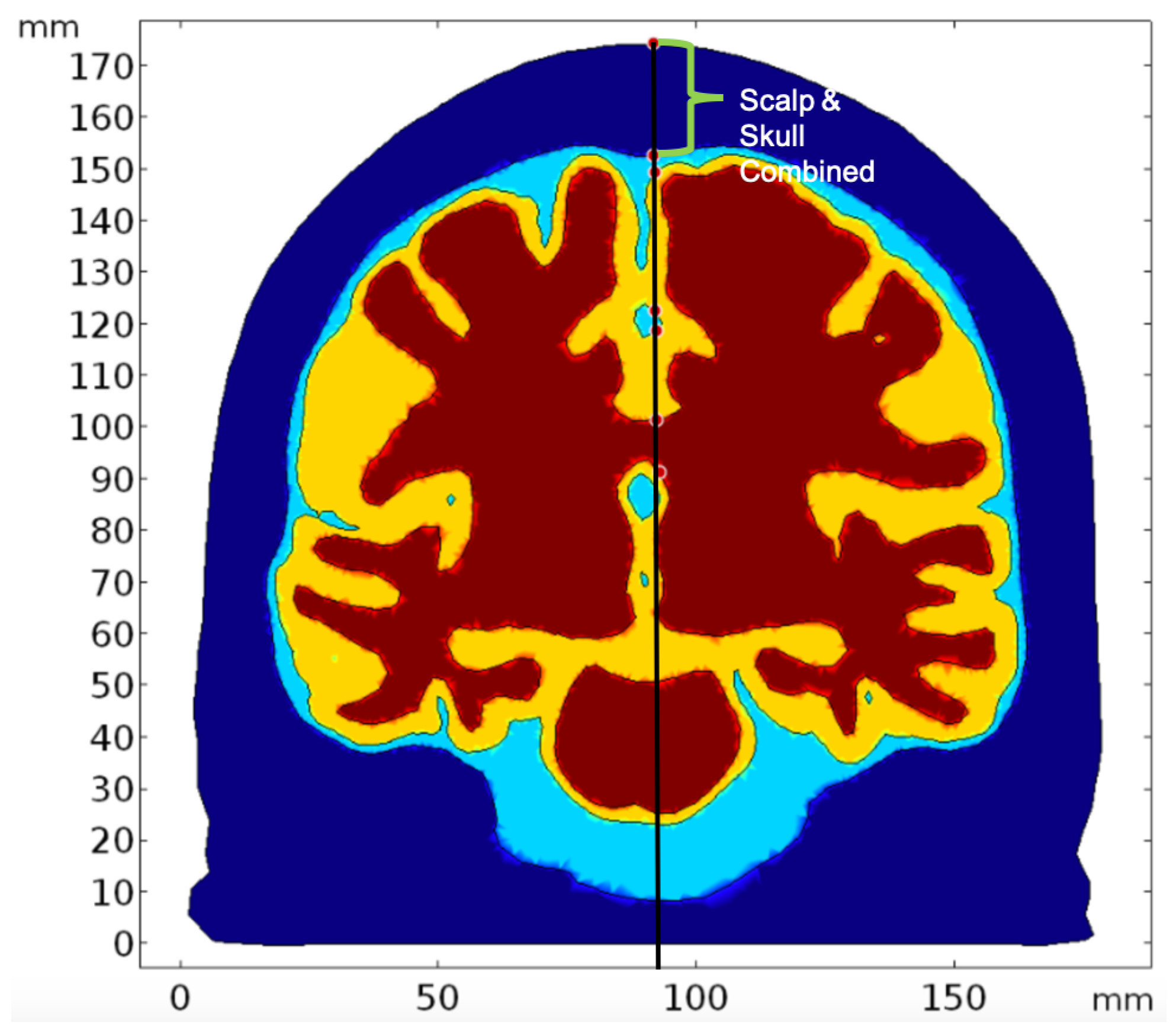
2.2. Geometry and Domain Assignment
- Domain 1: Combined scalp and skull
- Domain 2: Cerebrospinal Fluid
- Domain 3: Gray Matter
- Domain 4: White Matter
2.3. Simulation of Radiative Transfer Equation Using Diffusion Approximation
2.4. Optically Induced Thermal Effects Modeled Using Bioheat Transfer Mechanism
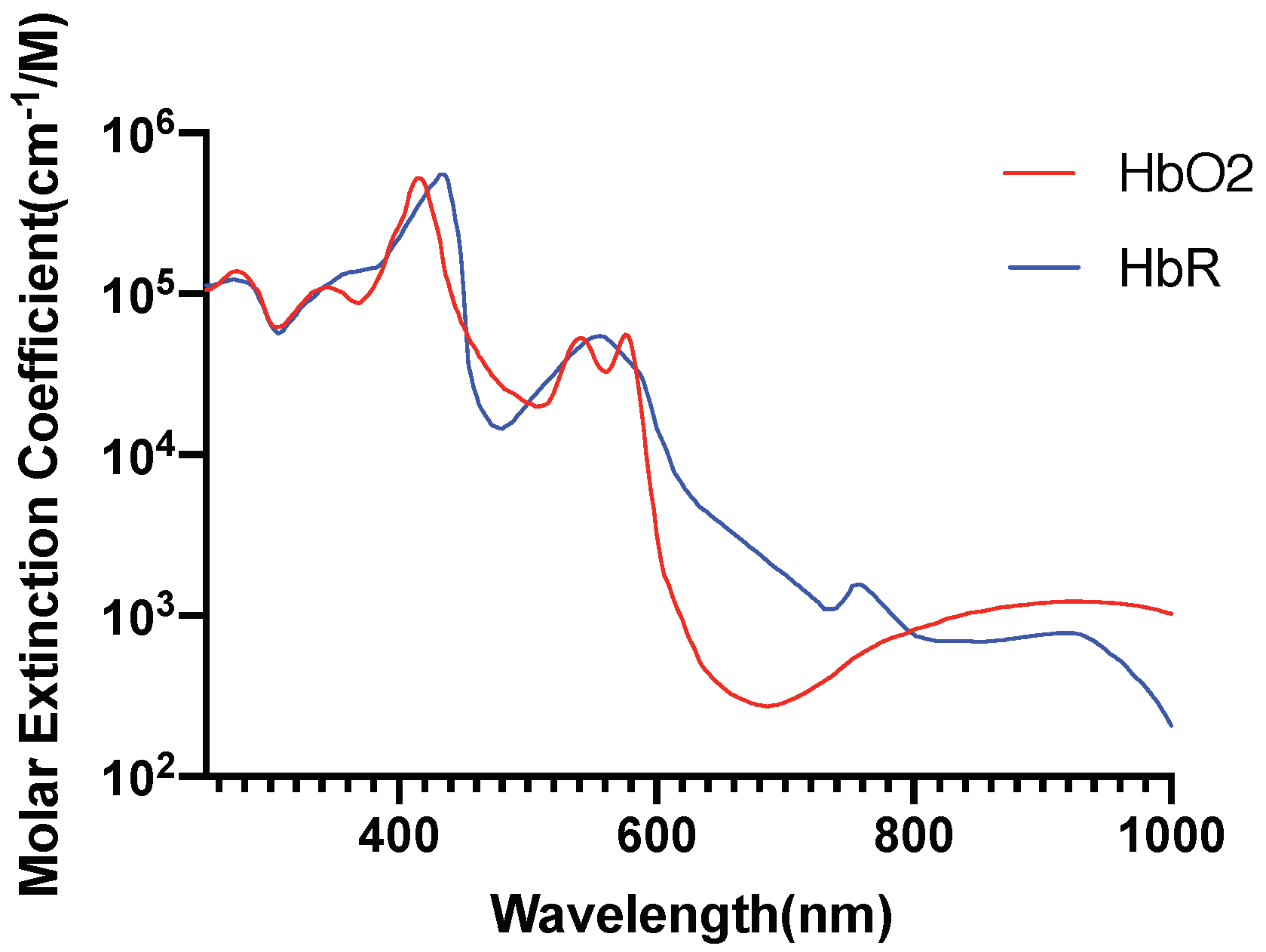
2.5. Investigation of Individual Chromophore Absorption in the Tissue
- Oxyhemoglobin
- Deoxyhemoglobin
- Cytochrome c oxidase(reduced and oxidized state)
- Lipid
2.6. Optical Parameters of Individual Chromophores
2.7. Finite Element Analysis
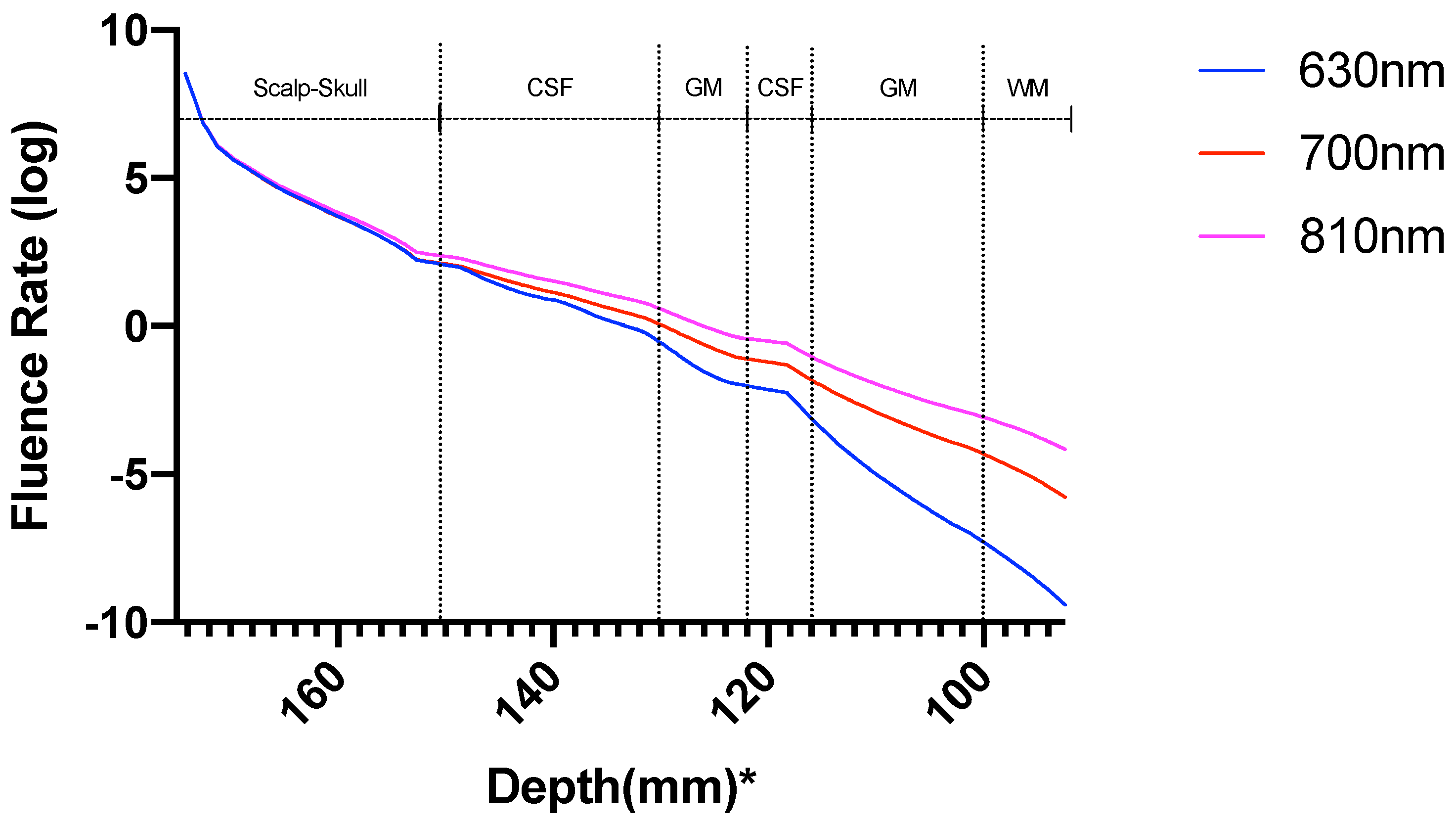
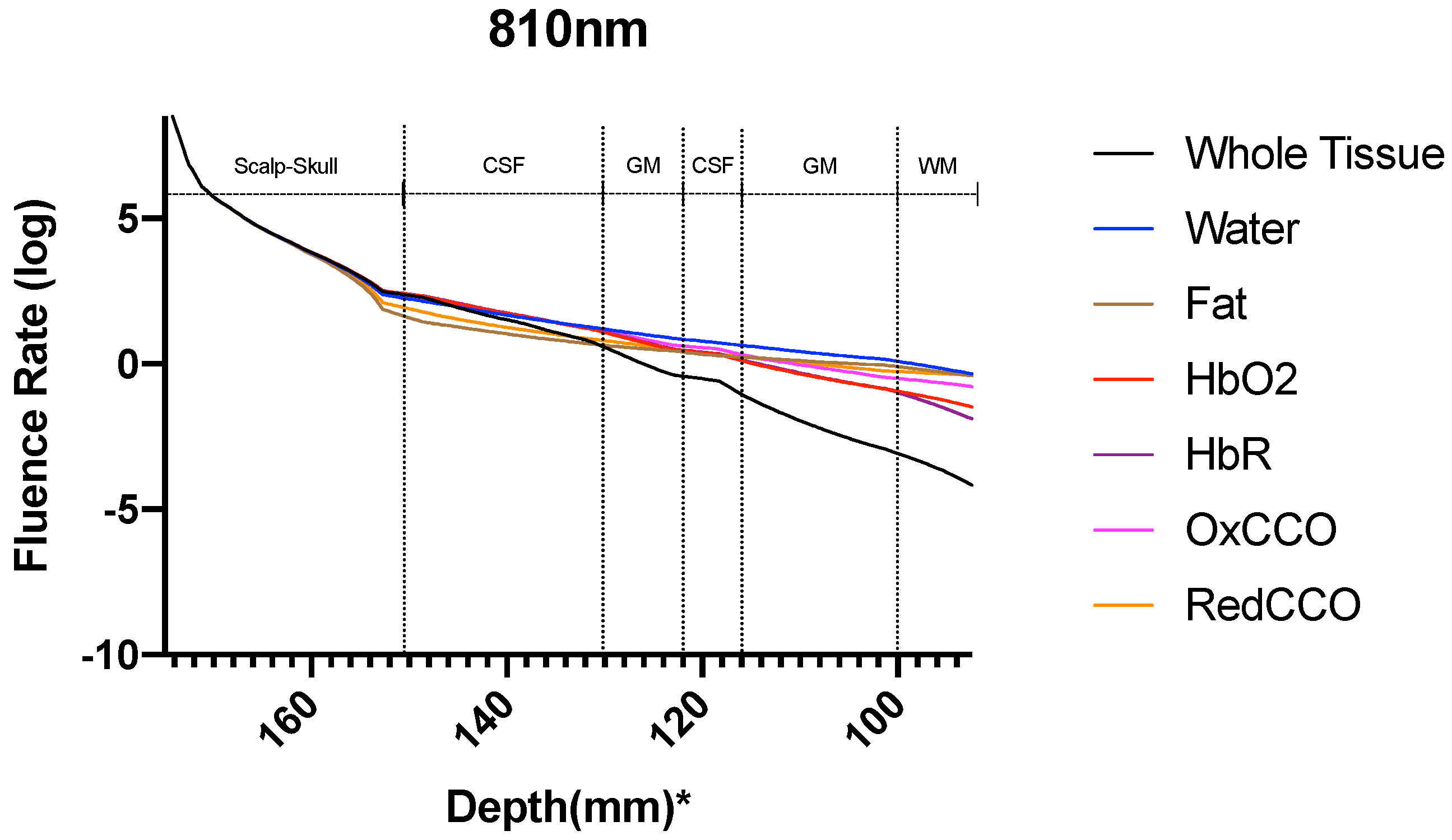
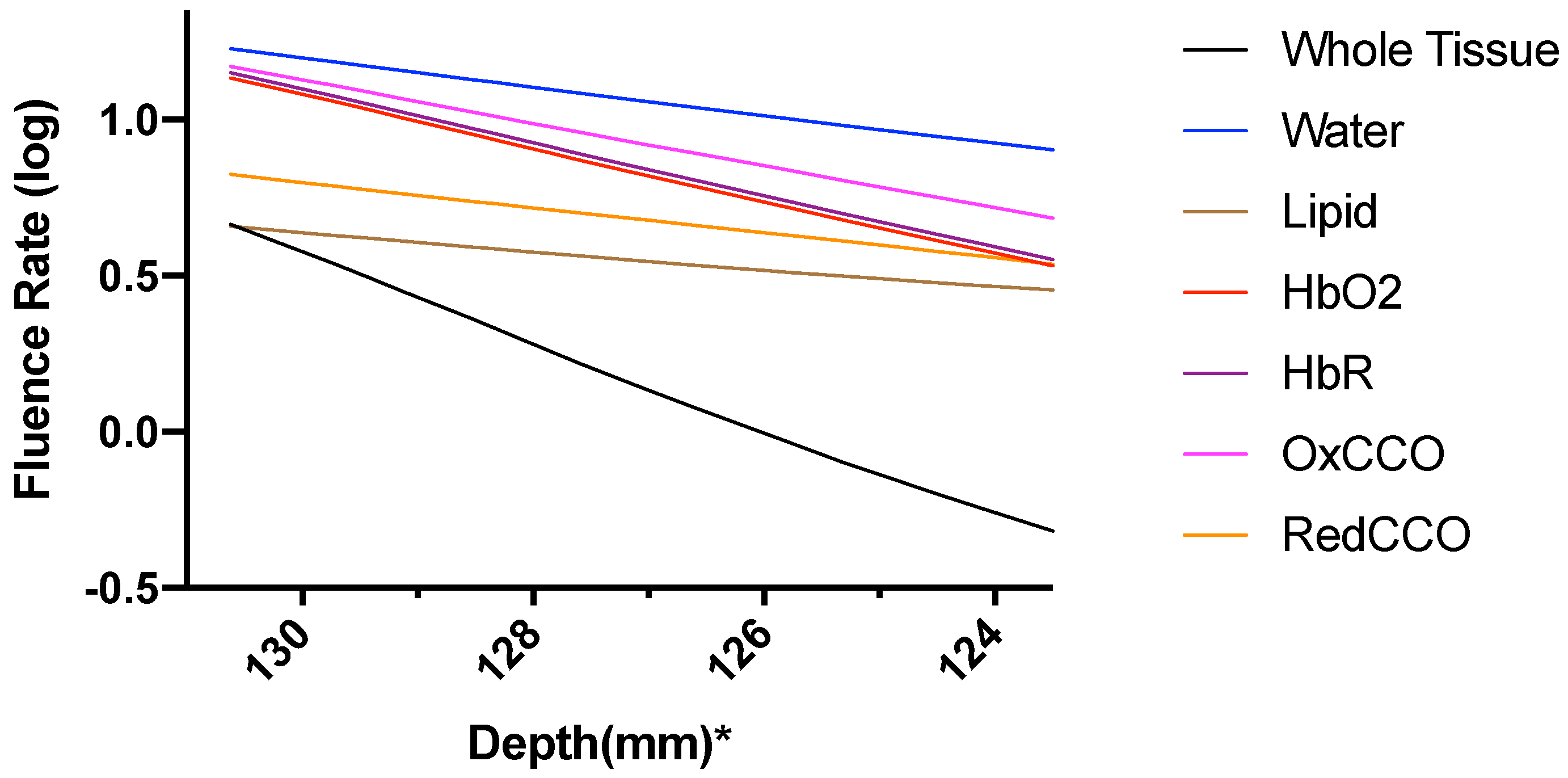
3. Results
3.1. Photothermal Effects
3.2. Photobiomodulation
4. Systematic Model Errors
- In our model, the brain has been assumed as a highly scattering medium which is not true for CSF which is a low scattering medium where RTE can produce erroneous results [57] (Comparison between RTE and Monte Carlo has been shown in Supplementary Material).
- Another error is related to computational limitation during the FEM modeling and discretization. There were limitations of accessible memory. Although enhancing resolution leads to better convergence of FEM results [58], but computational limitation restricted us to the standard COMSOL mesh refining process.
- The reflection effects due to light interaction have been excluded from the simulations to focus on light interaction with tissues due to absorption and scattering.
- The simulation of the Bioheat Transfer assumed that the heat loss at the skin surface is due to convection and radiative heat loss was considered insignificant at that temperature.
5. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PBM | Photobiomodulation |
| CCO | Cytochrome c oxidase |
| ROS | Reactive Oxygen Species |
| FEM | Finite Element Method |
| NIR | Near-Infrared |
| RTE | Radiative Transfer Equation |
| PDE | Partial Differential Equation |
| tNIRS | transcranial Near-Infrared Stimulation |
References
- Chaieb, L.; Antal, A.; Masurat, F.; Paulus, W. Neuroplastic effects of transcranial near-infrared stimulation (tNIRS) on the motor cortex. Front. Behav. Neurosci. 2015, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Bale, G.; Elwell, C.E.; Tachtsidis, I. From Jöbsis to the present day: A review of clinical near-infrared spectroscopy measurements of cerebral cytochrome-c-oxidase. J. Biomed. Opt. 2016, 21, 091307. [Google Scholar] [CrossRef] [PubMed]
- Wong-Riley, M.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef]
- Karu, T.I. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 2010, 62, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Cell biology: power Games. Nature 2006, 443, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Poyton, R.O.; Ball, K.A. Therapeutic photobiomodulation: Nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov. Med. 2011, 11, 154–159. [Google Scholar]
- Waypa, G.B.; Smith, K.A.; Schumacker, P.T. O2 sensing, mitochondria and ROS signaling: The fog is lifting. Mol. Asp. Med. 2016, 47, 76–89. [Google Scholar] [CrossRef]
- Sanderson, T.H.; Wider, J.M.; Lee, I.; Reynolds, C.A.; Liu, J.; Lepore, B.; Tousignant, R.; Bukowski, M.J.; Johnston, H.; Fite, A.; et al. Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain ischemia/reperfusion injury. Sci. Rep. 2018, 8, 3481. [Google Scholar] [CrossRef]
- Barrett, D.W.; Gonzalez-Lima, F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013, 230, 13–23. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation Affects Key Cellular Pathways of all Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef]
- Michel, B.; Bosshard, H.R. Spectroscopic analysis of the interaction between cytochrome c and cytochrome c oxidase. J. Biol. Chem. 1984, 259, 10085–10091. [Google Scholar] [PubMed]
- Thomson, A.; Brittain, T.; Greenwood, C.; Springall, J. Determination of the heme spin states in cytochrome c oxidase using magnetic circular dichroism. FEBS Lett. 1976, 67, 94–98. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci. Rep. 2016, 6, 33719. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337. [Google Scholar] [CrossRef] [PubMed]
- Haeussinger, F.B.; Heinzel, S.; Hahn, T.; Schecklmann, M.; Ehlis, A.C.; Fallgatter, A.J. Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PLoS ONE 2011, 6, e26377. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41. [Google Scholar] [PubMed]
- Wells, J.; Kao, C.; Mariappan, K.; Albea, J.; Jansen, E.D.; Konrad, P.; Mahadevan-Jansen, A. Optical stimulation of neural tissue in vivo. Opt. Lett. 2005, 30, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Baumhoff, P.; Maier, H.; Teudt, I.U.; Krüger, A.; Lenarz, T.; Kral, A. Nanosecond laser pulse stimulation of the inner ear—A wavelength study. Biomed. Opt. Express 2012, 3, 3332–3345. [Google Scholar] [CrossRef]
- Xu, A.G.; Qian, M.; Tian, F.; Xu, B.; Friedman, R.M.; Wang, J.; Song, X.; Sun, Y.; Chernov, M.M.; Cayce, J.M.; et al. Focal infrared neural stimulation with high-field functional MRI: A rapid way to map mesoscale brain connectomes. Sci. Adv. 2019, 5, eaau7046. [Google Scholar] [CrossRef]
- Cassano, P.; Petrie, S.R.; Hamblin, M.R.; Henderson, T.A.; Iosifescu, D.V. Review of transcranial photobiomodulation for major depressive disorder: Targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics 2016, 3, 031404. [Google Scholar] [CrossRef]
- Morries, L.D.; Cassano, P.; Henderson, T.A. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat. 2015, 11, 2159. [Google Scholar] [PubMed]
- Tian, F.; Hase, S.N.; Gonzalez-Lima, F.; Liu, H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg. Med. 2016, 48, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Naeser, M.A.; Saltmarche, A.; Krengel, M.H.; Hamblin, M.R.; Knight, J.A. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: Two case reports. Photomed. Laser Surg. 2011, 29, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Zivin, J.A.; Albers, G.W.; Bornstein, N.; Chippendale, T.; Dahlof, B.; Devlin, T.; Fisher, M.; Hacke, W.; Holt, W.; Ilic, S.; et al. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke 2009, 40, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, F.; Johnston, A.L.; Ravichandran, C.; Polcari, A.; Teicher, M.H.; Webb, R.H.; Hamblin, M.R. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: A pilot study of 10 patients with major depression and anxiety. Behav. Brain Funct. 2009, 5, 46. [Google Scholar] [CrossRef]
- Bunce, S.C.; Izzetoglu, M.; Izzetoglu, K.; Onaral, B.; Pourrezaei, K. Functional near-infrared spectroscopy. IEEE Eng. Med. Biol. Mag. 2006, 25, 54–62. [Google Scholar] [CrossRef]
- Wong-Riley, M.T. Bigenomic regulation of cytochrome c oxidase in neurons and the tight coupling between neuronal activity and energy metabolism. In Mitochondrial Oxidative Phosphorylation; Springer: Berlin, Germany, 2012; pp. 283–304. [Google Scholar]
- Von Lühmann, A.; Addesa, J.; Chandra, S.; Das, A.; Hayashibe, M.; Dutta, A. Neural interfacing non-invasive brain stimulation with NIRS-EEG joint imaging for closed-loop control of neuroenergetics in ischemic stroke. In Proceedings of the 8th International IEEE/EMBS Conference on Neural Engineering (NER), Shanghai, China, 25–28 May 2017; pp. 349–353. [Google Scholar]
- Collins, D.L.; Zijdenbos, A.P.; Kollokian, V.; Sled, J.G.; Kabani, N.J.; Holmes, C.J.; Evans, A.C. Design and construction of a realistic digital brain phantom. IEEE Trans. Med. Imaging 1998, 17, 463–468. [Google Scholar] [CrossRef]
- Tran, A.P.; Fang, Q. Fast and high-quality tetrahedral mesh generation from neuroanatomical scans. arXiv 2017, arXiv:1708.08954. [Google Scholar]
- Boissonnat, J.D.; Oudot, S. Provably good sampling and meshing of surfaces. Gr. Models 2005, 67, 405–451. [Google Scholar] [CrossRef]
- Si, H. TetGen, a Delaunay-based quality tetrahedral mesh generator. ACM Trans. Math. Softw. (TOMS) 2015, 41, 11. [Google Scholar] [CrossRef]
- L’Huillier, J.P.; Humeau, A. Use of the finite element method to study photon-tissue interactions in biological media. In Proceedings of the 17th IMACS Congress, Paris, France, 11–15 July 2005. [Google Scholar]
- Liemert, A.; Reitzle, D.; Kienle, A. Analytical solutions of the radiative transport equation for turbid and fluorescent layered media. Sci. Rep. 2017, 7, 3819. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q. Mesh-based Monte Carlo method using fast ray-tracing in Plücker coordinates. Biomed. Opt. Express 2010, 1, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Rasmussen, J.C.; Sevick-Muraca, E.M.; Wareing, T.A.; McGhee, J. Radiative transport-based frequency-domain fluorescence tomography. Phys. Med. Biol. 2008, 53, 2069. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavsky, A.; Schulze, P.; Yaroslavsky, I.; Schober, R.; Ulrich, F.; Schwarzmaier, H. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys. Med. Biol. 2002, 47, 2059. [Google Scholar] [CrossRef] [PubMed]
- Custo, A.; Wells, W.M., III; Barnett, A.H.; Hillman, E.M.; Boas, D.A. Effective scattering coefficient of the cerebral spinal fluid in adult head models for diffuse optical imaging. Appl. Opt. 2006, 45, 4747–4755. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef] [PubMed]
- Valvano, J. Bioheat Transfer Encyclopedia of Medical Devices and Instrumentation; Wiley: Austin, TX, USA, 2005. [Google Scholar]
- Welch, A.J.; Van Gemert, M.J. (Eds.) Optical-Thermal Response of Laser-Irradiated Tissue; Springer: Berlin, Germany, 2011; Volume 2. [Google Scholar]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009, 2, 201–207. [Google Scholar] [CrossRef]
- Datta, A.; Elwassif, M.; Bikson, M. Bio-heat transfer model of transcranial DC stimulation: Comparison of conventional pad versus ring electrode. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 670–673. [Google Scholar]
- Hamblin, M.R.; Demidova, T.N. Mechanisms of low level light therapy. In Mechanisms for Low-Light Therapy; International Society for Optics and Photonics: Bellingham, WA, USA, 2006; Volume 6140, p. 614001. [Google Scholar]
- Passarella, S.; Karu, T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J. Photochem. Photobiol. B Biol. 2014, 140, 344–358. [Google Scholar] [CrossRef]
- Pope, R.M.; Fry, E.S. Absorption spectrum (380–700 nm) of pure water. II. Integrating cavity measurements. Appl. Opt. 1997, 36, 8710–8723. [Google Scholar] [CrossRef]
- Van Veen, R.L.; Sterenborg, H.; Pifferi, A.; Torricelli, A.; Cubeddu, R. Determination of VIS-NIR absorption coefficients of mammalian fat, with time-and spatially resolved diffuse reflectance and transmission spectroscopy. In Biomedical Topical Meeting; Optical Society of America: Washington, DC, USA, 2004; p. SF4. [Google Scholar]
- Mason, M.G.; Nicholls, P.; Cooper, C.E. Re-evaluation of the near infrared spectra of mitochondrial cytochrome c oxidase: Implications for non invasive in vivo monitoring of tissues. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 1882–1891. [Google Scholar] [CrossRef]
- Prahl, S. Tabulated Molar Extinction Coefficient for Hemoglobin in Water. 1999. Available online: http://omlc.ogi.edu/spectra/hemoglobin/summary.html (accessed on 10 June 2019).
- Smith, R.C.; Baker, K.S. Optical properties of the clearest natural waters (200–800 nm). Appl. Opt. 1981, 20, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sogandares, F.M. The Spectral Absorption of Pure Water. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1991. [Google Scholar]
- Shi, L.; Sordillo, L.A.; Rodríguez-Contreras, A.; Alfano, R. Transmission in near-infrared optical windows for deep brain imaging. J. Biophotonics 2016, 9, 38–43. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.S.; Sampson, E.L. Lipid composition of the normal human brain: Gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 537–544. [Google Scholar] [PubMed]
- Hamberg, L.M.; Hunter, G.J.; Kierstead, D.; Lo, E.H.; González, R.G.; Wolf, G.L. Measurement of cerebral blood volume with subtraction three-dimensional functional CT. Am. J. Neuroradiol. 1996, 17, 1861–1869. [Google Scholar] [PubMed]
- Prahl, S. Optical Absorption of Hemoglobin. 1999. Available online: http://omlc.ogi.edu/spectra/hemoglobin (accessed on 10 June 2019).
- Johansson, J.D.; Wårdell, K. Intracerebral quantitative chromophore estimation from reflectance spectra captured during deep brain stimulation implantation. J. Biophotonics 2013, 6, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Boas, D.A. Monte Carlo simulation of photon migration in 3D turbid media accelerated by graphics processing units. Opt. Express 2009, 17, 20178–20190. [Google Scholar] [CrossRef] [PubMed]
- Arridge, S.; Schweiger, M.; Hiraoka, M.; Delpy, D. A finite element approach for modeling photon transport in tissue. Med. Phys. 1993, 20, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Normoyle, K.P.; Jackson, K.; Spitler, K.; Sharrock, M.F.; Miller, C.M.; Best, C.; Llano, D.; Du, R. Brain temperature and its fundamental properties: a review for clinical neuroscientists. Front. Neurosci. 2014, 8, 307. [Google Scholar] [CrossRef]
- Brooks, V.B. Study of brain function by local, reversible cooling. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin, Germany, 1983; Volume 95, pp. 1–109. [Google Scholar]
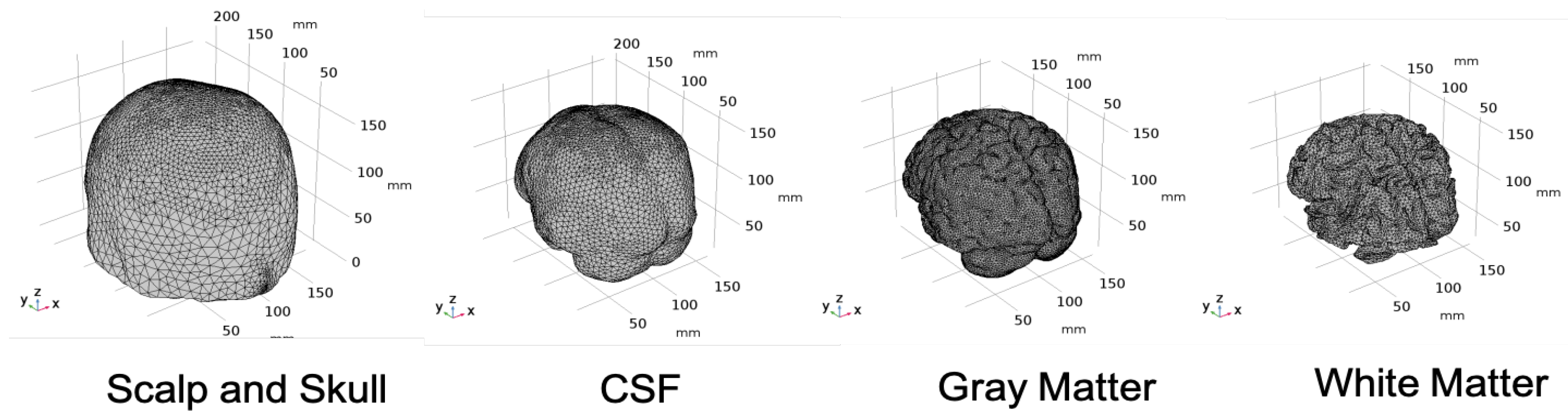
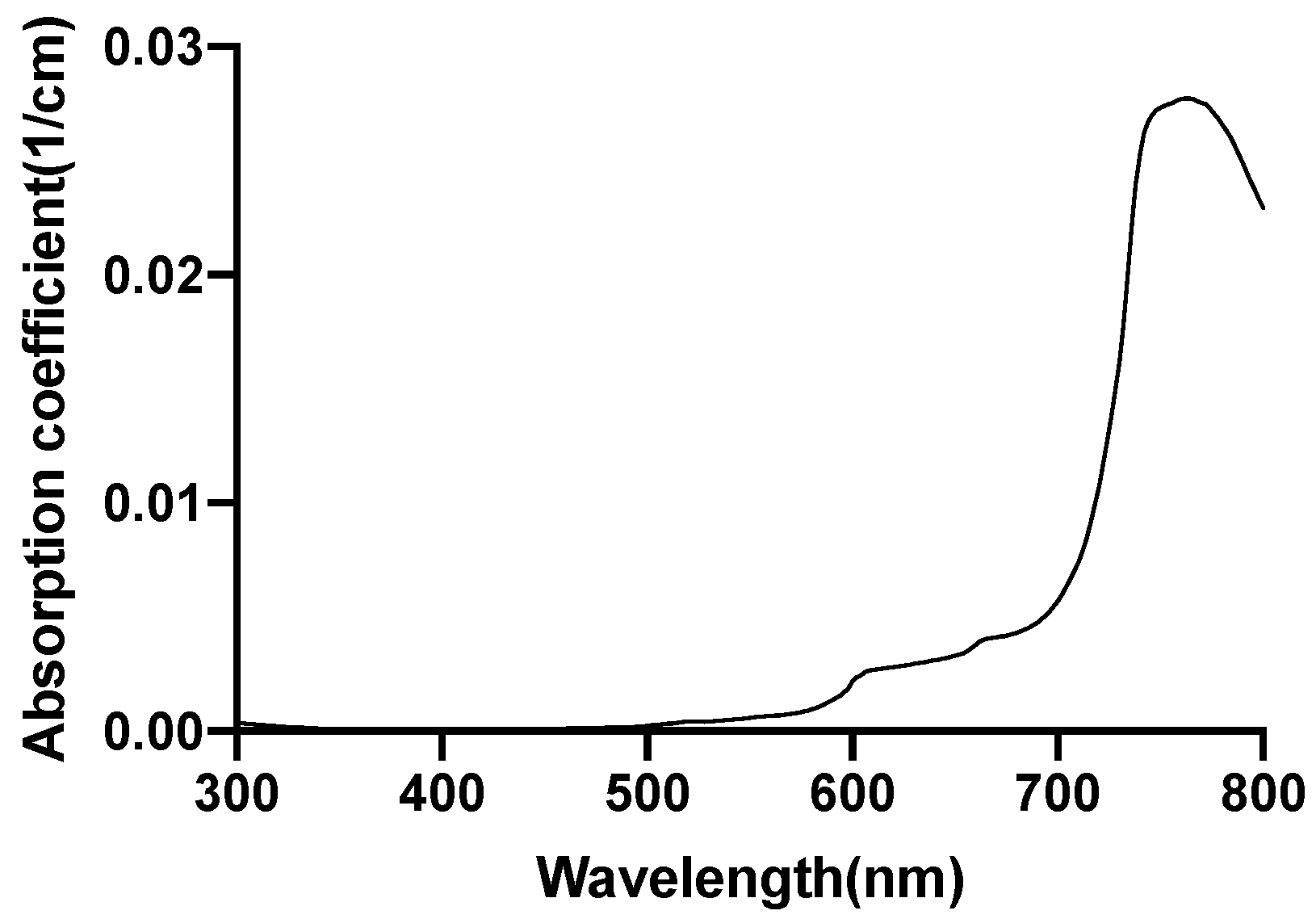
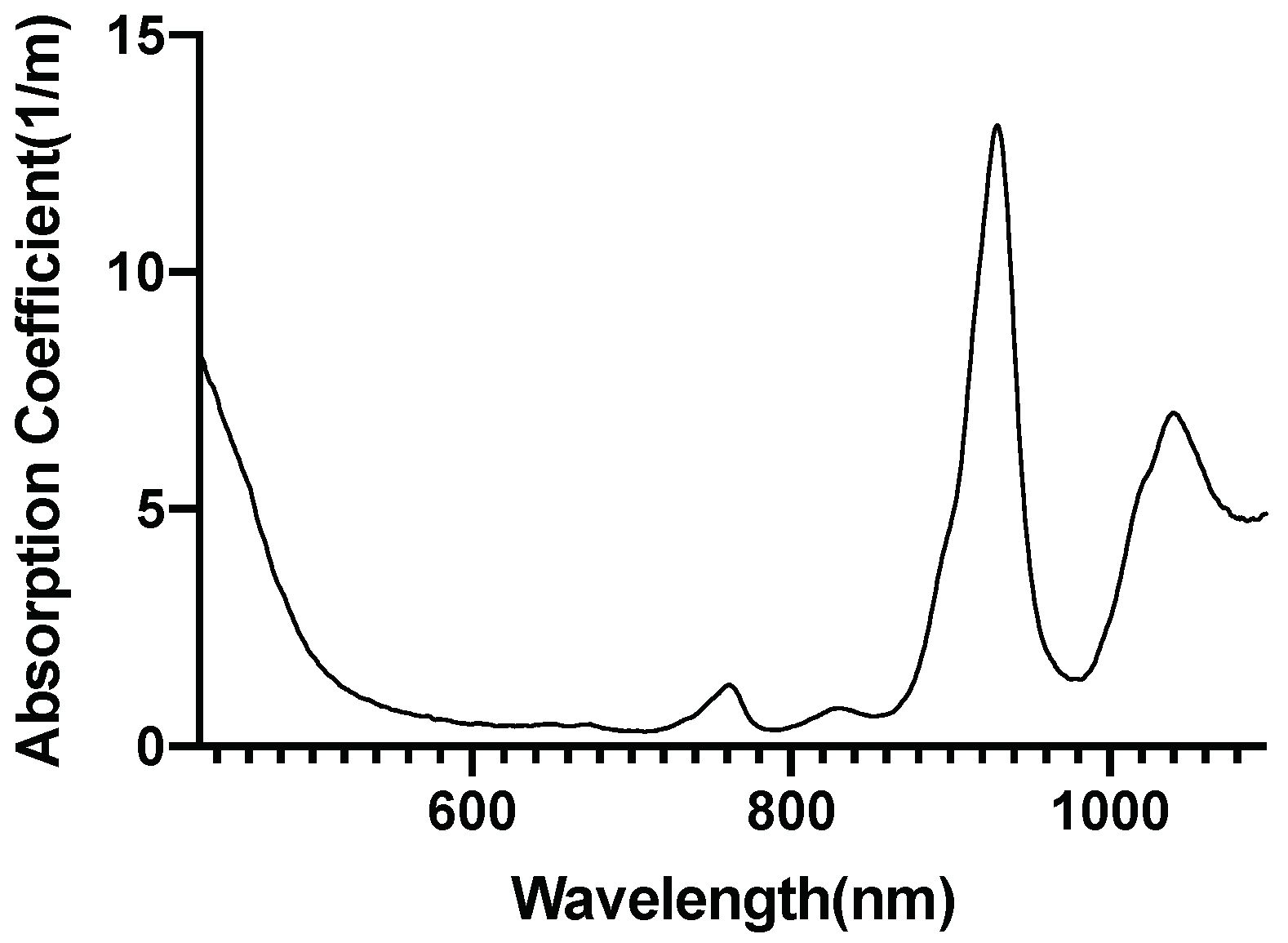
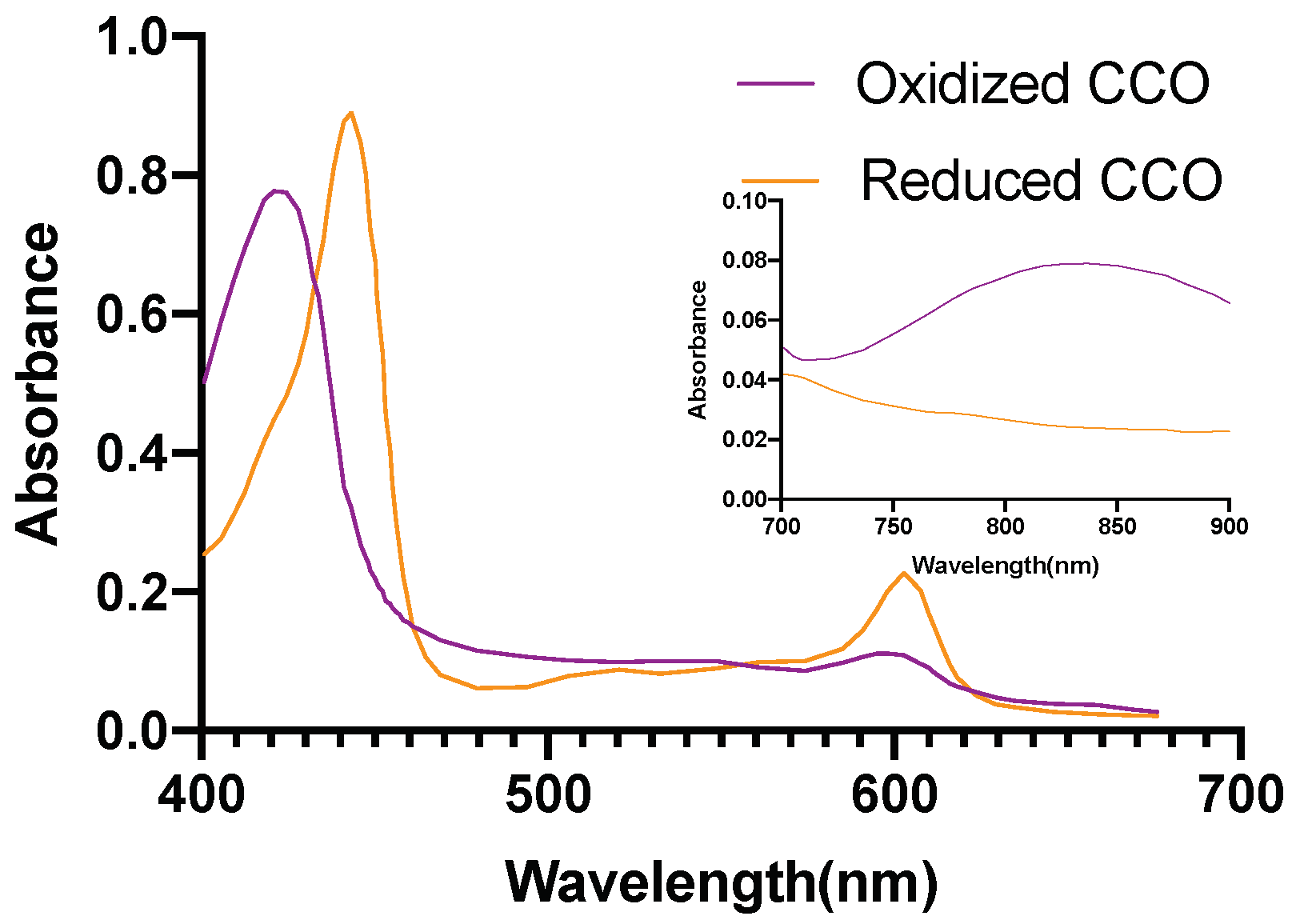
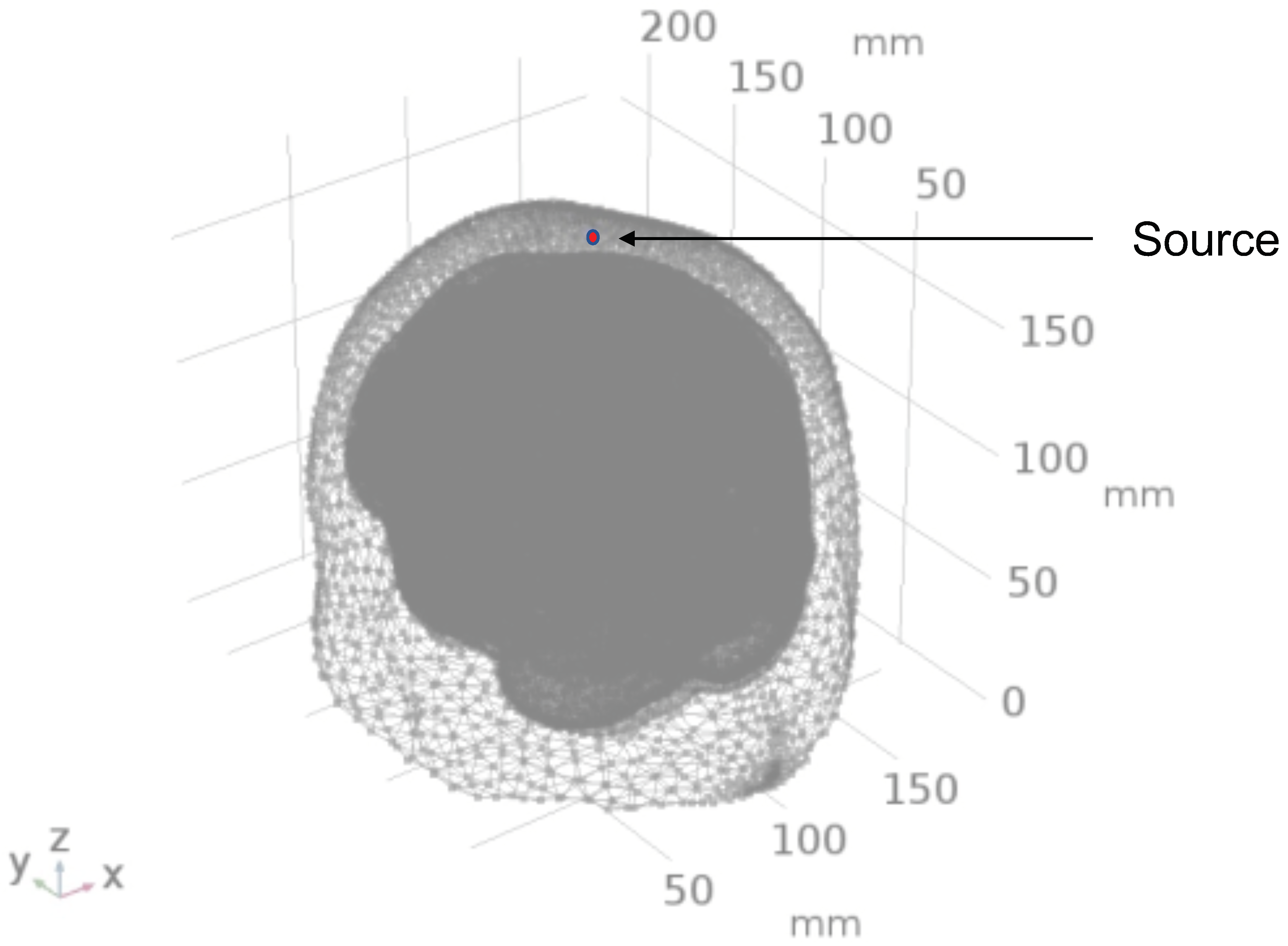

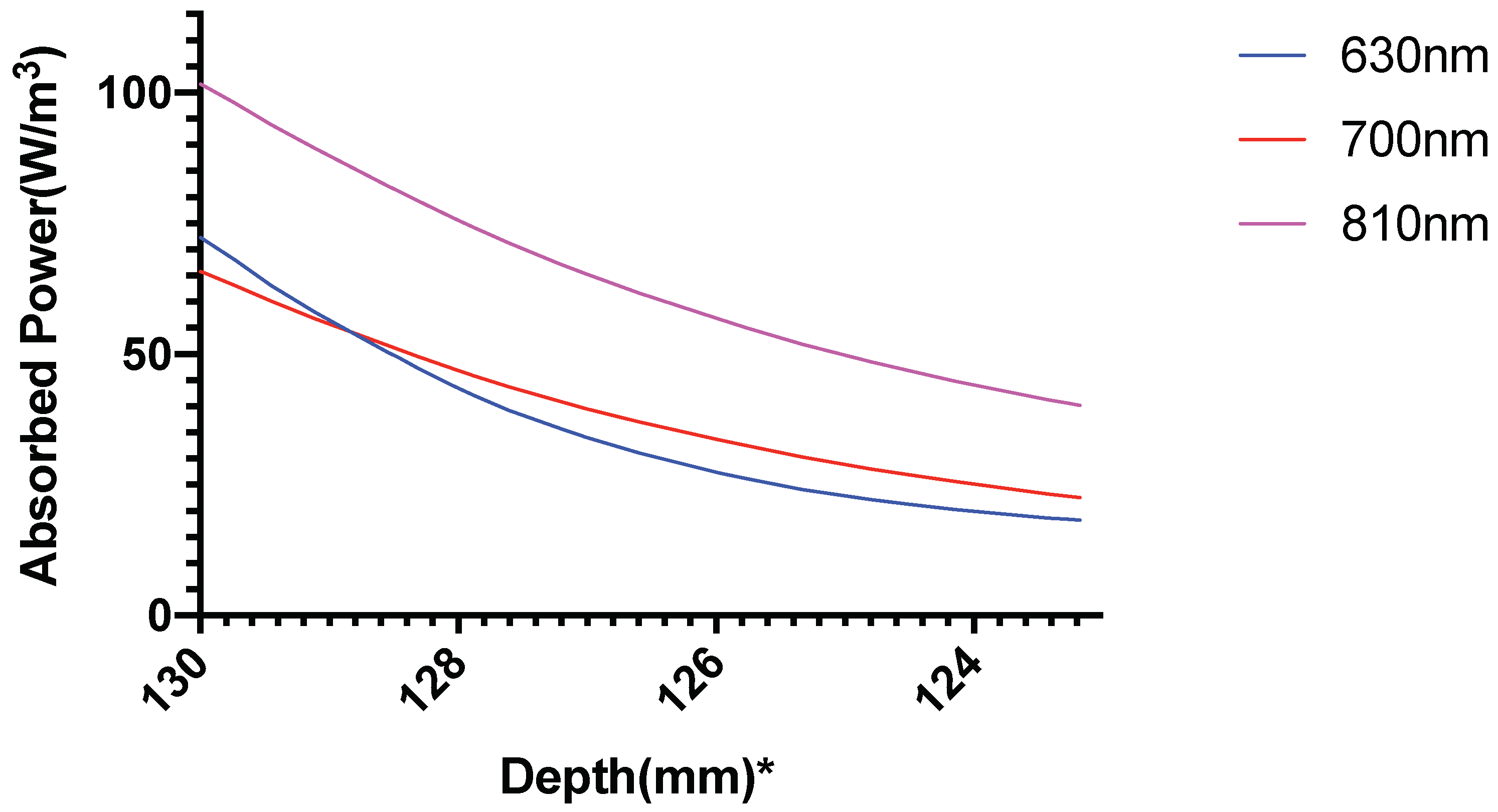
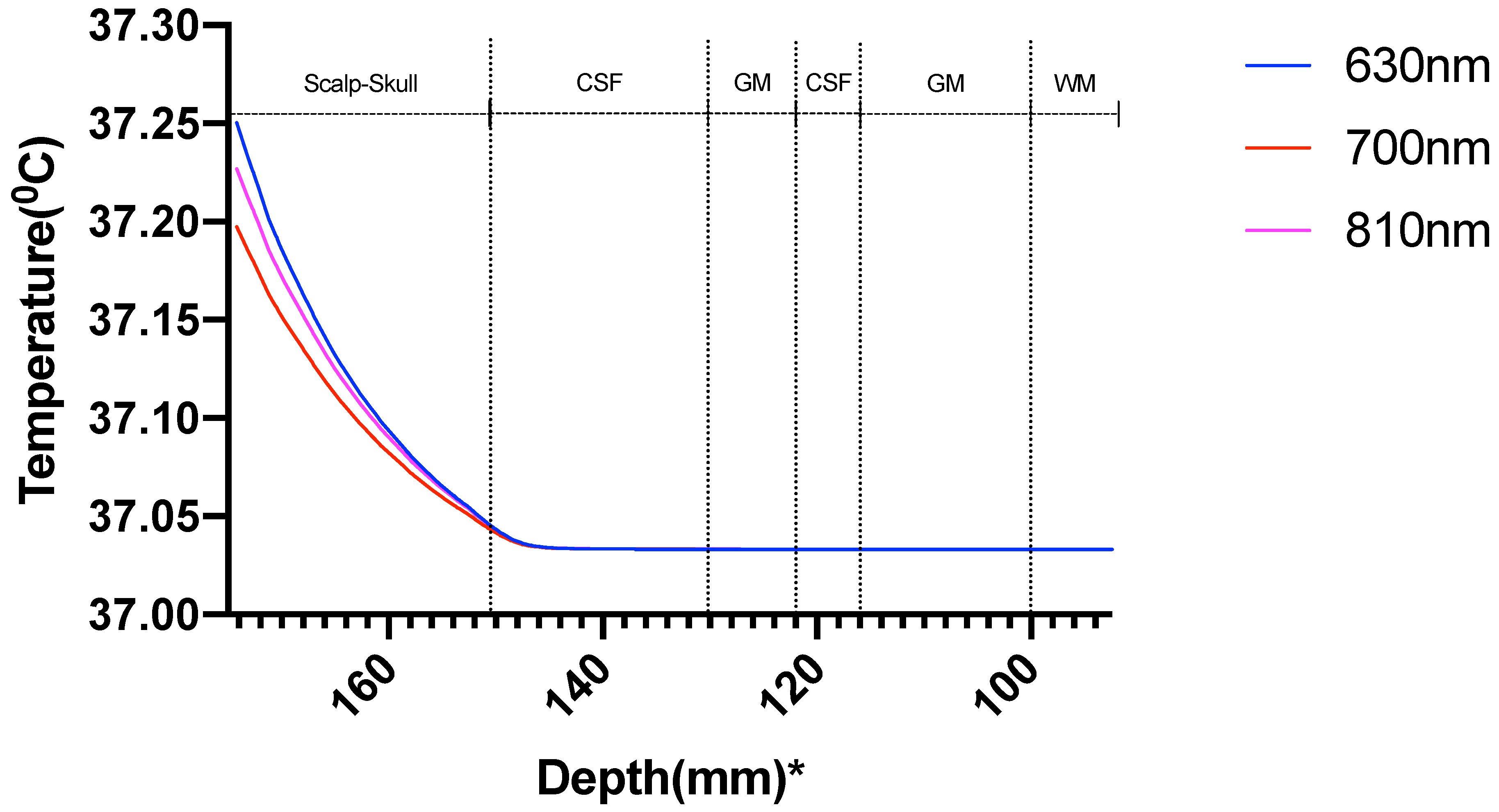
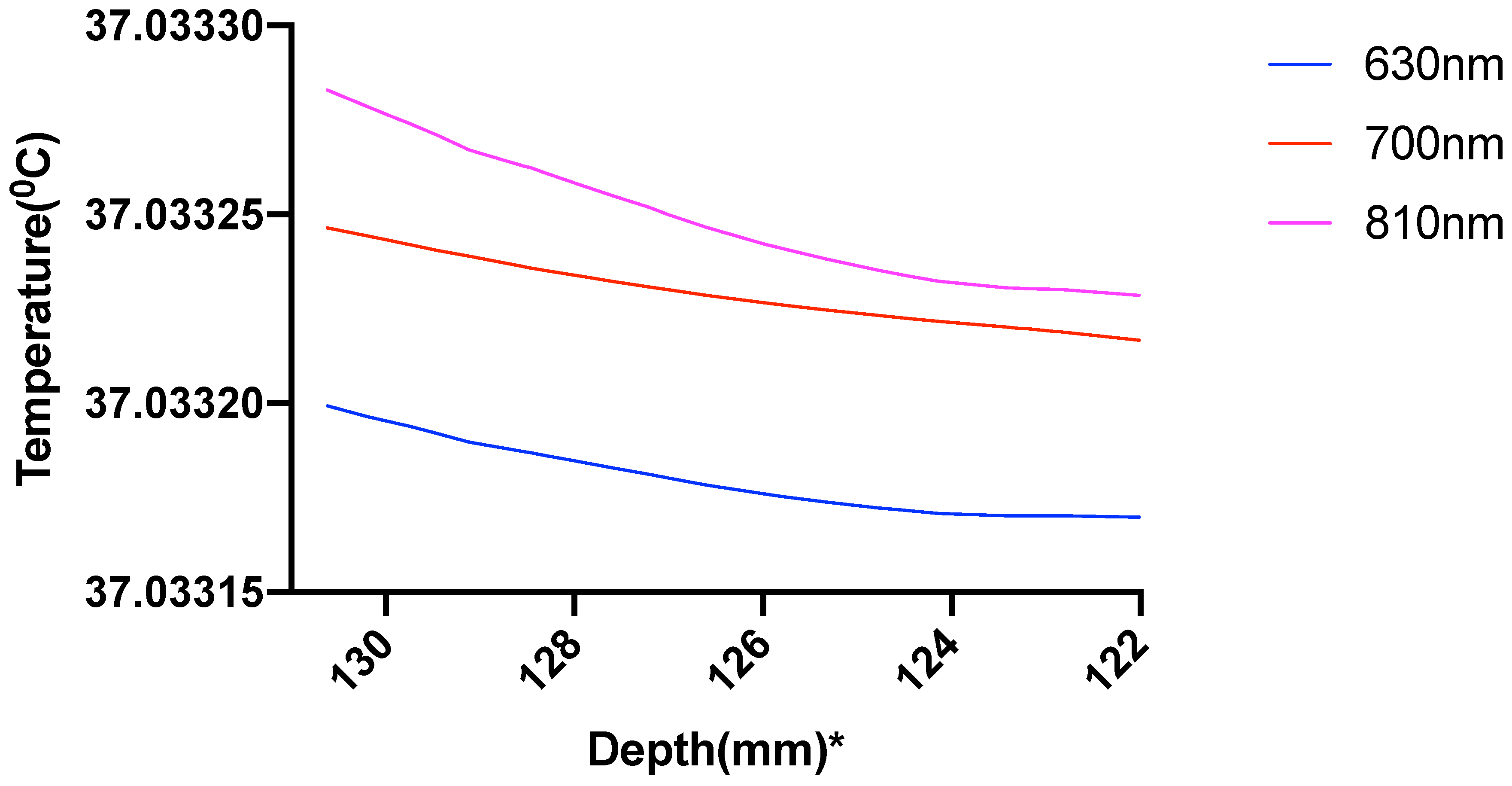
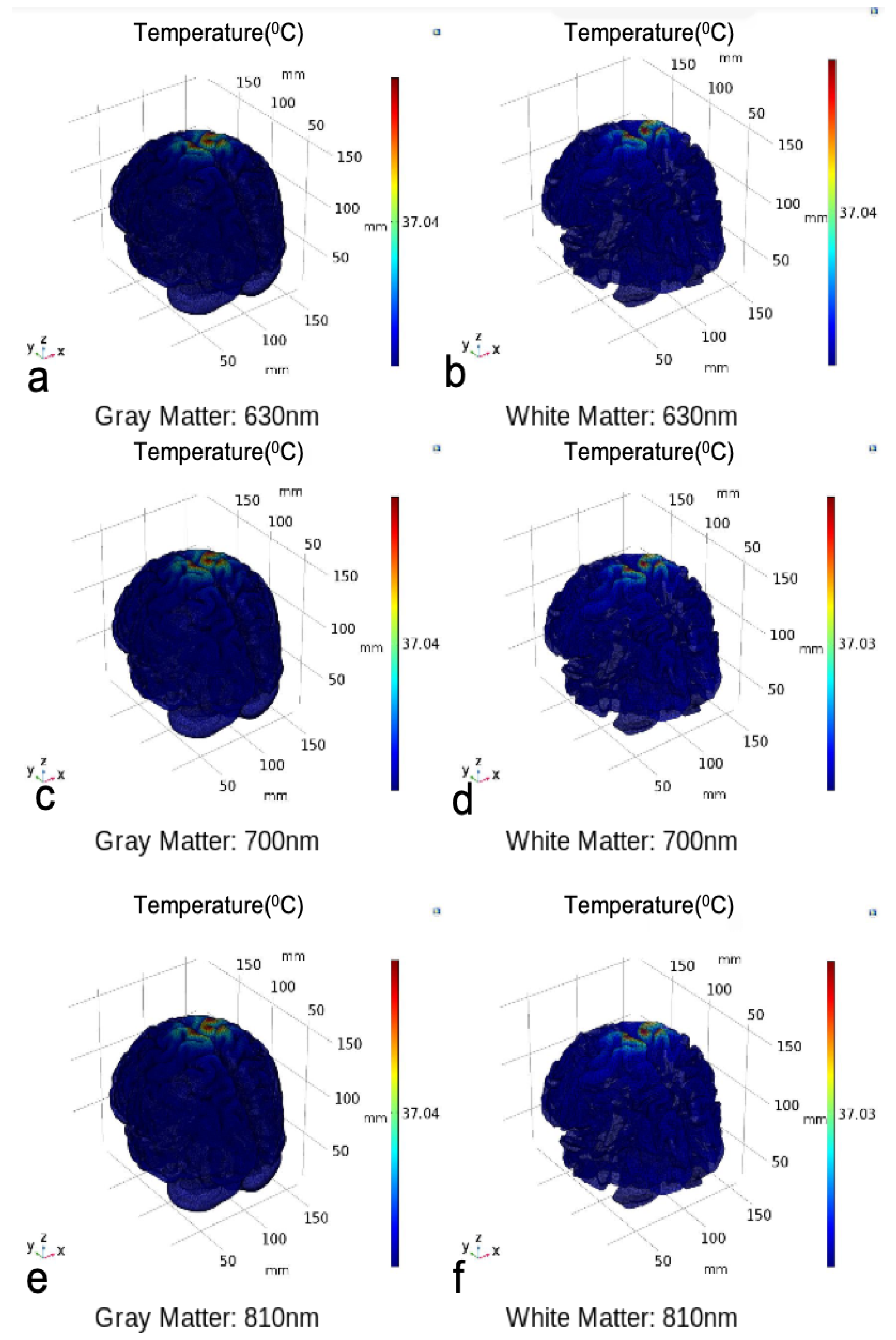
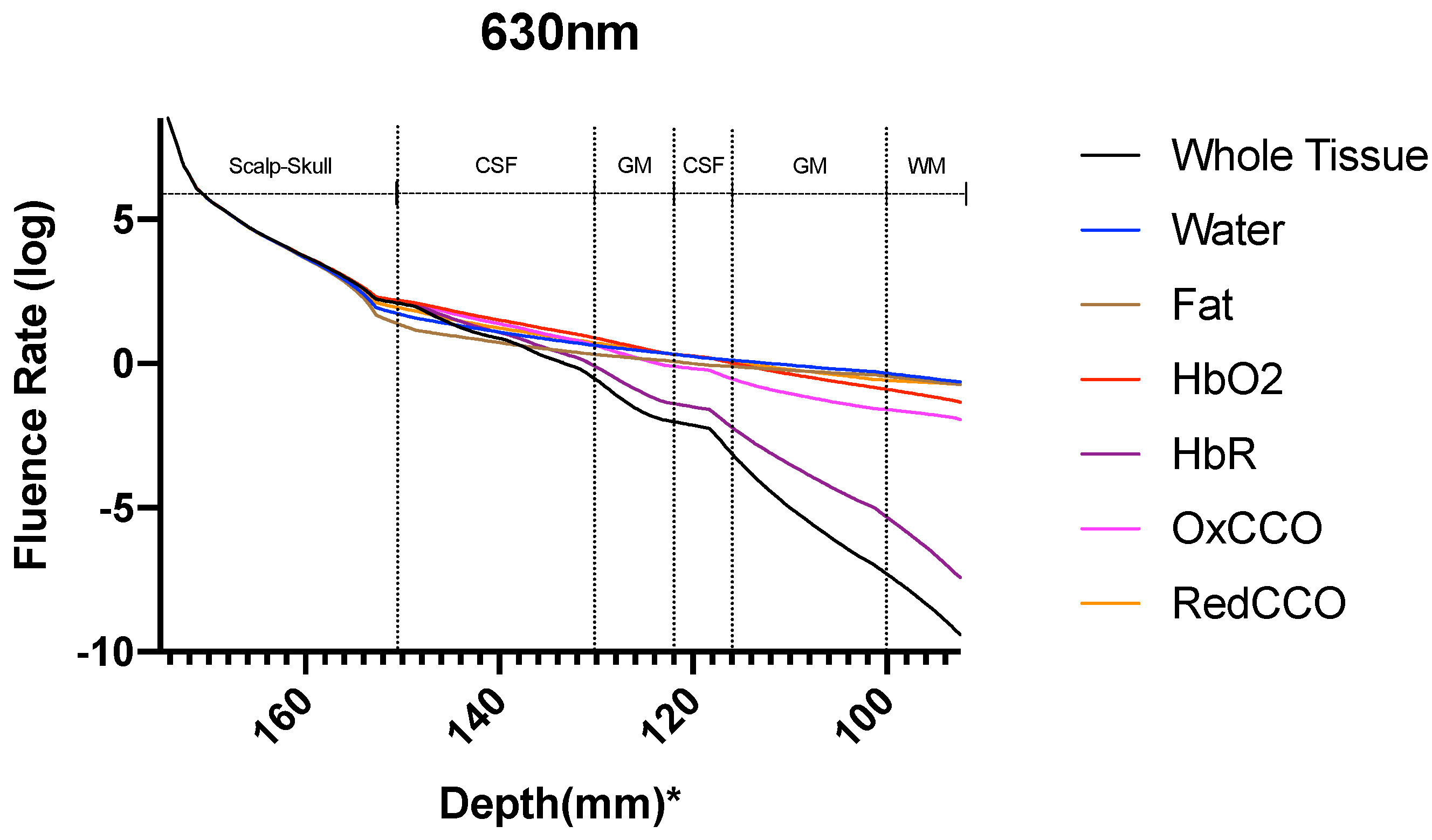
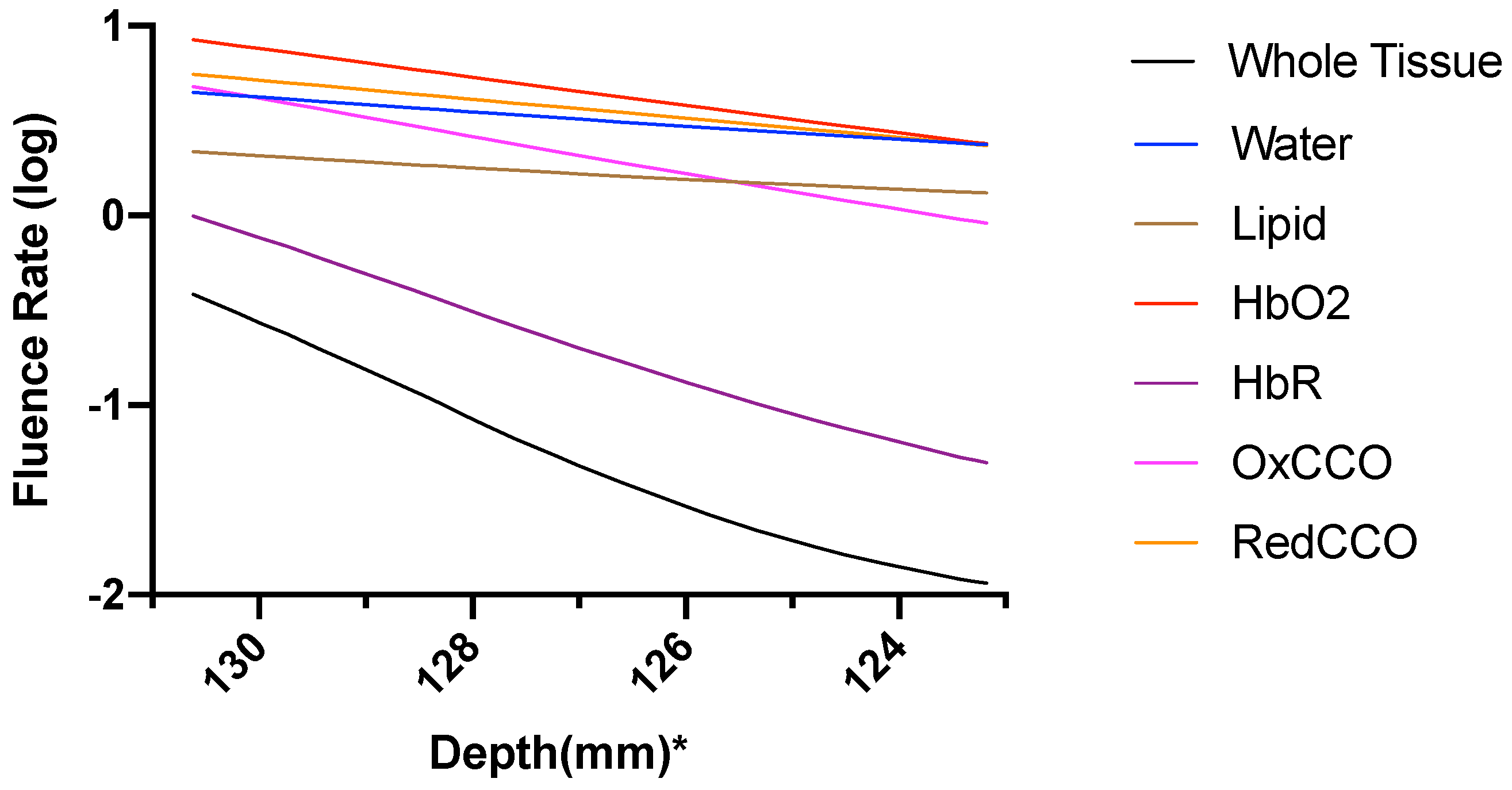
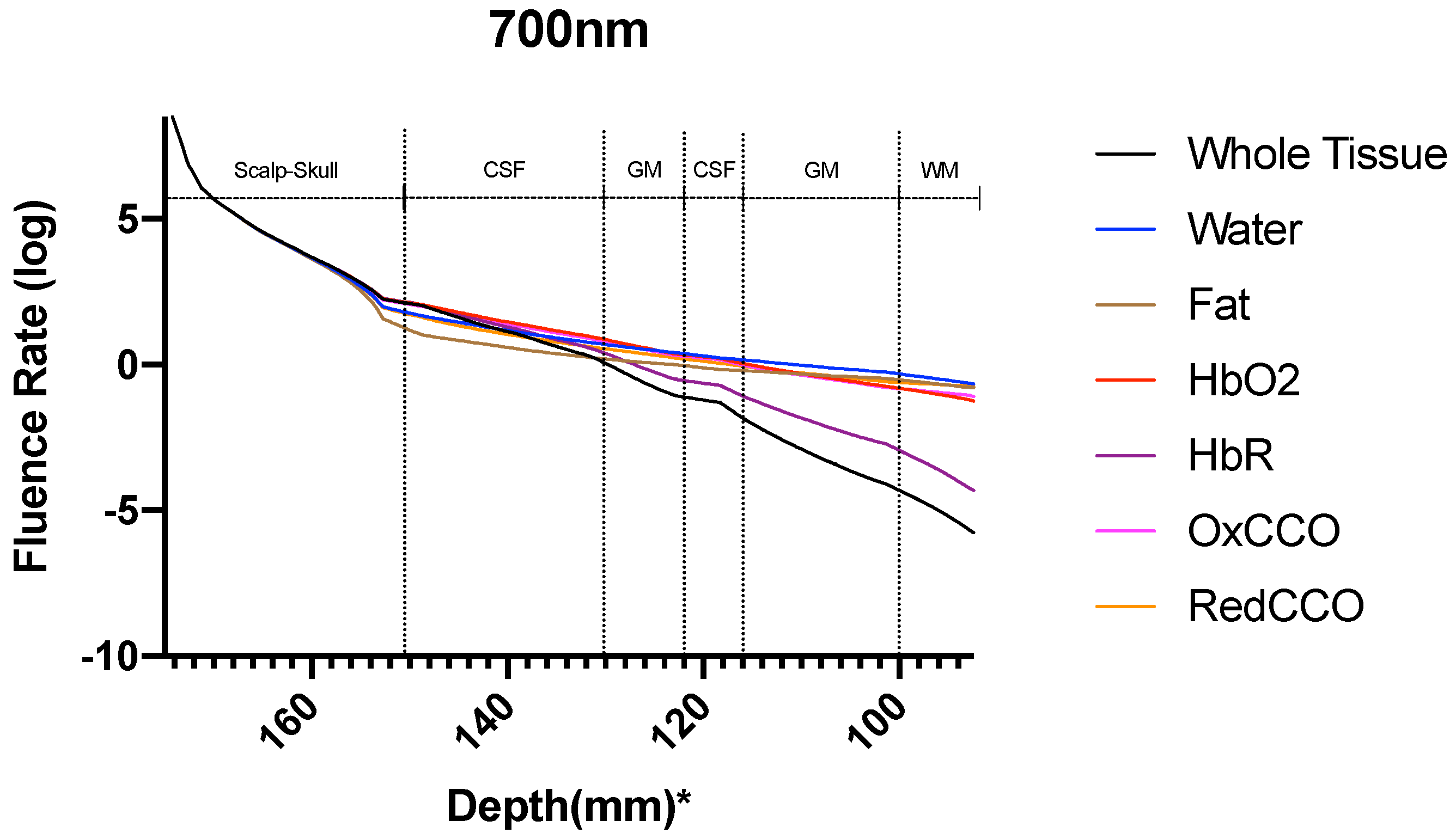
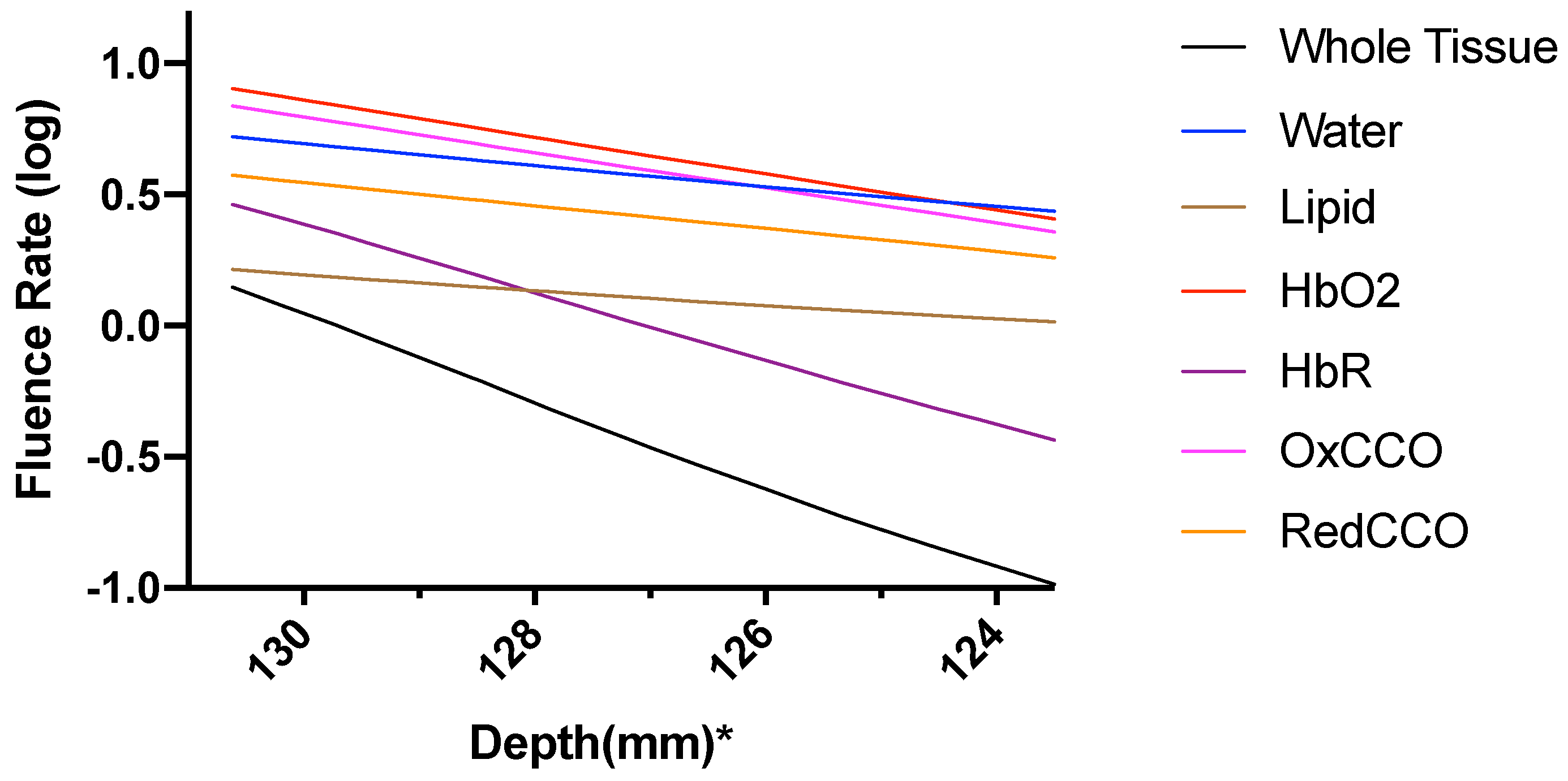
| Mesh Components | Number of Mesh Components | Aspect Ratio |
|---|---|---|
| Nodes | 58,131 | —– |
| Triangular Faces | 166,792 | 0.0003 |
| Tetrahedral Elements | 917,075 | 0.0000044 |
| Domains | Absorption Coefficient () | Reduced Scattering Coefficient () | ||||
|---|---|---|---|---|---|---|
| 630 nm | 700 nm | 810 nm | 630 nm | 700 nm | 810 nm | |
| Scalp and Skull | 19 | 13 | 16 | 858 | 900 | 760 |
| Cerebrospinal Fluid | 4 | 4 | 2.6 | 250 | 250 | 250 |
| Gray Matter | 127.25 | 62.91 | 57.09 | 990 | 880 | 746 |
| White Matter | 66.11 | 32.52 | 20.77 | 4400 | 4356 | 4070 |
| Tissues | Thermal Conductivity (W/m.°C) | Density () | Metabolic Heat () |
|---|---|---|---|
| Scalp | 0.342 | 1100 | 363 |
| Skull | 1.15 | 1990 | 70 |
| CSF | 0.61 | 0 | 0 |
| Brain | 0.57 | 0.08 | 10,437 |
| Tissues | Blood Specific Heat () | Blood Perfusion (1/s) | Blood Density () | Metabolic Heat Source () |
|---|---|---|---|---|
| Scalp | 3600 | 0.00143 | 1050 | 363 |
| Skull | 3600 | 0.000143 | 1050 | 70 |
| Cerebrospinal Fluid | 3600 | 0 | 1050 | 0 |
| Brain | 3600 | 0.08 | 1050 | 10,437 |
| Components | Absorption Coefficient () | |||||
|---|---|---|---|---|---|---|
| Gray Matter | White Matter | |||||
| 630 nm | 700 nm | 810 nm | 630 nm | 700 nm | 810 nm | |
| Water | 0.26 | 0.5 | 1.66 | 0.23 | 0.43 | 1.45 |
| Fat | 0.04 | 0.03 | 0.05 | 0.08 | 0.06 | 0.1 |
| Oxyhemoglobin | 14.06 | 12.17 | 22.44 | 3.37 | 2.92 | 5.38 |
| Deoxyhemoglobin | 73 | 34.2 | 15.48 | 59.92 | 28.11 | 12.72 |
| Oxidized Cytochrome c Oxidase | 35.64 | 14.04 | 16.2 | 2.34 | 0.9 | 1.1 |
| Reduced Cytochrome c Oxidase | 4.25 | 1.97 | 1.26 | 0.13 | 0.06 | 0.04 |
| Components | Absorption Coefficient () | |||||
|---|---|---|---|---|---|---|
| Gray Matter | White Matter | |||||
| 630 nm | 700 nm | 810 nm | 630 nm | 700 nm | 810 nm | |
| Whole Tissue | 127.25 | 62.91 | 57.09 | 66.11 | 32.52 | 20.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharya, M.; Dutta, A. Computational Modeling of the Photon Transport, Tissue Heating, and Cytochrome C Oxidase Absorption during Transcranial Near-Infrared Stimulation. Brain Sci. 2019, 9, 179. https://doi.org/10.3390/brainsci9080179
Bhattacharya M, Dutta A. Computational Modeling of the Photon Transport, Tissue Heating, and Cytochrome C Oxidase Absorption during Transcranial Near-Infrared Stimulation. Brain Sciences. 2019; 9(8):179. https://doi.org/10.3390/brainsci9080179
Chicago/Turabian StyleBhattacharya, Mahasweta, and Anirban Dutta. 2019. "Computational Modeling of the Photon Transport, Tissue Heating, and Cytochrome C Oxidase Absorption during Transcranial Near-Infrared Stimulation" Brain Sciences 9, no. 8: 179. https://doi.org/10.3390/brainsci9080179
APA StyleBhattacharya, M., & Dutta, A. (2019). Computational Modeling of the Photon Transport, Tissue Heating, and Cytochrome C Oxidase Absorption during Transcranial Near-Infrared Stimulation. Brain Sciences, 9(8), 179. https://doi.org/10.3390/brainsci9080179






